Abstract
Background
Dynamic intraventricular obstruction after transcatheter aortic valve implantation (TAVI) has been previously reported. There is a risk of haemodynamic collapse in the case of left ventricular outflow tract (LVOT) obstruction due to systolic anterior motion (SAM) of the mitral valve.
Case summary
An 83-year-old woman with aortic stenosis (AS) was referred to our hospital for TAVI. Transthoracic echocardiography revealed a severely calcified aortic valve with a peak velocity of 6.3 m/s across the valve. Acceleration of blood flow (peak velocity 2.6 m/s) at the LVOT due to a septal bulge was also seen. Transfemoral TAVI was performed, and a 29 mm Evolut PRO was implanted under general anaesthesia. After the implantation, a complete atrioventricular block with junctional rhythm developed, and refractory hypotension occurred immediately. Transoesophageal echocardiography revealed LVOT obstruction due to SAM of the mitral valve associated with severe mitral regurgitation (MR), which was not observed preoperatively. Fluid infusion and catecholamine administration were not effective. However, after performing temporary pacing from the right ventricular (RV) apex, the LVOT obstruction and severe MR improved. Her haemodynamics stabilized, and we could complete the procedure. A dual-chamber permanent pacemaker with beta-blocker administration as a longer-term treatment further improved the LVOT obstruction. The patient was finally discharged to a rehabilitation hospital.
Discussion
Alertness and recognition of potential LVOT obstruction after TAVI are important. Pacing from the RV apex, as well as dual-chamber pacing, comprise a less invasive and feasible therapeutic option in such cases.
Keywords: Transcatheter aortic valve implantation, Left ventricular outflow tract obstruction, Systolic anterior motion of the mitral valve, Right ventricular pacing, Case report
Learning points
It is important to determine the risk factors, mechanism, and management of dynamic intraventricular obstruction after transcatheter aortic valve implantation (TAVI).
Pacing from the right ventricular apex might be an effective therapeutic option for acute haemodynamic collapse after TAVI due to left ventricular outflow tract obstruction caused by systolic anterior motion of the mitral valve.
Dual-chamber pacemaker implantation with beta-blocker administration could further improve haemodynamics and may be an option for longer-term management in such cases.
Introduction
Transcatheter aortic valve implantation (TAVI) has become a promising procedure in high-risk surgical patients with aortic stenosis (AS).1 Similar to surgical aortic valve replacement (SAVR), dynamic intraventricular obstruction after TAVI has been reported.2,3
Since the haemodynamic profile of such cases is similar to those of patients with hypertrophic cardiomyopathy, alcohol septal ablation (ASA) has been reported as a bail-out therapeutic choice.4,5 However, some cases might not be eligible for ASA due to technical issues. We describe the case of a patient who we successfully treated with medication and dual-chamber pacing after recovery from acute haemodynamic collapse by temporary pacing from the right ventricular (RV) apex. We also present a literature review on significant intraventricular obstruction after TAVI.
