Abstract
Purpose:
To examine a) whether there are significant differences in the severity of symptoms of fatigue, sleep disturbance, or depression between patients with rectal cancer who develop co-occurring symptoms and those with no symptoms before and at the end of chemotherapy and radiation therapy (CRT); b) differences in gut microbial diversity between those with co-occurring symptoms and those with no symptoms; and c) whether before-treatment diversity measurements and taxa abundances can predict co-occurrence of symptoms.
Methods:
Stool samples and symptom ratings were collected from 31 patients with rectal cancer prior to and at the end of (24–28 treatments) CRT. Descriptive statistics were computed and the Mann-Whitney U test was performed for symptoms. Gut microbiome data were analyzed using R’s vegan package software.
Results:
Participants with co-occurring symptoms reported greater severity of fatigue at the end of CRT than those with no symptoms. Bacteroides and Blautia2 abundances differed between participants with co-occurring symptoms and those with no symptoms. Our random forest classification (unsupervised learning algorithm) predicted participants who developed co-occurring symptoms with 74% accuracy, using specific phylum, family, and genera abundances as predictors.
Conclusion:
Our preliminary results point to an association between the gut microbiota and co-occurring symptoms in rectal cancer patients and serves as a first step in potential identification of a microbiota-based classifier.
Keywords: gut microbiome, rectal cancer, co-occurrence of symptoms, chemotherapy and radiation therapy
Approximately 43,340 men and women will be diagnosed with rectal cancer (RC) in 2020 in the United States (Siegel et al., 2020). RC patients often receive preoperative neoadjuvant chemotherapy and radiation therapy (CRT; National Comprehensive Cancer Network [NCCN], 2016). Both treatment modalities can produce disruptive side effects with negative consequences on health-related quality of life (Gosselin et al., 2016). Such side effects include constipation or diarrhea, nausea, gastritis, cystitis, poor appetite, and cognitive impairments (Brunet et al., 2017). Patients with cancer undergoing CRT also report a high prevalence of severe behavioral comorbidities/symptoms including fatigue (85%), trouble sleeping (66%), and depression (24%–31%; Chongpison et al., 2016; Gosselin et al., 2016). Although the latter symptoms may be experienced independently, emerging evidence suggests that patients often experience two or more of these symptoms at a time, requiring adjustments in assessment and management approaches (Gosselin et al., 2016; Tantoy et al., 2016). Co-occurring symptoms may result in poorer quality of life and decreased rates of survival (Nho et al., 2017; Tantoy et al., 2016).
In the oncology arena, specifically in patients receiving concurrent treatment such as neoadjuvant CRT, it is uncommon for a patient to report a single symptom. Although the experience of co-occurring symptoms in patients with RC receiving neoadjuvant CRT is common, the literature describing these experiences is limited. In a recent study, post treatment symptoms reported by 275 patients with RC included co-occurring symptoms of being “worn out” (87%), feeling “tired” (85%), and “trouble sleeping” (66%), where feeling “tired” and having “trouble sleeping” were the most prevalent symptom described by the survivor subgroups (minimally symptomatic, n = 40; tired and trouble sleeping, n = 138; moderate symptoms, n = 42; and highly symptomatic, n = 55; Gosselin et al., 2016). Similar co-occurring symptoms have been reported in patients with mixed diagnoses of gastrointestinal cancers receiving cancer-treatments (Agasi-Idenburg et al., 2017; Tantoy et al., 2016, 2018). Studying symptom co-occurrence of fatigue, sleep disturbance, and depressive symptoms is important because it may represent a useful method in the early identification of subgroups of patients who may benefit from pro-active interventions (Gwede et al., 2008).
Although several hypotheses regarding the relationship between fatigue, sleep disturbance, and depressive symptoms have been proposed (e.g., neurotransmitter depletion, hypothalamic pituitary-adrenal axis dysregulation, neural immune activation; Li et al., 2018), there is no single hypothesis that can confirm the complex mechanism underlying the co-occurrence of these symptoms. Hence, innovative approaches to understand the mechanisms underlying the co-occurrence of symptoms especially related to cancer therapies, is worth pursuing. Recently, exploring the gut microbiome and its potential associations with behavioral comorbidities have been of great interest in a number of reviews, particularly in patients receiving chemotherapy and/or radiation therapy (Bajic et al., 2018; Jordan et al., 2018; Touchefeu et al., 2014). Briefly, it has been hypothesized that cancer treatments often have significant consequences on the intestinal barrier, which may lead to inflammation, enabling the translocation of bacteria and microbial-mediated metabolites into the systemic circulation (Jakobsson et al., 2010; Jordan et al., 2018). Some metabolites can make it to the brain, and others may induce aberrant activation of the immune system, i.e. cytokine-induced inflammatory reactions, that may in turn influence neural processes and shape behavioral symptoms (Jakobsson et al., 2010; Jordan et al., 2018; Rea et al., 2020). To our knowledge, researchers have not yet compared the varying degrees and/or types of dysbiosis related to the different types of cancer therapies received.
A major gap in the prevention and management of CRT-associated co-occurring symptoms is that there are no known targets for effective interventions. Previous research suggests that gut microbial perturbation/dysbiosis, a disruption in the balance, diversity, and function of symbiotic intestinal microbial communities (Perez et al., 2020) may be an important correlate of the symptom experience among patients with pelvic cancer undergoing treatments, perhaps mediated by the gut–brain axis (Bajic et al., 2018; Gonzalez-Mercado et al., 2019, 2020; Jordan et al., 2018). We previously demonstrated that gut microbial dysbiosis in RC patients was characterized by significantly lower alpha (within-sample) diversity at the end of CRT compared to before treatment using both Shannon diversity (community diversity) and the observed operational taxonomic units (OTUs [community richness]) for the RC sample as a whole (p < 0.05; Gonzalez-Mercado et al., 2020). In addition, Shannon diversity was associated with fatigue or sleep-disturbance scores (Gonzalez-Mercado et al., 2019, 2020). However, it is not yet known if there is a relationship between gut microbiome perturbation and co-occurrence of symptoms during CRT for RC. Therefore, the objectives of this proof-of-concept study were to examine a) whether there are significant differences in the severity of fatigue, sleep disturbance, or depression scores between patients with RC who develop co-occurring symptoms and those with no symptoms before and at the end of CRT; b) differences in gut microbial diversity and relative abundances of specific taxa between those with co-occurring symptoms and those with no symptoms; and, c) whether before-treatment diversity measurements and taxa abundances can predict co-occurrence of symptoms.
Methods
Study Population
Data for this proof-of-concept study are part of a larger study, the purpose of which is to determine whether gut dysbiosis is associated with symptoms in RC patients during CRT. Recruitment and data collection took place at three ambulatory radiation therapy facilities located in the southeastern United States from July 2017 to April 2018. We included data in the present study from participants of the parent study who completed study procedures before and at the end of CRT (N = 31). Patients were included in the parent study if they were at least 18 years of age, had newly diagnosed RC, and were scheduled to receive CRT. Exclusion criteria included history of other cancers, history of digestive disorders such as irritable bowel syndrome, diagnosed psychiatric and/or sleep disorders, comorbidities associated with sleep disturbance (e.g., sleep apnea), and use of insomnia medications, antibiotics, probiotics, prebiotics, steroids, or immune-suppressant agents within 1 month prior to each sample-collection time point. The Southeastern Academic Medical Center provided ethics approval prior to data collection and eligible participants provided written informed consent.
