Abstract
Rationale: Whether severe coronavirus disease (COVID-19) is a significant risk factor for the development of invasive fungal superinfections is of great medical interest and remains, for now, an open question.
Objectives: We aim to assess the occurrence of invasive fungal respiratory superinfections in patients with severe COVID-19.
Methods: We conducted the study on patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)–related pneumonia admitted to five ICUs in France who had respiratory and serum sampling performed for specific screening of fungal complications.
Measurements and Main Results: The study population included a total of 145 patients; the median age was 55 years old. Most of them were male (n = 104; 72%), were overweight (n = 99; 68%), and had hypertension (n = 83; 57%) and diabetes (n = 46; 32%). Few patients presented preexisting host risk factors for invasive fungal infection (n = 20; 14%). Their global severity was high; all patients were on invasive mechanical ventilation, and half (n = 73, 54%) were on extracorporeal membrane oxygenation support. Mycological analysis included 2,815 mycological tests (culture, galactomannan, β-glucan, and PCR) performed on 475 respiratory samples and 532 sera. A probable/putative invasive pulmonary mold infection was diagnosed in 7 (4.8%) patients and linked to high mortality. Multivariate analysis indicates a significantly higher risk for solid organ transplant recipients (odds ratio, = 4.66; interquartile range, 1.98–7.34; P = 0.004). False-positive fungal test and clinically irrelevant colonization, which did not require the initiation of antifungal treatment, was observed in 25 patients (17.2%).
Conclusions: In patients with no underlying immunosuppression, severe SARS-CoV-2–related pneumonia seems at low risk of invasive fungal secondary infection, especially aspergillosis.
Keywords: fungal infection, aspergillosis, COVID-19, SARS-CoV-2, aspergillus
At a Glance Commentary
Scientific Knowledge on the Subject
Whether coronavirus disease (COVID-19) is a significant risk factor for the development of invasive fungal superinfections remains an open question of great medical interest. We searched the PubMed database for articles published up to October 19, 2020, using the keywords “COVID-19” AND “Aspergillosis” OR “Aspergillus.” The most relevant occurrences available were brief series and two prospective studies dealing with aspergillosis, almost all of them claiming an alarming incidence of invasive aspergillosis (19.4–35%). We also found another series with low incidence of aspergillosis.
What This Study Adds to the Field
We report a cohort of 145 critically ill patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)–related pneumonia for whom a specific search for fungal superinfections has been conducted. An invasive pulmonary fungal infection was diagnosed in seven patients, amounting to a 4.8% incidence. Multivariate analysis indicates that solid organ transplant recipients appear particularly at risk for developing invasive aspergillosis. Contrary to what was previously reported, we provide relevant data indicating a low risk of invasive fungal complications in immunocompetent patients admitted to the ICU for severe COVID-19. In these patients, contamination/clinically irrelevant colonization is frequent (17.2% in our series) and should not lead to the initiation of antifungal treatment.
Approximately 5% of patients infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) experience severe lung damage due to viral replication, the ensuing cytokine storm, and complex inflammatory processes (1). This can lead to secondary infections early after the disease’s onset (2).
Most patients who develop severe forms of SARS-CoV-2 infection (coronavirus disease [COVID-19]) are immunocompetent individuals presenting with underlying chronic conditions, such as obesity, hypertension, diabetes, and chronic heart or kidney disease (3). None of these factors are usually associated with an increased risk of developing invasive pulmonary fungal infections. All of these occur almost exclusively in patients with well-defined inherited or acquired immunosuppression factors. These encompass different clinical situations such as neutropenia, high-dose and prolonged corticosteroid therapy, solid organ transplantation (SOT), hematological malignancies, cancer, or untreated HIV infection.
Previous data on coronavirus outbreaks due to the SARS-CoV (responsible for the severe acute respiratory syndrome outbreak in 2002–2003) or the Middle East respiratory syndrome coronavirus also indicate that immunocompromised patients do not present a higher risk of severe pulmonary disease when compared with the general population (4, 5). Nevertheless, even with no increased risk, SARS-CoV-2 can still infect immunocompromised patients, including those particularly exposed to fungal complications.
Importantly, invasive aspergillosis has been well described as a complication of severe viral-related pneumonia (6–8). A multicenter retrospective study reported an incidence of invasive aspergillosis amounting to 19% among 432 patients admitted to an ICU for influenza-related acute respiratory failure (7). In addition, in an autopsy series of patients who died in 2003 from SARS, 10% (2/20) had an invasive infection suggestive of aspergillosis (6). Finally, different authors, including ourselves, recently reported case reports and small series of invasive aspergillosis in immunocompetent critically ill patients with severe COVID-19 (9–14). In line with these first results mostly reporting a high incidence of invasive aspergillosis, Bartoletti and colleagues and White and colleagues also more recently reported a high incidence of aspergillosis in the setting of prospective studies (15, 16). On the opposite, Lamoth and colleagues reported a low incidence in a cohort of 80 patients with severe COVID-19 under mechanical ventilation (17).
As the medical community is confronted by this pandemic, it is important to determine whether patients infected with SARS-CoV-2 may be at particular risk of developing invasive fungal complications, especially those with a pulmonary tropism such as mold infections (mainly aspergillosis) and pneumocystosis. When mechanical ventilation–associated pneumonia was suspected in patients with severe COVID-19, the search for a fungal agent was conducted. Therefore, we report the results of a study that aimed to assess the occurrence of invasive pulmonary fungal infections (IFIs) and nonclinical relevant colonization in patients admitted to intensive care for severe COVID-19–related pneumonia.
Some of the results of this study have been previously briefly reported or discussed in the form of case report (10, 18) or letter (19, 20).
Methods
Design and Patients
We performed an analysis in five independent ICUs from a single center, the La Pitié-Salpêtrière hospital, a 1,850-bed tertiary care center in Paris, France. This hospital is one of the two reference centers in the Paris area (nine centers in France) designated for the coordination of the sanitary response to the epidemic and for the management of patients infected with SARS-CoV-2. The hospital’s organization was deeply changed during the study period, and 125 ICU beds were deployed and dedicated exclusively to the care of patients with severe COVID-19. All patients with a confirmed molecular diagnosis of SARS-CoV-2 infection admitted to our ICUs and who were tested for invasive fungal infections were included. The search for fungal infections was based on respiratory and/or serum sample analysis, which was done for all patients who were suspected of having mechanical ventilation–associated pneumonia and/or evidence of clinical disease progression.
Case Definition
For aspergillosis and, by extension, other mold infections, European Organization for Research and Treatment of Cancer (EORTC)/Mycosis Study Group (MSG) criteria were used for patients with immunosuppressive underlying conditions (21). Patients with no underlying immunosuppressive factors were classified as having either a putative invasive pulmonary mold infection (IPMI) or false-positive/clinically irrelevant colonization. All patients with one or more mycological test results (culture, galactomannan, Aspergillus PCR, or β-D-glucan) were analyzed as potential cases. They were considered as false-positive/clinically irrelevant colonization in the following circumstances: positive antigen testing or PCR with further negative controls and/or mold culture in respiratory samples with further negative samples without clinical degradation or underlying immunosuppressive condition. Moreover, a diagnosis of invasive infection was discarded for patients with favorable outcomes despite the absence of specific antifungal treatment.