Timeline
| Time | Events |
|---|---|
| 1 month earlier |
The patient was admitted to another hospital due to acute heart failure secondary to severe aortic stenosis. Her symptom was improved by oxygen and diuretics, then discharged. |
| 2 weeks earlier | She was referred to our hospital in consideration of transcatheter aortic valve implantation (TAVI). |
| 4 days earlier | Admission to our hospital. |
| Day 0 |
TAVI was performed. Haemodynamics collapsed after implantation of Evolut PRO. Severe left ventricular outflow tract obstruction attributable to systolic anterior motion of the mitral valve was observed. Temporary pacing from the right ventricle apex was effective. |
| Day 1 |
The patient experienced cardiopulmonary arrest due to self-removal of the temporary pacemaker because of delirium. A temporary pacing lead was inserted again, and return of spontaneous circulation was achieved 40 min from onset. |
| Day 7 | Extubation |
| Day 12 | A dual-chamber pacemaker was implanted. |
| Day 34 | The patient was discharged to a rehabilitation hospital due to post-resuscitation encephalopathy. |
| 9 months later | The patient was alive without cardiac symptom although she was in the nursing home because of the progression of cognitive decline and frailty. |
Case presentation
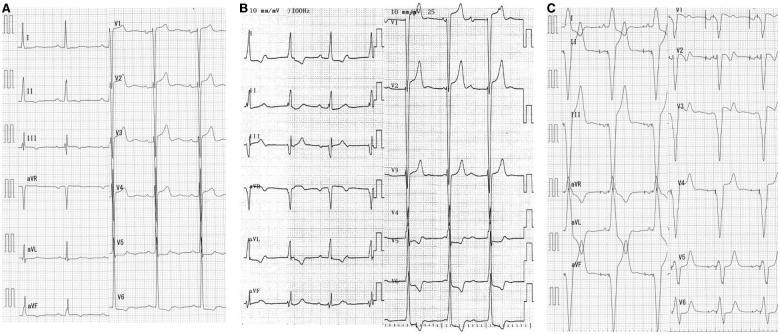
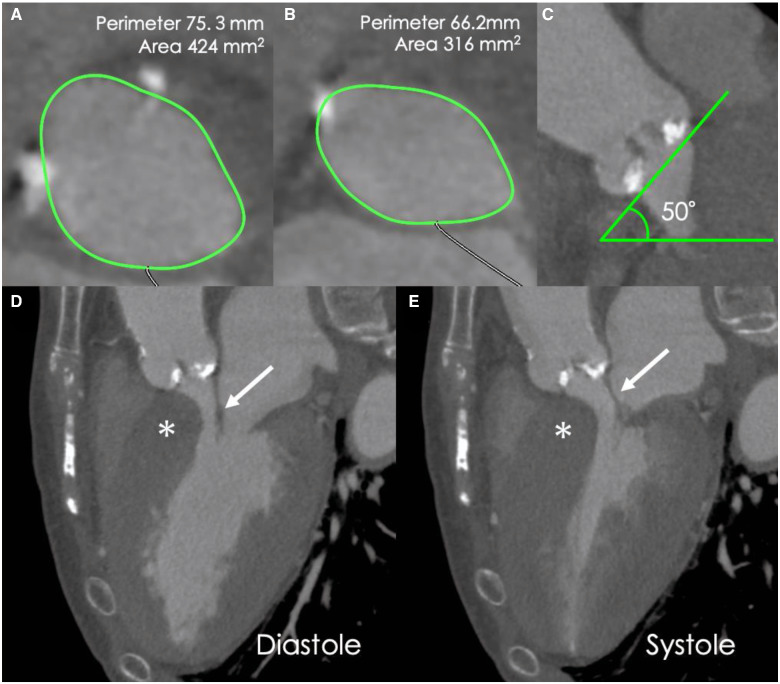
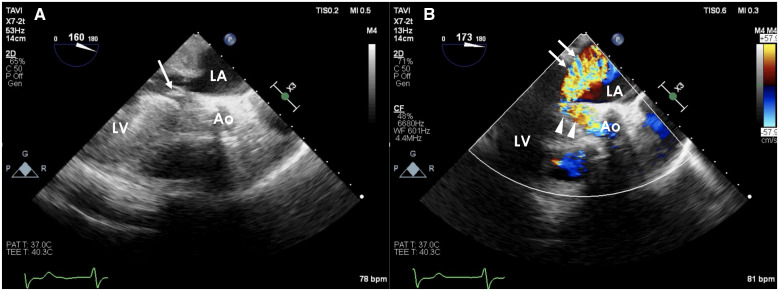
An 83-year-old woman was admitted to another hospital due to acute heart failure secondary to severe AS. Her initial condition was improved by medical treatment, however, dyspnoea on exertion (New York Heart Association functional Class II) was still observed. Therefore, she was referred to our hospital in consideration of intervention to AS. At the time of admission, she was haemodinamically stable. She had a systolic murmur of Levine IV/VI at the second left sternal border, clear lung sounds, and no oedema of the lower extremities. Transthoracic echocardiography (TTE) revealed a peak velocity of 6.3 m/s, a peak pressure gradient of 158.8 mmHg, and a mean gradient of 97.1 mmHg. Blood flow acceleration (peak velocity: 2.6 m/s) at the left ventricular outflow tract (LVOT) due to septal bulge was also seen. However, systolic anterior motion (SAM) of the mitral valve was not observed, and mitral regurgitation (MR) was mild (Supplementary material online, Videos S1 and S2). The left ventricular wall was concentrically hypertrophied with both the septum and posterior wall measured 19 mm. The LV cavity was relatively small, measuring 42 mm at end-diastole, and the ejection fraction was 66%. Electrocardiography showed 58 b.p.m. under normal sinus rhythm (Figure 