Abstract
Rationale: Sleep disorders are associated with hypertension and diabetes, which are primary risk factors for cardiovascular diseases and mortality. It is important to understand these associations in Hispanic/Latino individuals, in whom cardiovascular death is the leading cause of mortality.
Objectives: To investigate the prospective associations of sleep-disordered breathing (SDB) and insomnia with incident hypertension and diabetes among U.S. Hispanic/Latino people over 6 years of follow-up and to assess potential sex differences in these associations.
Methods: Data from 11,623 Hispanic/Latino participants in the Hispanic Community Health Study/Study of Latinos (visit 1, 2008–2011; visit 2, 2014–2017) were analyzed using survey logistic regression models, adjusting for potential confounders.
Measurements and Main Results: SDB (apnea–hypopnea index of 5 or more) and insomnia (Women’s Health Initiative Insomnia Rating Scale of 9 or more) were measured at baseline. Incident hypertension (stage 2 or greater) and diabetes were defined according to national guidelines. In the target population, 52.6% were women, with a mean age of 41.1 ± 14.9 years at baseline. SDB was associated with 1.54 higher adjusted odds of incident hypertension (95% confidence interval [CI], 1.18–2.00) and 1.33 higher odds of incident diabetes (95% CI, 1.05–1.67) compared with no SDB. Insomnia was associated with incident hypertension (odds ratio, 1.37; 95% CI, 1.11–1.69) but not with diabetes. The association between insomnia and incident hypertension was stronger among men than among women.
Conclusions: SDB was associated with incident hypertension and diabetes. Insomnia was associated with incident hypertension. These findings support the importance of sleep disorders as modifiable targets for disease prevention and reduction.
Keywords: sleep-disordered breathing, insomnia, hypertension, diabetes, Hispanic/Latino
At a Glance Commentary
Scientific Knowledge on the Subject
Sleep disorders are highly prevalent in the general population and are associated with hypertension and diabetes, which are primary risk factors for cardiovascular diseases and mortality. Although the majority of literature on sleep disorders and cardiometabolic diseases has focused on non-Hispanic white individuals, it is important to understand the associations in Hispanic/Latino populations who have not only high rates of hypertension and diabetes but also higher rates of sleep disturbances compared with non-Hispanic white populations. To our knowledge, no prior empirical studies have examined the prospective relationships between sleep disorders and incident hypertension and diabetes among U.S. Hispanic/Latino adults of diverse backgrounds.
What This Study Adds to the Field
This study presents the first empirical evidence on the prospective associations between the two most common sleep disorders (sleep-disordered breathing and insomnia) with two of the most important cardiovascular risk factors (hypertension and diabetes) among a large cohort (n = 11,623) of U.S. Hispanic/Latino adults. Results show that sleep-disordered breathing was significantly associated with incident hypertension and diabetes. Insomnia was significantly associated with incident hypertension, with a stronger association in men than in women. These findings suggest that sleep disorders represent underrecognized, undertreated, and modifiable targets for cardiometabolic disease prevention and reduction among U.S. Hispanic/Latino adults.
Hypertension and diabetes are among the leading global risk factors for cardiovascular diseases and premature mortality (1–3). It is estimated that approximately one of three adults in the United States has hypertension and one of nine has diabetes (1, 2). Detection, treatment, and control of hypertension and diabetes are adopted by the World Health Assembly in 2013 as global noncommunicable disease targets (4). Efforts are warranted to identify modifiable risk factors for hypertension and diabetes.
Sleep disorders (e.g., sleep-disordered breathing [SDB] and insomnia) are highly prevalent in the general population and are associated with an increased prevalence of hypertension and diabetes (5–21). Mechanisms include altered endocrine, metabolic, and immune system responses to sleep disturbances and curtailed sleep that result in increased blood pressure, decreased insulin sensitivity, and impaired glucose tolerance (22, 23). Research has also indicated sex differences in the relationships between suboptimal sleep and cardiometabolic diseases, but the evidence is inconclusive (24, 25).
Although the majority of literature on sleep disorders and cardiometabolic diseases focused on non-Hispanic white subjects, it is important to understand the associations in Hispanic/Latino subjects. The Hispanic/Latino population currently accounts for 17.8% (57.5 million) of the U.S. population and is expected to double within the next four decades (26). Compared with their non-Hispanic white counterparts of similar age, Hispanic/Latino individuals have higher risks of sleep disturbances (e.g., SDB), a similar risk of hypertension but a higher proportion of cases of uncontrolled blood pressure, and a higher risk of diabetes (27–31). The few prior studies on the roles of sleep disorders in the cardiometabolic health of Hispanic/Latino populations, though suggestive, are limited by cross-sectional designs, relatively small samples, and underrepresentation of various Hispanic/Latino heritage groups (7, 32–34). To our knowledge, no prior empirical studies have examined the prospective relationships between sleep disorders and incident hypertension and diabetes among U.S. Hispanic/Latino adults of diverse backgrounds.
This study investigated the prospective associations of the two most common sleep disorders (SDB and insomnia) (35, 36) with incident hypertension and diabetes and potential sex differences in the associations among a cohort of U.S. Hispanic/Latino subjects. Data for this study came from the HCHS/SOL (Hispanic Community Health Study/Study of Latinos), the largest study of cardiovascular risk factors and sleep traits in U.S. Hispanic/Latino individuals. Some of the results of these studies have been previously reported in the form of an abstract (37).
Methods
Study Design and Study Participants
The HCHS/SOL is a community-based cohort study of 16,415 self-identified Hispanic/Latino persons aged 18–74 years recruited from randomly selected households in four U.S. field centers (Bronx, New York; Chicago, Illinois; Miami, Florida; and San Diego, California). Details of the sampling methods and design have been published (38, 39). Briefly, the baseline study recruited participants between 2008 and 2011 from defined geographic areas to provide a representative sample of these target areas, including participants of Cuban, Dominican, Mexican, Puerto Rican, Central American, and South American backgrounds. Of all individuals who were screened and invited and who met eligibility criteria, 41.7% (n = 16,415) were enrolled. Visit 2 took place between 2014 and 2017 and reexamined 11,623 participants from the baseline sample, with an average follow-up time of 6.1 years (SD, 0.8 yr). The study was approved by the institutional review boards at each participating institution, and written informed consent was obtained from all participants.
Exposures of Interest
At baseline, sleep health was assessed by standardized questions and a validated type 3 home sleep apnea test that provided objective recordings of airflow (via nasal pressure), oximetry, position, and snoring (ARES Unicorder 5.2; B-Alert). Sleep records were scored at a central sleep reading center by trained scorers blinded to all other data (7). Apnea–hypopnea index (AHI3) was calculated based on the average number of all apneas plus hypopneas associated with a 3% desaturation per hour of sleep. SDB was defined as an AHI3 of ≥5. Interscorer and intrascorer reliability of the AHI3 using this device was excellent; compared with full polysomnography, the sensitivity and specificity for detecting an AHI3 ≥5 were shown to be 80% and 88%, respectively (7, 40).
Insomnia was evaluated using the Women’s Health Initiative Insomnia Rating Scale (WHIIRS), a standardized instrument of perceived insomnia symptoms that was developed and validated in a racially/ethnically diverse sample (41). Insomnia was defined as a score of 9 or greater, which corresponds with a high risk of insomnia.
We also explored subtypes of the sleep disorders because prior studies have indicated that SDB with sleepiness and insomnia with objective short sleep duration might represent subtypes associated with more detrimental effects on health (42, 43).
