Abstract
Rationale & Objectives
Due to unmeasured confounding, observational studies have limitations when assessing whether dialysis initiation reduces mortality compared with conservative therapy among adults with advanced chronic kidney disease (CKD). We addressed this issue in this meta-analysis.
Study Design
Meta-analysis with bias analysis for unmeasured confounding.
Setting & Study Population
Adults with stage 4 or 5 CKD who had initiated dialysis or conservative treatment.
Selection Criteria for Studies
Prospective or retrospective cohort studies comparing survival of dialysis versus conservatively managed patients were searched on MEDLINE and Embase from January 2009 to March 20, 2019.
Data Extraction
HRs of all-cause mortality associated with dialysis initiation compared with conservative treatment.
Analytical Approach
We pooled HRs using a random-effects model. We estimated the percentage of effect sizes more protective than HRs of 0.80 and severity of unmeasured confounding that could reduce this percentage to only 10%. Subgroup analysis was performed for studies with only older patients (aged ≥ 65 years).
Results
12 studies were included that involved 16,609 dialysis patients and 3,691 conservatively managed patients. A random-effects model suggested that dialysis initiation was associated with a mean mortality HR of 0.47 (95% CI, 0.34-0.64), in which 92% (95% CI, 50%-100%) of the true effects were more protective than HRs of 0.80. To reduce the percentage of HRs < 0.80 to 10%, unmeasured confounder(s) would need to be associated with both dialysis initiation and mortality by relative risks of 4.05 (95% CI, 2.39-4.15), which is equivalent to shifting each study’s estimated HR by 2.31-fold (95% CI, 1.51-2.36). Restricting studies to include only older patients did not modify the results.
Limitations
Limited number of studies and evidence on the absence of publication bias.
Conclusions
Our findings suggest that dialysis initiation considerably reduces mortality among adults with advanced CKD. Future bias-adjusted meta-analyses need to assess outcomes beyond mortality.
Index Words: Dialysis, conservative management, survival, mortality, unmeasured confounding, treatment effects
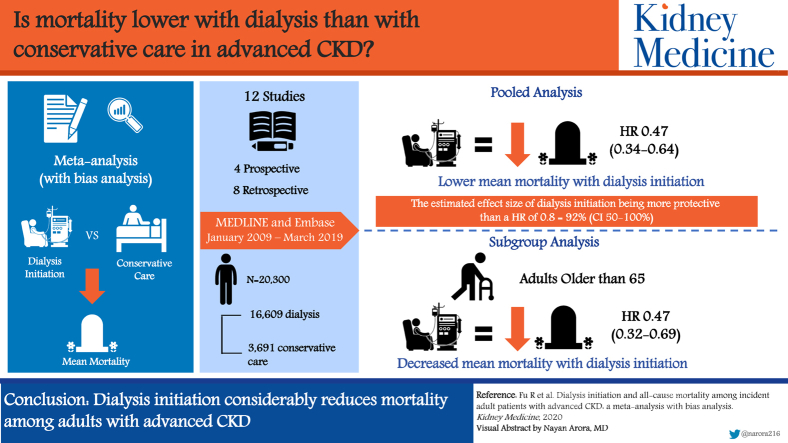
Graphical abstract
Plain-Language Summary.
We conducted a meta-analysis on observational studies in the past decade to understand the survival benefit of dialysis initiation for adults with incident advanced chronic kidney disease (CKD) and patients 65 years and older. A newly developed method was used to examine the effect of potential unmeasured confounders. Among 12 studies that involved 16,609 dialysis patients and 3,691 conservatively managed patients, dialysis initiation resulted in lower risk for mortality, a conclusion that also applied to older patients and was robust to potential unmeasured confounding. These findings suggest that at least in terms of avoiding mortality, all adults with advanced CKD regardless of age need to be adequately informed on the initiation of dialysis. Future meta-analyses need to establish the use of dialysis on outcomes beyond mortality.
Editorial, p. 18
Chronic kidney disease (CKD) affects 1 in 7 US adults and 1 in 10 Canadian adults.1,2 Because CKD in its early stages does not cause symptoms, patients often progress to advanced stages, commonly referred to as stages 4 and 5, without treatment.3 Whether to initiate dialysis for all incident patients who are not transplantation candidates has been a long-standing research interest. In theory, because dialysis actively supplements kidney function, its ability to avoid mortality should exceed conservative treatment, a nondialysis pathway with a focus on symptom control and advance planning.4 However, dialysis may cause complications and is associated with worse patient outcomes in the long run, especially for older patients with a high comorbidity burden.5 Hence, the survival benefit of dialysis initiation is unclear, at least for certain patient groups.
A recent meta-analysis of 3 observational studies concluded that dialysis initiation is associated with lower risk for mortality for adults 65 years and older with stage 5 CKD.6 In the absence of randomized controlled trials, results of these observational studies are hindered by confounding by indication because patients who avoid dialysis are driven by reasons that also affect their survival.7 Effects of confounders that are unmeasured, unknown, or incorrectly specified are difficult to remove and concerns of the unmeasured confounding in each study may translate to internal bias in a meta-analysis because pooling studies improves statistical precision but generally does not mitigate bias.8
Current guidelines for meta-analysis of observational studies do not require investigators to quantify the effects of unmeasured confounding on the pooled estimates.9,10 Previous methods aimed at enabling sensitivity analysis in a meta-analysis required strong assumptions on the nature of unmeasured confounders11 or sufficient understanding of Bayesian statistics.8,12 A recently proposed method may be able to address this gap in knowledge; it does not require statistical assumptions on the type of unmeasured confounders that are present in the meta-analyzed studies, produces interpretable results, and is straightforward to implement.13,14
The purpose of our study was to: (1) perform a meta-analysis of observational studies that compared all-cause mortality of adults with incident advanced CKD initiating dialysis versus conservative management and (2) assess the strength of the pooled dialysis effect subject to the severity of unmeasured confounding.
Methods
This meta-analysis was registered on the International Prospective Register of Systematic Reviews (CRD42019135633) and followed the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guideline.10
Search Strategy
A literature search of peer-reviewed English-language studies was performed on MEDLINE and Embase from January 2009 to March 20, 2019, to gather the most recent decade of evidence. The year 2009 was chosen because a recently published meta-analysis suggested that all high-quality studies pertaining to this topic were published in or after 2009.6 Reference lists of potentially eligible studies were also searched (Item S1). Two reviewers independently screened the titles, abstracts, and full text of studies to determine their eligibility. Disagreements were resolved through discussion.
Study Eligibility Criteria
We included prospective or retrospective cohort studies that examined all-cause mortality of adults (aged ≥ 18 years) with incident advanced CKD who had initiated dialysis versus conservative management. Advanced CKD was defined to be stage 4 or 5 CKD marked by estimated glomerular filtration rate (eGFR) < 30 mL/min/1.73 m2 of body surface area.3 We excluded studies that included nonincident patients who had been treated for CKD (using dialysis or conservative therapy), minors, and transplant recipients.