Mycological Analysis
Respiratory samples were subjected to cytospin and direct microscopic examination after silver staining and Giemsa coloration. All were put in culture on chromogenic agar (Chromagar; BioRad), Sabouraud dextrose agar (with and without cycloheximide), and Malt agar and were incubated for 7 days at 37°C then for 21 days at 25°C. Identification of fungal agents was achieved by mass spectrometry (Bruker Microflex and Mass Spectrometry Identification Database).
PCR
We performed real-time PCR on a 7500 Fast Real-Time PCR System (Applied Biosystems) to search for Aspergillus fumigatus, Pneumocystis jirovecii, and Mucorales DNA. DNA extraction was performed on 1 ml of respiratory sample or serum. All PCR assays were performed in duplicate, and a single positive well was considered a positive result. The A. fumigatus PCR assay targets a species-specific 67-bp segment of a 28S ribosomal RNA-coding DNA, as previously described (22). The P. jirovecii PCR assay targets the mitochondria1 large subunit ribosomal RNA gene (23). The Mucorales PCR was adapted from previously published data (24) and targeted several species among four genera.
Fungal Antigen Testing
The galactomannan (GM) index was determined by enzyme immunoassay (BioRad) according to the manufacturer’s recommendations. A result was considered positive after two determinations (performed on two different assays but on the same sample) showing both an index equal to or greater than 0.5 for serum and an index equal to or greater than 1 for respiratory samples. β-d-glucan research was performed in serum using the Fungitell assay in duplicate (Associates of Cape Cod) following the manufacturer’s recommendations. A positive result was defined as two consecutive tests above a cutoff of 80 pg/ml.
Statistical Analysis
Statistical analyses were performed using GraphPad Prism 5 and the R version 3.5.3 statistical software (R Foundation for Statistical Computing).
Continuous and categorical variables are presented as median (interquartile range [IQR]) or mean (SD) and number (percentage), respectively. Categorical variables were compared using the Fisher exact test, and the Mann-Whitney U test was used for continuous variables. Survival distributions were compared using the log-rank (Mantel-Cox) test. To explore the risk factors associated with occurrence of invasive pulmonary fungal infection, univariable and multivariable logistic regression models were used. The variables selected for the multivariate analysis were first those known to confer a risk of invasive fungal infection (corticosteroid therapy, SOT, and hematological malignancies) or fungal colonization (chronic obstructive pulmonary disease [COPD] and extracorporeal membrane oxygenation [ECMO]) and those for which a trend was observed in univariate analysis. Patients with missing data were excluded from analysis. A two-sided α of less than 0.05 was considered statistically significant.
Results
Patient Characteristics
Between March 6, 2020, and April 24, 2020, a total of 260 patients were admitted to the ICU for severe COVID-19. The median length of stay was 30 days (IQR, 15–50 d; mean, 36.8 d). Among these patients, 145 were specifically screened for fungal superinfections (Table 1). They were predominantly male (n = 104; 72%) and had a median age of 55 years (IQR, 48–64 yr). Most patients presented with hypertension (n = 83; 57%), were overweight/had obesity (n = 99; 68%), had diabetes mellitus (n = 46; 32%), were active smokers (n = 11; 8%), and had COPD (n = 9; 6%).
Table 1.
Characteristics of the Study Population
| Characteristics | All Patients (n = 145) | ||
|---|---|---|---|
| Demographic characteristics and underlying conditions | Age, median (interquartile range), yr |
55 (48–64) | |
| Sex, M, n (%) |
104 (72) | ||
| Active smoker, n (%) |
11 (8) | ||
| Hypertension, n (%) |
83 (57) | ||
| Cholesterol, n (%) |
32 (22) | ||
| Diabetes, n (%) |
46 (32) | ||
| Overweight (body mass index > 25 kg/m2), n (%) |
99 (68) | ||
| Chronic obstructive pulmonary disease, n (%) |
9 (6) | ||
| Preexisting risk factors for invasive fungal infection (n = 26/143) | Host factor, n (%)* |
20 (14) | |
| Hemopathy |
3 (2) | ||
| Hematopoietic stem cell allografft |
1 (0.7) | ||
| Solid organ transplant |
14 (10) | ||
| Corticosteroid therapy >0.3 mg/kg |
4 (3) | ||
| HIV infection, n (%) |
6 (4) | ||
| Specific COVID-19 therapy (n = 88/137) | HCQ, n (%) | HCQ | 57 (42) |
| HCQ/AZT | 9 (7) | ||
| Antiviral, n (%) | Lopinavir/ritonavir | 23 (17) | |
| Remdesivir | 4 (3) | ||
| Lopinavir/ritonavir and remdesivir | 4 (3) | ||
| Anti–IL-6, n (%) | Tocilizumab | 6 (4) | |
| Sarilumab | 3 (2) | ||
| Anti–IL-1, n (%) |
1 (0.7) | ||
| Corticosteroid therapy related to COVID-19 and/or ICU stay (n = 23/132) | Before ICU admission, n (%) |
2 (1.5) | |
| During ICU stay, n (%) |
22 (16.7) | ||
| ICU management and clinical characteristics; median [interquartile] | ICU stay (n = 134), median (interquartile range), d |
30 (15–50) | |
| SAPS II score (n = 109), median (interquartile range) |
47 (33–62) | ||
| Intubation period (n = 129), median (interquartile range), d |
27 (14–45) | ||
| Worst P/F (n = 135), median (interquartile range) |
60 (51–73) | ||
| Extracorporeal membrane oxygenation (n = 135), n (%) |
73 (54) | ||
| Vasopressor support (>1 mg/h of noradrenalin), n (%) |
94 (70) | ||
| Corticosteroid substitution (hydrocortisone succinate), n (%) |
21 (16) | ||
| Renal replacement therapy, n (%) |
41 (31) | ||
| Inflammatory markers; median [interquartile] | Neutrophil on lymphocyte ratio (n = 140), median (interquartile range) |
11 (8–17) | |
| C-reactive protein (n = 109), median (interquartile range), mg/L |
216 (130–304) | ||
| Ferritine (n = 119), median (interquartile range), mg/L |
2,005 (963–3,554) | ||
| IL-6 (n = 39), median (interquartile range), pg/ml |
248 (87–718) | ||
| Overall survival at d 30 after ICU’s admission, n (%) | 108 (74.5) | ||
Definition of abbreviations: AZT = azithromycine; COVID-19 = coronavirus disease; HCQ = hydroxychloroquine; P/F = PaO2/FiO2; SAPS = Simplified Acute Physiology Score.
As defined jointly by the European Organization for Research and Treatment of Cancer and Mycosis Study Group according to Donnelly and colleagues (21).
Among 143 patients for whom the information is available, 20 (14%) were immunocompromised and presented risk factors for IFI as defined conjointly by the EORTC/MSG (21). The main risk factor was corticosteroid and immunosuppressive drug administration, as 14 patients were SOT recipients, three patients had hematological malignancies, and three others had prolonged corticosteroid therapy for autoimmune diseases.
