Abstract
Diabetes-related complications are a significant source of morbidity and mortality worldwide. Diabetic kidney disease is a frequent microvascular complication and a primary cause of kidney failure in patients with diabetes. The glomerular filtration barrier is composed of 3 layers: the endothelium, glomerular basement membrane, and podocytes. Podocytes and the endothelium communicate through molecular crosstalk to maintain filtration at the glomerular filtration barrier. Chronic hyperglycemia affects all 3 layers of the glomerular filtration barrier, as well as the molecular crosstalk that occurs between the 2 cellular layers. One of the earliest events following chronic hyperglycemia is endothelial cell dysfunction. Early endothelial damage is associated with progression of diabetic kidney disease. However, current therapies are based in controlling glycemia and arterial blood pressure without targeting endothelial dysfunction. Disruption of the endothelial cell layer also alters the molecular crosstalk that occurs between the endothelium and podocytes. This review discusses both the physiologic and pathologic communication that occurs at the glomerular filtration barrier. It examines how these signaling components contribute to podocyte foot effacement, podocyte detachment, and the progression of diabetic kidney disease.
Index Words: Podocyte-endothelial crosstalk, diabetic nephropathy, microvascular, albuminuria, diabetic kidney disease
A challenging clinical problem
Diabetic kidney disease (DKD) is a frequent microvascular complication of diabetes and is the cause of kidney failure in ∼40% of people with diabetes.1 In 2015, it was estimated that more than 415 million people had diabetes worldwide, and it is expected that by 2040, the prevalence may increase to 642 million, with disproportionate growth in low- and middle-income countries.2 The recent surge of epidemiologic studies linking microvascular complications to diabetes highlights the urgent need to develop therapeutic alternatives that target microvascular complications to diminish these alarming figures.3,4 Few therapies are targeting endothelial damage or pathologic podocyte-endothelial crosstalk, both of which play a critical role in DKD progression.4,5
Cellular components of the glomerular filtration barrier
Glomeruli are composed of 3 distinct layers necessary for the filtration of plasmatic molecules and electrolytes: the endothelium, glomerular basement membrane (GBM), and podocytes (Fig 1A). The endothelium constitutes the first layer of the glomerular filtration barrier and is characterized by the presence of fenestrations, measuring between 70 and 100 nm in diameter.6 It is coated on the luminal surface by a gelatinous glycoprotein-rich structure called the glycocalyx that separates and protects endothelial cells from the flowing blood.7 Endothelial cells are separated from podocytes by the GBM, a complex mesh of extracellular matrix proteins, including type IV collagen, laminins, fibronectins, and proteoglycans.8 The components of the GBM are secreted from both podocytes and endothelial cells to form a hybrid basement membrane. The podocytes are an epithelial cell layer that can be divided into 3 segments: cell body, microtubule-rich major processes, and actin-based foot processes.9 The molecular interactions that occur at the slit diaphragm, an adhesive structure that joins individual foot processes, allow podocytes to control filtration inside glomeruli. Single-cell RNA sequencing experiments in mice revealed that endothelial cells make up between 2% and 3% of total kidney cells in wild-type mice, whereas podocytes make-up ∼0.2% of the total cellular portion of the kidney.10 In contrast, kidney single-cell transcriptomic analysis of human normal kidney biopsy specimens reveals a higher percentage (∼11.5%) of endothelial cells in the kidney.11 Inside the glomerulus, endothelial cells make up ∼12% of cells inside the glomerulus, while podocytes and mesangial cells are 80% and 2%, respectively.12
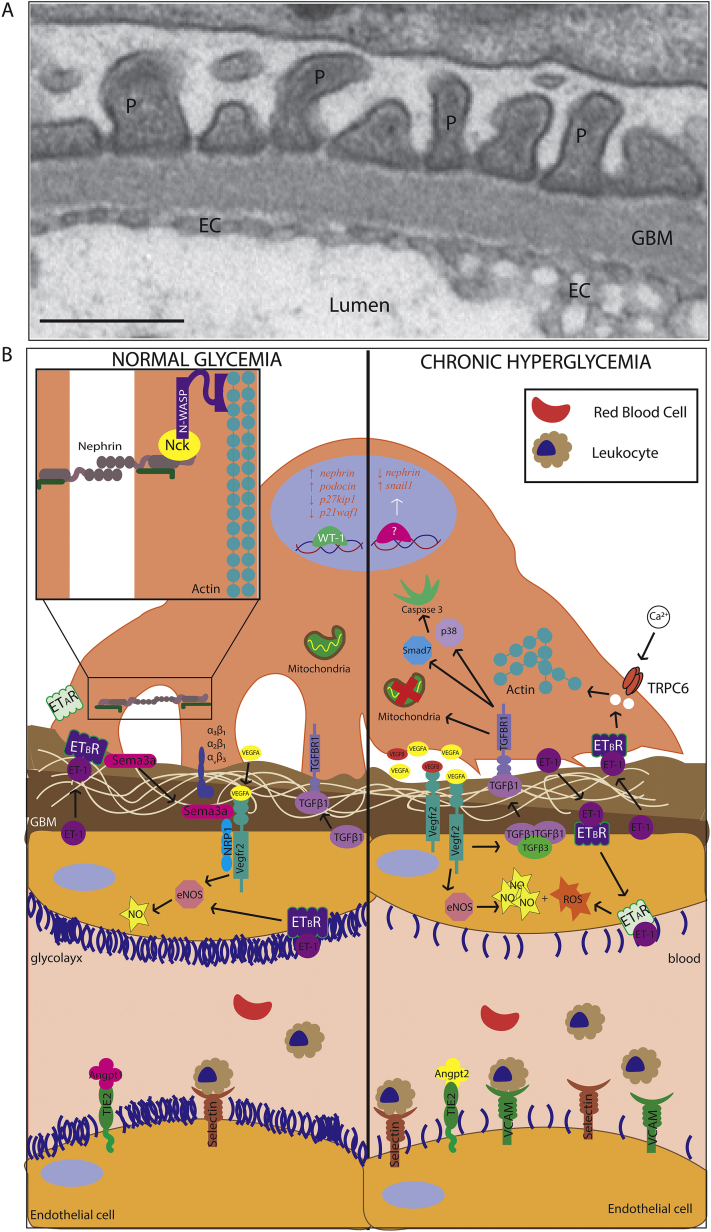
Figure 1.
Endothelial-podocyte crosstalk. (A) Transmission electron micrograph of a normal mouse glomerular filtration barrier shows podocyte (P) foot process, glomerular basement membrane (GBM), and endothelial cells (EC; scale bar: 500 nm). (B) Under physiologic conditions (left), various secreted factors are exchanged between podocytes and endothelial cells to allow for maintenance of the glomerular filtration barrier. During diabetic nephropathy (right), increased reactive oxygen species (ROS) production can lead to thinning of the glycocalyx of the endothelium, as well as cellular damage to both endothelial cells and podocytes. Overactivation of several pathways leads to endothelial dysfunction, podocyte foot effacement, and eventually podocyte detachment. Abbreviations: eNOS, endothelial nitric oxide synthase; ETAR, endothelin A receptor; TGFβ, transforming growth factor β; TRPC6, transient receptor potential canonical 6.
Both the endothelial and podocyte cellular layers interact directly with the GBM and each other through various secreted factors (Fig 1B). Recent studies also show that several cellular populations in the kidney can communicate through secreted exosomes.13 In particular, in vitro models have demonstrated that exosomes can be purified from glomerular endothelial cells and induce biological effects in podocytes.14 Exosomes can contain messenger RNA and microRNA and protein, allowing signaling to occur. Intriguingly, the quantity and contents of exosomes has been demonstrated to be altered in various kidney diseases, including DKD.14,15
Podocyte-endothelial cell crosstalk and regulation of the glomerular filtration barrier
Molecules secreted from both podocytes and endothelial cells help stabilize the slit diaphragm and maintain the structural integrity of both the endothelial and podocyte layers. One of the most well-established pathways involved in podocyte-endothelial cell crosstalk is the vascular endothelial growth factor (VEGF) signaling pathway. The VEGF family consists of 5 ligands (VEGF-A-D and placental growth factor) and 3 receptors (VEGFR1-3). In the kidney, podocytes are a primary source of VEGF-A. Podocyte-derived VEGF-A is necessary for the survival and function of glomerular endothelial cells.16 In the kidney, both loss and gain of podocyte-derived VEGF-A leads to endothelial dysfunction, podocyte foot effacement, and thickening of the GBM, demonstrating the importance of VEGF signaling to the glomerulus.16, 17, 18 Reduced VEGF levels can result in endothelial cell damage leading to podocyte loss and thickening of the GBM, whereas excessive VEGF production results in neovascularization leading to pathologic microangiopathy.