Assessment of Symptoms of Fatigue, Sleep Disturbance, and Depression
Participants completed two self-report measures for the assessment of symptoms of fatigue and sleep disturbances before and at the end of CRT: the 7-item Patient-Reported Outcome Measures Information System-Fatigue (PROMIS-F) and the 8-item Patient-Reported Outcome Measures Information System-Sleep Disturbance (PROMIS-SD). We administered the Hamilton Depression Rating Scale (HDRS; Hamilton, 1960) at the same time points to assess symptoms of depression. The ranges of possible scores for these instruments are 7–35 and 8–40 for the PROMIS-F and PROMIS-SD, respectively, and 0–50 for the HDRS. Higher scores on all three instruments are associated with more-severe symptoms. The adequacy of the measures’ psychometric properties is well documented (Bagby et al., 2004; Buysse et al., 2010; Cella et al., 2002).
To optimize the phenotypic characterization of study participants, we compared mean raw scores using the recommended raw-score meaningfully important differences (MIDs) ranges for research in cancer patients. Raw-score MIDs estimates for the 7-item PROMIS-F and other domains are in the range of 2–7 points (Kappelman et al., 2014; Yost et al., 2011). Previous research found that the MID on the HDRS are in the range of 3–7 points (Furukawa et al., 2007; Johnston et al., 2013). Therefore, we grouped participants based on an increase in PROMIS-F, PROMIS-SD, and HDRS scores of > 2 points from before to the end of CRT. In other words, we categorized patients with an increase of > 2 points in at least two of the symptom measures as having two or more co-occurring symptoms.
Fecal Samples
For each participant, we collected approximately 5 g of stool using a sterile plastic container for each of the two study time points. We stored stool samples at −4 °C and processed them in the lab within 24 hours of collection. The samples were divided into 250 mg portions and were immediately stored at −80 °C for batch DNA extraction.
DNA Extraction and 16SrDNA Profiling
Extraction of stool DNA for 16 S rRNA amplicon sequencing was conducted following the Power Soil DNA Isolation Kit (MoBio, Carlsbad, CA) protocol and used as the template for polymerase chain reaction (PCR) amplification. DNA concentrations were assessed by Qubit 3.0 Fluorometer (Life Technologies, Thermo Scientific, Wilmington, DE). DNA integrity was evaluated by loading 5 µl DNA on a 1% agarose gel and staining it with ethidium bromide. We sequenced the V3-V4 hypervariable region of the bacterial 16 S rRNA gene on the MiSeq Illumina platform following the standardized Illumina protocol (Illumina, personal email communication, April 15, 2018). The paired-end fastq files for each sample were imported to R (https://cran.r-project.org) and further analyzed with the DADA2 package v1.10.1 (Callahan et al., 2016) to determine amplicon sequence variants (ASVs). Briefly, the forward and reverse reads were trimmed by 20 and 30 bases from the end, respectively, and the expected errors was set to 2 for forward and 4 for reverse. Reads mapping to the phiX genome and reads with ambiguous bases were removed. From the filtered sequences, ASVs were called separately for the forward and reverse reads and subsequently merged in the next step. Finally, chimeras were removed and the ASVs were classified based on the Silva v132 database. ASV counts and their relative abundances in all samples were determined in R. Shannon diversity for each sample was calculated using the vegan R package. Pielou’s measure of evenness was calculated with the help of asbio R package and Faith’s phylogenetic diversity index was determined with picante, based on the ASV phylogenetic tree computed by Fasttree (Faith, 1992; Price et al., 2009). To identify bacterial genera significantly different across the target groups, linear discriminant analysis (LDA) was employed using linear discriminant analysis effect size (LEfSe; Segata) with a 1.5 score threshold based on ASV relative abundances (normalized to proportions) in the samples.
Statistical Analysis
We calculated descriptive statistics including frequency, percentages, means, and standard deviations (SDs), as appropriate, on demographic and disease characteristics of the patients. In addition, descriptive statistics for PROMIS-F, PROMIS-SD and HDRS scores for the sample as a whole and by symptom group were calculated. Participants were grouped into two subgroups for statistical analyses: those with no symptoms at the completion of therapy and those for whom two or more co-occurring symptoms developed from the start to the end of CRT.
Statistical and bioinformatics analyses were performed using the Statistics Package for Social Sciences (SPSS), version 24.0 for Windows, and R statistical software. To assess normality of the variables we used a Shapiro test with a significance threshold of p ≤ 0.05. We used a double-tailed t-test or nonparametric Mann-Whitney or Wilcoxon test with a significance level of 0.05 to assess statistical differences between the two-symptom groups. As an initial exploratory investigation with limited sample size, multiple or false discovery rate corrections were not applied to identify some trends in associations that can be used for validation in future studies.
To estimate the effect size of the differences in abundance of gut microbiota at the genus level between the two groups, LDA was used. The threshold on the logarithmic LDA score for discriminative features was 1.5. Further, the random forest function in the randomForest R package, a robust and accurate machine-learning approach with the capacity of measuring variable importance for classification, was used to test whether variables at baseline predicted development of two or more co-occurring symptoms at the end of treatment. The random forest function in R employs a cross-validation method to divide the dataset into different subsets automatically and creates an unbiased out-of-box (OOB) error estimate (Liaw & Wiener, 2002). The age and gender of participants, along with the relative abundances of all the ASVs (genera), phylum, class, order, and Shannon index were used as predictors, while categorization as no symptoms or two or more co-occurring symptoms was used as the dependent variable. We assessed variable importance for classification using the mean decrease in the Gini index based on 20,000 trees.
Results
Demographic and clinical characteristic of study participants are presented in Table 1. A total of 17 men and 14 women, with a mean age of 60.8 ± 11.40 years, completed study procedures. At the end of the CRT, 24 participants (77%) experienced two or more co-occurring symptoms, while 7 participants (23%) experienced no symptoms. Participants who developed two or more symptoms did not significantly differ by gender, tumor stage, infusion of 5-FU versus oral capecitabine, age, before treatment hemoglobin levels, number of radiotherapy days, or body mass index (BMI) from participants with no symptoms (p > 0.05).
Table 1.
