Abstract
Aim
Ventilator-associated pneumonia (VAP) is the most common intensive care unit (ICU)-acquired infection. The current study aimed to assess the efficacy of mechanical insufflation-exsufflation (MI-E) in preventing VAP in critically ill patients.
Materials and methods
This retrospective cohort study was conducted at the ICU of Chiba University Hospital between January 2014 and September 2017. The inclusion criteria were patients who required invasive mechanical ventilation ≥48 hours and those who underwent rehabilitation, including chest physical therapy (CPT). In 2015, the study institution started the use of MI-E in patients with impaired cough reflex. From January to December 2014, patients undergoing CPT were classified under the historical control group, and those who received treatment using MI-E from January 2015 to September 2017 were included in the intervention group. The patients received treatment using MI-E via the endotracheal or tracheostomy tube, with insufflation-exsufflation pressure of 15–40 cm H2O. The treatment frequency was one to three sessions daily, and a physical therapist who is experienced in using MI-E facilitated the treatment.
Results
From January 2015 to September 2017, 11 patients received treatment using MI-E. Of the 169 patients screened in 2014, 19 underwent CPT. The incidence of VAP was significantly different between the CPT and MI-E groups (84.2% [16/19] vs 26.4% [3/11], p = 0.011). After adjusting for covariates, a multivariate logistic regression analysis was performed, and results showed that the covariates were not associated with the incidence of VAP.
Conclusion
This retrospective cohort study suggests that the use of MI-E in critically ill patients is independently associated with a reduced incidence of VAP.
Clinical significance
Assessing the efficacy of MI-E to prevent VAP.
How to cite this article
Kuroiwa R, Tateishi Y, Oshima T, Inagaki T, Furukawa S, Takemura R, et al. Mechanical Insufflation-exsufflation for the Prevention of Ventilator-associated Pneumonia in Intensive Care Units: A Retrospective Cohort Study. Indian J Crit Care Med 2021;25(1):62–66.
Keywords: Airway clearance, Chest physical therapy, Critically ill patients, Mechanical insufflation-exsufflation, Ventilator-associated pneumonia
Introduction
Ventilator-associated pneumonia (VAP) is the most common nosocomial infection in intensive care units (ICUs).1 The colonization of the aerodigestive tract with pathogenic microorganisms and the aspiration of contaminated secretions are the risk factors of VAP. Thus, airway clearance is critical in preventing VAP,2 and it requires effective coughing. However, individuals with weak respiratory muscles and decreased cough reflex may experience difficulties in clearing their airways. Tracheal suctioning removes the secretions in some regions of the central airway. However, it cannot clear the mucus in the peripheral airways.3 Chest physical therapy (CPT) may help achieve a better airway clearance with effective coughing, but it cannot prevent VAP and other relevant ICU outcomes in patients with respiratory muscle weakness and decreased cough reflex.4,5
In contrast, mechanical insufflation-exsufflation (MI-E) can facilitate effective coughing. It consists of insufflation of the lungs with positive pressure and an active negative-pressure exsufflation that creates a peak and sustained flow that is high enough to provide adequate shear and velocity to loosen and move secretions toward the mouth for suctioning or expectoration.6,7 The current international guidelines for the management of neuromuscular diseases recommend this treatment.8,9 Based on a randomized controlled trial of ICU patients, Gonçalves et al. have reported that MI-E may reduce reintubation rates and consequently shorten postextubation ICU stay.10 However, data about the use of MI-E in critically ill patients are limited.
We hypothesized that airway clearance using MI-E prevents pneumonia and the propagation of bacteria in the bronchi due to microaspiration. Thus, the current study, which is a pilot study for a future prospective study about MI-E, aimed to assess the efficacy of MI-E in preventing VAP in critically ill patients in the ICU.
Materials and Methods
Study Design
This retrospective cohort study included patients admitted to the ICU of Chiba University Hospital between January 2014 and September 2017. Information was obtained from the electronic medical records, which routinely state the premorbid condition of the patients.
