Abstract
Rationale: Staphylococcus aureus and Pseudomonas aeruginosa often infect the airways in cystic fibrosis (CF). Because registry studies show higher prevalence of P. aeruginosa versus S. aureus in older patients with CF, a common assumption is that P. aeruginosa replaces S. aureus over time. In vitro, P. aeruginosa can outgrow and kill S. aureus. However, it is unknown how rapidly P. aeruginosa replaces S. aureus in patients with CF.
Methods: We studied a longitudinal cohort of children and adults with CF who had quantitative sputum cultures. We determined the abundance of P. aeruginosa and S. aureus in cfu/ml. We determined the duration and persistence of infections and measured longitudinal changes in culture positivity and abundance for each organism.
Measurements and Main Results: Between 2004 and 2017, 134 patients had ≥10 quantitative cultures, with median observation time of 10.15 years. One hundred twenty-four patients had at least one positive culture for P. aeruginosa, and 123 had at least one positive culture for S. aureus. Both species had median abundance of >106 cfu/ml. Culture abundance was stable over time for both organisms. There was an increase in the prevalence of S. aureus/P. aeruginosa coinfection but no decrease in S. aureus prevalence within individuals over time.
Conclusions: S. aureus and P. aeruginosa are abundant in CF sputum cultures. Contrary to common assumption, we found no pattern of replacement of S. aureus by P. aeruginosa. Many patients with CF have durable long-term coinfection with these organisms. New strategies are needed to prevent and treat these infections.
Keywords: Staphylococcus aureus, Pseudomonas aeruginosa sputum, cystic fibrosis, methicillin-resistant Staphylococcus aureus
At a Glance Commentary
Scientific Knowledge on the Subject
Staphylococcus aureus and Pseudomonas aeruginosa infections are common in cystic fibrosis. S. aureus is more prevalent in children and adolescents, whereas P. aeruginosa is more prevalent in adults.
What This Study Adds to the Field
Patients with cystic fibrosis have durable, highly abundant respiratory coinfections with S. aureus and P. aeruginosa. Contrary to common assumption, P. aeruginosa replacement of S. aureus within individuals is not routinely observed in longitudinal follow-up.
Respiratory infections with Staphylococcus aureus and Pseudomonas aeruginosa increase the risk of disease progression in cystic fibrosis (CF) (1–3). These organisms represent the two most prevalent CF respiratory pathogens (4). Registry studies indicate that S. aureus is most prevalent in younger patients, whereas P. aeruginosa is the most prevalent above age 24 (5). These cross-sectional data support a common, yet unproven, assumption that P. aeruginosa replaces S. aureus over time (6–12). Although this phenomenon of P. aeruginosa outcompeting S. aureus can be demonstrated in vitro (9, 12, 13), conclusive evidence of pathogen replacement in people with CF would require a longitudinal cohort study. It is possible that some differences in pathogen prevalence between older and younger patients might be explained by factors unrelated to direct bacterial competition. For instance, different birth cohorts were exposed to different treatments in early life. Older patients were born before eradication therapy for P. aeruginosa was available but were treated for S. aureus (14). Patients born in the era of inhaled antibiotic therapy now can suppress P. aeruginosa (15–17) but may not eliminate S. aureus, particularly methicillin-resistant S. aureus (MRSA) (18).
To measure how quickly P. aeruginosa replaces S. aureus in people with CF, we examined microbiology results from a longitudinal cohort of patients with CF. Because there could be doubt about the pathologic significance of S. aureus on oropharyngeal culture swab owing to its presence in healthy subjects (7, 19), we focused on quantitative cultures of sputum or BAL. With quantitation, long-term trends in the microbial population structure may become apparent. For instance, in a patient with both S. aureus and P. aeruginosa, one organism may begin to dominate.
Using this cohort, we determined the sequence of lower respiratory infections, the duration of infections, and the effect of incident infections on the presence and abundance of baseline pathogen. We hypothesized that P. aeruginosa would dominate and ultimately replace S. aureus in quantitative cultures from individuals with CF within one decade of follow-up.
Methods
Ethics Statement
The institutional review board of the University of Iowa approved this study (# 201905718). A waiver of informed consent was granted owing to the study being minimal in risk.
Study Design
This is a longitudinal, retrospective, single-center cohort study of adult and pediatric patients with CF who had chronic sputum production. The primary aim was to determine whether S. aureus prevalence would decrease over time in individuals with CF, coincident with pathogen replacement by P. aeruginosa. In examining the prevalence of respiratory microorganisms by age cohort in the Cystic Fibrosis Foundation (CFF) annual report (5), we noted an approximately 10% decrease in S. aureus prevalence with each decade. We estimated that 130 individuals, observed for a period of 10 years, would be required to detect this 10% reduction in S. aureus prevalence with α = 0.05 and power = 0.80 using a 1-sided McNemar’s exact test. Secondary endpoints included quantitative changes in sputum density of S. aureus and P. aeruginosa.
Inclusion and Exclusion Criteria
We included patients with CF who received care at the University of Iowa between 2004 and 2017. All subjects had diagnostic sweat chloride measurements or two CFTR mutations. For longitudinal analysis, we required each subject have at least 10 quantitative respiratory cultures of the lower respiratory tract (e.g., sputum or BAL). We excluded patients with CFTR-related metabolic syndrome. We excluded cultures that did not result in quantitation and cultures obtained after lung transplantation. In addition to microbiology data, we obtained electronic prescription data to determine whether infections were treated. Electronic prescriptions were available after 2009.
Definitions of MRSA and Methicillin-Sensitive S. aureus
We defined MRSA if it was explicitly named in the report, if there was phenotypic resistance to oxacillin or cefoxitin, or if there was molecular detection of PBP2a. We defined methicillin-sensitive S. aureus (MSSA) if it was declared on the report or if the S. aureus failed to meet above definitions of MRSA. A minority of S. aureus isolates could not be classified.
Definitions of Prevalence
Pathogen prevalence was the number of subjects with a culture positive for a given organism divided by the number of subjects cultured during the same calendar year. To determine microbial prevalence by age cohorts defined in the CFF annual report (5), we calculated the age of each subject as of the last day of the calendar year. We combined age <2 and ages 2–6 into a single group owing to few observations. Within each age category, we divided the number of subject-years that S. aureus or P. aeruginosa were present on quantitative culture by the number of subject-years a quantitative culture was obtained.
Duration and Persistence of Infections
The duration of infection was the time difference (in years) between the initial and final positive culture. The persistence of an organism was the percentage of quantitative cultures that were positive after the initial positive culture. The persistence was not calculated if an organism was only identified on the final culture for an individual. Similarly, we determined the time difference between the first and last cultures that were simultaneously positive for both S. aureus and P. aeruginosa (referred to as double-positive cultures). The persistence of coinfections was the percentage of cultures that were double-positive in between the initial and final double-positive cultures. For individuals with a single double-positive culture, the duration of coinfection was zero. If there were no intervening cultures between the first and last double-positive culture, the persistence of coinfection was not calculated.
Sequence of Appearance and Disappearance of Bacterial Species
For individuals with infections by multiple organisms, we calculated the time difference between the debut cultures of MSSA, MRSA, Haemophilus influenzae, and P. aeruginosa. To determine whether the appearance of a competitor species was temporally associated with disappearance of a preexisting species, we calculated the time difference between the last appearance of each organism versus the debut of a potential competitor.
