Abstract
Available tools to evaluate patients with central nervous system (CNS) tumors such as magnetic resonance imaging (MRI), cerebrospinal fluid (CSF) cytology, and brain biopsies, have significant limitations. MRI and CSF cytology have poor specificity and sensitivity, respectively, and brain biopsies are invasive. Circulating tumor DNA in CSF (CSF-ctDNA) could be used as a biomarker in patients with CNS tumors, but studies in this area are limited. We evaluated four CSF-ctDNA extraction methods and analyzed mutations in CSF-ctDNA with the Oncomine Pan-Cancer cell-free assay. CSF-ctDNA was extracted from 38 patients with primary or metastatic CNS tumors and 10 patients without CNS malignancy. Commercial ctDNA controls were used for assay evaluation. CSF-ctDNA yields ranged from 3.65 to 3120 ng. Mutations were detected in 39.5% of samples. TP53 was the most commonly mutated gene and copy number alterations were detected in CCND1, MYC, and ERBB2/HER2. Twenty-five percent of CSF-cytology–negative samples showed mutations in CSF-ctDNA. There was good concordance between mutations in CSF-ctDNA and matching tumors. The QIAamp Circulating Nucleic Acid Kit was the optimal method for extraction of CSF-ctDNA and the Oncomine cell-free DNA assay is suitable for detection of mutations in CSF-ctDNA. Analysis of CSF-ctDNA is more sensitive than CSF-cytology and has the potential to improve the diagnosis and monitoring of patients with CNS tumors.
Currently used methodologies to evaluate patients with central nervous system (CNS) tumors include magnetic resonance imaging, detection of malignant cells in cerebrospinal fluid (CSF-cytology), and tissue biopsies/resections, which have several limitations. Magnetic resonance imaging and CSF cytology have poor specificity and sensitivity, respectively, and brain biopsies are invasive. Therefore, there is a critical need for better methodologies to diagnose and evaluate patients with CNS malignancies. Liquid biopsies consist of analyzing circulating tumor cells or circulating nucleic acids in biofluids. In particular, several studies have addressed the utility of circulating tumor DNA (ctDNA) derived from plasma as a minimally invasive method of characterizing tumor mutations.1,2 However, studies have shown that plasma is suboptimal for the detection of ctDNA from CNS tumors.3,4 In contrast, CSF is a better source of cell-free DNA (cfDNA) to detect mutations derived from CNS tumors because of its proximity to the brain parenchyma.5, 6, 7 In addition, studies suggest that CSF ctDNA is more sensitive than CSF cytology for the evaluation of patients with CNS tumors.8,9 A recent study showed that CSF-ctDNA analysis can help clinical management by identifying actionable alterations and informing therapeutic decisions.10
Although the presence of ctDNA in CSF has been established previously, it is unclear which methodology is superior for optimal isolation of CSF-ctDNA.1,4,9,11,12 Similarly, it is unclear what the optimal sequencing assay is for identifying mutations in CSF-ctDNA. The Oncomine cell-free assay (Thermo Fisher Scientific, Waltham, MA) is a next-generation sequencing (NGS) panel evaluating alterations in the hotspot region of 52 cancer-associated genes, as well as copy number alterations in 12 genes. The recommended cfDNA input amount for the Oncomine assay is 20 ng. However, as low as 5 ng of cfDNA may be sufficient for evaluation of ctDNA with this assay (Thermo Fisher Scientific). Prior studies have performed successful NGS analysis of CSF-ctDNA starting with 0.75 to 7 mL of CSF to extract cfDNA with variable yields of approximately 1 to 100 ng.8,9,13,14 The objective of this study was to compare various methods for CSF-ctDNA isolation and to evaluate the utility of the Oncomine Pan-Cancer Cell-Free Assay (Oncomine) for the detection of CSF-ctDNA mutations.
CSF ctDNA from 38 patients with various types of primary and metastatic CNS tumors was also evaluated. CSF from patients with no history of CNS malignancy was used as control. Four different methods for CSF-ctDNA extraction were evaluated. Commercial cfDNA was used to evaluate the performance of the Oncomine assay. The sensitivity of CSF-ctDNA analysis with the Oncomine assay was compared with the results of CSF cytology. In addition, mutations detected in CSF-ctDNA were compared with those present in the corresponding tumor tissue.
Materials and Methods
CSF Samples
Thirty-eight CSF samples (Table 1) obtained from patients with a variety of primary or metastatic CNS tumors were selected for ctDNA analysis. The patient's diagnoses included the following: primary CNS tumors (n = 10), metastatic breast cancer (n = 17), lung cancer (n = 8), ovarian cancer (n = 1), ocular melanoma (n = 1), and uterine cancer (n = 1). CSF samples from patients without a history of CNS malignancy (n = 10) were used as controls (Table 1). Eight CSF samples were collected before treatment (Table 1) and the remaining 30 CSF samples were collected during treatment/follow-up evaluation. Patient ages ranged from 35 to 73 years. There were 27 females and 11 males. Demographic information for the control samples was not available. The study was approved by the Committee for the Protection of Human Subjects from the University of Texas Health Science Center at Houston and MD Anderson Cancer Center (Institutional Review Board PA16-0766, HSC-MS-17-0407).
Table 1.
