Abstract
Background
Nitrous oxide (N2O, laughing gas) is increasingly used as a recreational drug and is presumed relatively safe and innocent. It is often being used in combination with other substances, such as cannabis.
Case summary
A young adult attended the emergency room because of chest pain after recreational use of very high-dose nitrous oxide in combination with cannabis. Electrocardiography demonstrated ST-elevation in the anterior leads. Coronary angiography showed thrombus in the proximal and thrombotic occlusion of the distal left anterior descending coronary artery for which primary percutaneous coronary intervention was attempted. Thrombus aspiration was unsuccessful and the patient was further treated with a glycoprotein IIb/IIIa in addition to dual platelet therapy. Blood results showed low vitamin B12 and folic acid status with concomitant hyperhomocysteinaemia, a known cause of hypercoagulation. Transthoracic echocardiogram showed a moderately reduced left ventricular ejection fraction (LVEF). Three months later, an improvement in LVEF and no recurrent angina or symptoms of heart failure were noticed.
Discussion
We report a case of acute myocardial infarction secondary to very high-dose nitrous oxide abuse in combination with cannabis and possible hypoxia. We propose that severe hyperhomocysteinaemia secondary to nitrous oxide-induced vitamin B12 deficiency together with the vasoconstrictive effects of cannabis might pose a seriously increased risk for intracoronary, among others, thrombus formation. In conclusion, we contest the safety and innocence of recreational nitrous oxide (ab)use, notably in the context of other factors increasing the risk of coagulation.
Keywords: Nitrous oxide, Acute myocardial infarction, Hyperhomocysteinaemia, Case report
Learning points
Nitrous oxide is increasingly used as a recreational drug.
Nitrous oxide abuse can lead to hyperhomocysteinaemia due to irreversible inactivation of vitamin B12.
Hyperhomocysteinaemia is prothrombotic and can lead to arterial thrombosis causing acute myocardial infarction.
Introduction
Nitrous oxide (N2O) is known to humans since at least 1772, when Joseph Priestly was accredited to be the first to synthesize the gas. Its analgesic action was first described in 1800.1 Ever since, it has been used for various purposes ranging from medical use because of its analgesic properties, to its application as a mixing and foaming agent in the food industry, and finally for recreational use due to its hallucinogenic, euphoric, and relaxant properties. This led to the introduction of the term laughing gas.1,2 There has been a recent surge in the recreational appreciation of N2O, also known as ‘Hippy Crack’, its use equalling the use of ecstasy, cocaine, and ketamine.2 The increase of its use is likely to be related to its wide availability, rapid action, short duration, and presumed innocence. Yet, it leads to accumulation of serum homocysteine which is known to be prothrombotic and to impair fibrinolysis.3–5 N2O is often used in combination with other drugs, such as cannabis, which is known for its vasospastic properties, increasing the risks for cardiovascular events.6 We describe a case of acute myocardial infarction (MI) secondary to intoxication with N2O and cannabis.
Timeline
| T minus 24–48 h |
|
| T minus 12 h |
|
| T 0 |
|
| T plus 24 h |
|
| T plus 5 days |
|
| T plus 2 months (outpatient setting) |
|
Case presentation
A 27-year-old man of North African descent attended the emergency room of our tertiary percutaneous coronary intervention centre because of chest pain. Since 12 h, he had experienced severe retrosternal chest pain with sweating, nausea, and vomiting. Electrocardiography demonstrated ST-elevation in the anterior leads (Figure 1). Detailed history revealed that in the hours prior to presentation, he had been recreationally using high amounts of N2O by inhalation from balloons. For the last 24–48 h, he had been sleep-deprived and, while sitting in a car, he had inhaled approximately 3.3 kg (or 420 balloons) of N2O and smoked 4 joints of cannabis. The patient had not experienced recent trauma, had no co-morbidities and was not under any prescription. He had never experienced chest pain before, there were no signs of acute heart failure. Besides smoking nicotine and cannabis, he did not have any other cardiovascular risk factors. There was no family history of coronary disease or sudden cardiac death. Drug screening upon presentation was positive for tetrahydrocannabinol (THC). Patient admitted to regularly smoke cannabis but denied consumption of alcohol or any other recreational drugs.