Demographic and Clinical Characteristics of Study Participants (N = 31).
| Characteristics | 0 Symptoms (n = 7) |
> 2 Symptoms (n = 24) |
p-value* |
|---|---|---|---|
| Gender (M), n (%) | 3 (43) | 14 (58) | 0.48 |
| Stage, n (%) | |||
| II | 3 (43) | 9 (37) | 0.80 |
| III | 4 (57) | 15 (63) | |
| Chemotherapy, n (%) | |||
| 5-FU 225 mg/m2 over 24 hr | 4 (57) | 13 (54) | 0.89 |
| Oral capecitabine, 825 mg/m2 2×/day, 5 days/week | 3 (43) | 11 (46) | |
| Race, n (%) | |||
| Non-Hispanic White | 3 (43) | 11 (46) | |
| Hispanic White | 1 (14) | 11 (46) | |
| African American | 3 (43) | ||
| Asian | 2 (8) | ||
| Marital status, n (%) | |||
| Married/partnered | 4 (57) | 17 (70) | |
| Single/divorced | 3 (43) | 7 (30) | |
| Occupation, n (%) | |||
| Working | 2 (28.5) | 10 (42) | |
| Retired | 2 (28.5) | 6 (28) | |
| Disability | 3 (43) | 7 (30) | |
| Symptoms at the end of CRT, n (%) | |||
| None | 7 (100) | ||
| Fatigue + SD + Depression | 10 (42) | ||
| Fatigue + SD | 9 (37) | ||
| Fatigue + Depression | 4 (17) | ||
| SD + Depression | 1 (4) | ||
| Age, Median (IQR) | 56 (45, 68) | 61 (54, 72) | 0.29 |
| HgB, Median (IQR) | 13.3 (11.9, 14.9) | 13 (11.1, 14.8) | 0.12 |
| Number of RT days, Median (IQR) | 33 (27, 33) | 28 (28, 31) | 0.29 |
| BMI, Median (IQR) | 24 (18, 30) | 28 (24, 30) | 0.16 |
Note. BMI = body mass index; CRT = neoadjuvant chemotherapy and radiation therapy; Hgb = hemoglobin levels; RT = radiation therapy; SD = sleep disturbance.
*Represents p-value of a double-tailed Fisher or Mann-Whitney test.
Acute CRT Is Associated With Severe Fatigue in Participants With Co-Occurring Symptoms
The median raw scores on the PROMIS-F, PROMIS-SD, and HDRS before and at the end of CRT among participants with co-occurring symptoms and those with no symptoms are presented in Table 2. At the end of CRT, participants with co-occurring symptoms had significantly higher fatigue scores (more-severe fatigue) than those with no symptoms. A similar trend was observed for the sleep disturbance scores. However, there were no significant differences in depression scores after the initiation of CRT (p > 0.05) between participants with co-occurring symptoms compared to participants with no symptoms.
Table 2.
Comparison of Raw Scores for Fatigue (PROMIS-F), Sleep Disturbance (PROMIS-SD), and Depression (HDRS) Before and at the end of CRT Between Participants who had Developed Co-Occurring Symptoms by the end of CRT and those who Developed no Symptoms.
| Symptom Measure | 0 Symptoms (n = 7) Median (IQR) |
> 2 Symptoms (n = 24) Median (IQR) |
p-value* |
|---|---|---|---|
| PROMIS-F (possible range: 7–35) | |||
| Before CRT | 16 (13,26) | 15 (11, 22) | 0.50 |
| End of CRT | 14 (10,18) | 24 (20, 28) | 0.001 |
| PROMIS-SD (possible range: 8–40) | |||
| Before CRT | 23 (19, 34) | 22 (13, 28) | 0.27 |
| End of CRT | 21 (8, 29) | 28 (20, 34) | 0.09 |
| HDRS (possible range: 0–50) | |||
| Before CRT | 7 (5, 9) | 7 (9, 8) | 0.90 |
| End of CRT | 7 (5, 12) | 10 (4, 19) | 0.46 |
Note. CRT = chemotherapy and radiation therapy; HDRS = Hamilton Depression Rating Scale; PROMIS-F = Patient-Reported Outcome Measures Information System-fatigue; PROMIS-SD = Patient-Reported Outcome Measures Information System-sleep disturbance.
*Represents the p-value of a double-tailed Mann-Whitney test.
Gut Microbiome Differences Between Groups
We examined the alpha diversity indexes and taxonomic classification between participants who developed co-occurring symptoms by the end of CRT compared to those who developed no symptoms, shwon in Table 3. Further comparison analysis for alpha diversity showed a significantly lower Pielou index at the end of CRT (p ≤ 0.02) compared with before treatment for the participants who developed co-occurring symptoms. A similar trend was observed for lower abundance of ASVs, and lower Shannon and Faith diversity indexes at the end of CRT compared with before treatment for the participants who developed co-occurring symptoms. However, as an exploratory study, these significant findings were not corrected for multiple testing.
Table 3.
Within Group Comparison of Alpha Diversity Indices.
| Alpha Diversity Index | Before CRT Median (IQR) |
After CRT Median (IQR) |
p-value* |
|---|---|---|---|
| Shannon’s | |||
| 0 Symptoms | 4.36 (3.80, 4.78) | 4.16 (3.91, 4.59) | 1 |
| > 2 Symptoms | 4.69 (4.19, 4.97) | 4.36 (3.54, 4.57) | 0.08 |
| ASVs | |||
| 0 Symptoms | 137 (121, 268) | 165 (155, 227) | 0.67 |
| > 2 Symptoms | 221 (152, 280) | 173 (110, 226) | 0.09 |
| Pielou | |||
| 0 Symptoms | 0.85 (0.82, 0.86) | 0.85 (0.79, 0.86) | 0.73 |
| > 2 Symptoms | 0.86 (0.82, 0.89) | 0.83 (0.79, 0.85) | 0.02 |
| Faith | |||
| 0 Symptoms | 27.26 (12.98, 41.22 | 22.08 (12.45, 32.05) | 0.94 |
| > 2 Symptoms | 26.67 (16.54, 48.16) | 18.83 (12.72, 24.87) | 0.06 |
Note. CRT = chemotherapy and radiation therapy; ASVs = amplicon sequence variant; OTUs = operational taxonomic units.
*Represents the p-value of a Wilcoxon test (without correcting for multiple testing).
Comparisons of the taxonomic profiles at the phylum level between the 2 groups showed that before CRT, participants who developed co-occurring symptoms had a higher proportion of Bacteroidetes (median = 0.38) and a lower Firmicutes/Bacteroidetes ratio (median = 1.25) than those who developed no symptoms, but these differences were not significant (p > 0.05, Mann-Whitney; Table 4). Similarly, there were no significant differences at the phylum level between those with co-occurring symptoms and those with no symptom at the end of CRT.
Table 4.