Patients
Inclusion criteria were invasive mechanical ventilation (MV) for at least 48 hours and administration of rehabilitation, including CPT. The MI-E was initiated at the study institution in 2015. From January 2014 to December 2014, those receiving CPT constituted the historical control group, whereas those who received MI-E from January 2015 to September 2017 constituted the intervention group. The criterion for using MI-E was that the physiotherapist and physician judged the patient to have difficulty in expelling airway mucus by only chest physiotherapy due to impaired cough and considerable airway mucus.
Exclusion criteria included aspiration or community-acquired pneumonia occurring during provision of MV for less than 48 hours due to the definition of VAP, pneumothorax, pneumomediastinum, chronic obstructive pulmonary disease with bullae, severe cardiovascular failure defined as New York Heart Association functional class III or IV, active alveolar hemorrhage due to the risk of adverse events with MI-E,6 and injury or complication that did not allow CPT to be provided. Additionally, patients younger than 7 years of age were excluded due to the difference in airway structure that could affect airway clearance11 and increase risk of lung injury,12 and patients who received rehabilitation (including CPT) for less than 5 days for the same condition in the two groups were also excluded.
This study was conducted according to the principles established in the Declaration of Helsinki and approved by the Chiba University Ethics Committee, Approval No. 3089. The need for informed consent was obtained in the form of opt-out on the notice board by the Chiba University Ethics Committee owing to the retrospective observational design of the study.
Mechanical Insufflation-exsufflation Protocol
Patients received one or two sessions of MI-E with a Cough Assist E70 device (Philips Japan, Ltd., Tokyo) through the endotracheal or tracheostomy tube with both insufflation and exsufflation pressures set at a range of 15–40 cm H2O. For first-time users, low insufflation and exsufflation pressures were used initially (15–20 cm H2O), which were increased as needed by monitoring auscultatory clearing of rhonchi and improvements in oxyhemoglobin saturation.7 An insufflation/exsufflation time ratio of 2–3:2–3 seconds and a pause of 2 seconds between cycles were used. Five to ten cycles were applied in every session, with a chest wall thrust (manually assisted coughing) timed to the exsufflation cycle by the physical therapist. This was repeated several times or until secretions were sufficiently expelled.6 Mechanical insufflation-exsufflation was provided by a physical therapist with experience in the use of MI-E. Airway suctioning was performed at the end of each cycle and as needed during each rest period. Oxyhemoglobin saturation and heart rate were monitored during every treatment. Subjects were disconnected from the ventilator tubing to be connected to the tubing of the airway clearance device. Immediately after MI-E, mechanically ventilated and spontaneously breathing subjects were returned to their baseline ventilator status without any change in settings, mode, or fraction of inspired oxygen (FiO2).
Positioning of the patients depended on the most affected lung as revealed by auscultation, chest radiography, or chest computed tomography, and the patients were positioned such that the affected lung was positioned highest to allow gravity-assisted drainage.
Standard Medical Therapy and Chest Physiotherapy
All patients received standard medical therapy, including supplemental oxygen, as needed, CPT, therapeutic bronchoscopy (sputum removal for atelectasis), antibiotics and airway suctioning, and other therapies based on the discretion of the attending physician. The medical and nursing care based on the effective strategies of VAP bundle was provided to both groups during the study period. This strategies included strict infection control, alcohol-based hand disinfection, cuff pressure control, subglottic secretion drainage, use of microbiologic surveillance with timely availability of data about local multidrug-resistant pathogens, monitoring, and early removal of invasive devices and programs that reduce or change antibiotic prescribing practices.13 In addition, the patients underwent standard physical therapy, including range-of-motion exercises, head elevation exercises and positioning, and other physical therapies.
A physical therapist facilitated CPT, and this treatment comprised gravity-assisted drainage or positioning of patients by letting them lie on their side or assume a prone position horizontally on the bed for at least 20 minutes (with the most affected lung on chest radiography in the uppermost position), expiratory chest wall thrust, and airway suctioning via the endotracheal or tracheostomy tube interspersed via the treatment.