Characteristics of Patients and Samples
| Sample no. | Age, years | Sex | Volume, mL | Total yield, ng | Primary site | CSF cytology | MRI | Parenchymal metastases | LMD | ctDNA genetic alterations (AF% or CNV ratio) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1† | 48 | F | 1.8 | 9.5 | Brain‡ | Negative | Positive | Yes | No | None detected |
| 2 | 61 | M | 3 | 11.45 | Brain‡ | Negative | Positive | Yes | No | None detected |
| 3† | 54 | M | 2.3 | 16.5 | Brain‡ | Negative | Positive | Yes | No | None detected |
| 4† | 45 | M | 2.7 | 113.5 | Brain‡ | N/A | Positive | Yes | No | None detected |
| 5† | 69 | M | 1.5 | 13.5 | Brain‡ | Negative | Positive | Yes | No | None detected |
| 6 | 72 | F | 2.4 | 11.9 | Brain‡ | Negative | Negative | No | No | None detected |
| 7 | 53 | F | 3 | 14.85 | Brain§ | Positive | Positive | Yes | Yes | None detected |
| 8† | 44 | M | 1 | 11.25 | Brain¶ | Negative | Positive | Yes | No | None detected |
| 9 | 24 | M | 1.3 | 9 | Brain‖ | Negative | Positive | Yes | No | None detected |
| 10 | 19 | M | 2.5 | 110 | Brain¶ | Negative | Positive | Yes | No | None detected |
| 11 | 73 | F | 2.5 | 12.3 | Breast | Positive | Positive | No | Yes |
PIK3CA p.H1047R (15.2%) FGFR3 p.F384L (31.8%) FGFR2 p.Y375C (6.8%) |
| 12 | 71 | F | 4.9 | 8.45 | Breast | Positive | Positive | No | Yes | AKT1 p.E17K (31.8%) |
| 13 | 52 | F | N/A | 3.63 | Breast | Negative | Negative | No | No | None detected |
| 14 | 54 | F | 3 | 4.35 | Breast | Negative | Positive | Yes | Yes | FBXW7 p.R505C (7.9%) |
| 15 | 35 | F | 1.1 | 16.6 | Breast | Positive | Positive | No | No | None detected |
| 16 | 59 | F | 1 | 22.8 | Breast | Negative | Positive | No | Yes | ERBB2 ↑ (2.88) |
| 17 | 43 | F | N/A | 38.45 | Breast | Positive | Positive | No | Yes |
ESR1 p.D538G (78.5%) MAP2K1 p.F129L (0.38%) MYC ↑ (3.9) CCND1 ↑ (5.8) |
| 18 | 54 | F | 0.7 | 33 | Breast | Negative | Positive | Yes | Yes | None detected |
| 19 | 71 | F | 2 | 4.4 | Breast | Positive | Positive | No | Yes | AKT1 p.E17K (15.4%) |
| 20 | 71 | F | 3 | 8.4 | Breast | Negative | Positive | Yes | Yes | None detected |
| 21 | 59 | F | 3.6 | 42.25 | Breast | Negative | Positive | No | Yes | None detected |
| 22 | 44 | F | 4 | 24.6 | Breast | Negative | Positive | N/A | N/A |
TP53 p.V157F (2.8%) MYC ↑ (1.8) |
| 23 | 54 | F | 4 | 102 | Breast | Negative | Positive | Yes | Yes |
TP53 p.H193R (75.7%) ERBB2 ↑ (11.61) |
| 24 | 47 | F | 3 | 19.9 | Breast | Positive | Positive | Yes | Yes | TP53 p.R196∗ (50.5%) |
| 25 | 42 | F | 2.7 | 5.6 | Breast | Atypical | Negative | No | Yes | None detected |
| 26 | 71 | F | 4 | 48.25 | Breast | Positive | Positive | No | Yes |
AKT1 p.E17K (27.7%) CCND1 ↑ (1.96) |
| 27 | 51 | F | 4 | 73.5 | Breast | Positive | Positive | No | Yes | PIK3CA p.H1047R (24.0%) |
| 28 | 54 | F | 2.8 | 7.25 | Lung | Negative | Positive | Yes | Yes |
EGFR p.S768I (14.5%) EGFR p.L858R (11.9%) |
| 29 | 66 | M | 4 | 88 | Lung | Negative | Negative | No | No | None detected |
| 30 | 56 | F | 2 | 9.85 | Lung | Negative | Positive | No | Yes | None detected |
| 31† | 54 | M | 3 | 62.5 | Lung | N/A | Positive | Yes | Yes | None detected |
| 32† | 65 | M | 2.4 | 421.5 | Lung | Negative | Positive | Yes | No | TP53 p.G245C (21.6%) |
| 33† | 58 | F | 2.1 | 3120 | Lung | N/A | Positive | Yes | No | TP53 p.C176R (73.1%) |
| 34 | 65 | M | 3 | 36.4 | Lung | Negative | Positive | Yes | No | None detected |
| 35 | 53 | F | 3 | 10.95 | Lung | Negative | Positive | Yes | No | None detected |
| 36 | 47 | F | 3.1 | 104 | Melanoma | Negative | Positive | No | No | None detected |
| 37 | 64 | F | 3 | 4.95 | Ovary | Negative | Positive | Yes | Yes | None detected |
| 38 | 66 | F | 2.8 | 25 | Uterine | Positive | Positive | Yes | Yes | TP53 p.V272M (1.4%) |
| Sample no. | Volume, mL | Total yield, ng | Diagnosis | Parenchymal metastases | LMD |
|---|---|---|---|---|---|
| C1 | 1.43 | 71.5 | Hydrocephalus | No | No |
| C2 | 1.55 | 77.5 | Hydrocephalus | No | No |
| C3 | 1.73 | 86.5 | Hydrocephalus | No | No |
| C4 | 0.516 | 25.8 | Hydrocephalus | No | No |
| C5 | 0.343 | 17.15 | Hydrocephalus | No | No |
| C6 | 0.736 | 36.8 | Hydrocephalus | No | No |
| C7 | 1.19 | 59.5 | Hydrocephalus | No | No |
| C8 | 5.3 | 265 | Chiari malformation | No | No |
| C9 | 1.64 | 82 | Chiari malformation | No | No |
| C10 | 3.16 | 158 | Hydrocephalus | No | No |
↑, gene amplification; MAF%, Mutant Allele Frequency; CNV ratio, copy number ratio; CSF, cerebrospinal fluid; LMD, leptomeningeal disease; MRI, magnetic resonance imaging; N/A, cannot be evaluated.
Sample was collected before treatment.
Six glioblastomas.
Two ependymomas/grade II.
One supratentorial primitive neuroectodermal tumor.
One subependymal giant cell astrocytoma/grade I.
cfDNA Extraction from CSF
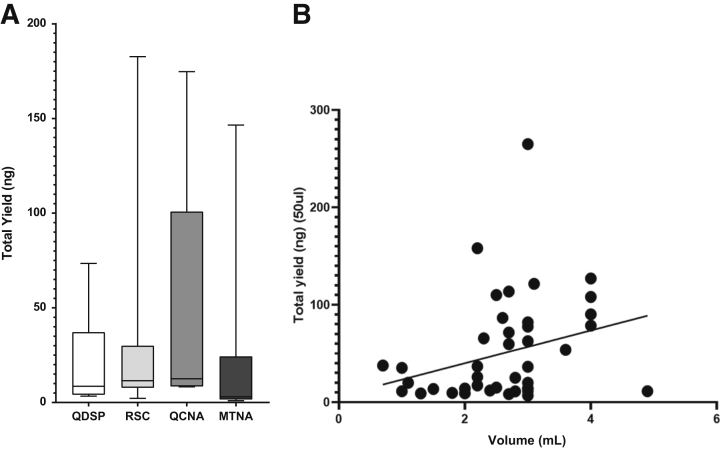
Ten additional CSF samples were selected for cell-free nucleic acid isolation using four different extraction kits [QIAamp Circulating Nucleic Acid (Qiagen, Hilden, Germany), Magmax Cell-free total Nucleic acid (Thermo Fisher Scientific), QIAsymphony DSP Circulating DNA (Qiagen), and Promega Maxwell RSC circulating cell-free DNA (Promega, Madison, WI)] with a starting volume of 1 mL for each kit. Extraction was performed according to the manufacturer's instructions. The final elution volume was 15 μL. Isolated nucleic acids were evaluated using Qubit (Thermo Fisher Scientific) and Tape Station (Agilent Technologies, Santa Clara, CA) to select the optimal extraction kit. The optimal method (QIAamp Circulating Nucleic Acid; Qiagen) was used to extract cfDNA from all remaining study samples for ctDNA analysis with the Oncomine assay (Figure 1).