Changes in Abundance of Baseline Infections following Incident Infection with Competing Species
In patients who were infected by both S. aureus and P. aeruginosa, we determined which infection was established first by identifying the calendar years that each organism debuted on quantitative culture. We monitored the abundance of each organism from 3 years before to 5 years after the appearance of the opposite species. Subjects whose first S. aureus and P. aeruginosa occurred in the same year were analyzed separately. To control for the potential overrepresentation of subjects having more follow-up cultures owing to more severe disease, we calculated the mean log10 cfu/ml of the established organisms on an annual basis. Because organism abundance for both species was not normally distributed, we used Spearman’s correlation to test for a relationship between culture density and time following detection of the opposite species.
Longitudinal Changes in Annual Culture Status
We determined positivity for S. aureus and P. aeruginosa for each year a subject had at least one quantitative culture. Owing to a decrease in the number of quantitative cultures in 2017 because of changes in center practice, we compared organism prevalence between each subject’s initial year of observation and the final year up to the year 2016 using exact McNemar’s test.
Statistics and Data Analysis
We used RStudio version 1.2.5001 (RStudio, Inc.) and SAS version 9.4 (SAS Institute) for data analysis and statistical tests. For nonnormal distributions, we used nonparametric statistical tests. We reported central tendency and variation with median and interquartile range. Box plots were made using R default settings.
Data Availability
Deidentified microbiologic data and source codes corresponding to results presented in this article will be made available upon publication and for up to 3 years after final publication. Written requests for data should be made by e-mail to the corresponding author. Data will be transferred contingent upon completion of a data transfer agreement.
Results
Subjects and Sources of Quantitative Cultures
To measure replacement of S. aureus by P. aeruginosa, we identified subjects with CF who had quantitative respiratory cultures. Between January 1, 2004, and December 31, 2017, our center cared for 337 patients with CF. The standard practice included quantitative sputum cultures (see online supplement 1 and 2). One hundred thirty-four patients had at least 10 quantitative cultures of either sputum or BAL. These patients were similar to the remainder of the center in terms of sex and genotype but were older and had more associated complications (Table 1). Patients with 10 quantitative cultures routinely produced sputum, had lower FEV1, and had higher P. aeruginosa prevalence (see online supplement 3–5).
Table 1.
Longitudinal Cohort of Subjects with CF Analyzed from January 1, 2004, to December 31, 2017
| Patients without Quantitative Cultures (Total = 66) | Patients with 1–9 Quantitative Cultures (Total = 137) | Patients with ≥10 Quantitative Cultures (Total = 134) | |
|---|---|---|---|
| Sex and genotypes, n (%) | |||
| Sex, F | 31 (47.0) | 68 (49.6) | 61 (45.5) |
| F508del/F508del | 32 (48.5) | 65 (47.4) | 79 (59.0) |
| F508del/other | 26 (39.4) | 57 (41.6) | 47 (35.1) |
| Age, yr, median (IQR) | |||
| Birth year | 2007 (1992–2013) | 1999 (1986–2007) | 1986 (1976–1997) |
| At first culture* | 3.40 (0.30–19.10) | 8.84 (0.31–23.72) | 19.34 (8.08–28.48) |
| At first quantitative culture | — | 11.21 (2.23–24.33) | 19.66 (8.69–28.48) |
| Follow-up duration, median (IQR) | |||
| Years between first and last cultures* | 1.25 (0.52–3.68) | 5.48 (2.66–9.72) | 11.98 (7.94–13.57) |
| Years between first and last quantitative cultures | — | 2.08 (0.00–4.47) | 10.15 (6.73–11.84) |
| Number of cultures* | 7 (3–14) | 14 (5–28) | 35 (26–41) |
| Number of quantitative cultures | — | 2 (1–5) | 25 (17–33) |
| Final clinical status, n (%) | |||
| Lung transplant | 0 (0) | 7 (5.1) | 24 (17.9) |
| Death without transplant | 1 (1.5) | 7 (5.1) | 14 (10.4) |
| Alive, no transplant, and cultured after January 1, 2016 | 53 (80.3) | 92 (67.2) | 92 (68.7) |
| Other (e.g., transfer of care) | 12 (18.2) | 31 (22.6) | 4 (3.0) |
| Culture results, median (IQR)* | |||
| Any S. aureus | 50 (75.8) | 120 (87.6) | 126 (94.0) |
| MSSA | 46 (69.7) | 100 (73.0) | 111 (82.8) |
| MRSA | 5 (7.6) | 64 (46.7) | 79 (59.0) |
| P. aeruginosa | 34 (51.5) | 101 (73.7) | 128 (95.5) |
| Either S. aureus or P. aeruginosa | 59 (89.4) | 133 (97.1) | 134 (100) |
| Both S. aureus and P. aeruginosa | 25 (37.9) | 88 (64.2) | 120 (89.6) |
| Simultaneous S. aureus and P. aeruginosa | 21 (31.8) | 71 (51.8) | 114 (85.1) |
Definition of abbreviations: CF = cystic fibrosis; IQR = interquartile range; MRSA = methicillin-resistant S. aureus; MSSA = methicillin-sensitive S. aureus; P. aeruginosa = Pseudomonas aeruginosa; S. aureus = Staphylococcus aureus.
Includes nonquantitative CF respiratory culture studies such as oropharyngeal swab in addition to quantitative cultures. All cultures obtained after lung transplant were excluded.
For patients with 10 quantitative cultures, their median starting age was 19.66 years, near the age cohort at which P. aeruginosa surpasses S. aureus in prevalence (5). The median follow-up was 10.15 years and 25 quantitative cultures. We used this longitudinal group to determine the duration and sequence of infections and to ascertain how quickly S. aureus is replaced by P. aeruginosa.
Prevalence and Abundance of S. aureus and P. aeruginosa in Quantitative Respiratory Cultures
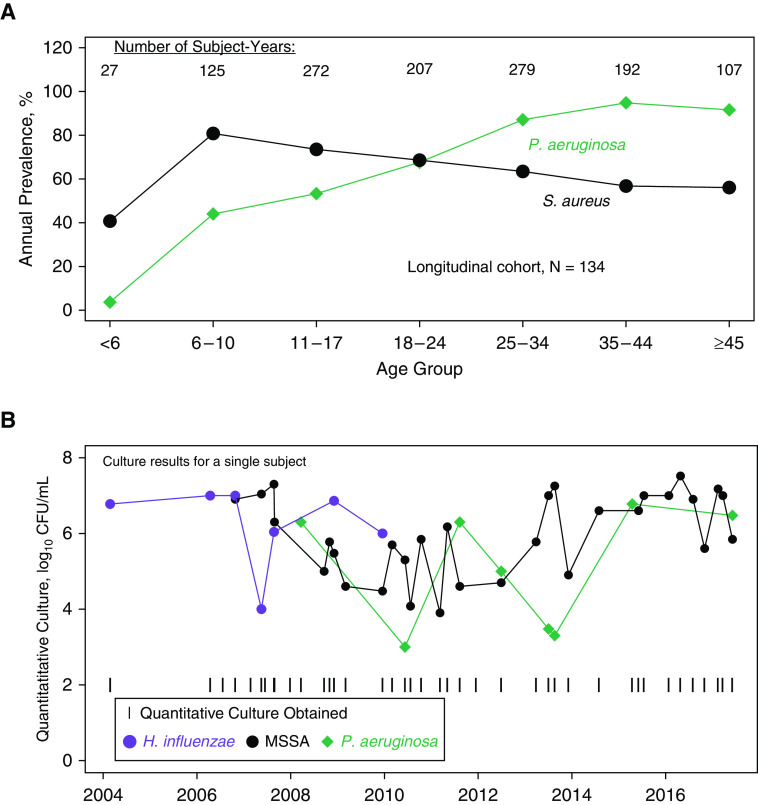
Most patients with quantitative respiratory cultures had at least one culture positive for S. aureus or P. aeruginosa (Table 1). Consistent with the CFF registry (5), S. aureus was more prevalent in younger patients and P. aeruginosa was more prevalent in older patients (Figure 1A). Although this suggested that P. aeruginosa might replace S. aureus over time, when we examined individual patients longitudinally (Figure 1B), we observed that some individuals had sustained and abundant loads of both S. aureus and P. aeruginosa, often for several years.
