To the Editor:
Severe coronavirus disease (COVID-19) is characterized by acute hypoxemic respiratory failure, usually with extensive consolidations and areas with ground glass on chest computed tomographic (CT) scans (1). Whether long-term respiratory sequelae persist in survivors of severe COVID-19 remains to be established. This report describes our findings of respiratory outcomes in mechanically ventilated survivors of COVID-19 at 3 months after hospital discharge.
Methods
We recorded clinical and follow-up data of all patients with COVID-19 treated at our ICU in the Maastricht Intensive Care COVID cohort (registered in the Netherlands Trial Register [NL8613]) (2). The institutional review board of Maastricht University Medical Center+ approved the study, and informed consent was obtained (METC2020–2287). During admission, ventilator strategies included lung-protective ventilation (Vt ≤ 6 ml/kg) and positive end-expiratory pressure titration using electrical impedance tomography. Prone positioning was considered when the PaO2/FiO2 ratio was less than 112.5 mm Hg (15 kPa) and maintained for at least 12 hours.
At 3 months after hospital discharge, survivors were screened at a multidisciplinary post-ICU outpatient clinic for respiratory outcomes with pulmonary function testing (PFT), including spirometry, lung volumes, and diffusing capacity for carbon monoxide adjusted for Hb, chest high-resolution CT (HRCT) imaging, and 6-minute-walk test (6-MWT). Two experienced radiologists systematically scored chest HRCT scans for the presence of pulmonary abnormalities, including ground-glass opacifications, reticulation, consolidations, bronchiectasis, atelectasis, presence of new emphysema, cystic changes, air trapping, extent of lobe involvement, and total lung involvement. The extent of lobe involvement was visually scored on a 0–5 scale, as follows: 0 = no involvement, 1 = 1–5%, 2 = 6–25%, 3 = 26–50%, 4 = 51–75%, and 5 = >75% involvement (3). The CT Severity Score (CTSS) was calculated by adding the lobar scores. HRCT scans were compared with scans performed at presentation (n = 33) at the emergency department or during admission (n = 5), depending on availability. All data are presented as median (interquartile range [IQR]). Correlations between CTSS, PFT results, and 6-MWT were assessed using Spearman’s rank correlation.
Results
During the first European pandemic wave between March and May 2020, the Maastricht Intensive Care COVID cohort included 94 patients. Fifty-two (55%) patients were alive 3 months after hospital discharge, and 48 of them (92%) participated in the follow-up clinic. The four missing patients attended follow-up elsewhere. Follow-up (IQR) occurred at a median of 120 (103–135) days after intubation and 90 (80–99) days after hospital discharge. Baseline characteristics are detailed in Table 1.
Table 1.
Baseline Characteristics, PFT, and HRCT Imaging Results at 3-Month Follow-Up
| Baseline Characteristics on Admission (N = 48) | |||
|---|---|---|---|
| Age, yr | 63.00 (55.00–68.00) | ||
| Sex, M | 33 (68.8) | ||
| BMI, kg/m2 | 27.68 (25.18–30.47) | ||
| Origin of admission | |
||
| Emergency department | 11 (22.9) | ||
| Hospital ward | 27 (56.2) | ||
| Transfer from another ICU | 10 (20.8) | ||
| Pre-ICU length of stay, d | 3.00 (1.00–5.00) | ||
| APACHE II score | 15.0 (13.0–17.3) | ||
| Preexisting lung disease | 3 (6.2) | ||
| Asthma | 3 (6.2) | ||
| COPD | 0 (0.0) | ||
| Smoking status | |
||
| Current smoker | 0 (0) | ||
| Former smoker | 23 (48) | ||
| Charlson Comorbidity Index | |
||
| 0 | 6 (12.5) | ||
| 1–2 | 24 (50.0) | ||
| 3–4 | 15 (31.2) | ||
| 5 or more | 3 (6.2) | ||
| Leukocyte count, 10−9/L | 9.25 (7.88–10.75) | ||
| C-reactive protein, mg/L | 191 (99–264) | ||
| D-dimer, μg/L | 1,343 (726–5,097) | ||
| PaO2/FiO2 ratio, mm Hg | 116 (92–156) | ||
| Prone positioned during ICU admission | 22 (45.8) | ||
| Pinsp, cm H2O | 26 (24–28) | ||