Comparison of the Relative Abundance (%) of the most Abundant Phyla Before and at the end of CRT Between Participants Developed Co-Occurring Symptoms by the end of CRT and those who Developed no Symptoms.
| Phylum | 0 Symptoms (n = 7) Median (IQR) |
> 2 Symptoms (n = 24) Median (IQR) |
p-value* |
|---|---|---|---|
| Bacteroidetes | |||
| Before CRT | 0.16 (0.008, 0.60) | 0.38 (0.02, 0.61) | 0.15 |
| End of CRT | 0.37 (0.08, 0.56) | 0.36 (0.0004, 0.66) | 0.67 |
| Firmicutes | |||
| Before CRT | 0.55 (0.31, 0.77) | 0.49 (0.25, 0.76) | 0.49 |
| End of CRT | 0.27 (0.21, 0.51) | 0.43 (0.26, 0.86) | 0.59 |
| Actinobacteria | |||
| Before CRT | 0.05 (0, 0.16) | 0.01 (0.0003, 0.23) | 0.49 |
| End of CRT | 0.007 (0, 0.11) | 0.018 (6.44 × 10−5, 0.17) | 0.24 |
| Proteobacteria | |||
| Before CRT | 0.10 (0, 0.30) | 0.05 (0.004, 0.52) | 0.97 |
| End of CRT | 0.12 (0.02, 0.22) | 0.055 (0.002, 0.46) | 0.24 |
| Firmicutes/Bacteroidetes Ratio | |||
| Before CRT | 3.24 (0.63, 98.36) | 1.25 (0.42, 25.28) | 0.18 |
| End of CRT | 0.97 (0.38, 10.14) | 0.22 (0.40, 804.63) | 0.55 |
Note. RT = chemotherapy and radiation therapy.
*Represents the p-value of a double-tailed Mann-Whitney test (without correcting for multiple testing).
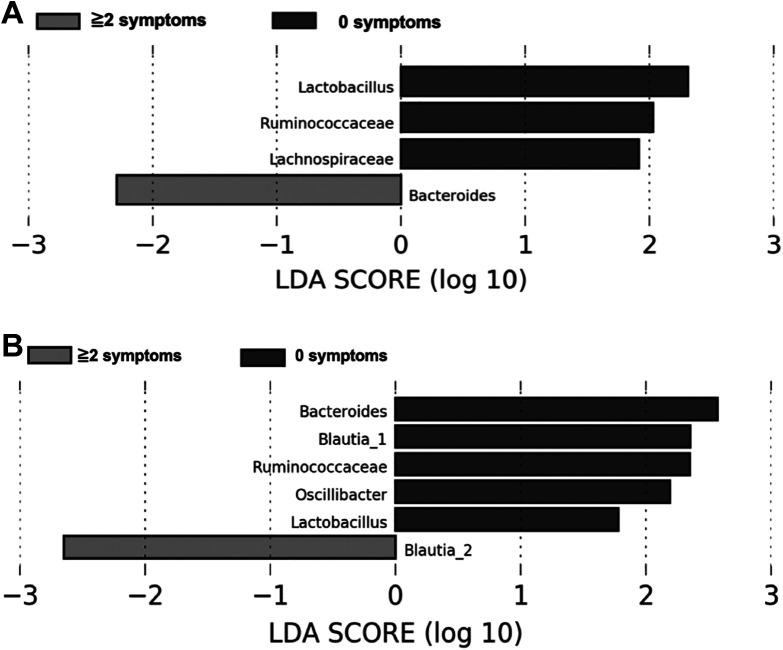
Further, exploratory LDA analyses were conducted to determine differentially abundant genera between both participant groups (Figure 1). Only two taxa, Bacteroides at before and Blautia2 at the end of CRT, were enriched in participants who developed two or more symptoms. In contrast, participants with no symptoms had enriched abundances of Lactobacillus, Ruminococcaceae, and Lachnospiraceae at the beginning of treatment (Figure 1A), and enriched abundances of Bacteroides, Blautia1, Ruminococcaceae, Oscillibacter, and Lactobacillus (Figure 1B) at the end of CRT. Blautia1 and Blautia2 refers to ASV_99 and ASV_188 which belonged to the genera Blautia; however, in our analysis, it could not be classified to species level.
Figure 1.
Taxa that Best Characterize Each Biological Class (those with co-occurring symptoms vs. those with no symptoms) at before (A) and at the end (B) of Chemotherapy and Radiation therapy (CRT) based on linear Discriminant Analysis (LDA) for effect size. Participants with co-Occurring Symptoms had Significant Enrichment for the Genera of Bacteroides at before and Blautia2 at the end of CRT. The 2 ASVs (ASV_99 and ASV_188) both belonged to the genus Blautia, but their Species Level Resolution could not be Obtained. Therefore, they have been coded as Blautia1 and Blautia2.
Gut Microbial Variables Before CRT Predict Co-Occurring Symptoms at the End of Treatment
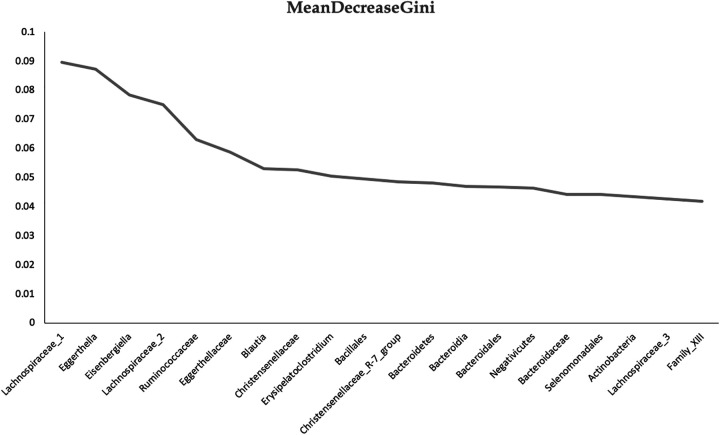
In an initial effort to investigate whether gut microbial variables before treatment predict co-occurring symptoms at the end of CRT, random forest classification (unsupervised learning algorithm) was used. We observed that this model could predict, with 74% accuracy, which participants developed co-occurring symptoms. The three most important variables for the classification of the groups identified by the algorithm, measured by the mean decrease of the Gini index, were the levels of Lachnospiraceae, Eghertella, and Eisenbergiella (Figure 2).
Figure 2.
Before Treatment Variable Importance Based on Random Forest Classification of co-Occurring Symptoms Developed by the end of Chemotherapy and Radiation Therapy (CRT).
Discussion
Because of the small sample sizes, our results are exploratory in nature and should be interpreted with caution. Nonetheless, this proof-of-concept pilot study builds on prior evidence from our group that CRT-induced comorbidities/symptoms may be associated with gut-microbial dysbiosis (Gonzalez-Mercado et al., 2020; Gonzalez-Mercado et al., 2019). In the present study, we compared the severity of fatigue, sleep disturbance, and depression scores before and at the end of CRT between participants who developed co-occurring symptoms by the end of CRT (n = 24) and those who developed no symptom (n = 7). In addition, we investigated differences in the fecal microbiome composition between the two groups of participants and the utility of predictive models incorporating microbiome data in predicting end-of-treatment co-occurring symptoms.