The primary receptor for VEGF-A, VEGFR2 (FLK-1/KDR), is expressed by both endothelial and podocyte cells, indicating that both cell types may be responsive to local changes in VEGF-A concentration. Genetic and pharmacologic removal of the VEGF-A ligand clearly disrupts both podocyte and endothelial cell function. However, podocyte-specific inhibition of VEGF-A signaling through deletion of the VEGFR2 in podocytes does not impair podocyte function or alter podocyte morphology.17 These studies suggest that the disruption of podocyte function after VEGF-A depletion arises from endothelial cell dysfunction and a disruption of podocyte-endothelial crosstalk, rather than impaired VEGF-A signaling in podocytes.
In contrast to VEGF signaling, which is necessary for maintenance of the glomerular filtration barrier, transforming growth factor β (TGFβ) signaling is largely detrimental to both podocytes and endothelial cells in the glomerulus.19 TGFβ1 belongs to the TGFβ superfamily that comprises several members, including TGFβ1-3, activins, bone morphogenetic proteins (BMPs), and growth differentiation factor ligands and their receptors.20 Upon ligand binding to a complex of TGFβ receptors (TGFβRs), the Smad proteins can be phosphorylated and translocate to the nucleus to modify gene transcription.21 While during kidney development, many BMP and TGFβ ligands are expressed, in adult human glomeruli, ligands of the TGFβ family have little to no expression.22, 23, 24 Multiple studies indicate that activating TGFβ signals can lead to podocyte apoptosis and foot effacement, decreasing VEGF production and ultimately leading to endothelial cell death.19,25 In many cell types, TGFβ signaling has been implicated in epithelial- (or endothelial)-to-mesenchymal transitions.26 Both in vitro and in vivo studies indicate that TGFβ1 treatment can dedifferentiate or induce epithelial-to-mesenchymal transitions in podocytes.27 Endothelial cells may also be affected by the production of TGFβ1. One in vivo study demonstrated that removal of the TGFβR, TGFβRII, in endothelial cells was sufficient to inhibit endothelial-to-mesenchymal transition from occurring in 2 kidney disease models.28
Other soluble molecules have also been shown to mediate the crosstalk between endothelial cells and podocytes. Angiopoietin 1, which is produced by podocytes, can promote microvascular growth through the Tie2 receptor expressed in glomerular endothelial cells.29 Interestingly, angiopoietin 2, a natural antagonist of Tie2, has been found to be upregulated in DKD, and its podocyte-specific overexpression can lead to endothelial apoptosis and albuminuria.30,31 Stromal cell–derived factor 1, which is produced by podocytes, can elicit signaling in neighboring endothelial cells through the CXCR4 receptor.32 Endothelial-specific inactivation of CXCR4 has been shown to adversely affect renal angiogenesis, and inactivation of stromal cell–derived factor 1 diabetic mice has been shown to prevent albuminuria.33 Finally, Sema3a, a member of the semaphorin family, is produced by podocytes and can bind to the receptor neuropilin 1 expressed in glomerular endothelial cells, where it can modulate VEGF/VEGFR2 signaling. Podocyte-specific overexpression of Sema3a has been shown to result in endothelial apoptosis, whereas its deletion is accompanied by endothelial overgrowth.34,35
These examples demonstrate how secreted signals exchanged between podocytes and endothelial cells regulate both the survival and maintenance of these 2 cellular populations and the GBM.
Early glomerular endothelial cell alterations in diabetes
Chronic hyperglycemia is the principal cause of kidney-related diabetic microangiopathy.36 Metabolic dysregulation, reactive oxygen species (ROS) production, polyol pathway activation, and advanced glycation end product (AGE) formation have all been reported to contribute to the progression of microvascular complications in diabetes.37 The earliest event resulting from the activation of these pathways is endothelial dysfunction. Importantly, early endothelial damage is associated with the progression of DKD.38,39
Under physiologic conditions, glucose is taken up by endothelial cells through various glucose transporters. Endothelial cells express primarily glucose transporter 1 (GLUT1), and the expression of GLUT1 in endothelial cells has not been reported to change during hyperglycemic conditions.40 Despite a low dependency on the mitochondria, endothelial cells, which rely primarily on anaerobic glycolysis for energy production,41 demonstrate mitochondrial defects under diabetic conditions that contribute to microvascular dysfunction. Both mitochondrial fission and a switch to glycolysis have been reported to lead to a loss of barrier function and a loss of podocytes during hyperglycemic conditions.42,43 Endothelial cells also experience higher ROS levels due to mitochondrial dysfunction from hyperglycemia.42,43 Interestingly, in a model of glomerulosclerosis, scavenging of mitochondrial ROS produced by endothelial cells could prevent podocytes loss,44 suggesting that ROS production in one cell type may affect the function of the others.
In addition to the damage caused by metabolic dysregulation and ROS accumulation, chronic hyperglycemia can induce vascular dysfunction by decreasing glycocalyx thickness.45 This event has been associated with microalbuminuria in patients with DKD.46 Increased plasma levels of both hyaluronan, a key glycosaminoglycan abundantly present in the glycocalyx, and the enzyme that degrades hyaluronan, hyaluronidase, have been observed in patients with diabetes.47 Plasma levels of other glycocalyx components, such as hemagglutinin glycoprotein, heparan sulfate, and syndecan, have also been observed to increase during chronic hyperglycemia.48 The increased levels of these factors in plasma is thought to be a consequence of glycocalyx degradation.49 Hyperglycemic conditions also produce ROS that can alter glycosaminoglycans, in particular hyaluronan, ultimately disrupting its polymerization.50,51
Finally, endothelial damage is also strongly involved in promoting the expression of inflammatory molecules such as cytokines and CP-1 that affect podocytes.52 The proinflammatory environment in the diabetic kidney also leads to an increase of the adhesion molecules intercellular adhesion molecule 1, vascular cell adhesion molecule 1, P-selectin, and E-selectin on the surface of endothelial cells.53, 54, 55 A recent study demonstrated that expression of the adhesion marker vascular cell adhesion molecule 1 can be directly regulated by secreted cytokines and chemokines such as CCL2 coming from podocytes.56
Functionally, the mentioned studies are supported by data demonstrating that increased expression of various adhesion molecules is associated with increased infiltration of distinct immune cell populations in a rat model of diabetes.57 The increased expression of leukocyte adhesion molecules on endothelial cells also correlated with increased proteinuria in this model.57 Furthermore, single-cell or single-nucleus transcriptomic studies in both humans and mice demonstrated increased immune cell infiltration in diabetic kidneys.58,59 In mice, M1 macrophages were found to be the predominant immune cell type inside the glomerulus, whereas in human kidney biopsy samples, T cells represented 49% of the infiltrating leukocytes in the diabetic kidney.58,59 It is important to note that the mouse model used was an endothelial nitric oxide (NO) synthase (eNOS) knock-out streptozocin-induced model of diabetes.58 Because eNOS is globally reduced, this loss may itself affect the immune system when compared with wild-type mice.
Podocyte alterations in diabetes
Endothelial dysfunction is typically accompanied by podocyte foot effacement or detachment and a progressive decrease in estimated glomerular filtration rate when poor glycemic control and inadequate blood pressure are present.4,60 We discuss some of the mechanisms leading to podocyte alterations associated with DKD, which are summarized in Table 1.25,61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77
Table 1.
Factors Involved in Podocyte Foot Effacement and Detachment During DKD
| Molecule | Changes in DKD | Role | Reference |
|---|---|---|---|
| Podocyte Effacement | |||
| Nephrin | Downregulated | Disruption of SD proteins Insulin resistance in podocytes |
61, 62, 70 |
| TRPC6 and PLCγ1 | Upregulated | Increase of calcium influx into podocytes leading to reorganization of actin filaments and SD disruption | 63-69 |
| ZO-1 | Upregulated | Podocyte foot reorganization | 69 |
| Sns | Downregulated | Actin rearrangement Dysregulation of insulin pathway in podocytes |
70 |
| Podocyte Detachment | |||
| Glycemia | Increased | Podocyte and mesangial cell hypertrophy resulting in loss of adhesion to the GBM | 73-75 |
| WT1 | Downregulated | Development of proteinuria and kidney disease progression | 71, 72 |
| ROS | Increased | Mitochondrial stress leading to podocyte apoptosis | 77 |
| α3β1 integrin | Downregulated | Focal podocyte detachment from the GBM | 76 |
| p38MAPK and Smad7 | Upregulated | Induction of apoptosis through caspase3 activation | 25 |
Abbreviations: DKD, diabetic kidney disease; GBM, glomerular basement membrane; p38MAPK, p38 mitogen-activated protein kinase; PLCγ1, phospholipase Cγ1; ROS, reactive oxygen species; SD, slit diaphragm; TRPC6, transient receptor potential canonical 6; WT1, Wilms tumor 1; ZO-1, zonula occludens 1.