Figure 1.
Comparison of DNA extraction kits. A: Bar graphs showing total yield (in nanograms) of cell-free DNA extracted from the same 10 samples using four different extraction kits. Yield (in nanograms) ranged from 0.95 to 182.70. The highest yield was obtained with the QIAmp Circulating Nucleic Acid kit (QCNA). B: A trend was observed when comparing the volume of CSF versus the total yield of circulating tumor DNA (P = 0.06; r2 = 0.08). Samples 32 and 33 (outliers) were not considered for this analysis. MTNA, Magmax Cell-free Total Nucleic Acid; QDSP, QIAsymphony DSP Circulating DNA; RSC, cell-free DNA extraction Maxwell RSC system.
DNA Extraction from Formalin-Fixed, Paraffin-Embedded Tissue and Comparison of Tissue and CSF Results
Corresponding tumor tissue blocks were available for 7 of the 38 patients (patients 13, 14, 17, 18, 22, 31, and 33) with CNS tumors included in the study. Ten 5-μm slides were used to extract DNA. Hematoxylin and eosin slides were evaluated histologically by board-certified pathologists (L.Y.B. and H.C.), and the tumor-enriched region was marked for microdissection. AllPrep DNA/RNA formalin-fixed, paraffin-embedded (Qiagen) was used to isolate DNA for NGS according to the manufacturer's instructions. Deparaffination was performed using Hemo-De solution (Thermo Fisher Scientific) for 10 minutes, and air drying for 10 to 15 minutes before microdissection. The final elution was performed in 2 steps (25 μL and 15 μL). For the remaining 10 patients who were included in the comparison of tissue versus CSF ctDNA, the mutations were obtained from the patient's electronic medical record. Only genes that were examined in both tissue and CSF were included in the concordance analysis.
Targeted NGS Assay
NGS was performed using the Oncomine Pan-cancer cell-free assay. At least 1.3 ng ctDNA was used for library preparation. Libraries were quantified via Tape station (Agilent), and templating was performed with the Ion 540 Kit-Chef (Thermo Fisher Scientific). Samples were sequenced at high depth on the Ion S5 XL sequencer and analyzed with Torrent Suite Software v.5.2 and Ion Reporter versions 5.6 and 5.10 (Thermo Fisher Scientific) using Human Genome Build 19 as the reference. The workflow for Oncomine TagSeq Pan-Cancer Liquid Biopsy w2.1 was used with default parameters. Oncomine variant annotator version 2.4 was used for variant annotation. The mean sequencing depth for all samples was 23,160x. Copy number variation (CNV) sensitivity was set to medium. Some CNVs were identified in control CSF samples without a history of cancer (range, 1.30- to 1.69-fold), these were considered nonspecific alterations. Therefore, a cut-off value of 1.7-fold or higher was set up to report a CNV alteration. Low allele frequency single-nucleotide variants (SNVs) were identified in the CSF from control patients (range, 0.07% to 0.20%). Therefore, a cut-off value of 0.3% was set to report a SNV. The recommended DNA input to achieve 0.1% SNV limit of detection is 20 ng, but not all CSF samples yielded 20 ng (Supplemental Figure S1). The range of cfDNA input for library preparation was 1.3 to 20 ng. The libraries were prepared and sequenced following the manufacturer's instructions.
Digital Droplet PCR
The digital droplet PCR AKT1 p.E17K assay (dHsaCP2000031) and PIK3CA p.H1047R assay (dHsaCP20000077) were used to confirm a subset of mutations identified with the Oncomine Pan-cancer cell-free assay. Samples were run in duplicate with positive, negative, and no-template controls. Two microliters of the sample were used for setting up the digital droplet PCR reaction with 2 ng input. Droplets were generated using the Bio-Rad (Hercules, CA) automatic droplet generator, after which PCR amplification was performed according to the manufacturer's instructions. At least 10,000 droplets were required for droplet generation to be considered successful. Droplets were read with the QX200 droplet digital PCR system (Bio-Rad) and analyzed using QuantaSoft software version 1.7 (Bio-Rad).9
Results
Comparison of CSF-ctDNA Extraction Methods
To evaluate which commercially available cfDNA extraction kit provides the maximum yield and optimal sample quality, 10 CSF samples were used to extract cfDNA using an initial CSF volume of 1 mL for each kit. Two automated kits [QIAsymphony DSP Circulating DNA (Qiagen) and cfDNA extraction Maxwell RSC system (Promega)], and two manual kits [QIAamp Circulating Nucleic Acid (QCNA; Qiagen), Magmax Cell-free Total Nucleic Acid (Thermo Fisher Scientific)] were tested using the same 10 CSF samples. On average, the Magmax Cell-free Total Nucleic Acid kit gave the lowest yield while the QCNA kit gave the highest yield (Figure 1A). In addition, higher ctDNA concentrations were obtained with the QCNA (Supplemental Table S1). On average, the cfDNA yield from 1 mL CSF was 21.0 ng from the Magmax Cell-free Total Nucleic Acid kit, 47.4 ng from the QCNA kit, 32.0 ng from the cfDNA extraction Maxwell RSC system, and 21.2 ng from QIAsymphony DSP Circulating DNA kit. Therefore, the QCNA kit was selected to extract patient samples because it produced a higher ctDNA yield. CSF ctDNA for the 38 study samples was extracted using the QCNA kit. The volume of CSF used for extraction ranged from 0.7 to 4.9 mL. The yield of cfDNA obtained ranged from 3.65 to 3120 ng. Although not significant, a trend was observed between larger CSF volumes and cfDNA yield (Table 1 and Figure 1B).