Our preliminary results support previous studies among other cancer populations that have identified fatigue, sleep disturbance, and depression as symptoms within the head/neck-cancer-specific cluster in patients undergoing radiotherapy (Chiang et al., 2018) or as part of distinct symptom clusters among breast cancer survivors undergoing chemotherapy (Sullivan et al., 2018). Further, clinicians need to be aware of the possibility of worsening of fatigue, sleep disturbance, and depression among those who present with co-occurring symptoms and consider using the guidelines from the National Comprehensive Cancer Network for the assessment and treatment of symptoms such as fatigue (Berger et al., 2015), early referral to mental health professionals, and treatment of depression during the cancer trajectory. Future, larger studies examining symptom clusters (e.g., based on the dimensions of occurrence and/or severity of symptoms) and evaluating the stability of these clusters over time using cluster analyses (e.g., exploratory factor analysis) are needed (Sullivan et al., 2018). Such studies may inform clinicians’ development of comprehensive care plans to target these debilitating symptoms (Chiang et al., 2018). Further research, perhaps using animal models or from a large sample, is needed to investigate if the co-occurrence of symptoms is an additive or compounding effect of the disease process, the physiological response to the combined treatments, or behavioral consequences of these symptoms among each other.
The pathobiology of cancer-treatment-associated co-occurring symptoms is not completely understood, but it is considered to be multifactorial, with the immune system, gut–brain axis, intestinal permeability, and imbalances in the gut microbiota thought to play roles (Jordan et al., 2018; Lynch et al., 2016; Rea et al., 2020). Our pilot taxa analysis found that participants who developed co-occurring symptoms by the end of CRT had a reduced microbial diversity at the end of treatment compared to their baseline levels. This finding is important because it suggests that patients who are at increased risk of co-occurring symptoms during CRT for RC may have a less-diverse gut microbiome at the end of CRT. It is, of course, possible that dysbiosis results from the disease itself. For example, meta-analyses have suggested strong associations between the gut microbiota and colon tumorigenesis (Sze & Schloss, 2018). Additional mechanistic studies, perhaps using mucosal biopsy samples or fecal transplantation, are needed to determine whether gut-microbiota dysbiosis drives and/or is a consequence of co-occurring symptoms as well as if chemotherapy and radiotherapy alone or in combination are worse than other treatments with respect to dysbiosis. Furthermore, there is a potential role for chemoprevention in CRT-associated co-occurring symptoms by modulating the disruption of the gut microbiota (Chen et al., 2018; Jordan et al., 2018; Zhong et al., 2015).
Understanding the interplay among microbes and symptoms is challenging, but also promising. It varies among individuals and is susceptible to changes driven by dietary factors (Chen et al., 2018; Jordan et al., 2018). Our pilot study found no significant differences at the phylum level between those with co-occurring symptoms and those with no symptoms, which is likely due to the small sample size. Our LDA analyses, however, revealed that before-treatment participants with co-occurring symptoms were enriched in microbes classified to the genus Bacteroides. This finding is in line with our previous work showing higher relative abundances of Bacteroides in the stool of fatigued participants undergoing CRT (Gonzalez-Mercado et al., 2020). Bacteroides, including B. fragilis, plays an important role in systemic inflammation and neurodegeneration processes (Zhao & Lukiw, 2018). B. fragilis strains are also associated with colorectal cancer, and related to negative health outcomes, such as bacteremia, colitis, diarrhea, sepsis, and systemic infection (Zhao & Lukiw, 2018). In comparison, two of the three most abundant genera in participants with no symptoms belong to butyrate-producing Clostridia from the Ruminococcaceae and Lachnospiraceae families. Increasing evidence associates butyrate-producing Clostridia with an anti-inflammatory immune response and a healthy gut (Schwab et al., 2014; Wong et al., 2006). In fact, butyrate production has been found to be associated to reduction in pro-inflammatory cytokines (e.g., tumor necrosis factor alpha, Interleukin-6, and IL-8) while it induces anti-inflammatory cytokines like IL-10; Larasati et al., 2019; Park et al., 2007). Based on our results, it may be hypothesized that participants with persistent co-occurring symptoms during cancer treatment have higher inflammatory-related gut bacteria before treatment. These findings also have important implications for future studies such as the incorporation of a direct biomarker of mucosal inflammation (e.g., fecal calprotection; Forbes et al., 2016; Schoepfer et al., 2012) and/or the metabolites short chain fatty acids (e.g., butyrate). Observations from such studies will provide important information regarding the associations of dysbiosis and severity of gut inflammation.
At the end of treatment, Blautia was differentially abundant in both groups. Interestingly, higher abundances of the genera Blautia and Bacteroides have been observed in colorectal cancer animal models after the administration on 5FU compared to controls (Yuan et al., 2018). Blautia has also being found to be associated to colorectal cancer (Baxter et al., 2014) and with most severe clinical stage and histoprognostic grade in breast cancer patients (Luu et al., 2017). In contract, increased abundance of strains belonging to the Blautia genus are associated with improved overall survival in acute Graft-versus-Host Disease after allogeneic blood/marrow transplantation (Jenq et al., 2015).
Participants in the present study with no symptoms continued to have significant enrichment of microbes in the Ruminococcaceae and Lactobacillus genera (Figure 1A and B). These findings are important because, as previously stated, prior research has found that some strains of Ruminococcaceae were associated with favorable anti-inflammatory effects on the gut (Schwab et al., 2014; Wong et al., 2006). Additionally, this finding is interesting because lactic acid producing bacteria (e.g., Lactococcus lactis) are generally used as probiotics and considered beneficial to the host because they have immunomodulative, anti-cancer, and anti-inflammatory activities (Chen et al., 2019). Future experimental and mechanistic studies are needed to determine whether there are multiple metagenomic pathways (e.g., inflammation, immune dysfunction) causing/contributing to co-occurring symptoms that can be exacerbated by bacteria and/or their metabolites; also to be considered is the possibility that some cancer treatment modalities are worse than others with respect to dysbiosis. This knowledge will contribute to our understanding of the microbiological underpinnings of co-occurring symptoms and may help improve interventional therapies.
In this pilot study, we also found preliminary evidence that it is possible to predict co-occurrence of symptoms at the end of CRT based solely on the taxa within before-treatment fecal samples. We identified the levels of Lachnospiraceae, Eghertella, and Eisenbergiella (also from the family of Lachnospiraceae) as the three most important variables that increased our ability to predict which patients would have no symptom and which would have co-occurring symptoms. This pilot study sets the stage for future validation and optimization of the classifiers/model in a well-designed longitudinal study with a larger validation sample. The ability to predict increased risk of co-occurring symptoms or to identify a particular microbiome signature that may increase risk for developing symptoms has clinical relevance since early identification of symptoms is critical to patient adherence to treatment. The findings from the present study may also facilitate the development of therapeutic strategies targeting modifiable risk factors (e.g., dietary, cognitive–behavioral stress and self-management) that lead to a reduction of co-occurring symptoms.