Figure 1.
(A) Average annual prevalence of Pseudomonas aeruginosa and Staphylococcus aureus between 2004 and 2017, stratified by age cohorts (in years) defined in the Cystic Fibrosis Foundation Annual Report. Data are from subjects who had at least 10 quantitative cultures (N = 134). Consistent with registry-wide data from the same time interval, P. aeruginosa prevalence is greater than S. aureus prevalence among adult patients. (B) Quantitative culture results for a representative single subject. H. influenzae was present early. MSSA and P. aeruginosa were subsequently identified and remained stable for nearly 10 years. H. influenzae = Haemophilus influenzae; MSSA = methicillin-sensitive Staphylococcus aureus.
To systematically compare the abundance of common CF pathogens, we determined their culture density in cfu/ml (Table 2). As expected, bacteria were significantly more abundant than fungi. Most bacteria, including P. aeruginosa and S. aureus, had median culture density of more than 106 cfu/ml.
Table 2.
Microorganism Abundance by Species
| Organism | log10 cfu/ml [Median (IQR)] | Number of Studies* (Total = 3,522) |
|---|---|---|
| P. aeruginosa | 6.7 (5.9–7.0) | 2,267 |
| S. aureus† | 6.5 (5.3–7.0) | 2,046 |
| MRSA | 6.6 (5.7–7.0) | 1,084 |
| MSSA | 6.3 (5.0–7.0) | 972 |
| H. influenzae | 6.3 (5.5–7.0) | 209 |
| Other bacteria‡ | 6.5 (5.8–7.0) | 1,803 |
| Fungus | 3.5 (3.0–4.0) | 99 |
Definition of abbreviations: CF = cystic fibrosis; H. influenzae = Haemophilus influenzae; IQR = interquartile range; MRSA = methicillin-resistant S. aureus; MSSA = methicillin-sensitive S. aureus; P. aeruginosa = Pseudomonas aeruginosa; S. aureus = Staphylococcus aureus.
Subjects with CF who had at least one quantitative culture positive for the indicated species and quantified in cfu/ml. All subjects had at least 10 quantitative culture studies between January 1, 2004, and December 31, 2017.
Some S. aureus cultures could not be classified as either MRSA or MSSA. Occasionally, MRSA and MSSA were present on the same culture.
Sum of all bacteria other than P. aeruginosa, S. aureus, and H. influenzae.
Simultaneous Abundance of S. aureus and P. aeruginosa
We considered the possibility that P. aeruginosa would dominate S. aureus when both were present. One hundred seven patients had simultaneous growth of S. aureus and P. aeruginosa. From these patients, we identified 1,195 double-positive cultures. The median density of P. aeruginosa (in log10 cfu/ml) was 6.52 and S. aureus was 6.42 (Figure 2A). Although the difference was statistically significant by Wilcoxon signed-rank test, we did not consider the absolute difference in bacterial abundance to be biologically significant. To determine whether some of the cultures display dominance of one pathogen, we compared the abundance of P. aeruginosa and S. aureus by scatter plot (Figure 2B). We found that some double-positive cultures had dominance of either S. aureus or P. aeruginosa. However, most cultures had high density of both species, and few cultures had low abundance of both pathogens. Our findings were similar when we analyzed MRSA and MSSA cultures separately (see online supplement 6).
Figure 2.
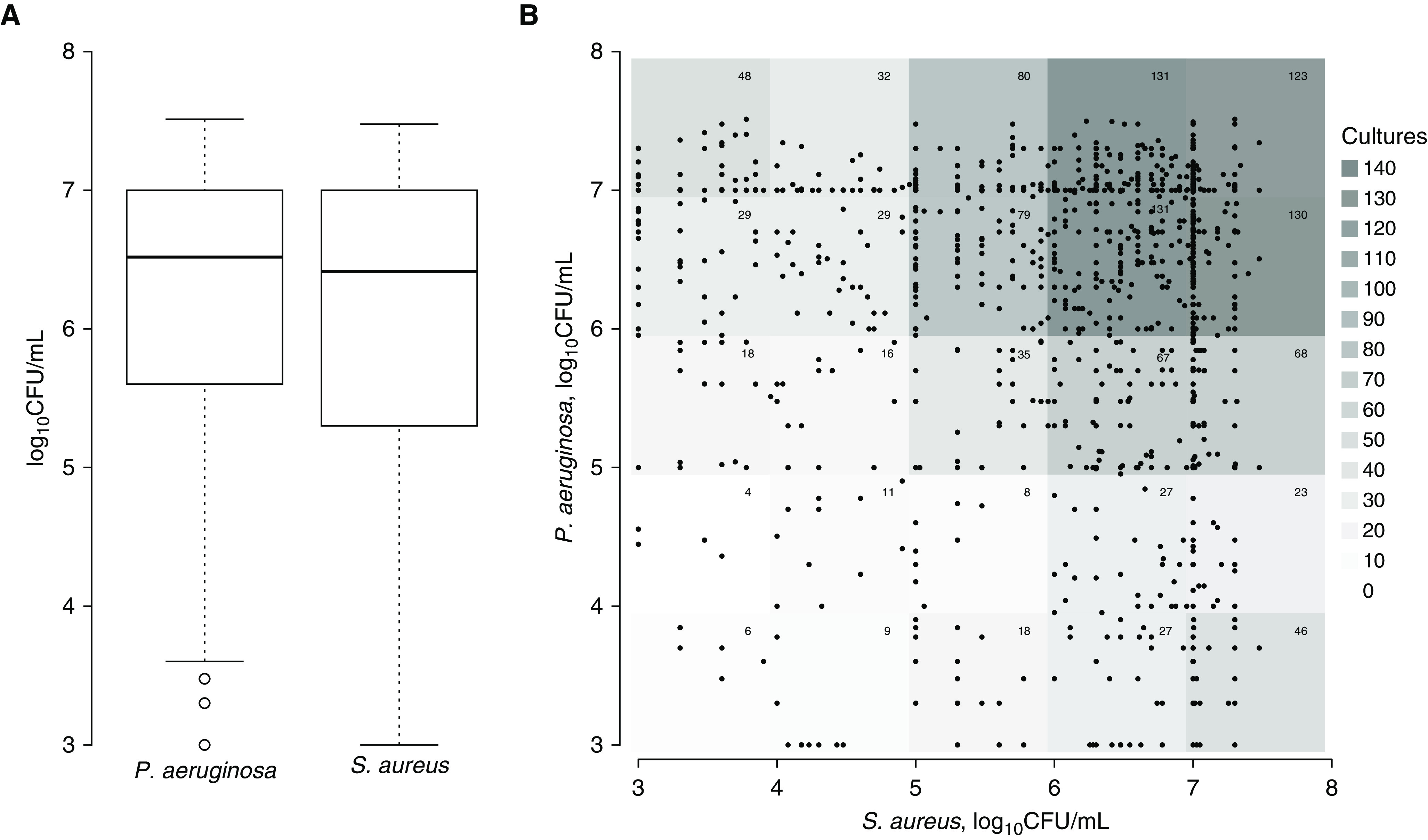
(A) Abundance of Pseudomonas aeruginosa and Staphylococcus aureus in patients with simultaneous positive cultures. P. aeruginosa was slightly more abundant than S. aureus (medians, 6.52 vs. 6.42; P = 0.02 by Wilcoxon signed-rank test), but the difference may be biologically insignificant. (B) Scatter diagram displaying abundance of S. aureus and P. aeruginosa in double-positive cultures. Because many points are superimposed, the number of cultures within a 1-log range of titer are indicated at the top right corner of each log unit and graphically depicted by the background shading. Some double-positive cultures had dominant P. aeruginosa, whereas others had dominant S. aureus. Few had low abundance of both organisms. There was weak negative correlation between S. aureus and P. aeruginosa abundance (Spearman rho = −0.067, P = 0.02). n = 107 patients, 1,195 double-positive cultures.