Data Extraction
Two reviewers independently extracted the following information: authors, publication year, country, study design, type of cohort, definition of study entry (time zero), indication of dialysis initiation, description of the conservative management program, and duration of patient follow-up. At study entry, we recorded patient age, percentage of women, CKD stages, dialysis types, and eGFR.
The same reviewers independently extracted the hazard ratio (HR) and associated 95% CI of all-cause mortality associated with dialysis initiation estimated at the end of study. For studies that performed more than 1 regression adjusted for different sets of confounders, we extracted the most adjusted HR. When studies applied a quasi-experimental design, we extracted the corresponding HR. For studies that compared different modalities of dialysis separately with conservative care, we used a within-study inverse-variance weighted fixed-effects model to pool these HRs to form a single input. The same technique was applied to 1 study that performed year-based subgroup analysis to generate an overall HR for the entire follow-up period. For studies that only reported Kaplan-Meier curves, we reached out to authors to request the availability of HRs and received 1 reply from 3 requests. For the remaining studies, we first reconstructed the raw time-to-event data from the Kaplan-Meier curves using the software DigitizeIt (Ingo Bormann: https://www.digitizeit.de/eula.html).15 These data and other information were used to estimate the HR and its 95% CI.16
Standard Meta-analysis
We used a random-effects model to pool HRs on the log-HR scale to estimate an average treatment effect of initiating dialysis to avoid mortality. This model was fit through restricted maximum likelihood with the Hartung-Knapp standard error adjustment due to the small number of studies and potentially considerable heterogeneity.17 Absence of publication bias was assessed by the contour-enhanced funnel plot that displayed contour lines indicating the standard threshold for statistical significance (P ≤ 0.05 or P > 0.05).18
Evidence Strength Given Heterogenous Effects
We used nonparametric methods to characterize the strength of evidence in the presence of heterogeneity.13,19 First, we estimated the proportion of effect sizes more protective than an HR of 0.80 (HR < 0.80), which is approximately equivalent to an 14% reduction in the relative risk (RR) for mortality due to dialysis compared with conservative treatment (using the approximation RR=1-0.5ˆsqrt(0.8))/(1-0.5ˆsqrt(1/0.8))=0.86).20 We then estimated the proportion of effect sizes with HR > 1, representing harmful rather than protective associations of dialysis initiation with mortality.
To explore causes of heterogeneity, we performed subgroup analysis based on categorical moderating variables. The estimated average log-HRs of subgroups were pooled in a fixed-effects model and compared using a Wald test. Mean patient age and percentage of women were entered into separate linear metaregression models to further explore causes of heterogeneity.
Risk of Bias Assessment
We rated the level of confounder adjustment in each study using a score between 0 and 4 (Item S2).21 Studies with a score ≥ 3 represented relatively low risk for confounding bias. We then used the Newcastle-Ottawa Scale for cohort studies to assess the overall quality of evidence on the basis of selection, comparability, and outcome.22
Bias Analysis on the Effects of Unmeasured Confounding
To assess the extent to which unmeasured confounding might have affected results, we applied nonparametric methods to conduct sensitivity analyses.13,14 We converted the HR estimates to the log-RR scale using a transformation that accommodates common outcomes.20
We operationalized the hypothetical severity of unmeasured confounding in each study as a multiplicative bias factor.14 We estimated the minimum strength of bias factor that, if present in all studies, would “explain away” the pooled results of random-effects meta-analysis in the sense of reducing the proportion of studies with true effects more protective than HR of 0.80 (HR<0.80) to only 10%.23 We then estimated the minimum strength of the bias factor such that half of all studies would have concluded that dialysis initiation was harmful rather than protective. This bias factor would have to increase the proportion of studies with true effects concluding HRs > 1.0 to 50%. To further aid interpretation, we expressed confounding severity in terms of the risk ratios by which an unmeasured confounder(s) in each study would need to be associated with both dialysis initiation and mortality risk. This metric is a meta-analytic extension of the E-value for single studies.24,25
Subgroup Analysis
First, we restricted studies to include only patients 65 years or older in their cohort. We repeated all procedures, including the random-effects meta-analysis and bias analysis, on this set of studies to confirm whether starting dialysis led to differential survival outcomes for older patients. Next, we considered all outcomes beyond mortality that were assessed in at least 2 of the included studies. Effects of dialysis initiation on these outcomes were discussed qualitatively and, when possible, pooled using a random-effects model.
Results
Study Selection
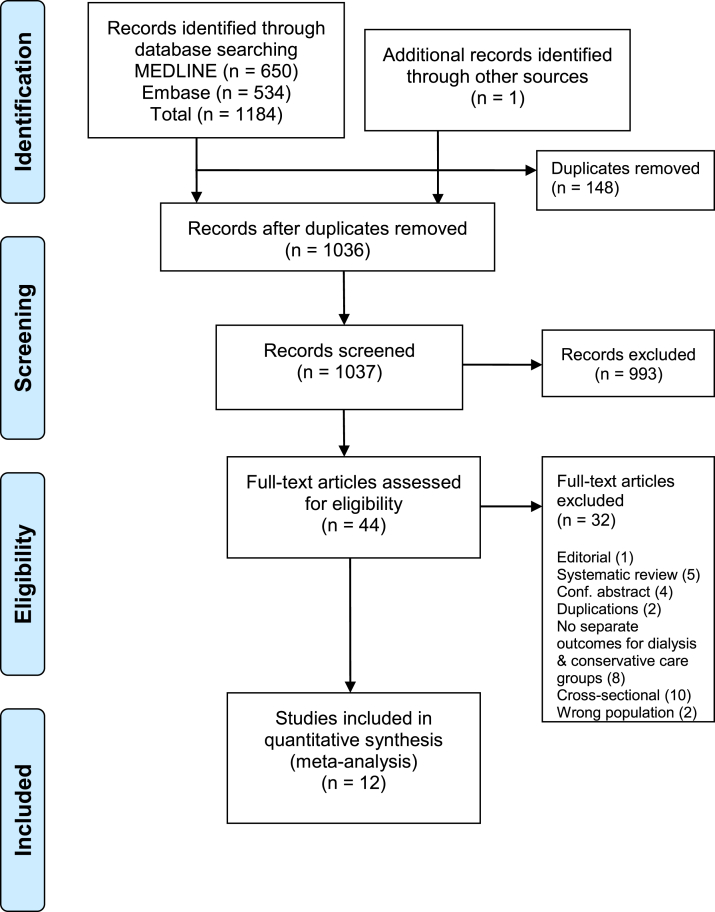
An initial search yielded 1,184 records, of which 1,036 were unique (Fig 1). Screening of titles and abstracts identified 44 studies eligible for full-text assessment (Item S1). One additional study was identified after exploring the included reference lists. Exclusion was performed for studies that were editorials (1 study), reviews or conference abstracts (9 studies), cross-sectional (10 studies), did not report outcomes separately for dialysis and conservative treatment groups (8 studies), and inclusion of nonincident patients or transplant recipients (2 studies). After exclusions, 12 studies remained for the meta-analysis.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flow diagram. Literature search was performed on MEDLINE and Embase on March 30, 2019.