Podocyte Foot Effacement
Podocyte foot effacement in DKD is a major cause of proteinuria. Disruption of the slit diaphragm (SD) is a feature of most instances of podocyte foot effacement. Disruption of glomerular SD proteins, such as nephrin in DKD, has been experimentally demonstrated to play a role in albuminuria.61,62 One study histologically compared nephrin expression in 15 patients with type 2 diabetic versus 12 nondiabetic control patients. The authors identified a statistically significant decrease in nephrin and podocin expression in 100% of patients with DKD diagnosed and exhibiting either micro- or macroalbuminuria, leading them to conclude that nephrin could be used as an indicator of early kidney damage in DKD.78 Patients with DKD also exhibit decreased messenger RNA and protein expression of nephrin, which has been strongly correlated to albuminuria in patients with DKD.61,62
Chronic hyperglycemia can also lead to disruption of nephrin and the SD through increased expression of the transient receptor potential canonical 6 (TRPC6) calcium channel.63 TRPC6 binds to phospholipase Cγ1 (PLCγ1) and mediates the influx of calcium.64 Nephrin-mediated activation of PLCγ1, a known regulator of calcium signalling, also leads to increased entry of calcium into podocytes.65 This increased calcium influx causes reorganization of actin filaments that ends in SD disruption.66, 67, 68 Intriguingly, a recent study also demonstrates that zonula occludens-1 (ZO-1) on the foot process of podocytes is reorganized after calcium influx under hyperglycemic conditions in a TRPC6-dependent manner, suggesting that TRPC6 may directly contribute to foot-process effacement.69
Nephrin also plays an important role regulating podocyte insulin sensitivity.79 Knockdown of nephrin in podocytes blocks the ability of these cells to respond to insulin and impairs the ability of podocytes to uptake glucose.79 The cytoplasmic domain of nephrin enables the docking of glucose transporters GLUT1 and GLUT4 in the plasma membrane of podocytes.79 Surprisingly, insulin sensitivity effects of nephrin expression are conserved even in an insect model of chronic hyperglycemia. Expression of the nephrin-like protein Sns in Drosophila was reduced under conditions of chronic hyperglycemia. Loss of Sns expression also led to actin rearrangement, loss of the nephrocyte diaphragm function, and dysregulation of the insulin signaling pathway. Using this fly model of chronic hyperglycemia, the authors identified a novel transcription factor negatively regulating nephrin expression.70 Reduction of Drosophila Knot or mouse Ebf2 conferred resistance to glucose and stabilization of Sns and nephrin at the slit diaphragm in nephrocytes and podocytes, respectively.70 This and other studies suggest that loss of nephrin during conditions of chronic hyperglycemia contributes to podocyte insulin resistance. However, some studies have also reported elevations in nephrin expression and location in patients with types 1 and 2 diabetes,61 which may be related to the duration of diabetes and hypertension.
Podocyte Detachment
In addition to podocyte foot effacement, podocyte detachment has been observed in histology samples of patients with diabetes.71,72 Podocyte detachment or loss can be measured by examining the number of Wilms tumor 1 (WT-1)–expressing cells. A reduction in WT-1–positive podocyte cell number and density per glomerulus has been linked to the development of both proteinuria and kidney disease progression.71,72
Various mechanisms have been described regarding the way in which podocytes can detach from the GBM. One of the earliest phenomena taking place in podocyte detachment is podocyte hypertrophy.80 Under conditions of chronic hyperglycemia, while endothelial and mesangial cells proliferate, podocytes are unable to proliferate and may increase in size to cover the glomerular tuft.73,74 In some instances, chronic hyperglycemia can also induce hypertrophy of both the podocyte and mesangial cells.75 Hypertrophic mesangial cells are not able to properly synthesize the extracellular matrix, causing worsened hypertrophy of podocytes.81 When the stress is removed, podocytes fail to return to their normal size.82 This adaptive response of podocytes leads to both molecular and structural changes to the podocyte and eventually results in deterioration of their function and adhesion to the GBM. Podocytes may increase the number of occludin-based tight junctions to counteract the loss of their function during hypertrophy. Increasing the occludin-type tight junctions temporarily prevents podocyte foot effacement but is not sustainable over the long term. Eventually, the loss of the SD and junctions leads to detachment of the podocyte from the GBM.83,84 Moreover, mechanical forces such as increased glomerular pressure that are associated with hyperfiltration may also exacerbate podocyte hypertrophy and disrupt the connection between podocytes and GBM.73,82 Importantly, hyperfiltration is frequently observed in diabetic patients.85,86
At the molecular level, the physical disruption of podocyte-GBM attachment is often observed as a disruption of integrin signaling. Numerous studies in cultured podocytes have shown that exposure to high glucose levels reduces α3β1 integrin expression.87,88 This reduction in α3β1 integrin has also been reported in both human patients with DKD and streptozotocin-induced diabetic rats.87 Loss or reduction of α3β1 integrin can lead to focal detachment of podocytes from the GBM and ultimately separation of the podocyte from the GBM.76 Another common cause for a decrease in podocyte number is cellular apoptosis. Podocytes exposed to high glucose levels increase ROS production and activate p38 mitogen-activated protein kinase (p38MAPK) signaling, a mediator of mitochondrial stress. Both these signaling events can lead to the initiation of podocyte apoptosis in situ.77 During DKD, TGFβ, a potent inducer of podocyte apoptosis, is upregulated and may also activate p38MAK signaling. Both Smad7 and p38MAPK may act downstream of TGFβ to induce apoptosis through caspase3 activation.25
Pathologic podocyte–endothelial cell crosstalk in dkd
Multiple secreted factors that facilitate the physiologic crosstalk between podocytes and endothelial cells are affected in hyperglycemic conditions (Fig 1B and Table 214,44,58,59,62,72,89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101).
Table 2.
Molecules Involved in Pathologic Crosstalk Between Podocytes and Endothelial Cells During Diabetic Nephropathy
| Molecules | Pathologic Processes | Potential Clinical Therapy | References |
|---|---|---|---|
| VEGF-A, VEGF-B, VEGF-C | VEGF-A: angiogenesis of microvessels, decrease in glycocalyx, modified GBM VEGF-B: fatty acid transport (lipid accumulation) VEGF-C: reduces VEGF-A–induced albumin permeability |
Anti-VEGF antibodies, small molecule inhibitors (SU5416), angiostatin, endostatin | 89, 90, 93-96 |
| TGFβ1, TGFβ3, BMP7, LTBP1 | TGFβ1: endothelial cell apoptosis, renal microangiopathy, epithelial-mesenchymal transition of podocytes, endothelial-mesenchymal transition TGFβ3: podocyte-mesangial cell crosstalk BMP7: exogenous BMP7 improves GFR LTBP1: regulates targeting of TGFβ complexes |
Neutralizing antibodies, antisense oligonucleotides, small molecule inhibitors (SISI3) | 14, 58, 59, 62, 72, 91, 99-101 |
| ET-1 | Mitochondrial oxidative stress, endothelial cell dysfunction, loss of slit diaphragm organization | Endothelin receptor antagonists (Sitaxentan, Atrasentan) | 44, 92, 97, 98 |
Note: All molecules are upregulated.
Abbreviations: BMP7, bone morphogenetic protein 7; ET-1, endothelin 1; GBM, glomerular basement membrane; GFR, glomerular filtration rate; LTBP1, latent-transforming growth factor β-binding protein 1; TGFβ, transforming growth factor β; VEGF, vascular endothelial growth factor.
