One of the main challenges in the management of patients with asthma is the variability of associated symptoms and airflow limitation over time. Graduation of different severities of the disease still refers to treatment intensity of a regularly applied controller mediation and the related treatment efficacy (1, 2). However, the stepwise treatment approach is not a one-way street and includes regular reassessment of symptoms, exacerbations, side-effects, lung function, and patient satisfaction, consequently encouraging physicians to “step down” to find the lowest possible treatment intensity appropriate to achieve asthma control (1).
Four new monoclonal antibodies have recently been introduced as add-on therapies for patients that have uncontrolled asthma under high-dose inhaled corticosteroid (ICS) therapy together with a second controller medication, usually a long-acting β2-agonist (LABA) (3). Carefully administered to the right selection of patients, these drugs demonstrate a reduction in annualized exacerbation frequency and the need for continuous oral steroids (3–5). In patients clinically benefiting from such antibody therapies, reports that would clearly support disease-modifying potentials of these medications are missing, and it may still be too early for studies that would support the safe discontinuation of an applied antibody therapy after a certain period of successful add-on treatment.
Escalation on the one hand and deescalation or discontinuation of asthma treatment on the other not only address questions of disease severity but also the concept of disease remission. In pediatric asthma care, this concept is widely accepted, and responsible physicians are trained to carefully reevaluate their patients with respect to the general presence or absence of asthma symptoms and whether diagnostic asthma criteria are still met (6). With regard to puberty in particular, boys are likely to lose their symptoms and go into a phase of disease remission, whereas girls tend to experience new-onset disease during this period (Figure 1) (6). In adult patient care, reevaluation of diagnostic criteria is less common. In a carefully conducted study by Aaron and colleagues (7), investigators tried to reestablish asthma diagnosis in a population-based sample of ∼17,000 Canadian households and finally included a sample of 613 participants with asthma that had been physician diagnosed within the previous 5 years. Among the 410 participants in whom the diagnosis could eventually be confirmed, only 86 patients (22.5%) demonstrated guideline-concordant reversibility of airflow limitation at the first study visit; others subsequently had their ICS/LABA controller medication tapered and finally discontinued and were challenged with methacholine up to five times in the upcoming 12 months to at least once meet lung function diagnostic criteria for asthma. Among those 203 participants that repeatedly did not fulfill diagnostic criteria for asthma within the study period, a considerably large proportion of 16% was found to have documented evidence of variable airflow limitation or bronchial hyperresponsiveness within the previous 5 years.
Figure 1.
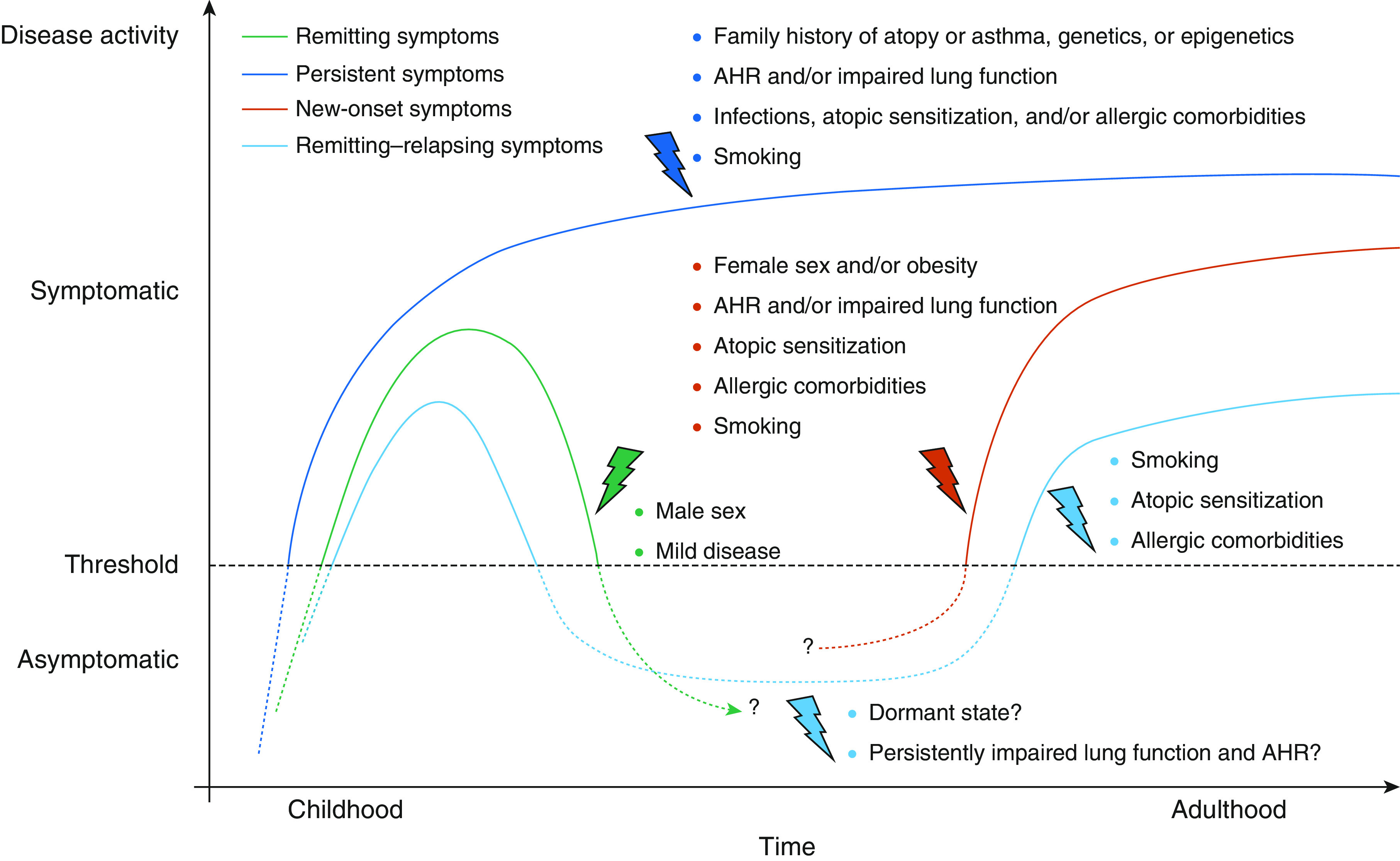