| PEEP, cm H2O | 14 (12–14) | ||
| VT/kg bodyweight, ml/kg | 5.46 (4.98–6.02) | ||
| Dynamic compliance, ml/cm H2O | 37.25 (29.80–48.85) | ||
| Received steroids during admission* | 12 (25.0) | ||
| IMV duration, d | 18.5 (9.0–28.5) | ||
| ICU length of stay, d | 20.5 (10.8–33.3) | ||
| Hospital length of stay, d | 32.0 (21.0–40.0) | ||
| Hospital discharge location | |
||
| Home | 7 (14.9) | ||
| Nursing home | 1 (2.1) | ||
| Rehabilitation center | 39 (83.0) | ||
| Rehabilitation center length of stay, d† | 14.0 (7.0–27.3) | ||
| ECMO during admission | 3 (6.2) | ||
| PFT (N = 43) | Absolute Value | Percentage of Predicted | Below LLN |
|---|---|---|---|
| FEV1, L | 2.9 (2.6–3.5) | 95.0 (77.0–104.5) | 11 (25.6) |
| FEV1/VC, % | 79.9 (76.1–86.6) | — | 0 (0.0) |
| FVC, L | 3.6 (3.1–4.2) | 87.0 (70.0–106.0) | 16 (37.2) |
| RV, L | 2.0 (1.6–2.2) | 88.0 (70.0–103.0) | 9 (20.9) |
| TLC, L | 5.6 (4.6–6.7) | 84.0 (71.5–102.5) | 23 (53.5) |
| DlCOc, L‡ | 5.4 (4.6–6.3) | 61.0 (50.0–69.0) | 36 (87.8) |
| 6-MWT, m§ | 480.0 (386.0–536.0) | 81.5 (69.5–99.5) | — |
| MRC Dyspnea score | |
||
| Grade 0–1 (none/mild) | 27 (62.8) | ||
| Grade 2–3 (moderate) | 14 (32.5) | ||
| Grade 4–5 (severe) | 2 (4.7) | ||
| HRCT Imaging Results (N = 46) | |||
|---|---|---|---|
| Fibrosis | 42 (91.3) | ||
| Ground glass | 41 (89.1) | ||
| Atelectasis | 15 (32.6) | ||
| Dominant pattern | |
||
| Reticular | 31 (67.4) | ||
| Ground glass | 13 (28.3) | ||
| No abnormalities | 2 (4.3) | ||
| Decreased attenuation | 25 (54.3) | ||
| Due to small-airways disease | 21 (45.7) | ||
| Due to new emphysema | 12 (25.0) | ||
| CTSS | 11.0 (5.0–15.0) | ||
Definition of abbreviations: 6-MWT = 6-minute-walk test; APACHE = Acute Physiology And Chronic Health Evaluation; BMI = body mass index; COPD = chronic obstructive pulmonary disease; CTSS = Computed Tomographic Severity Score; DlCOc = diffusing capacity for carbon monoxide adjusted for Hb; ECMO = extracorporeal membrane oxygenation; HRCT = high-resolution chest computed tomographic; IMV = invasive mechanical ventilation; LLN = lower limit of normal; MRC Dyspnea = Medical Research Council Dyspnea questionnaire; PEEP = positive end-expiratory pressure; PFT = pulmonary function testing; Pinsp = inspiratory pressure in bilevel pressure-controlled ventilation; RV = residual volume.
Data are presented as median (interquartile range) or n (%) unless indicated otherwise. For laboratory results and ventilator settings, the worst value for the first 24 hours of admission was recorded.
Defined as receiving steroid treatment for at least 2 days or more.
Three patients were still admitted to a rehabilitation center at the moment of follow-up.
DlCOc failed in two patients.
Two patients were on supplemental oxygen while performing the 6-MWT.
We found diminished TLC and diffusion capacity in 23 and 36 participants, respectively, but no airway obstruction on PFT (Table 1), whereas five participants had no abnormalities. The median 6-MWT result was 482 m (82% of predicted distance). Two participants were on home supplemental oxygen, and four participants experienced a significant saturation drop during this test (>4% drop).
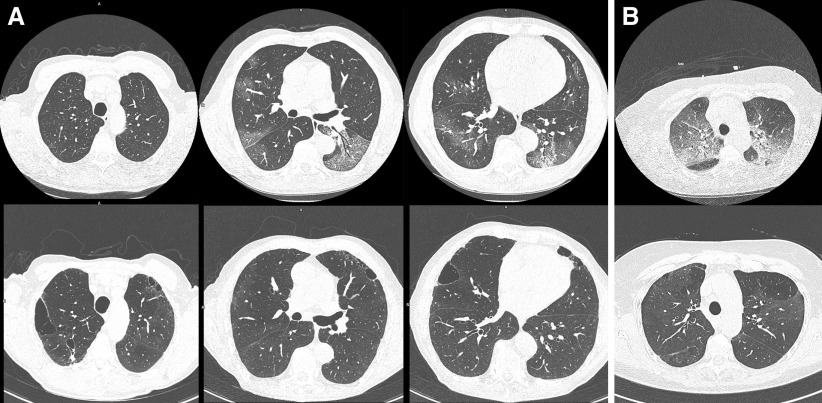
Only two participants had no signs of COVID-19–related abnormalities at follow-up HRCT scan. HRCT scans showed ground-glass opacities in 89% (n = 41) of cases. Signs of reticulation, including course fibrous bands either with or without obvious parenchymal distortion, bronchiectasis, and bronchiolectasis, were seen in 67% (n = 31) of cases and were assumed to represent fibrosis. One-quarter of the survivors showed new emphysematous destruction or cavitation that was not present at baseline scan or showed obvious deterioration of preexistent emphysema (Figure 1). Some air trapping was common, but it was not a dominant feature. Traction bronchiectasis was rare and not a dominant feature at follow-up.