Clinical data have demonstrated a significant increase in circulating VEGF levels in diabetic patients.102 It has been demonstrated by multiple groups that persistent high glucose levels stimulate podocytes to produce VEGF-A.103,104 Multiple factors can lead to altered VEGF-A secretion and availability from the podocytes. WT-1, a podocyte-specific transcription factor, can regulate the gene expression of various genes involved in the pathogenesis of DKD including VEGF-A.105 Furthermore, VEGF in the diabetic kidney can also be increased by the accumulation of AGEs during chronic hyperglycemia.106,107 AGE accumulation in all tissues has been shown to lead to VEGF overproduction in vivo and in vitro.75 Additionally, modifications of the extracellular matrix components, such as the reduction of heparan sulfate proteoglycans (HSPGs) in DKD, also affect the availability and affinity of growth factor ligands such as VEGF-A. HSPGs situated on the cell surface or in the extracellular matrix can mediate receptor-ligand interactions of VEGF-A, basic fibroblast growth factor, and heparin-binding epidermal growth factor.108 Increased expression and activity of heparanases in DKD decreases HSPG levels, ultimately reducing growth factor signaling in the cell populations that express these receptors.108
The excessive levels of VEGF-A produced by podocytes induce the endothelium to undergo angiogenesis, leading to the formation of immature microvessels in the glomerulus.89,109,110 Increased circulating VEGF also leads to alterations of the GBM and a decrease in the glycocalyx, which further promotes leaky permeable vessels in DKD.90 At the molecular level, the overproduction of VEGF in DKD also leads to oxidative stress in both endothelial cells and podocytes.111 VEGF signaling in endothelial cells increases eNOS activity.91 Although in physiologic conditions, activation of VEGFR2 will activate PI3K and Akt, which phosphorylate and activate eNOS and increase NO production,91 in chronic hyperglycemia, VEGFR2 overactivation increases ROS production, in particular superoxide (O2-), worsening endothelial damage.72,112 ROS molecules can also decrease the bioavailability of NO by forming the damaging reactive nitrogen species, nitrate (NO3−).72 ROS and reactive nitrogen species production have also been associated with podocyte damage and foot effacement.113 In agreement with these data, increased eNOS activity can promote podocyte detachment and is also associated with increased microalbuminuria levels in diabetic patients.62
VEGF-A is not the only ligand of the VEGF family that is increased in models of diabetes. A recent study demonstrated that VEGF-B is also increased in 3 separate rodent models of diabetes. VEGF-B upregulation in diabetic mice increased fatty acid transport across the endothelium, ultimately resulting in lipid accumulation within the podocyte. Removal of VEGF-B could restore the function of the glomerular filtration barrier.114 Furthermore, increasing expression of VEGF-C, secreted by podocytes, could also reduce VEGF-A–induced albumin permeability. This effect was dependent on the presence of the glycocalyx on endothelial cells and was mediated by VEGFR2 and VEGFR3.115
In addition to the striking effects on the VEGF ligands, hyperglycemic conditions also increase expression of the TGFβ family of ligands.14,58 TGFβ1, TGFβ3, and BMP7 are all reported to be increased in models of DKD.14,58 Expression of LTBP1 (latent-transforming growth factor beta-binding protein 1), a regulator of TGFβ signaling, has also been reported to be altered in human DKD.59 Exosomes isolated from glomerular endothelial cells treated with high glucose were found to induce an increase in TGFβ1, both locally and in podocytes exposed to the purified exosomes.14 Autocrine TGFβ signaling by endothelial cells promotes endothelial cell apoptosis and ultimately worsens kidney microangiopathy in DKD.72,116 Increased TGFβ production can also be detrimental to podocytes. When treated with endothelial exosomes, podocytes also went through an epithelial to mesenchymal transition that was associated with a loss of barrier function.14 Other studies have also indicated that activation of the TGFβ receptor, TGFβR1, in podocytes was associated with increased release of endothelin 1 (ET-1) from podocytes, podocyte apoptosis, and endothelial dysfunction.44 Endothelin A receptor (ETAR) activation in the endothelium by podocyte-derived ET-1 caused mitochondrial oxidative stress and endothelial cell dysfunction, ultimately leading to podocyte drop out and apoptosis. These effects could be prevented by both ROS scavenging and inhibition of ETAR.44 In agreement with these data, increased ET-1 levels have also been found in plasma of patients with diabetes type 2.117 Podocytes detect the increased ET-1 levels through the ETBR receptor. Activation of ETBR allows rapid entry of calcium into the podocytes, which leads to rearrangement and loss of the organization of the actin filaments in the SD.92 Activation of the ETAR in podocytes also regulates the glomerular filtration barrier through indirect modifications of the components of the SD in response to endothelial-derived ET-1.118 These perturbations to the actin cytoskeleton and the SD have a direct impact on the glomerular filtration barrier.118 Intriguingly, a recent study indicates that ETAR expression can be induced on cultured murine glomerular endothelial cells in response to podocyte-derived ET-1 induced by TGFβR1 activation.119 Activation of ETAR, by decreasing the thickness of the endothelial glycocalyx and promoting mitochondrial ROS production in endothelial cells, leads to endothelial damage, which can in turn result in albuminuria in patients with diabetes.119
As DKD progresses, podocytes respond to signals from the endothelium as described and eventually detach and drop out.120 When this occurs, endothelial cells are deprived of essential growth factors such as VEGF-A. Without these essential growth factors, endothelial cells will also fail to survive exacerbating the vicious cycle of pathologic endothelial-podocyte crosstalk.
Preclinical or clinical use of molecules targeting endothelial dysfunction or podocyte-endothelial crosstalk in dkd
The identification of clinically relevant biomarkers of the crosstalk between endothelial cells and podocytes is hampered by issues that need to be addressed. To date, there is no single test that can identify endothelial cell dysfunction in patients. Endothelial dysfunction is typically characterized by a reduction in endothelium-dependent vasodilation, primarily due to a decline in endothelial-derived NO. Forearm reactive hyperemia, a measure of microvascular vasodilation that is mediated in part by NO, is impaired in patients with chronic kidney disease and kidney failure and is associated with albuminuria, although it cannot be necessarily associated with podocyte dysfunction. However, dysfunctional endothelial cells will release factor(s) that mediate damage and depletion of adjacent podocytes in response to stress. As such, a combination of functional measurements of vascular function combined with systemic or local evaluation of such stress-induced factors, including TGFβ or ET-1, in patients with diabetes at risk for developing chronic kidney disease could provide strategies to assess crosstalk events that underlie observed increases in glomerular permeability to albumin in kidney disease.
Although intensive control of glucose and blood pressure remain the clinical gold standards to deter the progression of DKD, new treatments that aim to prevent endothelial damage or restore endothelial function could be an effective strategy for preventing or even reversing DKD. Several drugs have been evaluated for their potential to alleviate vascular dysfunction in the glomerular endothelium. Administration of neutralizing monoclonal anti-VEGF antibodies to type 1 and type 2 diabetic animals decreases albuminuria and glomerular hypertrophy, indicating the efficacy of anti-VEGF therapy against DKD.93,121 Furthermore, SU5416, a pan-VEGFR tyrosine kinase inhibitor, has also been reported to reduce albuminuria in type 2 diabetic mice.94 Other molecules targeting VEGF signaling in the glomerular endothelium are also being evaluated in preclinical studies, including angiostatin, a proteolytic fragment of plasminogen,95 and endostatin, which interacts with α5β1 integrin, leading to the inhibition of focal adhesion kinase and subsequent inhibition of VEGF-induced MAPKs.96
Preclinical and clinical studies have shown that several endothelin receptor antagonists, including sitaxentan97 and atrasentan,98 can prevent proteinuria and have nephroprotective potential in experimental models of DKD. Several strategies targeting TGFβ signaling, including neutralizing antibodies, antisense oligonucleotides, or inhibitors such as SISI3 can also prevent endothelial-mesenchymal transition and renal fibrosis in experimental models of nephropathy.99, 100, 101 Gliquidone can ameliorate the diabetic symptoms of DKD through inhibiting Notch signaling in the endothelium, improving antioxidative response and delaying renal interstitial fibrosis.122 Finally, COMP-Ang1, a potent and selective angiopoietin agonist, has been shown to reduce albuminuria, mesangial expansion, thickening of the GBM, and podocyte foot-process broadening and effacement in a mouse model of DKD.123
Conclusions
Numerous cross-sectional studies have shown that albuminuria in DKD is linked to endothelial and podocyte dysfunction and loss. There is still much to explore considering the numerous signaling pathways involved in endothelial-podocyte interactions, which makes this challenging clinical problem much more complex. To date, the crosstalk between endothelial cells and podocytes can only be assessed indirectly through the expression of mediators. Improved methods to identify signaling pathways and regulators have greatly improved our understanding of glomerular cross-communication in vivo and should lead to the identification of new targets for the prevention and treatment of glomerular diseases to help reduce the growing number of patients with diabetes who may require dialysis.
