To the Editor:
The global coronavirus disease (COVID-19) pandemic has seen a significant increase in the use of preprint services to enable the widespread dissemination of research findings (1). However, whether such facilities influence practice change is currently unknown. Here, we describe the impact of the preprint release and eventual publication of the RECOVERY (Randomised Evaluation of COVID-19 Therapy) trial (2) on corticosteroid use in clinical practice in Australian ICUs. The RECOVERY trial tested the efficacy of dexamethasone (6 mg by mouth or intravenously for up to 10 d) in hospitalized patients with clinically suspected or confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and demonstrated a reduction in 28-day mortality, particularly in those receiving either mechanical ventilation or oxygen. Before these results, the Australian and New Zealand Intensive Care Society COVID-19 guidelines recommended against the routine use of corticosteroids (3).
SPRINT-SARI (Short Period Incidence Study of Severe Acute Respiratory Infection) Australia is a multicenter, prospective, observational database, with nearly complete coverage of all patients with laboratory-confirmed COVID-19 admitted to the ICU. Seventy-eight (n = 78) sites are participating, most of which are tertiary or metropolitan public hospitals, across all states and territories in Australia. Fifty-three (n = 53) contributed data to this analysis, including 15 sites with over 20 ICU beds.
A preprint of the RECOVERY trial results was released on June 22, 2020 (4). The final paper was published on July 17, 2020 (2), without any major changes. We compared corticosteroid use in adult patients in the SPRINT-SARI Australia database before preprint, after preprint, and after publication. The percentage of patients receiving corticosteroids per week was calculated as the number of patients receiving this therapy during their hospital stay (any dose or type and for any duration or indication) divided by the total number of patients with COVID-19 admitted to the ICU that week. Differences in proportions, with 95% confidence intervals (95% CIs), are provided. A Fisher exact test was used for comparisons across periods. All analyses were performed in R version 4.0.2 (R Foundation for Statistical Computing).
A total of 461 patients with confirmed COVID-19 were included, from February 27 to November 11, 2020. Baseline characteristics, additional therapies, and clinical outcomes in each time period are shown in Table 1. Most of the patients were male, were overweight, and presented to the hospital with fever, cough, and shortness of breath. Just over half were 60 years of age or older. Mechanical ventilation was used in 37.7% of patients on Day 1 and in 55.3% of patients at any point during the ICU stay. Overall in-hospital mortality was 13.2%. Although illness severity at admission was similar across patients, those admitted in the period before the preprint release were older, more often males, and leaner; received high-flow nasal cannula treatment, noninvasive ventilation, or remdesevir less often; and received hydroxychloroquine more often. The duration of mechanical ventilation and ICU and hospital length of stay were longer in the preprint period, albeit ICU and hospital mortality were similar.
Table 1.
Demographic, Illness Severity, Treatment, and Outcome Characteristics in SPRINT-SARI Australia before and after Preprint Release of the RECOVERY Trial
| Overall (n = 461) | Before Preprint (n = 204) | After Preprint* (n = 257) | P Value† | ||
|---|---|---|---|---|---|
| Age, yr | 61.0 (51.0–70.0) | 63.5 (53.8–72.0) | 58.0 (50.0–68.0) | 0.002 | |
| <60 | 215 (46.6) | 78 (38.2) | 137 (53.3) | 0.005 | |
| 60–69 | 119 (25.8) | 56 (27.5) | 63 (24.5) | ||