Outcomes of Interest
Prevalent (stage 2 or greater) hypertension was defined as a systolic blood pressure ≥140 mm Hg, a diastolic blood pressure ≥90 mm Hg, or the receipt of antihypertensive medication within 4 weeks before the visit (44). Incident hypertension was defined as not having hypertension at baseline and having hypertension at visit 2.
Diabetes was defined based on the American Diabetes Association definition as a fasting plasma glucose ≥126 mg/dl, 2-hour postload plasma glucose ≥200 mg/dl, or HbA1c ≥6.5% (45), with an additional criterion of self-reported use of antidiabetic medication within 4 weeks before the visit. We used the universal term “diabetes” because the analyses did not discriminate between type 1 and type 2 diabetes. Incident diabetes was defined as not having diabetes at baseline and having diabetes at visit 2.
Covariates
In line with prior work, we controlled for potential confounders measured at baseline, including sociodemographic factors, health behaviors, and adiposity, which are considered to be important risk factors for both sleep disorders and incident metabolic diseases (7, 46, 47). The adjusted sociodemographic factors included age, sex, Hispanic/Latino background, marital status (married/living with a partner, single, separated/divorced/widower), and education (no high school diploma or General Educational Development credential [GED], at most a high school diploma or GED, and greater than high school diploma or GED). Health behaviors included alcohol use (never, former, and current) and smoking (never, former, and current) status. Adiposity was measured by body mass index (BMI) derived from weight and height (underweight/normal, overweight, and obese) and waist circumference, which was measured using standardized protocols (39). We also controlled for time between visits and indicators for field centers to account for potential systematic differences across sites.
Statistical Analyses
Descriptive statistics are presented for the overall sample and for the two analytic samples (one for incident hypertension and the other for incident diabetes), accounting for the complex survey design and sampling weights. For the main analyses, survey logistic regression models were used to estimate the odds of 6-year incident hypertension and diabetes associated with SDB and insomnia, controlling for covariates. A sequential modeling approach was used, with model 1 including only the sleep disorders (entering simultaneously into the model); model 2 also adjusting for sociodemographics, health behaviors, time between visits, and indicators for field centers; and model 3 also adjusting for BMI and waist circumference. Interaction terms between the sleep disorders and sex were introduced to evaluate potential effect modification. We performed sex-stratified analyses when the interaction term was statistically significant (P < 0.05).
Prespecified sensitivity analyses explored alternative ways to model SDB and insomnia by introducing both variables as continuous (instead of as binary) variables, by introducing SDB as a categorical variable defined according to clinical cutoffs (with an AHI ≥5, ≥15, and ≥30 indicating mild, moderate, and severe SDB, respectively), and by introducing insomnia according to a different cutoff (WHIIRS of 10 or more instead of 9 or more). Sleep subphenotypes were explored by evaluating SDB with comorbid sleepiness (Epworth Sleepiness Scale of 11 or more) (48) by looking at those with neither SDB nor sleepiness, those with SDB only, those with sleepiness only, and those with both SDB and sleepiness. For insomnia, we evaluated insomnia with comorbid self-reported short sleep duration (6 h or less) by looking at those with neither insomnia nor short sleep duration, those with insomnia only, those with short sleep duration only, and those with both insomnia and short sleep duration. We evaluated the combination of SDB and insomnia both by testing for statistical interaction between these terms and by modeling each term alone and in combination. We reran the analyses by adjusting additional covariates, including years lived in the United States, household income, physical activity, depressive symptoms, prevalent diabetes at baseline (when modeling incident hypertension), and prevalent hypertension at baseline (when modeling incident diabetes). In post hoc exploratory analyses, we explored the mechanisms between the sleep disorders and the cardiometabolic outcomes by including the following potential mediators in the analyses: change in BMI between visit 1 and visit 2, average heart rate during sleep at baseline (a marker of sympathetic nervous system activity [SNA]) (49), and baseline levels of high sensitivity CRP (C-reactive protein).
All analyses were conducted in R version 3.5.3 (https://www.r-project.org/) using survey packages. All tests were two sided, with a significance level of 5%.
Results
Descriptive Results
Of the 11,623 participants, 1,424 (12.3%) did not undergo a sleep study or had insufficient sleep data for analyses. An additional 93 (0.8%) participants were excluded for missing data on covariates. For incident hypertension analyses, participants who had prevalent hypertension at visit 1 (n = 3,139) or had missing data on hypertension (n = 2) were excluded, yielding an analytic sample of 6,965. For incident diabetes analyses, participants who had prevalent diabetes at visit 1 (n = 2,062) or had missing data on diabetes (n = 21) were excluded, yielding an analytic sample of 8,023 (see Figure E1 in the online supplement).
Table 1 presents overall descriptive statistics by incident hypertension (excluding those with hypertension at baseline or those with missing data on hypertension) and by incident diabetes (excluding those with diabetes at baseline or those with missing data on diabetes). Overall, approximately half of the participants were women, and the mean age at baseline was 41.1 years (SD, 14.9 yr). Six main Hispanic/Latino heritage groups were represented. SDB was more common in men, whereas insomnia was more common in women. Hypertension incidence was similar in women and men, whereas diabetes incidence was slightly higher in men than in women.
Table 1.
Descriptive Characteristics of HCHS/SOL (2008–2017)
| All Panel Participants |