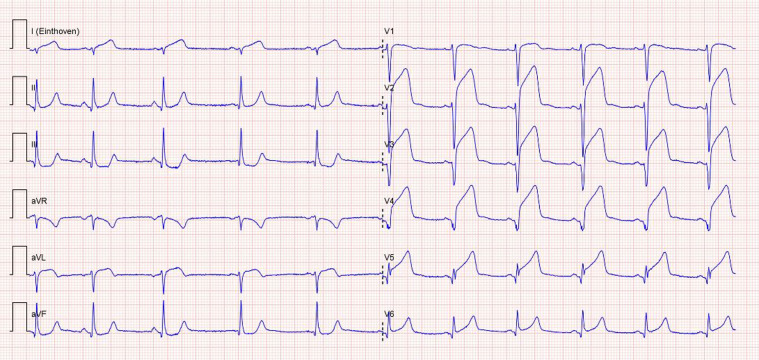
Figure 1.
Electrocardiogram at admission showing sinus rhythm with elevated ST-segments in the anterior leads (V2–V6) with reciprocal ST-depression in the inferior leads (II, III, and aVF), indicating anteroseptal myocardial infarction.
As per hospital protocol, he received 180 mg ticagrelor orally, 300 mg aspirin intravenously (i.v.) and 5000 EH heparin i.v. and was referred for immediate coronary angiography (CAG). This showed a normal aspect of the left main, right coronary and circumflex artery. The distal left anterior descending artery (LAD) was totally occluded, and thrombus was angiographically visible in the proximal segment, with maintained flow (TIMI 3) (Figure 2A and Supplementary material online, Movies S1 and S2). Despite successful wire passage into the distal LAD and multiple balloon dilatations, no recanalization was achieved. Therefore, mechanical thrombectomy by means of AngioJet was attempted without distal recanalization, nor angiographical resolution of the proximal thrombus. Optical coherence tomography of the proximal LAD confirmed the presence of residual thrombus but excluded atherosclerotic media thickening. Plaque/endothelial erosion underneath the thrombus could not be excluded (Figure 2B and Supplementary material online, Movie S3). Because of a minimal lumen area of 10.75 mm2 no further intervention was undertaken. The procedure was discontinued, and the patient received tirofiban i.v. for 48 h, on top of standard dual antiplatelet therapy (DAPT: ticagrelor and aspirin).
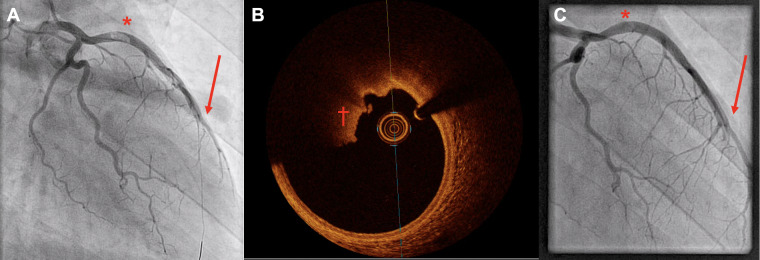
Figure 2.
(A) Primary coronary angiogram (left anterior oblique cranial view) showing thrombus in the proximal left anterior descending artery (*). Guidewire in the distal left anterior descending artery; however, it remains occluded (arrow). (B) Optical coherence tomography during re-coronary angiography showing thrombus approximately 90° (†), without evidence of further endothelial damage. (C) Re-coronary angiography (left anterior oblique cranial view) 5 days later with still evidence of thrombus in the proximal left anterior descending artery (*) and occluded distal left anterior descending artery (arrow).