Figure 1.
Representative high-resolution chest computed tomographic (HRCT) images of two of the survivors. (A) HRCT imaging performed at admission (upper row) and at 3-month follow-up (lower row). Chest computed tomographic (CT) imaging at admission shows typical bilateral subpleural ground-glass opacities. No signs of previous emphysema were detected. However, follow-up HRCT imaging shows obvious emphysematous destruction. (B) CT image at presentation at emergency department with evident ground areas with reticulation (crazy paving). Follow-up reveals diffuse areas of persistent ground glass without reticulation, as well as areas with low density in previously normal areas, possibly due to hypoperfusion.
Total severity scores for the 3-month follow-up scans ranged from 0 to 25, with a median score of 11. Participants with limited residual changes mainly showed subpleural parenchymal bands or small plate atelectasis. No predilection for a certain part of the lungs was noted. Residual lesions were predominantly located in the areas that showed crazy paving (ground glass with reticulation) at presentation, whereas areas with consolidations observed during admission appeared to be spared at 3 months. Diffusion capacity was significantly correlated with both TLC (ρ = 0.56; P < 0.001) and 6-MWT (ρ = 0.53, P < 0.001) but not with CTSS.
Discussion
We assessed respiratory sequelae of invasively mechanically ventilated patients with COVID-19 detailing both pulmonary function and HRCT scan results at 3 months after hospital discharge. Key findings were high prevalence of diminished diffusion capacity and TLC and fibrotic changes on HRCT images. These findings add to previous studies reporting a lower prevalence of pulmonary dysfunction in patients with COVID-19. However, these reports were based on less severely ill patients with COVID-19 who were not supported by invasive mechanical ventilation (4) or who were ventilated for a shorter duration (5). As such, our data represent the more severe spectrum of COVID-19 disease.
Based on our HRCT findings, fibrosis (evident from reticular pattern found in the majority of participants) and ground-glass opacifications were the dominant pathophysiological hallmarks observed. Notably, ground-glass opacifications were still present at 3 months after hospital discharge, mainly with a subpleural distribution similar to a nonspecific interstitial pneumonia pattern or a diffuse distribution with lower density than in areas of ground glass seen at baseline imaging. Whether these are signs of fibrosis or of ongoing inflammation is speculative, but this may become clear from follow-up imaging studies. Finally, we noted new emphysematous abnormalities both in areas showing a so-called vacuole sign at baseline imaging as well as in areas outside the areas with infiltration. The former might be explained by direct parenchymal destruction caused by infection; the latter finding could be a manifestation of ventilator-induced injury.
Long-term pulmonary effects have been documented in patients with acute respiratory distress syndrome (ARDS) and, to a lesser extent, in respiratory syndromes caused by coronaviruses such as severe acute respiratory syndrome (SARS) and Middle East Respiratory Syndrome (MERS) (6–8). Comparisons with our findings in COVID-19 should be interpreted with caution, as populations and follow-up strategies varied highly. In general, fibrotic changes on chest imaging were described in survivors of ARDS, SARS, and MERS (6, 7, 9). Fibrosis in our cohort was diffusely distributed in the lungs, which contrasted with the usually described localization in the anterior parts of the lungs in severe ARDS (9). To our knowledge, the new areas of emphysema we observed seem specific for COVID-19 compared with SARS or MERS (10).
In addition, the sparse data available suggest similar predominant reduction in diffusion capacity in survivors of COVID-19 compared with survivors of ARDS, SARS, and MERS (6–8). Whether this reduction in diffusion capacity is aggravated by the vascular and thrombotic complications witnessed in patients with COVID-19 remains to be investigated (11). Furthermore, as reported for ARDS, physical capacity was clearly diminished in our cohort, which correlated as expected with reduced lung function (8).
Strengths of our follow-up cohort include the inclusion of only mechanically ventilated patients and the high follow-up response rate. An important limitation is the lack of a direct comparison between survivors of COVID-19 and survivors of non–COVID-19 ARDS, which remains speculative at this point. Other limitations include the single-center character and the lack of baseline information on pulmonary function before the infection. Moreover, at the time of study initiation, the high incidence of pulmonary embolism in COVID-19 was not (yet) acknowledged and therefore not systematically screened for.