Study Characteristics
Six studies were conducted in Europe,26, 27, 28, 29, 30, 31 followed by 4 that were set in Asia,32, 33, 34, 35 1 in North America,36 and another one37 in Australia (Table 1). More studies were retrospective (n = 8) than prospective (n = 4) and used single-center data (n = 7) rather than multicenter data (n = 5). At study entry, the percentage of women ranged from 32.5% to 56.3% and average patient age ranged from 60.2 to 80.9 years. Baseline mean eGFR ranged from 6.4 to 16 mL/min/1.73 m2. Indications of dialysis initiation were based on thresholds of eGFR and serum creatinine levels, as well as on the onset of symptoms including hyperkalemia and acidosis. Only 1 study reported eGFRs of patients at dialysis initiation (mean, 8.3; SD, 2.8 mL/min/1.73 m2).29 All but 2 studies focused on adults 65 years or older. Eight studies considered an exclusive cohort of patients with stage 5 CKD, 3 also included patients with stage 4 CKD, and 1 included only patients with stage 4 CKD. Seven studies gave a description of the conservative management program (Table S1).26, 27, 28, 29,34,35,37 Apart from the ongoing medical treatment, conservatively managed patients had access to a range of services with a focus on symptom control and advance care planning by a multidisciplinary team that included primary care physicians, dieticians, palliative care specialists, and social workers. Home visits and access to a telephone hotline were also described.
Table 1.
Summary Characteristics of the 12 Studies
| Study | Country | Study Design | Age, y (range) | Women | CKD Stages | Dialysis Type | Baseline eGFR, mL/min/1.73 m2 | Indication of Dialysis Initiation | Definition of Study Entry | Follow-up Duration |
|---|---|---|---|---|---|---|---|---|---|---|
| Brown et al37 (2015) | Australia | Single-center prospective | 71.6 ± 12.7 (65+) | 36.7% | 4/5 | HD, PD | 16 ± 7.7 | eGFR < 15 | First attendance to predialysis or CM clinic after modality decision had been made | ≥1 y |
| Chandna et al28 (2016) | UK | Multicenter retrospective | 80.9 ± 4.0 (75+) | 32.8% | 5 | HD, PD | 13.3 ± 1.4 | eGFR of 10-15 | Date of stage 5 CKD diagnosis | ≥3 y |
| Da Silva-Gane et al26 (2012) | UK | Single-center prospective | 60.3 ± 13.8 (18+) | 32.5% | 4/5 | HD, PD | 13.8 ± 3.9 | Persistent uremic symptoms, volume overload, hyperkalemia or acidosis | Recruitment date (before the planning meeting) | 31.9 mo (25.1) |
| Hussain et al27 (2013) | UK | Single-center retrospective | 79.0 ± 5.8 (70+) | 43.5% | 4 | Unspecified | <20 | Unspecified | First date of eGFR < 20 | Unspecified |
| Kwok et al35 (2016) | China | Single-center retrospective | 78.4 ± 7.0 (65-101) | 56.3% | 5 | HD, PD | 10.1 ± 2.9 | Serum creatinine > 350 or >400 μmol/L for diabetic or nondiabetic patients | Date of renal advance care planning meeting | ≥1 y |
| Raman et al31 (2018) | UK | Single-center prospective | 80.8 ± 3.4 (75+) | 37.7% | 5 | HD, PD | 13.1 ± 2.2 | Unspecified | Date of stage 5 CKD diagnosis | 35.1 ± 22.1 mo |
| Reindl-Schwaighofer et al30 (2017) | Austria | Multicenter retrospective | 74.2 ± 5.8 (65+) | 47.0% | 5 | HD | <10 | Unspecified | Dialysis group: date of first dialysis; CKM group: date of eGFR < 10 | Unspecified |
| Shih et al33 (2014) | Taiwan | Multicenter retrospective | 79.4 ± 7.0 (70+) | 55.3% | 5 | Unspecified | <15 | Unspecified | First prescription of erythropoiesis-stimulating agents (proxy date of stage 5 CKD diagnosis) | 1,026 ± 880 d |
| Shum et al34 (2014) | China | Single-center retrospective | 73.8 ± 5.4 (65-90) | 49.7% | 5 | PD | 6.4 ± 1.9 | eGFR < 15 | Date of stage 5 CKD diagnosis | 1.96 y (0.9-3.6) |
| Tam-Tham et al36 (2018) | Canada | Multicenter retrospective | 79.1 ± 6.7 (65+) | 51.4% | 5 | HD, PD | 7.8 ± 1.5 | eGFR < 10 | Confirmed date of eGFR < 10 | ≥1 y |
| Teo et al32 (2010) | Singapore | Multicenter prospective | 60.2 ± 12.8 (20+) | 49.1% | 5 | HD, PD | Unknown | Serum creatinine > 880 μmol/L | Date of stage 5 CKD diagnosis | 1 y |
| Verberne et al29 (2016) | Netherlands | Single-center retrospective | 78.2 ± 4.4 (70+) | 37.6% | 4/5 | HD, PD | 13.9 ± 4.7 | Discussion started when eGFR < 20 | Date of modality decision | Unspecified |
Note: Values reported as mean ± standard deviation. eGFR < 15 mL/min/1.73 m2 is used to determine stage 5 CKD. Group mean ± standard deviation values are pooled using the Cochrane formulas for combining groups.
Abbreviations: CKD, chronic kidney disease; CM, conservative management; eGFR, estimated glomerular filtration rate (in mL/min/1.73 m2); HD, hemodialysis; PD, peritoneal dialysis; UK, United Kingdom.
There were 16,609 dialysis patients compared with 3,691 patients managed conservatively (Table 2). Two studies used propensity score matching. Except for 1 study that concluded dialysis initiation was associated with increased risk for mortality,33 the remaining 11 studies agreed that dialysis was associated with reduced mortality.
Table 2.