The study population was characterized by very severe presentations; all patients were intubated and placed on mechanical ventilation (n = 145; 100%) and presented severe respiratory failure (worst PaO2/FiO2: median, 60; IQR, 51–73) and elevated Simplified Acute Physiology Score II score (median, 47; IQR, 33–62) Consequently a high number of patients (n = 73/135; 54%) were placed on venovenous ECMO (vv-ECMO) support. Most of the study population needed vasopressor support with more than 1 mg/h of norepinephrine equivalent (n = 94; 70%), and a third required renal replacement therapy (n = 41; 31%). Most of the patients (n = 112; 77%) had an absolute lymphocyte count below 1,000/mm3. Overall survival at Day 30 after ICU admission was 74.5% (108/145).
Mycological Test Results
A total of 475 respiratory samples (347 BAL, 120 tracheal aspiration, and 8 distal protected specimens) were sent to the mycology laboratory (mean of 3.3 per patient) (Table E1 in the online supplement) and submitted to direct examination/culture, galactomannan index determination, and PCR targeting A. fumigatus, P. jiroveccii, and/or Mucorales. A total of 532 sera were sampled (mean, 3.7 samples per patient) and tested for galactomannan, β-d-glucan, and/or A. fumigatus and Mucorales.
Regarding both respiratory and serum samples, 106 patients (73.1%) had negative tests, 22 patients (15.2%) had one positive test, 6 (4.1%) had two positive tests, and 11 (7.6%) three or more positive tests. Among those 39 patients with at least one mycological positive test, two had isolated positive β-glucan in the setting of candidiasis, and five were excluded from further analysis because of lacking data (four patients died within 48 h after a single positive mycological test without any other performed test and any way to confirm or infirm the fungal hypothesis, and one patient was transferred to another hospital and lost to follow-up) (Figure 1). Finally, according to clinical evaluation and mycological markers, 25 patients were found to have false-positive/clinically irrelevant colonization, and seven patients were diagnosed with probable or putative invasive pulmonary mold infections (IPMIs).
Figure 1.
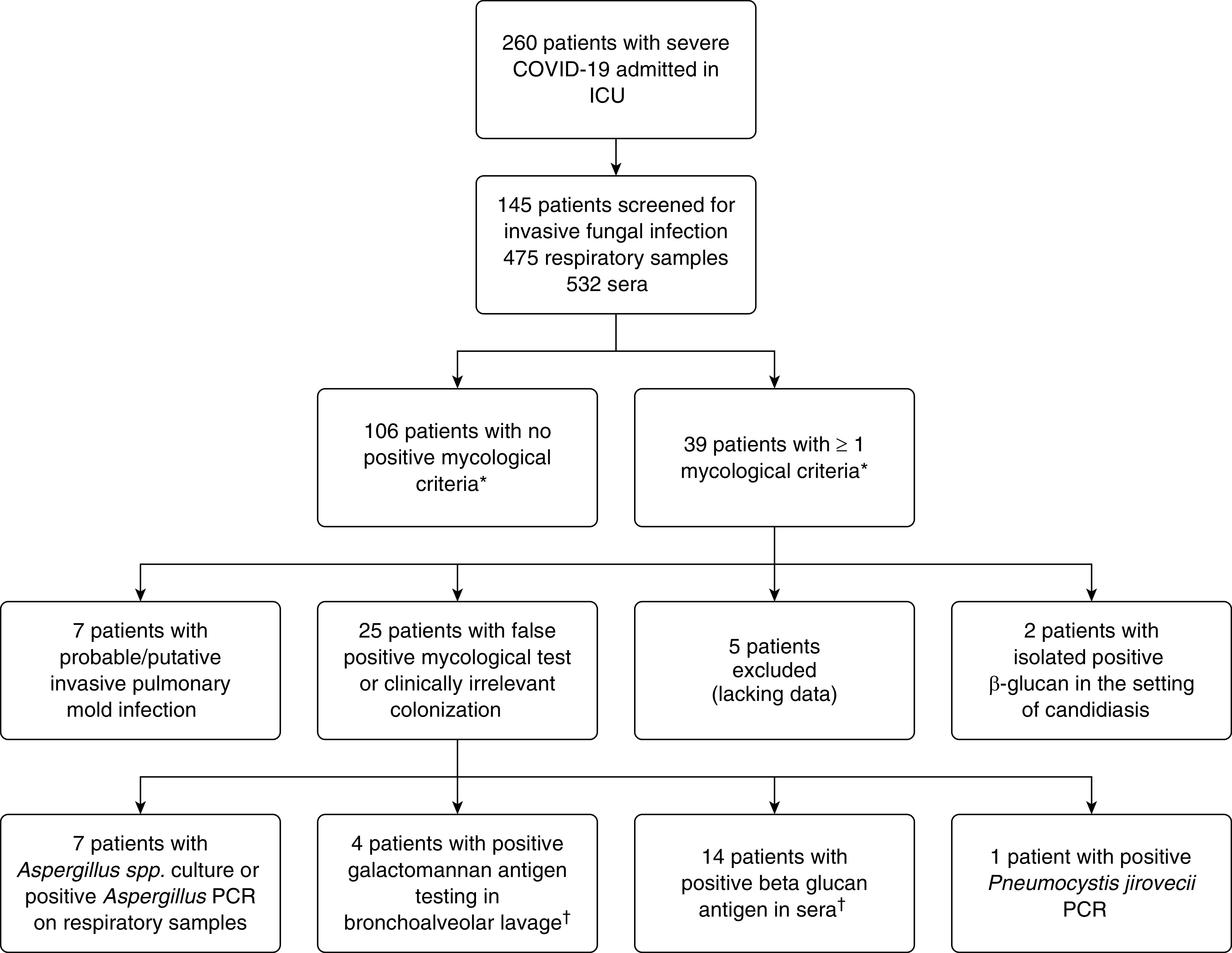
Study flowchart. *Presence of Candida spp. in respiratory tract or cutaneous samples were not included. †One patient had a single positive β-glucan and a positive galactomannan on a BAL sampled 10 days later. COVID-19 = coronavirus disease.
Characteristics of Patients with IPMIs
A diagnosis of putative/probable IPMI was retained in seven patients (4.8%), including six with aspergillosis and one with fusariosis (Table 2). Taking into account the patients’ underlying conditions, pulmonary mold infection occurred in 20% (4/20) of the patients with one EORTC/MSG host factor, whereas it was found in 2.4% (3/123) of patients without a EORTC/MSG host factor (P = 0.007 by Fisher exact test). Among the four patients with EORTC/MSG host criteria who developed an invasive mold infection, three were SOT recipients (two kidney; one liver), and one received corticosteroid therapy (>0.3 mg/kg/d for 6 wk).
Table 2.