Article Information
Authors’ Full Names and Academic Degrees
Cindy Lora Gil, MSc, MD, Erika Hooker, PhD, and Bruno Larrivée, PhD.
Support
This work was supported by a grant-in-aid from the Heart and Stroke Foundation of Canada and from the Canadian Institutes of Health Research (363450). Dr Larrivée was a recipient of a New Investigator Award from the Heart and Stroke Foundation of Canada.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Acknowledgements
CLG and EH had an equal contribution.
Peer Review
Received May 26, 2020. Evaluated by 1 external peer reviewer, with direct editorial input from an Associate Editor and the Editor-in-Chief. Accepted in revised form October 18, 2020.
Footnotes
Complete author and article information provided before references.
References
- 1.Alicic R.Z., Rooney M.T., Tuttle K.R. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol. 2017;12:2032–2045. doi: 10.2215/CJN.11491116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gheith O., Farouk N., Nampoory N., Halim M.A., Al-Otaibi T. Diabetic kidney disease: world wide difference of prevalence and risk factors. J Nephropharmacol. 2015;5:49–56. [PMC free article] [PubMed] [Google Scholar]
- 3.Orasanu G., Plutzky J. The pathologic continuum of diabetic vascular disease. J Am Coll Cardiol. 2009;53(suppl 5):S35–S42. doi: 10.1016/j.jacc.2008.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daehn I.S. Glomerular endothelial cell stress and cross-talk with podocytes in early diabetic kidney disease. Front Med (Lausanne) 2018;5:76. doi: 10.3389/fmed.2018.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sena C.M., Pereira A.M., Seiça R. Endothelial dysfunction — a major mediator of diabetic vascular disease. Biochim Biophys Acta. 2013;1832:2216–2231. doi: 10.1016/j.bbadis.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 6.Sörensson J., Fierlbeck W., Heider T. Glomerular endothelial fenestrae in vivo are not formed from caveolae. J Am Soc Nephrol. 2002;13:2639–2647. doi: 10.1097/01.asn.0000033277.32822.23. [DOI] [PubMed] [Google Scholar]
- 7.Jourde-Chiche N., Fakhouri F., Dou L. Endothelium structure and function in kidney health and disease. Nat Rev Nephrol. 2019;15:87–108. doi: 10.1038/s41581-018-0098-z. [DOI] [PubMed] [Google Scholar]
- 8.Shimomura H., Spiro R.G. Studies on macromolecular components of human glomerular basement membrane and alterations in diabetes: decreased levels of heparan sulfate proteoglycan and laminin. Diabetes. 1987;36:374–381. doi: 10.2337/diab.36.3.374. [DOI] [PubMed] [Google Scholar]
- 9.Greka A., Mundel P. Cell biology and pathology of podocytes. Ann Rev Physiol. 2012;74:299–323. doi: 10.1146/annurev-physiol-020911-153238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park J., Shrestha R., Qiu C. Single-cell transcriptomics of the mouse kidney reveals potential cellular targets of kidney disease. Science. 2018;360:758–763. doi: 10.1126/science.aar2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu H., Malone A.F., Donnelly E.L. Single-cell transcriptomics of a human kidney allograft biopsy specimen defines a diverse inflammatory response. J Am Soc Nephrol. 2018;29:2069–2080. doi: 10.1681/ASN.2018020125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karaiskos N., Rahmatollahi M., Boltengagen A. A single-cell transcriptome atlas of the mouse glomerulus. J Am Soc Nephrol. 2018;29:2060–2068. doi: 10.1681/ASN.2018030238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Medeiros T., Myette R.L., Almeida J.R., Silva A.A., Burger D. Extracellular vesicles: cell-derived biomarkers of glomerular and tubular injury. Cell Physiol Biochem. 2020;54:88–109. doi: 10.33594/000000207. [DOI] [PubMed] [Google Scholar]
- 14.Wu X., Gao Y., Xu L. Exosomes from high glucose-treated glomerular endothelial cells trigger the epithelial-mesenchymal transition and dysfunction of podocytes. Sci Rep. 2017;7:9371. doi: 10.1038/s41598-017-09907-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abe H., Sakurai A., Ono H. Urinary exosomal mRNA of WT1 as diagnostic and prognostic biomarker for diabetic nephropathy. J Med Invest. 2018;65:208–215. doi: 10.2152/jmi.65.208. [DOI] [PubMed] [Google Scholar]
- 16.Eremina V., Sood M., Haigh J. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J Clin Invest. 2003;111:707–716. doi: 10.1172/JCI17423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sison K., Eremina V., Baelde H. Glomerular structure and function require paracrine, not autocrine, VEGF–VEGFR-2 signaling. J Am Soc Nephrol. 2010;21:1691–1701. doi: 10.1681/ASN.2010030295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Veron D., Villegas G., Aggarwal P.K. Acute podocyte vascular endothelial growth factor (VEGF-A) knockdown disrupts alphaVbeta3 integrin signaling in the glomerulus. PLoS One. 2012;7 doi: 10.1371/journal.pone.0040589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghayur A., Margetts P.J. Transforming growth factor-beta and the glomerular filtration barrier. Kidney Res Clin Pract. 2013;32:3–10. doi: 10.1016/j.krcp.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hata A., Chen Y.-G. TGF-β signaling from receptors to smads. Cold Spring Harb Perspect Biol. 2016;8:a022061. doi: 10.1101/cshperspect.a022061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.ten Dijke P., Arthur H.M. Extracellular control of TGFβ signalling in vascular development and disease. Nat Rev Mol Cell Biol. 2007;8:857–869. doi: 10.1038/nrm2262. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto T., Noble N.A., Cohen A.H. Expression of transforming growth factor-β isoforms in human glomerular diseases. Kidney Int. 1996;49:461–469. doi: 10.1038/ki.1996.65. [DOI] [PubMed] [Google Scholar]
- 23.Ito Y., Goldschmeding R., Kasuga H. Expression patterns of connective tissue growth factor and of TGF-β isoforms during glomerular injury recapitulate glomerulogenesis. Am J Physiol Renal Physiol. 2010;299:F545–F558. doi: 10.1152/ajprenal.00120.2009. [DOI] [PubMed] [Google Scholar]
- 24.Blank U., Seto M.L., Adams D.C., Wojchowski D.M., Karolak M.J., Oxburgh L. An in vivo reporter of BMP signaling in organogenesis reveals targets in the developing kidney. BMC Dev Biol. 2008;8:86. doi: 10.1186/1471-213X-8-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schiffer M., Bitzer M., Roberts I.S.D. Apoptosis in podocytes induced by TGF-β and Smad7. J Clin Invest. 2001;108:807–816. doi: 10.1172/JCI12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu J., Lamouille S., Derynck R. TGF-β-induced epithelial to mesenchymal transition. Cell Res. 2009;19:156–172. doi: 10.1038/cr.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ying Q., Wu G. Molecular mechanisms involved in podocyte EMT and concomitant diabetic kidney diseases: an update. Ren Fail. 2017;39:474–483. doi: 10.1080/0886022X.2017.1313164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xavier S., Vasko R., Matsumoto K. Curtailing endothelial TGF-β signaling is sufficient to reduce endothelial-mesenchymal transition and fibrosis in CKD. J Am Soc Nephrol. 2015;26:817–829. doi: 10.1681/ASN.2013101137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jeansson M., Gawlik A., Anderson G. Angiopoietin-1 is essential in mouse vasculature during development and in response to injury. J Clin Invest. 2011;121:2278–2289. doi: 10.1172/JCI46322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davis B., Dei Cas A., Long D.A. Podocyte-specific expression of angiopoietin-2 causes proteinuria and apoptosis of glomerular endothelia. J Am Soc Nephrol. 2007;18:2320–2329. doi: 10.1681/ASN.2006101093. [DOI] [PubMed] [Google Scholar]