Performance Characteristics of the Oncomine-cfDNA Assay
cfDNA Input for Library Preparation
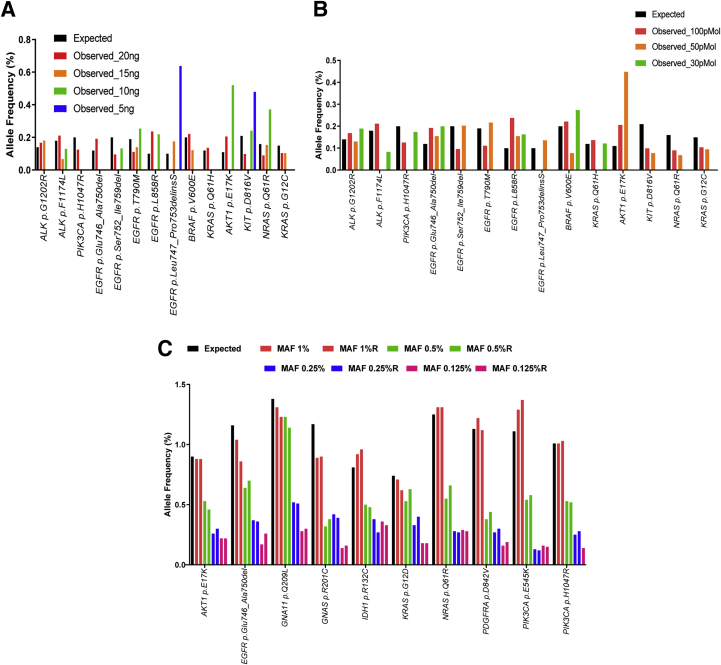
The recommended amount of ctDNA input for the Oncomine-cfDNA panel is 20 ng. A Seraseq ctDNA Complete Mutation Mix AF 0.1% (Seracare, Milford, MA) synthetic ctDNA control sample was used to determine if lower input amounts of cfDNA were sufficient to maintain a limit of detection of 0.1% for SNVs as the panel guidelines suggest. The expected mutant allele frequency (MAF) in the commercial control (provided by the manufacturer) was compared using 20 ng, 15 ng, 10 ng, and 5 ng of starting cfDNA. Dilutions were made up by adding nuclease-free water. As the cfDNA input decreased, the number of mutations identified also decreased. At 20 ng of input, 14 of 15 (93.3%) mutations were detected; while at 15 ng, 10 ng, and 5 ng, 8 of 15 (53.3%), 7 of 15 (46.7%), and 2 of 15 (13.3%) mutations were detected, respectively (Figure 2A).
Figure 2.
Performance of the Oncomine cell-free DNA assay. A: The commercially available Seraseq circulating tumor DNA (ctDNA) CompleteMutation Mix AF0.1% was used in varying amounts (20, 15, 10, and 5 ng) for library preparation. The mutant allele frequency (MAF) (%) for the various mutations detected is depicted in the y axis. At 20 ng of input, 14 of 15 (93.3%) mutations were detected; while at 15 ng, 10 ng, and 5 ng, 8 of 15 (53.3%), 7 of 15 (46.7%), and 2 of 15 (13.3%) mutations were detected, respectively. B: MAF (%) of variants detected in Seraseq ctDNA Complete Mutation Mix AF0.1% with varying library inputs of 100 pMol, 50 pMol, and 30 pMol. A starting amount of 20 ng was used for library preparation and various amounts of library input were used for sequencing. At 100 pmol of input, 13 of 15 (86.7%) mutations were identified; while at 50 pmol and 30 pmol, 10 of 15 (66.7%) and 7 of 15 (46.7%) of the mutations were identified, respectively. C: Seraseq ctDNA Reference Material v2 AF1% was serially diluted (1%, 0.5%, 0.25%, and 0.125%). The experiment was performed in duplicate. Twenty nanograms of ctDNA and 100 pmol of library were used for sequencing. The MAF of the variants detected is shown in the y axis. Ten mutations were detected for all dilutions. APC p.1450∗, CTNNB1 p.T41A, EGFR p.T790M, EGFR p.L858R, ERBB2 p.Glu770_Ala771 ins AlaTyrValMet, FLT3 p.D835Y, GNAQ p.Q209P, KIT p.D816V, RET p.M918T, TP53 p.R248Q, TP53 p.R175H, and TP53 p.R273H were missed at 0.125% dilution, and BRAF p.V600E was missed at 0.25% and 0.125% dilutions. This can be attributed to having an allele frequency lower than the limit of detection. TP53 p.S90fs was not detected because it is not covered by the panel.
DNA Library Input for NGS
The recommended ctDNA library input for the Oncomine-cfDNA panel is 100 pmol. The optimal library input for maximum mutation detection was determined using the Seraseq ctDNA Complete Mutation Mix AF 0.1% synthetic ctDNA control. All of the libraries were made using the 20 ng ctDNA input. The expected MAF (provided by the manufacturer) was compared between the different DNA library input amounts (100 pMol, 50 pMol, and 30 pMol). It was observed that as the library input decreased, the number of mutations identified also decreased. At 100 pMol of input, 13 of 15 (86.7%) mutations were identified; while at 50 pMol and 30 pMol, 10 of 15 (66.7%) and 7 of 15 (46.7%) of the mutations were identified, respectively (Figure 2B). Because a reduction in mutation detection is seen at lower library inputs and a lower sample input, 100 pMol library input and 20 ng sample input were considered optimal for the analysis of CSF ctDNA.
cfDNA Dilution Study
The indicated limit of detection for MAF for the Oncomine-cfDNA panel is 0.1% with 20 ng input and 100 pMol library input. To test this we used the Seraseq ctDNA Reference Material v2 AF1% (Seracare) synthetic ctDNA control. Serial dilutions (1, 1:2, 1:4, and 1:8) were made using Seraseq ctDNA Reference Material. The dilutions were performed in duplicate and libraries were made and sequenced using the Oncomine-cfDNA panel with 20 ng sample input and 100 pMol library input. The expected allele frequency (provided by the manufacturer) was compared among the various dilutions: 1%, 0.5%, 0.25%, and 0.125%. It was observed that 23 of 24 (95.8%) mutations were detected in all of the diluted samples. All of the duplicates showed the same mutations with comparable MAF (Figure 2C).
Analysis of CSF cfDNA in Control Samples
NGS was performed using CSF-cfDNA obtained from patients with no history of CNS malignancy (n = 10). Low allele frequency SNVs (0.07% to 0.21%) and low-level amplifications (1.3- to 1.65-fold) were identified in 4 of the 10 control samples (Supplemental Table S2). Therefore, a cut-off value of 0.25% allele frequency for SNVs and 1.7-fold for CNVs were used as part of the criteria to report alterations in CSF ctDNA from patients with a history of CNS cancer.
