Abstract
The psychedelic alkaloid ibogaine has anti-addictive properties in both humans and animals.1 Unlike most substance use disorder (SUD) medications, anecdotal reports suggest that ibogaine possesses the potential to treat patients addicted to a variety of substances including opiates, alcohol, and psychostimulants. Like other psychedelic compounds, its therapeutic effects are long-lasting,2 which has been attributed to its ability to modify addiction-related neural circuitry through activation of neurotrophic factor signaling.3,4 However, several safety concerns have hindered the clinical development of ibogaine including its toxicity, hallucinogenic potential, and proclivity for inducing cardiac arrhythmias. Here, we apply the principles of function-oriented synthesis (FOS) to identify the key structural elements of its potential therapeutic pharmacophore, enabling us to engineer tabernanthalog (TBG)—a water soluble, non-hallucinogenic, non-toxic analog of ibogaine that can be prepared in a single step. TBG promoted structural neural plasticity, reduced alcohol- and heroin-seeking behavior, and produced antidepressant-like effects in rodents. This work demonstrates that through careful chemical design, it is possible to modify a psychedelic compound to produce a safer, non-hallucinogenic variant with therapeutic potential.
Keywords: psychedelic, psychoplastogen, neural plasticity, ibogaine, function-oriented synthesis, drug design, neuropsychiatric disorder, substance use disorder, addiction, alcohol use disorder, opioid use disorder, antidepressant, depression, serotonin, 5-HT2A receptor
Ibogaine is the most abundant of the numerous alkaloids produced by Tabernanthe iboga,5 and though it has not been tested in double-blind placebo-controlled clinical trials, anecdotal reports and open-label studies suggest that it can reduce symptoms of drug withdrawal, reduce drug cravings, and prevent relapse.1,6 Unfortunately, ibogaine suffers from major issues that severely limit its potential as a therapeutic. First, access to large quantities has been limited by overexploitation of the plant from which it is derived as well as the lack of a scalable, enantioselective, total synthesis.6 Currently, there are only three synthetic routes to racemic ibogaine with longest linear sequences of 9–16 steps and overall yields ranging from 0.1–4.8%.6 Second, ibogaine’s safety profile is unacceptable. From a physicochemical perspective, it is very non-polar, which leads to its accumulation in adipose tissue7 and contributes to its known cardiotoxicity through inhibition of hERG potassium channels. 8,9 Several deaths have been linked to ibogaine’s cardiotoxicity,10,11 and it produces long-lasting hallucinations (> 24 h). While ibogaine was once sold in France as a medicine for treating neuropsychiatric diseases, it was removed from the market due to its adverse effects.1
Though ibogaine’s exact mechanism of action has not yet been fully elucidated, evidence suggests that it might alter addiction-related circuitry by promoting neural plasticity. First, ibogaine has been shown to increase glial cell line-derived neurotrophic factor (GDNF) expression in the ventral tegmental area (VTA), and intra-VTA infusion of ibogaine reduces alcohol-seeking behavior in rodents.3 A more recent study demonstrated that ibogaine impacts brain-derived neurotrophic factor (BDNF) and GDNF signaling in multiple brain regions implicated in the behavioural effects of addictive drugs.4 Recently, we demonstrated that noribogaine, an active metabolite of ibogaine,12 is a potent psychoplastogen13 that increases cortical neuron dendritic arbor complexity.14 Other psychoplastogens, such as lysergic acid diethylamide (LSD) and psilocin (the active metabolite of psilocybin) have also been shown in anecdotal and open-label studies to decrease drug use in the clinic, similar to ibogaine.15 We hypothesize that the ability of psychoplastogens to promote structural and functional neural plasticity in addiction-related circuitry might explain their abilities to reduce drug-seeking behavior for weeks to months following a single administration. Moreover, by modifying neural circuitry rather than simply blocking the targets of a particular addictive substance, psychoplastogens like ibogaine could have the potential to be broadly applicable anti-addictive agents.
