Abstract
We present a novel approach for the separation and recovery of Pt and Pd leached from a spent automotive catalyst relying on conventional and polymerized supported ionic liquid phases (SILPs and polySILPs, respectively). A variety of parameters with possible effects on the separation behavior, namely, acidity and concentration of the platinum group metal (PGM) containing solution, as well as different SILP and polySILP loadings, were evaluated for the separation of PGMs in the presence of high concentrations of Al, Fe, Zn, and Ce. The polySILP material demonstrated the ability to separate the PGMs from major accompanying interferences in a single separation step, while problems arising from ionic liquid leaching in the case of SILPs could be avoided. Moreover, the use of supported ionic liquid phases allowed the drastic reduction of the amount of required ionic liquid compared to conventional liquid–liquid separation, while avoiding problems arising from emulsion formation. Subsequent stripping experiments lead to further purification of the PGMs and finally desorption from the solid material into a pure solution. Eventually, the concept of chemisorbed polySILPs provides a new and convenient approach for the recycling of platinum group metals.
Keywords: Platinum group metals, Automotive catalysts, Solid supported ionic liquid phases, Polymerized ionic liquids
Short abstract
Supported ionic liquid technology presents an environmentally benign alternative for fast and simple recovery and separation of platinum group metals from end-of-life car catalysts, thereby, contributing to a sustainable future.
Introduction
Platinum, palladium, and rhodium are the three platinum group metals (PGMs) that currently hold the greatest financial and industrial significance. Largely owing to their distinctive properties, they are employed in a broad array of applications including jewelry, electrical and electronic equipment, and dental materials; however, they primarily dominate the automotive industry as indispensable components of catalytic converters.1,2 The introduction of catalytic converters in the automotive market in 1975 and their subsequent widespread use was propelled by the increasing concern about the adverse environmental effects of exhaust emissions generated by automotive vehicles.3 PGMs are therefore of significant importance in the industry with a predominantly elevated and continuously increasing demand in the automotive sector; however, at the same time, their supply is precarious, especially as far as Europe is concerned.4,5 Thus, the recycling of PGMs is of paramount importance since it signifies conservation of the already limited primary resources and consequent stability in their market price.6,7 Although detailed data on commercial recycling procedures are absent from the literature, it is known that the leaching of PGMs from secondary raw materials is based on hydrometallurgical processes. However, these processes have drawbacks, the most notable of which is the use of aqua regia and the environmental implications it entails.8 Further refining and separation of PGMs is typically based on solvent extraction in the presence of carefully refined ligands.9 The speed, simplicity, and wide applicability of solvent extractions have resulted in their widespread industrial use for metal recovery.10 However, the extensive use of liquid-based separations does not imply lack of any shortcomings. Phase separation is dependent on the kinetics of the chemical and mass-transfer processes which determine the overall separation rate. The rate can be further influenced by the formation of emulsions during the mixing process of the phases. Additionally, the generated amount of the output organic waste is significant.11
As an alternative, liquid-based separation employing ionic liquids has emerged in the last years. The extensive potential that ionic liquids offer for metal separation has chiefly emerged from the distinctive and unique properties they possess, such as negligible vapor pressure, wide solubility range, and nonflammability, as well as their tunable nature by simple variation of the building anions and cations.12−14 Phosphonium-based ionic liquids have been reported to be effective candidates for the extraction and separation of PGMs from their mixtures in HCl solutions.15−17 Although there is a considerable number of publications on PGM extraction and separation from their model solutions, there is a very limited number of publications dealing with ionic liquid-based separations employed on car catalyst leach liquors.18−20 While ionic liquids have clearly demonstrated their potential and benefits for PGM separations, some limitations of conventional liquid–liquid extraction could not be overcome. This includes mass-transfer limitations due to high viscosities of ionic liquids but also problems arising from emulsion formation. In fact, many ionic liquids that have been identified as promising candidates for liquid–liquid separation are also powerful amphiphiles, which drastically complicates their implementation in continuous processes. These aspects, in combination with the still significant cost associated with ionic liquids, often result in their dilution with conventional solvents, such as heptane.21
A new concept that emerged along with the expanding interest in ionic liquids was their immobilization on solid support materials which entails the deposition of a thin ionic liquid layer on a solid surface. Supported ionic liquid phases (SILPs) are an attractive alternative approach to exploiting the advantages that ionic liquids have to offer, while at the same time circumventing accompanying problems of liquid-based processes, such as mass-transport limitations and use of excess amount of ionic liquids.22 The fields that have predominantly benefited from the novelty introduced by the SILP concept are catalysis,23−25 whereas metal separations have received less attention. Rare earth element separation with phosphonium, imidazolium, and quaternary ammonium salt-based SILPs has been reported in the literature.26,27 Supported phases impregnated with phosphonium-based and quaternary ammonium-based ionic liquids have been employed for the selective recovery of post-transition metals from transition metal-containing solutions.28,29
Herein, we present a novel separation approach for PGMs leached in hydrochloric acid by employing supported (polymerized)-ionic liquid phases (polySILPs). Although supported ionic liquid phase technology has been used for the separation of various elements, there is no literature available on its application for PGM separation. Additionally, polymerized supported ionic liquid phases have only been reported so far in catalysis applications but have never been applied to metal separation. Aiming for a rapid and efficient PGM recovery with high separation from the accompanying elements leached from the car catalyst material, we introduce a fast and simple separation procedure with reduced consumption of chemical reagents compared to liquid-based separations.
Materials and Methods
Chemicals and Starting Materials
All reagents employed in the method development were of analytical grade, unless otherwise stated. Concentrated HCl 37% was purchased from Merck, Germany. Stock solutions of Pt, Pd, and Rh 1000 ppm in 5% HCl and Pd 10.000 ppm in 10% HCl were obtained from Sigma-Aldrich, Germany, and used for the preparation of the PGM model system as well as the calibration standard solutions. Stock solution of Indium 1000 ppm in 2%–3% HNO3 was also obtained from Sigma-Aldrich, Germany. P66614Cl was purchased from Iolitec, Germany. Organic solvents used for the preparation of SILPs and polySILPs were of the highest purity. MeOH, EtOH, CH2Cl2, and toluene were obtained from Merck, Germany. Silica 60 (size 40–60 μm, specific surface area 480–540 m2/g) was also obtained from Merck, Germany. The compounds (3-mercaptopropyl) trimethoxysilane (>96%), 4-vinylbenzyl chloride (>90%), 4-(diphenylphosphino) styrene (97%), and 2,2′-azobis(2-methylpropionitrile) (AIBN) (98%) were all purchased from Sigma-Aldrich, Germany. The certified reference material, ERM-EB504, was also purchased from Sigma-Aldrich, Germany. High purity water was supplied by an Easipure water system (Thermo, USA, resistivity 18 MΩ cm). The car catalyst material employed in this work was provided by Monolithos Ltd. (Athens, Greece). The grinding size of the provided catalyst powder was less than 0.16 mm.
Synthesis of polySILP 10%
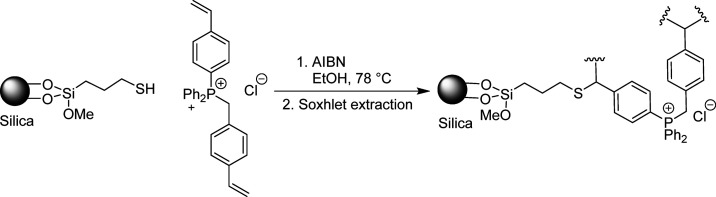
Silica 60 (Merck, size 40–60 μm, specific surface area 480–540 m2/g) was predried in the oven at 400 °C for 16 h and stored under argon atmosphere. Silica-60 (10 ± 0.10 g) was refluxed in toluene (150 mL) with (3-mercaptopropyl)trimethoxysilane (15 mL) under argon atmosphere for 24 h. The reaction mixture was cooled to RT and filtered. The solid was subsequently washed with MeOH and dried under high vacuum (10–2 mbar) for 24 h. The loading of the obtained product was 0.9 mmol/g. (3-Mercaptopropyl)trimethoxysilane grafted silica-60 (5 ± 0.1 g) was mixed with dry EtOH (30 mL), diphenyl(4-vinylbenzyl)(4-vinylphenyl)phosphonium chloride (0.5 g; 1.12 mmol; 10 wt %), and AIBN (16 mol % with respect to the double bonds). The mixture was stirred at RT for 2 h. The EtOH was evaporated (40 °C, 20 mbar), and the solid was dried under high vacuum (10–2 mbar). Subsequently, degassed dry EtOH (50 mL) was added to the recovered physisorbed solid under an argon atmosphere. The suspension was stirred at 78 °C for 20 h. The solvent was evaporated (40 °C, 20 mbar), and the solid was extracted with MeOH via Soxhlet extraction. In the final step, the solid was dried under vacuum (0.7 mbar). The polymer was obtained with a loading of 70 mg/g. The procedure was accordingly adapted for the preparation of 20% and 50% polySILPs, and loadings of 138 and 387 mg/g, respectively, were obtained (Figure 1).