Clinical and Mycological Characteristics of Seven ICU Patients with Severe COVID-19 Who Developed Subsequent Invasive Respiratory Mold Infections
| Patients | 1 | 2 | 3 | 4 | 5 | 6 | 7 | ||
|---|---|---|---|---|---|---|---|---|---|
| Demographic characteristics |
Sex | M | M | M | M | M | F | M | |
| Age, yr | 75 | 49 | 57 | 40 | 50 | 62 | 72 | ||
| Host factors for IFI related to underlying immunosuppression | None | Kidney transplantation | None | None | Kidney transplantation | Liver transplantation | Steroids (predinose >0.3 mg/kg/d) | ||
| Underlying chronic diseases | Hypertension/obesity | Hypertension/obesity | Hypertension/diabetes/obesity | Hypertension/obesity/tabagism | Dyslipidemia/obesity | Hypertension/diabetes/obesity | Hypertension/dyslipidemia | ||
| Clinical characteristics |
Time from illness onset to ICU, d | 7 | 7 | 7 | 2 | 10 | 6 | 2 | |
| Ventilation | Invasive mechanical ventilation | Invasive mechanical ventilation | Invasive mechanical ventilation | vv-ECMO | vv-ECMO | Invasive mechanical ventilation | vv-ECMO | ||
| ICU, d | 8 | 15 | 32 | 30 | 77 | 18 | 63 | ||
| Orotracheal intubation, d | 8 | 15 | 28 | 30 | 77 | 18 | 63 | ||
| Amine support | Yes | Yes | No | Yes | Yes | No | No | ||
| Dialysis | No | Yes | Yes | No | Yes | Yes | Yes | ||
| Worst P/F | 140 | 77 | 50 | 58 | 80 | 40 | 52 | ||
| Specific COVID-19 therapies | No | HCQ/LPV | No | No | No | HCQ | HCQ | ||
| Mycology laboratory findings | Respiratory samples | Respiratory sample | Tracheal aspiration | BAL and DPS | BAL | BAL | BAL | Tracheal aspiration | BAL and DPS |
| Number of respiratory samples with ≥1 positive mycological test | 2 | 2 | 5 | 0 | 8 | 1 | 1 | ||
| Culture | A. fumigatus | A. fumigatus | F. proliferatum | Negative | A. fumigatus | A. fumigatus | A. fumigatus | ||
| Galactommanane index | Negative | 1.5 | 1.7 | 0.7 | 3.2 | Not performed | Negative | ||
| A. fumigatus PCR copies/ml (Log10) | 3,670 (3.6) | 1,500,000 (6.2) | Negative | Negative | 1,160,000 (6.1) | 7,000,000 (6.8) | Negative | ||
| Mucorales PCR | Rhizopus/Mucor* | Negative | Negative | Negative | Rhizopus/Mucor† | Negative | Negative | ||
| Serum | Galactomannan index | Negative | Negative | Negative | 1.19 | Negative | Negative | Negative | |
| β-d-glucan, pg/ml | Negative | Negative | Negative | 520 | Negative | Negative | > 523 | ||
| A. fumigatus PCR copies/ml (Log10) | Negative | Negative | Not performed | 1,740 (3.2) | Negative | 1,164 (3.1) | Negative | ||
| Mucorales PCR | Rhizopus/Mucor* | Negative | Negative | Negative | Rhizopus/Mucor† | Negative | Negative | ||
| Invasive fungal infection characteristics |
Diagnosis | Invasive pulmonary aspergillosis and mucormycosis, putative | Invasive pulmonary aspergillosis, probable | Invasive pulmonary fusariosis, putative | Invasive pulmonary aspergillosis, putative (and candidemia) | Invasive pulmonary aspergillosis and mucormycosis, probable | Invasive pulmonary aspergillosis, probable | Invasive pulmonary aspergillosis, probable | |
| Time from ICU admission to diagnosis, d | 3 | 9 | 10 | 10 | 21 | 8 | 62 | ||
| Antifungal therapy | No | Voriconazole (400 mg b.i.d.) and caspofungin (70 mg/d) (D 14–D 17) | Liposomal amphotericine B (7 mg/kg/d) (D 13–D 34) and caspofungin (70 mg/d) (D 15–D 30) | Voriconazole (400 mg b.i.d.) (D 11–D 18) then caspofungin (70 mg/d) (D 18–D 28) | Voriconazole (400 mg b.i.d.) (D 22–D 58) then amphotericin B (1 mg/kg/d) (D 24–D 37) then caspofungin (70 mg/d) (D 37–D 44) then isavuconazole (200 mg/d) (D 58–D 72) | Voriconazole (400 mg b.i.d.) and caspofungin (70 mg/d) (D 9–D 12) then amphotericin B (1 mg/kg/d) (D 12–D 14) | Caspofungine (70 mg/d) (D 57–D 62) then voriconazole (300 mg b.i.d.) (D 63–D 79) | ||
| Outcome, D 30 after diagnosis | Dead | Dead | Alive | Alive | Alive | Dead | Dead | ||
Definition of abbreviations: b.i.d. = twice daily; COVID-19 = coronavirus disease; DPS = distal protected specimen; HCQ = hydroxychloroquine; IFI = invasive fungal infection; LPV = lopinavir; P/F = PaO2/FiO2; vv-ECMO = venovenous extracorporeal membrane oxygenation.
Rhizopus/Mucor PCR was weakly positive (1/3 reacting well) in a single serum of two tested and positive (3/3 reacting well) in one BAL of two tested.
Rhizopus/Mucor PCR was weakly positive (1/3 reacting well) in one BAL of 10 tested and one serum of two tested.
In a multivariate model analysis, SOT and corticosteroid therapies were related to an increased risk for developing IPMI (odds ratio [OR], 4.66; IQR, 1.98–7.34; P = 0.004 and OR, 8.55; IQR, 6.8–10.3; P = 0.01, respectively), whereas no association was observed for other underlying conditions such as hypertension, cholesterol, diabetes, or being overweight/obese (Table 3). The length of ICU stay, of mechanical ventilation (days), the worst PaO2/FiO2, or the requirement for vv-ECMO support were also not associated with an increased incidence of fungal superinfections, but the Simplified Acute Physiology Score II score was higher for patients who developed IPMI. Of note, no patient with COPD developed IPMI. Importantly, no patient who received corticosteroids specifically for COVID-19 care (mainly dexamethasone 20 mg/d for 10 d) developed subsequent IPMI. IPMI occurred at a median of 7 days (IQR, 2–56 d) after ICU admission.
Table 3.