In order to develop simplified, and potentially safer analogs, we first needed to understand which of ibogaine’s structural features were critical for its psychoplastogenic effects. Our approach mirrored that taken by Wender and colleagues in their seminal FOS studies on the structurally complex marine natural product bryostatin 1.16 Here, we report our efforts to engineer simplified analogs of iboga alkaloids (ibogalogs) that lack ibogaine’s toxicity and hallucinogenic effects but maintain its behavioural effects in rodent models of drug self-administration and relapse.
Function-Oriented Synthesis of Ibogalogs
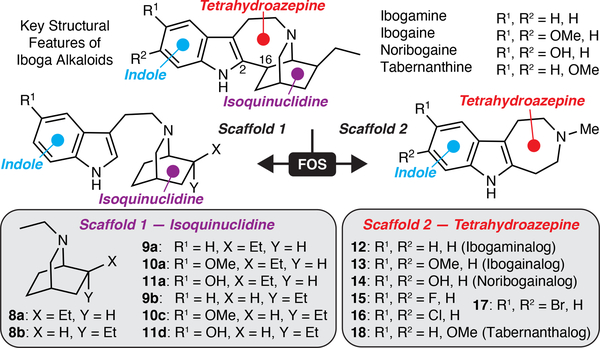
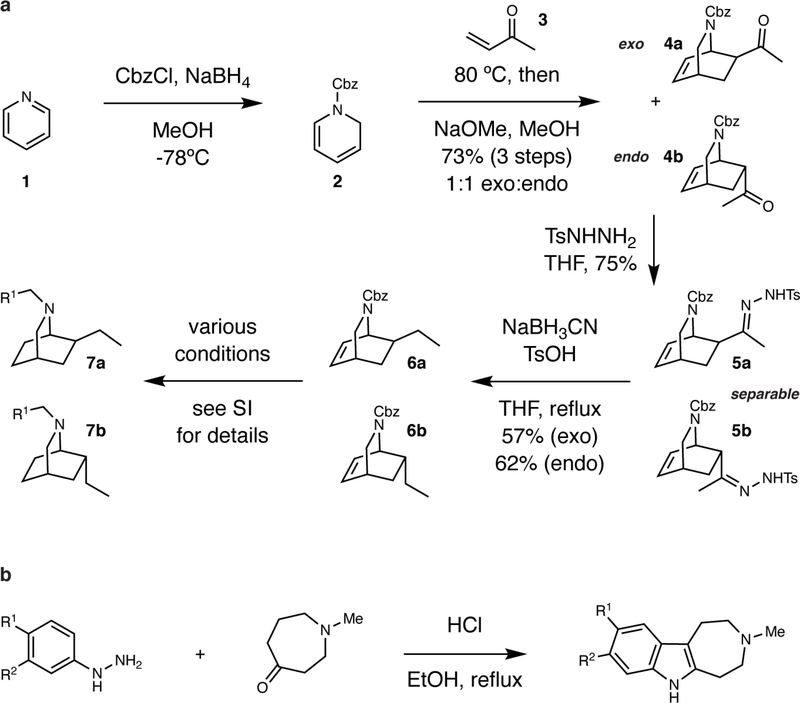
The hallmarks of ibogaine’s structure include an indole, a 7-membered tetrahydroazepine, and a bicyclic isoquinuclidine (Fig. 1). We reasoned that systematic deletion of these key structural elements would reveal the essential features of ibogaine’s psychoplastogenic pharmacophore. By adapting chemistry developed by Sames and co-workers,17 we were able to access a series of isoquinuclidine-containing compounds (8–11) that lacked the tetrahydroazepine and/or indole characteristic of ibogaine (Extended Data Fig. 1a). Ibogalog 8a lacks both the indole and tetrahydroazepine rings characteristic of ibogaine, while 9a–11a only lack the tetrahydroazepine. Ibogamine, ibogaine, and noribogaine only differ from 9a, 10a, and 11a by the presence of the C2–C16 bond (LeMen and Taylor convention), respectively.