Analysis of CSF ctDNA from Patients with CNS Malignancies
Circulating ctDNA from CSF samples was extracted using the QCNA extraction kit. Ten samples from patients without a history of cancer were used as controls; no mutations were detected in the CSF of control patients. The Oncomine-cfDNA panel was used to analyze CSF ctDNA and at least one genetic alteration was identified in 15 of 38 (39.5%) samples. Overall, 18 SNVs involving eight genes were identified. TP53 was the most commonly mutated gene observed in six patients, and six CSF samples showed the presence of copy number alterations in CCND1, MYC, and ERBB2 (Table 1). Of the 17 CSF samples derived from patients with metastatic breast cancer, 11 samples showed at least one genetic alteration (11 of 17; 64.7%). Thirteen SNVs were found with common mutations in TP53, AKT1, and PIK3CA. Three of eight (37.5%) CSF samples derived from patients with metastatic lung cancer had at least one genetic mutation. Four SNVs were identified in TP53 and EGFR (Table 1). Ten CSF samples derived from patients with primary CNS tumors were examined but no mutations were identified (0 of 10). A TP53 mutation was identified in the patient with metastatic uterine carcinoma. No mutations were identified in 23 of 38 CSF samples (Table 1).
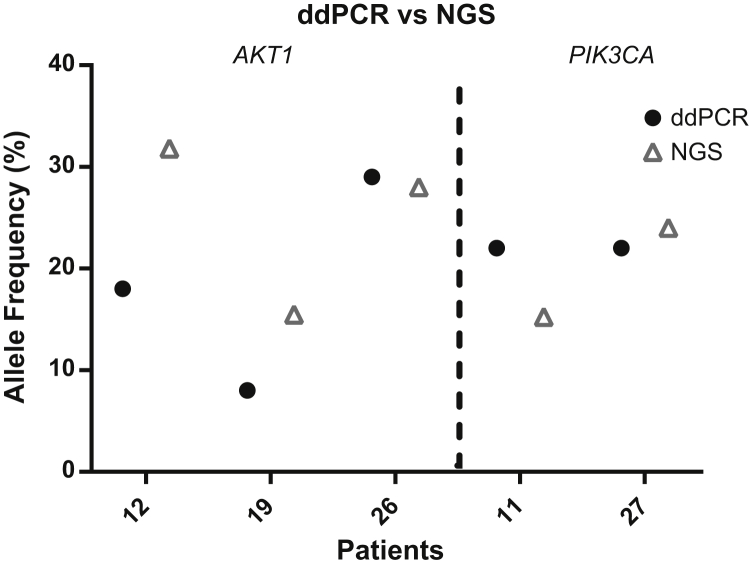
To confirm some of the results obtained with the Oncomine-cfDNA assay, five CSF samples, three with AKT1 p.E17K and two with PIK3CA p.H1047R mutations, also were analyzed using digital droplet PCR. All mutations (5 of 5) also were identified by digital droplet PCR, with comparable MAF (Figure 3).
Figure 3.
Orthogonal confirmation by digital droplet PCR (ddPCR). Five CSF samples with mutations in PIK3CA and AKT1 detected by next-generation sequencing (NGS) were analyzed by ddPCR with similar results.
Because the mean sequencing depth can influence the detection of variants with low allele frequency, the relationship between sequencing depth and mutation detection in the samples from patients with a magnetic resonance image positive for malignancy at the time of CSF collection was evaluated. A mutation was detected in CSF ctDNA in 26.3% of samples with a mean sequencing depth of <10,000×. In contrast, mutations were detected in 52.6% of samples with a mean sequencing depth of >10,000×. A relationship between mean sequencing depth and CSF volume was not observed. However, as expected, a correlation was observed between cfDNA input and mean sequencing depth, with an average depth of 38,321× for samples with 20 ng cfDNA input and 18,132× for samples with <20 ng cfDNA input.
Copy Number Variants
Six CNVs were identified in CSF-ctDNA from five samples with a ratio greater than 1.7-fold. The five samples were from patients with metastatic breast cancer. The variants included two MYC amplifications, two CCND1 amplifications, and two ERBB2 amplifications (Table 1).
Concordance between Mutations in CSF ctDNA and Tumor Tissue
Of the 15 patients in whom mutations were detected in CSF-ctDNA, 8 patients also had the results of sequencing analysis from tumor tissue available in their electronic medical record and an Oncomine-cfDNA panel. A comparison of the mutations identified in tumor tissue with the mutations identified in CSF-ctDNA showed partial concordance (Table 2 and Supplemental Table S3). Complete concordance was seen in two patients, and six patients showed partial concordance between mutations identified in tumor tissue and CSF-ctDNA. Sixty-seven percent (10 of 15) of the mutations detected in tissue also were seen in CSF-ctDNA; although 33% (5 of 15) of the mutations detected in CSF-ctDNA were not detected in tumor tissue. Sample 28 had three EGFR mutations identified in tissue whereas two of three mutations also were identified in CSF-ctDNA analysis (Table 2). Copy number variants were detected in four samples, a total of five CNVs were identified with a ratio above the established cut-off value of 1.7-fold. Three CNVs were identified in three tissue samples, which also were detected in CSF. Two CNVs were detected in CSF ctDNA but not reported in the tumor tissue.
Table 2.
Comparison of Genomic Alterations Detected in Tissue and CSF ctDNA
| ID | Primary tumor | Tissue analyzed | Tissue alterations | CSF ctDNA alterations |
|---|---|---|---|---|
| 14∗ | Breast | Breast |
FBXW7 p.R505C CTNNB1 p.S45F HRAS p. G12C ERBB2 p.R896C |
FBXW7 p.R505C |
| 16 | Breast | Breast | ERBB2 ↑ | ERBB2 ↑ |
| 17∗ | Breast | Pericardium | ESR1 p.D538G |
ESR1 p.D538G MAP2K1 p.F129L MYC ↑ CCND1 ↑ |
| 22∗ | Lung | Brain |
TP53 p.V157F MYC ↑ |
FBXW7 p.R505C TP53 p.V157F MYC ↑ |
| 23 | Breast | Breast | ERBB2 ↑ | ERBB2 ↑ |
| 27 | Breast | Bone marrow | TP53 p.P48R |
PIK3CA p.H1047R TP53 p.P48R |
| 28 | Lung | Lung |
EGFR p.S768I EGFR p.L858R EGFR p.T790M |
EGFR p.S768I EGFR p.L858R |
| 33∗ | Lung | Brain |
KIT p.T500_S501insSA TP53 p.C176R |
TP53 p.C176R |
Bold entries in Table indicate genetic variations showing concordance between tissue and CSF ctDNA.
↑, gene amplification.
CSF, cerebrospinal fluid; ctDNA, circulating tumor DNA.
Tissue sample sequenced with the Oncomine Pancancer Assay. ERBB2 amplification in the tissue was detected by fluorescence in situ hybridization.