Figure 1.
Polymerization of the monomer on the silica surface.
Leaching Procedure
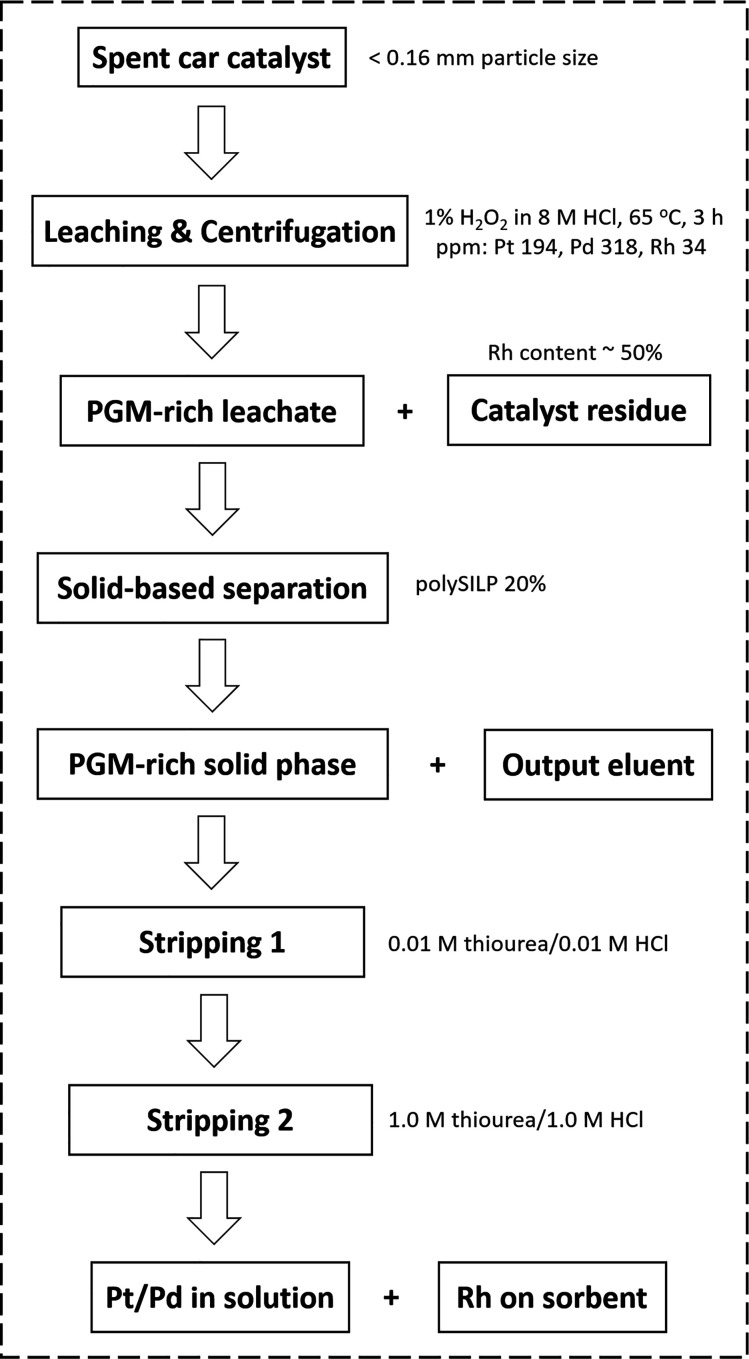
For the leaching, 0.20 ± 0.01 g grinded car catalyst were mixed with 1.00 ± 0.010 g of 1% H2O2 in 8 M HCl (solid:liquid 1:5), and the mixture was stirred in a clear glass screw cap vial at 65 °C for 3 h. The mixture was subsequently centrifuged for 30 min at 13500 rpm for the sedimentation of the remaining solid car catalyst material. The liquid phase was recovered and appropriately diluted prior to PGM quantification by ICP-OES (inductively couple plasma-optical emission spectroscopy) analysis. The % leaching efficiency was calculated based on eq 1
| 1 |
Solid–Liquid Separation Procedure
For the S–L separation, an in-house column packed with 0.50 ± 0.01 g of solid sorbent material (SILP or polySILP) was prepared, wherein it was immobilized by addition of glass wool. On top of the column, 2.50 ± 0.010 g of diluted leachate (1:7 dilution with H2O) was pipetted and forced through it via application of constant air flow generating a flow rate of 3 mL/min. The output leachate was collected and appropriately diluted prior to PGM quantification by ICP-OES analysis. The % retention of the metals was calculated according to eq 2
| 2 |
Stripping Procedure
The stripping was performed in two steps with acidified thiourea solutions of different concentration levels. The first step was performed with 0.01 M thiourea in 0.01 M HCl as the stripping agent. A total of 10 mL of the stripping agent was required to achieve the reported desorption percentages. The second step was performed with 1.0 M thiourea in 1.0 M HCl as the stripping agent, and a total of 30 mL of the stripping agent was required to achieve the reported desorption percentages. The % stripped metals was calculated according to eq 3
 |
3 |
and
Measurement Procedure
Calibration functions for ICP-OES analysis were determined using a set of six standards in the range of 500–12000 ppb for the PGMs and a set of five calibration standards in the range of 1000–30000 ppb for the other quantified elements (Al, Fe, Zn, Pb, Mn, Sr, Cu, Ni, Cr, B, Mg, Ce, La, Ti, Mo). The standard and sample solutions were appropriately diluted in a 5% HCl diluent. The standard and sample solutions corresponding to the stripping experiments were diluted in a HCl/thiourea diluent, where the concentration of HCl and thiourea were accordingly adjusted to achieve matrix matching between standards and samples.
Five procedural replicas of each sample were prepared, and each replica was measured five times. Blank solutions with compositions identical to the diluent were used for the determination of limits of detection and quantification. Indium was used as internal standard, and the recorded element specific signals were corrected using the emission line at 230.606 nm.
Results and discussion
Design and Characterization of SILP and polySILP Materials
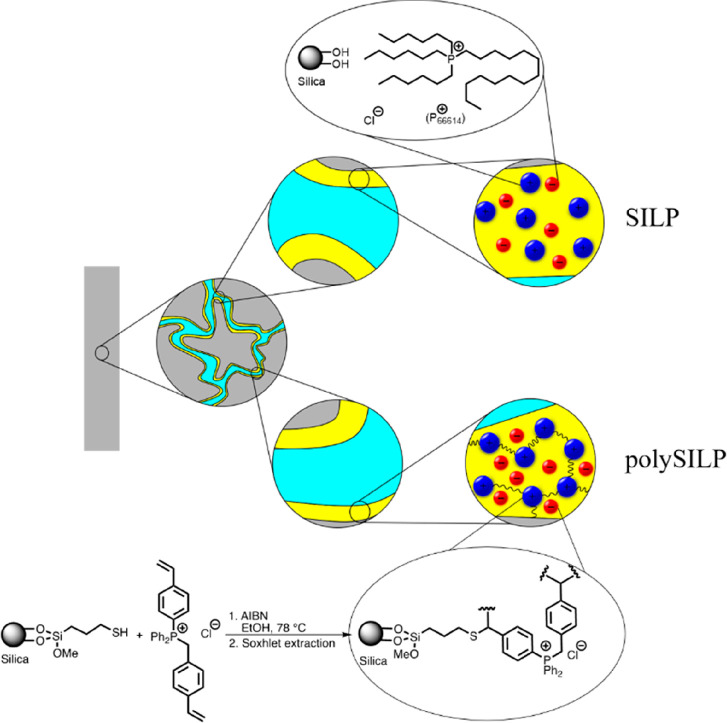
On the basis of previous studies on the liquid–liquid extraction of PGMs from acidic media, the hydrophobic ionic liquid P66614Cl was selected for the preparation of SILP materials via physisorption on silica support (Figure 2). Phosphonium-based ionic liquids have already been applied to PGM recovery and separation from model solutions16,30,31 and car catalyst acidic liquor.19 In general, hydrophobic ionic liquids with strongly coordinating anions, such as the chloride anion, demonstrate high affinity toward the chelation of metal anions.32,33 Moreover, phosphonium-based ionic liquid cations typically exhibit higher thermal and electrochemical stabilities compared to the widely investigated nitrogen- and imidazolium-based ionic liquids and are, as in the case of the employed P66614Cl, already often liquid at room temperature.34,35 The physisorption of the ionic liquid layer on the silica support in the case of SILP materials is based entirely on van der Waals forces which are weaker compared to chemical interactions; therefore, there is the possibility of the physisorbed ionic liquid to leach out of the solid support. While this problem is rarely faced in well-established catalytic processes in the gas phase, losses of ionic liquid from SILP materials is a considerable problem for catalysis in the liquid phase, particularly in the case of continuous processing.36
Figure 2.
Concept, structure, and preparation of SILP and polySILP materials as solid sorbent materials for PGM recovery.