- 31.Sun H., Zheng J., Chen S., Zeng C., Liu Z., Li L. Enhanced expression of ANGPTL2 in the microvascular lesions of diabetic glomerulopathy. Nephron Exp Nephrol. 2007;105:e117–e123. doi: 10.1159/000100493. [DOI] [PubMed] [Google Scholar]
- 32.Takabatake Y., Sugiyama T., Kohara H. The CXCL12 (SDF-1)/CXCR4 axis is essential for the development of renal vasculature. J Am Soc Nephrol. 2009;20:1714–1723. doi: 10.1681/ASN.2008060640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sayyed S.G., Hägele H., Kulkarni O.P. Podocytes produce homeostatic chemokine stromal cell-derived factor-1/CXCL12, which contributes to glomerulosclerosis, podocyte loss and albuminuria in a mouse model of type 2 diabetes. Diabetologia. 2009;52:2445–2454. doi: 10.1007/s00125-009-1493-6. [DOI] [PubMed] [Google Scholar]
- 34.Reidy K.J., Villegas G., Teichman J. Semaphorin3a regulates endothelial cell number and podocyte differentiation during glomerular development. Development. 2009;136:3979–3989. doi: 10.1242/dev.037267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Villegas G., Tufro A. Ontogeny of semaphorins 3A and 3F and their receptors neuropilins 1 and 2 in the kidney. Mech Dev. 2002;119(suppl 1):S149–S153. doi: 10.1016/s0925-4773(03)00108-4. [DOI] [PubMed] [Google Scholar]
- 36.Leung W.K., Gao L., Siu P.M., Lai C.W. Diabetic nephropathy and endothelial dysfunction: current and future therapies, and emerging of vascular imaging for preclinical renal-kinetic study. Life Sci. 2016;166:121–130. doi: 10.1016/j.lfs.2016.10.015. [DOI] [PubMed] [Google Scholar]
- 37.Brasacchio D., Okabe J., Tikellis C. Hyperglycemia induces a dynamic cooperativity of histone methylase and demethylase enzymes associated with gene-activating epigenetic marks that coexist on the lysine tail. Diabetes. 2009;58:1229–1236. doi: 10.2337/db08-1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stehouwer C.D.A., Andreas Fischer H.R., Van Kuijk A.W.R., Polak B.C.P., Donker A.J.M. Endothelial dysfunction precedes development of microalbuminuria in IDDM. Diabetes. 1995;44:561–564. doi: 10.2337/diab.44.5.561. [DOI] [PubMed] [Google Scholar]
- 39.Clausen P., Feldt-Rasmussen B., Jensen G., Jensen J. Endothelial haemostatic factors are associated with progression of urinary albumin excretion in clinically healthy subjects: a 4-year prospective study. Clin Science (London, England) 1999;97:37–43. [PubMed] [Google Scholar]
- 40.Kaiser N., Sasson S., Feener E.P. Differential regulation of glucose transport and transporters by glucose in vascular endothelial and smooth muscle cells. Diabetes. 1993;42:80–89. doi: 10.2337/diab.42.1.80. [DOI] [PubMed] [Google Scholar]
- 41.De Bock K., Georgiadou M., Schoors S. Role of PFKFB3-driven glycolysis in vessel sprouting. Cell. 2013;154:651–663. doi: 10.1016/j.cell.2013.06.037. [DOI] [PubMed] [Google Scholar]
- 42.Imasawa T., Obre E., Bellance N. High glucose repatterns human podocyte energy metabolism during differentiation and diabetic nephropathy. FASEB J. 2017;31:294–307. doi: 10.1096/fj.201600293R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang W., Wang Y., Long J. Mitochondrial fission triggered by hyperglycemia is mediated by ROCK1 activation in podocytes and endothelial cells. Cell Metab. 2012;15:186–200. doi: 10.1016/j.cmet.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Daehn I., Casalena G., Zhang T. Endothelial mitochondrial oxidative stress determines podocyte depletion in segmental glomerulosclerosis. J Clin Invest. 2014;124:1608–1621. doi: 10.1172/JCI71195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lemkes B.A., Nieuwdorp M., Hoekstra J.B.L., Holleman F. The glycocalyx and cardiovascular disease in diabetes: should we judge the endothelium by its cover? Diabetes Technol Ther. 2012;14(suppl 1) doi: 10.1089/dia.2012.0011. S-3. [DOI] [PubMed] [Google Scholar]
- 46.Nieuwdorp M., Mooij H.L., Kroon J. Endothelial glycocalyx damage coincides with microalbuminuria in type 1 diabetes. Diabetes. 2006;55:1127–1132. doi: 10.2337/diabetes.55.04.06.db05-1619. [DOI] [PubMed] [Google Scholar]
- 47.Nieuwdorp M., Holleman F., de Groot E. Perturbation of hyaluronan metabolism predisposes patients with type 1 diabetes mellitus to atherosclerosis. Diabetologia. 2007;50:1288–1293. doi: 10.1007/s00125-007-0666-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shakya S., Wang Y., Mack J.A., Maytin E.V. Hyperglycemia-induced changes in hyaluronan contribute to impaired skin wound healing in diabetes: review and perspective. Int J Cell Biol. 2015;2015:701738. doi: 10.1155/2015/701738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yilmaz O., Afsar B., Ortiz A., Kanbay M. The role of endothelial glycocalyx in health and disease. Clin Kidney J. 2019;12:611–619. doi: 10.1093/ckj/sfz042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kennett E.C., Davies M.J. Degradation of matrix glycosaminoglycans by peroxynitrite/peroxynitrous acid: Evidence for a hydroxyl-radical-like mechanism. Free Radic Biol Med. 2007;42:1278–1289. doi: 10.1016/j.freeradbiomed.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 51.Kennett E.C., Davies M.J. Glycosaminoglycans are fragmented by hydroxyl, carbonate, and nitrogen dioxide radicals in a site-selective manner: implications for peroxynitrite-mediated damage at sites of inflammation. Free Radic Biol Med. 2009;47:389–400. doi: 10.1016/j.freeradbiomed.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 52.Chow F.Y., Nikolic-Paterson D.J., Ozols E., Atkins R.C., Rollin B.J., Tesch G.H. Monocyte chemoattractant protein-1 promotes the development of diabetic renal injury in streptozotocin-treated mice. Kidney Int. 2006;69:73–80. doi: 10.1038/sj.ki.5000014. [DOI] [PubMed] [Google Scholar]
- 53.Navarro-González J.F., Mora-Fernández C., de Fuentes M.M., García-Pérez J. Inflammatory molecules and pathways in the pathogenesis of diabetic nephropathy. Nat Rev Nephrol. 2011;7:327–340. doi: 10.1038/nrneph.2011.51. [DOI] [PubMed] [Google Scholar]
- 54.Hirata K., Shikata K., Matsuda M. Increased expression of selectins in kidneys of patients with diabetic nephropathy. Diabetologia. 1998;41:185–192. doi: 10.1007/s001250050888. [DOI] [PubMed] [Google Scholar]
- 55.Clausen P., Jacobsen P., Rossing K., Jensen J.S., Parving H.H., Feldt-Rasmussen B. Plasma concentrations of VCAM-1 and ICAM-1 are elevated in patients with type 1 diabetes mellitus with microalbuminuria and overt nephropathy. Diabet Med. 2000;17:644–649. doi: 10.1046/j.1464-5491.2000.00347.x. [DOI] [PubMed] [Google Scholar]
- 56.Alghamdi T., Batchu S.N., Hadden M.J. Histone H3 serine 10 phosphorylation facilitates endothelial activation in diabetic kidney disease. Diabetes. 2018;67:2668–2681. doi: 10.2337/db18-0124. [DOI] [PubMed] [Google Scholar]
- 57.Fan L., He X., Muroya Y., Fan F., Roman R.J. Increased renal expression of adhesion molecules and inflammation in diabetic nephropathy. FASEB J. 2019;33:573–577. [Google Scholar]
- 58.Fu J., Akat K.M., Sun Z. Single-cell RNA profiling of glomerular cells shows dynamic changes in experimental diabetic kidney disease. J Am Soc Nephrol. 2019;30:533–545. doi: 10.1681/ASN.2018090896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wilson P.C., Wu H., Kirita Y. The single-cell transcriptomic landscape of early human diabetic nephropathy. Proc Natl Acad Sci U S A. 2019;116:19619–19625. doi: 10.1073/pnas.1908706116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Siddiqi F.S., Advani A. Endothelial-podocyte crosstalk: the missing link between endothelial dysfunction and albuminuria in diabetes. Diabetes. 2013;62:3647–3655. doi: 10.2337/db13-0795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Doublier S., Salvidio G., Lupia E. Nephrin expression is reduced in human diabetic nephropathy: evidence for a distinct role for glycated albumin and angiotensin II. Diabetes. 2003;52:1023–1030. doi: 10.2337/diabetes.52.4.1023. [DOI] [PubMed] [Google Scholar]