Comparison of CSF-ctDNA Analysis with CSF Cytology
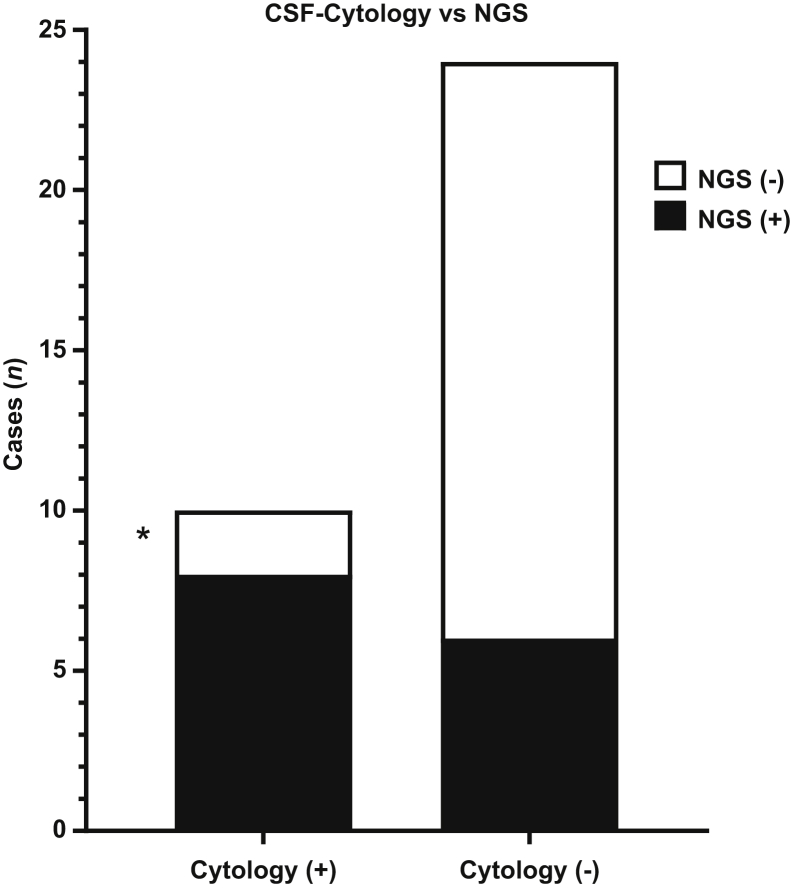
To compare the sensitivity of the Oncomine-cfDNA assay with the results of CSF cytology two × two contingency table analysis was used. Three of the 38 CSF samples had no CSF cytology results available and were excluded from the analysis (Table 1). Mutations in CSF-ctDNA were detected in all but two (samples 7 and 15) of the CSF samples with a positive CSF cytology result (8 of 10; 80%). In addition, mutations in CSF-ctDNA also were detected in 6 of 24 (25%) CSF samples with a negative CSF cytology result (Figure 4).
Figure 4.
Comparison of cerebrospinal fluid (CSF) cytology and CSF circulating tumor DNA (ctDNA) analysis. Mutations were identified in 8 of 10 (80%) samples with a positive CSF cytology result. In addition, mutations in CSF-ctDNA were identified in 6 of 24 (25%) CSF samples with a negative CSF cytology result. Asterisk indicates low input.
Discussion
Four different cfDNA extraction methods were compared using CSF samples (two automated and two manual methods). In our opinion, the QCNA extraction method provided the best overall performance in terms of yield and DNA integrity. One limitation of this extraction method was that it is a manual method with less throughput capacity than the automated methods. Although the Magmax Cell-free Total Nucleic Acid kit is recommended by the manufacturer for use with the Oncomine-cfDNA assay, we found the performance of this method for CSF-ctDNA extraction to be less optimal than the QCNA.
It is apparent from our results that the volume of the initial CSF plays an important role in the cfDNA yield. The CSF volume used in our study ranged from 0.7 to 4.9 mL and cfDNA yields ranged from 3.65 to 3120 ng. Seventy-six percent (76%) of samples yielded cfDNA amounts below the 20 ng recommended as input for the Oncomine-cfDNA assay (Supplemental Figure S1). Our experiments show that input amounts below 20 ng affect the ability of this assay to detect mutations in CSF-ctDNA and thus will affect assay sensitivity. It is possible that larger CSF volumes will increase the ability of the assay to detect mutations in CSF-ctDNA.
The performance characteristics of the Oncomine pan-cancer cell-free assay for the detection of genomic alterations in ctDNA derived from the CSF of patients with CNS malignancies was analyzed. CSF-ctDNA from 38 patients with various types of primary CNS tumors, metastatic CNS tumors, and controls (patients without a history of CNS malignancy) were evaluated. Overall, mutations in CSF-ctDNA were identified in 15 of 38 CSF samples from patients with CNS tumors, consistent with prior results showing that mutations in CSF-ctDNA can be identified in approximately 40% to 60% of samples.8,13 Variants were identified in 4 of 10 CSF samples from controls. The majority of the variants in control samples were CNVs (ratio, ≤1.65) and mutations with a MAF of 0.21% or less (Supplemental Table S2). These variants could be easily identified and removed with the established cut-off values, therefore, the Oncomine-cfDNA assay can achieve high specificity. Our results show that the Oncomine-cfDNA panel can detect alterations successfully (SNV and CNV) in CSF-ctDNA with good specificity. However, the sensitivity of the assay is affected significantly by the low amounts of cfDNA obtained from many CSF samples.
The literature shows that mutations in CSF can be detected in approximately 50% of patients with a CNS tumor.13,15 Although there might be some contribution from the tumor's proximity to a CSF space, the reasons for this limitation in sensitivity in previous studies remain unclear. It is possible that assays with the ability to detect mutations with a low MAF could improve the sensitivity of CSF-ctDNA analysis in patients with CNS tumors. The Oncomine-cfDNA assay detected 3 mutations with a MAF of <3% in CSF-ctDNA [TP53 p.V157F (2.8%), TP53 p.V272M (1.4%), and MAP2K1 p.F129L (0.38%)]. It is possible that these mutations would have been missed by less-sensitive NGS assays. Concordant CNVs in CSF and tissue were identified in 3 cases. Although this supports the idea that CNVs can be detected by analysis of CSF-ctDNA, the sample size was small and additional studies are required to evaluate the utility of the CNV detection in the CSF of patients with primary or metastatic CNS malignancies.