In order to overcome this foreseeable problem, the concept of polySILPs was introduced. The preparation of polymerized ionic liquids on silica support (polySILP) was performed in a “grafting-from-surface” approach, relying on surface modification of silica with reactive thiol groups, followed by deposition of a thin ionic liquid layer and subsequent radical polymerization (Figure 2). It is envisioned that this type of silica–polymer hybrid material will improve stability toward leaching of the ionic liquid compared to conventional supported ionic liquid phases. Due to the commercial availability of precursor molecules, we switched, from the tetraalkylphosphonium cations used in the SILP materials, toward an aryl-functionalized species in the case of polySILPs, since the corresponding monomer, diphenyl(4-vinylbenzyl)(4-vinylphenyl)phosphonium chloride, provides an easily accessible precursor for the preparation of a cross-linked polymer layer on the mesoporous support of the polySILP. The synthetic process of the polySILP materials, which is described below in the example of polySILP 10% (w/w), was adapted from literature procedures.37,38
SILP materials with physisorbed P66614Cl and polySILP materials relying on the diphenyl(4-vinylbenzyl)(4-vinylphenyl)phosphonium chloride monomer with different loadings [10%–50% (w/w)] were prepared. The amount of ionic liquid monomer corresponding to the polySILP target loading was initially physisorbed on S-grafted silica and subsequently polymerized by addition of a radical initiator (AIBN). Nevertheless, lower than the target loadings were achieved which can be attributed to the inevitable deceleration of the polymerization process. For the polySILP target loadings of 10%, 20%, and 50%, actual loadings of 7%, 14%, and 39% were obtained, respectively. Apart from the gravimetric data, a number of characterization techniques, such as thermogravimetric analysis (TGA), diffuse reflectance infrared Fourier transform-infrared spectroscopy (Drift-IR), scanning electron microscopy (SEM), and Brunauer–Emmett–Teller (BET) analysis were conducted for the systematic characterization of these materials. Prior to each analysis, the samples were dried overnight to a residual pressure of 0.1 mbar in order to eliminate interferences due to the absorbed water molecules.
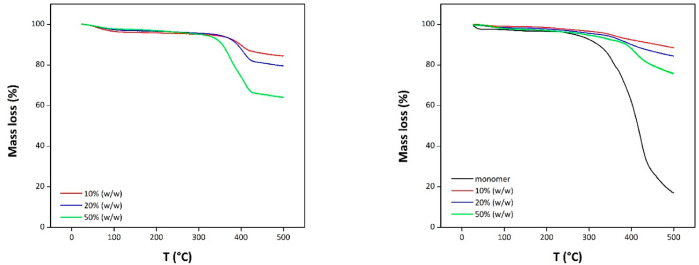
The thermal decomposition temperatures of these materials were found to be higher than 350 °C (Figure 3). Moreover, in the case of polySILPs, the weight loss allowed calculation of the actual loading after radical polymerization and removal of residual monomers via Soxhlet extraction.
Figure 3.
TGA curves of SILP (left) and polySILP (right) with loadings of 10%, 20%, and 50% (w/w). TGA was performed on a Netzsch STA 449 F1 system. The temperature was increased from 25 to 500 °C with a rate of 5 °C/min. Nitrogen gas flow was set to 40 mL/min.
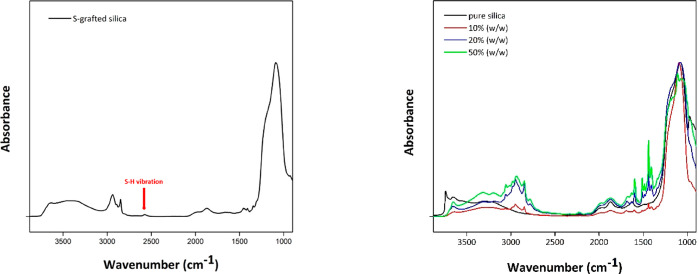
Successful loading of the polymer on the silica is evident from the IR spectra (Figure 4), where peaks in the region of 2500–3500 cm–1 and in the region of 1400–2000 cm–1 suggest the presence of polymerized ionic liquid on the surface. The symmetric and asymmetric stretches of the double bonds (C=C) are exhibited as peaks in the 1400–2000 cm–1 region, while the presence of =CH bonds is evident by the visible peaks in the 2500–3500 cm–1 region. The absence of the characteristic thiol group (S–H) peak at 2550 cm–1, which is present in the S-grafted silica (Figure 4, left), further indicates the successful attachment and polymerization of the ionic liquid monomer on the S-grafted silica surface.
Figure 4.
Drift-IR spectra of S-grafted silica (left) and pure silica and polySILP loadings of 10%, 20%, and 50% (w/w) (right). Recorded with a Bruker Vertex 80FTIR spectrophotometer using a narrow band MCT (mercury–cadmium–telluride) detector measuring diffuse reflectance. 256 scans were collected for each spectrum with 4 cm–1 resolution.
Additionally, the comparison of the attenuated total reflection-infrared spectroscopy (ATR-IR) spectrum of polySILP 20% and PGM-loaded polySILP 20% (Figure S2) indicates that the S atom does not coordinate with any of the PGMs. The C–S stretching vibration appears at 692 cm–1 in both spectra, meaning that a coordination extraction mechanism is very unlikely.
The SEM data recorded for polySILP 20%, both pure and after every process step (loading, stripping) (Figures S6–S9), do not reveal any modification on the physical appearance of the sorbent material surface. Unfortunately, the detection limit of the SEM measurements did not allow the quantification of the elements absorbed on the polySILP, since it is approximately 100 times higher than the concentration of the absorbed elements.
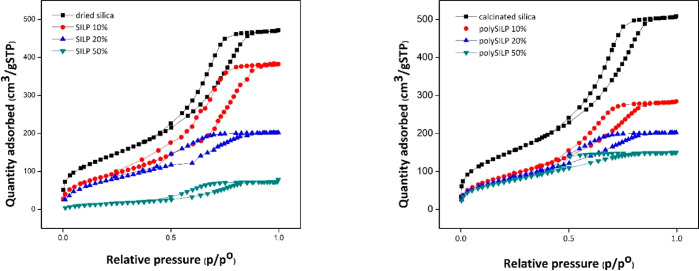
Nitrogen physisorption experiments were acquired with an ASAP 2020 from Micromeritics GmbH at 77K. Prior to the measurement, samples were degassed in vacuum at 180 °C for 6 h to remove moisture and adsorbed gases. For elucidation of the specific surface area and pore size distribution, the Brunauer–Emmett–Teller (BET) model and Barret–Joyner–Halenda (BJH) method were used, respectively. The mesoporous character of the supported ionic liquid materials, which has been determined via BET analysis, is responsible for their higher surface area as opposed to other conventionally employed solid sorbent materials (Figure 5).
Figure 5.
BET curve for dried silica and SILP (left) and calcinated silica and polySILP (right) with different loadings. Acquired with an ASAP 2020 from Micromeritics GmbH at 77K.
It is evident by the obtained BET data (Table 1) that increasing ionic liquid loading on the solid material decreases its specific surface area, as indicated by the decreasing size of the average pore diameter. The suitable loading for the target application should be selected in a way that both the mesoporous character of the material and the ionic liquid properties are effectively exploited.
Table 1. Structural Parameters Calculated for N2 Absorption–Desorption Isotherms.
| Sample | BET surface areaa (m2/g) | Pore volumeb (cm3/g) | Average pore diameterc (nm) |
|---|---|---|---|
| Pure silica | 496.08 | 0.76 | 5.9 |
| Calcinated silica | 525.61 | 0.81 | 6.0 |
| SILP 10% | 326.22 | 0.61 | 5.9 |
| SILP 20% | 243.63 | 0.42 | 5.4 |
| SILP 50% | 58.37 | 0.11 | 4.4 |
| polySILP 10% | 323.06 | 0.46 | 5.1 |
| polySILP 20% | 289.63 | 0.30 | 4.2 |
| polySILP 50% | 263.43 | 0.20 | 3.3 |
Calculated by the BET equation.
BJH pore desorption volume.
Desorption average pore diameter.
The introduction of ionic liquid on the silica surface leads to a considerable decrease in the specific surface area, both in the case of SILPs and polySILPs, as well as a small decrease in the average pore size. Blocking of the micropores within the silica by formation of a thin ionic liquid coating in the interior of the mesopore surface could explain this observation.
Additionally, increasing ionic liquid loading leads to a further decrease in the surface area, which is considerable in the case of SILP, while in the case of polySILP a drastic decrease in the pore diameter is observed. Our hypothesis is that in the case of SILPs, the ionic liquid does not only form uniform coatings on the silica substrate but probably starts filling the pores instead, which does not have an effect on the pore diameter but does have a drastic effect on the surface area. In contrast, the ionic liquid in polySILPs is further deposited on the internal coating, thus decreasing the pore diameter.