Figure 1. Function-oriented synthesis of ibogalogs.
(a) Key structural features of ibogaine, related alkaloids, and ibogalogs.
Key Structural Elements of Ibogaine’s Psychoplastogenic Pharmacophore
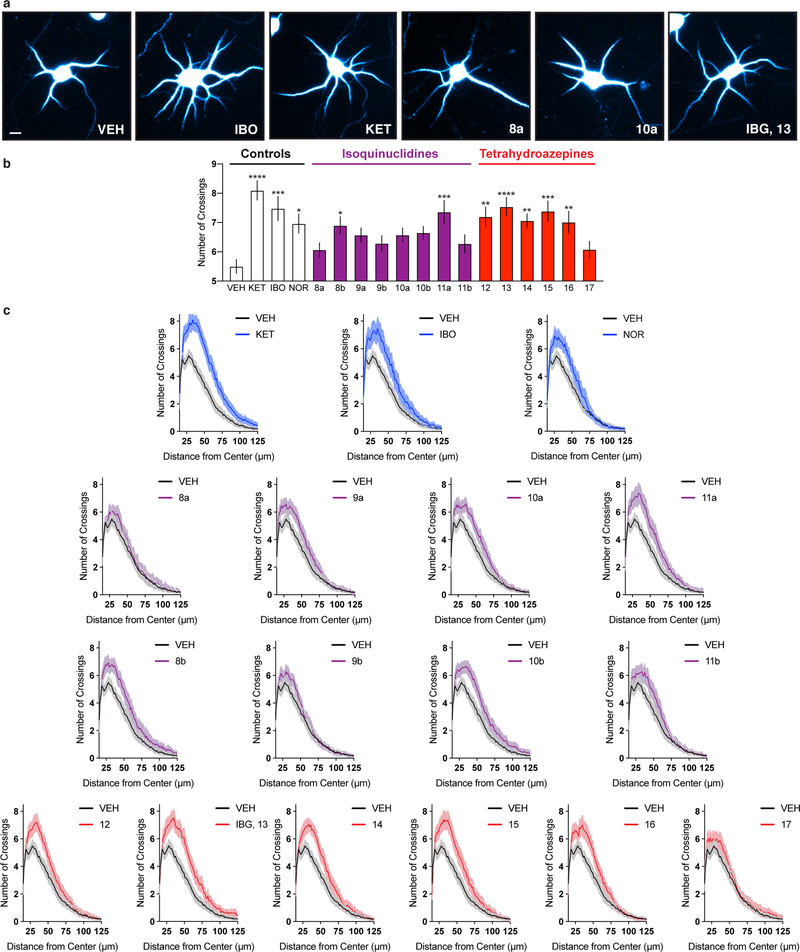
Except for 11a, ibogalogs containing the isoquinuclidine but lacking the tetrahydroazepine ring (8–10 and 11b) were either weak psychoplastogens or did not promote neuronal growth compared to the vehicle (VEH) control (Extended Data Fig. 2). In contrast, the majority of ibogalogs lacking the isoquinuclidine but retaining the tetrahydroazepine (12–16) were efficacious (Extended Data Fig. 2). Indole substitution at C5 with either fluorine (15) or chlorine (16) was tolerated, but a more sterically demanding bromine substituent (17) was not. We found that IBG (13) performed comparably to ibogaine despite having a simplified chemical structure. We prioritized IBG for further development as we reasoned that its reduced lipophilicity (cLogP = 2.61) relative to ibogaine (cLogP = 4.27) would make formulation less challenging and also reduce the potential for cardiotoxicity, as lipophilicity is a known contributing factor to hERG channel inhibition. The attractiveness of IBG as a potential therapeutic was underscored by its improved CNS multiparameter optimization (MPO) score18 (IBG MPO = 5.2; Ibogaine MPO = 3.8; optimal MPO = 6.0) coupled with the fact that it can be synthesized in a single step.