It is apparent from our results that there are differences in the frequency of detecting mutations in CSF-ctDNA from patients with different tumor types. For example, no mutations were detected in CSF samples from patients with primary CNS tumors, whereas mutations were detected in 11 of 17 (64.7%) patients with metastatic breast cancer. This difference likely is multifactorial and is influenced by factors intrinsic to the biology of the primary or metastatic CNS tumors, and by factors intrinsic to the Oncomine-cfDNA assay. For example, one factor limiting the ability of the Oncomine-cfDNA assay to detect mutations in the CSF of patients with primary CNS tumors is that some of the genes frequently mutated in primary CNS tumors such as TERT promoter (TERTp), histone H3 (H3F3A), are not investigated by the assay. In addition to larger CSF volumes, using an NGS assay that investigates mutations in genes frequently altered in primary CNS tumors (such as TERTp and H3F3A)11,12,16 will increase the sensitivity of CSF-ctDNA analysis for patients with primary CNS malignancies. The Oncomine-cfDNA assay is better suited for the detection of genetic alterations in metastatic CNS tumors. This is evident by a detection rate of mutations in 64.7% and 37.5% of CSF samples from patients with metastatic breast cancer and lung cancer, respectively.
The mutations detected in CSF-ctDNA were compared with mutations present in matching tumor tissue from the same patients. Overall, there is relatively good concordance between mutations in CSF-ctDNA and tumor tissue, however, several discrepancies were identified. These discrepancies might reflect heterogeneity in the alterations present in primary lesions/systemic metastatic lesions versus CNS lesions because some of the tumor tissues used for comparison were outside the CNS.17,18 However, discrepancies were identified even when CNS tumor tissue was used for comparison, these cases might be owing to intratumor heterogeneity within the CNS tumor tissue or limited sensitivity of the NGS assay, which can be influenced by the amount of ctDNA.19 It also is possible that the low input of cfDNA used for some cases diminishes the sensitivity of the assay to detect all of the mutations present in CSF-ctDNA. Higher concordance between tissue and CSF-ctDNA is likely to be observed if larger cfDNA input is used for NGS analysis of CSF-ctDNA.
Previous studies have reported good concordance between mutations detected in CSF-ctDNA and tissue from primary or metastatic CNS tumors.3,13 In the present study mutations in CSF-ctDNA with a primary CNS tumor were not observed, therefore, only CSF tissue concordance could be examined in the setting of metastatic lesions. Our results show good CSF tissue concordance, which is superior to that reported in recent studies analyzing ctDNA in the plasma of patients with primary CNS tumors (ie, glioblastoma) in which none of the mutations in the tumor tissue were detected in plasma ctDNA.20 These data and existing literature support the idea that CSF is superior to plasma for the analysis of circulating tumor DNA in patients with CNS malignancies.3,13
The Oncomine assay detected mutations in all patients with positive CSF-cytology with the exception of patient 7 (an ependymoma) and patient 15 (primary breast cancer metastasized to the brain). The lack of mutations detected in this sample could be attributed to a false-negative result of the NGS assay, owing to low cfDNA input amount (<5 ng) or to the absence of mutations in the investigated genes. Alternatively, the possibility of a false-positive CSF-cytology result also should be considered. The genes included in the Oncomine assay usually are not altered in ependymomas and a re-review of the CSF cytology confirmed the presence of tumor cells in this case. Therefore, this was interpreted as a false-negative result of the Oncomine assay, likely owing to inadequate coverage of genes altered in this particular tumor type.
In conclusion, our results show that the QCNA is an optimal method for the extraction of CSF-ctDNA and the Oncomine-cfDNA assay is suitable for the detection of mutations in CSF-ctDNA from patients with the most common metastatic CNS tumors (ie, breast and lung) including SNV and CNV such as ERBB2 amplification. In addition, our results indicate that the analysis of CSF-ctDNA can be more sensitive than CSF cytology for the evaluation of patients with CNS malignancies and it could be a good complement in the evaluation of CSF from patients with suspected CNS disorders. Our results indicate that larger volumes of CSF to isolate enough amount of cfDNA (≥20 ng) are required for optimal assay sensitivity. Use of CSF-cfDNA for mutational analysis is informative and has the potential to improve the care of patients with primary or metastatic CNS tumors. This approach may allow for minimally invasive diagnosis and monitoring of patients by facilitating molecular characterization of tumors without the need for obtaining brain tumor tissue.
Footnotes
Supported by the National Cancer Institute, NIH, award K08CA241651 (L.Y.B.).
Disclosures: None declared.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Supplemental material for this article can be found at http://doi.org/10.1016/j.jmoldx.2020.10.013.
Contributor Information
Yoshua Esquenazi, Email: yoshua.esquenazilevy@uth.tmc.edu.
Leomar Y. Ballester, Email: leomar.y.ballester@uth.tmc.edu.
Supplemental Data
Pie chart depicting the percentage of samples with range of cfDNA input for library preparations.
References
- 1.Siravegna G., Marsoni S., Siena S., Bardelli A. Integrating liquid biopsies into the management of cancer. Nat Rev Clin Oncol. 2017;14:531–548. doi: 10.1038/nrclinonc.2017.14. [DOI] [PubMed] [Google Scholar]