Outcome Measures
Primary outcome was incidence of VAP, defined as pneumonia in a patient who was on MV for at least 48 hours. The incidence of VAP was determined using the clinical pulmonary infection score (CPIS); a CPIS >6 indicates the presence of pneumonia.14 Clinical pulmonary infection score is based on body temperature, blood leukocyte count, tracheal secretion, oxygenation (PaO2/FiO2), pulmonary radiography, and positive tracheal aspirate culture. To determine VAP incidence, CPIS was calculated from the date of bacterial culture testing of specimens collected by endotracheal aspiration or bronchoalveolar lavage.
Secondary outcome measures, including the incidence of VAP, was determined using the guidelines of the American Thoracic Society and the Infectious Disease Society of America (ATS/IDSA),15 infection-related ventilator-associated complication (IVAC),16 MV duration, length of ICU stay, mortality, number of VAPs (CPIS)/MV duration, bronchoscopy, number of bronchoscopies/MV duration, number of days of antibiotic use in patients with suspicious VAP, number of days with antibiotic use/duration of MV, number of bronchial obstructions (defined as an oxyhemoglobin saturation <90%), and number of bronchial obstructions/MV duration.
Covariates Affecting VAP
In terms of the covariates affecting VAP, we selected secretion score and severity of illness; the latter is equivalent to the acute physiology and chronic health evaluation score II (APACHE II) score in previous studies.17–19 The aspiration of secretions containing bacterial pathogens in the lower respiratory tract is the main cause of VAP.17 Several studies have considered the severity of illness as an important risk factor for VAP.18 The risk increases by 1–3% daily in a patient requiring MV.19 Therefore, the increased duration of MV was considered a potential risk factor for VAP.
Statistical Analysis
Descriptive statistics were presented as absolute numbers, percentages, medians, and ranges for both groups. Ventilator-associated pneumonia and other categorical variables in the groups were compared using the Fisher's exact test, and ordinal variables were compared using the Mann–Whitney U test.
A multivariable logistic regression model was used to analyze the groups for each variable, which included secretion score, APACHE II score as confounding factors for VAP, and MV duration as a mediator for VAP. Fit of the logistic model was assessed using the Hosmer–Lemeshow test. The statistical analysis was performed using the JMP software (version 13.0; SAS Institute Japan, Tokyo, Japan). p values < 0.05 were considered significant.
Results
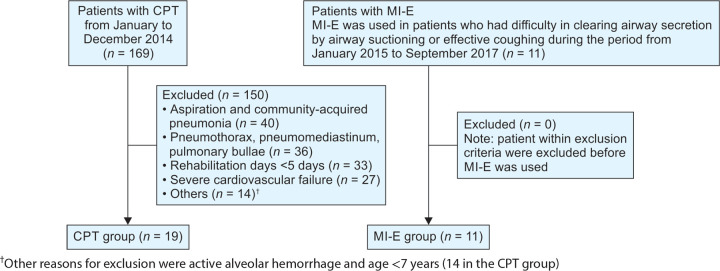
A total of 30 patients, including 11 and 19 patients in the MI-E and CPT groups, respectively, fulfilled all the entry criteria (Flowchart 1). The two groups were well matched for age, sex, body mass index, and PaO2/FiO2 (Table 1).
Flowchart 1.
Flow diagram of the process of the study. CPT, chest physical therapy; MI-E, mechanical insufflation-exsufflation
Table 1.