| 70–79 | 105 (22.8) | 60 (29.4) | 45 (17.5) | ||
| >80 | 22 (4.8) | 10 (4.9) | 12 (4.7) | ||
| Sex, M, n (%) | 295 (64.0) | 141 (69.1) | 154 (59.9) | 0.052 | |
| APACHE II | 14.0 (10.0–18.0) | 14.0 (10.0–18.0) | 14.0 (10.0–18.0) | 0.970 | |
| Days between hospital and ICU admission | 0.4 (0.1–2.0) | 0.4 (0.1–2.6) | 0.3 (0.1–1.5) | 0.210 | |
| Days of symptoms at hospital admission | 6.2 (3.7–9.2) | 6.0 (3.4–9.0) | 7.0 (4.1–9.3) | 0.177 | |
| Body mass index, kg/m2 | 29.7 (25.6–34.7) | 28.8 (24.6–32.2) | 30.5 (26.7–35.7) | 0.001 | |
| Coexisting disorders, n (%) | |||||
| Diabetes | 136 (30.8) | 56 (27.5) | 80 (33.6) | 0.195 | |
| Obesity | 119 (27.0) | 51 (25.0) | 68 (28.7) | 0.445 | |
| Use of ACEi or ARB | 91 (20.6) | 51 (25.1) | 40 (16.8) | 0.042 | |
| Chronic cardiac failure | 66 (14.9) | 40 (19.6) | 26 (10.9) | 0.016 | |
| Asthma | 60 (13.6) | 22 (10.8) | 38 (16.0) | 0.148 | |
| Smoker | 55 (12.5) | 27 (13.2) | 28 (11.9) | 0.773 | |
| Chronic pulmonary disease‡ | 33 (7.5) | 16 (7.8) | 17 (7.1) | 0.922 | |
| Symptoms, n (%) | |||||
| Fever | 338 (78.2) | 165 (85.1) | 173 (72.7) | 0.003 | |
| Cough | 308 (71.3) | 152 (78.4) | 156 (65.5) | 0.005 | |
| Shortness of breath | 318 (73.6) | 132 (68.0) | 186 (78.2) | 0.024 | |
| Respiratory support at ICU admission, n (%) | |||||
| No support | 41 (9.8) | 26 (14.1) | 15 (6.4) | <0.001 | |
| Low-flow oxygen | 68 (16.2) | 39 (21.2) | 29 (12.3) | ||
| HFNC and/or NIV | 152 (36.3) | 39 (21.2) | 113 (48.1) | ||
| Invasive mechanical ventilation | 158 (37.7) | 80 (43.5) | 78 (33.2) | ||
| Interventions, n (%) | |||||
| Drugs | |||||
| Antibiotics | 392 (91.2) | 176 (91.2) | 216 (91.1) | 0.999 | |
| Steroids | 288 (66.1) | 57 (29.5) | 231 (95.1) | <0.001 | |
| Hydroxychloroquine | 37 (8.6) | 35 (18.3) | 2 (0.8) | <0.001 | |
| Oseltamivir | 1 (0.2) | 1 (0.5) | 0 (0.0) | 0.913 | |
| Lopinavir–ritonavir | 13 (3.0) | 9 (4.7) | 4 (1.7) | 0.125 | |
| Remdesevir | 134 (29.1) | 2 (1.0) | 132 (51.4) | <0.001 | |
| Organ support§ | |||||
| Mechanical ventilation | 247 (55.3) | 119 (58.3) | 128 (52.7) | 0.270 | |
| Inotropic or vasopressor | 224 (52.6) | 111 (57.2) | 113 (48.7) | 0.098 | |
| Neuromuscular blocking agent | 169 (39.5) | 86 (44.3) | 83 (35.5) | 0.077 | |
| HFNC | 261 (60.7) | 83 (42.8) | 178 (75.4) | <0.001 | |
| Prone positioning | 146 (34.2) | 56 (28.9) | 90 (38.6) | 0.044 | |
| Renal replacement therapy | 45 (10.5) | 25 (12.9) | 20 (8.6) | 0.199 | |
| NIV | 52 (12.2) | 14 (7.2) | 38 (16.4) | 0.006 | |
| Other cardiac procedures | 22 (5.1) | 12 (6.2) | 10 (4.3) | 0.502 | |
| Tracheostomy | 33 (7.7) | 13 (6.7) | 20 (8.6) | 0.587 | |
| Inhaled nitric oxide | 27 (6.3) | 13 (6.7) | 14 (6.0) | 0.917 | |
| Extracorporeal membrane oxygenation | 12 (2.8) | 1 (0.5) | 11 (4.7) | 0.021 | |
| Clinical outcomes | |||||
| Duration of ventilation, d | 10.0 (5.0–16.0) | 12.0 (7.0–14.0) | 8.0 (4.0–17.0) | 0.020 | |
| ICU length of stay, d | 6.7 (2.8–15.6) | 8.0 (3.1–18.0) | 6.0 (2.5–11.6) | 0.006 | |
| Truncated at extraction, d | 6.8 (2.8–15.8) | 8.0 (3.1–18.0) | 6.0 (2.6–12.7) | 0.015 | |
| Hospital length of stay, d | 15.1 (8.6–25.6) | 17.2 (8.8–30.2) | 14.3 (8.6–21.4) | 0.029 | |
| Truncated at extraction, d | 15.1 (8.9–26.7) | 17.3 (8.9–30.5) | 14.8 (8.9–23.1) | 0.114 | |
| ICU mortality, n (%) | 57 (12.4) | 30 (14.7) | 27 (10.5) | 0.223 | |
| Hospital mortality, n (%) | 61 (13.2) | 30 (14.7) | 31 (12.1) | 0.488 |
Definition of abbreviations: ACEi = angiotensin-converting enzyme inhibitor; APACHE = Acute Physiology and Chronic Health Evaluation; ARB = angiotensin II receptor blocker; COVID-19 = coronavirus disease; HFNC = high-flow nasal cannula; NIV = noninvasive ventilation; RECOVERY = Randomised Evaluation of COVID-19 Therapy; SPRINT-SARI = Short Period Incidence Study of Severe Acute Respiratory Infection.