1A). Computed tomography showed the annular perimeter at 75.3 mm and an annular area at 424 mm2. The basal septum was bulged and led to narrowing of the LVOT (Figure 2). Coronary angiography revealed no obstruction. We decided to perform TAVI due to the patient’s age and frailty (Society of Thoracic Surgeons score of 4.34%, clinical frailty scale of 6). Owing to severe calcification leading to LVOT, a self-expanding valve was selected. Transcatheter aortic valve implantation was performed using a transfemoral approach under general anaesthesia, and a 29-mm Evolut PRO (Medtronic, Minneapolis, MN, USA) was implanted after balloon dilatation. Immediately after implantation, a complete atrioventricular (AV) block with junctional rhythm (over 60 b.p.m.) developed (Figure 1B). Shortly after, her haemodynamics collapsed and refractory hypotension occurred. Fluid infusion (over 500 ml) and intravenous administration of dopamine (5 μg/kg/min) were not effective. Bolus infusion of norepinephrine and phenylephrine slightly increased the patient’s blood pressure only temporarily. Transoesophageal echocardiography demonstrated normal functioning of the implanted valve and revealed LVOT obstruction attributable to SAM of the mitral valve associated with severe MR (Figure 3, Supplementary material online, Videos S3 and S4). However, after performing temporary pacing from the RV apex at 80 b.p.m., which was higher than the junctional rhythm, the LVOT obstruction and MR decreased. Thereafter, the haemodynamics stabilized. The patient was successfully extubated and transferred to the intensive care unit.
Figure 1.
Electrocardiographic findings. (A) Baseline (58 b.p.m.). (B) After implantation of the transcatheter heart valve. Complete atrioventricular block with junctional rhythm (65 b.p.m.). (C) After permanent pacemaker placement (60 b.p.m.).
Figure 2.
Pre-procedural cardiac computed tomography images. (A) Annular assessment. (B) Left ventricular outflow tract assessment. (C) The angulation of the aorta was 50°. (D, E) Long-axis computed tomography images of the diastolic and systolic phases showing interventricular septal bulge (*). Asymmetric septal hypertrophy or systolic anterior motion of the anterior mitral leaflet are not observed. Anterior mitral leaflet (arrow).
Figure 3.
Intraoperative transoesophageal echocardiography after transcatheter aortic valve implantation. (A) Left ventricular outflow tract obstruction with systolic anterior motion of the anterior mitral valve leaflet (arrow). (B) Severe mitral regurgitation (arrow) and turbulent flow of the left ventricular outflow tract (arrowhead). The mitral regurgitation jet is blowing towards the posterior side.