Characteristics of the study subjects according to two groups
| CPT (n = 19) | MI-E (n = 11) | p | |
|---|---|---|---|
| Age, median (range), years | 59 (18–87) | 52 (10–83) | 0.636 |
| Male/female, n | 14/5 | 7/4 | 0.687 |
| Height, median (range), cm | 159 (149.1–180) | 165 (132.5–175) | 0.343 |
| Weight, mean (SD), kg | 58.2(15.4) | 56.9 (16.3) | 0.843 |
| BMI, mean (SD) | 22.3 (5.6) | 21.2 (4.5) | 0.537 |
| APACHE II score, mean (SD) | 29.9 (9) | 23.3 (8.4) | 0.148 |
| GCS, median (range) | 3 (3–10) | 5 (3–15) | 0.151 |
| Admission PaO2/FiO2, median (range) | 185 (47–503) | 198 (80–517) | 0.533 |
| Secretion score†, median (range) | 12 (1–63) | 21 (9–34) | 0.080 |
| Type of admission | |||
| Medical, n | 13 | 6 | |
| Postoperative, n | 4 | 1 | |
| Trauma, n | 2 | 4 | |
CPT, chest physical therapy; MI-E, mechanical insufflation-exsufflation; SD, standard deviation; BMI, body mass index; APACHE II, acute physiology and chronic health evaluation score; GCS, Glasgow Coma Scale
Secretion score was defined as that obtained using the number of tracheal suctions in 24 hours and the amounts of secretion, which were classified as small (1 point), moderate (2 points), and large (3 points). Secretion score was calculated on the date of the starting day of MI-E in the MI-E group. The average of the starting date for MI-E was 5.6 days after starting rehabilitation. Therefore, the secretion score for CPT group was calculated on day 5 after starting rehabilitation
The incidence of VAP (CPIS) was significantly different between the CPT and MI-E groups (84.2% [16/19] vs 26.4% [3/11], p = 0.011). The duration of antibiotic use (days)/MV was significantly lower in the MI-E group than in the CPT group (Table 2).
Table 2.
Comparison of outcomes in both groups
| Outcomes | CPT (n = 19) | MI-E (n = 11) | p |
|---|---|---|---|
| Primary outcome | |||
| VAP (CPIS), n (%) | 16 (84.2) | 3 (26.4) | 0.011 |
| Secondary outcomes | |||
| VAPs (CPIS)/MV duration, median (range), % | 6.7 (0–14.3) | 0 (0–0.4) | <0.001 |
| VAP (ATS/IDSA), n (%) | 9 (47.4) | 2 (18.2) | 0.140 |
| IVAC, n (%) | 6 (31.6) | 1 (9.1) | 0.215 |
| VAP (CPIS) ≤ 4 days, n (%) | 10 (52.6) | 0 (0) | 0.091 |
| VAP (CPIS) > 4 days, n (%) | 9 (47.4) | 3 (26.4) | 0.263 |
| Tracheostomy, n (%) | 12 (63.2) | 7 (63.6) | >0.999 |
| Bronchoscopy, n (%) | 17 (89.5) | 8 (72.7) | 0.327 |
| Bronchoscopy, median (range) | 2 (0–26) | 2 (0–10) | 0.913 |
| Bronchoscopy/MV duration, median (range), % | 11 (0–38) | 14 (0–53) | 0.880 |
| Bronchial obstruction, median (range) | 1 (0–12) | 0 (0-2) | 0.052 |
| Bronchial obstruction/MV duration, median (range), % | 6.5 (0–70) | 0.6 (0–12.5) | 0.052 |
| Antibiotic use, median (range), days | 11 (5–86) | 8 (4–37) | 0.142 |
| Days antibiotic uses/MV duration, mean (SD), % | 76.5 (22.7–100) | 40 (5–100) | 0.035 |
| Rehabilitation days, median (range), days | 8 (5–63) | 10 (5–134) | 0.217 |
| MV duration, median (range), days | 15 (8–108) | 15 (7–180) | 0.829 |
| ICU duration, median (range), days | 17 (6–226) | 12 (2–53) | 0.131 |
| Mortality, n (%) | 5 (26.3) | 0 (0) | 0.129 |
CPT, chest physical therapy; MI-E, mechanical insufflation-exsufflation; VAP, ventilator-associated pneumonia; CPIS, clinical pulmonary infection score; ATS/IDSA, American Thoracic Society and the Infectious Disease Society of America; IVAC, infection-related ventilator-associated complication; MV, mechanical ventilation; SD, standard deviation
The univariate analysis showed that there was no statistically significant difference between the secretion score and APACHE II; however, the airway secretion tended to be more in the MI-E group and the severity tended to be higher in the CPT group. Based on the univariate analysis results, the covariates affecting VAP were secretion score, APACHE II, and MV duration, and logistic regression analysis was performed. The multivariate logistic regression analysis showed that none of the covariates were associated with the incidence of VAP (Table 3).