The first high-sensitive troponin T and CK-MB levels were 0.418 μg/L and 77 μg/L, peaking at 5.35 μg/L (upper limit of reference range: 0.014 μg/L) and >300 μg/L (upper limit of reference range: 7.6 μg/L). Transthoracic echocardiogram (TTE) showed a reduced (30–40%) left ventricular ejection fraction (LVEF) with apical and mid septal akinesia, a normal right ventricular function and no valvular abnormalities (Supplementary material online, Movie S4). Migration of a venous thromboembolism due to intracardiac right-left shunt was excluded by using right-sided contrast. Vitamin B12 (114 pmol/L; reference range: 160–700 pmol/L), folic acid (7.7 nmol/L; 8.8–40 nmol/L), and homocysteine (205 μmol/L; <15 μmol/L) levels were measured upon admission.
After 5 days, serum homocysteine levels had decreased to 86 μg/L. Additional analysis excluded the presence of a methylenetetrahydrofolate reductase (MTHFR)-gene polymorphism. To evaluate the effect of the initiated antiplatelet therapy CAG was repeated after 7 days. The size of the thrombus in the proximal LAD was slightly reduced, but the distal LAD remained occluded. (Figure 2C and Supplementary material online, Movie S5). After initiation of heart failure medication, he was discharged with the advice to take DAPT for 1 year, discontinue drug abuse and take oral folic acid and vitamin B12 supplementation. Upon discharge persistent ST-elevation with negative T- (V2–V6) and pathological Q-waves (V2–V6) in the anterior leads was shown on the electrocardiogram (Figure 3).
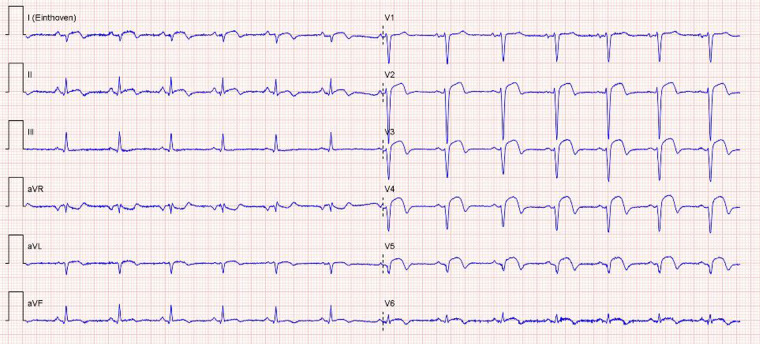
Figure 3.
Electrocardiogram at discharge showing sinus rhythm with pathological Q- and negative T-waves (V2–V6) in anterior leads, consisted with normal electrocardiogram evolution after anteroseptal myocardial infarction.
After 3 months, a repeat TTE showed improvement of LVEF (53%), with remaining anteroseptal wall motion abnormalities (Supplementary material online, Movie S6). He did not report recurrent angina or symptoms of heart failure.
Discussion
We describe a case of acute MI after chronic and high-dose recreational N2O inhalation in combination with cannabis. As proposed 173 years ago by Rudolf Virchow, a triad of stasis, endothelial dysfunction and hypercoagulability predisposes to thrombosis. Our patient fulfilled this triad.
N2O has been shown to interfere with the methionine and folate cycles by directly inhibiting methionine synthase due to irreversible inactivation of its co-enzyme vitamin B12, with consequent increase of homocysteine.7 Because no MTHFR-mutation was found and folic acid status was only slightly reduced, N2O seems the most plausible explanation for the low B12 and high serum homocysteine levels. Homocysteine has been linked to cardiovascular disease due to reduced vascular elasticity, endothelial dysfunction/inflammation with increased uptake of ox-LDL and foam cell production, and to platelet and coagulation activation, impaired fibrinolysis and consequently to accelerated atherosclerosis and thrombosis.3–5,8 Data from the Multi‐ethnic Study of Atherosclerosis (MESA) cohort recently showed that high homocysteine levels are associated with increased incidence and progression of coronary artery calcium score.9 Moreover, elevated plasma homocysteine levels were shown to induce dysfibrinogenaemia and the formation of clots that are abnormally resistant to fibrinolysis.3 A few cases of thromboembolism in the setting of nitrous oxide use and abuse have been described earlier (Table 1) with at least two cases11,12 of arterial thrombus formation in the setting of hyperhomocysteinaemia (65 μmol/L and >50 μmol/L respectively). In our case, we decided to treat the thrombus with DAPT and added tirofiban, a glycoprotein IIb/IIIa inhibitor. This is in line with our standard of care in case of MI with high thrombus load and/or distal embolization. In retrospect and taking other cases11,12 of successful thrombus dissolution into account it might be argued that anticoagulation therapy would have been a better choice in the setting of hyperhomocysteinaemia. Future studies might resolve this question.