TBG is a Safer, Non-Hallucinogenic 5-HT2A Agonist
Inspection of IBG’s structure reveals its similarity to the potent hallucinogen and 5-HT2A agonist 5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT). Pioneering structure-activity relationship (SAR) studies conducted by Glennon and co-workers demonstrated that, unlike 5-MeO-DMT and other known hallucinogens, 6-MeO-DMT did not substitute for the hallucinogen 2,5-dimethoxy-4-methylamphetamine (DOM) at any dose in rodents trained to discriminate DOM from saline.19 Moreover, our group has shown that 6-MeO-DMT does not produce a head-twitch response (HTR)20—a well-established rodent behavioral proxy for hallucinations induced by 5-HT2A agonists.21 Therefore, we synthesized the 6-methoxyindole-fused tetrahydroazepine 18. As 18 resembles the iboga alkaloid tabernanthine, we refer to this compound as tabernanthalog (TBG). Like IBG, TBG can be synthesized in a single step enabling us to rapidly access large quantities (>1 g).
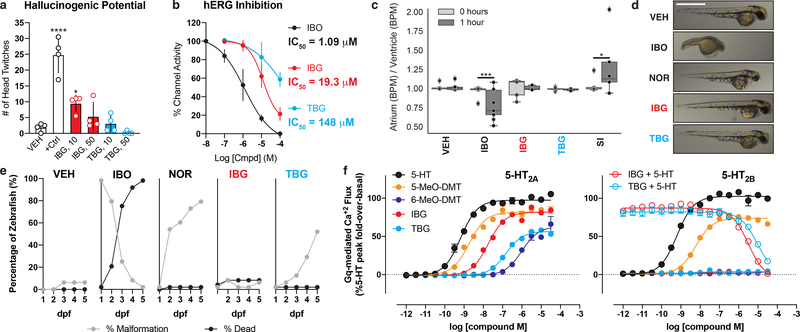
To evaluate the hallucinogenic potential of IBG and TBG, we tested them in the HTR assay using 5-MeO-DMT (10 mg/kg) as a positive control (Fig. 2a). While 5-MeO-DMT produces a robust HTR, its conformationally restricted analog IBG exhibits significantly reduced hallucinogenic potential. As hypothesized, the 6-methoxy substituent of TBG rendered it devoid of hallucinogenic potential as measured by the HTR assay. For these in vivo studies, we used the fumarate salts of IBG and TBG. Unlike ibogaine hydrochloride, they are readily soluble in 0.9% saline up to 40 mg/mL (Extended Data Table 1).
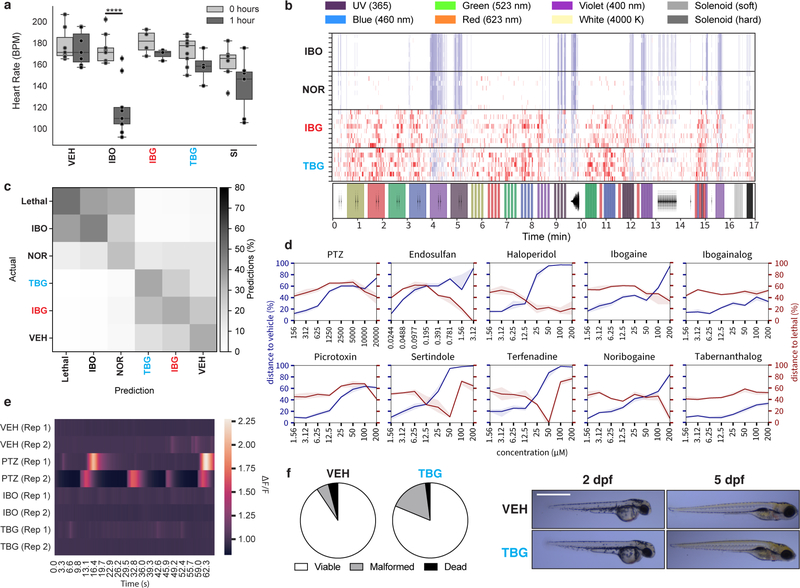
Figure 2. TBG is a safer analog of iboga alkaloids.