Longitudinal asthma cohort studies have identified genetic background, family history of asthma and atopy, infections early in life, allergic disease, and lung function as risk factors for persistent symptoms. In children and adolescents, male sex is one of the factors associated with remission of symptoms, while in adulthood female sex is associated with new-onset disease. The archetypical phenotype of persistent asthma that starts in childhood and is permanently present throughout life represents only a minority of patients with asthma. Reprinted with permission from Reference 6. AHR = airway hyperresponsiveness.
In this issue of the Journal, Psallidas and colleagues (pp. 296–306) present a study for which they, first of all, have to be congratulated (8). Their study design was high risk and strived for nothing less than a paradigm shift in asthma treatment. Although the efficacy results for the investigated inhaled TLR9 (Toll-like receptor 9) agonist AZD1419 have to be considered negative, the general conclusions of the study are noteworthy and may help to better understand asthma care delivery and asthma clinal trial design—and they therefore might be kept in mind in a sense of “fail early, fail fast, but always fail forward.”
TLR9 recognizes the CpG DNA oligonucleotide motifs typical of bacterial and viral DNA, and its activation leads to production of type I IFN and induction of type 1 (T1)-associated immune responses (9). Investigators of the present study hypothesized that a once-weekly inhaled application of the TLR9 agonist AZD1419 in patients with features indicative of T2 airway inflammation might experience a “rebalancing” of their T2/T1-associated immune responses toward environmental allergens, which they assumed to translate into an effective asthma treatment. Patients were preselected according to the presence of blood eosinophilia (>250/μl prior to inclusion and >150/μl at the screening visit) and were otherwise thought to have stable, persistent asthma that was treated with mostly moderate doses of ICS together with LABA. The most striking—and risky—feature of the study design was the updosing of the study drug in the first 12 weeks of the trial and subsequent tapering of 1) LABA and 2) ICS, followed by 40 weeks of observation, thereby striving to replace the daily inhaled ICS/LABA controller therapy for a weekly inhaled TLR9 agonist monotherapy. Consequently, the primary endpoint was chosen to be the time to loss of asthma control as defined by symptoms, exacerbations, lung function, or physician assessment. Investigators assumed that at Week 52, only 20% of asthmatics in the placebo group, which goes along with a total absence of any controller medication for more than 40 weeks, remained controlled, whereas at least 60% in the treatment arm of the study were supposed to have controlled asthma. Although the performance of AZD1419 clearly remained below the expectations with respect to asthma control, a surprising proportion of 40% of patients in the placebo arm did not experience any signs of uncontrolled asthma and might be considered to be in a (temporary) state of “disease remission.” In those subjects losing asthma control, the time point of time to loss of asthma control was preceded by increasing levels of fractional exhaled nitric oxide as early as 20 days in advance, potentially confirming previous studies using fractional exhaled nitric oxide to guide asthma therapy (10, 11).