Data are shown as the median (quartile 25% to quartile 75%) or n (%). Percentages may not total 100 because of rounding.
Including the period after final publication
P values from Wilcoxon rank-sum test for continuous variables and Fisher exact test for categorical variables.
Not considering asthma.
Assessed daily until ICU discharge.
There was an increase in the cumulative number, and in the proportion, of patients receiving corticosteroids after the release of the preprint (Figure 1). For the period prior, corticosteroids were used in 29.5% of patients, which increased to 92.0% after the preprint release (absolute difference, 62.5% [95% CI, 51.3% to 73.6%]; P < 0.001). After formal publication of the trial results, 95.8% of patients received corticosteroids, a proportion similar to that observed in the preprint period (absolute difference, 3.8% [95% CI, −5.4% to 13.1%]; P = 0.275). Based on the degree of respiratory support at ICU admission, corticosteroid use increased from 4.0% to 66.7%, 7.7% to 100.0%, 35.9% to 100.0%, and 48.6% to 93.1% after the preprint release in those requiring no support, low-flow oxygen, high-flow nasal cannula administration or noninvasive ventilation, and mechanical ventilation, respectively. Corticosteroid use in the post-preprint period increased from 45.2% to 95.8% in centers with 10 or fewer ICU beds, from 28.9% to 88.9% in centers with 11 to 20 beds, and from 25.8% to 96.4% in centers with more than 20 beds.
Figure 1.
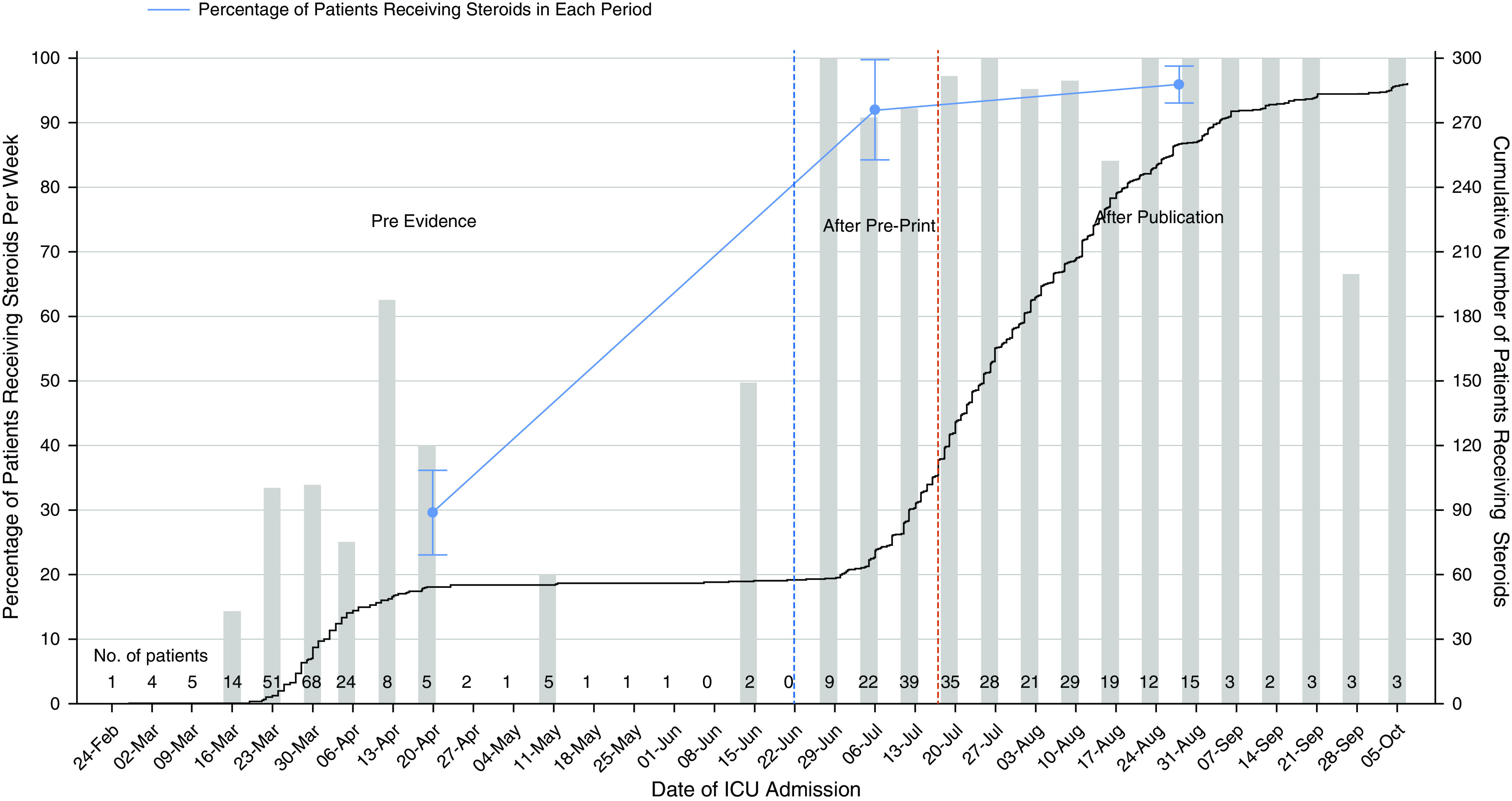
Patients receiving corticosteroids over time in Australian ICUs. The black line represents the cumulative number of patients receiving corticosteroids over time. The blue line represents the percentage (95% confidence interval) of patients receiving corticosteroids before any evidence was available (earlier than June 22, 2020), after the preprint release (between June 22, 2020, and July 17, 2020), and after the peer-reviewed publication (after July 17, 2020). The gray bars are the percentage of patients receiving corticosteroids per week. The number of patients with coronavirus disease (COVID-19) admitted to the ICU each week is reported along the x-axis. The total number of COVID-19 cases impacts the cumulative count in the post-preprint and postpublication time periods and should be taken into consideration when interpreting this figure. Pre = before preprint release.