All samples show type IV isotherms with type H2(b) hysteresis, which is indicative of the presence of mesopores and minor pore blocking with wide size distributions of pore and neck widths, which is typical of mesocellular silica foams.39 The hysteresis curves of SILPs indicate partially filled pores, whereas the polySILP curves indicate that the pores are uniformly coated with the exception of entrance and exit points, where thicker coatings form bottlenecks.
Overall, in the case of SILPs, a thin ionic liquid layer is formed which eventually starts filling up the pores instead of further contributing to the coating. The ionic liquid in the case of polySILPs adds to the interior coating in a layer-type fashion, but eventually the coating becomes nonuniform and thicker on the entrance and exit points, thus forming bottlenecks.
Leaching of a Spent Car Catalyst Sample
Before addressing leaching and separation of PGMs, the spent car catalyst used in this study was fully characterized. It was digested with the aid of a mixture of mineral acids in a microwave oven (Multiwave 3000, Anton Paar, Germany), prior to measurement, for the complete dissolution of the ceramic material, which primarily comprises the car catalyst. The quantification was performed by ICP-OES (Radial iCAP6500, Thermo Scientific, USA) after appropriate sample dilution. The fitness for purpose of the analytical measurement method was initially verified with the aid of the certified reference material ERM-EB504.
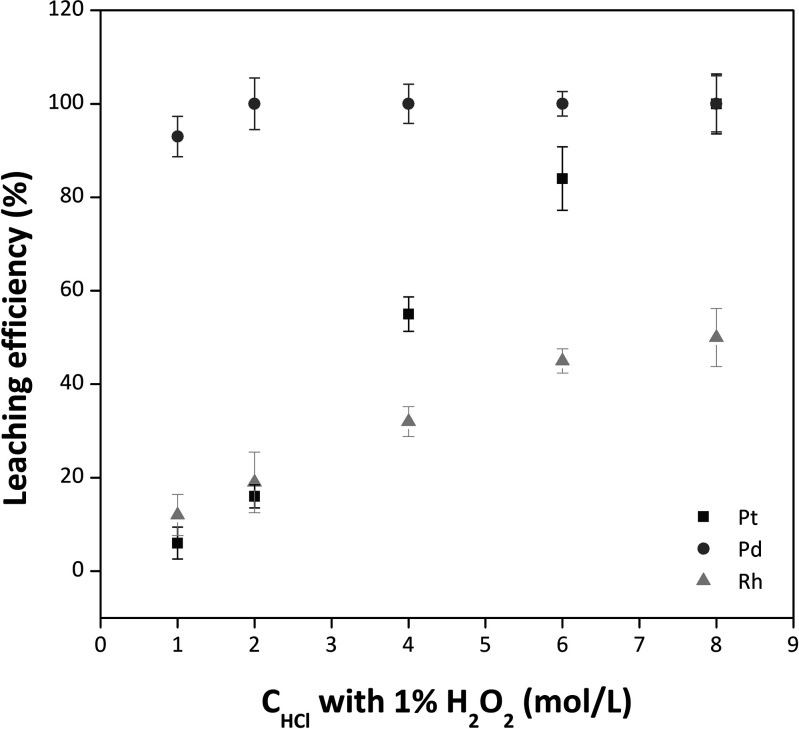
While different protocols for the leaching of PGMs from spent car catalysts exist in the literature, the combination of hydrochloric acid and hydrogen peroxide as an oxidant provides particularly attractive and, by way of comparison, benign conditions. Here, we employed a protocol for the leaching process developed by Harjanto et al.40 The grinded car catalyst was mixed with 1% H2O2 in HCl in a solid:liquid ratio of 1:5 and extracted at 65 °C for 3 h. The mixture was subsequently centrifuged for the sedimentation and separation of the solid car catalyst material, and the recovered leachate was diluted prior to PGM quantification by ICP-OES analysis. As expected, the leaching efficiencies of Pt and Rh are proportional to the HCl concentration, and a concentration of 8 M is required to obtain the maximum extraction efficiency under the employed experimental conditions (Figure 6).
Figure 6.
Effect of HCl concentration on PGM leaching efficiency. Conditions: car catalyst, 0.16 mm, 0.20 ± 0.01 g; 1% H2O2 in 8 M HCl, 1.00 ± 0.010 g; temperature, 65 °C; time, 3 h.
On the contrary, the extraction of Pd is almost independent of the HCl concentration, since any HCl concentration over 2 M yields quantitative extraction of Pd. Additional experiments were performed at higher temperatures (80, 120 °C) and longer reaction times (4, 6 h) in order to enhance the extraction of Rh; however, there was no observed effect on its extraction efficiency. The concentrations of all leached elements in the original catalyst and in the recovered acidic leachate (1% H2O2 in 8 M HCl) can be found in Table S1.
Evaluation of Supported Ionic Liquid Phases for Solid–Liquid Separation
The solid–liquid separation with SILP and polySILP materials was originally investigated with a model solution to study the retention of PGMs on the supported ionic liquids. According to the results obtained from the leaching of the car catalyst material, model PGM solutions were prepared reflecting the PGM concentration levels of the actual leachate. An acidic solution with 194 ppm Pt, 318 ppm Pd, and 34 ppm Rh (to which we refer as the model solution throughout the text) was used (where ppm = mg/L), and the PGM retention behavior was investigated under a variety of different parameters. The effect of different HCl concentrations of the model solution (while the PGM concentration was kept constant) on the PGM retention on the solid material is presented on the example of polySILP 20% in Figure 7.
Figure 7.
Retention behavior of PGMs from model solution of different HCl concentrations on a 20% (w/w) polySILP. Conditions: polySILP 20%, 0.50 ± 0.01 g; model solution, 2.50 ± 0.010 g; flow rate, 3 mL/min.
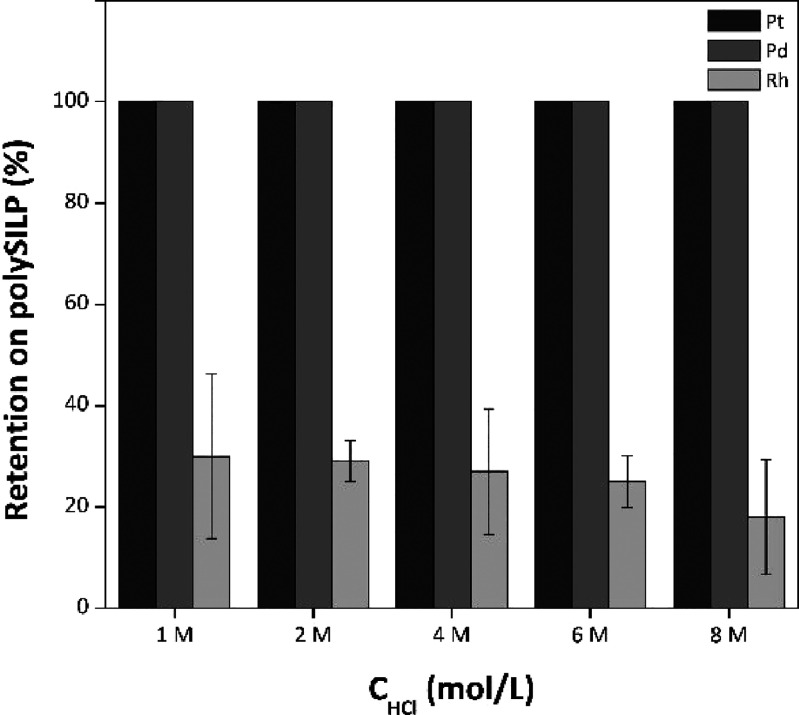
In both cases of SILP 20% (Figure S11) and polySILP 20% (Figure 7) materials, quantitative retention of Pt and Pd on the sorbent is observed, and it exhibits independency from the acidity of the model PGM solution. On the contrary, the retention of Rh is dependent on the HCl concentration of the model PGM solution; increasing HCl concentration yields a decrease in the retention of Rh, which implies that partial separation of Pt and Pd from Rh is possible by simply tuning the acidity of the PGM solution.
Similar behavior is observed in the case that SILPs and polySILPs with loadings of 10% and 50% (Figures S10 and S12 and Figures S13 and S15, respectively) are employed. The effect of the HCl concentration on the separation of Pt and Pd from Rh in biphasic systems has been previously reported; Pt and Pd are extracted from a wide range of HCl concentrations into an amine-based extractant, whereas Rh can only be extracted when low acidic concentrations are employed.41 Additionally, the dependency of Rh retention on various anion exchangers on the HCl acid concentration has been previously demonstrated.42,43
The dependency of Rh retention on the HCl concentration can be attributed to the presence of different Rh species at different concentrations and their respective difference in extractability. Increasing [HCl] leads to an increase in the concentration of aquated Rh–chlorocomplexes, which pose higher metal ion charge densities as opposed to the nonaquated ones. Extraction efficiency via the anion exchange mechanism is proportional to the metal ion charge density, while higher charge density also implies lower solvation energy of the respective complexes. At lower acidities, which implies a concurrently higher amount of water in solution, the aquated species [RhCl4(H2O)]− and [RhCl5(H2O)]2– are the dominant and most probably the extractable ones, while RhCl36– is most probably not extracted. Aside from the high charge density of the latter species, the steric demands imposed by their complexation further hinder their extraction.16,44−46
Further studies on the effect of the dilution of the 8 M HCl model PGM solution (both acidity and PGM concentration are modified via dilution) on the retention on the solid material showed that partial separation of Pt and Pd from Rh may be observed, as shown on the examples of SILP and polySILP with 20% loading (Figures S17 and S20). In both cases of SILP and polySILP materials, quantitative retention of Pt and Pd on the column is observed, and it exhibits independency from the dilution of the model PGM solution. On the contrary, the retention of Rh is dependent on the dilution, as a lower dilution (i.e., a higher HCl concentration) yields a decrease in the retention of Rh. Similar behavior is observed in the case that SILPs and polySILPs with loadings of 10% and 50% are employed (Figures S16 and S18 and Figures S19 and S21, respectively).