Table 1.
Reported cases of thromboembolism after nitrous oxide inhalation
| Author(s) | Faguer et al.10 | Faguer et al.10 | Indraratna et al.11 | den Uil et al.12 | Sun et al.13 | Pratt et al.14 | Molina et al.15 | Current study |
|---|---|---|---|---|---|---|---|---|
| Year | 2016 | 2016 | 2017 | 2018 | 2019 | 2020 | 2020 | — |
| Location | DVT | DVT | Coronary | Aortic | DVT and PE | DVTa | DVT and PE | Coronary |
| Sex | F | F | M | M | M | F | M | M |
| Age | 36 | 30 | 28 | 32 | 29 | 21 | 23 | 27 |
| N2O-use | Acuteb | Acuteb | Acute | Chronic | Chronic | Acute on chronic | Acute | Acute on chronic |
| Medication | Prophylactic LMWH | Prophylactic LMWH | None | None | Vitamin B12 | None | None | None |
| Other drugs | Not reported | Not reported | None | None | None | None | Not reported | Cannabis |
| Tobacco | Not reported | Not reported | Active smoker | No | Active smoker | Active smoker | Not reported | Active smoker |
| Homocysteine level (μmol/L)c | 153 | 99 | 65 | >55 | 24 | >65 | 104 | 205 |
| Vitamin B12 level (pmol/L)d | Normal | Normal | Unknown | 116e | 734 | Normal | <150 | 114 |
| Folic acid level (nmol/L)f | Normal | Normal | Unknown | Unknown | 14.4 | Normal | Unknown | 7.7 |
| MTHFR-polymorphism | Unknown | Unknown | Considered unlikely | Unknown | Unknown | Yes, homozygous | Unknown | No |
| Platelet aggregation | None | None | Aspirin + clopidogrel | Clopidogrel | None | None | None | Aspirin + ticagrelor + tirofiban |
| Anticoagulation | Therapeutic enoxaparin | Intravenous thrombolysis | Tenectepase + enoxaparin | Continuous heparin, warfarin, rivaroxaban | Alteplase + ‘anticoagulant’ | Enoxaparin | tPA | Bolus heparin |
| Supplementation started | None reported | None reported | None reported | None | Folic acid | Cobalamine + folic acid | None reported | Cobalamine + folic acid |
| Remarks | — | — | — | — | — | Pregnant | COVID-19 + | — |
Involving the cerebral sinus venous and jugular vein thrombosis.
In the setting of sickle cell disease therapy for which prophylactic LWMH was used.
Reference value: <15 μmol/L.
Reference value: 160–700 pmol/L.
Although the authors report nmol/L, we assume they mean pmol/L.
Reference value: 8.8–40 nmol/L.
DVT, deep vein thrombosis; F, female; LMWH, low molecular weight heparin; M, male; MTHFR, methylenetetrahydrofolate reductase; PE, pulmonary embolism; tPA, tissue plasminogen activator.