It is likely that the observed HRCT scan and PFT abnormalities will—at least partially—resolve over time, as was shown in long-term evaluations of survivors of SARS, MERS, and ARDS (6, 8, 12). Nevertheless, the long-term detrimental impact of these pulmonary sequalae on patient health and quality of life in survivors of ARDS is well established (13). Whether respiratory effects of COVID-19 hold similar implications has yet to be investigated. As such, our findings support long-term respiratory follow-up of mechanically ventilated patients with COVID-19.
Conclusions
The majority of invasively mechanically ventilated survivors of COVID-19 still had abnormal pulmonary function tests and residual changes on HRCT scans at 3 months after hospital discharge. Diminished diffusion capacity, diminished TLC, and fibrosis on HRCT were the dominant features. Our findings warrant intensive respiratory follow-up of mechanically ventilated patients with COVID-19.
Footnotes
Originally Published in Press as DOI: 10.1164/rccm.202010-3823LE on December 16, 2020
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Ferrando C, Suarez-Sipmann F, Mellado-Artigas R, Hernandez M, Gea A, Arruti E, et al. COVID-19 Spanish ICU Network Clinical features, ventilatory management, and outcome of ARDS caused by COVID-19 are similar to other causes of ARDS Intensive Care Med 2020462200–2211.[Published erratum appears in Intensive Care Med 1–3.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tas J, van Gassel RJJ, Heines SJH, Mulder MMG, Heijnen NFL, Acampo-de Jong MJ, et al. Serial measurements in COVID-19-induced acute respiratory disease to unravel heterogeneity of the disease course: design of the Maastricht Intensive Care COVID cohort (MaastrICCht) BMJ Open. 2020;10:e040175. doi: 10.1136/bmjopen-2020-040175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gietema HA, Müller NL, Fauerbach PV, Sharma S, Edwards LD, Camp PG, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) investigators. Quantifying the extent of emphysema: factors associated with radiologists’ estimations and quantitative indices of emphysema severity using the ECLIPSE cohort. Acad Radiol. 2011;18:661–671. doi: 10.1016/j.acra.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 4.Frija-Masson J, Debray MP, Gilbert M, Lescure FX, Travert F, Borie R, et al. Functional characteristics of patients with SARS-CoV-2 pneumonia at 30 days post-infection. Eur Respir J. 2020;56:2001754. doi: 10.1183/13993003.01754-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramani C, Davis EM, Kim JS, Provencio JJ, Enfield KB, Kadl A.Post-ICU COVID-19 outcomes: a case series Chest[online ahead of print] 21 Aug 202010.1016/j.chest.2020.08.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hui DS, Wong KT, Ko FW, Tam LS, Chan DP, Woo J, et al. The 1-year impact of severe acute respiratory syndrome on pulmonary function, exercise capacity, and quality of life in a cohort of survivors. Chest. 2005;128:2247–2261. doi: 10.1378/chest.128.4.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park WB, Jun KI, Kim G, Choi JP, Rhee JY, Cheon S, et al. Correlation between pneumonia severity and pulmonary complications in Middle East respiratory syndrome. J Korean Med Sci. 2018;33:e169. doi: 10.3346/jkms.2018.33.e169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, Diaz-Granados N, Al-Saidi F, et al. Canadian Critical Care Trials Group. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348:683–693. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 9.Desai SR, Wells AU, Rubens MB, Evans TW, Hansell DM. Acute respiratory distress syndrome: CT abnormalities at long-term follow-up. Radiology. 1999;210:29–35. doi: 10.1148/radiology.210.1.r99ja2629. [DOI] [PubMed] [Google Scholar]
- 10.Antonio GE, Wong KT, Chu WC, Hui DS, Cheng FW, Yuen EH, et al. Imaging in severe acute respiratory syndrome (SARS) Clin Radiol. 2003;58:825–832. doi: 10.1016/S0009-9260(03)00308-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel BV, Arachchillage DJ, Ridge CA, Bianchi P, Doyle JF, Garfield B, et al. Pulmonary angiopathy in severe COVID-19: physiologic, imaging, and hematologic observations. Am J Respir Crit Care Med. 2020;202:690–699. doi: 10.1164/rccm.202004-1412OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilcox ME, Patsios D, Murphy G, Kudlow P, Paul N, Tansey CM, et al. Radiologic outcomes at 5 years after severe ARDS. Chest. 2013;143:920–926. doi: 10.1378/chest.12-0685. [DOI] [PubMed] [Google Scholar]
- 13.Heyland DK, Groll D, Caeser M. Survivors of acute respiratory distress syndrome: relationship between pulmonary dysfunction and long-term health-related quality of life. Crit Care Med. 2005;33:1549–1556. doi: 10.1097/01.ccm.0000168609.98847.50. [DOI] [PubMed] [Google Scholar]