- 62.Welsh G.I., Saleem M.A. Nephrin—signature molecule of the glomerular podocyte? J Pathol. 2010;220:328–337. doi: 10.1002/path.2661. [DOI] [PubMed] [Google Scholar]
- 63.Sonneveld R., van der Vlag J., Baltissen M.P.A. Glucose specifically regulates TRPC6 expression in the podocyte in an AngII-dependent manner. Am J Pathol. 2014;184:1715–1726. doi: 10.1016/j.ajpath.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 64.Kanda S., Harita Y., Shibagaki Y. Tyrosine phosphorylation–dependent activation of TRPC6 regulated by PLC-γ1 and nephrin: effect of mutations associated with focal segmental glomerulosclerosis. Mol Biol Cell. 2011;22:1824–1835. doi: 10.1091/mbc.E10-12-0929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Harita Y., Kurihara H., Kosako H. Phosphorylation of nephrin triggers Ca2+ signaling by recruitment and activation of phospholipase C-γ1. J Biol Chem. 2009;284:8951–8962. doi: 10.1074/jbc.M806851200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reiser J., Polu K.R., Möller C.C. TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nat Genet. 2005;37:739–744. doi: 10.1038/ng1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Winn M.P., Conlon P.J., Lynn K.L. A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science. 2005;308:1801–1804. doi: 10.1126/science.1106215. [DOI] [PubMed] [Google Scholar]
- 68.Eckel J., Lavin P.J., Finch E.A. TRPC6 enhances angiotensin II-induced albuminuria. J Am Soc Nephrol. 2011;22:526–535. doi: 10.1681/ASN.2010050522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lu X.Y., Liu B.C., Cao Y.Z. High glucose reduces expression of podocin in cultured human podocytes by stimulating TRPC6. Am J Physiol Renal Physiol. 2019;317:F1605–F1611. doi: 10.1152/ajprenal.00215.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Na J., Sweetwyne M.T., Park A.S.D., Susztak K., Cagan R.L. Diet-induced podocyte dysfunction in drosophila and mammals. Cell Rep. 2015;12:636–647. doi: 10.1016/j.celrep.2015.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Su J., Li S.J., Chen Z.H. Evaluation of podocyte lesion in patients with diabetic nephropathy: Wilms’ tumor-1 protein used as a podocyte marker. Diabetes Res Clin Pract. 2010;87:167–175. doi: 10.1016/j.diabres.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 72.Tufro A., Veron D. VEGF and podocytes in diabetic nephropathy. Semin Nephrol. 2012;32:385–393. doi: 10.1016/j.semnephrol.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fukuda A., Chowdhury M.A., Venkatareddy M.P. Growth-dependent podocyte failure causes glomerulosclerosis. J Am Soc Nephrol. 2012;23:1351–1363. doi: 10.1681/ASN.2012030271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nagata M., Schärer K., Kriz W. Glomerular damage after uninephrectomy in young rats. I. Hypertrophy and distortion of capillary architecture. Kidney Int. 1992;42:136–147. doi: 10.1038/ki.1992.271. [DOI] [PubMed] [Google Scholar]
- 75.Young B.A., Johnson R.J., Alpers C.E. Cellular events in the evolution of experimental diabetic nephropathy. Kidney Int. 1995;47:935–944. doi: 10.1038/ki.1995.139. [DOI] [PubMed] [Google Scholar]
- 76.Kreidberg J.A., Donovan M.J., Goldstein S.L. Alpha 3 beta 1 integrin has a crucial role in kidney and lung organogenesis. Development. 1996;122:3537–3547. doi: 10.1242/dev.122.11.3537. [DOI] [PubMed] [Google Scholar]
- 77.Susztak K., Raff A.C., Schiffer M., Böttinger E.P. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes. 2006;55:225–233. [PubMed] [Google Scholar]
- 78.Jim B., Ghanta M., Qipo A. Dysregulated nephrin in diabetic nephropathy of type 2 diabetes: a cross sectional study. PLoS One. 2012;7:e36041. doi: 10.1371/journal.pone.0036041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Coward R.J.M., Welsh G.I., Koziell A. Nephrin is critical for the action of insulin on human glomerular podocytes. Diabetes. 2007;56:1127–1135. doi: 10.2337/db06-0693. [DOI] [PubMed] [Google Scholar]
- 80.Li J.J., Kwak S.J., Jung D.S. Podocyte biology in diabetic nephropathy. Kidney Int Suppl. 2007;72:S36–S42. doi: 10.1038/sj.ki.5002384. [DOI] [PubMed] [Google Scholar]
- 81.Ziyadeh F.N. The extracellular matrix in diabetic nephropathy. Am J Kidney Dis. 1993;22:736–744. doi: 10.1016/s0272-6386(12)80440-9. [DOI] [PubMed] [Google Scholar]
- 82.Kriz W., Lemley K.V. A potential role for mechanical forces in the detachment of podocytes and the progression of CKD. J Am Soc Nephrol. 2015;26:258–269. doi: 10.1681/ASN.2014030278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shirato I., Sakai T., Kimura K., Tomino Y., Kriz W. Cytoskeletal changes in podocytes associated with foot process effacement in Masugi nephritis. Am J Pathol. 1996;148:1283–1296. [PMC free article] [PubMed] [Google Scholar]
- 84.Kriz W., Shirato I., Nagata M., LeHir M., Lemley K.V. The podocyte’s response to stress: the enigma of foot process effacement. Am J Physiol Renal Physiol. 2012;304:F333–F347. doi: 10.1152/ajprenal.00478.2012. [DOI] [PubMed] [Google Scholar]
- 85.Jerums G., Premaratne E., Panagiotopoulos S., MacIsaac R.J. The clinical significance of hyperfiltration in diabetes. Diabetologia. 2010;53:2093–2104. doi: 10.1007/s00125-010-1794-9. [DOI] [PubMed] [Google Scholar]
- 86.Magee G.M., Bilous R.W., Cardwell C.R., Hunter S.J., Kee F., Fogarty D.G. Is hyperfiltration associated with the future risk of developing diabetic nephropathy? A meta-analysis. Diabetologia. 2009;52:691–697. doi: 10.1007/s00125-009-1268-0. [DOI] [PubMed] [Google Scholar]
- 87.Chen H.C., Chen C.A., Guh J.Y., Chang J.M., Shin S.J., Lai Y.H. Altering expression of α3β1 integrin on podocytes of human and rats with diabetes. Life Sci. 2000;67:2345–2353. doi: 10.1016/s0024-3205(00)00815-8. [DOI] [PubMed] [Google Scholar]
- 88.Han S.H., Yang S., Jung D.S. Gene expression patterns in glucose-stimulated podocytes. Biochem Biophys Res Commun. 2008;370:514–518. doi: 10.1016/j.bbrc.2008.03.121. [DOI] [PubMed] [Google Scholar]
- 89.Østerby R., Nyberg G. New vessel formation in the renal corpuscles in advanced diabetic glomerulopathy. J Diabetic Complications. 1987;1:122–127. doi: 10.1016/s0891-6632(87)80069-7. [DOI] [PubMed] [Google Scholar]
- 90.Oltean S., Qiu Y., Ferguson J.K. Vascular endothelial growth factor-a165b is protective and restores endothelial glycocalyx in diabetic nephropathy. J Am Soc Nephrol. 2015;26:1889-1904. doi: 10.1681/ASN.2014040350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Feliers D., Chen X., Akis N., Choudhury G.G., Madaio M., Kasinath B.S. VEGF regulation of endothelial nitric oxide synthase in glomerular endothelial cells. Kidney Int. 2005;68:1648–1659. doi: 10.1111/j.1523-1755.2005.00575.x. [DOI] [PubMed] [Google Scholar]
- 92.Lenoir O., Milon M., Virsolvy A. Direct action of endothelin-1 on podocytes promotes diabetic glomerulosclerosis. J Am Soc Nephrol. 2014;25:1050–1062. [Google Scholar]
- 93.Flyvbjerg A., Dagnæs-Hansen F., De Vriese A.S., Schrijvers B.F., Tilton R.G., Rasch R. Amelioration of long-term renal changes in obese type 2 diabetic mice by a neutralizing vascular endothelial growth factor antibody. Diabetes. 2002;51:3090–3094. doi: 10.2337/diabetes.51.10.3090. [DOI] [PubMed] [Google Scholar]
- 94.Sung S.H., Ziyadeh F.N., Wang A., Pyagay P.E., Kanwar Y.S., Chen S. Blockade of vascular endothelial growth factor signaling ameliorates diabetic albuminuria in mice. J Am Soc Nephrol. 2006;17:3093–3104. doi: 10.1681/ASN.2006010064. [DOI] [PubMed] [Google Scholar]