Summary of Study-Specific HRs, Adjusted Covariates, Sample Sizes, and Other Survival Data
| Study | HR of All-Cause Mortality | Adjusted Covariates and/or Calculation Method | Modality (N), Survival Data |
|---|---|---|---|
| Brown et al37 (2015) | HR, 0.31 (95% CI, 0.21-0.47) | Results provided by authors after being contacted by email | Dialysis (164): median survival 46 mo, mean survival 36 mo, total deaths 37; CM (122): median survival 16 mo, mean survival 20 mo, total deaths 68 |
| Chandna et al28 (2016) | HR, 0.53 (95% CI, 0.39-0.73) | Age, sex, ethnicity, and comorbid conditions | Dialysis (92): median survival 38.2 (95% CI, 27.7-46.4) mo; CM (158): median survival 23.1 (95% CI, 19.8-26.6) mo |
| Da Silva-Ganen et al26 (2012) | HR, 0.44 (95% CI, 0.22-0.92) | Age, sex, weight, comorbid conditions, functional impairment and eGFR; calculated using propensity scores pooled from HD and PD | HD/PD (124): median survival 1,317 d (in HD group); CM (30): median survival 913 d |
| Hussain et al27 (2013) | HR, 0.46 (95% CI, 0.32-0.68) | Estimated from Kaplan-Meier curves | Dialysis (164): median survival 1,695 d; CM (142): median survival 804 d |
| Kwok et al35 (2016) | HR, 0.22 (95% CI, 0.17-0.30) | Estimated from Kaplan-Meier curves | Dialysis (126): median survival 44.6 (95% CI, 37.3-51.9) mo; total deaths 67; CM (432): median survival 10.0 (95% CI, 8.3-11.7) mo; total deaths 387 |
| Raman et al31 (2018) | HR, 0.61 (95% CI, 0.41-0.91) | Age, comorbid conditions and living alone | Dialysis (123); CM (81); survival data not available |
| Reindl-Schwaighofer et al30 (2017) | HR, 0.23 (95% CI, 0.18-0.29) | Age, sex, and comorbid conditions | HD (8622): median survival 26.9 (95% CI, 25.8-28.0) mo; CM (174): median survival 1.1 (95% CI, 0.4-0.8) mo |
| Shih et al33 (2014) | HR, 1.16 (95% CI, 1.07-1.25) | Age, sex, income, type of residence, comorbid conditions, primary renal disease, and use of medications; calculated using propensity scores | Dialysis (6,292); CM (2,049); survival data not available |
| Shum et al34 (2014) | HR, 0.46 (95% CI, 0.31-0.68) | Age, comorbid conditions, and functional impairment | PD (157): median survival 3.75 (95% CI, 2.49-5.25) y; CM (42): median survival 2.35 (95% CI, 1.13-3.71) y |
| Tam-Tham et al36 (2018) | HR, 0.67 (95% CI, 0.53-0.83) | Age, sex, eGFR, type of residence, ethnicity, medications, and comorbid conditions; pooled from year-based subgroup analysis | Dialysis (500): median survival 3.0 (IQR, 1.6-4.5) y; CM (338): median survival 0.79 (IQR, 0.3-1.8) y |
| Teo et al32 (2010) | HR, 0.44 (95% CI, 0.22-0.86) | Age, sex, race, type of therapy center, and left ventricular ejection fraction | PD (41): survived for 1 y = 32; CM (16): survived for 1 year = 5 |
| Verberne et al29 (2016) | HR, 0.62 (95% CI, 0.42-0.92) | Age and comorbid conditions | Dialysis (204): median survival 3.1 (IQR, 1.5-6.9) y; CM (107): median survival 1.5 (IQR, 0.7-3.0) y |
Abbreviations: CM, conservative management; eGFR, estimated glomerular filtration rate; HD, hemodialysis; HR, hazard ratio; IQR, interquartile range; PD, peritoneal dialysis.
Basic Results
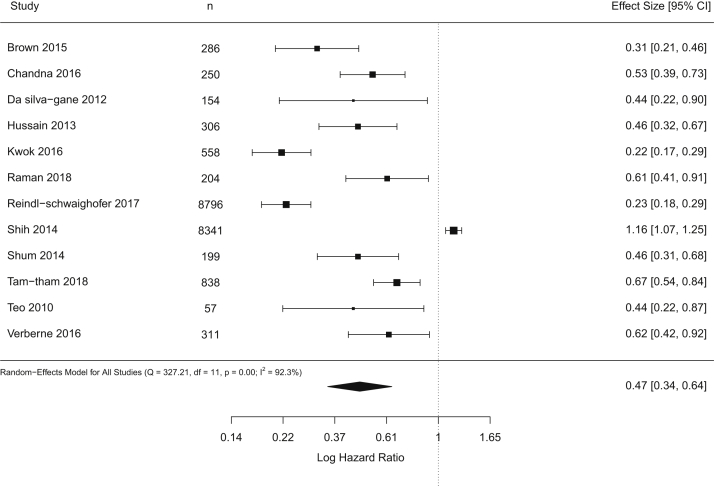
A forest plot in Figure 2 shows results of random-effects meta-analysis. This analysis yielded a pooled HR of 0.47 (95% CI, 0.34-0.64), suggesting that dialysis initiation was associated with considerably reduced mortality. The estimated standard deviation of the true effects on the log-HR scale was 0.47 (95% CI, 0.07-0.66) with significant heterogeneity with I2 of 92%. We were unable to rule out the possibility of publication bias (Fig S1).
Figure 2.
Forest plot shows results of standard random-effects meta-analysis.
Subgroup analysis and metaregression revealed no significant effects (Items S3 and S4). Four studies had relatively low risk for confounding bias (Item S2). The remaining studies did not control for patient age (3 studies), comorbid conditions (4 studies), or patient race and/or sex (7 studies). The overall quality of studies was high; however, most studies did not use a sufficiently representative patient cohort (Item S2).
We estimated the percentage of effect sizes more protective than HR of 0.80 to be 92% (95% CI, 50%-100%). This suggests that in most study settings, dialysis initiation was associated with meaningfully large reductions in mortality. The percentage of effect sizes with an HR > 1 was low (8%; 95% CI, 0%-25%), providing little evidence for harmful effects of dialysis initiation on survival.