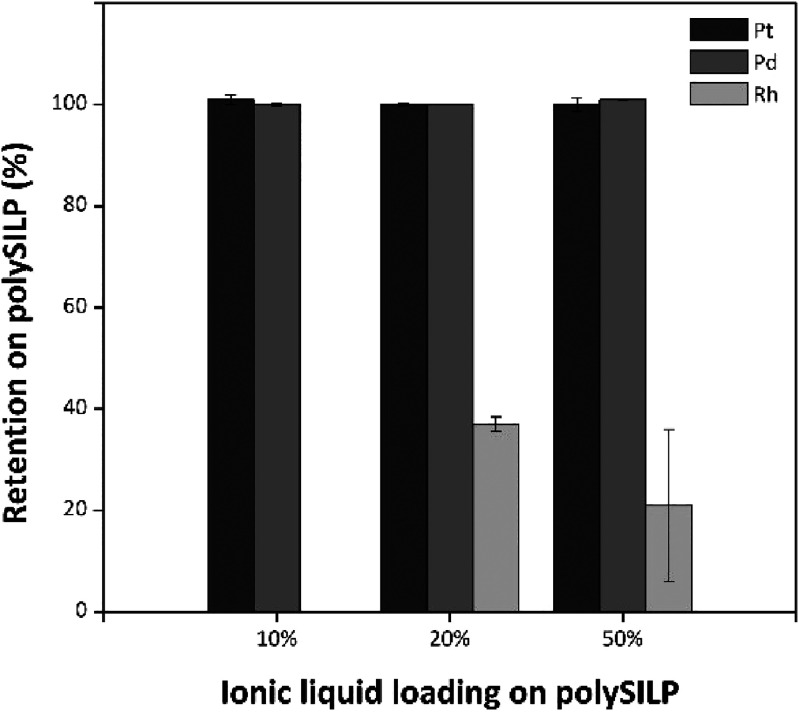
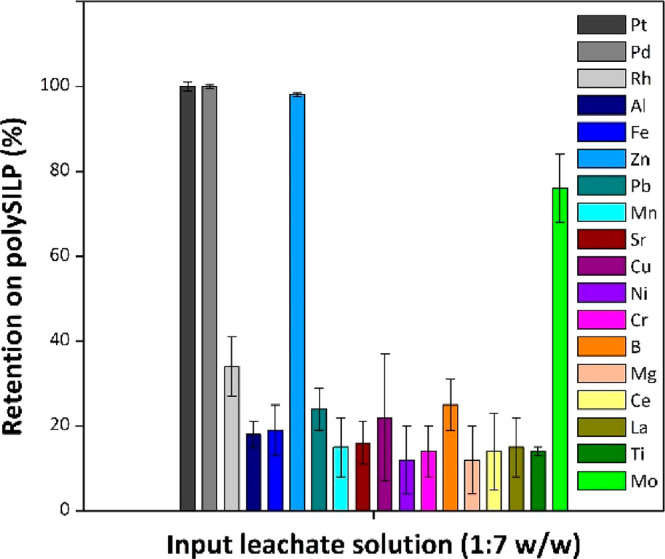
Eventually, the effect of the solid material loading on the retention of PGMs was studied on the example of a model solution diluted at a 1:7 w/w ratio with H2O. In both cases of SILP (Figure S22) and polySILP (Figure 8) materials, the quantitative retention of Pt and Pd on the solid material is not affected by the ionic liquid loading of the solid material. The retention of Rh, however, follows the trend of the ionic liquid loading on the solid material; an increase in the loading results in higher retention of Rh on the solid material. The difference in the retention behavior of the PGMs might therefore be exploited in order to achieve partial separation of Pt and Pd from Rh by simply adjusting the ionic liquid loading on the solid material.
Figure 8.
Retention behavior of PGMs from model solution diluted 1:7 w/w on polySILP with different ionic liquid loadings. Conditions: polySILP various loadings, 0.50 ± 0.01 g; model solution 1:7 w/w, 2.50 ± 0.010 g; flow rate, 3 mL/min.
After optimization of the sorption behavior of the model solution and identification of the most suitable supported ionic liquid phase, we addressed the development of a separation process for an authentic PGM containing solution. The effectiveness of the solid materials was tested on a real leachate sample in order to determine the retention behavior of PGMs as well as their recovery efficiency in the presence of other elements comprising the catalyst matrix. For this purpose, supported ionic liquid phases with 20% (w/w) loading were selected. PGMs along with accompanying elements present in the catalyst matrix were leached according to the optimized conditions, and the obtained leachate was diluted with H2O on a 1:7 w/w ratio prior to the solid-based separation.
The retention profiles of PGMs from the leachate on SILP (Figure S23) and polySILP 20% (Figure 9) are similar to the ones exhibited when model solutions are employed; quantitative retention of Pt and Pd and partial retention of Rh.
Figure 9.
Retention behavior of PGMs and accompanying elements from automotive catalyst leachate on polySILP 20% (w/w). Conditions: polySILP 20%, 0.50 ± 0.01 g; catalyst leachate 1:7 w/w, 2.50 ± 0.010 g; flow rate, 3 mL/min.
Concerning the major interfering elements, Al, Ce, Fe, and Zn, it seems that both SILP and polySILP have the capacity for quantitative retention of Zn. SILP also shows high affinity for Fe, contrary to polySILP, where only partial Fe retention (20%) is observed. Furthermore, partial separation of the PGMs from Al and Ce is obtained in both cases. Overall, polySILP 20% is preferable to SILP 20% for the solid-based separation process, given the fact that partial separation of PGMs from the majority of the main interfering elements is obtained in a single separation step.
Stripping of Pt and Pd from polySILP Material
With the loading of PGMs on the solid material optimized, we addressed the separation of Pt and Pd from the interfering elements and their recovery. For this purpose, a stepwise stripping procedure was developed. The individual steps of the entire retention and separation process are summarized in the flowsheet presented in Figure 10.
Figure 10.
Flowsheet of PGM leaching and recovery process.
For the desorption of the PGMs from the solid adsorbent material, various concentrations of the selected stripping agent were applied in a sequential order. Acidified thiourea solution (2 M HCl/1 M thiourea) has been reported as an effective stripping agent for the selective removal of Pt and Pd from a silica-based anion exchanger in the presence of Fe, Cu, and Ni.47 The same stripping agent (0.1 M HCl/0.1 M thiourea) has demonstrated the ability for quantitative desorption of Pt and Pd from a Dowex anion exchanger.48
Employing acidified thiourea as desorption agent generates considerable matrix effects; thus, all the stripped elements were quantified by a matrix matching approach, i.e., applying acidified thiourea calibration standards.
The stripping was performed in two steps with acidified thiourea solutions of different concentration levels. The first stripping step was performed with 0.01 M thiourea in 0.01 M HCl as the stripping agent. The majority of the interfering elements were successfully removed, whereas no PGMs, except for 4 ± 2% Pt, were desorbed from the solid sorbent material. Trace elements Mn, Ni, and Mo were only partially removed in this stripping step, while further stripping efforts with additional fractions of the stripping agent did not have any effect on stripping the PGMs or further stripping the remaining traces of interfering elements. Treatment of the loaded solids with 0.02 M thiourea in 0.02 M HCl, as well as 0.05 M thiourea in 0.05 M HCl, generated comparable results in terms of desorption; hence, the lowest concentration (0.01 M thiourea in 0.01 M HCl) was selected for this stripping cycle.
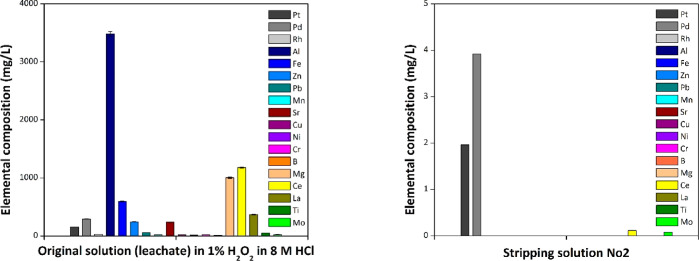
For the recovery of the PGMs from the solid material, a second stripping step was performed with a stripping solution of 1.0 M thiourea in 1.0 M HCl. Pt and Pd were successfully removed from the column with a desorption of 92 ± 3% and 100 ± 2%, respectively. The two elements were recovered in the stripping solution with 99% purity and recoveries of 86% for Pt and 96% for Pd (Figure 11, right). The desorption of Rh was attempted with various stripping agents previously reported in the literature; 6 M HCl, 5 M NH3,49 1 M thiourea,47 and 1 M NaClO3 in 5 M HCl.43 Nevertheless, none of these stripping agents was effective for the desorption of Rh from the polySILP. Rh is retained on the column along with traces of Cr, Ni, and Mo.