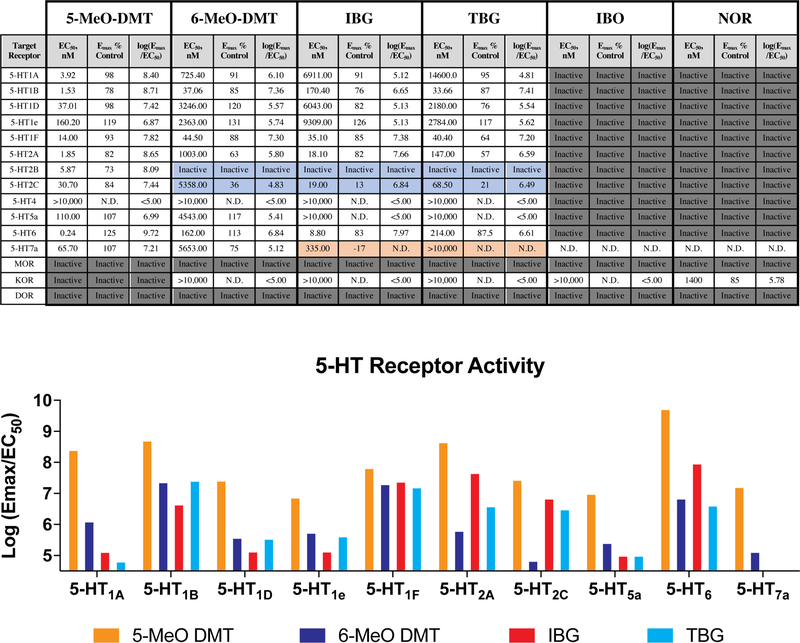
(a) Mouse HTR assays demonstrate that TBG is not hallucinogenic. The doses (mg/kg) of IBG and TBG are indicated. +Ctrl = 5-MeO-DMT (10 mg/kg). (b) Inhibition of hERG channels expressed in HEK293 cells. Error bars represent SD. (c) Unlike ibogaine, IBG and TBG do not increase the risk for arrhythmias in larval zebrafish. Sertindole (SI) was used as a positive control. (d) Representative images of zebrafish treated with compounds (100 μM) for 2 dpf. Scale bar = 1 mm. (e) Compound-induced malformation and death over time (n = 48 zebrafish for all treatment groups). (f) Activities at 5-HT2A and 5-HT2B receptors as measured by Gq-mediated calcium flux. Data represent percent 5-HT fold-over-basal response. Exact N numbers for each experimental condition are reported in the Source Data and Supplementary Table 1. Specific statistical tests, information on reproducibility, and exact p values are reported in the Methods and Supplementary Table 1.
The lipophilicity of ibogaine not only poses practical issues for its administration, it is likely a major factor contributing to its toxicity and adverse cardiac effects. Ibogaine inhibits hERG channels with an IC50 of 1 μM (Fig. 2b). In contrast, IBG and TBG are approximately 10- and 100-fold less potent than ibogaine, respectively, indicating a lower potential for cardiotoxicity. Administration of ibogaine to immobilized larval zebrafish decreased heart rate (Extended Data 3a, Video S1) and increased the likelihood for inducing arrhythmias as measured by the ratio of atrium to ventricle beats per minute (BPM) (Fig. 2c). Neither IBG nor TBG induced these undesirable phenotypes.