Incident Hypertension Analyses Sample |
Incident Diabetes Analyses Sample |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Overall | Women | Men | Overall | Women | Men | Overall | Women | Men | |
| n | 10,106 | 6,434 | 3,672 | 6,965 | 4,405 | 2,560 | 8,023 | 5,105 | 2,918 |
| Age, yr, mean (SD) | 41.1 (14.9) | 41.7 (15.0) | 40.3 (14.6) | 36.9 (13.0) | 37.2 (13.1) | 36.5 (13.0) | 39.0 (14.1) | 39.5 (14.3) | 38.4 (13.9) |
| Sex, % | |||||||||
| F | 52.6 | 100.0 | 0.0 | 52.4 | 100.0 | 0.0 | 52.1 | 100.0 | 0.0 |
| M | 47.4 | 0.0 | 100.0 | 47.6 | 0.0 | 100.0 | 47.9 | 0.0 | 100.0 |
| Ethnic background, % | |||||||||
| Mexican | 40.7 | 41.8 | 39.4 | 44.8 | 46.4 | 43.0 | 40.8 | 41.8 | 39.7 |
| Dominican | 9.8 | 11.0 | 8.4 | 9.2 | 10.4 | 8.0 | 9.8 | 11.1 | 8.4 |
| Central American | 7.5 | 7.5 | 7.5 | 7.9 | 7.9 | 7.9 | 7.7 | 7.7 | 7.6 |
| Cuban | 18.0 | 16.3 | 20.0 | 15.2 | 13.8 | 16.8 | 18.1 | 16.5 | 19.8 |
| Puerto Rican | 15.3 | 14.9 | 15.9 | 13.4 | 12.3 | 14.6 | 14.3 | 13.6 | 15.1 |
| South American | 4.7 | 5.0 | 4.4 | 5.0 | 5.2 | 4.7 | 5 | 5.3 | 4.7 |
| More than one/other heritage | 4.0 | 3.5 | 4.4 | 4.4 | 4.0 | 5.0 | 4.3 | 3.9 | 4.7 |
| Marital status, % | |||||||||
| Married or living with a partner | 50.3 | 48.4 | 52.5 | 50.0 | 50.5 | 49.4 | 49.3 | 48.2 | 50.5 |
| Single | 34.1 | 30.8 | 37.9 | 38.0 | 33.9 | 42.7 | 36.7 | 32.8 | 40.8 |
| Separated, divorced, or widowed | 15.6 | 20.9 | 9.7 | 12.0 | 15.7 | 7.9 | 14 | 18.9 | 8.7 |
| Education, % | |||||||||
| No high school diploma or GED | 32.3 | 32.8 | 31.8 | 30.0 | 29.6 | 30.5 | 29.8 | 29.6 | 30.1 |
| At most a high school diploma or GED | 28.0 | 26.8 | 29.2 | 29.2 | 28.1 | 30.3 | 28.8 | 27.4 | 30.3 |
| Greater than high school diploma or GED | 39.7 | 40.4 | 39.0 | 40.8 | 42.3 | 39.2 | 41.4 | 43 | 39.7 |
| Smoking status, % | |||||||||
| Never | 64.0 | 72.3 | 54.8 | 65.6 | 73.7 | 56.7 | 64.8 | 72.8 | 56.1 |
| Former | 17.1 | 12.8 | 21.9 | 14.8 | 11.3 | 18.6 | 15.8 | 12 | 20 |
| Current | 18.9 | 14.9 | 23.3 | 19.6 | 15.0 | 24.7 | 19.4 | 15.2 | 23.9 |
| Drinking status, % | |||||||||
| Never | 18.7 | 25.8 | 10.7 | 17.1 | 22.9 | 10.7 | 18.1 | 24.4 | 11.2 |
| Former | 30.1 | 33.5 | 26.4 | 29.3 | 33.0 | 25.2 | 28.7 | 32.3 | 24.7 |
| Current | 51.2 | 40.7 | 62.9 | 53.6 | 44.1 | 64.0 | 53.3 | 43.3 | 64.1 |
| BMI, kg/m2, % | |||||||||
| <25 (underweight and normal) | 23.4 | 23.4 | 23.3 | 27.2 | 27.7 | 26.7 | 25.5 | 26.0 | 24.9 |
| 25–30 (overweight) | 37.1 | 33.9 | 40.6 | 37.1 | 34.0 | 40.5 | 38.1 | 35.1 | 41.2 |
| ≥30 (obese) | 39.5 | 42.7 | 36.0 | 35.7 | 38.2 | 32.8 | 36.5 | 38.9 | 33.8 |
| Waist circumference, cm, mean (SD) | 97.3 (14.2) | 96.5 (14.6) | 98.1 (13.7) | 95.5 (13.9) | 94.8 (14.4) | 96.3 (13.3) | 95.9 (13.5) | 95.0 (13.8) | 96.9 (13.2) |
| Time between visits, yr, mean (SD) | 6.1 (0.8) | 6.1 (0.9) | 6.1 (0.8) | 6.2 (0.9) | 6.1 (0.9) | 6.2 (0.8) | 6.1 (0.9) | 6.1 (0.9) | 6.1 (0.8) |
| SDB, % | |||||||||
| No | 73.9 | 80.2 | 66.9 | 80.9 | 87.5 | 73.7 | 77.9 | 84.3 | 70.9 |
| Yes | 26.1 | 19.8 | 33.1 | 19.1 | 12.5 | 26.3 | 22.1 | 15.7 | 29.1 |
| Insomnia, % | |||||||||
| No | 67.4 | 62.4 | 72.9 | 70.1 | 65.9 | 74.8 | 68.7 | 63.6 | 74.3 |
| Yes | 32.6 | 37.6 | 27.1 | 29.9 | 34.1 | 25.2 | 31.3 | 36.4 | 25.7 |
| Incident hypertension, % | |||||||||
| No | 89.1 | 89.2 | 89.0 | 85.7 | 85.8 | 85.7 | 90.0 | 89.8 | 90.2 |
| Yes | 10.9 | 10.8 | 11.0 | 14.3 | 14.2 | 14.3 | 10.0 | 10.2 | 9.8 |
| Incident diabetes, % | |||||||||
| No | 92.4 | 92.8 | 92.0 | 94.0 | 93.9 | 94.1 | 91.1 | 91.4 | 90.7 |
| Yes | 7.6 | 7.2 | 8.0 | 6.0 | 6.1 | 5.9 | 8.9 | 8.6 | 9.3 |
Definition of abbreviations: BMI = body mass index; GED = General Educational Development credential; HCHS/SOL = Hispanic Community Health Study/Study of Latinos; SDB = sleep-disordered breathing.
The numbers of participants were unweighted. The percentages were weighted. Weighted descriptive statistics of the following three samples are presented: all participants who participated in both examinations, participants in the incident hypertension analyses sample (excluding those with prevalent hypertension at visit 1 or those who had missing data on hypertension), and participants in the incident diabetes analyses sample (excluding those with prevalent diabetes at visit 1 or who had missing data on diabetes).
Sleep Disorders and Incident Hypertension
Table 2 presents the adjusted odd ratios (ORs) of the associations between sleep disorders and incident hypertension. In fully adjusted models, SDB was significantly associated with increased odds of incident hypertension (OR, 1.54; 95% confidence interval [CI], 1.18–2.00). Insomnia was also significantly associated with higher odds of incident hypertension (OR, 1.37; 95% CI, 1.11–1.69). There was no evidence that a sex difference existed in the association between SDB and incident hypertension (P = 0.70 for the interaction term; Figure 1). A significant interaction between insomnia and sex was found for incident hypertension (P = 0.03 for the interaction term; Figure 1). In sex-stratified analyses, insomnia was associated with incident hypertension among men (OR, 1.87; 95% CI, 1.31–2.68). In contrast, no association was observed between insomnia and incident hypertension among women.
Table 2.
Effect Estimates from Survey Logistic Regression Models for the Association between Sleep Disorders and 6-Year Incident Hypertension (n = 6,965)/Diabetes (n = 8,023) in HCHS/SOL (2008–2017)
| Model 1 |
Model 2 |
Model 3 |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Hypertension | ||||||
| SDB | 3.19* | 2.60–3.90 | 1.93* | 1.52–2.45 | 1.54† | 1.18–2.00 |
| Insomnia | 1.73* | 1.44–2.08 | 1.43† | 1.15–1.76 | 1.37† | 1.11–1.69 |
| Diabetes | ||||||
| SDB | 2.97* | 2.41–3.66 | 2.06* | 1.66–2.56 | 1.33‡ | 1.05–1.67 |
| Insomnia | 1.39‡ | 1.12–1.74 | 1.22 | 0.96–1.55 | 1.13 | 0.89–1.45 |
Definition of abbreviations: CI = confidence interval; HCHS/SOL = Hispanic Community Health Study/Study of Latinos; OR = odds ratio; SDB = sleep-disordered breathing.
Model 1 included only SDB and insomnia. Model 2 further controlled for sociodemographics, health behaviors, time between visits, and indicators for field centers. Model 3 further controlled for body mass index and waist circumference. Statistically significant (P < 0.05) effect estimates are bolded.
P < 0.001.
P < 0.01.
P < 0.05.
Figure 1.
