Abstract
How to cite this article: Prakash J, Bhattacharya PK, Priye S, Kumar N. Post-COVID-19 Pulmonary Fibrosis: A Lifesaving Challenge. Indian J Crit Care Med 2021;25(1):104–105.
Keywords: Consequences, Coronavirus disease 2019, Pulmonary fibrosis
Editor Sir,
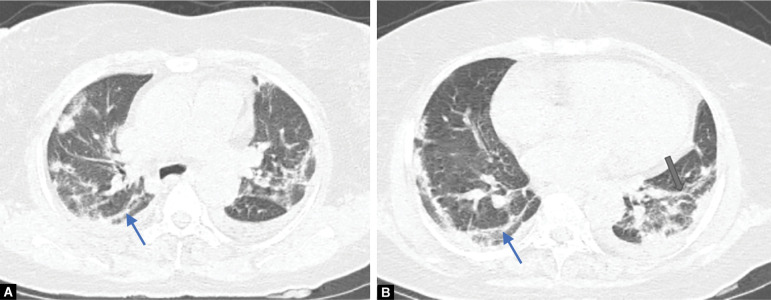
Coronavirus disease 2019 (COVID-19) is an ongoing pandemic worldwide which was first recognized in Wuhan, China, in December 2019 and infected around 35 million people and 1,039,406 people have died to date.1 Computed tomography (CT) scans revealed bilateral ground-glass opacities with or without consolidation and lower lobe preference in all patients in the first large series of hospitalized patients (n = 138) with COVID-19.2 We noticed that most patients with acute respiratory distress syndrome (ARDS) or severe pneumonia were found to have pulmonary fibrosis during follow-up after the cure of COVID-19. The role of antifibrotic medication is still under observation.3 A PubMed search was performed using the phrases “pulmonary fibrosis,” “COVID-19,” we got two literatures of functional data or pulmonary fibrosis imaging from post-COVID-19 patients.3,4 The components through which ARDS causes lung damage are not fully known yet, however, conceivable contributors include a cytokine discharge disorder set off by the viral antigen-induced, drug-induced pulmonary toxicity, high airway pressure, and hyperoxia-prompted intense lung damage following mechanical ventilation. Until now, about 2.6 million individuals worldwide have survived from COVID-19, however, there remains concerned that a few organs, including the lungs, may have a long-term impairment following infection (Fig. 1). Functional data or imagings are still not available for patients with COVID-19.
Figs 1A and B.
HRCT showing patchy opacities with small areas of ground-glass opacities and fibrotic bands seen predominantly in the periphery of both lungs with apicobasal gradient
The characteristics of pulmonary fibrosis include failed alveolar re-epithelialization, fibroblast activation, excessive collagen deposition, and other extracellular matrix (ECM) components that disrupt the normal lung architecture.5 Progression of pulmonary fibrosis contributes to interstitial matrix expansion, compression, and normal lung parenchyma degradation, further weakening the capillaries and may lead to ventilatory failure.6 Recent studies have indicated that the primary culprit for most lung fibrotic processes is alveolar-epithelial injury and generation of active myofibroblast foci. A collection of growth factors and cytokines such as monocyte chemoattractant protein-1 (MCP-1), interleukin-1β (IL-1β) and interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), and transforming growth factor-β1 (TGF-β1) are overexpressed and released by the cells upon identification of damage to lung tissue. Type II alveolar epithelial cells are the most important sources of these fibrogenic factors and promote hyperproliferation of type II alveolar epithelial cells that recruit fibroblasts into the fibrotic loci and induce the transdifferentiation/activation of myofibroblast fibroblasts. Myofibroblasts play a role in excessive ECM accumulation and may result from increased or decreased ECM synthesis or degradation, or both.5 Collagen expression and deposition contribute to the accumulation of ECM. Different forms of collagen, such as, form I, type III, and type VI, have been identified in fibrotic lung injuries. The plasminogen activator/plasmin system plays an important role in ECM degradation, among several factors.
No data to date reports the incidence or prognosis of post-COVID pulmonary fibrosis. It is important to make preparations now to quickly determine whether the survivor population is developing pulmonary fibrosis. Our findings or personal views to understand the long-term pulmonary effects of COVID-19 involve well-designed studies post-COVID-19.
Footnotes
Source of support: Nil
Conflict of interest: None
References
- 1.WHO Coronavirus Disease (COVID-19) Dashboard. https://covid19.who.int https://covid19.who.int
- 2.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spagnolo P, Balestro E, Aliberti S, Cocconcelli E, Biondini D, Casa GD. Pulmonary fibrosis secondary to COVID-19: a call to arms? Lancet Respir Med. 2020;8(8):750–752. doi: 10.1016/S2213-2600(20)30222-8. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.George PM, Wells AU, Jenkins RG. Pulmonary fibrosis and COVID-19: the potential role for antifibrotic therapy. Lancet Respir Med. 2020;8(8):807–815. doi: 10.1016/S2213-2600(20)30225-3. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sime PJ, O'Reilly KM. Fibrosis of the lung and other tissues: new concepts in pathogenesis and treatment. Clin Immunol. 2001;99(3):308–319. doi: 10.1006/clim.2001.5008. DOI: [DOI] [PubMed] [Google Scholar]
- 6.Razzaque MS, Taguchi T. Pulmonary fibrosis: cellular and molecular events. Pathol Int. 2003;53(3):133–145. doi: 10.1046/j.1440-1827.2003.01446.x. DOI: [DOI] [PubMed] [Google Scholar]