Abstract
Cardioembolic stroke in a patient with peripartum cardiomyopathy (PPCM) patient is rare despite a higher incidence of thromboembolic events. We report a case of acute right middle cerebral artery territory cardioembolic stroke in a postpartum female as the initial presenting feature of PPCM. The patient was thrombolyzed with intravenous alteplase and had an almost complete neurological recovery.
How to cite this article: Nasa P, Mortada M, Ali A, Malhotra V, Koul K, Singh A. Cardioembolic Stroke with Peripartum Cardiomyopathy: An Unusual Presentation. Indian J Crit Care Med 2021;25(1):97–99.
Keywords: Cardioembolic stroke, Peripartum cardiomyopathy, Stroke with peripartum cardiomyopathy
Introduction
We present a case of acute right middle cerebral artery territory stroke who was thrombolyzed within the window period with intravenous alteplase and made a complete neurological recovery. The patient on evaluation was found to have peripartum cardiomyopathy (PPCM) and cardioembolic stroke.
Case Description
A 39-year-old woman with no other comorbidities presented with left-sided numbness and weakness, facial asymmetry, and difficulty in speech for one hour. She delivered two months back her fourth live child. On neurological examination, she had dysarthria, left-sided hypoesthesia, upper motor neuron seventh nerve palsy, and left hemiparesis; National Institutes of Health Stroke Scale (NIHSS) score 8. On examination, she was dyspneic, tachypnea (respiratory rate 24/minute), pulse 104/minute, SpO2 89%, respiratory examination showed bilateral crepitations. Her bedside transthoracic echocardiography (TTE) showed global hypokinesia of left ventricle (LV) with ejection fraction (EF) 30%, mild-to-moderate mitral regurgitation. She was started on noninvasive ventilation and oxygen for acute heart failure. Her other laboratory investigations are in Table 1. The computed tomography (CT) brain showed no abnormality and she was thrombolyzed with intravenous alteplase. Her transesophageal echocardiography showed no intracardiac thrombus (Fig. 1). She was started on other anti-heart failure medications, candesartan, bisoprolol, and aldactone in the next 72 hours. The enoxaparin at 1 mg/kg body weight 12 hourly was started after 24 hours of thrombolysis and repeat CT scan showed no hemorrhage. The magnetic resonance imaging (MRI) brain after 72 hours of thrombolysis showed multiple focal areas of acute ischemic infarct in the right centrum semiovale and frontal parieto-temporal region with normal carotid and cerebral vessels Doppler (Figs 2 and 3). Her thrombophilia profile was normal. She was started on warfarin after 5 days and enoxaparin was stopped after International Normalized Ratio (INR) increased above 2. She was discharged on the 8th day with warfarin, candesartan, bisoprolol, and aldactone and necessary education on INR monitoring. She was followed for 5 months and warfarin was stopped after 4 months and serial TTE showed resolution of cardiomyopathy.
Table 1.
Significant laboratory investigations
| Hemoglobin (g/dL) | 12.0 |
| Total leukocyte count (×109/L) | 6.82 |
| Platelets (×109/L) | 263 |
| Hemoglobin A1c (HbA1c) | 5.5% |
| Serum blood urea (mg/dL) | 44 |
| Serum creatinine (umol/L) | 81.2 |
| Serum cholesterol (mmol/L) | 5.3 |
| HDL (mmol/L) | 1.45 |
| LDL (mmol/L) | 2.30 |
| Thyroid-stimulating hormone, serum (μIU/mL) | 1.988 |
| B-type natriuretic peptide (pg/mL) | 473.84 |
| Sodium, serum (mmol/L) | 139 |
| Potassium, serum (mmol/L) | 4.5 |
HDL, high-density lipoproteins; LDL, low-density lipoprotein
Fig. 1.

Transesophageal echocardiography showed global hypokinesia of left ventricle and no intracardiac thrombus
Fig. 2.
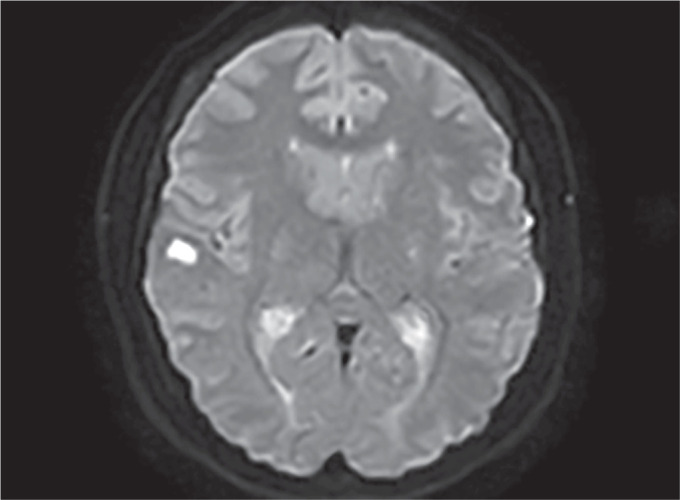
MRI diffusion sequence showing one of the infarct areas in right frontal-parieto-temporal region
Fig. 3.