So, did AZD1419 fail as a drug? First, a proportion of 40% of patients currently not requiring any asthma medication in the placebo group might be too large to detect any differences and indicates that disease remission in asthma might be an issue that is larger than expected and potentially interferes with clinical trial design. Second, the patients studied were 57 years old on average, with an average age of asthma diagnosis at 43 years. There were slightly more females (55% AZD1419 vs. 58% placebo) in the study, and most patients were overweight. All these factors combined might resemble a distinct asthma phenotype (i.e., female, obese, late onset) that has been identified in several cluster analyses (12, 13). Therefore, the negative results of this study might not readily be transferable to other asthma phenotypes such as the “classic” childhood-onset, atopic phenotype with one or more allergic comorbidities, in whom airway hyperresponsiveness to airborne particles might be the driving pathological feature. Maybe physicians taking take of children with asthma might reconsider the potentials of TLR9 agonists in their respective setting. For physicians taking care of adult patients with asthma, the study by Psallidas and colleagues has delivered many noteworthy insights with respect to disease remission and safe deescalation of established inhaled controller medications but no new drug.
Footnotes
Originally Published in Press as DOI: 10.1164/rccm.202008-3161ED on October 8, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Global Initiative for Asthma. Global strategy for asthma management and prevention. 2020 [accessed 2020 Aug 9]. Available from: https://ginasthma.org/wp-content/uploads/2020/04/GINA-2020-full-report_-final-_wms.pdf.
- 2.Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343–373. doi: 10.1183/09031936.00202013. [Published erratum appears in Eur Respir J 43:1216.] [DOI] [PubMed] [Google Scholar]
- 3.McGregor MC, Krings JG, Nair P, Castro M. Role of biologics in asthma. Am J Respir Crit Care Med. 2019;199:433–445. doi: 10.1164/rccm.201810-1944CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Israel E, Reddel HK. Severe and difficult-to-treat asthma in adults. N Engl J Med. 2017;377:965–976. doi: 10.1056/NEJMra1608969. [DOI] [PubMed] [Google Scholar]
- 5.Holguin F, Cardet JC, Chung KF, Diver S, Ferreira DS, Fitzpatrick A, et al. Management of severe asthma: a European Respiratory Society/American Thoracic Society guideline. Eur Respir J. 2020;55:1900588. doi: 10.1183/13993003.00588-2019. [DOI] [PubMed] [Google Scholar]
- 6.Fuchs O, Bahmer T, Rabe KF, von Mutius E. Asthma transition from childhood into adulthood. Lancet Respir Med. 2017;5:224–234. doi: 10.1016/S2213-2600(16)30187-4. [DOI] [PubMed] [Google Scholar]
- 7.Aaron SD, Vandemheen KL, FitzGerald JM, Ainslie M, Gupta S, Lemière C, et al. Canadian Respiratory Research Network. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA. 2017;317:269–279. doi: 10.1001/jama.2016.19627. [DOI] [PubMed] [Google Scholar]
- 8.Psallidas I, Backer V, Kuna P, Palmér R, Necander S, Aurell M, et al. A phase 2a, double-blind, placebo-controlled randomized trial of inhaled TLR9 agonist AZD1419 in asthma. Am J Respir Crit Care Med. 2021;203:296–306. doi: 10.1164/rccm.202001-0133OC. [DOI] [PubMed] [Google Scholar]
- 9.Huang X, Yang Y. Targeting the TLR9-MyD88 pathway in the regulation of adaptive immune responses. Expert Opin Ther Targets. 2010;14:787–796. doi: 10.1517/14728222.2010.501333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, et al. American Thoracic Society Committee on Interpretation of Exhaled Nitric Oxide Levels (FENO) for Clinical Applications. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184:602–615. doi: 10.1164/rccm.9120-11ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petsky HL, Cates CJ, Kew KM, Chang AB. Tailoring asthma treatment on eosinophilic markers (exhaled nitric oxide or sputum eosinophils): a systematic review and meta-analysis. Thorax. 2018;73:1110–1119. doi: 10.1136/thoraxjnl-2018-211540. [DOI] [PubMed] [Google Scholar]
- 12.Haldar P, Pavord ID, Shaw DE, Berry MA, Thomas M, Brightling CE, et al. Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med. 2008;178:218–224. doi: 10.1164/rccm.200711-1754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moore WC, Meyers DA, Wenzel SE, Teague WG, Li H, Li X, et al. National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med. 2010;181:315–323. doi: 10.1164/rccm.200906-0896OC. [DOI] [PMC free article] [PubMed] [Google Scholar]