Duration and Persistence of Infections by Bacterial Pathogen
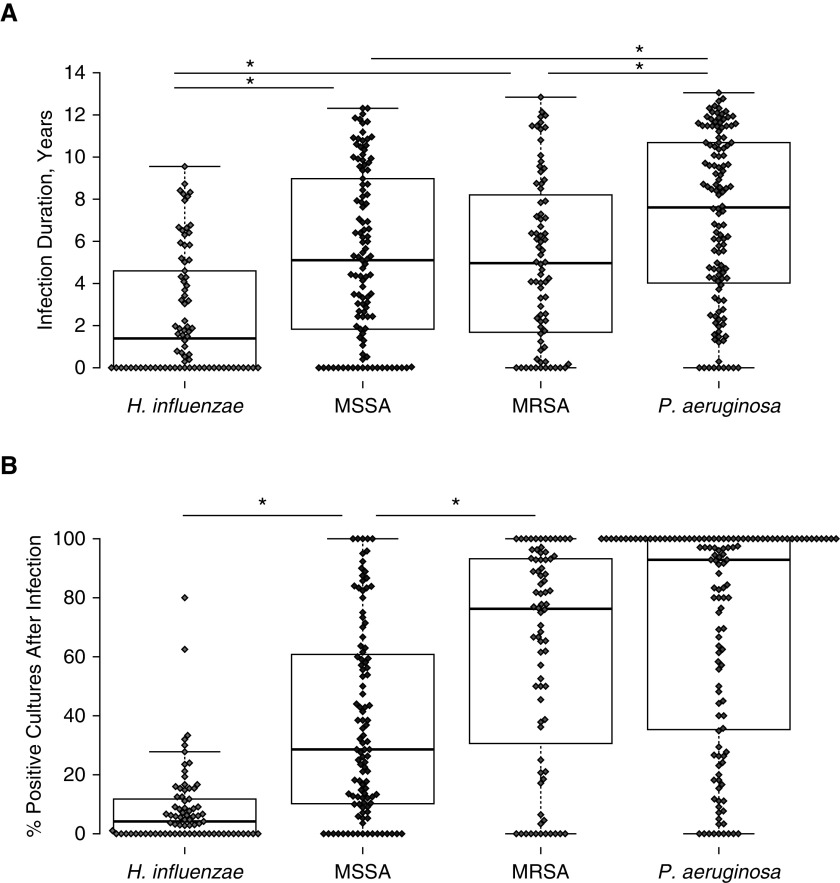
A hallmark of CF pathogens is their ability to establish chronic bacterial infections of the airways. Using our longitudinal cohort, we measured the chronicity of bacterial infections by two methods: duration and persistence. We defined duration as the time from initial to final positive culture. Because the duration of infection does not represent how frequently the pathogen was cultured and could be biased by how late the organism appeared during the observation period, we defined persistence as the percentage of cultures that were positive following an initial positive culture for that organism.
We compared the duration and persistence of the benchmark pathogen P. aeruginosa with MRSA, MSSA, and H. influenzae (Figure 3). P. aeruginosa infections had the longest duration (median 7.6 yr) and the greatest persistence (median of 92.9%). By contrast, H. influenzae infections were brief (median 1.4 yr) and had low persistence. S. aureus was between these two extremes, with a median duration of approximately 5 years. The persistence of S. aureus infections was associated with methicillin resistance; 28.6% of subsequent cultures were positive for MSSA after incident infection, whereas 76.2% of subsequent cultures were positive for MRSA.
Figure 3.
(A) Duration of infections within individual patients between January 1, 2004, and December 31, 2017. Dots represent the time (in years) from the first positive culture to the last positive culture for the organisms listed at bottom. N = 134 subjects; 78 Haemophilus influenzae, 109 methicillin-sensitive Staphylococcus aureus (MSSA), 76 methicillin-resistant Staphylococcus aureus (MRSA), 124 Pseudomonas aeruginosa. (B) Persistence of bacterial species after initial positive infection. Dots represent percentage of cultures that are positive for the organism listed after the first positive culture. Dots are not displayed if the subject’s only positive culture for an organism was the final culture. N = 134 subjects; 77 H. influenzae, 107 MSSA, 76 MRSA, 124 P. aeruginosa. *P < 0.05 by Wilcoxon signed-rank test.
Sequence of Bacterial Appearance and Disappearance
H. influenzae and S. aureus are often considered early CF pathogens and P. aeruginosa a later pathogen. This sequence could occur by multiple mechanisms. Early S. aureus infections could be replaced by P. aeruginosa, or chronic P. aeruginosa infections may represent a barrier to acquisition of new S. aureus infections. Because of this, we hypothesized that P. aeruginosa would generally appear later than S. aureus. We calculated the sequence in which four bacterial species appeared, including H. influenzae, MSSA, MRSA, and P. aeruginosa. We measured the time of the first documented infection with each pathogen following the appearance of a reference “early” pathogen (see online supplement 7). In our cohort, the sequence of pathogen appearance varied by individual. Some patients had P. aeruginosa for several years before they cultured MSSA, whereas others had MSSA for years before P. aeruginosa. MRSA, an emerging pathogen of the early 2000s, appeared later than all other pathogens examined.
We predicted that pathogen replacement events would be temporally linked to the appearance of a competitor species. To identify these replacement events, we measured the time each organism last appeared relative to the debut of a potential competitor (see online supplement 8). The only example of this temporal association was the disappearance of H. influenzae approximately 1 year after the appearance of MRSA. However, because H. influenzae displays the weakest persistence, this temporal relationship could be coincidental. S. aureus, often assumed to be replaced by P. aeruginosa, was identified at least 5 years after the first culture of P. aeruginosa. These data either could be consistent with long-term coinfections with S. aureus and P. aeruginosa or might indicate that P. aeruginosa infections do not prevent S. aureus acquisition or reinfection.