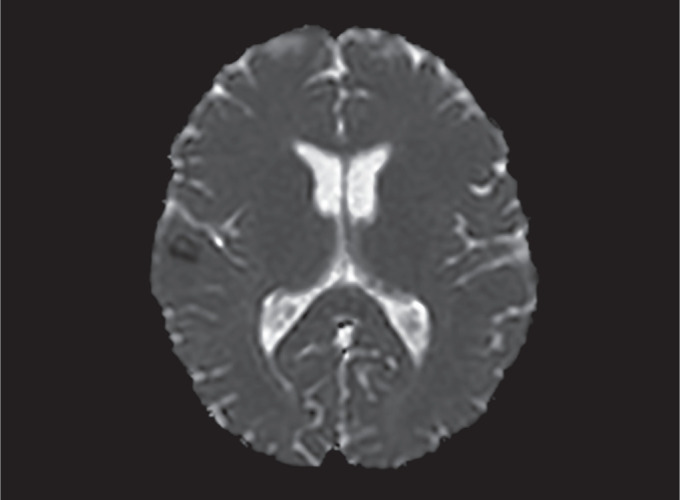
MRI showing apparent-diffusion coefficient (ADC) sequence taken from the same area at the same time as in Figure 2
Discussion
Acute ischemic stroke during a peripartum period is uncommon and is usually associated with either hypertensive disorder of pregnancy or preexisting chronic hypertension.1 Cardioembolic stroke in PPCM is reported very rarely.2–4 The PPCM is diagnosed as LV systolic dysfunction (LVEF < 45%), in the last month of pregnancy to 5 months following delivery, and when no other cause is identified for cardiomyopathy.5 The etiopathogenesis of PPCM is unclear.6 There is a higher incidence of systemic and pulmonary thromboembolic events in patients of PPCM.5 The hypercoagulable state of the peripartum period itself, enlarged cardia, endothelial injury, and additionally, immobility may be contributing to the increased propensity of thromboembolism.7
The majority of the PPCM is postpartum within the first week of delivery and symptoms like orthopnea, paroxysmal nocturnal dyspnea. Our case was unique where the patient presented almost 2 months after delivery with initial symptoms of ischemic stroke along with heart failure. There are no recommendations available for the management of stroke in such patients. There is also controversy regarding the use of anticoagulation to prevent stroke recurrence in patients with PPCM because of the risk of intracranial hemorrhage (ICH).1 Recent guidelines recommended anticoagulation therapy can be used within 4–14 days after stroke in the setting of atrial fibrillation with cardioembolic stroke.7 The guidance in the case of cardiomyopathy is still unclear. In our case, the patient has a low risk of hemorrhage with smaller infarcts and a high risk of recurrence, so we considered starting anticoagulation early with close monitoring for any ICH. The consensus management of PPCM recommends anticoagulation during pregnancy and up to two months postpartum along with heart failure medications till recovery of left ventricle function.8
Our patient presented within one hour onset of left side weakness and in absence of any absolute contraindications; was thrombolyzed using intravenous alteplase. The diagnosis of PPCM was made after establishing no previous cause of cardiomyopathy as uncomplicated vaginal delivery 2 months back. The patient MRI findings of multiple focal infarcts, normal carotid vessels Doppler, and absence of no other atherosclerotic or thrombophilia risk factors favored cardioembolic stroke. In our patient, we did not find any intracardiac thrombus on TEE which may be absent in many such cases after a thromboembolic event. The patient was managed with anticoagulation and heart failure medications, till recovery of heart functions.
In summary, acute ischemic cardioembolic stroke is a rare presentation of PPCM and requires standard management of heart failure with anticoagulation. This case highlights the importance of consideration of anticoagulation to prevent thromboembolism in patients with PPCM.
Footnotes
Source of support: Nil
Conflict of interest: None
References
- 1.Del Zotto E, Giossi A, Volonghi I, Costa P, Padovani A, Pezzini A. Ischemic stroke during pregnancy and puerperium. Stroke Res Treat. 2011;2011:606780. doi: 10.4061/2011/606780. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hodgman MT, Pessin MS, Homans DC. Cerebral embolism as the initial manifestation of peripartum cardiomyopathy. Neurology. 1982;32(6):668–671. doi: 10.1212/WNL.32.6.668. DOI: [DOI] [PubMed] [Google Scholar]
- 3.Málaga G, Quintana J, Posadas D, Quispe J, Rotta A. Early peripartum cardiomyopathy complicated with cardioembolic stroke: a case report. Rev Med Hered. 2010;21(2):97–102. Spanish. [Google Scholar]
- 4.Dyken ME, Biller J. Peripartum cardiomyopathy and stroke. Cerebrovasc Dis. 1994;4(4):325–328. doi: 10.1159/000108502. DOI: [DOI] [Google Scholar]
- 5.Kolte D, Khera S, Aronow WS, Palaniswamy C, Mujib M, Ahn C, et al. Temporal trends in incidence and outcomes of peripartum cardiomyopathy in the United States: a nationwide population-based study. J Am Heart Assoc. 2014;3(3):e001056. doi: 10.1161/JAHA.114.001056. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sliwa K, Hilfiker-Kleiner D, Petrie MC, Mebazaa A, Pieske B, Buchmann E, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of peripartum cardiomyopathy: a position statement from the Heart Failure Association of the European Society of Cardiology working group on peripartum cardiomyopathy. Eur J Heart Fail. 2010;12(8):767–778. doi: 10.1093/eurjhf/hfq120. DOI: [DOI] [PubMed] [Google Scholar]
- 7.Warner JJ, Harrington RA, Sacco RL, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke. Stroke. 2019;50(12):3331–3332. doi: 10.1161/STROKEAHA.119.027708. DOI: [DOI] [PubMed] [Google Scholar]
- 8.Arany Z, Elkayam U. Peripartum cardiomyopathy. Circulation. 2016;133(14):1397–1409. doi: 10.1161/CIRCULATIONAHA.115.020491. DOI: [DOI] [PubMed] [Google Scholar]