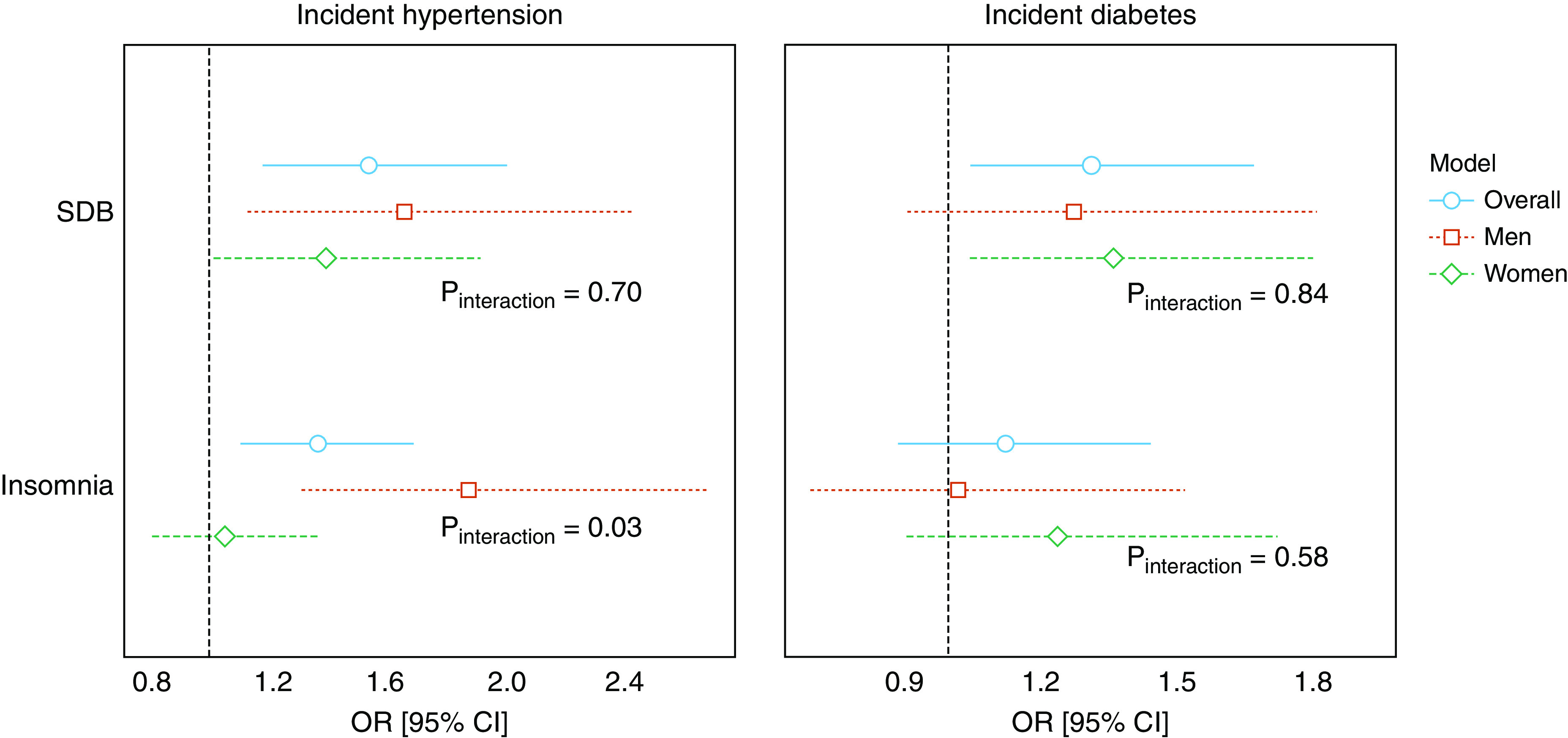
Sex-stratified analyses on the associations between sleep disorders and 6-year incident hypertension (n = 6,965) or diabetes (n = 8,023) from the Hispanic Community Health Study/Study of Latinos (2008–2017). P values of interaction terms between the respective sleep disorder and sex are presented. Survey logistic regression analyses adjusted for sociodemographics, health behaviors, body mass index, waist circumference, time between visits, and indicators for field centers. CI = confidence interval; OR = odds ratio; SDB = sleep-disordered breathing.
Sleep Disorders and Incident Diabetes
Table 2 presents the regression results of the associations between each sleep disorder and incident diabetes. When only the sleep disorders were included in model 1, both SDB and insomnia were significantly associated with incident diabetes. After adjusting for covariates, the association between SDB and incident diabetes remained (OR, 1.33; 95% CI, 1.05–1.67) whereas the association between insomnia and incident diabetes did not (OR, 1.13; 95% CI, 0.89–1.45). We observed no evidence that sex modified the associations between either sleep disorder with incident diabetes (Figure 1).
Sensitivity Analyses
We reran the analyses using alternative ways to model SDB and insomnia, including an assessment of their joint effects. For hypertension, there was no evidence that increasing severity of SDB category beyond an AHI ≥5 was associated with increasing odds of incident disease in fully adjusted models (Table E1). In contrast, odds of incident diabetes were highest for individuals with the most severe SDB category (AHI ≥30). When we examined subtypes of SDB and insomnia, we found no evidence that comorbid SDB and sleepiness (vs. SDB only) or comorbid insomnia and short sleep duration (vs. insomnia only) differed regarding associations with incident hypertension or diabetes compared with the simpler phenotypes (Table E2). The interaction term between SDB and insomnia had a P value of 0.94 in the model estimating incident hypertension and a P value of 0.24 in the model estimating incident diabetes. However, the comorbid SDB and insomnia phenotype was associated with both incident hypertension and diabetes. Compared with insomnia alone, a stronger OR was observed for this combined phenotype for incident hypertension but not for incident diabetes (Table E3). Similar results to the primary analyses were found when the analyses were adjusted for additional covariates (e.g., physical activity).
Results of the post hoc exploratory analyses show that including the change in BMI from visit 1 to visit 2 in the final model did not change the significance of the observed associations between the sleep disorders and the cardiometabolic outcomes, although the magnitude of the associations decreased modestly (e.g., by 6.5% for the OR relating SDB to hypertension; Table E4). Including average heart rate during sleep in the final model also did not materially alter the main effects model (e.g., effect estimate was reduced by 3.9% for the OR relating SDB to hypertension; Table E4). No changes in the results were observed for models including CRP (data not shown). There is thus limited evidence that change in BMI, average heart rate during sleep, or CRP level mediated the associations between the sleep disorders and the cardiometabolic diseases.
Discussion
To the best of our knowledge, this study presents the first empirical evidence on the prospective associations between SDB and insomnia with 6-year incident hypertension and diabetes among U.S. Hispanic/Latino subjects. Results show that SDB was associated with both incident hypertension and diabetes, whereas insomnia was associated with incident hypertension. The association between insomnia and incident hypertension was stronger among men compared with women, despite women reporting more insomnia symptoms.
Prior studies documenting the role of SDB and insomnia on cardiometabolic disorders have focused predominantly on non-Hispanic white populations (5, 6, 8, 9, 50); for instance, data from the Sleep Heart Health Study demonstrated that SDB was cross-sectionally associated with hypertension (9), and a prospective association was shown with data from the Wisconsin Sleep Cohort Study (6). A few recent studies focused on Hispanic/Latino populations (7, 32). Using visit 1 data from HCHS/SOL, Redline and colleagues reported that SDB was associated with prevalent hypertension and diabetes, whereas Ramos and colleagues reported that insomnia was not associated with prevalent hypertension (7, 32). These findings used cross-sectional designs that limited the assessment of causality (7, 32). Our study expands the current literature by investigating the associations of SDB and insomnia with incident hypertension and diabetes in a prospective large cohort of U.S. Hispanic/Latino individuals, showing that SDB was an antecedent risk factor for the development of both hypertension and diabetes and that insomnia, particularly in men, was associated with incident hypertension.