To compare the acute behavioral effects of ibogaine, IBG, and TBG, we treated larval zebrafish with these compounds across a range of doses (1–200 μM), recorded their behavioral responses to a battery of light and acoustic stimuli,20,22 and analyzed the resulting behavioral profiles (Extended Data 3b). Treatment with ibogaine and noribogaine produced behavioral profiles similar to the lethal control (Extended Data 3b–c). In contrast, IBG- and TBG-treated zebrafish produced behavioral profiles more similar to the vehicle control. With increasing concentration, ibogaine, noribogaine, and the hERG inhibitors haloperidol, sertindole, and terfenadine become more phenotypically distinct from the vehicle control, while more closely resembling the lethal control (eugenol, 100 μM) (Extended Data 3d). In contrast, IBG and TBG did not produce this phenotype.
As it is unclear whether or not ibogaine causes seizures at very high doses,1,23 we next used larval zebrafish expressing GCaMP5 to assess seizurogenic potential. Neither ibogaine nor TBG produced excessive neural activity as was observed following treatment with the known seizure-inducing compound pentylenetetrazole (PTZ) (Extended Data 3e, Video S2).
Finally, we compared the morphological effects of ibogaine, IBG, and TBG using a well-established zebrafish developmental toxicity assay.24 Ibogaine (100 μM) significantly increased malformations and mortality at 2 and 5 days post-fertilization (dpf), respectively (Fig. 2d–e). At both timepoints, the proportion of viable to non-viable fish was significantly different from vehicle control (p < 0.0001). Ibogaine-treated zebrafish suffered from numerous malformations. Noribogaine treatment resulted in greater survival, but the majority of zebrafish exhibited yolk sac and/or pericardial edemas. In