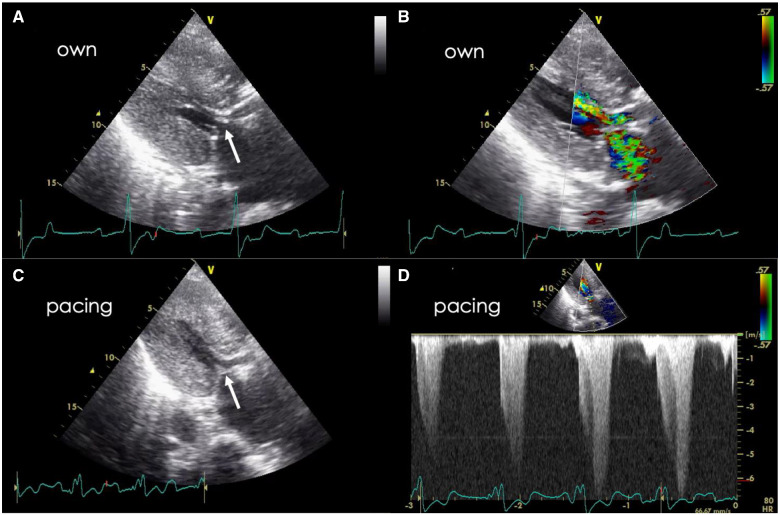
There, hypotension was reproducibly induced by interrupting the pacing. Transthoracic echocardiography revealed SAM of the mitral valve under junctional own beat, which was improved by RV pacing (Figure 4, Supplementary material online, Videos S5 and S6). When accidental self-removal of the pacing lead due to delirium occurred, the haemodynamics deteriorated, although junctional rhythm was observed and led to cardiopulmonary arrest. A temporal pacing lead was reinserted emergently while performing resuscitation; thereafter, a return of spontaneous circulation was achieved.
Figure 4.
Transthoracic echocardiography after transcatheter aortic valve implantation. (A, B) Under own junctional rhythm. Left ventricular outflow tract obstruction with systolic anterior motion of the mitral valve (arrow). The anterior mitral leaflet is thickened. (C) Systolic anterior motion of the anterior mitral leaflet (arrow) and left ventricular outflow tract obstruction are decreased by right ventricular pacing. (D) The peak flow velocity and pressure gradient are 6.8 m/s and 185 mmHg, respectively, even under right ventricular pacing.
Since severe obstruction was still observed (peak velocity 6.8 m/s) under RV pacing, a beta-blocker (bisoprolol) was administered. However, significant acceleration (peak velocity 3.5 m/s) remained, and the complete AV block was persistent. Therefore, a dual-chamber permanent pacemaker was implanted. The permanent RV lead was placed at the apex because it showed better haemodynamic effects than those of septal pacing (Supplementary material online, S7). After adjustment of AV synchrony and medication (bisoprolol: 5 mg, enalapril: 5 mg, and amlodipine: 2.5 mg), TTE revealed mild MR with no evidence of SAM, and the LVOT obstruction was almost resolved. The patient developed post-resuscitation encephalopathy, therefore she was finally discharged to a rehabilitation hospital 34 days after TAVI. At the 9-month follow-up, she had no cardiac symptoms although she was in the nursing home because of the progression of cognitive decline and frailty.
Discussion
Dynamic intraventricular obstruction has been reported to occur in approximately 15% of patients undergoing SAVR for AS.6 Its predictive factors were small LV diameter, asymmetrical hypertrophy, high ejection fraction, and high valve gradients.7 In contrast to hypertrophic obstructive cardiomyopathy, wherein obstruction is usually located in the LVOT, systolic obstruction after aortic valve replacement tends to be seen in the mid-ventricle because most cases involve concentric hypertrophy.6,7 However, asymmetrical hypertrophy, which is also seen in patients with AS and a septal bulge as in our case, might induce LVOT obstruction after TAVI. Dynamic LVOT obstruction is a complex phenomenon arising from the interplay of subaortic hypertrophy, LV ejection fraction, venturi effects, and anterior positioning of the mitral valve apparatus. It is associated with SAM of the anterior mitral leaflet, which causes significant MR. The treatment strategy usually focuses on maintaining LV filling pressure, slowing the heart rate to increase the diastolic filling time, and decreasing inotropy. Conservative therapies often improve the haemodynamics in patients with mid-ventricular obstruction.2,8,9 However, since LVOT obstruction can further deteriorate the haemodynamics, some cases reportedly require bail-out treatment (Table 1).3–5,10–13 Concomitant septal myectomy in high-risk patients undergoing SAVR has been recommended.14 However, surgical resection might not be feasible for patients undergoing TAVI due to patient comorbidities and frailty. Currently, ASA has been reported to be a bail-out therapeutic choice to manage severe intraventricular gradient after TAVI.4,5,10–12 Alcohol septal ablation is less invasive than surgical resection. However, up to 20% of patients might not have an appropriate septal artery.15 Moreover, ASA requires experienced operators and equipment. Dual-chamber pacing is reported as a therapeutic alternative to hypertrophic obstructive cardiomyopathy and is less invasive than myectomy or ASA.16 A possible mechanism for its therapeutic effect has been suggested that initiation of electrical impulse at the RV apex alters the systolic contraction sequence of the basal septum. Although the pacing is not considered as the primary therapy for obstruction, cohort studies have indicated that pacing effectively reduces the LVOT gradient and symptoms.16,17 In fact, RV apex pacing was essential in managing the acute haemodynamic collapse in our case. In addition, a dual-chamber pacemaker was also effective in reducing the residual LVOT gradient. A previous paper reported that disordered AV synchrony following TAVI caused intraventricular obstruction.9 Increasing the LV filling pressure by maintaining AV synchrony is important to reduce the gradient. Thus, dual-chamber pacing might be considered if there is no haemodynamic improvement with a single RV pacing.