Limitations of this proof-of-concept study include the small sample size with respect to the number of predictors tested. This was evident from Table 3 as the results were significant only if no corrections for multiple testing was applied. However, considering that this is a ‘proof of concept’ study, the findings are still important for future research. Another limitation was the use of an instrument for depression that includes items for fatigue and insomnia that may have increased the participants’ HDRS scores, creating a redundancy in measuring the same symptom rather than describing true “co-occurrence” of these symptoms. Future co-occurrence research may consider using other validated measures of depressive symptoms such as the PROMIS depression forms that emphasize the affective and cognitive manifestations of depression rather than the somatic symptoms related to depression such as appetite, fatigue and sleep (Schalet et al., 2016). Further, a limitation of the present study is the use of fecal samples, which may not fully represent the structure of the mucosal microbiota (Flynn et al., 2018; Sze & Schloss, 2018). However, because obtaining repeated tissue biopsy samples in a clinical setting may not be feasible, studies that rely on fecal samples are commonly used to study microbial communities (Shaw et al., 2016; Sze & Schloss, 2018; Thomas et al., 2016). Another important limitation is our inability to classify beyond the genus level. Notably, studies using other molecular analyses (e.g., metagenomics and/or using real-time PCR to quantify certain bacteria) are needed to confirm and determine the role of certain groups of bacteria in the development of co-occurring symptoms. Additional considerations include the fact that, while other variables were assessed (e.g., diet, diarrhea), we did not include them in the current analyses, and in some cases, clinical data were not available to us (tumor characteristics [e.g., location, size; Thomas et al., 2016] and surgical recovery [e.g., ischemia/reperfusion; Hoehn et al., 2016]), and their influence on the gut microbiome remains a possibility. Nonetheless, the importance of findings and new knowledge gain outweighs the limitations.
Conclusion
Within the limitations of this small, pilot study, our findings reveal important associations of gut-microbiota dysbiosis and distinct microorganisms with co-occurring symptoms among patients with RC undergoing CRT. Genus-level profiles revealed correlations between the genera Bacteroides and Blautia2 and co-occurring symptoms. Our preliminary results associating changes to the gut microbiota with co-occurring symptoms in patients with RC undergoing CRT represent a first step in the identification of a microbiota-based signature and/or biomarkers and the subsequent development of treatment interventions. As future research continues to support that gut dysbiosis contributes to the pathobiology of CRT-induced co-occurring symptoms, it is possible that development of therapeutic interventions to target the modification of the intestinal microbiota (e.g., probiotics) can help ameliorate symptoms.
Acknowledgments
The authors would like to express their special appreciation to all the staff at Tampa General Hospital Cancer Center, the Cancer Care team at Advent Health Tampa, and the St. Joseph’s Hospital Cancer Institute for the collaborative clinical recruitment support. This study could not have been completed without the participants, for whom we are most grateful. In addition, we thank the staff of the College of Nursing’s Biobehavioral Laboratory at University of South Florida for the 16 S rRNA gene sequencing support.
Footnotes
Data Availability: The data used to support the findings of this study are available from the corresponding author, upon request. Additionally, the sequences will be deposited in a data base upon study completion.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This article was made possible by the National Institute of Nursing Research (NINR) of the National Institutes of Health (NIH) under award number F32NR016618. Research reported in this publication was supported by the University of Puerto Rico NIH–funded awards 2U54MD007587 and CA096297/CA096300. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
ORCID iDs: Velda J. González-Mercado  https://orcid.org/0000-0002-7236-0182
https://orcid.org/0000-0002-7236-0182
Wendy A. Henderson  https://orcid.org/0000-0003-3924-7118
https://orcid.org/0000-0003-3924-7118
Maureen Groer  https://orcid.org/0000-0002-5526-2927
https://orcid.org/0000-0002-5526-2927
References
- Agasi-Idenburg S. C., Thong M. S., Punt C. J., Stuiver M. M., Aaronson N. K. (2017). Comparison of symptom clusters associated with fatigue in older and younger survivors of colorectal cancer. Supportive Care in Cancer, 25(2), 625–632. 10.1007/s00520-016-3451-4 [DOI] [PubMed] [Google Scholar]
- Bagby R. M., Ryder A. G., Schuller D. R., Marshall M. B. (2004). The Hamilton depression rating scale: Has the gold standard become a lead weight? American Journal of Psychiatry, 161(12), 2163–2177. 10.1176/appi.ajp.161.12.2163 [DOI] [PubMed] [Google Scholar]
- Bajic J. E., Johnston I. N., Howarth G. S., Hutchinson M. R. (2018). From the bottom-up: Chemotherapy and gut-brain axis dysregulation. Frontiers in Behavioral Neuroscience, 12, 104 10.3389/fnbeh.2018.00104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter N. T., Zackular J. P., Chen G. Y., Schloss P. D. (2014). Structure of the gut microbiome following colonization with human feces determines colonic tumor burden. Microbiome, 2, 20 10.1186/2049-2618-2-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger A. M., Mooney K., Alvarez-Perez A., Breitbart W. S., Carpenter K. M., Cella D., Cleeland C., Dotan E., Eisenberger M. A., Escalante C. P., Jacobsen P. B., Jankowski C., LeBlanc T., Ligibel J. A., Loggers E. T., Mandrell B., Murphy B. A., Palesh O., Pirl W. F.…Smith C. (2015). Cancer-related fatigue, version 2.2015. Journal of the National Comprehensive Cancer Network, 13(8), 1012–1039. 10.6004/jnccn.2015.0122 [DOI] [PMC free article] [PubMed]
- Brunet J., Burke S., Grocott M. P., West M. A., Jack S. (2017). The effects of exercise on pain, fatigue, insomnia, and health perceptions in patients with operable advanced stage rectal cancer prior to surgery: A pilot trial. BMC Cancer, 17(1), 153 10.1186/s12885-017-3130-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse D. J., Yu L., Moul D. E., Germain A., Stover A., Dodds N. E., Johnston K. L., Shablesky-Cade M. A., Pilkonis P. A. (2010). Development and validation of patient-reported outcome measures for sleep disturbance and sleep-related impairments. Sleep, 33(6), 781–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan B. J., McMurdie P. J., Rosen M. J., Han A. W., Johnson A. J., Holmes S. P. (2016). DADA2: High-resolution sample inference from Illumina amplicon data. Nature Methods, 13(7), 581–583. 10.1038/nmeth.3869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella D., Eton D. T., Lai J. S., Peterman A. H., Merkel D. E. (2002). Combining anchor and distribution-based methods to derive minimal clinically important differences on the functional assessment of cancer therapy (FACT) anemia and fatigue scales. Journal of Pain and Symptom Management, 24(6), 547–561. [DOI] [PubMed] [Google Scholar]