Comparison between Patients with IPMI and Those without during Severe COVID-19–related Pneumonia
| No IPMI (n = 138) | IPMI (n = 7) | Univariable OR (95% CI) | P Value | Multivariable OR (95% CI) | P Value | ||
|---|---|---|---|---|---|---|---|
| Demographic characteristics and underlying conditions | Age, median (IQR), yr | 54.4 (48–64) | 57.7 (49–67) | — | 0.55 | — | — |
| Sex M, n (%) | 98 (71) | 6 (86) | 2.44 (0.3–115.3) | 0.67 | — | — | |
| Active smoker, n (%) | 10 (7) | 1 (14) | 2.06 (0.04–19.9) | 0.44 | — | — | |
| Hypertension, n (%) | 77 (56) | 6 (86) | 4.56 (0.5–214.8) | 0.23 | — | — | |
| Cholesterol, n (%) | 30 (22) | 2 (29) | 1.41 (0.1–9.1) | 0.65 | — | — | |
| Diabetes, n (%) | 44 (32) | 2 (29) | 0.84 (0.08–5.4) | NS | — | — | |
| Overweight (body mass index >25 kg/m2), n (%) | 93 (67) | 6 (86) | 2.57 (0.3–121.6) | 0.67 | — | — | |
| Chronic obstructive pulmonary disease, n (%) | 9 (7) | 0 (0) | — | — | — | — | |
| Risk factors for invasive fungal infection | Preexisting host factor, n (%)* | 16 (11) | 4 (57) | 9.72 (1.5–72.5) | 0.007 | 7.54 (5.7–9.4) | 0.03 |
| Hemopathy, n (%) | 3 (2) | 0 (0) | — | 1 | — | — | |
| Hematopoietic stem cell allograft | 1 (0.7) | 0 (0) | — | 1 | — | — | |
| Solid organ transplant, n (%) | 11 (8) | 3 (43) | 8.28 (1.1–56.1) | 0.02 | 4.66 (1.98–7.34) | 0.004 | |
| Long-term (>3 wk) corticosteroid therapy >0.3 mg/kg, n (%) | 3 (2) | 1 (14) | 7.16 (0.12–106.9) | 0.18 | 8.16 (5.5–10.8) | 0.1 | |
| Long-term (>3 wk) corticosteroid therapy (any dose), n (%) | 15 (11) | 4 (57) | 10.43 (1.6–78.2) | 0.006 | 8.55 (6.8–10.3) | 0.01 | |
| Corticosteroid therapy linked to COVID-19 care, n (%) | 22 (16) | 0 (0) | — | — | — | — | |
| Inflammatory markers | Neutrophil on lymphocyte ratio, median (IQR) | 11 (8–18) | 13 (11–14) | — | 0.84 | — | — |
| C-reactive protein, median (IQR), mg/L | 217 (130–304) | 213 (114–298) | — | 0.72 | — | — | |
| Ferritine, median (IQR), mg/L | 1,956 (946–3,527) | 2,609 (1,762–3,803) | — | 0.43 | — | — | |
| IL-6, median (IQR), pg/ml | 250 (87–718) | 181 (143–414) | — | 0.87 | — | — | |
| ICU management and clinical characteristics | ICU stay, median (IQR), d | 30 (15–50) | 24 (14–62) | — | 0.89 | — | — |
| SAPS II, median (IQR) | 46 (31–60) | 73 (54–85) | — | 0.01 | — | 0.02 | |
| Intubation period, median (IQR), d | 27 (15–44) | 25 (11–55) | — | 0.98 | — | — | |
| Worst P/F, median (IQR) | 60 (52–72) | 58 (51–79) | — | 0.94 | — | — | |
| Extracorporeal membrane oxygenation, n (%) | 70 (54) | 3 (43) | 0.62 (0.09–3.8) | 0.70 | — | — | |
| Vasopressor support (>1 mg/h of noradrenalin), n (%) | 88 (70) | 6 (86) | 2.64 (0.3–125.3) | 0.67 | — | — | |
| Renal replacement therapy, n (%) | 37 (29) | 4 (57) | 3.21 (0.5–23.0) | 0.20 | — | — | |
| Hydroxychloroquine, n (%) | 61 (44) | 5 (71) | 3.71 (0.6–40.4) | 0.25 | — | — | |
| Corticosteroid substitution (hydrocortisone succinate) , n (%) | 18 (14) | 3 (43) | 4.42 (0.6–28.6) | 0.06 | 4.43 (2.8–6.1) | 0.09 | |
| Survival at D 30 after admission, n (%) | 104 (76) | 4 (57) | 2.57 ( 0.4–18.4) | 0.2 | — | — | |
Definition of abbreviations: CI = confidence interval; COVID-19 = coronavirus disease; IPMI = invasive pulmonary mold infection; IQR = interquartile range; OR = odds ratio; P/F = PaO2/FiO2; SAPS = Simplified Acute Physiology Score.
Statistically significant values appear in bold.
As defined jointly by the European Organization for Research and Treatment of Cancer and Mycosis Study Group according to Donnelly and colleagues (21).
Characteristics of the False-Positive/Clinically Irrelevant Colonization Cases
A total of 25 of 39 patients with at least one positive mycological criterion (64.1% of these patients and 17.2% of the entire cohort) were considered as having false-positive tests or clinically irrelevant colonization. Seven patients had isolated Aspergillus species culture or positive Aspergillus PCR on respiratory samples, and four patients had positive GM (i.e., GM index above 1) on BAL samples. For two of these four patients, no other BAL was performed until they were discharged from the ICU after a favorable outcome. As the microscopic examinations, fungal culture and Aspergillus PCR were negative, and because their conditions improved without antifungal treatment, they were considered as false positive. For the two other patients, a second BAL performed 6 days later tested negative for GM. Again, microscopic examinations, fungal culture. and Aspergillus PCR were also negative, and these patients were not considered as having an invasive aspergillosis. Finally, 14 patients had isolated positive β-glucan in sera (including one with a positive GM on a BAL sampled 10 d later) (Figure 1 and Table E2 for the details of mycological results). In these patients, further antigen testing and fungal cultures were systematically found negative in ulterior control samples. ECMO support was not related to IPMI but was more frequently associated with fungal colonization and/or clinically irrelevant mycological colonization, although this association did not reach statistical significance (P = 0.08 by Fisher exact test).
Outcome of Patients with Positive Mycological Criteria
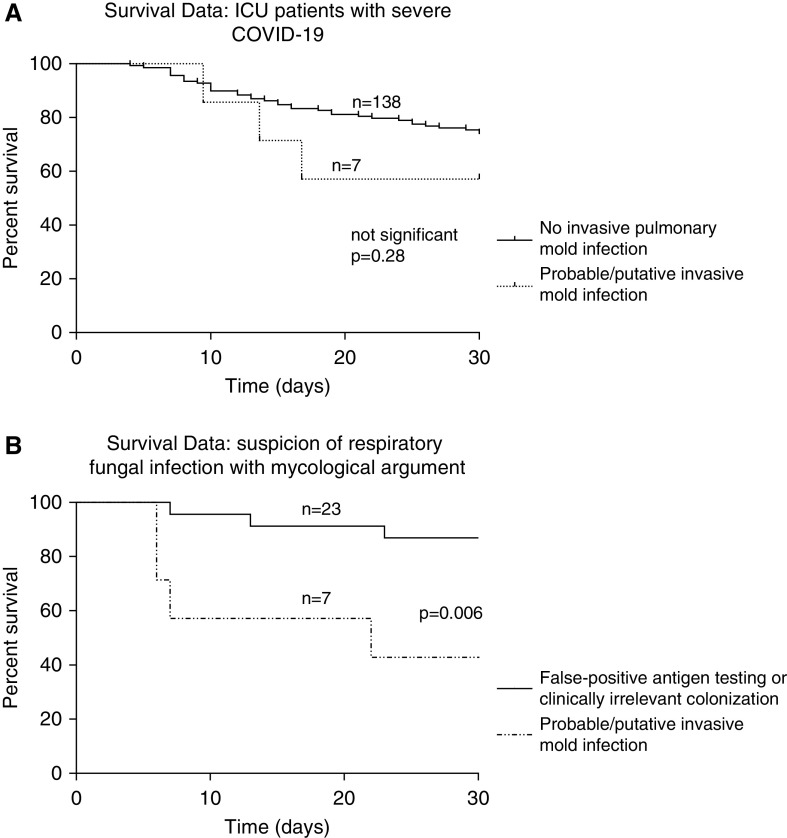
Overall survival on Day 30 of ICU admission was 74.5% (108/145) and was higher for patients who did not exhibit invasive mold infection (Figure 2A). Regarding the patients with one or more positive mycological criteria, the survival at Day 30 after the first positive fungal test was 42.8% (3/7) for patients with IPMI, whereas it was 86.9% (20/23 for whom data is available) for patients with no final diagnosis of invasive fungal infection (P = 0.006; OR, 15.3; 95% CI of ratio, 2.17–108.9 with log-rank [Mantel-Cox] test) (Figure 2B and Table 4). IPMI was therefore associated with a higher risk of mortality in comparison with false-positive/clinically irrelevant colonization.