Table 3.
Association between each variable for VAP (CPIS) and group in multivariable logistic regression model
| Variables | Odds ratios (95% CI) | p |
|---|---|---|
| Model 1 | ||
| APACHE II | 1.04 (0.9–1.1) | 0.987 |
| CPT/MI-E | 0.08 (0.01–0.57) | 0.012 |
| Model 2 | ||
| Secretion score | 1.04 (0.93–1.15) | 0.457 |
| CPT/MI-E | 0.08 (0.01–0.59) | 0.006 |
| Mediators | ||
| MV duration | 1.01 (0.99–1.03) | 0.501 |
| CPT/MI-E | 0.06 (0.01–0.41) | 0.001 |
VAP, ventilator-associated pneumonia; CPIS, clinical pulmonary infection score; CPT, chest physical therapy; APACHE II, acute physiology and chronic health evaluation score; MI-E, mechanical insufflation-exsufflation; MV, mechanical ventilation
Discussion
This retrospective cohort study assessed the efficacy of MI-E in preventing VAP in critically ill patients in the ICU, and the results showed that MI-E, which enhances airway clearance, was associated with a reduced incidence of VAP (CPIS). The CPT group had a high incidence of VAP compared with the common ICU in previous study.20 In the current study, the MI-E group included patients with weak coughing reflex. Thus, the historical control group comprised patients with a similar characteristic. Therefore, the incidence of VAP might be higher in the two groups than in those admitted in a common ICU.
Based on these results, a multivariate logistic regression analysis was performed after adjusting for covariates affecting VAP. The models were utilized to analyze the two groups using each variable of VAP by considering the small sample size and the effect of multicollinearity. Our analysis revealed that none of these covariates were associated with the incidence of VAP (CPIS). In terms of the risk factor of VAP, the microaspiration of subglottic secretion is a cause of VAP.13 Thus, the results of the current study might support our hypothesis that the use of MI-E for airway clearance prevents pneumonia and the propagation of bacteria in the bronchi due to aspiration. Furthermore, the duration of antibiotic use (days)/MV was found to be significantly lower in the MI-E group than in the CPT group. Thus, MI-E might also reduce the cost of therapy in these patients.
This study was not blinded and may have been affected by the Hawthorne effect; however, three measures were taken to control it: the study was observational in nature, comparing conditions before and after the use of MI-E; the primary outcome, VAP (CPIS), was an objective outcome; and subjects should be less affected by the Hawthorne effect because they were ventilated with low levels of consciousness or sedation.
The current study had several limitations. First, a relatively small sample size was included, particularly in the MI-E group. Mechanical insufflation-exsufflation has been primarily used in patients with impaired coughing, such as those with neuromuscular diseases.21 Meanwhile, this study used MI-E in patients with weak cough reflex, including those with neurological disorders. Therefore, the proportion of patients included in the MI-E group was small. From the CPT group, a historical group was established to reduce selection bias caused by the use of MI-E, and the CPT and MI-E groups had similar characteristics based on the exclusion criteria. Although the CPT group included a historical control group, the medical system in the ICU of our hospital did not change significantly from 2014 to 2017. In addition, the two groups had common characteristics, and only few differences were observed. Furthermore, the patients in our study were selected from groups who were at higher risk of VAP, not from a common ICU. Nevertheless, the incidence of VAP (CPIS) was significantly lower in the MI-E group than in the CPT group, and this result supports the notion that MI-E can be used as a potential measure to prevent VAP in critically ill patients. The results were assessed using the logistic regression model after adjusting for covariates. However, these findings may be affected by the small sample size. Therefore, a large-scale prospective research must be conducted in the future to validate the results of the current study.