- Chen L., Jiang B., Zhong C., Guo J., Zhang L., Mu T., Zhang Q., Bi X. (2018). Chemoprevention of colorectal cancer by black raspberry anthocyanins involved the modulation of gut microbiota and SFRP2 demethylation. Carcinogenesis, 39(3), 471–481. 10.1093/carcin/bgy009 [DOI] [PubMed] [Google Scholar]
- Chen X. H., Wang A., Chu A. N., Gong Y. H., Yuan Y. (2019). Mucosa-associated microbiota in gastric cancer tissues compared with non-cancer tissues. Frontiers in Microbiology, 10, 1261 10.3389/fmicb.2019.01261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang S. H., Ho K. Y., Wang S. Y., Lin C. C. (2018). Change in symptom clusters in head and neck cancer patients undergoing postoperative radiotherapy: A longitudinal study. European Journal of Oncology Nursing, 35, 62–66. 10.1016/j.ejon.2018.01.014 [DOI] [PubMed] [Google Scholar]
- Chongpison Y., Hornbrook M. C., Harris R. B., Herrinton L. J., Gerald J. K., Grant M., Bulkley J. E., Wendel C. S., Krouse R. S. (2016). Self-reported depression and perceived financial burden among long-term rectal cancer survivors. Psychooncology, 25(11), 1350–1356. 10.1002/pon.3957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faith D. P. (1992). Conservation evaluation and phylogenetic diversity. Biological Conservation, 61(1), 1–10. 10.1016/0006-3207(92)91201-3 [DOI] [Google Scholar]
- Flynn K. J., Ruffin M. T., Turgeon D. K., Schloss P. D. (2018). Spatial variation of the native colon microbiota in healthy adults. Cancer Prevention Research (Philadelphia, PA), 11(7), 393–402. 10.1158/1940-6207.capr-17-0370 [DOI] [PubMed] [Google Scholar]
- Forbes J. D., Van Domselaar G., Bernstein C. N. (2016). Microbiome survey of the inflamed and noninflamed gut at different compartments within the gastrointestinal tract of inflammatory bowel disease patients. Inflammatory Bowel Diseases, 22(4), 817–825. 10.1097/mib.0000000000000684 [DOI] [PubMed] [Google Scholar]
- Furukawa T. A., Akechi T., Azuma H., Okuyama T., Higuchi T. (2007). Evidence-based guidelines for interpretation of the Hamilton rating scale for depression. Journal of Clinical Psychopharmacology, 27(5), 531–534. 10.1097/JCP.0b013e31814f30b1 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Mercado V. J., Perez-Santiago J., Lyon D., Dilan-Pantojas I., Henderson W., McMillan S., Groer M., Kane B., Marrero S., Pedro E., Saligan L. N. (2020). The role of gut microbiome perturbation in fatigue induced by repeated stress from chemoradiotherapy: A proof of concept study. Advances in Medicine, 2020, 10.1155/2020/6375876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Mercado V. J., Sakar A., Penedo F. J., Pérez-Santiago J., Mcmillan S., Marrero S. J, Marrero-Falcón M. A., Munro C. L. (2019). Gut microbiota perturbation is associated with acute sleep disturbance among rectal cancer patients. Journal of Sleep Research, 2019(00), e12915 10.1111/jsr.12915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosselin T. K., Beck S., Abbott D. H., Grambow S. C., Provenzale D., Berry P., Kahn K. L., Malin J. L. (2016). The symptom experience in rectal cancer survivors. Journal of Pain and Symptom Management, 52(5), 709–718. 10.1016/j.jpainsymman.2016.05.027 [DOI] [PubMed] [Google Scholar]
- Gwede C. K., Small B. J., Munster P. N., Andrykowski M. A., Jacobsen P. B. (2008). Exploring the differential experience of breast cancer treatment-related symptoms: A cluster analytic approach. Supportive Care in Cancer, 16(8), 925–933. 10.1007/s00520-007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. (1960). A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry, 23, 56–62. 10.1136/jnnp.23.1.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehn R. S., Seitz A. P., Jernigan P. L., Gulbins E., Edwards M. J. (2016). Ischemia/reperfusion injury alters sphingolipid metabolism in the gut. Cellular Physiology and Biochemistry, 39(4), 1262–1270. 10.1159/000447831 [DOI] [PubMed] [Google Scholar]
- Jakobsson S., Ahlberg K., Taft C., Ekman T. (2010). Exploring a link between fatigue and intestinal injury during pelvic radiotherapy. The Oncologist, 15(9), 1009–1015. 10.1634/theoncologist.2010-0097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenq R. R., Taur Y., Devlin S. M., Ponce D. M., Goldberg J. D., Ahr K. F., Littmann E. R., Ling L., Gobourne A. C., Miller L. C., Docampo M. D., Peled J. U., Arpaia N., Cross J. R., Peets T. K., Lumish M. A., Shono Y., Dudakov J. A., Poeck H.…van den Brink M. R. (2015). Intestinal blautia is associated with reduced death from graft-versus-host disease. Biology of Blood and Marrow Transplantation: Journal of the American Society for Blood and Marrow Transplantation, 21(8), 1373–1383. 10.1016/j.bbmt.2015.04.016 [DOI] [PMC free article] [PubMed]
- Johnston B. C., Patrick D. L., Thorlund K., Busse J. W., da Costa B. R., Schunemann H. J., Guyatt G. H. (2013). Patient-reported outcomes in meta-analyses-part 2: Methods for improving interpretability for decision-makers. Health and Quality of Life Outcomes, 11, 211 10.1186/1477-7525-11-211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan K. R., Loman B. R., Bailey M. T., Pyter L. M. (2018). Gut microbiota-immune-brain interactions in chemotherapy-associated behavioral comorbidities. Cancer, 124(20), 3990–3999. 10.1002/cncr.31584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappelman M. D., Long M. D., Martin C., DeWalt D. A., Kinneer P. M., Chen W., Lewis J. D., Sandler R. S. (2014). Evaluation of the patient-reported outcomes measurement information system in a large cohort of patients with inflammatory bowel diseases. Clinical Gastroenterology and Hepatology, 12(8), 1315–1323 e1312. 10.1016/j.cgh.2013.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larasati R. A., Harbuwono D. S., Rahajeng E., Pradipta S., Nuraeni H. S., Susilowati A., Wibowo H. (2019). The role of butyrate on monocyte migration and inflammation response in patient with type 2 diabetes mellitus. Biomedicines, 7(4). 10.3390/biomedicines7040074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Hao Y., Fan F., Zhang B. (2018). The role of microbiome in insomnia, circadian disturbance and depression. Frontiers in Psychiatry, 9, 669 10.3389/fpsyt.2018.00 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liaw A, Wiener M. (2002). Classification and regression by randomForest. R News, 2(3), 18–22. [Google Scholar]
- Luu T. H., Michel C., Bard J. M., Dravet F., Nazih H., Bobin-Dubigeon C. (2017). Intestinal proportion of Blautia sp. is associated with clinical stage and histoprognostic grade in patients with early-stage breast cancer. Nutrition and Cancer, 69(2), 267–275. 10.1080/01635581.2017.1263750 [DOI] [PubMed] [Google Scholar]