Sensitivity Analysis for Unmeasured Confounding
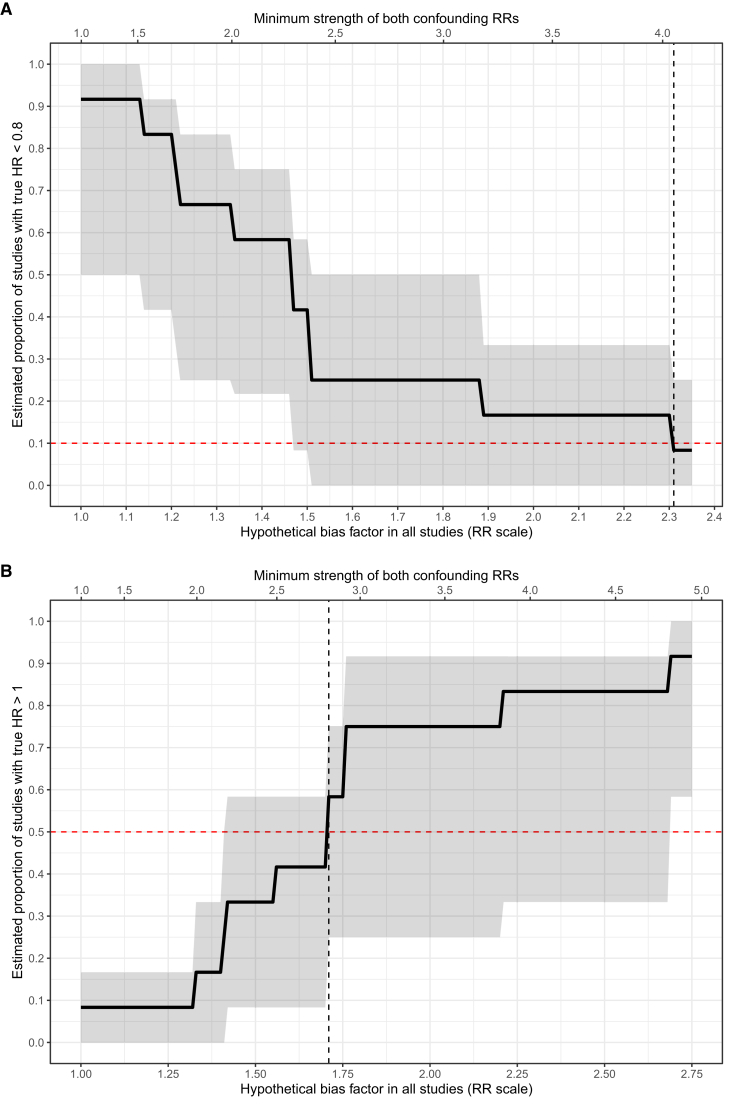
To reduce the percentage of effects more protective than HR of 0.80 from 92% to 10%, we estimated that a bias factor of at least 2.31 (95% CI, 1.51-2.36) would be required in each study (upper part of Fig 3). This means that unmeasured confounding would have needed to shift each study’s point estimate away from the null by 2.31-fold on the RR scale. For instance, in a study with a point estimate representing a 131% increase in the RR for mortality due to dialysis initiation (RR, 2.31), the true point estimate would need to be at most 1.0 after accounting for unmeasured confounding, which indicates no effects of dialysis, to explain away the scientific importance of the overall results.
Figure 3.
Estimated proportion of true effects more protective than hazard ratio (HR) of 0.80 (upper) and harmful rather than protective effects (lower) as a function of hypothetical unmeasured confounding severity in each study. In each diagram, the lower and upper x-axes describe confounding severity, respectively, in terms of the bias factor in each study and the risk ratio by which hypothetical unmeasured confounder(s) would need to be associated with both dialysis initiation and mortality risk. Red horizontal line represents the threshold at which <10% of effects are more protective than HR of 0.80 (upper) or at least 50% of effects indicate harm, rather than benefits (lower); black vertical line is the estimated bias factor or, alternatively, confounding strength at which this occurs. The shaded bands represent 95% bootstrapped CIs.
Using the E-value metric, this bias factor is equivalent to an unmeasured confounder(s) in each study that affects both dialysis initiation and mortality by risk ratios of at least 4.05 each (95% CI, 2.39-4.15). This means that conditional on measured confounding, there would need to be unmeasured confounder(s) in each study that modifies both the RR for starting dialysis (vs conservative care) and the RR for mortality by 4-fold each, a highly unlikely scenario in biomedical research.25 In this context, most studies adjusted for at least a few of the clinically important confounders, such as patient age and other comorbid conditions. We therefore believe it is implausible that there was residual confounding, above and beyond these measured confounders, strong enough to explain away the results as above.
We repeated the analysis for HR > 1.0, representing harmful rather than beneficial effects of dialysis initiation (lower part of Fig 3). To increase the percentage of studies with harmful effects to 50% would require a bias factor of at least 1.71 (95% CI, 1.41-1.76) in each study. This corresponds to unmeasured confounding strengths on the risk ratio scale of at least 2.81 (95% CI, 2.17-2.92) in each study. Hence, our findings of a protective effect of dialysis initiation on mortality appear to be robust to unmeasured confounding of realistic severity.
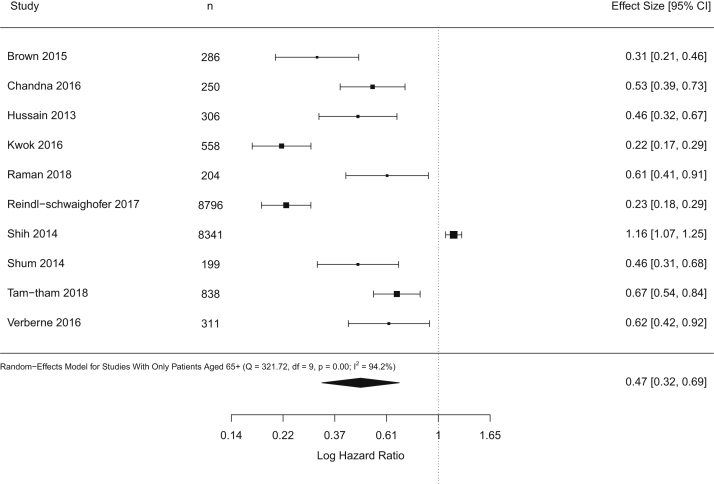
Subgroup Analysis on Adults 65 Years or Older
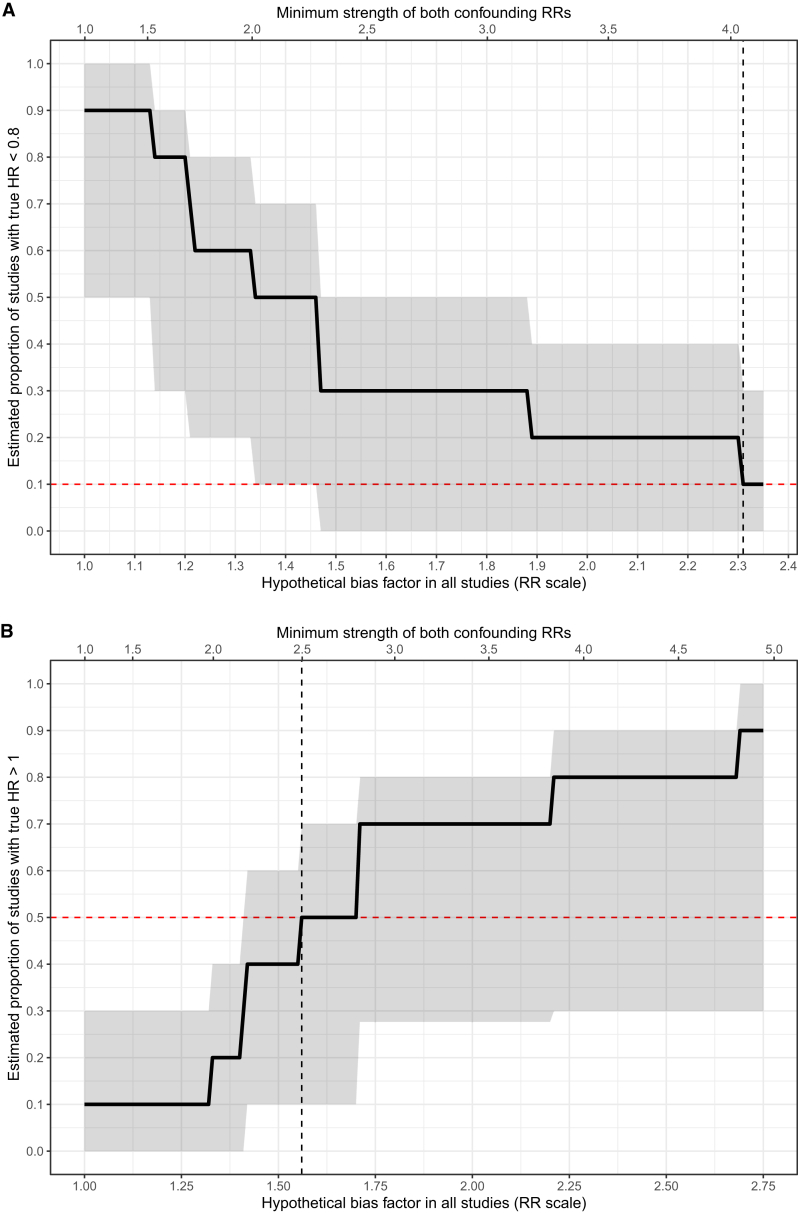
Among the 10 studies that focused on patients 65 years or older, Figure 4 presents the pooled HR for dialysis initiation on mortality (0.47; 95% CI, 0.32-0.69). Heterogeneity was high (I2 = 94%) and was not a result of measured confounders (Item S5). We estimated that 90% (95% CI, 40%-100%) of the true effect estimates were more protective than HRs of 0.80. To reduce this proportion to just 10%, a bias factor of 2.31 (95% CI, 1.47-2.36) would be required in each study, which would be equivalent to having an unmeasured confounder(s) associated with both dialysis initiation and mortality with RRs of 4.05 each (95% CI, 2.30-4.15). To have half the studies with truly harmful rather than protective effects of dialysis, we estimated that a bias factor of at least 1.56 (95% CI, 1.33-1.71) would be required in each study. This would be equivalent to having an unmeasured confounder(s) associated with both dialysis initiation and mortality with RR of 2.49 (95% CI, 1.99-2.81; Fig 5). Hence, we conclude based on this set of studies that the protective effect of starting dialysis for patients 65 years or older on mortality also appears robust to unmeasured confounding.
Figure 4.
Forest plot shows the results of standard random-effects meta-analysis for incident patients 65 years or older.
Figure 5.