Figure 2.
Survival data for ICU patients with severe coronavirus disease (COVID-19). (A) Survival data for 145 patients at Day 30 after ICU admission. (B) Survival data for 30 patients at Day 30 after a first positive mycological test. Patients with a diagnosis of probable/putative invasive mold infection had worse outcomes than patients without infection (log-rank [Mantel-Cox] test).
Table 4.
Characteristics of Patients with IPMIs and of Those with Clinically Irrelevant Mycological Findings
| Characteristics | Clinical Irrelevant (n = 25) | IPMI (n = 7) | P Value | |
|---|---|---|---|---|
| Demographic characteristics and underlying conditions | Age, median (IQR), yr | 56 (45–61) | 57.7 (49–67) | 0.55 |
| Sex, M, n (%) | 21 (84) | 6 (86) | 1 | |
| Active smoker, n (%) | 1 (4) | 1 (14) | 0.39 | |
| Hypertension, n (%) | 13 (52) | 6 (86) | 0.19 | |
| Cholesterol, n (%) | 4 (16) | 2 (29) | 0.59 | |
| Diabetes, n (%) | 10 (40) | 2 (29) | 0.70 | |
| Overweight (body mass index >25 kg/m2), n (%) | 18 (72) | 6 (86) | 0.64 | |
| Chronic obstructive pulmonary disease, n (%) | 0 (0) | 0 (0) | NA | |
| Risk factors for IPMI | Host factor, n (%)* | 0 (0) | 4 (57) | 0.001 |
| Hemopathy, n (%) | 0 (0) | 0 (0) | NA | |
| Hematopoietic stem cell allograft, n (%) | 0 (0) | 0 (0) | NA | |
| Solid organ transplant, n (%) | 0 (0) | 3 (43) | 0.007 | |
| Long-term (>3 wk) corticosteroid therapy >0.3 mg/kg, n (%) | 0 (0) | 1 (14) | 0.22 | |
| Long-term (>3 wk) corticosteroid therapy (any dose), n (%)† | 0 (0) | 4 (57) | 0.001 | |
| Corticosteroid therapy linked to COVID-19 care, n (%) | 8 (32) | 0 (0) | 0.15 | |
| Inflammatory markers | Neutrophil on lymphocyte ratio, median (IQR) | 10 (9–13) | 13 (11–14) | 0.43 |
| C-reactive protein, median (IQR), mg/L | 240 (160–334) | 213 (114–298) | 0.54 | |
| Ferritine, median (IQR), mg/L | 2,193 (1,223–4,605) | 2,609 (1,762–3,803) | 0.93 | |
| IL-6, median (IQR), pg/ml | 524 (222–3,098) | 181 (143–414) | 0.49 | |
| ICU management and clinical characteristics | ICU stay, median (IQR), d | 53 (40–63) | 24 (14–62) | 0.16 |
| SAPS II, median (IQR) | 54 (42–66) | 73 (54–85) | 0.10 | |
| Intubation period, median (IQR), d | 42 (33–59) | 25 (11–55) | 0.37 | |
| Worst P/F, median (IQR) | 58 (51–62) | 58 (51–79) | 0.96 | |
| Extracorporeal membrane oxygenation, n (%) | 20 (80) | 3 (43) | 0.08 | |
| Vasopressor support (>1 mg/h of noradrenalin), n (%) | 15 (60) | 6 (86) | 0.37 | |
| Renal replacement therapy, n (%) | 7 (29) | 4 (57) | 0.20 | |
| Corticosteroid substitution (hydrocortisone hemisuccinate), n (%) | 3 (13) | 3 (43) | 0.10 | |
| Survival data | At D 30 after admission, n (%) | 23/25 (92) | 4/7 (57) | 0.003 |
| At D 30 after the first positive fungal test, n (%) | 20/23 (86.9) | 3/7 (42.8) | 0.006 | |
Definition of abbreviations: COVID-19 = coronavirus disease; IPMI = invasive pulmonary mold infection; IQR = interquartile range; P/F = PaO2/FiO2; SAPS = Simplified Acute Physiology Score.
Statistically significant values appear in bold.
As defined jointly by the European Organization for Research and Treatment of Cancer and Mycosis Study Group according to Donnelly and colleagues (21).
Any dosage and duration of treatment combined, including those not retained in the European Organization for Research and Treatment of Cancer and Mycosis Study Group criteria.
Discussion
Our results indicate that patients with severe SARS-CoV-2–related pneumonia and no underlying immunosuppression seem at low risk of pulmonary invasive fungal secondary infection, especially aspergillosis.
Our study has limitations. It is retrospective and single-centered. A prospective design would have allowed us to be exhaustive on certain clinical criteria. However, it should be noted that the number of samples sent to the mycology laboratory would not necessarily have been higher. It should also be noted that although this is a single-center study, it involves five distinct and independent units, some of which have specific features, such as the exclusive orientation toward the use of ECMO support. Finally, owing to the number of patients, the results of secondary analysis remain exploratory and are not destined to produce a predictive model for the incidence of fungal superinfection.
To this day and to the best of our knowledge, the incidence of invasive fungal superinfections in this population remains unclear. Studies available mainly included aspergillosis case reports/small series and two prospective series also dealing with aspergillosis, seven of which reported an alarming incidence of more than 20% (9–16). These first published results raise several concerns regarding at least two points. First was the causal relationship between COVID-19 and aspergillosis (i.e., whether SARS-CoV-2 infection by itself promotes invasive forms of aspergillosis). Indeed, several patients among the reported cases had underlying chronic respiratory disease such as COPD or asthma and were receiving inhaled or systemic corticosteroid therapy, different conditions that are known to favor Aspergillus implantation in the respiratory tract. Consequently, careful attention should be paid to clearly differentiate aspergillosis as a subsequent complication of severe COVID-19–related pneumonia from aspergillosis in patients with underlying predisposing conditions.
The second point is the clinical relevance of the presence of Aspergillus in the respiratory tract of a patient with severe COVID-19–related pneumonia and the question of the invasiveness of the process, as we recently discussed in response to van Arkel and colleagues (20). Moreover, some patients present favorable outcomes without any antifungal treatment (9). For the same reason, from our point of view, the relevance of a single fungal antigen as a diagnostic criterium should be subject to caution in patients with clinical improvement. In the present study, many patients could have been classified as having putative invasive aspergillosis. Assuming the fact that an invasive infectious process that resolves spontaneously when the underlying condition improves is not considered invasive, these cases were not retained in the present study.