The plausibility for a causal relationship between SDB and cardiometabolic diseases is supported by evidence from intervention studies. Prior research showed that SDB treatment reduces nighttime and daytime blood pressure in patients with SDB (51). Some experimental and small clinical intervention data also showed that SDB treatment improves insulin sensitivity and glucose levels, although more data from large trials are needed (52). For instance, one randomized controlled trial involving 39 individuals with prediabetes showed that 2 weeks of 8-hour nightly continuous positive airway pressure (CPAP) treatment improved glucose metabolism compared with placebo (17). Two 6-month randomized controlled trials reported inconsistent findings on the effect of CPAP on glycemic control in patients with diabetes; a study involving 298 patients found no effect of CPAP treatment on HbA1c, whereas a study involving 50 patients did find an effect (18, 19). SDB, which is characterized by repetitive airflow interruptions during sleep, results in intermittent hypoxia and arousals that cause sleep fragmentation and increased SNA, a mechanism for hypertension and diabetes (53, 54). SDB also induces inflammatory and hormonal responses, including insulin resistance, elevations of inflammatory cells, and endothelial dysfunction. Wide intrathoracic swings in systemic blood and arterial pulmonary pressures could further contribute to vascular damage (49, 55). In addition, although obesity is an established risk factor for SDB, individuals with SDB are also prone to more weight gain (56), which might increase their risk of hypertension and diabetes (57, 58).
Insomnia has been associated with increases in markers of systematic inflammation, known risk factors for hypertension and diabetes (1, 59, 60). Furthermore, patients with insomnia have increased heart rate and chronic activation of the hypothalamic–pituitary–adrenal axis, which have been linked to metabolic disorders such as diabetes (61). Although there are strong biological bases linking insomnia to adverse cardiometabolic health, prior literature has not consistently shown associations between insomnia with incident hypertension or diabetes. A recent systematic analysis found that insomnia with short sleep duration or arousal is associated with hypertension. However, based on a majority of case–control studies, those with and without insomnia did not differ in blood pressure (62). Similarly, although meta-analyses show consistent associations between short sleep duration and incident diabetes (63), associations between insomnia in the absence of short sleep with diabetes are less clear (50). Findings from the current large prospective study provide new data showing that insomnia in U.S. Hispanic/Latino individuals was associated with incident hypertension but not diabetes and that these associations were not modified by self-reported sleep duration. It is possible that objectively measured sleep duration may have allowed an improved phenotypic characterization of an “insomnia–short sleep” risk factor (50). The severity of insomnia symptoms in a community-based cohort may also not reflect the extent of metabolic disfunction in clinical settings where patients seek help for more severe sleep disorders and are better characterized with clinical interviews.
As discussed earlier, there are multiple potential mechanisms linking sleep disorders to cardiometabolic disease. Although the aim of this study was not to identify physiological mechanisms, we conducted several post hoc exploratory analyses modeling change in BMI, baseline heart rate during sleep (a marker of SNA), and CRP level. These analyses did not change the significance of the associations between the sleep disorders and the cardiometabolic outcomes and only slightly to modestly decreased magnitude of the associations. Our findings provide a framework for designing future research to address which physiological mediators link sleep disorders to later cardiometabolic diseases.
Sex differences abound in human health and diseases, possibly because of hormonal factors, gene expression, and behavioral and sociocultural factors. Our data found that the association between insomnia and incident hypertension was stronger among men than women despite a higher prevalence of insomnia in women. Although small experimental studies indicated that acute inflammatory responses to sleep disruption may be stronger in women (64, 65), prior epidemiological studies reported a stronger relationship between insomnia and higher levels of CRP and a stronger relationship between insomnia and mortality among men (66, 67). Self-rated health has been reported to associate more strongly with mortality in men than women (68) despite women usually reporting worse self-rated health (69). It is possible that women, the primary consumers of health services and information, might be more likely to know about their health and report health concerns/problems (69). Men, on the other hand, might only report symptoms with a high level of disturbance. Thus, insomnia, based on self-reported symptoms, might reflect a greater severity of sleep disturbance in men compared with women despite comparable scores. Our data suggest a need for further studies of sex differences that address potential reporting differences and link perceived symptoms with objective changes in sleep.
Recent data suggested that sleepiness reported by predominantly white, older individuals with SDB marked individuals at increased cardiovascular risk (70). Our exploratory analysis did not support differences in the associations between SDB and cardiometabolic outcomes in the presence of sleepiness. This suggests that in middle-aged U.S. Hispanic/Latino individuals, sleepiness should not be relied on to identify individuals at high risk for SDB-associated hypertension and diabetes. On the other hand, comorbid SDB and insomnia was associated with a higher OR of hypertension compared with insomnia only, suggesting increased hypertension risk among individuals with both intermittent desaturation (SDB) and a hyperarousal phenotype (insomnia), supporting further work to identify susceptibility to chronic diseases by SDB subphenotypes.
Our data did not show evidence of a dose–response association between SDB severity and incident hypertension, suggesting that mild levels of SDB confer increased hypertension risk in this sample. In contrast, risk for incident diabetes was highest among those with severely elevated AHI levels, consistent with prior research suggesting that more severe levels of hypoxemia confer greater metabolic dysfunction (15).
Strengths of the study include the large and diverse Hispanic/Latino sample, which was representative of four distinct and diverse geographical areas; the prospective design; the objective measure of SDB and standardized measure of insomnia; standardized assessments of hypertension and diabetes; and the consideration of multiple potential confounders.
This study also has several limitations. First, the home sleep apnea test device did not allow the evaluation of arousal or sleep architecture, which may lead to an underestimation of disease severity both because of overestimation of sleep time and underrecognition of hypopneas unassociated with desaturation. Another limitation is that the current insomnia analyses were based on validated assessment of insomnia symptoms but not on a clinical diagnosis (which uses symptoms and a diagnostic interview). The WHIIRS was developed in a sample of postmenopausal women, and the results should be interpreted cautiously with other age groups and with men. Prior research has suggested that minority populations might underreport sleep disturbances (71), possibly because of social desirability (a tendency not to encode a negative event), stress, stereotype threat, acculturation, attitudes, et cetera. If there was underreporting of insomnia symptoms unrelated to study outcomes, the results may be biased toward the null. However, the WHIIRS was developed in a racially/ethnically diverse group, and psychometric work on a sample of almost 70,000 participants demonstrated that the scale has excellent reliability and validity. Factor analysis revealed that the scale has a highly stable factor structure across age and racial/ethnic groups. WHIIRS items correspond with the majority of insomnia characteristics noted in the major nosologies, and differences in sleep latency, sleep efficiency, and waking after sleep onset measured by a wrist activity monitor were reflected by corresponding differences in WHIIRS scores (41, 72). Short sleep was also based on self-report, which might be subject to misclassification (73). Third, although the analyses used prospective data and carefully controlled for potential confounders, the observational design constrained our ability to identify causality and to detect mediating mechanisms. Future research that includes more detailed information on the time of onset of incident hypertension and diabetes would help quantify the extent to which individuals with sleep disorders have an accelerated rate of developing new hypertension or diabetes.
Furthermore, the HCHS/SOL participants were largely recruited from urban areas, and thus, the results might not generalize to populations in rural areas. On a related note, the ethnic background distribution of individuals in HCHS/SOL does not mirror that in the United States. For instance, 41% of the individuals in HCHS/SOL are of Mexican origin, whereas 63% of the Hispanic/Latino population in the United States is of Mexican origin. Because there might be marked heterogeneity and systematic differences in the sleep patterns and cardiovascular risk factors among subgroups of Hispanic/Latino subjects (74, 75), the results might not generalize to the Hispanic/Latino populations outside of the target areas of HCHS/SOL. Additional research with larger numbers of outcomes within each background group is needed to address potential heterogeneity in associations between disturbed sleep and incident cardiometabolic disease. Because there is no non-Hispanic control group, the results might not be directly compared with the observations made in other racial/ethnic groups.