The second limitation of this study was the use of CPIS because it is unclear whether this is an accurate approach for the diagnosis of VAP. Currently, VAP is diagnosed using several approaches. The definition of the Centers for Disease Control and Prevention was designed for surveillance and quality improvement at the population level and not for the diagnosis and treatment decisions at bedside.15 The guidelines of the ATS/IDSA might be useful clinical criteria. However, there are no radiological criteria for VAP, and the chest radiography results of patients who are on MV are extremely difficult to interpret. Therefore, the ATS/IDSA panel recognizes the lack of a gold standard approach for the diagnosis of VAP.15 While the approach for accurate diagnosis of VAP is unclear, some reports suggest that CPIS may be a useful screening tool for assessing the risk of pneumonia.22 This study used three diagnostic criteria for VAP, including CPIS, and found a statistically significant difference in CPIS only. The MI-E group tended to have a lower incidence of VAP by other diagnostic criteria, and MI-E may prevent VAP. However, the current study was a pilot study with a small sample size and could not be justified by these results alone. Therefore, prospective studies with more accurate diagnostic criteria with larger sample sizes are needed in the future.
Conclusion
In this retrospective cohort study, the use of MI-E was independently associated with a reduced incidence of VAP (CPIS). However, the current study only provides data for clinical practice and the small sample size may result in several potential biases. Future randomized controlled trials with a sufficient sample size are required to confirm the efficacy of MI-E in critically ill patients.
Acknowledgments
Thank you to all of our colleagues. We also would like to thank Philips Japan, Ltd., for their helpful support. The authors would like to thank Enago (www.enago.jp) for the English language review.
Footnotes
Source of support: Nil
Conflict of interest: The device of MI-E was provided by Philips Japan, Ltd. The provider had no role in this study.
References
- 1.Zand F, Zahed L, Mansouri P, Dehghanrad F, Bahrani M, Ghorbani M. The effects of oral rinse with 0.2% and 2% chlorhexidine on oropharyngeal colonization and ventilator associated pneumonia in adults’ intensive care units. J Crit Care. 2017;40:318–322. doi: 10.1016/j.jcrc.2017.02.029. DOI: [DOI] [PubMed] [Google Scholar]
- 2.Rello J, Ollendorf DA, Oster G, Vera-Llonch M, Bellm L, Redman R, et al. Epidemiology and outcomes of ventilator-associated pneumonia in a large US database. Chest. 2002;122(6):2115–2121. doi: 10.1378/chest.122.6.2115. DOI: [DOI] [PubMed] [Google Scholar]
- 3.Nakagawa NK, Franchini ML, Driusso P, de Oliveira LR, Saldiva PH, Lorenzi-Filho G. Mucociliary clearance is impaired in acutely ill patients. Chest. 2005;128(4):2772–2777. doi: 10.1378/chest.128.4.2772. DOI: [DOI] [PubMed] [Google Scholar]
- 4.Wang MY, Pan L, Hu XJ. Chest physiotherapy for the prevention of ventilator-associated pneumonia: a meta-analysis. Am J Infect Control. 2019;47(7):755–760. doi: 10.1016/j.ajic.2018.12.015. DOI: [DOI] [PubMed] [Google Scholar]
- 5.Spapen HD, De Regt J, Honoré PM. Chest physiotherapy in mechanically ventilated patients without pneumonia-a narrative review. J Thorac Dis. 2017;9(1):E44–E49. doi: 10.21037/jtd.2017.01.32. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Homnick DN. Mechanical insufflation-exsufflation for airway mucus clearance. Respir Care. 2007;52(10):1296–1305. discussion 306-7. [PubMed] [Google Scholar]
- 7.Vianello A, Corrado A, Arcaro G, Gallan F, Ori C, Minuzzo M, et al. Mechanical insufflation-exsufflation improves outcomes for neuromuscular disease patients with respiratory tract infections. Am J Phys Med Rehabil. 2005;84(2):83–88. doi: 10.1097/01.phm.0000151941.97266.96. DOI: discussion 9-91. [DOI] [PubMed] [Google Scholar]