Table 1.
Summary of published case reports of significant intraventricular obstruction after TAVI
| Age (years) | Sex | Pre TTE | Implanted valve | Onset | Symptoms | TTE findings | Treatment | |
|---|---|---|---|---|---|---|---|---|
| Suh et al.2 | 82 | F |
AVPG(p) 121 mmHg MVO (PG 12 mmHg) |
23 mm Sapien (TF) |
1 day later | Hypotension | MVO |
Fluid Beta-blocker |
| Takeda et al.13 | 91 | M |
AVPG(p) 152 mmHg Septal bulge |
26 mm Sapien (TA) |
Immediately | Not mentioned |
LVOTO (PG 50 mmHg) SAM of the mitral valve MR severe |
Beta-blocker Cibenzoline Pacemaker* |
| Soraja et al.4 | 89 | M | AVPG(m) 65 mmHg |
26 mm Sapien (TA) |
1 month later | Exertional dyspnoea |
LVOTO (PG 55 mmHg) SAM of the mitral valve |
Beta-blocker ASA* |
| Gerckens et al.11 | 88 | F | AVPG(p) 115 mmHg |
23 mm Sapien (TF) |
12 h later | Hemodynamic collapse |
MVO (PG 108 mmHg) SAM of the mitral valve MR moderate |
Fluid, Pacemaker Beta-blocker Emergent ASA* |
| Krishnaswamy et al.10 | 91 | F |
AVPG(m) 58 mmHg ASH LVOTO (PG 12.5 mmHg) |
23 mm Sapien (TF) |
Immediately | Hemodynamic collapse |
LVOTO (PG 120 mmHg) SAM of the mitral valve MR severe |
Emergent ASA* |
| Alfonso et al.8 | 79 | F | AVPG(m) 82 mmHg |
26 mm CoreValve (TF) |
Immediately | Asymptomatic | MVO (PG 120 mmHg) | Asymptomatic |
| Ibrahim et al.9 | 81 | F | AVPG(p) 109 mmHg |
29 mm CoreValve (DA) |
Immediately | Not mentioned | MVO |
Induced by sinus arrest →spontaneous restoration |
| Leya et al.3 | 87 | F |
AVPG(m) 45 mmHg Sigmoid septum |
26 mm CoreValve (TF) |
Immediately after BAV | Hemodynamic collapse |
LVOTO+MVO SAM of the mitral valve MR severe |
AV pacemaker Beta-blocker Valve in valve (26 mm CoreValve)* |
| Yanagiuchi et al.12 | 83 | M |
ASH, SAM of the mitral valve |
26 mm Sapien 3 (TF) |
Immediately | Hypotension | LVOTO (PG 121 mmHg) | ASA (ad hoc)* |
| Kitahara et al.5 | 86 | F |
AVPG(p) 64 mmHg ASH, SAM of the mitral valve →Disappeared after beta-blocker initiation |
23 mm Sapien 3 (TF) |
15 min later | Hemodynamic collapse |
LVOTO PG >50 mmHg SAM of the mitral valve MR severe |
Fluid Beta-blocker infusion Emergent ASA* |
ASA, alcohol septal ablation; ASH, asymmetrical septal hypertrophy; AVPG, aortic valve pressure gradient; BAV, balloon aortic valvuloplasty; DA, direct aortic; LVOTO, left ventricular outflow tract obstruction; (m), mean; MR, mitral regurgitation; MVO, mid-ventricular obstruction; (p), peak; PG, pressure gradient; SAM, systolic anterior motion; TA, trans apical; TAVI, transcatheter aortic valve implantation; TF, transfemoral; TTE, transthoracic echocardiography.