- 95.Zhang S.X., Wang J.J., Lu K., Mott R., Longeras R., Ma J. Therapeutic potential of angiostatin in diabetic nephropathy. J Am Soc Nephrol. 2006;17:475–486. doi: 10.1681/ASN.2005020217. [DOI] [PubMed] [Google Scholar]
- 96.Ichinose K., Maeshima Y., Yamamoto Y. Antiangiogenic endostatin peptide ameliorates renal alterations in the early stage of a type 1 diabetic nephropathy model. Diabetes. 2005;54:2891–2903. doi: 10.2337/diabetes.54.10.2891. [DOI] [PubMed] [Google Scholar]
- 97.Dhaun N., MacIntyre I.M., Kerr D. Selective endothelin-A receptor antagonism reduces proteinuria, blood pressure, and arterial stiffness in chronic proteinuric kidney disease. Hypertension. 2011;57:772–779. doi: 10.1161/HYPERTENSIONAHA.110.167486. [DOI] [PubMed] [Google Scholar]
- 98.Heerspink H.J.L., Parving H.H., Andress D.L. Atrasentan and renal events in patients with type 2 diabetes and chronic kidney disease (SONAR): a double-blind, randomised, placebo-controlled trial. Lancet. 2019;393:1937–1947. doi: 10.1016/S0140-6736(19)30772-X. [DOI] [PubMed] [Google Scholar]
- 99.Moon J.-A., Kim H.-T., Cho I.-S., Sheen Y.Y., Kim D.-K., IN- IN-1130, a novel transforming growth factor-β type I receptor kinase (ALK5) inhibitor, suppresses renal fibrosis in obstructive nephropathy. Kidney Int. 2006;70:1234–1243. doi: 10.1038/sj.ki.5001775. [DOI] [PubMed] [Google Scholar]
- 100.Li J., Qu X., Yao J. Blockade of endothelial-mesenchymal transition by a smad3 inhibitor delays the early development of streptozotocin-induced diabetic nephropathy. Diabetes. 2010;59:2612–2624. doi: 10.2337/db09-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Petersen M., Thorikay M., Deckers M. Oral administration of GW788388, an inhibitor of TGF-β type I and II receptor kinases, decreases renal fibrosis. Kidney Int. 2008;73:705–715. doi: 10.1038/sj.ki.5002717. [DOI] [PubMed] [Google Scholar]
- 102.Hovind P., Tarnow L., Oestergaard P.B., Parving H.-H. Elevated vascular endothelial growth factor in type 1 diabetic patients with diabetic nephropathy. Kidney Int Suppl. 2000;57:S56–S61. [PubMed] [Google Scholar]
- 103.Iglesias-de la Cruz M.C., Ziyadeh F.N., Isono M. Effects of high glucose and TGF-β1 on the expression of collagen IV and vascular endothelial growth factor in mouse podocytes. Kidney Int. 2002;62:901–913. doi: 10.1046/j.1523-1755.2002.00528.x. [DOI] [PubMed] [Google Scholar]
- 104.Hoshi S., Nomoto K., Kuromitsu J., Tomari S., Nagata M. High glucose induced VEGF expression via PKC and ERK in glomerular podocytes. Biochem Biophys Res Commun. 2002;290:177–184. doi: 10.1006/bbrc.2001.6138. [DOI] [PubMed] [Google Scholar]
- 105.McCarty G., Awad O., Loeb D.M. WT1 protein directly regulates expression of vascular endothelial growth factor and is a mediator of tumor response to hypoxia. J Biol Chem. 2011;286:43634–43643. doi: 10.1074/jbc.M111.310128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tsuchida K., Makita Z., Yamagishi S. Suppression of transforming growth factor beta and vascular endothelial growth factor in diabetic nephropathy in rats by a novel advanced glycation end product inhibitor, OPB-9195. Diabetologia. 1999;42:579–588. doi: 10.1007/s001250051198. [DOI] [PubMed] [Google Scholar]
- 107.Wendt T.M., Tanji N., Guo J. RAGE drives the development of glomerulosclerosis and implicates podocyte activation in the pathogenesis of diabetic nephropathy. Am J Pathol. 2003;162:1123–1137. doi: 10.1016/S0002-9440(10)63909-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.van der Vlag J., Buijsers B. The glomerular endothelium in diabetic nephropathy: role of heparanase. In: Roelofs J.J., Vogt L., editors. Diabetic Nephropathy: Pathophysiology and Clinical Aspects. Springer Int Publishing; 2019. pp. 153–170. [Google Scholar]
- 109.Guo M., Ricardo S.D., Deane J.A., Shi M., Cullen-McEwen L., Bertram J.F. A stereological study of the renal glomerular vasculature in the db/db mouse model of diabetic nephropathy. J Anat. 2005;207:813–821. doi: 10.1111/j.1469-7580.2005.00492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liu E., Morimoto M., Kitajima S. Increased expression of vascular endothelial growth factor in kidney leads to progressive impairment of glomerular functions. J Am Soc Nephrol. 2007;18:2094–2104. doi: 10.1681/ASN.2006010075. [DOI] [PubMed] [Google Scholar]
- 111.Majumder S., Advani A. VEGF and the diabetic kidney: more than too much of a good thing. J Diabetes Complications. 2017;31:273–279. doi: 10.1016/j.jdiacomp.2016.10.020. [DOI] [PubMed] [Google Scholar]
- 112.Zheng G.H., Shan Q., Mu J.J. Purple sweet potato color attenuates kidney damage by blocking VEGFR2/ROS/NLRP3 signaling in high-fat diet-treated mice. Oxid Med Cell Longev. 2019;2019:5189819. doi: 10.1155/2019/5189819. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 113.Ratliff B.B., Abdulmahdi W., Pawar R., Wolin M.S. Oxidant mechanisms in renal injury and disease. Antioxid Redox Signal. 2016;25:119–146. doi: 10.1089/ars.2016.6665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Falkevall A., Mehlem A., Palombo I. Reducing VEGF-B signaling ameliorates renal lipotoxicity and protects against diabetic kidney disease. Cell Metab. 2017;25:713–726. doi: 10.1016/j.cmet.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 115.Onions K.L., Gamez M., Buckner N.R. VEGFC reduces glomerular albumin permeability and protects against alterations in VEGF receptor expression in diabetic nephropathy. Diabetes. 2019;68:172–187. doi: 10.2337/db18-0045. [DOI] [PubMed] [Google Scholar]
- 116.Pozzi A., Zent R. Integrins in kidney disease. J Am Soc Nephrol. 2013;24:1034. doi: 10.1681/ASN.2013010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ak G., Buyukberber S., Sevinc A. The relation between plasma endothelin-1 levels and metabolic control, risk factors, treatment modalities, and diabetic microangiopathy in patients with Type 2 diabetes mellitus. J Diabetes Complications. 2001;15:150–157. doi: 10.1016/s1056-8727(01)00137-4. [DOI] [PubMed] [Google Scholar]
- 118.Morigi M., Buelli S., Zanchi C. Shigatoxin-induced endothelin-1 expression in cultured podocytes autocrinally mediates actin remodeling. Am J Pathol. 2006;169:1965–1975. doi: 10.2353/ajpath.2006.051331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ebefors K., Wiener R.J., Yu L. Endothelin receptor-A mediates degradation of the glomerular endothelial surface layer via pathologic crosstalk between activated podocytes and glomerular endothelial cells. Kidney Int. 2019;96:957–970. doi: 10.1016/j.kint.2019.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Baelde H.J., Eikmans M., Lappin D.W.P. Reduction of VEGF-A and CTGF expression in diabetic nephropathy is associated with podocyte loss. Kidney Int. 2007;71:637–645. doi: 10.1038/sj.ki.5002101. [DOI] [PubMed] [Google Scholar]
- 121.Vriese A.S.D., Tilton R.G., Elger M., Stephan C.C., Kriz W., Lameire N.H. Antibodies against vascular endothelial growth factor improve early renal dysfunction in experimental diabetes. J Am Soc Nephrol. 2001;12:993–1000. doi: 10.1681/ASN.V125993. [DOI] [PubMed] [Google Scholar]
- 122.Tian H., Yang J., Xie Z., Liu J. Gliquidone alleviates diabetic nephropathy by inhibiting notch/snail signaling pathway. Cell Physiol Biochem. 2018;51:2085–2097. doi: 10.1159/000495827. [DOI] [PubMed] [Google Scholar]
- 123.Lee S., Kim W., Moon S.O. Renoprotective effect of COMP-angiopoietin-1 in db/db mice with type 2 diabetes. Nephrol Dial Transplant. 2006;22:396–408. doi: 10.1093/ndt/gfl598. [DOI] [PubMed] [Google Scholar]