Estimated proportion of true effects more protective than hazard ratio (HR) of 0.80 (upper) and harmful rather than protective effects (lower) as a function of hypothetical unmeasured confounding severity in studies of adults 65 years and older. Abbreviation: RR, relative risk.
Subgroup Analysis on Outcomes Beyond Mortality
Five studies assessed a total of 2 outcomes beyond mortality (Item S6). Annual duration of hospitalization was discussed in 3 studies.31,32,34 A random-effects model found that annual hospital days did not differ between dialysis recipients and conservatively managed patients (standardized mean difference, −0.02; 95% CI, −1.39 to 1.36; I2 = 89%). Two studies used the 36-Item Short Form Health Survey (SF-36) instruments to observe improvements in quality of life.26,37 Brown et al37 found no differences in physical (P = 0.12) and mental (P = 0.78) health scores at 12 months between those who had initiated dialysis versus conservative care. Similarly, Da Silva-Gane et al26 found a lack of association between dialysis initiation and monthly change in physical or mental health scores (both P > 0.05).
Discussion
This meta-analysis is a comprehensive investigation on the effects of dialysis initiation on all-cause mortality among incident adult patients with advanced CKD. Two major findings emerged. First, we found dialysis initiation to be associated with a reduction in risk for mortality in all adult incident patients and patients 65 years or older. Second, these survival benefits due to dialysis were robust to potential unmeasured confounding.
In our study, initiating dialysis was associated with reduced mortality for all incident adults with advanced CKD and for patients 65 years or older. For both age groups, we found that unless there was strong unmeasured confounding in each study above and beyond measured confounders, dialysis initiation would remain an important factor in lowering the risk for mortality with clinically meaningful effect sizes. However, as the contour-enhanced funnel plot in Fig S1 illustrates, all studies in the meta-analysis were significant. The relatively small number of studies and lack of any nonsignificant studies precluded quantitative assessment of publication bias; we therefore cannot rule out the possibility that the observed point estimate is inflated due to publication bias.
Our results are in congruence with a previous meta-analysis that found survival advantages associated with dialysis initiation in older patients with stage 5 CKD.6 Moreover, we were able to show that this effect appears robust to unmeasured confounding, which is a stronger conclusion with implications in evidence-based dialysis practice. Our findings suggest that at least in terms of avoiding all-cause mortality, all stage 4-5 incident adult patients, regardless of their age, need to be adequately informed on the commencement of dialysis. However, results of our subgroup analysis provided some evidence that dialysis initiation may not reduce the length of hospitalization. These findings imply that the use of dialysis to improve outcomes beyond survival is not established and considerations on these factors are required to aid patients and families during the decision-making process.
Residual confounding caused by confounders that are unmeasured, unknown, or incorrectly specified is difficult to mitigate. Our study is the first meta-analysis to rigorously assess the possible effects of unmeasured confounders in dialysis outcomes research. We demonstrated how to quantify the extent to which unmeasured confounder(s) could shift the pooled effect estimates to the null.14 Unlike rating methods that qualitatively assess the severity of this bias,38,39 the method we used gives statistically meaningful results and allows for quantitative interpretations on exactly how much unmeasured confounding would be able to modify the pooled estimates.14,25 As a result, instead of just downgrading the evidence, we are able to more directly and quantitatively characterize statistical robustness. We therefore believe that supplementing a standard meta-analysis with a bias analysis of the effects of unmeasured confounding has important benefits.21,23 Nevertheless, observational studies, even when meta-analyzed with sensitivity analyses, still cannot replace randomized controlled trials to confidently rule out the possibility of spurious results due to unmeasured confounding. Our analyses suggest promising results in observational studies that appear robust to unmeasured confounding and conducting randomized trials on this topic would therefore be warranted if ethically feasible.