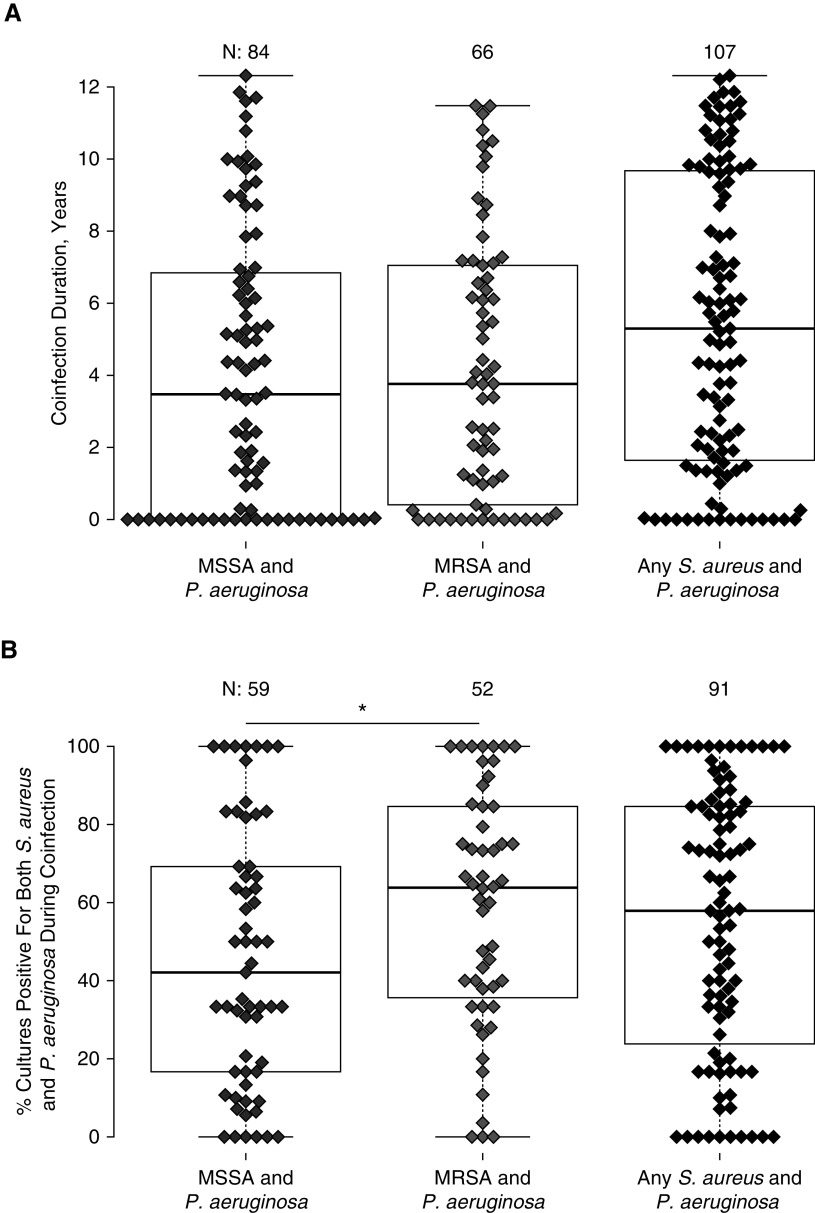
Duration of S. aureus/P. aeruginosa Coinfections
We measured the duration of S. aureus/P. aeruginosa coinfections. One hundred seven patients from the longitudinal cohort had at least one double-positive culture. When we examined MSSA and MRSA infections separately, the median duration of coinfection was between 3 and 4 years (Figure 4). Because MRSA appeared later in time, this could underestimate the duration of MRSA infections. Because 43 subjects had both MRSA and MSSA, the median duration of coinfection with P. aeruginosa and either phenotype of S. aureus was 5.3 years. MRSA was more likely to appear consistently in quantitative respiratory cultures than MSSA (Figure 4B).
Figure 4.
(A) Duration of positivity for Staphylococcus aureus and Pseudomonas aeruginosa between January 1, 2004, and December 31, 2017. Dots represent time from initial to final cultures that were simultaneously positive for Pseudomonas aeruginosa and methicillin-sensitive Staphylococcus aureus (MSSA), methicillin-resistant Staphylococcus aureus (MRSA), or either phenotype of S. aureus. The number of individuals per group is shown at top. Forty-three individuals had both MSSA and MRSA during the study. (B) Percentage of cultures that were double-positive for S. aureus and P. aeruginosa among subjects with ≥2 double-positive cultures. Dots represent the percentage of cultures between the first and last double-positive culture that were simultaneously positive for P. aeruginosa and the subtype of S. aureus listed at bottom. The number of individuals per group is given at the top. Initial and final cultures were excluded from this statistic, as they are double-positive by definition. Subjects having only two double-positive cultures obtained consecutively were excluded. MRSA had greater persistence compared with MSSA. *P < 0.05 by Wilcoxon signed-rank test.
Changes in Abundance of Baseline Pathogens following Incident Infection with a Potential Competing Species
We hypothesized that S. aureus abundance would decrease following appearance of P. aeruginosa. We examined 42 subjects with baseline S. aureus who developed P. aeruginosa infection in a later calendar year. To control for the potential bias of oversampling from patients with more frequent follow up, we calculated an annualized average of culture density. We measured S. aureus abundance for up to 3 years before and 5 years after P. aeruginosa infection (Figures 5A and 5B). From Year 0 to Year 5, the average S. aureus titer was stable. We performed similar analysis for P. aeruginosa. Twenty-nine patients had baseline P. aeruginosa and developed S. aureus infection in a later calendar year. Among these patients, the P. aeruginosa culture density was stable following S. aureus infection. Among patients who first had S. aureus and P. aeruginosa in the same year, both species were stable in the following years (see online supplement 9). Titers tended to be higher for MRSA than MSSA in patients coinfected with P. aeruginosa (see online supplement 10).
Figure 5.
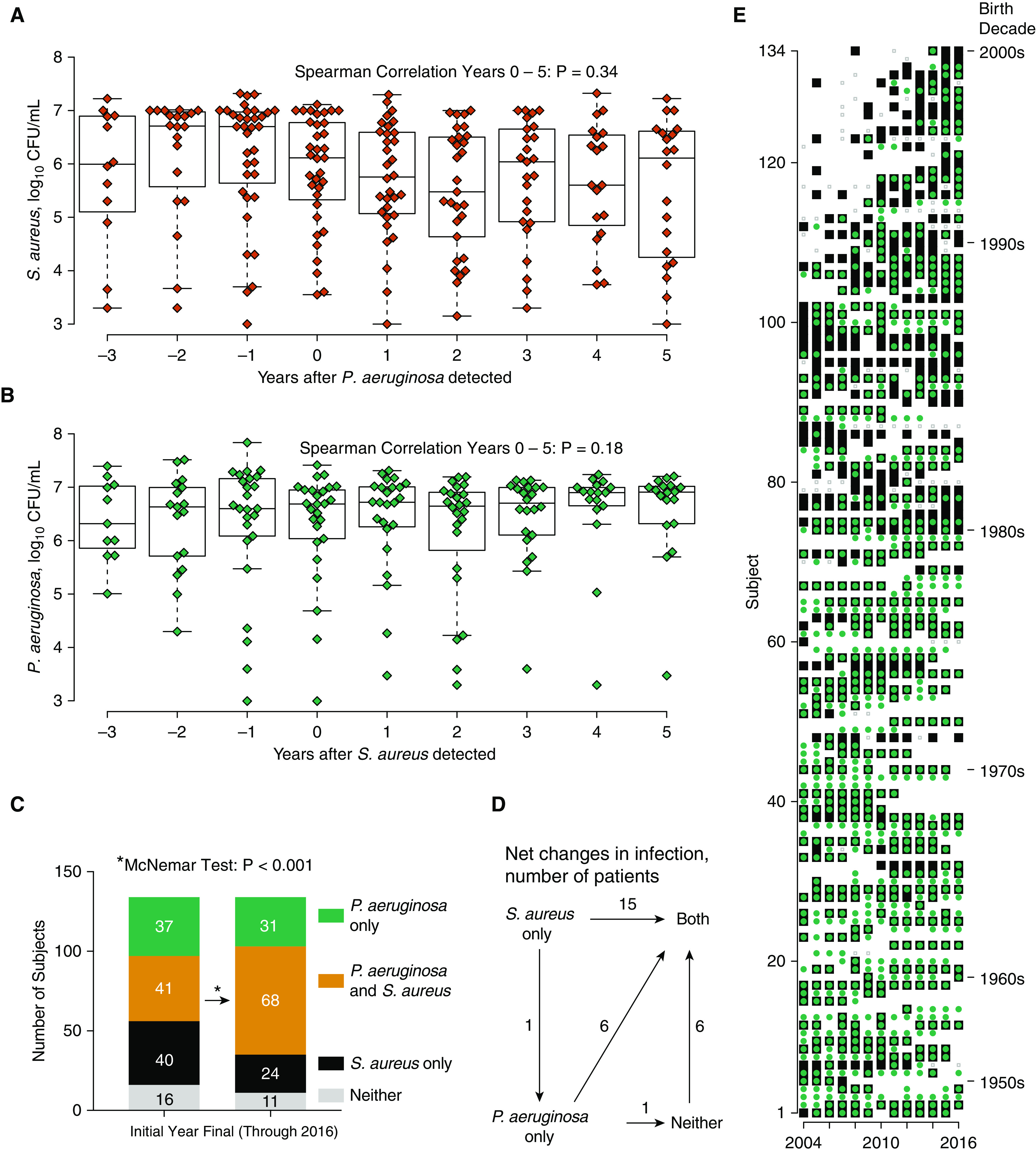
(A) Changes in Staphylococcus aureus abundance following incident Pseudomonas aeruginosa infection. Data show annualized mean culture density of S. aureus (on log10 scale) for 42 patients who had S. aureus before P. aeruginosa. For years 0–5, Spearman rho = −0.075, P = 0.34. (B) Changes in P. aeruginosa abundance following incident S. aureus infection. Data represent mean density of P. aeruginosa for 29 patients who had P. aeruginosa before S. aureus. For Years 0–5, Spearman rho = 0.12, P = 0.18. (C) Annual prevalence of S. aureus, P. aeruginosa, and dual infection between each subject’s initial and final year up to 2016. A statistically significant increase in dual infection was detected. Neither organism decreased in prevalence when examined individually. (D) Data represent net changes in infection status for individuals between their first and final year up to 2016. Transition from S. aureus to P. aeruginosa was rare and was less common in this cohort than the transition from either organism alone to dual infection. (E) Longitudinal trends in S. aureus and P. aeruginosa positivity for the cystic fibrosis cohort. Each row is a subject, displayed in order by year of birth. The youngest individual within a birth decade is indicated at the right. The x-axis shows years in which a subject had a quantitative culture. Symbols represent positivity for S. aureus (black squares), P. aeruginosa (green circles), dual infection (green circle inside a black square), or absence of both (gray dots).
Trends in Positivity for S. aureus and P. aeruginosa within Individuals