To our knowledge, this is the first report to quantify the impact of a preprint release on clinical practice across an entire country. More specifically, the RECOVERY preprint findings were widely adopted by ICU clinicians in Australia, leading to significant practice change, with minimal further modification after eventual peer-reviewed publication. It is interesting to speculate whether this was in addition influenced by the widespread availability of this intervention, clinical familiarity, and minimal cost implications. Indeed, although corticosteroids have been extensively studied in critically ill populations (resulting in a well-established safety profile) (5), such rapid adoption of other higher-risk interventions remains a significant cause for concern (6).
It is important to emphasize that many factors are likely to have encouraged the rapid translation of the RECOVERY results into practice in Australia, beyond simply the preprint availability of the data. Indeed, the unique situation of a global viral pandemic, coupled with the eagerness of clinicians to use therapies for COVID-19 and the size and quality of the RECOVERY trial, are all likely to have hastened this process. To more thoroughly quantify the impact of preprint release, a more detailed assessment, including within other fields of research, is required, but this is beyond the scope of the current report.
We acknowledge the following limitations. First, the type, dose, and duration of corticosteroid therapy were not captured as part of SPRINT-SARI Australia. Second, a detailed description of corticosteroid use in other locations (e.g., the emergency department or general ward) is not possible, although the median duration of hospitalization before ICU admission was 0.4 days (Table 1). Finally, the indication for corticosteroid therapy was not captured, and the subgroup receiving no respiratory support at ICU admission was small (n = 15, Table 1), which limits our ability to assess whether the RECOVERY findings were “overgeneralized.”
In conclusion, preprint release of the RECOVERY trial findings led to an almost immediate dramatic change in corticosteroid use in critically ill patients with COVID-19 across Australia. Although the rapid translation of medical evidence is beneficial in cases in which the intervention is of proven benefit, preprint release of trial findings before peer review creates a risk of inappropriate global translation. Fortunately, in this scenario, independent findings from other research groups identified a similar result (7), and potentially thousands of critically ill patients with COVID-19 were advantaged globally through the rapid dissemination of these results.
Footnotes
Supported by the Department of Health, Australia (standing deed SON60002733 for SPRINT-SARI [Short Period Incidence Study of Severe Acute Respiratory Infection] Australia).
Author Contributions: A.J.C.B., A.S.N., T.T., T.B., C.F., and A.A.U. were involved in study design, data acquisition, statistical analysis, and manuscript preparations.