A major limitation of our study is that we did not conduct meta-analysis on quality-of-life outcomes. This was due to the paucity of studies that reported such outcomes and lack of consensus on the type of statistical procedures in these studies (Item S6). Second, we did not assess the timing of initiating dialysis because randomized controlled trials had been performed to evaluate this topic.40 Third, we did not differentiate dialysis by dose, frequency, or duration, which may introduce even higher heterogeneity into our findings. Instead, we assessed whether the type of dialysis affected the results and did not find any evidence (Items S3 and S5). Fourth, we did not further investigate patients 75 years and older due to the very limited number of studies that examined this patient group (2 studies). Fifth, most included studies were based in Europe and Asia, representing regions with longer life expectancy than some other parts of the world.41 Fifth, we could not rule out the possibility of publication bias due to the small number of studies and lack of any study presenting nonsignificant effects that precluded quantitative assessment of publication bias. Hence, careful assessment of publication bias will be an important task for future work as more studies become available. Last, our sensitivity analyses considered hypothetical unmeasured confounding for which the severity was the same across all studies, though potentially arising from different unmeasured confounder(s).14 Nevertheless, regardless of how much unmeasured confounding might have varied across studies, to shift the pooled HR of 0.47 to the null would require unmeasured confounder(s) associated on average, across studies, with both dialysis initiation and mortality by risk ratios of 2.76-fold each; and to shift the CI of the pooled point estimate to the null would require confounder(s) associated by risk ratios of 2.07-fold each.14 These results corroborate the main findings.
Our study has several strengths. Our results, informed by sensitivity analyses, strongly suggest that dialysis initiation results in lower mortality in adults with advanced CKD, which provides evidence on a causal conclusion stronger than a statistically significant association. In addition, we demonstrate to detail how to perform such bias analysis with R codes, which hopefully can inspire future researchers to explore unmeasured confounding in meta-analyses of observational studies.
This meta-analysis found significant survival advantages due to dialysis initiation for adults with advanced CKD, including those 65 years and older. Findings on patients 75 years and older and on outcomes beyond mortality were limited, which highlights the need for future investigations to provide a more comprehensive understanding of the benefits of dialysis initiation.
Article Information
Authors’ Full Names and Academic Degrees
Rui Fu, MSc, Nigar Sekercioglu, MD, PhD, Maya B. Mathur, PhD, Rachel Couban, MISt, and Peter C. Coyte, PhD.
Authors’ Contributions
Research idea and study design: RF, NS; literature search: RC; study screening and data extraction: RF, NS; statistical analysis: MBM, RF; supervision and mentorship: PCC. Each author contributed important intellectual content during manuscript drafting or revision, accepts personal accountability for the author’s own contributions, and agrees to ensure that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
None.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Data Sharing Statement
All data, R codes and results files are freely available on Open Science Framework (OSF) under the project “Dialysis initiation and mortality meta-analysis” available from https://osf.io/v2yu5/.
Peer Review
Received April 21, 2020. Evaluated by 3 external peer reviewers, with direct editorial input from the Editor-in-Chief. Accepted in revised form September 13, 2020.
Footnotes
Complete author and article information provided before references.
Figure S1: Contour-enhanced funnel plot to assess publication bias in effect estimates in all-cause mortality.
Item S1: Electronic search strategies and results.
Item S2: Assessing level of confounder adjustment and risk of bias.
Item S3: Explore heterogeneity using subgroup analysis.
Item S4: Metaregression by patient age and sex.
Item S5: Explore heterogeneity in studies with only older patients using subgroup analysis and meta-regression.
Item S6: Results of analysis on outcomes beyond mortality.
Table S1: Description of conservative management strategy used in each study
Supplementary Material
Figure S1; Items S1-S6; Table S1
References
- 1.Centers for Disease Control and Prevention Chronic kidney disease in the United States, 2019. https://www.cdc.gov/kidneydisease/pdf/2019_National-Chronic-Kidney-Disease-Fact-Sheet.pdf Published March 13, 2019. Accessed October 15, 2019.
- 2.The Kidney Foundation of Canada Facing the facts: include highlights from the Canadian Organ Replacement Register. https://www.kidney.ca/file/Facing-the-Facts-2018.pdf Published online 2018. Accessed October 14, 2019.
- 3.Levey A.S., Coresh J. Chronic kidney disease. Lancet. 2012;379(9811):165–180. doi: 10.1016/S0140-6736(11)60178-5. [DOI] [PubMed] [Google Scholar]
- 4.Charra B., Calemard E., Ruffet M. Survival as an index of adequacy of dialysis. Kidney Int. 1992;41(5):1286–1291. doi: 10.1038/ki.1992.191. [DOI] [PubMed] [Google Scholar]
- 5.McIntyre C.W., Rosansky S.J. Starting dialysis is dangerous: how do we balance the risk? Kidney Int. 2012;82(4):382–387. doi: 10.1038/ki.2012.133. [DOI] [PubMed] [Google Scholar]
- 6.Wongrakpanich S., Susantitaphong P., Isaranuwatchai S., Chenbhanich J., Eiam-Ong S., Jaber B.L. Dialysis therapy and conservative management of advanced chronic kidney disease in the elderly: a systematic review. Nephron. 2017;137(3):178–189. doi: 10.1159/000477361. [DOI] [PubMed] [Google Scholar]
- 7.Tam-Tham H., Thomas C.M. Does the evidence support conservative management as an alternative to dialysis for older patients with advanced kidney disease? Clin J Am Soc Nephrol. 2016;11(4):552–554. doi: 10.2215/CJN.01910216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCandless L.C. Meta-analysis of observational studies with unmeasured confounders. Int J Biostat. 2012;8(2) doi: 10.2202/1557-4679.1350. [DOI] [PubMed] [Google Scholar]
- 9.Moher D., Liberati A., Tetzlaff J., Altman D.G., The PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stroup D.F., Berlin J.A., Morton S.C. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 11.Turner R.M., Spiegelhalter D.J., Smith G.C.S., Thompson S.G. Bias modelling in evidence synthesis. J R Stat Soc Ser A Stat Soc. 2009;172(1):21–47. doi: 10.1111/j.1467-985X.2008.00547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Welton N.J., Ades A.E., Carlin J.B., Altman D.G., Sterne J.A.C. Models for potentially biased evidence in meta-analysis using empirically based priors. J R Stat Soc Ser A Stat Soc. 2009;172(1):119–136. [Google Scholar]
- 13.Mathur M.B., VanderWeele T.J. Robust metrics and sensitivity analyses for meta-analyses of heterogeneous effects. Epidemiology. 2020;31:356–358. doi: 10.1097/EDE.0000000000001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mathur M.B., VanderWeele T.J. Sensitivity analysis for unmeasured confounding in meta-analyses. Published online February 6, 2019. J Am Stat Assoc. 2020;115(529):163–172. doi: 10.1080/01621459.2018.1529598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bormann I. DigitizeIt. 2016 Accessed October 14, 2019, https://www.digitizeit.de/ [Google Scholar]
- 16.Guyot P., Ades A., Ouwens M.J., Welton N.J. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2012;12:9. doi: 10.1186/1471-2288-12-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hartung J., Knapp G. A refined method for the meta-analysis of controlled clinical trials with binary outcome. Stat Med. 2001;20(24):3875–3889. doi: 10.1002/sim.1009. [DOI] [PubMed] [Google Scholar]
- 18.Peters J.L., Sutton A.J., Jones D.R., Abrams K.R., Rushton L. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J Clin Epidemiol. 2008;61(10):991–996. doi: 10.1016/j.jclinepi.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 19.Mathur M.B., VanderWeele T.J. New metrics for meta-analyses of heterogeneous effects. Stat Med. 2019;38(8):1336–1342. doi: 10.1002/sim.8057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.VanderWeele T.J. On a square-root transformation of the odds ratio for a common outcome. Epidemiology. 2017;28(6):e58–e60. doi: 10.1097/EDE.0000000000000733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morrison A.E., Zaccardi F., Khunti K., Davies M.J. Causality between non-alcoholic fatty liver disease and risk of cardiovascular disease and type 2 diabetes: a meta-analysis with bias analysis. Liver Int. 2019;39(3):557–567. doi: 10.1111/liv.13994. [DOI] [PubMed] [Google Scholar]
- 22.Wells G., Shea B., O’Connell D. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp Accessed November 7, 2020.