- Lynch K. D., Dickinson K., Hsiao C. P., Lukkahatai N., Gonzalez-Marrero V., McCabe M., Saligan L. N. (2016). Biological basis for the clustering of symptoms. Seminars in Oncology Nursing, 32(4), 351–360. 10.1016/j.soncn.2016.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Comprehensive Cancer Network. (2016). Clinical practice guidelines in oncology; Rectal Cancer. https://www.nccn.org/Store/Login/Register.aspx?returnurl=https%3a%2f%2fwww.nccn.org%2fprofessionals%2fphysician_gls%2fpdf%2frectal.pdf
- Nho J. H., Reul Kim S., Nam J. H. (2017). Symptom clustering and quality of life in patients with ovarian cancer undergoing chemotherapy. European Journal of Oncology Nursing, 30, 8–14. 10.1016/j.ejon.2017.07.007 [DOI] [PubMed] [Google Scholar]
- Park J. S., Lee E. J., Lee J. C., Kim W. K., Kim H. S. (2007). Anti-inflammatory effects of short chain fatty acids in IFN-gamma-stimulated RAW 264.7 murine macrophage cells: Involvement of NF-kappaB and ERK signaling pathways. International Immunopharmacology, 7(1), 70–77. 10.1016/j.intimp.2006.08.015 [DOI] [PubMed] [Google Scholar]
- Perez N. B., Dorsen C., Squires A. (2020). Dysbiosis of the gut microbiome: A concept analysis. Journal of Holistic Nursing, 38(2), 223–232. 10.1177/0898010119879527 [DOI] [PubMed] [Google Scholar]
- Price M. N., Dehal P. S., Arkin A. P. (2009). FastTree: Computing large minimum evolution trees with profiles instead of a distance matrix. Molecular Biology and Evolution, 26(7), 1641–1650. 10.1093/molbev/msp077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea K., Dinan T. G., Cryan J. F. (2020). Gut microbiota: A perspective for psychiatrists. Neuropsychobiology, 79(1), 50–62. 10.1159/000504495 [DOI] [PubMed] [Google Scholar]
- Schalet B. D., Pilkonis P. A., Yu L., Dodds N., Johnston K. L., Yount S., Riley W., Cella D. (2016). Clinical validity of PROMIS depression, anxiety, and anger across diverse clinical samples. Journal of Clinical Epidemiology, 73, 119–127. 10.1016/j.jclinepi.2015.08.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoepfer A. M., Vavricka S., Zahnd-Straumann N., Straumann A., Beglinger C. (2012). Monitoring inflammatory bowel disease activity: Clinical activity is judged to be more relevant than endoscopic severity or biomarkers. Journal of Crohn’s & Colitis, 6(4), 412–418. 10.1016/j.crohns.2011.09.008 [DOI] [PubMed] [Google Scholar]
- Schwab C., Berry D., Rauch I., Rennisch I., Ramesmayer J., Hainzl E., Heider S., Decker T., Kenner L., Müller M., Strobl B., Wagner M., Schleper C., Loy A., Urich T. (2014). Longitudinal study of murine microbiota activity and interactions with the host during acute inflammation and recovery. The ISME Journal, 8(5), 1101–1114. 10.1038/ismej.2013.223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw K. A., Bertha M., Hofmekler T., Chopra P., Vatanen T., Srivatsa A., Prince J., Kumar A., Sauer C., Zwick M. E., Satten G. A., Kostic A. D., Mulle J. G., Xavier R. J., Kugathasan S. (2016). Dysbiosis, inflammation, and response to treatment: A longitudinal study of pediatric subjects with newly diagnosed inflammatory bowel disease. Genome Medicine, 8(1), 75 10.1186/s13073-016-0331-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R. L., Miller K. D., Jemal A. (2020). Cancer statistics, 2020. CA: A Cancer Journal for Clinicians, 70(1), 7–30. 10.3322/caac.21590 [DOI] [PubMed] [Google Scholar]
- Sullivan C. W., Leutwyler H., Dunn L. B., Cooper B. A., Paul S. M., Levine J. D., Hammer M., Conley Y. P., Miaskowski C. A. (2018). Stability of symptom clusters in patients with breast cancer receiving chemotherapy. Journal of Pain and Symptom Management, 55(1), 39–55. 10.1016/j.jpainsymman.2017.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze M. A., Schloss P. D. (2018). Leveraging existing 16 S rRNA gene surveys to identify reproducible biomarkers in individuals with colorectal tumors. MBio, 9(3). 10.1128/mBio.00630-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tantoy I. Y., Cataldo J. K., Aouizerat B. E., Dhruva A., Miaskowski C. (2016). A review of the literature on multiple co-occurring symptoms in patients with colorectal cancer who received chemotherapy alone or chemotherapy with targeted therapies. Cancer Nursing, 39(6), 437–445. 10.1097/ncc.0000000000000343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tantoy I. Y., Cooper B. A., Dhruva A., Cataldo J., Paul S. M., Conley Y. P., Hammer M., Wright F., Dunn L. B., Levine J. D., Miaskowski C. (2018). Changes in the occurrence, severity, and distress of symptoms in patients with gastrointestinal cancers receiving chemotherapy. Journal of Pain Symptom Management, 55(3), 808–834. 10.1016/j.jpainsymman.2017.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A. M., Jesus E. C., Lopes A., Aguiar S., Jr, Begnami M. D., Rocha R. M., Carpinetti P. A., Camargo A. A., Hoffmann C., Freitas H. C., Silva I. T., Nunes D. N., Setubal J. C., Dias-Neto E. (2016). Tissue-associated bacterial alterations in rectal carcinoma patients revealed by 16 S rRNA community profiling. Frontiers in Cellular and Infection Microbiology, 6, 179 10.3389/fcimb.2016.00179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touchefeu Y., Montassier E., Nieman K., Gastinne T., Potel G., Bruley des Varannes S., Le Vacon F., de La Cochetiere M. F. (2014). Systematic review: The role of the gut microbiota in chemotherapy- or radiation-induced gastrointestinal mucositis—Current evidence and potential clinical applications. Alimentary Pharmacology & Therapeutics, 40(5), 409–421. 10.1111/apt.12878 [DOI] [PubMed] [Google Scholar]
- Wong J. M., de Souza R., Kendall C. W., Emam A., Jenkins D. J. (2006). Colonic health: Fermentation and short chain fatty acids. Journal of Clinical Gastroenterology, 40(3), 235–243. 10.1097/00004836-200603000-00015 [DOI] [PubMed] [Google Scholar]
- Yost K. J., Eton D. T., Garcia S. F., Cella D. (2011). Minimally important differences were estimated for six patient-reported outcomes measurement information system-cancer scales in advanced-stage cancer patients. Journal of Clinical Epidemiology, 64(5), 507–516. 10.1016/j.jclinepi.2010.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L., Zhang S., Li H., Yang F., Mushtaq N., Ullah S., Shi Y., An C., Xu J. (2018). The influence of gut microbiota dysbiosis to the efficacy of 5-Fluorouracil treatment on colorectal cancer. Biomedicine & Pharmacotherapy, 108, 184–193. 10.1016/j.biopha.2018.08.165 [DOI] [PubMed] [Google Scholar]
- Zhao Y., Lukiw W. J. (2018). Bacteroidetes neurotoxins and inflammatory neurodegeneration. Molecular Neurobiology, 55(12), 9100–9107. 10.1007/s12035-018-1015-y [DOI] [PubMed] [Google Scholar]
- Zhong Y., Nyman M., Fak F. (2015). Modulation of gut microbiota in rats fed high-fat diets by processing whole-grain barley to barley malt. Molecular Nutrition & Food Research, 59(10), 2066–2076. 10.1002/mnfr.201500187 [DOI] [PubMed] [Google Scholar]