In summary, this study presents some of the first prospective evidence on the associations of SDB and insomnia with incident hypertension and diabetes in U.S. Hispanic/Latino individuals. Results show that SDB was associated with both 6-year incident hypertension and diabetes, whereas insomnia was associated with incident hypertension. Moreover, the association between insomnia and incident hypertension was stronger in men compared with women. Given the fact that sleep disorders are largely undiagnosed and undertreated (7), they might represent modifiable targets for disease prevention and reduction among U.S. Hispanic/Latino populations. Culturally informed interventions focusing on detecting and treating sleep disorders might improve cardiometabolic health among U.S. Hispanic/Latino individuals.
Acknowledgments
Acknowledgment
The authors thank the staff and participants of the Hispanic Community Health Study/Study of Latinos for their important contributions.
Footnotes
The Hispanic Community Health Study/Study of Latinos is a collaborative study supported by contracts from the NHLBI to the University of North Carolina (HHSN268201300001I/N01-HC-65233), the University of Miami (HHSN268201300004I/N01-HC-65234), the Albert Einstein College of Medicine (HHSN268201300002I/N01-HC-65235), the University of Illinois at Chicago (HHSN268201300003I/N01-HC-65236 Northwestern University), and San Diego State University (HHSN268201300005I/N01-HC-65237). The following institutes, centers, and offices have contributed to the Hispanic Community Health Study/Study of Latinos through a transfer of funds to the NHLBI: the National Institute on Minority Health and Health Disparities, the National Institute on Deafness and Other Communication Disorders, the National Institute of Dental and Craniofacial Research, the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Neurological Disorders and Stroke, and the NIH Institution-Office of Dietary Supplements. The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the NHLBI, the NIH, or the U.S. Department of Health and Human Services. S.R. was partially supported by the NIH (grant R35 HL135818). The funding source had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the report for publication.
Author Contributions: X.L. and S.R. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: X.L. and S.R. Acquisition, analysis, or interpretation of data: all authors. Drafting of the manuscript: X.L. and S.R. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: X.L. and D.S.-A. Obtaining funding: N.S., M.D., and S.R. Administrative, technical, or material support: D.S.-A., M.D., and S.R. Study supervision: S.R.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201912-2330OC on August 6, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Dariush M, Emelia B, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics- 2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.National Center for Health Statistics (US) Hyattsville, MD: National Center for Health Statistics (US); 2017. Health, United States, 2016: with chartbook on long-term trends in health. [accessed 2018 Mar 27]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK453378/ [PubMed] [Google Scholar]
- 3.Global Burden of Metabolic Risk Factors for Chronic Diseases Collaboration. Cardiovascular disease, chronic kidney disease, and diabetes mortality burden of cardiometabolic risk factors from 1980 to 2010: a comparative risk assessment. Lancet Diabetes Endocrinol. 2014;2:634–647. doi: 10.1016/S2213-8587(14)70102-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. Geneva, Switzerland: World Health Organization; Global action plan for the prevention and control of noncommunicable diseases 2013-2020. 2013 [accessed 2018 Mar 28]. Available from: http://apps.who.int/iris/bitstream/handle/10665/94384/9789241506236_eng.pdf?sequence=1. [Google Scholar]
- 5.Marin JM, Agusti A, Villar I, Forner M, Nieto D, Carrizo SJ, et al. Association between treated and untreated obstructive sleep apnea and risk of hypertension. JAMA. 2012;307:2169–2176. doi: 10.1001/jama.2012.3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 7.Redline S, Sotres-Alvarez D, Loredo J, Hall M, Patel SR, Ramos A, et al. The Hispanic Community Health Study/Study of Latinos. Sleep-disordered breathing in Hispanic/Latino individuals of diverse backgrounds. Am J Respir Crit Care Med. 2014;189:335–344. doi: 10.1164/rccm.201309-1735OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bixler EO, Vgontzas AN, Lin H-M, Ten Have T, Leiby BE, Vela-Bueno A, et al. Association of hypertension and sleep-disordered breathing. Arch Intern Med. 2000;160:2289–2295. doi: 10.1001/archinte.160.15.2289. [DOI] [PubMed] [Google Scholar]
- 9.Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S, et al. Study for the SHH. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study: Sleep Heart Health Study. JAMA. 2000;283:1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 10.Ayas NT, Taylor CM, Laher I. Cardiovascular consequences of obstructive sleep apnea. Curr Opin Cardiol. 2016;31:599–605. doi: 10.1097/HCO.0000000000000329. [DOI] [PubMed] [Google Scholar]
- 11.Mokhlesi B, Finn LA, Hagen EW, Young T, Hla KM, Van Cauter E, et al. Obstructive sleep apnea during REM sleep and hypertension: results of the Wisconsin Sleep Cohort. Am J Respir Crit Care Med. 2014;190:1158–1167. doi: 10.1164/rccm.201406-1136OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ayas NT, Hirsch AAJ, Laher I, Bradley TD, Malhotra A, Polotsky VY, et al. New frontiers in obstructive sleep apnoea. Clin Sci (Lond) 2014;127:209–216. doi: 10.1042/CS20140070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pamidi S, Tasali E. Obstructive sleep apnea and type 2 diabetes: is there a link? Front Neurol. 2012;3:126. doi: 10.3389/fneur.2012.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reichmuth KJ, Austin D, Skatrud JB, Young T. Association of sleep apnea and type II diabetes: a population-based study. Am J Respir Crit Care Med. 2005;172:1590–1595. doi: 10.1164/rccm.200504-637OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kendzerska T, Gershon AS, Hawker G, Tomlinson G, Leung RS. Obstructive sleep apnea and incident diabetes: a historical cohort study. Am J Respir Crit Care Med. 2014;190:218–225. doi: 10.1164/rccm.201312-2209OC. [DOI] [PubMed] [Google Scholar]
- 16.Harsch IA, Schahin SP, Radespiel-Tröger M, Weintz O, Jahreiss H, Fuchs FS, et al. Continuous positive airway pressure treatment rapidly improves insulin sensitivity in patients with obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2004;169:156–162. doi: 10.1164/rccm.200302-206OC. [DOI] [PubMed] [Google Scholar]
- 17.Pamidi S, Wroblewski K, Stepien M, Sharif-Sidi K, Kilkus J, Whitmore H, et al. Eight hours of nightly continuous positive airway pressure treatment of obstructive sleep apnea improves glucose metabolism in patients with prediabetes: a randomized controlled trial. Am J Respir Crit Care Med. 2015;192:96–105. doi: 10.1164/rccm.201408-1564OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shaw JE, Punjabi NM, Naughton MT, Willes L, Bergenstal RM, Cistulli PA, et al. The effect of treatment of obstructive sleep apnea on glycemic control in type 2 diabetes. Am J Respir Crit Care Med. 2016;194:486–492. doi: 10.1164/rccm.201511-2260OC. [DOI] [PubMed] [Google Scholar]