contrast, both IBG and TBG treatments (100 μM) resulted in significantly fewer non-viable fish than ibogaine (p < 0.0001 for ibogaine vs. IBG and ibogaine vs. TBG at both 2 and 5 dpf). Importantly, reducing the concentration of TBG from 100 to 66 μM resulted in a proportion of viable to non-viable fish that was statistically indistinguishable from vehicle control after 5 dpf (p = 0.3864, Extended Data 3f).
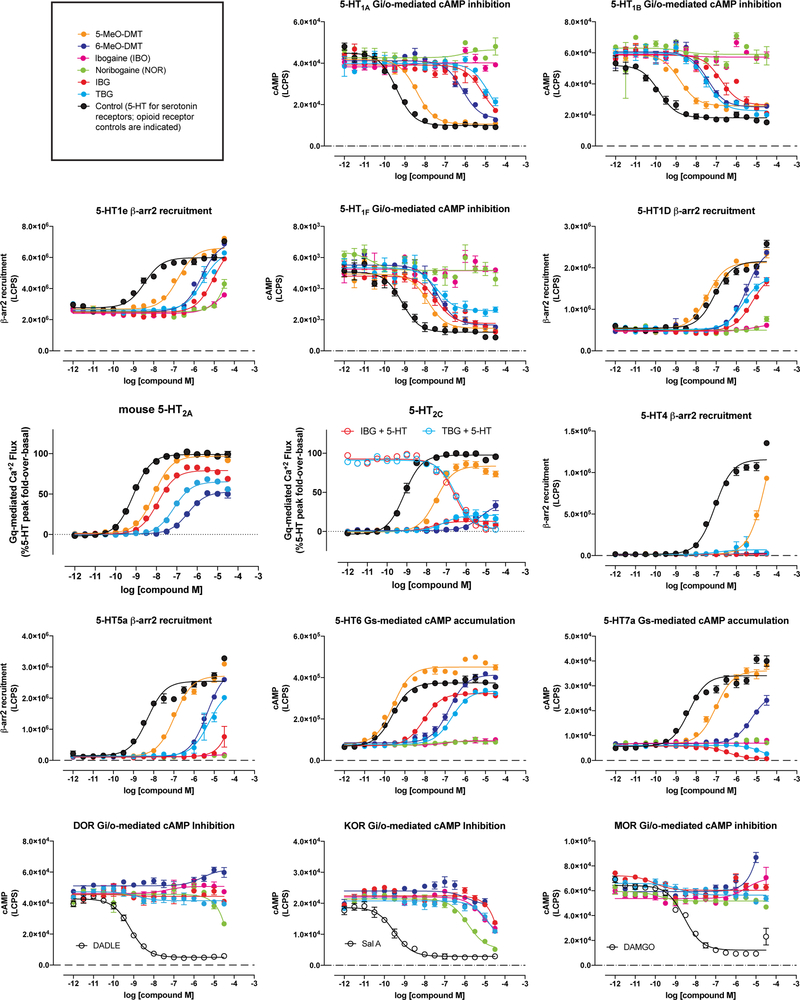
To validate the targets of IBG and TBG, we performed a panel of serotonin (5-HT) and opioid receptor functional assays assessing canonical GPCR signaling. Unlike noribogaine, IBG and TBG showed weak or no opioid agonist activity (Extended Data Figs. 4 and 5). However, IBG and TBG demonstrated potent agonist activity at human (Fig. 2f) and mouse 5-HT2A receptors (Extended Data Fig. 4). Many 5-HT2A agonists, such as 5-MeO-DMT, are also agonists of 5-HT2B receptors, which can lead to cardiac valvulopathy.25 In contrast, IBG and TBG act as antagonists at 5-HT2B receptors (Fig. 2f). When profiled across the 5-HT receptorome, both IBG and TBG displayed more selective, and potentially safer profiles as compared to the less conformationally restricted 5-MeO-DMT (Extended Data Figs. 4 and 5). A full safety screen across 81 potential targets revealed that TBG exhibits high selectivity for 5-HT2 receptors (Extended Data Table 2).
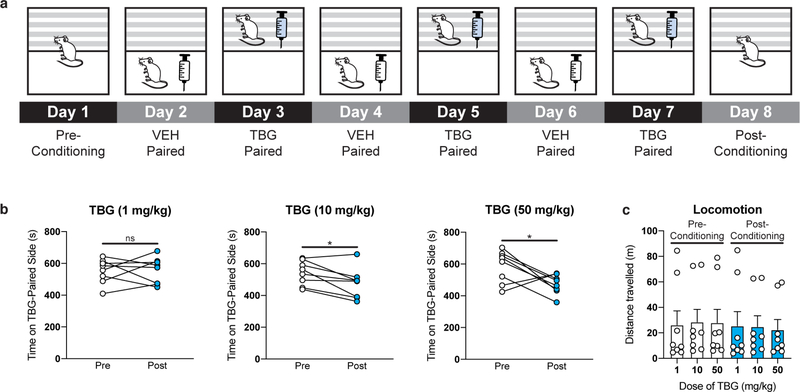
To determine if TBG has rewarding effects, we performed a conditioned place preference assay (CPP) in mice (Extended Data Fig. 6). A low dose of TBG (1 mg/kg) did not have any effect on place preference (p = 0.8972). Higher doses produced a modest conditioned place aversion (CPA) (p = 0.0199 for 10 mg/kg; p = 0.0489 for 50 mg/kg), suggesting that TBG has a low potential for abuse.