- 8.Bott J, Blumenthal S, Buxton M, Ellum S, Falconer C, Garrod R, et al. Guidelines for the physiotherapy management of the adult, medical, spontaneously breathing patient. Thorax. 2009;64(Suppl 1):i1–i51. doi: 10.1136/thx.2008.110726. DOI: [DOI] [PubMed] [Google Scholar]
- 9.Wang CH, Bonnemann CG, Rutkowski A, Sejersen T, Bellini J, Battista V, et al. Consensus statement on standard of care for congenital muscular dystrophies. J Child Neurol. 2010;25(12):1559–1581. doi: 10.1177/0883073810381924. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonçalves MR, Honrado T, Winck JC, Paiva JA. Effects of mechanical insufflation-exsufflation in preventing respiratory failure after extubation: a randomized controlled trial. Crit Care. 2012;16(2):R48. doi: 10.1186/cc11249. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oberwaldner B. Physiotherapy for airway clearance in paediatrics. Eur Respir J. 2000;15(1):196–204. doi: 10.1183/09031936.00.15119600. DOI: [DOI] [PubMed] [Google Scholar]
- 12.Morrow B, Zampoli M, van Aswegen H, Argent A. Mechanical insufflation-exsufflation for people with neuromuscular disorders. Cochrane Database Syst Rev. 2013;12(12):CD010044. doi: 10.1002/14651858.CD010044.pub2. DOI: [DOI] [PubMed] [Google Scholar]
- 13.Gunasekera P. Ventilator-associated pneumonia. BJA Education. 2016;16(6):198–202. doi: 10.1093/bjaed/mkv046. DOI: [DOI] [Google Scholar]
- 14.Liu C, Zhang YT, Peng ZY, Zhou Q, Hu B, Zhou H, et al. Aerosolized amikacin as adjunctive therapy of ventilator-associated pneumonia caused by multidrug-resistant Gram-negative bacteria: a single-center randomized controlled trial. Chin Med J (Engl) 2017;130(10):1196–1201. doi: 10.4103/0366-6999.205846. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):e61–e111. doi: 10.1093/cid/ciw353. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention National Healthcare Safety Network. https://www.cdc.gov/nhsn/pdfs/pscmanual/10-vae_final.pdf. [Accessed June 12, 2019]. https://www.cdc.gov/nhsn/pdfs/pscmanual/10-vae_final.pdf ( )
- 17.Muscedere J, Rewa O, McKechnie K, Jiang X, Laporta D, Heyland DK. Subglottic secretion drainage for the prevention of ventilator-associated pneumonia: a systematic review and meta-analysis. Crit Care Med. 2011;39(8):1985–1991. doi: 10.1097/CCM.0b013e318218a4d9. DOI: [DOI] [PubMed] [Google Scholar]
- 18.Wolkewitz M, Vonberg R, Grundmann H, Beyersmann J, Gastmeier P, Bärwolff S, et al. Risk factors for the development of nosocomial pneumonia and mortality on intensive care units: application of competing risks models. Crit Care. 2008;12(2):R44. doi: 10.1186/cc6852. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cook DJ, Walter SD, Cook RJ, Griffith LE, Guyatt GH, Leasa D, et al. Incidence of and risk factors for ventilator-associated pneumonia in critically ill patients. Ann Intern Med. 1998;129(6):433–440. doi: 10.7326/0003-4819-129-6-199809150-00002. DOI: [DOI] [PubMed] [Google Scholar]
- 20.Kalanuria AA, Ziai W, Zai W, Mirski M. Ventilator-associated pneumonia in the ICU. Crit Care. 2014;18(2):208. doi: 10.1186/cc13775. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Auger C, Hernando V, Galmiche H. Use of mechanical insufflation-exsufflation devices for airway clearance in subjects with neuromuscular disease. Respir Care. 2017;62(2):236–245. doi: 10.4187/respcare.04877. DOI: [DOI] [PubMed] [Google Scholar]
- 22.Wongsurakiat P, Tulatamakit S. Clinical pulmonary infection score and a spot serum procalcitonin level to guide discontinuation of antibiotics in ventilator-associated pneumonia: a study in a single institution with high prevalence of nonfermentative gram-negative bacilli infection. Ther Adv Respir Dis. 2018;12:1753466618760134. doi: 10.1177/1753466618760134. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]