Because P. aeruginosa is thought to replace S. aureus in the CF airway, we followed individual patients over time to see if their annual culture positivity for P. aeruginosa increased and S. aureus decreased. There was inadequate coverage in 2017 because of a shift in clinical practice, with discontinuation of quantitative cultures (see online supplement 2 and 11). Therefore, we compared the initial culture year to the last culture year through 2016 for 134 individuals with longitudinal culture results, Figure 5C. In this longitudinal analysis, S. aureus showed a trend toward increasing (from 60.4% to 68.7%). There was a statistically significant increase in P. aeruginosa (from 58.2% to 73.9%). Coinfections with S. aureus and P. aeruginosa were common and rose substantially over time, from 30.6% to 50.7% (Table 3). In summary, the net change in infection state for the cohort was in the direction of coinfection, rather than replacement of S. aureus by P. aeruginosa (Figure 5D). Although we observed some reversions from coinfected status back to S. aureus or P. aeruginosa only, these were relatively less common (see online supplement 12). Replacement of S. aureus by P. aeruginosa occurred in two individuals, whereas S. aureus replaced P. aeruginosa in one individual. The annual infection state for each member of the cohort is depicted graphically in Figure 5E. Both pathogens were persistently identified in individuals with CF.
Table 3.
Longitudinal Culture Positivity for S. aureus and P. aeruginosa
| Organism | Initial Year [n (%)] | Final through 2016 [n (%)] | OR (95% CI)* | P Value† |
|---|---|---|---|---|
| S. aureus | 81 (60.4) | 92 (68.7) | 1.5 (0.87–2.57) | 0.17 |
| P. aeruginosa | 78 (58.2) | 99 (73.9) | 4.5 (1.86–10.9) | 0.0003 |
| S. aureus and P. aeruginosa | 41 (30.6) | 68 (50.7) | 2.6 (1.48–4.52) | 0.0007 |
Definition of abbreviations: CI = confidence interval; OR = odds ratio; P. aeruginosa = Pseudomonas aeruginosa; S. aureus = Staphylococcus aureus.
Odds ratio using Mantel-Haenszel method and 95% CI.
P value calculated by Exact McNemar’s test, based on N = 134 individuals.
Treatment of S. aureus and P. aeruginosa
A potential explanation for sustained growth of S. aureus in people with CF would be a medical decision to forgo antistaphylococcal treatment. Therefore, we obtained electronic prescription records following implementation of Epic in 2009. We found widespread prescription of antibiotics directed against both S. aureus and P. aeruginosa (see online supplement 13 and 14). Thus, both organisms demonstrate sustained persistence despite intensive antimicrobial therapy.
Discussion
In this longitudinal CF cohort study, we report trends in quantitative microbial burden with extensive follow-up. We found that both S. aureus and P. aeruginosa are abundant and persistent in the CF airway. Compared with H. influenzae, S. aureus has similar abundance on quantitative cultures but persists longer within individuals. Although S. aureus is commonly considered an early pathogen relative to P. aeruginosa, the sequence of infections is variable. Baseline infections with either species persist for years following the appearance of the opposite species, and both organisms are abundant in coinfected patients. Contrary to common assumption, we rarely observed replacement of S. aureus by P. aeruginosa within individuals, despite an average follow-up time of 10 years. We found a trend toward accumulation of both species over time, despite apparent attempts to treat both pathogens.
Comparison with Previous Studies
There is growing recognition of the prevalence of S. aureus and P. aeruginosa coinfection in CF (4). Molecular analysis of these bacteria shows that the same pulsed-field types can remain for years (20, 21). Our data show that these organisms are also stable in terms of quantitative abundance. The abundance of either S. aureus or P. aeruginosa predicts low microbial diversity and neutrophilic inflammation in early CF lung disease (22). Coinfection with these organisms may be consequential for patients (23–29). Adults with S. aureus and P. aeruginosa experience worse outcomes than those with S. aureus alone (27). MRSA is recognized as a risk factor for poorer CF outcomes (3, 30, 31). Compared with MSSA, MRSA is associated with worse outcomes in combination with P. aeruginosa (25). Our data show that MRSA infections were more persistent than MSSA, suggesting a potential mechanism for this difference.
The recent development of CFTR modulator therapies raises hope that these drugs could correct the underlying host defense defects in CF, thereby preventing or clearing chronic pulmonary infections. However, epidemiologic studies show that chronic bacterial infections persist in patients with CF despite CFTR modulator therapy (32–35). This indicates a continued need to determine mechanisms of bacterial persistence.
Advantages of This Study
Our study addresses unanswered questions about coinfections with S. aureus and P. aeruginosa. It was not clear from previous literature which of these two species dominates in coinfections, how long coinfections last, how often these organisms are isolated from the same cultures, and whether one organism is eventually replaced. Our study focused on sputum and BAL because S. aureus can be found in the oropharynx of healthy children without CF (19). Although sputum is expectorated through the mouth, multiple studies show that sputum has greater sensitivity and specificity for predicting endobronchial pathogens versus oropharyngeal swabs (36–38). Thus, sputum is considered by some to be a “credible surrogate” for BAL (39). Moreover, quantitation allows us to determine the abundance of both organisms over time.