- 2.Perakis S., Speicher M.R. Emerging concepts in liquid biopsies. BMC Med. 2017;15:1–12. doi: 10.1186/s12916-017-0840-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Mattos-Arruda L., Mayor R., Ng C.K.Y., Weigelt B., Martínez-Ricarte F., Torrejon D., Oliveira M., Arias A., Raventos C., Tang J., Guerini-Rocco E., Martínez-Saéz E., Lois S., Marín O., De La Cruz X., Piscuoglio S., Towers R., Vivancos A., Peg V., Cajal S.R.Y., Carles J., Rodon J., González-Cao M., Tabernero J., Felip E., Sahuquillo J., Berger M.F., Cortes J., Reis-Filho J.S., Seoane J. Cerebrospinal fluid-derived circulating tumour DNA better represents the genomic alterations of brain tumours than plasma. Nat Commun. 2015;6:1–6. doi: 10.1038/ncomms9839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pan C., Diplas B.H., Chen X., Wu Y., Xiao X., Jiang L., Geng Y., Xu C., Sun Y., Zhang P., Wu W., Wang Y., Wu Z., Zhang J., Jiao Y., Yan H., Zhang L. Molecular profiling of tumors of the brainstem by sequencing of CSF-derived circulating tumor DNA. Acta Neuropathol. 2019;137:297–306. doi: 10.1007/s00401-018-1936-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y., Springer S., Zhang M., McMahon K.W., Kinde I., Dobbyn L., Ptak J., Brem H., Chaichana K., Gallia G.L., Gokaslan Z.L., Groves M.L., Jallo G.I., Lim M., Olivi A., Quinones-Hinojosa A., Rigamonti D., Riggins G.J., Sciubba D.M., Weingart J.D., Wolinsky J.P., Ye X., Oba-Shinjo S.M., Marie S.K.N., Holdhoff M., Agrawal N., Diaz L.A., Papadopoulos N., Kinzler K.W., Vogelstein B., Bettegowda C. Detection of tumor-derived DNA in cerebrospinal fluid of patients with primary tumors of the brain and spinal cord. Proc Natl Acad Sci U S A. 2015;112:9704–9709. doi: 10.1073/pnas.1511694112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bettegowda C., Sausen M., Leary R.J., Kinde I., Yuxuan W., Agrawal N. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med. 2014;6:224ra24. doi: 10.1126/scitranslmed.3007094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pan W., Gu W., Nagpal S., Gephart M.H., Quake S.R. Brain tumor mutations detected in cerebral spinal fluid. Clin Chem. 2015;61:514–522. doi: 10.1373/clinchem.2014.235457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pentsova E.I., Shah R.H., Tang J., Boire A., You D., Briggs S., Omuro A., Lin X., Fleisher M., Grommes C., Panageas K.S., Meng F., Selcuklu S.D., Ogilvie S., Distefano N., Shagabayeva L., Rosenblum M., DeAngelis L.M., Viale A., Mellinghoff I.K., Berger M.F. Evaluating cancer of the central nervous system through next-generation sequencing of cerebrospinal fluid. J Clin Oncol. 2016;34:2404–2415. doi: 10.1200/JCO.2016.66.6487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ballester L.Y., Glitza Oliva I.C., Douse D.Y., Chen M.M., Lan C., Haydu L.E., Huse J.T., Roy-Chowdhuri S., Luthra R., Wistuba I.I., Davies M.A. Evaluating circulating tumor DNA from the cerebrospinal fluid of patients with melanoma and leptomeningeal disease. J Neuropathol Exp Neurol. 2018;77:628–635. doi: 10.1093/jnen/nly046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.von Baumgarten L., Kumbrink J., Jung A., Reischer A., Flach M., Liebmann S., Metzeler K.H., Holch J.W., Niyazi M., Thon N., Straube A., von Bergwelt-Baildon M., Heinemann V., Kirchner T., Westphalen C.B. Therapeutic management of neuro-oncologic patients - potential relevance of CSF liquid biopsy. Theranostics. 2020;10:856–866. doi: 10.7150/thno.36884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martínez-Ricarte F., Mayor R., Martínez-Sáez E., Rubio-Pérez C., Pineda E., Cordero E., Cicuéndez M., Poca M.A., López-Bigas N., y Cajal S.R., Vieito M., Carles J., Tabernero J., Vivancos A., Gallego S., Graus F., Sahuquillo J., Seoane J. Molecular diagnosis of diffuse gliomas through sequencing of cell-free circulating tumor DNA from cerebrospinal fluid. Clin Cancer Res. 2018;24:2812–2819. doi: 10.1158/1078-0432.CCR-17-3800. [DOI] [PubMed] [Google Scholar]
- 12.Huang T.Y., Piunti A., Lulla R.R., Qi J., Horbinski C.M., Tomita T., James C.D., Shilatifard A., Saratsis A.M. Detection of histone H3 mutations in cerebrospinal fluid-derived tumor DNA from children with diffuse midline glioma. Acta Neuropathol Commun. 2017;5:28. doi: 10.1186/s40478-017-0436-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller A.M., Shah R.H., Pentsova E.I., Pourmaleki M., Briggs S., Distefano N., Zheng Y., Skakodub A., Mehta S.A., Campos C., Hsieh W.-Y., Selcuklu S.D., Ling L., Meng F., Jing X., Samoila A., Bale T.A., Tsui D.W.Y., Grommes C., Viale A., Souweidane M.M., Tabar V., Brennan C.W., Reiner A.S., Rosenblum M., Panageas K.S., DeAngelis L.M., Young R.J., Berger M.F., Mellinghoff I.K. Tracking tumour evolution in glioma through liquid biopsies of cerebrospinal fluid. Nature. 2019;565:654–658. doi: 10.1038/s41586-019-0882-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zorofchian S., Lu G., Zhu J.J., Duose D.Y., Windham J., Esquenazi Y., Ballester L.Y. Detection of the MYD88p.L265P mutation in the CSF of a patient with secondary central nervous system lymphoma. Front Oncol. 2018;8:1–6. doi: 10.3389/fonc.2018.00382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mattox A.K., Yan H., Bettegowda C. The potential of cerebrospinal fluid-based liquid biopsy approaches in CNS tumors. Neuro Oncol. 2019;21:1509–1518. doi: 10.1093/neuonc/noz156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Juratli T.A., Stasik S., Zolal A., Schuster C., Richter S., Daubner D., Juratli M.A., Thowe R., Hennig S., Makina M., Meinhardt M., Lautenschlaeger T., Schackert G., Krex D., Thiede C. TERT promoter mutation detection in cell-free tumor-derived DNA in patients with IDH wild-type glioblastomas: a pilot prospective study. Clin Cancer Res. 2018;24:5282–5291. doi: 10.1158/1078-0432.CCR-17-3717. [DOI] [PubMed] [Google Scholar]
- 17.De Mattos-Arruda L., Ng C.K.Y., Piscuoglio S., Gonzalez-Cao M., Lim R.S., De Filippo M.R., Fusco N., Schultheis A.M., Ortiz C., Viteri S., Arias A., Macedo G.S., Oliveira M., Gomez P., Teixidó C., Nuciforo P., Peg V., Saura C., Cajal S.R.Y., Casas F.T., Weigelt B., Cortes J., Seoane J., Reis-Filho J.S. Genetic heterogeneity and actionable mutations in HER2-positive primary breast cancers and their brain metastases. Oncotarget. 2018;9:20617–20630. doi: 10.18632/oncotarget.25041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paik P.K., Shen R., Won H., Rekhtman N., Wang L., Sima C.S., Arora A., Seshan V., Ladanyi M., Berger M.F., Kris M.G. Next-generation sequencing of stage IV squamous cell lung cancers reveals an association of PI3K aberrations and evidence of clonal heterogeneity in patients with brain metastases. Cancer Discov. 2016;5:610–621. doi: 10.1158/2159-8290.CD-14-1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fisher R., Pusztai L., Swanton C. Cancer heterogeneity: implications for targeted therapeutics. Br J Cancer. 2013;108:479–485. doi: 10.1038/bjc.2012.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bagley S., Nabavizadeh S.A., Mays J., Till J.E., Ware J., Levy S., Sarchiapone W., Hussain J., Prior T.J., Guiry S., Christensen T., Yee S.S., Nasrallah M., Morrissette J.J., Binder Z., O’Rourke D.M., Cucchiara A.J., Brem S., Desai A.S., Carpenter E.L. Clinical utility of plasma cell-free DNA in adult patients with newly diagnosed glioblastoma: a pilot prospective study. Clin Cancer Res. 2020;26:397–407. doi: 10.1158/1078-0432.CCR-19-2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Pie chart depicting the percentage of samples with range of cfDNA input for library preparations.