- 19.Martínez-Cerón E, Barquiel B, Bezos A-M, Casitas R, Galera R, García-Benito C, et al. Effect of continuous positive airway pressure on glycemic control in patients with obstructive sleep apnea and type 2 diabetes: a randomized clinical trial. Am J Respir Crit Care Med. 2016;194:476–485. doi: 10.1164/rccm.201510-1942OC. [DOI] [PubMed] [Google Scholar]
- 20.Aronsohn RS, Whitmore H, Van Cauter E, Tasali E. Impact of untreated obstructive sleep apnea on glucose control in type 2 diabetes. Am J Respir Crit Care Med. 2010;181:507–513. doi: 10.1164/rccm.200909-1423OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mokhlesi B, Grimaldi D, Beccuti G, Abraham V, Whitmore H, Delebecque F, et al. Effect of one week of 8-hour nightly continuous positive airway pressure treatment of obstructive sleep apnea on glycemic control in type 2 diabetes: a proof-of-concept study. Am J Respir Crit Care Med. 2016;194:516–519. doi: 10.1164/rccm.201602-0396LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buxton OM, Cain SW, O’Connor SP, Porter JH, Duffy JF, Wang W, et al. Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci Transl Med. 2012;4:129ra43. doi: 10.1126/scitranslmed.3003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leproult R, Van Cauter E. Role of sleep and sleep loss in hormonal release and metabolism. Endocr Dev. 2010;17:11–21. doi: 10.1159/000262524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roca GQ, Redline S, Claggett B, Bello N, Ballantyne CM, Solomon SD, et al. Sex-specific association of sleep apnea severity with subclinical myocardial injury, ventricular hypertrophy, and heart failure risk in a community-dwelling cohort: the atherosclerosis risk in communities-Sleep Heart Health Study. Circulation. 2015;132:1329–1337. doi: 10.1161/CIRCULATIONAHA.115.016985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gottlieb DJ, Redline S, Nieto FJ, Baldwin CM, Newman AB, Resnick HE, et al. Association of usual sleep duration with hypertension: the Sleep Heart Health Study. Sleep. 2006;29:1009–1014. doi: 10.1093/sleep/29.8.1009. [DOI] [PubMed] [Google Scholar]
- 26.Vespa J, Medina L, Armstrong DM. Washington, DC: US Census Bureau; 2020. Demographic turning points for the United States: population projections for 2020 to 2060. Current population reports. [accessed 2020 Mar 6]. Available from: https://www.census.gov/content/dam/Census/library/publications/2020/demo/p25-1144.pdf. [Google Scholar]
- 27.Chen X, Wang R, Zee P, Lutsey PL, Javaheri S, Alcántara C, et al. Racial/ethnic differences in sleep disturbances: the Multi-Ethnic Study of Atherosclerosis (MESA) Sleep. 2015;38:877–888. doi: 10.5665/sleep.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roane BM, Johnson L, Edwards M, Hall J, Al-Farra S, O’Bryant SE. The link between sleep disturbance and depression among Mexican Americans: a Project FRONTIER study. J Clin Sleep Med. 2014;10:427–431. doi: 10.5664/jcsm.3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fryar CD, Ostchega Y, Hales CM, Zhang G, Kruszon-Moran D. Hypertension prevalence and control among adults: United States, 2015-2016. NCHS Data Brief. 2017:1–8. [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention. National diabetes statistics report. Atlanta, GA: US Department of Health and Human Services; 2017. [Google Scholar]
- 31.Sorlie PD, Allison MA, Avilés-Santa ML, Cai J, Daviglus ML, Howard AG, et al. Prevalence of hypertension, awareness, treatment, and control in the Hispanic Community Health Study/Study of Latinos. Am J Hypertens. 2014;27:793–800. doi: 10.1093/ajh/hpu003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramos AR, Weng J, Wallace DM, Petrov MR, Wohlgemuth WK, Sotres-Alvarez D, et al. Sleep patterns and hypertension using actigraphy in the Hispanic Community Health Study/Study of Latinos. Chest. 2018;153:87–93. doi: 10.1016/j.chest.2017.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cespedes EM, Hu FB, Redline S, Rosner B, Alcantara C, Cai J, et al. Comparison of self-reported sleep duration with actigraphy: results from the Hispanic Community Health Study/Study of Latinos Sueño Ancillary Study. Am J Epidemiol. 2016;183:561–573. doi: 10.1093/aje/kwv251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Full KM, Schmied EA, Parada H, Cherrington A, Horton LA, Ayala GX. The relationship between sleep duration and glycemic control among Hispanic adults with uncontrolled type 2 diabetes. Diabetes Educ. 2017;43:519–529. doi: 10.1177/0145721717724564. [DOI] [PubMed] [Google Scholar]
- 35.Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6:97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- 36.Ohayon MM. Epidemiological overview of sleep disorders in the general population. Sleep Med Res. 2011;2:1–9. [Google Scholar]
- 37.Li X, Sotres-Alvarez D, Sofer T, Gallo LC, Aviles-Santa L, Perreira KM, et al. Associations of sleep disordered breathing and insomnia with incident hypertension and diabetes: a prospective analysis of data from the Hispanic Community Health Study/Study of Latinos. Am J Respir Crit Care Med. 2020;201:A5605. doi: 10.1164/rccm.201912-2330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lavange LM, Kalsbeek WD, Sorlie PD, Avilés-Santa LM, Kaplan RC, Barnhart J, et al. Sample design and cohort selection in the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol. 2010;20:642–649. doi: 10.1016/j.annepidem.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sorlie PD, Avilés-Santa LM, Wassertheil-Smoller S, Kaplan RC, Daviglus ML, Giachello AL, et al. Design and implementation of the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol. 2010;20:629–641. doi: 10.1016/j.annepidem.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Louis J, Auckley D, Miladinovic B, Shepherd A, Mencin P, Kumar D, et al. Perinatal outcomes associated with obstructive sleep apnea in obese pregnant women. Obstet Gynecol. 2012;120:1085–1092. doi: 10.1097/AOG.0b013e31826eb9d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Levine DW, Kripke DF, Kaplan RM, Lewis MA, Naughton MJ, Bowen DJ, et al. Reliability and validity of the Women’s Health Initiative Insomnia Rating Scale. Psychol Assess. 2003;15:137–148. doi: 10.1037/1040-3590.15.2.137. [DOI] [PubMed] [Google Scholar]
- 42.Huang T, Lin BM, Stampfer MJ, Tworoger SS, Hu FB, Redline S. A population-based study of the bidirectional association between obstructive sleep apnea and type 2 diabetes in three prospective U.S. cohorts. Diabetes Care. 2018;41:2111–2119. doi: 10.2337/dc18-0675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bathgate CJ, Fernandez-Mendoza J. Insomnia, short sleep duration, and high blood pressure: recent evidence and future directions for the prevention and management of hypertension. Curr Hypertens Rep. 2018;20:52. doi: 10.1007/s11906-018-0850-6. [DOI] [PubMed] [Google Scholar]
- 44.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 45.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33:S62–S69. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buxton OM, Marcelli E. Short and long sleep are positively associated with obesity, diabetes, hypertension, and cardiovascular disease among adults in the United States. Soc Sci Med. 2010;71:1027–1036. doi: 10.1016/j.socscimed.2010.05.041. [DOI] [PubMed] [Google Scholar]
- 47.Lederer DJ, Bell SC, Branson RD, Chalmers JD, Marshall R, Maslove DM, et al. Control of confounding and reporting of results in causal inference studies: guidance for authors from editors of respiratory, sleep, and critical care journals. Ann Am Thorac Soc. 2019;16:22–28. doi: 10.1513/AnnalsATS.201808-564PS. [DOI] [PubMed] [Google Scholar]