The overall incidence of fungal respiratory complications (4.8%) was much lower in our study population compared with what was previously reported. Unlike influenza, SARS-CoV-2 does not seem to promote invasive fungal infection in critically ill patients. In this new disease, the precise pathophysiology of the lung damage remains unclear but seems to rely primarily on collateral cytokine-induced inflammatory injury rather than on direct viral replication in the low respiratory tract. This may partially explain the differences observed with influenza. In addition, the population affected by the first wave of the SARS-CoV-2 pandemic and requiring intensive care is also different, displaying a higher proportion of younger patients and fewer immunocompromised patients than what is usually reported with seasonal influenza (7). Also, the number of patients receiving corticosteroids was lower in our series than in others (25). Indeed, owing to the deceptive data regarding immunomodulatory approaches in influenza, corticosteroids were initially not administered to our patients with severe COVID-19. To this day, as the second epidemic wave is raging over Europe, critically ill patients are receiving corticosteroids more frequently. It will therefore be interesting to compare the incidence of fungal superinfections between the two periods.
Invasive aspergillosis is now a well-recognized complication that can affect immunocompetent patients during an ICU stay, whether or not it is associated with influenza pneumonia and ECMO support (26, 27). In a recent study, 7% of patients receiving vv-ECMO had putative aspergillosis, and 7% had Aspergillus colonization (28). In our series, however, vv-ECMO support was not related to IPMI, and its potential association with fungal colonization and/or clinically irrelevant mycological test warrants further study. Incidentally, the high proportion of patients on vv-ECMO support in our population (54%) is explained by the fact that the study was performed in an expert center for this specific technique, which is used as a rescue therapy in severe COVID-19 (29).
The diagnosis of invasive aspergillosis remains challenging, especially in critically ill patients with no underlying host risk factors. In this setting, clinical criteria are usually sidelined, and imaging criteria, when available, can be difficult to interpret. Computed tomographic scan and magnetic resonance imaging have over time become important components of the diagnosis of fungal infections. In our series, almost all patients lacked these analyses except for one with probable aspergillosis who had cavitary lesions compatible with an invasive fungal process. Histology, which could be the irrefutable diagnostic element, is almost never obtainable. The classifications therefore give pride of place to mycological criteria, as illustrated by the recent inclusion of antigen testing, which adds diagnostic sensibility but may reduce positive predictive value (22, 30, 31).
The difficulties in classifying the cases is well illustrated by the variety of diagnostic criteria established and used in recent studies. To this day, for patients with COVID-19–associated pulmonary aspergillosis, there are almost as many criteria as there are publications (9, 11, 14, 16, 17). We acknowledge that those criteria must include the more recent mycological tests (for example, PCR), but they should also take into account the increasing amount of false-positive and transient colonization detected, amounting to clinically irrelevant case identification (with spontaneous favorable outcome or test negativation for instance). We have included those considerations in our case classification, as we believe they are more accurate and clinically pertinent.
In our series, 25 patients were considered as having transient respiratory tract colonization or false-positive antigen. Half of those patients (n = 14) only had positive β-glucan testing, which is known to lack specificity and also has poor sensitivity for filamentous infections.
Galactomannan detection in the BAL has become an important tool for the diagnosis of invasive aspergillosis in immunocompetent patients in the ICU. If we focus on BAL samples, only 11 of 333 samples (3.3%) were positive (i.e., an index strictly above 1) for eight patients (5.5%). Among them, three had also a positive fungal culture in BAL and were classified as having a probable or putative invasive mold infection (IPMI group). Four other patients were considered false positive, and one patient was excluded from the analysis because he died 2 days after GM-positive BAL result without any control, any other positive test, or any way to confirm or infirm the fungal hypothesis. We acknowledge that these five patients could have been classified as having putative aspergillosis according to the modified AspICU criteria (7) or the criteria for influenza-associated aspergillosis (32), but because they finally improved or had negative control on subsequent samples without appropriate antifungal treatment, we believe that this diagnostic should not be considered. However, if we had chosen to classify these cases, the incidence of pulmonary mold infections would have risen to 8.3%, which remains relatively low.
Efforts should be made to investigate cases as efficiently as possible to promptly retain or rule out a diagnosis of invasive fungal infections in ICU patients with severe COVID-19–associated pneumonia. A special attention should be paid to patients presenting with already recognized risk factors, especially SOT recipients. Furthermore, as most mycological criteria lack specificity but play a key role in the diagnosis of fungal superinfections in the ICU, they should be multiplied and carefully analyzed because they can lead to overdiagnosis and excessive use of antifungal therapy.
Conclusions
In conclusion, the current data indicate a low risk for patients with severe forms of COVID-19–associated pneumonia to develop a secondary invasive pulmonary fungal infection. As the outbreak continues to spread, other reports are required to confirm these results.
Footnotes
Author Contributions: A.F. designed the study, participated in data collection and analysis, performed statistical analysis, and wrote the paper. A.L. participated in the study design and data collection and analysis, performed statistical analysis, and participated in writing. J.M., S.D., J.-M.C., A.M. and C.-E.L. participated in the patients’ care and clinical data collection and analysis. C.P. participated in the study design and data collection and analysis. A.-G.M. performed virology analysis. M.B. participated in study design, data collection and analysis, and writing. All authors contributed to the writing of the paper.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202009-3400OC on December 2, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ HLH Across Speciality Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323:1775–1776. doi: 10.1001/jama.2020.4683. [Published erratum appears in JAMA 323:1619.] [DOI] [PubMed] [Google Scholar]
- 4.D’Antiga L. Coronaviruses and immunosuppressed patients: the facts during the third epidemic. Liver Transpl. 2020;26:832–834. doi: 10.1002/lt.25756. [DOI] [PubMed] [Google Scholar]