Originally Published in Press as DOI: 10.1164/rccm.202009-3661LE on December 3, 2020
Author disclosures are available with the text of this letter at www.atsjournals.org.
Contributor Information
Collaborators: for the SPRINT-SARI Australia Investigators, Adam Visser, Adrian Mattke, Adrian Regli, Alan Rashid, Alexis Tabah, Alison Walker, Allen Cheng, Amanda Corley, Andrew Udy, Anil Ramnani, (Isabel) Anne Leditschke, Anthony Eidan, Bart DeKeulenaer, Baumik Mevavala, Ben Mulholland, Benjamin Reddi, Brent Richards, Cameron Knott, Cara Moore, Carmel Delzoppo, Catherine Boschert, Catherine Tacon, Claire Corrigan, Craig French, Danielle Austin, David Brewster, David Cooper, David Crosbie, David Hawkins, Edda Jessen, Eduardo Martinez, Edward Fysh, Edward Litton, Felix Oberender, Forbes McGain, Gavin Salt, Glenn Eastwood, Gopal Taori, Hannah Thompson, Hayden White, Hergen Buscher, Ian Seppelt, Ifrah Khan, Janelle Young, Jayshree Lavana, Jeremy Cohen, Jessica Lugsdin, Jill Garlick, Jim Buttery, John Botha, John Santamaria, Jonathan Barrett, Kasha Singh, Kevin Laupland, Khaled El-Khawas, Kristine Estensen, Kush Deshpande, Kyle White, Leigh Fitzpatrick, Lewis Campbell, Mahesh Ramanan, Manoj Saxena, Marie Draper, Marion Kainer, Mark Kol, Mark Page, Mark Plummer, Martin Sterba, Matthew Anstey, Matthew Brain, Matthew Maiden, Myrene Kilminster, Naomi Hammond, Neeraj Bhadange, Nicole Humphreys, Paras Jain, Paul Azzi, Paul Secombe, Paula Lister, Peter Chan, Peter McCanny, Phillip Britton, Pierre Janin, Rashmi Runiyar, Ravi Krishnamurthy, Ravikiran Sonawane, Ravindranath Tiruvoipati, Rebecca Jessup, Richard Totaro, Rinaldo Bellomo, Ritesh Sanghavi, Samantha Bates, Sandra Peake, Shailesh Bihari, Shane George, Sharon Waterson, Simon Erickson, Steve Webb, Subhash Arora, Subodh Ganu, Thomas Rozen, Toni McKenna, Umesh Kadam, Vineet Nayyar, Wei Han Choy, and Wisam Albassam
References
- 1.Majumder MS, Mandl KD. Early in the epidemic: impact of preprints on global discourse about COVID-19 transmissibility. Lancet Glob Health. 2020;8:e627–e630. doi: 10.1016/S2214-109X(20)30113-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, et al. RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with COVID-19: preliminary report. N Engl J Med. [online ahead of print] 17 Jul 2020; DOI: 10.1056/NEJMoa2021436. [Google Scholar]
- 3.Australian and New Zealand Intensive Care Society COVID-19 guidelines: version 1 Melbourne, Australia: Australian and New Zealand Intensive Care Society; 2020[updated 2020 Mar 16; accessed 2020 Nov 12]. Available from: https://www.anzics.com.au/wp-content/uploads/2020/03/ANZICS-COVID-19-Guidelines-Version-1.pdf [Google Scholar]
- 4.Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, et al. RECOVERY Collaborative Group Effect of dexamethasone in hospitalized patients with COVID-19: preliminary report [preprint] medRxiv 2020[accessed 2020 Sep 27]. Available from: 10.1101/2020.06.22.20137273 [DOI]
- 5.Venkatesh B, Finfer S, Cohen J, Rajbhandari D, Arabi Y, Bellomo R, et al. ADRENAL Trial Investigators; Australian–New Zealand Intensive Care Society Clinical Trials Group. Adjunctive glucocorticoid therapy in patients with septic shock. N Engl J Med. 2018;378:797–808. doi: 10.1056/NEJMoa1705835. [DOI] [PubMed] [Google Scholar]
- 6.Bauchner H, Fontanarosa PB. Randomized clinical trials and COVID-19: managing expectations. JAMA. 2020;323:2262–2263. doi: 10.1001/jama.2020.8115. [DOI] [PubMed] [Google Scholar]
- 7.Sterne JAC, Murthy S, Diaz JV, Slutsky AS, Villar J, Angus DC, et al. WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324:1330–1341. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]