Limitations
Our findings should be interpreted in the context of the high local MRSA prevalence, which may impair S. aureus eradication. Future studies involving larger cohorts with lower MRSA could reveal S. aureus attrition. Our focus on quantitative cultures limits this study to patients with chronic sputum production. Thus, we cannot describe potential interactions between S. aureus and P. aeruginosa in younger patients or the sequence of infections that occur in the first years after birth. The dynamic range of our quantitative cultures may not capture the most or least abundant infections. However, unlike molecular metagenomic techniques, quantitative cultures are relatively easier to interpret. We did not correlate coinfection with clinical outcomes such as FEV1, as these comparisons have been made by others. We also did not calculate the changes in pathogen abundance following treatment with antibiotics or CFTR modulators. Finally, our database did not include information about infections with small colony variant S. aureus, which are associated with antibiotic resistance and poorer outcomes (26).
Conclusions
S. aureus and P. aeruginosa establish stable, long-term coinfections in people with CF. Both organisms are abundant in sputum and BAL. New strategies are needed to prevent and treat infections by these CF pathogens.
Acknowledgments
Acknowledgment
The authors thank Harry S. Porterfield and Benjamin Doyle for assistance. They also thank Joseph Zabner, George O’Toole, and Michael Welsh for providing critical review.
Footnotes
Supported by NHLBI K08 HL136927 (A.J.F.); the Cystic Fibrosis Foundation (CFF): CFF FISCHE16I0 (A.J.F.), LIMOLI18F5 (D.H.L.), and LIMOLI19R3 (D.H.L.); and the CFF Research and Development Program, Iowa (D.A.S.).
Author Contributions: Designed study: A.J.F. Collected data: A.J.F., M.M.L., L.J.M., Z.E.K., and T.A.P. Analyzed data: A.J.F., S.B.S., L.J.M., D.A.S., and D.H.L. Wrote initial draft: A.J.F. Edited paper: A.J.F., S.B.S., M.M.L., T.A.P., D.A.S., and D.H.L.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202004-1322OC on August 4, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Sly PD, Gangell CL, Chen L, Ware RS, Ranganathan S, Mott LS, et al. AREST CF Investigators. Risk factors for bronchiectasis in children with cystic fibrosis. N Engl J Med. 2013;368:1963–1970. doi: 10.1056/NEJMoa1301725. [DOI] [PubMed] [Google Scholar]
- 2.Caudri D, Turkovic L, Ng J, de Klerk NH, Rosenow T, Hall GL, et al. The association between Staphylococcus aureus and subsequent bronchiectasis in children with cystic fibrosis. J Cyst Fibros. 2018;17:462–469. doi: 10.1016/j.jcf.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Dasenbrook EC, Checkley W, Merlo CA, Konstan MW, Lechtzin N, Boyle MP. Association between respiratory tract methicillin-resistant Staphylococcus aureus and survival in cystic fibrosis. JAMA. 2010;303:2386–2392. doi: 10.1001/jama.2010.791. [DOI] [PubMed] [Google Scholar]
- 4.Salsgiver EL, Fink AK, Knapp EA, LiPuma JJ, Olivier KN, Marshall BC, et al. Changing epidemiology of the respiratory bacteriology of patients with cystic fibrosis. Chest. 2016;149:390–400. doi: 10.1378/chest.15-0676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cystic Fibrosis Foundation. Patient registry annual data report. 2016 [accessed 2020 Nov 6]. Available from: https://www.cff.org/research/researcher-resources/patient-registry/2016-patient-registry-annual-data-report.pdf. [Google Scholar]
- 6.Barnabie PM, Whiteley M. Iron-mediated control of Pseudomonas aeruginosa-Staphylococcus aureus interactions in the cystic fibrosis lung. J Bacteriol. 2015;197:2250–2251. doi: 10.1128/JB.00303-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lyczak JB, Cannon CL, Pier GB. Lung infections associated with cystic fibrosis. Clin Microbiol Rev. 2002;15:194–222. doi: 10.1128/CMR.15.2.194-222.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen AT, Oglesby-Sherrouse AG. Interactions between Pseudomonas aeruginosa and Staphylococcus aureus during co-cultivations and polymicrobial infections. Appl Microbiol Biotechnol. 2016;100:6141–6148. doi: 10.1007/s00253-016-7596-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pallett R, Leslie LJ, Lambert PA, Milic I, Devitt A, Marshall LJ. Anaerobiosis influences virulence properties of Pseudomonas aeruginosa cystic fibrosis isolates and the interaction with Staphylococcus aureus. Sci Rep. 2019;9:6748. doi: 10.1038/s41598-019-42952-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beaudoin T, Yau YCW, Stapleton PJ, Gong Y, Wang PW, Guttman DS, et al. Staphylococcus aureus interaction with Pseudomonas aeruginosa biofilm enhances tobramycin resistance. NPJ Biofilms Microbiomes. 2017;3:25. doi: 10.1038/s41522-017-0035-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Brien S, Fothergill JL. The role of multispecies social interactions in shaping Pseudomonas aeruginosa pathogenicity in the cystic fibrosis lung. FEMS Microbiol Lett. 2017;364:fnx128. doi: 10.1093/femsle/fnx128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Filkins LM, Graber JA, Olson DG, Dolben EL, Lynd LR, Bhuju S, et al. Coculture of Staphylococcus aureus with Pseudomonas aeruginosa drives S. aureus towards fermentative metabolism and reduced viability in a cystic fibrosis model. J Bacteriol. 2015;197:2252–2264. doi: 10.1128/JB.00059-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Limoli DH, Warren EA, Yarrington KD, Donegan NP, Cheung AL, O’Toole GA. Interspecies interactions induce exploratory motility in Pseudomonas aeruginosa. Elife. 2019;8:e47365. doi: 10.7554/eLife.47365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loening-Baucke VA, Mischler E, Myers MG. A placebo-controlled trial of cephalexin therapy in the ambulatory management of patients with cystic fibrosis. J Pediatr. 1979;95:630–637. doi: 10.1016/s0022-3476(79)80785-4. [DOI] [PubMed] [Google Scholar]
- 15.Ramsey BW, Pepe MS, Quan JM, Otto KL, Montgomery AB, Williams-Warren J, et al. Cystic Fibrosis Inhaled Tobramycin Study Group. Intermittent administration of inhaled tobramycin in patients with cystic fibrosis. N Engl J Med. 1999;340:23–30. doi: 10.1056/NEJM199901073400104. [DOI] [PubMed] [Google Scholar]
- 16.Gibson RL, Emerson J, McNamara S, Burns JL, Rosenfeld M, Yunker A, et al. Cystic Fibrosis Therapeutics Development Network Study Group. Significant microbiological effect of inhaled tobramycin in young children with cystic fibrosis. Am J Respir Crit Care Med. 2003;167:841–849. doi: 10.1164/rccm.200208-855OC. [DOI] [PubMed] [Google Scholar]