- 23.Baumeister S.E., Leitzmann M.F., Linseisen J., Schlesinger S. Physical activity and the risk of liver cancer: a systematic review and meta-analysis of prospective studies and a bias analysis. J Natl Cancer Inst. 2019;111:1142–1151. doi: 10.1093/jnci/djz111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ding P., VanderWeele T.J. Sensitivity analysis without assumptions. Epidemiology. 2016;27(3):368–377. doi: 10.1097/EDE.0000000000000457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.VanderWeele T.J., Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med. 2017;167(4):268–274. doi: 10.7326/M16-2607. [DOI] [PubMed] [Google Scholar]
- 26.Da Silva-Gane M., Wellsted D., Greenshields H., Norton S., Chandna S.M., Farrington K. Quality of life and survival in patients with advanced kidney failure managed conservatively or by dialysis. Clin J Am Soc Nephrol. 2012;7(12):2002–2009. doi: 10.2215/CJN.01130112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hussain J.A., Mooney A., Russon L. Comparison of survival analysis and palliative care involvement in patients aged over 70 years choosing conservative management or renal replacement therapy in advanced chronic kidney disease. Palliat Med. 2013;27(9):829–839. doi: 10.1177/0269216313484380. [DOI] [PubMed] [Google Scholar]
- 28.Chandna S.M., Carpenter L., Da Silva-Gane M., Warwicker P., Greenwood R.N., Farrington K. Rate of decline of kidney function, modality choice, and survival in elderly patients with advanced kidney disease. Nephron. 2016;134(2):64–72. doi: 10.1159/000447784. [DOI] [PubMed] [Google Scholar]
- 29.Verberne W.R., Geers A.B.M.T., Jellema W.T., Vincent H.H., van Delden JJM, Bos W.J.W. Comparative survival among older adults with advanced kidney disease managed conservatively versus with dialysis. Clin J Am Soc Nephrol. 2016;11(4):633–640. doi: 10.2215/CJN.07510715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reindl-Schwaighofer R., Kainz A., Kammer M., Dumfarth A., Oberbauer R. Survival analysis of conservative vs. dialysis treatment of elderly patients with CKD stage 5. PLoS One. 2017;12(7) doi: 10.1371/journal.pone.0181345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raman M., Middleton R.J., Kalra P.A., Green D. Outcomes in dialysis versus conservative care for older patients: a prospective cohort analysis of stage 5 chronic kidney disease. PLoS One. 2018;13(10) doi: 10.1371/journal.pone.0206469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Teo B.W., Ma V., Xu H., Li J., Lee E.J. Profile of hospitalisation and death in the first year after diagnosis of end-stage renal disease in a multi-ethnic Asian population. Ann Acad Med Singapore. 2010;39(2):79–87. [PubMed] [Google Scholar]
- 33.Shih C.-J., Chen Y.-T., Ou S.-M., Yang W.-C., Kuo S.-C., Tarng D.-C. The impact of dialysis therapy on older patients with advanced chronic kidney disease: a nationwide population-based study. BMC Med. 2014;12(1):169. doi: 10.1186/s12916-014-0169-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shum C., Tam K., Chak W. Outcomes in older adults with stage 5 chronic kidney disease: comparison of peritoneal dialysis and conservative management. J Gerontol Biol Sci Med Sci. 2014;69(3):308–314. doi: 10.1093/gerona/glt098. [DOI] [PubMed] [Google Scholar]
- 35.Kwok W.-H., Yong S.-P., Kwok O.-L. Outcomes in elderly patients with end-stage renal disease: comparison of renal replacement therapy and conservative management. Hong Kong J Nephrol. 2016;19:42–56. [Google Scholar]
- 36.Tam-Tham H., Quinn R.R., Weaver R.G. Survival among older adults with kidney failure is better in the first three years with chronic dialysis treatment than not. Kidney Int. 2018;94(3):582–588. doi: 10.1016/j.kint.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 37.Brown M.A., Collett G.K., Josland E.A., Foote C., Li Q., Brennan F.P. CKD in elderly patients managed without dialysis: survival, symptoms, and quality of life. Clin J Am Soc Nephrol. 2015;10(2):260–268. doi: 10.2215/CJN.03330414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deeks J.J., Dinnes J., D’Amico R. Evaluating non-randomised intervention studies. Health Technol Assess. 2003;7(27) doi: 10.3310/hta7270. iii-x, 1-173. [DOI] [PubMed] [Google Scholar]
- 39.Guyatt G., Oxman A.D., Akl E.A. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383–394. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 40.Slinin Y., Greer N., Ishani A. Timing of dialysis initiation, duration and frequency of hemodialysis sessions, and membrane flux: a systematic review for a KDOQI clinical practice guideline. Am J Kidney Dis. 2015;66(5):823–836. doi: 10.1053/j.ajkd.2014.11.031. [DOI] [PubMed] [Google Scholar]
- 41.United Nations . UN; 2019. World Mortality 2019: Data Booklet. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1; Items S1-S6; Table S1