Most effective treatment.
Based on our literature review, one article has already reported the effectiveness of pacing in LVOT obstruction after TAVI.13 However, we concluded that our case was valuable as the first report to clearly demonstrate the crucial difference in outcomes with and without pacing (Figure 4, Supplementary material online, Videos S5 and S6).
Leya et al.3 reported a case in which severe LVOT obstruction was successfully managed by implanting a second self-expandable valve (‘valve in valve’). Retaining the radial force at the LVOT might be a solution for LVOT obstruction. However, no report has compared balloon-expandable and self-expandable prosthetic valves. In our review, LVOT obstruction developed regardless of valve type (Table 1). From another perspective, prophylactic MitraClip (Abbott, Davis, CA, USA) implantation prior to TAVI was reported to be useful in preventing exacerbation of LVOT obstruction due to SAM.18 Further studies are warranted to clarify the effectiveness of such novel approaches.
Alertness and recognition of possible LVOT obstruction after TAVI are important. Pacing from the RV apex and dual-chamber pacing comprise a less invasive and feasible therapeutic option in such cases.
Lead author biography

Nana Endo, MD, is a senior resident of cardiology in Tokyo Women’s Medical University. She graduated from Tokyo Women’s Medical University in 2014. She finished junior resident program in 2016. After completing resident program of emergency medicine, she started her career as cardiologist since 2017. Membership: Japanese Circulation Society. Japanese Association of Cardiovascular Intervention and Therapeutics.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Supplementary Material
Acknowledgements
We would like to thank Editage (www.editage.jp) for English language editing.
Slide sets: A fully edited slide set detailing these cases and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case report including images and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: H.O. and J.Y. report that they belong to the division (Clinical Research division for Cardiovascular Catheter Intervention) financially supported by donations from Abbott, Boston Scientific, Medtronic, and Terumo. Other authors have no conflict of interest.
Funding: None declared.
References
- 1. Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG. et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011;364:2187–2198. [DOI] [PubMed] [Google Scholar]
- 2. Suh WM, Witzke CF, Palacios IF.. Suicide left ventricle following transcatheter aortic valve implantation. Cathet Cardiovasc Intervent 2010;76:616–620. [DOI] [PubMed] [Google Scholar]
- 3. Leya F, Tuchek JM, Coats W.. Abnormal distortion of aortic corevalve bioprosthesis with suicide left ventricle, aortic insufficiency, and severe mitral regurgitation during transcatheter aortic valve replacement. Cathet Cardiovasc Intervent 2016;88:1181–1187. [DOI] [PubMed] [Google Scholar]
- 4. Sorajja P, Booker JD, Rihal CS.. Alcohol septal ablation after transaortic valve implantation: the dynamic nature of left outflow tract obstruction. Cathet Cardiovasc Intervent 2013;81:387–391. [DOI] [PubMed] [Google Scholar]