- 5.Korean Society of Infectious Diseases and Korea Centers for Disease Control and Prevention. Analysis on 54 mortality cases of coronavirus disease 2019 in the Republic of Korea from January 19 to March 10, 2020. J Korean Med Sci. 2020;35:e132. doi: 10.3346/jkms.2020.35.e132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hwang DM, Chamberlain DW, Poutanen SM, Low DE, Asa SL, Butany J. Pulmonary pathology of severe acute respiratory syndrome in Toronto. Mod Pathol. 2005;18:1–10. doi: 10.1038/modpathol.3800247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schauwvlieghe AFAD, Rijnders BJA, Philips N, Verwijs R, Vanderbeke L, Van Tienen C, et al. Dutch-Belgian Mycosis study group. Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: a retrospective cohort study. Lancet Respir Med. 2018;6:782–792. doi: 10.1016/S2213-2600(18)30274-1. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz IS, Friedman DZP, Zapernick L, Dingle TC, Lee N, Sligl W, et al. High rates of influenza-associated invasive pulmonary aspergillosis may not be universal: a retrospective cohort study from Alberta, Canada. Clin Infect Dis. 2020;71:1760–1763. doi: 10.1093/cid/ciaa007. [DOI] [PubMed] [Google Scholar]
- 9.Alanio A, Dellière S, Fodil S, Bretagne S, Mégarbane B. Prevalence of putative invasive pulmonary aspergillosis in critically ill patients with COVID-19. Lancet Respir Med. 2020;8:e48–e49. doi: 10.1016/S2213-2600(20)30237-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blaize M, Mayaux J, Nabet C, Lampros A, Marcelin AG, Thellier M, et al. Fatal invasive aspergillosis and coronavirus disease in an immunocompetent patient. Emerg Infect Dis. 2020;26:1636–1637. doi: 10.3201/eid2607.201603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koehler P, Cornely OA, Böttiger BW, Dusse F, Eichenauer DA, Fuchs F, et al. COVID-19 associated pulmonary aspergillosis. Mycoses. 2020;63:528–534. doi: 10.1111/myc.13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nasir N, Farooqi J, Mahmood SF, Jabeen K. COVID-19-associated pulmonary aspergillosis (CAPA) in patients admitted with severe COVID-19 pneumonia: an observational study from Pakistan. Mycoses. 2020;63:766–770. doi: 10.1111/myc.13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prattes J, Valentin T, Hoenigl M, Talakic E, Reisinger AC, Eller P.Invasive pulmonary aspergillosis complicating COVID-19 in the ICU - a case report Med Mycol Case Rep[online ahead of print] 11 May 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Arkel ALE, Rijpstra TA, Belderbos HNA, van Wijngaarden P, Verweij PE, Bentvelsen RG. COVID-19-associated pulmonary aspergillosis. Am J Respir Crit Care Med. 2020;202:132–135. doi: 10.1164/rccm.202004-1038LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bartoletti M, Pascale R, Cricca M, Rinaldi M, Maccaro A, Bussini L, et al. PREDICO study group Epidemiology of invasive pulmonary aspergillosis among COVID-19 intubated patients: a prospective study Clin Infect Dis[online ahead of print] 28 Jul 2020 [Google Scholar]
- 16.White PL, Dhillon R, Cordey A, Hughes H, Faggian F, Soni S, et al. A national strategy to diagnose COVID-19 associated invasive fungal disease in the ICU Clin Infect Dis[online ahead of print] 29 Aug 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lamoth F, Glampedakis E, Boillat-Blanco N, Oddo M, Pagani J-L. Incidence of invasive pulmonary aspergillosis among critically ill COVID-19 patients. Clin Microbiol Infect. 2020;26:1706–1708. doi: 10.1016/j.cmi.2020.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poignon C, Blaize M, Vezinet C, Lampros A, Monsel A, Fekkar A. Invasive pulmonary fusariosis in an immunocompetent critically ill patient with severe COVID-19. Clin Microbiol Infect. 2020;26:1582–1584. doi: 10.1016/j.cmi.2020.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blaize M, Mayaux J, Luyt C-E, Lampros A, Fekkar A.COVID-19 related respiratory failure and lymphopenia do not seem associated with pneumocystosis Am J Respir Crit Care Med[online ahead of print] 17 Sep 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fekkar A, Poignon C, Blaize M, Lampros A. Fungal infection during COVID-19: does Aspergillus mean secondary invasive aspergillosis? Am J Respir Crit Care Med. 2020;202:902–903. doi: 10.1164/rccm.202005-1945LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Donnelly JP, Chen SC, Kauffman CA, Steinbach WJ, Baddley JW, Verweij PE, et al. Revision and update of the consensus definitions of invasive fungal disease from the European organization for research and treatment of cancer and the mycoses study group education and research consortium. Clin Infect Dis. 2020;71:1367–1376. doi: 10.1093/cid/ciz1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Imbert S, Meyer I, Palous M, Brossas JY, Uzunov M, Touafek F, et al. Aspergillus PCR in bronchoalveolar lavage fluid for the diagnosis and prognosis of aspergillosis in patients with hematological and non-hematological conditions. Front Microbiol. 2018;9:1877. doi: 10.3389/fmicb.2018.01877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meliani L, Develoux M, Marteau-Miltgen M, Magne D, Barbu V, Poirot JL, et al. Real time quantitative PCR assay for Pneumocystis jirovecii detection. J Eukaryot Microbiol. 2003;50:651. doi: 10.1111/j.1550-7408.2003.tb00670.x. [DOI] [PubMed] [Google Scholar]
- 24.Millon L, Larosa F, Lepiller Q, Legrand F, Rocchi S, Daguindau E, et al. Quantitative polymerase chain reaction detection of circulating DNA in serum for early diagnosis of mucormycosis in immunocompromised patients. Clin Infect Dis. 2013;56:e95–e101. doi: 10.1093/cid/cit094. [DOI] [PubMed] [Google Scholar]
- 25.Du Y, Tu L, Zhu P, Mu M, Wang R, Yang P, et al. Clinical features of 85 fatal cases of COVID-19 from Wuhan: a retrospective observational study. Am J Respir Crit Care Med. 2020;201:1372–1379. doi: 10.1164/rccm.202003-0543OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loughlin L, Hellyer TP, White PL, McAuley DF, Conway Morris A, Posso RB, et al. Pulmonary aspergillosis in patients with suspected ventilator-associated pneumonia in UK ICUs. Am J Respir Crit Care Med. 2020;202:1125–1132. doi: 10.1164/rccm.202002-0355OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blot SI, Taccone FS, Van den Abeele AM, Bulpa P, Meersseman W, Brusselaers N, et al. AspICU Study Investigators. A clinical algorithm to diagnose invasive pulmonary aspergillosis in critically ill patients. Am J Respir Crit Care Med. 2012;186:56–64. doi: 10.1164/rccm.201111-1978OC. [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez-Goncer I, Thomas S, Foden P, Richardson MD, Ashworth A, Barker J, et al. Invasive pulmonary aspergillosis is associated with adverse clinical outcomes in critically ill patients receiving veno-venous extracorporeal membrane oxygenation. Eur J Clin Microbiol Infect Dis. 2018;37:1251–1257. doi: 10.1007/s10096-018-3241-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Falcoz PE, Monnier A, Puyraveau M, Perrier S, Ludes PO, Olland A, et al. Extracorporeal membrane oxygenation for critically ill patients with COVID-19-related acute respiratory distress syndrome: worth the effort? Am J Respir Crit Care Med. 2020;202:460–463. doi: 10.1164/rccm.202004-1370LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Vlieger G, Lagrou K, Maertens J, Verbeken E, Meersseman W, Van Wijngaerden E. Beta-D-glucan detection as a diagnostic test for invasive aspergillosis in immunocompromised critically ill patients with symptoms of respiratory infection: an autopsy-based study. J Clin Microbiol. 2011;49:3783–3787. doi: 10.1128/JCM.00879-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pfeiffer CD, Fine JP, Safdar N. Diagnosis of invasive aspergillosis using a galactomannan assay: a meta-analysis. Clin Infect Dis. 2006;42:1417–1427. doi: 10.1086/503427. [DOI] [PubMed] [Google Scholar]
- 32.Verweij PE, Rijnders BJA, Brüggemann RJM, Azoulay E, Bassetti M, Blot S, et al. Review of influenza-associated pulmonary aspergillosis in ICU patients and proposal for a case definition: an expert opinion. Intensive Care Med. 2020;46:1524–1535. doi: 10.1007/s00134-020-06091-6. [DOI] [PMC free article] [PubMed] [Google Scholar]