- 48.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 49.Geovanini GR, Wang R, Weng J, Tracy R, Jenny NS, Goldberger AL, et al. Elevations in neutrophils with obstructive sleep apnea: the Multi-Ethnic Study of Atherosclerosis (MESA) Int J Cardiol. 2018;257:318–323. doi: 10.1016/j.ijcard.2017.10.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vgontzas AN, Liao D, Pejovic S, Calhoun S, Karataraki M, Bixler EO. Insomnia with objective short sleep duration is associated with type 2 diabetes: a population-based study. Diabetes Care. 2009;32:1980–1985. doi: 10.2337/dc09-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Norman D, Loredo JS, Nelesen RA, Ancoli-Israel S, Mills PJ, Ziegler MG, et al. Effects of continuous positive airway pressure versus supplemental oxygen on 24-hour ambulatory blood pressure. Hypertension. 2006;47:840–845. doi: 10.1161/01.HYP.0000217128.41284.78. [DOI] [PubMed] [Google Scholar]
- 52.Weinstock TG, Wang X, Rueschman M, Ismail-Beigi F, Aylor J, Babineau DC, et al. A controlled trial of CPAP therapy on metabolic control in individuals with impaired glucose tolerance and sleep apnea. Sleep. 2012;35:617–625B. doi: 10.5665/sleep.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Palatini P. Role of elevated heart rate in the development of cardiovascular disease in hypertension. Hypertension. 2011;58:745–750. doi: 10.1161/HYPERTENSIONAHA.111.173104. [DOI] [PubMed] [Google Scholar]
- 54.Shigetoh Y, Adachi H, Yamagishi S, Enomoto M, Fukami A, Otsuka M, et al. Higher heart rate may predispose to obesity and diabetes mellitus: 20-year prospective study in a general population. Am J Hypertens. 2009;22:151–155. doi: 10.1038/ajh.2008.331. [DOI] [PubMed] [Google Scholar]
- 55.Gaspar LS, Álvaro AR, Moita J, Cavadas C. Obstructive sleep apnea and hallmarks of aging. Trends Mol Med. 2017;23:675–692. doi: 10.1016/j.molmed.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 56.Phillips BG, Kato M, Narkiewicz K, Choe I, Somers VK. Increases in leptin levels, sympathetic drive, and weight gain in obstructive sleep apnea. Am J Physiol Heart Circ Physiol. 2000;279:H234–H237. doi: 10.1152/ajpheart.2000.279.1.H234. [DOI] [PubMed] [Google Scholar]
- 57.Juhaeri SJ, Stevens J, Chambless LE, Tyroler HA, Rosamond W, Nieto FJ, et al. Associations between weight gain and incident hypertension in a bi-ethnic cohort: the Atherosclerosis Risk in Communities Study. Int J Obes Relat Metab Disord. 2002;26:58–64. doi: 10.1038/sj.ijo.0801846. [DOI] [PubMed] [Google Scholar]
- 58.Colditz GA, Willett WC, Rotnitzky A, Manson JE. Weight gain as a risk factor for clinical diabetes mellitus in women. Ann Intern Med. 1995;122:481–486. doi: 10.7326/0003-4819-122-7-199504010-00001. [DOI] [PubMed] [Google Scholar]
- 59.Irwin MR, Olmstead R, Carroll JE. Sleep disturbance, sleep duration, and inflammation: a systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol Psychiatry. 2016;80:40–52. doi: 10.1016/j.biopsych.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brunner EJ, Kivimäki M, Witte DR, Lawlor DA, Davey Smith G, Cooper JA, et al. Inflammation, insulin resistance, and diabetes: Mendelian randomization using CRP haplotypes points upstream. PLoS Med. 2008;5:e155. doi: 10.1371/journal.pmed.0050155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vgontzas AN, Bixler EO, Lin HM, Prolo P, Mastorakos G, Vela-Bueno A, et al. Chronic insomnia is associated with nyctohemeral activation of the hypothalamic-pituitary-adrenal axis: clinical implications. J Clin Endocrinol Metab. 2001;86:3787–3794. doi: 10.1210/jcem.86.8.7778. [DOI] [PubMed] [Google Scholar]
- 62.Jarrin DC, Alvaro PK, Bouchard M-A, Jarrin SD, Drake CL, Morin CM. Insomnia and hypertension: a systematic review. Sleep Med Rev. 2018;41:3–38. doi: 10.1016/j.smrv.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 63.Itani O, Jike M, Watanabe N, Kaneita Y. Short sleep duration and health outcomes: a systematic review, meta-analysis, and meta-regression. Sleep Med. 2017;32:246–256. doi: 10.1016/j.sleep.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 64.Irwin MR, Wang M, Ribeiro D, Cho HJ, Olmstead R, Breen EC, et al. Sleep loss activates cellular inflammatory signaling. Biol Psychiatry. 2008;64:538–540. doi: 10.1016/j.biopsych.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Irwin MR, Carrillo C, Olmstead R. Sleep loss activates cellular markers of inflammation: sex differences. Brain Behav Immun. 2010;24:54–57. doi: 10.1016/j.bbi.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liukkonen T, Räsänen P, Ruokonen A, Laitinen J, Jokelainen J, Leinonen M, et al. C-reactive protein levels and sleep disturbances: observations based on the Northern Finland 1966 Birth Cohort study. Psychosom Med. 2007;69:756–761. doi: 10.1097/PSY.0b013e318157cb96. [DOI] [PubMed] [Google Scholar]
- 67.Fernandez-Mendoza J, Vgontzas AN, Liao D, Shaffer ML, Vela-Bueno A, Basta M, et al. Insomnia with objective short sleep duration and incident hypertension: the Penn State Cohort. Hypertension. 2012;60:929–935. doi: 10.1161/HYPERTENSIONAHA.112.193268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McCallum J, Shadbolt B, Wang D. Self-rated health and survival: a 7-year follow-up study of Australian elderly. Am J Public Health. 1994;84:1100–1105. doi: 10.2105/ajph.84.7.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Idler EL. Discussion: gender differences in self-rated health, in mortality, and in the relationship between the two. Gerontologist. 2003;43:372–375. [Google Scholar]
- 70.Mazzotti DR, Keenan BT, Lim DC, Gottlieb DJ, Kim J, Pack AI. Symptom subtypes of obstructive sleep apnea predict incidence of cardiovascular outcomes. Am J Respir Crit Care Med. 2019;200:493–506. doi: 10.1164/rccm.201808-1509OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Palesh OG, Roscoe JA, Mustian KM, Roth T, Savard J, Ancoli-Israel S, et al. Prevalence, demographics, and psychological associations of sleep disruption in patients with cancer: University of Rochester Cancer Center-Community Clinical Oncology Program. J Clin Oncol. 2010;28:292–298. doi: 10.1200/JCO.2009.22.5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Levine DW, Kaplan RM, Kripke DF, Bowen DJ, Naughton MJ, Shumaker SA. Factor structure and measurement invariance of the Women’s Health Initiative Insomnia Rating Scale. Psychol Assess. 2003;15:123–136. doi: 10.1037/1040-3590.15.2.123. [DOI] [PubMed] [Google Scholar]
- 73.Jackson CL, Patel SR, Jackson WB, II, Lutsey PL, Redline S. Agreement between self-reported and objectively measured sleep duration among white, black, Hispanic, and Chinese adults in the United States: Multi-Ethnic Study of Atherosclerosis. Sleep. 2018;41:zsy057. doi: 10.1093/sleep/zsy057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Patel SR, Sotres-Alvarez D, Castañeda SF, Dudley KA, Gallo LC, Hernandez R, et al. Social and health correlates of sleep duration in a US Hispanic population: results from the Hispanic Community Health Study/Study of Latinos. Sleep. 2015;38:1515–1522. doi: 10.5665/sleep.5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Daviglus ML, Talavera GA, Avilés-Santa ML, Allison M, Cai J, Criqui MH, et al. Prevalence of major cardiovascular risk factors and cardiovascular diseases among Hispanic/Latino individuals of diverse backgrounds in the United States. JAMA. 2012;308:1775–1784. doi: 10.1001/jama.2012.14517. [DOI] [PMC free article] [PubMed] [Google Scholar]