Figure 11.
Elemental composition (mg/L) of the leachate solution (left) and the recovered stripping solution after the second stripping step (right). Conditions retention (left): polySILP 20%, 0.50 ± 0.01 g; catalyst leachate 1:7 w/w, 2.50 ± 0.010 g; flow rate, 3 mL/min. Conditions stripping cycle 2 (right): polySILP 20%, 0.50 ± 0.01 g after stripping cycle 1; 1.0 M thiourea/1.0 M HCl, 60.0 ± 0.10 g; flow rate, 3 mL/min.
Stability, Capacity, and Recyclability of Supported Ionic Liquid Phases
The stability of the supported ionic liquid phases used for the separation is of crucial importance, since leaching of the ionic liquid out of the silica would mean decreasing capacity of the solid toward PGM retention, which implies limited reuse possibility. The stability against leaching was evaluated based on the P content detected by ICP-OES measurements in the output eluents collected after the retention and stripping experiments.
The merit of polySILP materials compared to conventional SILP systems is obvious when assessing the leaching behavior of both materials, as significantly lower phosphor contents were found in solution for the polymerized supported ionic liquid phase, indicating that a lesser amount of ionic liquid is leaching out of the sorbent material in the case of polySILPs, i.e., 10 times less than in the case of SILPs (Table 2).
Table 2. Stability of SILP and polySILP Expressed in % Leaching of Ionic Liquid.
| Solid material | Loading (%) | Retention 1:7 | Stripping 0.01/0.01 | Stripping 1.0/1.0 |
|---|---|---|---|---|
| SILP | 10 | 0.010 | 0.038 | 0.027 |
| 20 | 0.011 | 0.048 | 0.031 | |
| 50 | 0.018 | 0.068 | 0.038 | |
| polySILP | 10 | 0.002 | 0.003 | 0.004 |
| 20 | 0.001 | 0.002 | 0.001 | |
| 50 | 0.004 | 0.009 | 0.002 |
In general, PGMs have the tendency to form stable anionic chlorocomplexes in acidic chloride solutions.40,50 The retention of the PGMs on the supported ionic liquid phases can be attributed to their complexation with the chloride anions, as it has been previously reported for the liquid–liquid extraction of platinum group metals with ionic liquids such as P66614Cl15,51 but also for anion exchange resins.37 The polymerized phosphonium-based salt can act as an anion exchanger;52 it is thus presumed that the retention of the PGMs on polySILP is based on the Cl– substitution by the anionic PGM chlorocomplexes (anion exchange mechanism).
In this regard, the comparison of polySILP materials with the commercially available resins that have been reported as efficient absorbents for platinum group metals, such as Amberlite,49 is of crucial importance. The sorbent material was loaded with leachate. The ensuing eluted fractions (5 mL each) were collected, and their PGM content was quantified by ICP-OES. The sorbent loading and fraction collection were interrupted as soon as the quantification results of 10 consecutive fractions were reproducible. Our studies showed a strongly increased short-term capacity of polySILP for both Pt and Pd compared to Amberlite IRA-400 (Figure 12); the observed behavior implies that polySILP retains more PGM amount/time unit, which can directly be translated to faster release of high PGM amounts to the market, which is highly desirable from an industrial point of view. This advantage coupled with the ability of the polySILP column to be recycled without losing any of its retention capacity renders polySILP a much more efficient approach for the timely satisfaction of the increasingly demanding PGM market. The complete breakthrough curves beyond the breaking point are also provided (Figure S23).
Figure 12.
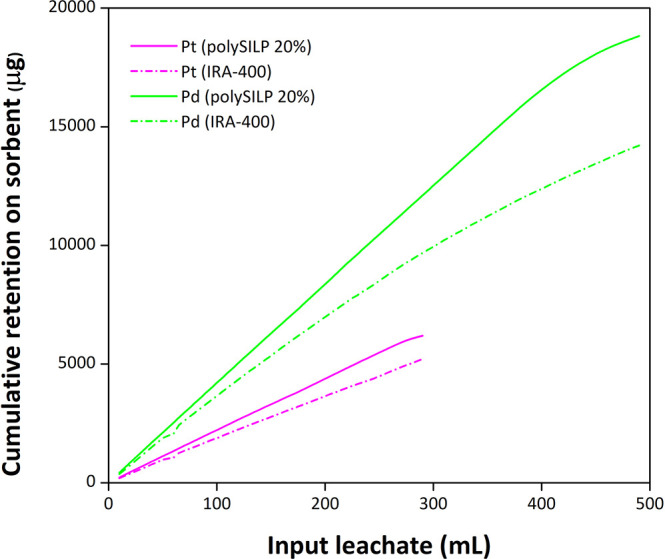
Cumulative retention capacity for Pt and Pd on a 20% (w/w) polySILP vs Amberlite IRA-400. Conditions: polySILP 20%, 0.50 ± 0.01 g; total catalyst leachate 1:7 w/w, 690 ± 50 g; flow rate, 3 mL/min; Amberlite IRA-400, 0.50 ± 0.01 g; total catalyst leachate 1:7 w/w, 670 ± 50 g; flow rate, 3 mL/min.
An additional advantage of polySILP is that it does not require equilibration prior to use, which leads to shorter experimental time and reduced generated waste. On the contrary, Amberlite can retain higher amounts of both Pt and Pd before it reaches its maximum capacity; however, this is a slow process considering that it needs to be loaded with more than double the amount of leachate that polySILP 20% requires until it loses its retention capacity. Additionally, it has to be equilibrated and washed with solution multiple times its volume before loading.
We should also mention the ease and simplicity of removing Pt and Pd; in the case of polySILPs, Pt and Pd can be desorbed in a single stripping step employing acidified thiourea at RT, whereas in the case of Amberlite a two-step process with NH3 (two different NH3 concentrations consecutively applied) at elevated temperatures is required for the simultaneous removal of Pt and Pd.49
Eventually, the possibility of reusing polySILP 20% for subsequent separation experiments was evaluated. After stripping the PGMs and the accompanying elements from the solid phase, according to the process previously discussed in detail, polySILP 20% was loaded with a new fraction of acidic car catalyst leachate. The recycled column exhibited the same retention capacity for the PGMs and the accompanying elements (Figure S22). The reproducible retention behavior of the recycled column additionally verifies the stability of the IL bound on the sorbent material. Nevertheless, stripping the recycled loaded solid with 0.01 M thiourea/0.01 M HCl did not yield comparable results to stripping fresh solid material, as a considerable amount of Pd and Pt were stripped along with the interfering elements, therefore, not enabling their efficient separation and recovery.
It has already been demonstrated in previously published research53,54 that sufficient washing of solid sorbent materials between stripping and retention steps is necessary for regeneration of the sorbent material. Therefore, polySILP 20% was removed from the column after completion of the second stripping step and washed three times, for 8 h each, with various solvents, namely, H2O, 0.1 M HCl, and 1.0 M HCl. Furthermore, the absence of residual thiourea after these washing steps was verified via ATR-IR (Figure S3). Nevertheless, while the retention of Pt and Pd remained excellent, the separation performance suffered with the washed and recycled sorbent material, since the majority of Pd (70%) and a considerable amount of Pt (30%) were desorbed from the solid together with the accompanying elements. Our future studies will aim for a more refined analysis of the recovered polySILP material to account for this modified stripping behavior, aiming to improve the separation of Pd and Pt from the accompanying elements with the recycled material.
Conclusions
A novel supported ionic liquid-based method for the separation of platinum group metals was developed. Supported ionic liquid phases and polymerized supported ionic liquid phases were synthesized and compared in terms of separation performance. Here, polySILPs exhibited higher separation efficiency of PGMs from accompanying interfering elements, as well as higher stability toward leaching of the ionic liquid out of the solid material compared to SILPs. Contrary to conventionally used silica bead-based sorbent materials, polySILPs are markedly characterized by their high porosity and no requirement of preconditioning. Separation of Pt and Pd from Rh and other interfering elements and recovery of Pt and Pd in solution were successfully performed with acidified thiourea solutions. Recoveries of Pt and Pd of 86% and 96%, respectively, in the stripping solution were obtained, with a combined 99% purity. While the retention of Pd and Pt on the recycled polySILP remained excellent, the separation of Pt and Pd from interfering elements was not possible, indicating that further improvements for the recycling of the spent material are required. Our future studies will address an improved recycling procedure for the solid material and aim to investigate further the separation of Pt and Pd from each other as well as the possibility of stripping Rh. On the basis of efficiency, speed, and simplicity of the developed separation method, we envision its application to different types of PGM-containing matrices.