- 5. Kitahara H, Mastuura K, Sugiura A, Yoshimura A, Muramatsu T, Tamura Y. et al. Recurrence of left ventricular outflow tract obstruction requiring alcohol septal ablation after transcatheter aortic valve implantation. Case Rep Cardiol 2018;2018:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. López Ayerbe J, Evangelista Masip A, Armada Romero E, Mateos González M, González Alujas MT, García Del Castillo H. et al. Predictive factors of abnormal dynamic intraventricular gradient after valve replacement in severe aortic stenosis. Rev Esp Cardiol 2002;55:127–134. [DOI] [PubMed] [Google Scholar]
- 7. Tsuruta H, Hayashida K, Yashima F, Yanagisawa R, Tanaka M, Arai T. et al. Incidence, predictors, and midterm clinical outcomes of left ventricular obstruction after transcatheter aortic valve implantation. Catheter Cardiovasc Interv 2018;92:E288–E298. [DOI] [PubMed] [Google Scholar]
- 8. Alfonso F, Domínguez L, Rivero F, Benedicto A, Trillo R.. Severe intraventricular dynamic gradient following transcatheter aortic valve implantation: suicide ventricle? EuroIntervention 2015;11:e1. [DOI] [PubMed] [Google Scholar]
- 9. Ibrahim H, Barker CM, Reardon MJ, Kleiman NS.. Suicide left ventricle due to conduction disturbance following transcatheter aortic valve replacement and reversal with restoration of sinus rhythm: is there life after death? J Invasive Cardiol 2015;27:E107–E109. [PubMed] [Google Scholar]
- 10. Krishnaswamy A, Tuzcu EM, Svensson LG, Kapadia SR.. Combined transcatheter aortic valve replacement and emergent alcohol septal ablation. Circulation 2013;128:e366–e368. [DOI] [PubMed] [Google Scholar]
- 11. Gerckens U, Pizzulli L, Raisakis K.. Alcohol septal ablation as a bail-out procedure for suicide left ventricle after transcatheter aortic valve implantation. J Invasive Cardiol 2013;25:E114–E117. [PubMed] [Google Scholar]
- 12. Yanagiuchi T, Tada N, Mizutani Y, Matsumoto T, Sakurai M, Ootomo T.. Feasibility assessment of alcohol septal ablation in transcatheter aortic valve replacement using multidetector computed tomography. JACC Cardiovasc Interv 2017;10:e7–e9. [DOI] [PubMed] [Google Scholar]
- 13. Takeda Y, Nakatani S, Kuratani T, Mizote I, Sakata Y, Torikai K. et al. Systolic anterior motion of the mitral valve and severe mitral regurgitation immediately after transcatheter aortic valve replacement. J Echocardiogr 2012;10:143–145. [DOI] [PubMed] [Google Scholar]
- 14. Kayalar N, Schaff HV, Daly RC, Dearani JA, Park SJ.. Concomitant septal myectomy at the time of aortic valve replacement for severe aortic stenosis. Ann Thorac Surg 2010;89:459–464. [DOI] [PubMed] [Google Scholar]
- 15. Singh M, Edwards WD, Holmes DR, Tajik AJ, Nishimura RA.. Anatomy of the first septal perforating artery: a study with implications for ablation therapy for hypertrophic cardiomyopathy. Mayo Clin Proc 2001;76:799–802. [PubMed] [Google Scholar]
- 16. Fananapazir L, Epstein ND, Curiel RV, Panza JA, Tripodi D, McAreavey D.. Long-term results of dual-chamber (DDD) pacing in obstructive hypertrophic cardiomyopathy. Evidence for progressive symptomatic and hemodynamic improvement and reduction of left ventricular hypertrophy. Circulation 1994;90:2731–2742. [DOI] [PubMed] [Google Scholar]
- 17. Kappenberger L, Linde C, Daubert C, McKenna W, Meisel E, Sadoul N, the PIC Study Group et al. Pacing in hypertrophic obstructive cardiomyopathy. A randomized crossover study. PIC Study Group. Eur Heart J 1997;18:1249–1256. [DOI] [PubMed] [Google Scholar]
- 18. Bode MF, Ahmed AA, Baron SJ, Labib SB, Gadey G.. The use of MitraClip to prevent posttranscatheter aortic valve replacement left ventricular "suicide". Catheter Cardiovasc Interv 2020;doi: 10.1002/ccd.29100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.