- 17.Tiddens HAWM, De Boeck K, Clancy JP, Fayon M, Arets HGM, Bresnik M, et al. Open label study of inhaled aztreonam for Pseudomonas eradication in children with cystic fibrosis: the ALPINE study. J Cyst Fibros. 2015;14:111–119. doi: 10.1016/j.jcf.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 18.Dezube R, Jennings MT, Rykiel M, Diener-West M, Boyle MP, Chmiel JF, et al. Eradication of persistent methicillin-resistant Staphylococcus aureus infection in cystic fibrosis. J Cyst Fibros. 2019;18:357–363. doi: 10.1016/j.jcf.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 19.Rosenfeld M, Bernardo-Ocampo C, Emerson J, Genatossio A, Burns J, Gibson R. Prevalence of cystic fibrosis pathogens in the oropharynx of healthy children and implications for cystic fibrosis care. J Cyst Fibros. 2012;11:456–457. doi: 10.1016/j.jcf.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 20.Kahl BC, Duebbers A, Lubritz G, Haeberle J, Koch HG, Ritzerfeld B, et al. Population dynamics of persistent Staphylococcus aureus isolated from the airways of cystic fibrosis patients during a 6-year prospective study. J Clin Microbiol. 2003;41:4424–4427. doi: 10.1128/JCM.41.9.4424-4427.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Warren AE, Boulianne-Larsen CM, Chandler CB, Chiotti K, Kroll E, Miller SR, et al. Genotypic and phenotypic variation in Pseudomonas aeruginosa reveals signatures of secondary infection and mutator activity in certain cystic fibrosis patients with chronic lung infections. Infect Immun. 2011;79:4802–4818. doi: 10.1128/IAI.05282-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jorth P, Ehsan Z, Rezayat A, Caldwell E, Pope C, Brewington JJ, et al. Direct lung sampling indicates that established pathogens dominate early infections in children with cystic fibrosis. Cell Rep. 2019;27:1190–1204, e3. doi: 10.1016/j.celrep.2019.03.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Limoli DH, Yang J, Khansaheb MK, Helfman B, Peng L, Stecenko AA, et al. Staphylococcus aureus and Pseudomonas aeruginosa co-infection is associated with cystic fibrosis-related diabetes and poor clinical outcomes. Eur J Clin Microbiol Infect Dis. 2016;35:947–953. doi: 10.1007/s10096-016-2621-0. [DOI] [PubMed] [Google Scholar]
- 24.Sagel SD, Gibson RL, Emerson J, McNamara S, Burns JL, Wagener JS, et al. Inhaled Tobramycin in Young Children Study Group; Cystic Fibrosis Foundation Therapeutics Development Network. Impact of Pseudomonas and Staphylococcus infection on inflammation and clinical status in young children with cystic fibrosis. J Pediatr. 2009;154:183–188. doi: 10.1016/j.jpeds.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hubert D, Reglier-Poupet H, Sermet-Gaudelus I, Ferroni A, Le Bourgeois M, Burgel PR, et al. Association between Staphylococcus aureus alone or combined with Pseudomonas aeruginosa and the clinical condition of patients with cystic fibrosis. J Cyst Fibros. 2013;12:497–503. doi: 10.1016/j.jcf.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 26.Wolter DJ, Emerson JC, McNamara S, Buccat AM, Qin X, Cochrane E, et al. Staphylococcus aureus small-colony variants are independently associated with worse lung disease in children with cystic fibrosis. Clin Infect Dis. 2013;57:384–391. doi: 10.1093/cid/cit270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahlgren HG, Benedetti A, Landry JS, Bernier J, Matouk E, Radzioch D, et al. Clinical outcomes associated with Staphylococcus aureus and Pseudomonas aeruginosa airway infections in adult cystic fibrosis patients. BMC Pulm Med. 2015;15:67. doi: 10.1186/s12890-015-0062-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maliniak ML, Stecenko AA, McCarty NA. A longitudinal analysis of chronic MRSA and Pseudomonas aeruginosa co-infection in cystic fibrosis: a single-center study. J Cyst Fibros. 2016;15:350–356. doi: 10.1016/j.jcf.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 29.Schwerdt M, Neumann C, Schwartbeck B, Kampmeier S, Herzog S, Görlich D, et al. Staphylococcus aureus in the airways of cystic fibrosis patients - a retrospective long-term study. Int J Med Microbiol. 2018;308:631–639. doi: 10.1016/j.ijmm.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 30.Dasenbrook EC, Merlo CA, Diener-West M, Lechtzin N, Boyle MP. Persistent methicillin-resistant Staphylococcus aureus and rate of FEV1 decline in cystic fibrosis. Am J Respir Crit Care Med. 2008;178:814–821. doi: 10.1164/rccm.200802-327OC. [DOI] [PubMed] [Google Scholar]
- 31.Ren CL, Morgan WJ, Konstan MW, Schechter MS, Wagener JS, Fisher KA, et al. Investigators and Coordinators of the Epidemiologic Study of Cystic Fibrosis. Presence of methicillin resistant Staphylococcus aureus in respiratory cultures from cystic fibrosis patients is associated with lower lung function. Pediatr Pulmonol. 2007;42:513–518. doi: 10.1002/ppul.20604. [DOI] [PubMed] [Google Scholar]
- 32.Harris JK, Wagner BD, Zemanick ET, Robertson CE, Stevens MJ, Heltshe SL, et al. Changes in airway microbiome and inflammation with ivacaftor treatment in patients with cystic fibrosis and the G551D mutation. Ann Am Thorac Soc. 2020;17:212–220. doi: 10.1513/AnnalsATS.201907-493OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hisert KB, Heltshe SL, Pope C, Jorth P, Wu X, Edwards RM, et al. Restoring cystic fibrosis transmembrane conductance regulator function reduces airway bacteria and inflammation in people with cystic fibrosis and chronic lung infections. Am J Respir Crit Care Med. 2017;195:1617–1628. doi: 10.1164/rccm.201609-1954OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heltshe SL, Mayer-Hamblett N, Burns JL, Khan U, Baines A, Ramsey BW, et al. GOAL (the G551D Observation-AL) Investigators of the Cystic Fibrosis Foundation Therapeutics Development Network. Pseudomonas aeruginosa in cystic fibrosis patients with G551D-CFTR treated with ivacaftor. Clin Infect Dis. 2015;60:703–712. doi: 10.1093/cid/ciu944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singh SB, McLearn-Montz AJ, Milavetz F, Gates LK, Fox C, Murry LT, et al. Pathogen acquisition in patients with cystic fibrosis receiving ivacaftor or lumacaftor/ivacaftor. Pediatr Pulmonol. 2019;54:1200–1208. doi: 10.1002/ppul.24341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramsey BW, Wentz KR, Smith AL, Richardson M, Williams-Warren J, Hedges DL, et al. Predictive value of oropharyngeal cultures for identifying lower airway bacteria in cystic fibrosis patients. Am Rev Respir Dis. 1991;144:331–337. doi: 10.1164/ajrccm/144.2.331. [DOI] [PubMed] [Google Scholar]
- 37.Rosenfeld M, Emerson J, Accurso F, Armstrong D, Castile R, Grimwood K, et al. Diagnostic accuracy of oropharyngeal cultures in infants and young children with cystic fibrosis. Pediatr Pulmonol. 1999;28:321–328. doi: 10.1002/(sici)1099-0496(199911)28:5<321::aid-ppul3>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 38.Eyns H, Piérard D, De Wachter E, Eeckhout L, Vaes P, Malfroot A. Respiratory bacterial culture sampling in expectorating and non-expectorating patients with cystic fibrosis. Front Pediatr. 2018;6:403. doi: 10.3389/fped.2018.00403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ronchetti K, Tame JD, Paisey C, Thia LP, Doull I, Howe R, et al. The CF-Sputum Induction Trial (CF-SpIT) to assess lower airway bacterial sampling in young children with cystic fibrosis: a prospective internally controlled interventional trial. Lancet Respir Med. 2018;6:461–471. doi: 10.1016/S2213-2600(18)30171-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Deidentified microbiologic data and source codes corresponding to results presented in this article will be made available upon publication and for up to 3 years after final publication. Written requests for data should be made by e-mail to the corresponding author. Data will be transferred contingent upon completion of a data transfer agreement.