Acknowledgments
The European Commission is gratefully acknowledged. This project has received funding from the European Union’s Horizon 2020 Research and innovation Programme under Grant Agreement No. 730224 (PLATIRUS). The authors are grateful to all project partners for discussions and input and, in particular, to Monolithos Ltd. (Athens, Greece) for providing the spent car catalyst material used in this work. The authors thank Fabian Scharinger for his assistance in designing the graphical abstract.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acssuschemeng.0c07384.
Synthesis procedure for SILPs, DRIFT-IR spectra for SILPs and ATR-IR spectra of polySILPs, SEM images of SILPs and polySILPs, retention behaviors of PGMs from model solutions and real leachate on SILPs and polySILPs, breakthrough curves for polySILP 20% and Amberlite IRA-400, characterization of car catalyst material, and ICP-OES analysis parameters (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Johnson Matthey PGM Market Report 2018, 2018. https://matthey.com/en/news/2018/pgm-market-report-2018-out-now (accessed December 2020).
- Johnson Matthey PGM Market Report 2019, 2019. https://matthey.com/en/news/2019/pgm-market-report-may-2019-out-now
- Shelef M.; Graham G. W. Why Rhodium in Automotive Three-Way Catalysts?. Catal. Rev.: Sci. Eng. 1994, 36 (3), 433–457. 10.1080/01614949408009468. [DOI] [Google Scholar]
- Zientek M. L.; Causey J. D.; Parks H. L.; Miller R. J.. Platinum-Group Elements in Southern Africa: Mineral Inventory and an Assessment of Undiscovered Mineral Resources: Chapter Q in Global Mineral Resource Assessment; Scientific Investigations Report 2010-5090Q; U.S. Geological Survey: Reston, VA, 2014; p 142. 10.3133/sir20105090Q. [DOI]
- Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions on the 2017 List of Critical Raw Materials for the EU, 2017. https://ec.europa.eu/transparency/regdoc/rep/1/2017/EN/COM-2017-490-F1-EN-MAIN-PART-1.PDF (accessed December 2020).
- Alonso E.; Field F. R.; Kirchain R. E.. A Case Study of the Availability of Platinum Group Metals for Electronics Manufacturers. In Proceedings of the 2008 IEEE International Symposium on Electronics and the Environment, IEEE Computer Society, 2008; pp 1–6. 10.1109/ISEE.2008.4562902. [DOI]
- Bardi U.; Caporali S. Precious Metals in Automotive Technology: An Unsolvable Depletion Problem?. Minerals 2014, 4 (2), 388–398. 10.3390/min4020388. [DOI] [Google Scholar]
- Jha M. K.; Lee J.-c.; Kim M.-s.; Jeong J.; Kim B.-S.; Kumar V. Hydrometallurgical recovery/recycling of platinum by the leaching of spent catalysts: A review. Hydrometallurgy 2013, 133, 23–32. 10.1016/j.hydromet.2012.11.012. [DOI] [Google Scholar]
- Bernfeld G. J.; A.J B.; Edwards R. I.; Köpf H.; Köpf-Maier P.; Raub C. J.; te Riele W. A. M.; Simon F.; Westwood W.. Gmelin Handbook of Inorganic and Organometallic Chemistry; Springer, 1985. [Google Scholar]
- El-Nadi Y. A. Solvent Extraction and Its Applications on Ore Processing and Recovery of Metals: Classical Approach. Sep. Purif. Rev. 2017, 46 (3), 195–215. 10.1080/15422119.2016.1240085. [DOI] [Google Scholar]
- Cantwell F. F.; Losier M.. Chapter 11 Liquid—liquid Extraction. In Comprehensive Analytical Chemistry; Elsevier, 2002; Vol. 37, pp 297–340. 10.1016/S0166-526X(02)80048-4. [DOI] [Google Scholar]
- Freemantle M.An Introduction to ionic liquids; RSC Publishing, 2010; ISBN 978-1-84755-161-0. [Google Scholar]
- Tan Z.-C.; Welz-Biermann U.; Yan P.-F.; Liu Q.-S.; Fang D.-W.. Thermodynamic Properties of Ionic Liquids - Measurements and Predictions. In Ionic Liquids: Theory, Properties, New Approaches; IntechOpen, 2011. 10.5772/15222. [DOI] [Google Scholar]
- Rebelo L. P. N.; Lopes J. N. C.; Esperança J. M. S. S.; Guedes H. J. R.; Łachwa J.; Najdanovic-Visak V.; Visak Z. P. Accounting for the Unique, Doubly Dual Nature of Ionic Liquids from a Molecular Thermodynamic and Modeling Standpoint. Acc. Chem. Res. 2007, 40 (11), 1114–1121. 10.1021/ar7000556. [DOI] [PubMed] [Google Scholar]
- Nguyen V. T.; Lee J.-c.; Chagnes A.; Kim M.-s.; Jeong J.; Cote G. Highly selective separation of individual platinum group metals (Pd, Pt, Rh) from acidic chloride media using phosphonium-based ionic liquid in aromatic diluent. RSC Adv. 2016, 6 (67), 62717–62728. 10.1039/C6RA09328K. [DOI] [Google Scholar]
- Svecova L.; Papaiconomou N.; Billard I. Quantitative extraction of Rh(iii) using ionic liquids and its simple separation from Pd(ii). Dalton Transactions 2016, 45 (38), 15162–15169. 10.1039/C6DT02384C. [DOI] [PubMed] [Google Scholar]
- Cieszynska A.; Wisniewski M. Extraction of palladium(II) from chloride solutions with Cyphos®IL 101/toluene mixtures as novel extractant. Sep. Purif. Technol. 2010, 73 (2), 202–207. 10.1016/j.seppur.2010.04.001. [DOI] [Google Scholar]
- Firmansyah M. L.; Kubota F.; Yoshida W.; Goto M. Application of a Novel Phosphonium-Based Ionic Liquid to the Separation of Platinum Group Metals from Automobile Catalyst Leach Liquor. Ind. Eng. Chem. Res. 2019, 58 (9), 3845–3852. 10.1021/acs.iecr.8b05848. [DOI] [Google Scholar]
- Firmansyah M. L.; Kubota F.; Goto M. Selective Recovery of Platinum Group Metals from Spent Automotive Catalysts by Leaching and Solvent Extraction. J. Chem. Eng. Jpn. 2019, 52 (11), 835–842. 10.1252/jcej.19we093. [DOI] [Google Scholar]
- Marinho R. S.; Afonso J. C.; da Cunha J. W. S. D. Recovery of platinum from spent catalysts by liquid–liquid extraction in chloride medium. J. Hazard. Mater. 2010, 179 (1), 488–494. 10.1016/j.jhazmat.2010.03.029. [DOI] [PubMed] [Google Scholar]
- Han D.; Row K. Recent Applications of Ionic Liquids in Separation Technology. Molecules 2010, 15, 2405–26. 10.3390/molecules15042405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemus J.; Palomar J.; Gilarranz M. A.; Rodriguez J. J. Characterization of Supported Ionic Liquid Phase (SILP) materials prepared from different supports. Adsorption 2011, 17 (3), 561–571. 10.1007/s10450-011-9327-5. [DOI] [Google Scholar]
- Mehnert C. P.; Cook R. A.; Dispenziere N. C.; Afeworki M. Supported Ionic Liquid Catalysis – A New Concept for Homogeneous Hydroformylation Catalysis. J. Am. Chem. Soc. 2002, 124 (44), 12932–12933. 10.1021/ja0279242. [DOI] [PubMed] [Google Scholar]
- Riisager A.; Jørgensen B.; Wasserscheid P.; Fehrmann R. First application of supported ionic liquid phase (SILP) catalysis for continuous methanol carbonylation. Chem. Commun. 2006, 9, 994–996. 10.1039/b516314e. [DOI] [PubMed] [Google Scholar]
- Kukawka R.; Pawlowska-Zygarowicz A.; Dzialkowska J.; Pietrowski M.; Maciejewski H.; Bica K.; Smiglak M. Highly Effective Supported Ionic Liquid-Phase (SILP) Catalysts: Characterization and Application to the Hydrosilylation Reaction. ACS Sustainable Chem. Eng. 2019, 7 (5), 4699–4706. 10.1021/acssuschemeng.8b04357. [DOI] [Google Scholar]
- Liu Y.; Zhu L.; Sun X.; Chen J.; Luo F. Silica Materials Doped with Bifunctional Ionic Liquid Extractant for Yttrium Extraction. Ind. Eng. Chem. Res. 2009, 48 (15), 7308–7313. 10.1021/ie900468c. [DOI] [Google Scholar]