Despite the fact that the association between elevated homocysteine levels and cardiovascular disease has long been known, its causality has remained a matter of debate.4,5 A meta-analysis of observational prospective cohort studies showed a significantly higher risk (odds ratio 1.16) for ischaemic heart disease in subjects with the highest quintile of homocysteine levels.16 Likewise, higher risks of cardiovascular disease have been shown in (meta)-analyses of observational studies in people with higher homocysteine levels secondary to MTHFR-mutations,17 end-stage renal disease,18 perioperative nitrous oxide use,19 folate deficiency,20,21 and cystathionine β-synthase deficiency.22
Our patient also admitted chronic use of cannabis. Cannabis is the most widely used illicit drug worldwide. Its main active substance, THC, affects cannabinoid receptors 1 and 2 (CB1R and CB2R respectively). CB2R has anti-inflammatory properties. CB1R activation induces inflammation and oxidative stress. The resulting endothelial dysfunction, smooth muscle-cell hyperreactivity, and vasoconstriction6 has been associated with cannabis-induced coronary vasospasm and MI.23 The cardiovascular effects of cannabis depend on several factors, including the content amount of THC. The higher the THC content, the higher the likelihood of CB1R‐mediated cardiovascular effects.6 There has been a dramatic increase in potency of cannabis over the past decade, attributable to an increase in THC content from around 2–3% to 20%. A recent study found that use of cannabis is present in 6% of patients with MI younger than 50 years.24
Taken together, stasis (sitting in his car), hyperhomocysteinaemia and the chronic use of cannabis together are likely to have fulfilled Virchow’s triad and thus have contributed to the ‘thrombolysis resistant’ thrombus in the LAD of our patient.
Conclusion
Due to its influence on homocysteine levels, chronic recreational use of N2O might pose a serious risk for ischaemic vascular events. Notably, in combination with other risks factors, like cannabis, it may explain the development of a large MI due to a thrombus in the proximal and distal LAD in an otherwise healthy young adult.
Lead author biography

Thomas Oomens was born in Kollum, the Netherlands in 1988. He recieved his medical training at the Vrije Universiteit of Amsterdam. Currently he is a resident in cardiology at the OLVG hospital in Amsterdam, the Netherlands.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case report including images and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: None declared.
Funding: None declared.
Supplementary Material
Contributor Information
Thomas Oomens, Department of Cardiology, OLVG, Oosterpark 9, 1091 AC Amsterdam, The Netherlands.
Robert K Riezebos, Department of Cardiology, OLVG, Oosterpark 9, 1091 AC Amsterdam, The Netherlands.
Giovanni Amoroso, Department of Cardiology, OLVG, Oosterpark 9, 1091 AC Amsterdam, The Netherlands.
Remko S Kuipers, Department of Cardiology, OLVG, Oosterpark 9, 1091 AC Amsterdam, The Netherlands.
References
- 1. Banks A, Hardman JG.. Nitrous oxide. Contin Educ Anaesth Crit Care Pain 2005;5:145–148. [Google Scholar]
- 2. Randhawa G, Bodenham A.. The increasing recreational use of nitrous oxide: history revisited. Br J Anaesth 2016;116:321–324. [DOI] [PubMed] [Google Scholar]
- 3. Sauls DL, Wolberg AS, Hoffman M.. Elevated plasma homocysteine leads to alterations in fibrin clot structure and stability: implications for the mechanism of thrombosis in hyperhomocysteinemia. J Thromb Haemost 2003;1:300–306. [DOI] [PubMed] [Google Scholar]
- 4. Harker LA, Slichter SJ, Scott CR, Ross R.. Vascular injury and arterial thrombosis. N Engl J Med 1974;291:537–543. [DOI] [PubMed] [Google Scholar]
- 5. Chambers JC, Kooner JS.. Homocysteine–an innocent bystander in vascular disease? Eur Heart J 2001;22:717–719. [DOI] [PubMed] [Google Scholar]
- 6. Pacher P, Steffens S, Haskó G, Schindler TH, Kunos G.. Cardiovascular effects of marijuana and synthetic cannabinoids: the good, the bad, and the ugly. Nat Rev Cardiol 2018;15:151–166. [DOI] [PubMed] [Google Scholar]
- 7. Christensen B, Ueland PM.. Methionine synthase inactivation by nitrous oxide during methionine loading of normal human fibroblasts. Homocysteine remethylation as determinant of enzyme inactivation and homocysteine export. J Pharmacol Exp Ther 1993;267:1298–1303. [PubMed] [Google Scholar]
- 8. Cianciolo G, De Pascalis A, Di Lullo L, Ronco C, Zannini C, La Manna G.. Folic acid and homocysteine in chronic kidney disease and cardiovascular disease progression: which comes first? Cardiorenal Med 2017;7:255–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Karger AB, Steffen BT, Nomura SO, Guan W, Garg PK, Szklo M.. Association between homocysteine and vascular calcification incidence, prevalence, and progression in the MESA cohort. J Am Heart Assoc 2020;9:e013934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Faguer S, Ruiz J, Mari A.. Massive hyperhomocysteinaemia as a complication of nitrous oxide inhalation. Br J Clin Pharmacol 2016;81:391–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Indraratna P, Alexopoulos C, Celermajer D, Alford K.. Acute ST-elevation myocardial infarction, a unique complication of recreational nitrous oxide use. Heart Lung Circ 2017;26:e41–e43. [DOI] [PubMed] [Google Scholar]
- 12. den Uil SH, Vermeulen EGJ, Metz R, Rijbroek A, de Vries M.. Aortic arch thrombus caused by nitrous oxide abuse. J Vasc Surg Cases Innov Tech 2018;4:80–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sun W, Liao J-P, Hu Y, Zhang W, Ma J, Wang G-F.. Pulmonary embolism and deep vein thrombosis caused by nitrous oxide abuse: a case report. World J Clin Cases 2019;7:4057–4062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pratt DN, Patterson KC, Quin K.. Venous thrombosis after nitrous oxide abuse, a case report. J Thromb Thrombolysis 2020;49:501–503. [DOI] [PubMed] [Google Scholar]
- 15. Molina MF, Al Saud AA, Al Mulhim AA, Liteplo AS, Shokoohi H.. Nitrous oxide inhalant abuse and massive pulmonary embolism in COVID-19. Am J Emerg Med 2020;38:1549.e1–1549.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Homocysteine Studies Collaboration. Homocysteine and risk of ischemic heart disease and stroke: a meta-analysis. JAMA 2002;288:2015–2022. [DOI] [PubMed] [Google Scholar]
- 17. Klerk M, Verhoef P, Clarke R, Blom HJ, Kok FJ, Schouten EG. et al. MTHFR 677C–>T polymorphism and risk of coronary heart disease: a meta-analysis. JAMA 2002;288:2023–2031. [DOI] [PubMed] [Google Scholar]
- 18. Qin X, Huo Y, Xie D, Hou F, Xu X, Wang X.. Homocysteine-lowering therapy with folic acid is effective in cardiovascular disease prevention in patients with kidney disease: a meta-analysis of randomized controlled trials. Clin Nutr 2013;32:722–727. [DOI] [PubMed] [Google Scholar]
- 19. Badner NH, Beattie WS, Freeman D, Spence JD.. Nitrous oxide-induced increased homocysteine concentrations are associated with increased postoperative myocardial ischemia in patients undergoing carotid endarterectomy. Anesth Analg 2000;91:1073–1079. [DOI] [PubMed] [Google Scholar]
- 20. Li Y, Huang T, Zheng Y, Muka T, Troup J, Hu FB.. Folic acid supplementation and the risk of cardiovascular diseases: a meta-analysis of randomized controlled trials. J Am Heart Assoc 2016;5:e003768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang Y, Jin Y, Wang Y, Li L, Liao Y, Zhang Y. et al. The effect of folic acid in patients with cardiovascular disease: a systematic review and meta-analysis. Medicine (Baltimore) 2019;98:e17095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mudd SH, Havlik R, Levy HL, McKusick VA, Feinleib M.. A study of cardiovascular risk in heterozygotes for homocystinuria. Am J Hum Genet 1981;33:883–893. [PMC free article] [PubMed] [Google Scholar]
- 23. Rezkalla SH, Sharma P, Kloner RA.. Coronary no-flow and ventricular tachycardia associated with habitual marijuana use. Ann Emerg Med 2003;42:365–369. [DOI] [PubMed] [Google Scholar]
- 24. DeFilippis EM, Singh A, Divakaran S, Gupta A, Collins BL, Biery D. et al. Cocaine and marijuana use among young adults with myocardial infarction. J Am Coll Cardiol 2018;71:2540–2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.