- Sun X.; Ji Y.; Chen J.; Ma J. Solvent impregnated resin prepared using task-specific ionic liquids for rare earth separation. J. Rare Earths 2009, 27 (6), 932–936. 10.1016/S1002-0721(08)60365-8. [DOI] [Google Scholar]
- Navarro R.; Garcia E.; Saucedo I.; Guibal E. Platinum(IV) Recovery from HCl Solutions using Amberlite XAD-7 Impregnated with a Tetraalkyl Phosphonium Ionic Liquid. Sep. Sci. Technol. 2012, 47 (14–15), 2199–2210. 10.1080/01496395.2012.697522. [DOI] [Google Scholar]
- Van Roosendael S.; Regadío M.; Roosen J.; Binnemans K. Selective recovery of indium from iron-rich solutions using an Aliquat 336 iodide supported ionic liquid phase (SILP). Sep. Purif. Technol. 2019, 212, 843–853. 10.1016/j.seppur.2018.11.092. [DOI] [Google Scholar]
- Regel-Rosocka M.; Rzelewska M.; Baczynska M.; Janus M.; Wiśniewski M. Removal of Pd(II) from Aqueous Chloride Solutions with Cyphos Phosphonium Ionic Liquids as Metal Ion Carriers for Liquid-Liquid Extraction and Transport Across Polymer Inclusion Membranes. Physicochem. Probl. Miner. Process. 2015, 51, 621–631. 10.5277/ppmp150221. [DOI] [Google Scholar]
- Kumar J. R.; Choi I.-H.; Lee J.-Y. Precious Metals Extraction Processing in Chloride Media by Using Ionic Liquids as Novel Extractant Systems. Korean Chem. Eng. Res. 2017, 55, 503–509. 10.9713/KCER.2017.55.4.503. [DOI] [Google Scholar]
- Zhou Y.; Boudesocque S.; Mohamadou A.; Dupont L. Extraction of Metal Ions with Task Specific Ionic Liquids: Influence of a Coordinating Anion. Sep. Sci. Technol. 2015, 50 (1), 38–44. 10.1080/01496395.2014.952747. [DOI] [Google Scholar]
- Mehdi H.; Binnemans K.; Van Hecke K.; Van Meervelt L.; Nockemann P. Hydrophobic ionic liquids with strongly coordinating anions. Chem. Commun. 2010, 46 (2), 234–236. 10.1039/B914977E. [DOI] [PubMed] [Google Scholar]
- Barsanti A. C.; Chiappe C.; Ghilardi T.; Pomelli C. S. Functionalized phosphonium based ionic liquids: properties and application in metal extraction. RSC Adv. 2014, 4 (73), 38848–38854. 10.1039/C4RA04723K. [DOI] [Google Scholar]
- Fraser K. J.; MacFarlane D. R. Phosphonium-Based Ionic Liquids: An Overview. Aust. J. Chem. 2009, 62 (4), 309–321. 10.1071/CH08558. [DOI] [Google Scholar]
- Riisager A.; Fehrmann R.; Haumann M.; Wasserscheid P. Supported Ionic Liquid Phase (SILP) Catalysis: An Innovative Concept for Homogeneous Catalysis in Continuous Fixed-Bed Reactors. Eur. J. Inorg. Chem. 2006, 2006 (4), 695–706. 10.1002/ejic.200500872. [DOI] [Google Scholar]
- Gruttadauria M.; Liotta L. F.; Salvo A. M. P.; Giacalone F.; La Parola V.; Aprile C.; Noto R. Multi-Layered, Covalently Supported Ionic Liquid Phase (mlc-SILP) as Highly Cross-Linked Support for Recyclable Palladium Catalysts for the Suzuki Reaction in Aqueous Medium. Adv. Synth. Catal. 2011, 353 (11–12), 2119–2130. 10.1002/adsc.201100186. [DOI] [Google Scholar]
- Bivona L. A.; Giacalone F.; Vaccaro L.; Aprile C.; Gruttadauria M. Cross-Linked Thiazolidine Network as Support for Palladium: A New Catalyst for Suzuki and Heck Reactions. ChemCatChem 2015, 7 (16), 2526–2533. 10.1002/cctc.201500408. [DOI] [Google Scholar]
- Thommes M.; Kaneko K.; Neimark A. V.; Olivier J. P.; Rodriguez-Reinoso F.; Rouquerol J.; Sing K. S. W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87 (9–10), 1051. 10.1515/pac-2014-1117. [DOI] [Google Scholar]
- Harjanto S.; Cao Y.; Shibayama A.; Naitoh I.; Nanami T.; Kasahara K.; Okumura Y.; Liu K.; Fujita T. Leaching of Pt, Pd and Rh from Automotive Catalyst Residue in Various Chloride Based Solutions. Mater. Trans. 2006, 47 (1), 129–135. 10.2320/matertrans.47.129. [DOI] [Google Scholar]
- Khattak M. A.; Magee R. J. Extraction of platinum metals by high-molecular-weight amines. rhodium(III) systems. Anal. Chim. Acta 1969, 45 (2), 297–304. 10.1016/S0003-2670(01)95601-6. [DOI] [Google Scholar]
- Fontàs C.; Hidalgo M.; Salvadó V. Adsorption and Preconcentration of Pd(II), Pt(IV), and Rh(III) using Anion-Exchange Solid-Phase Extraction Cartridges (SPE). Solvent Extr. Ion Exch. 2009, 27 (1), 83–96. 10.1080/07366290802544635. [DOI] [Google Scholar]
- Alam M. S.; Inoue K.; Yoshizuka K. Ion exchange/adsorption of rhodium(III) from chloride media on some anion exchangers. Hydrometallurgy 1998, 49 (3), 213–227. 10.1016/S0304-386X(98)00024-3. [DOI] [Google Scholar]
- Benguerel E.; Demopoulos G. P.; Harris G. B. Speciation and separation of rhodium (III) from chloride solutions: a critical review. Hydrometallurgy 1996, 40 (1), 135–152. 10.1016/0304-386X(94)00086-I. [DOI] [Google Scholar]
- Firmansyah M. L.; Kubota F.; Goto M. J. Chem. Technol. Biotechnol. 2018, 93 (6), 1714–1721. 10.1002/jctb.5544. [DOI] [Google Scholar]
- Svecova L.; Papaïconomou N.; Billard I. Rh(III) Aqueous Speciation with Chloride as a Driver for Its Extraction by Phosphonium Based Ionic Liquids. Molecules 2019, 24 (7), 1391. 10.3390/molecules24071391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer J.; Driessen W. L.; Koch K. R.; Reedijk J. Highly Selective and Efficient Recovery of Pd, Pt, and Rh from Precious Metal-Containing Industrial Effluents with Silica-Based (Poly)Amine Ion Exchangers. Sep. Sci. Technol. 2005, 39 (1), 63–75. 10.1081/SS-120027401. [DOI] [Google Scholar]
- Kovacheva P.; Djingova R. Ion-exchange method for separation and concentration of platinum and palladium for analysis of environmental samples by inductively coupled plasma atomic emission spectrometry. Anal. Chim. Acta 2002, 464 (1), 7–13. 10.1016/S0003-2670(02)00428-2. [DOI] [Google Scholar]
- Gaita R.; Al-Bazi S. J. An ion-exchange method for selective separation of palladium, platinum and rhodium from solutions obtained by leaching automotive catalytic converters. Talanta 1995, 42 (2), 249–255. 10.1016/0039-9140(94)00246-O. [DOI] [PubMed] [Google Scholar]
- Jimenez de Aberasturi D.; Pinedo R.; Ruiz de Larramendi I.; Ruiz de Larramendi J. I.; Rojo T. Recovery by hydrometallurgical extraction of the platinum-group metals from car catalytic converters. Miner. Eng. 2011, 24 (6), 505–513. 10.1016/j.mineng.2010.12.009. [DOI] [Google Scholar]
- Kubota F.; Shigyo E.; Yoshida W.; Goto M. Extraction and Separation of Pt and Pd by an Imidazolium-Based Ionic Liquid Combined with Phosphonium Chloride. Solvent Extr. Res. Dev., Jpn. 2017, 24 (2), 97–104. 10.15261/serdj.24.97. [DOI] [Google Scholar]
- Sun Q.; Ma S.; Dai Z.; Meng X.; Xiao F.-S. A hierarchical porous ionic organic polymer as a new platform for heterogeneous phase transfer catalysis. J. Mater. Chem. A 2015, 3 (47), 23871–23875. 10.1039/C5TA07267K. [DOI] [Google Scholar]
- Navarro R.; Saucedo I.; Gonzalez C.; Guibal E. Amberlite XAD-7 impregnated with Cyphos IL-101 (tetraalkylphosphonium ionic liquid) for Pd(II) recovery from HCl solutions. Chem. Eng. J. 2012, 185–186, 226–235. 10.1016/j.cej.2012.01.090. [DOI] [Google Scholar]
- Vincent T.; Parodi A.; Guibal E. Immobilization of Cyphos IL-101 in biopolymer capsules for the synthesis of Pd sorbents. React. Funct. Polym. 2008, 68 (7), 1159–1169. 10.1016/j.reactfunctpolym.2008.04.001. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.