Effect of TBG on Neural Plasticity
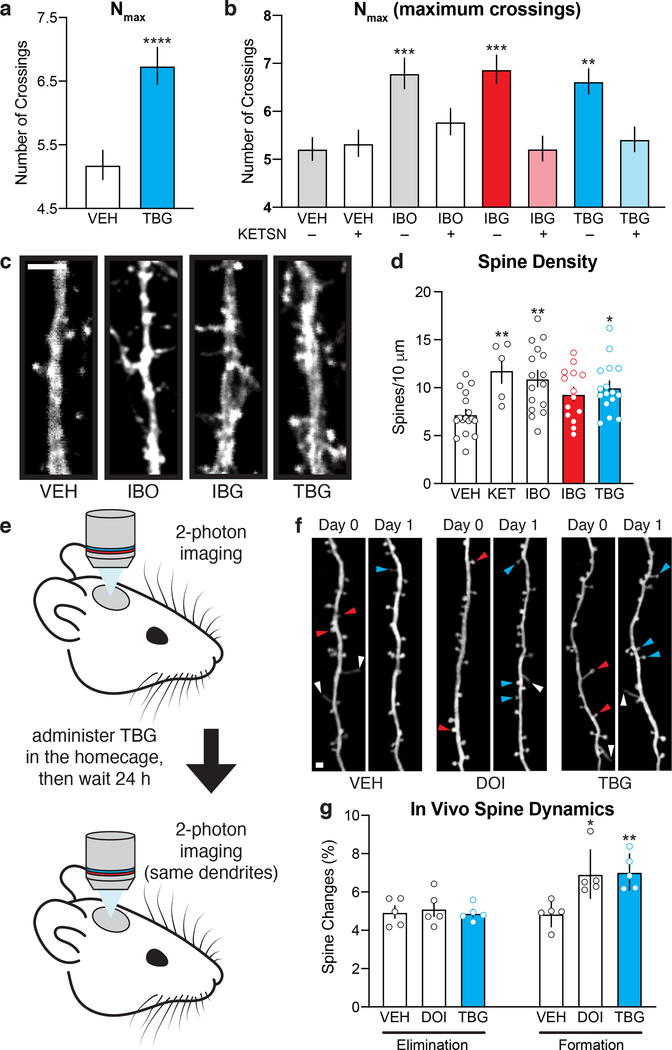
Having demonstrated the improved safety profile of TBG relative to ibogaine, we next assessed its effects on structural plasticity. Treating rat embryonic cortical neurons with TBG increased dendritic arbor complexity as measured by Sholl analysis (Fig. 3a), and this effect appears to be 5-HT2A-dependent, as it was blocked by pretreatment with the 5-HT2A antagonist ketanserin (Fig. 3b).
Figure 3. TBG promotes neural plasticity.
(a) Maximum numbers of crossings (Nmax) of Sholl plots obtained from rat embryonic cortical neurons (DIV6). (b) The effects of TBG on dendritic growth are blocked by the 5-HT2A antagonist ketanserin. (c) Representative images of secondary branches of rat embryonic cortical neurons (DIV20) after treatment with ibogalogs for 24 h. Scale bar = 2 μm. (d) TBG increases dendritic spine density on rat embryonic cortical neurons (DIV20) after treatment for 24 h. (e) Schematic illustrating the design of transcranial 2-photon imaging experiments. (f) Representative images of the same dendritic segments from mouse primary sensory cortex before (Day 0) and after (Day 1) treatment. Blue, red, and white arrowheads represent newly formed spines, eliminated spines, and filopodia, respectively. Scale bar = 2 μm. (g) DOI and TBG increase spine formation but have no effect on spine elimination. Exact N numbers for each experimental condition are reported in the Source Data and Supplementary Table 1. Specific statistical tests, information on reproducibility, and exact p values are reported in the Methods and Supplementary Table 1.
In addition to promoting dendritic growth, TBG also increases dendritic spine density to a comparable extent as ibogaine in mature cortical cultures (DIV20) (Fig. 3c–d). Next, we used transcranial 2-photon imaging (Fig. 3e) to assess the effects of TBG on spine dynamics in mouse sensory cortex. Both TBG and the hallucinogenic 5-HT2A agonist 2,5-dimethoxy-4-iodoamphetamine (DOI) increased spine formation without impacting spine elimination 24 h after treatment (Fig. 3f–g). Similar results were observed in more anterior parts of the cortex and mirrored the effects of ketamine reported previously.26
Effect of TBG on Forced Swim Test Behavior After Stress
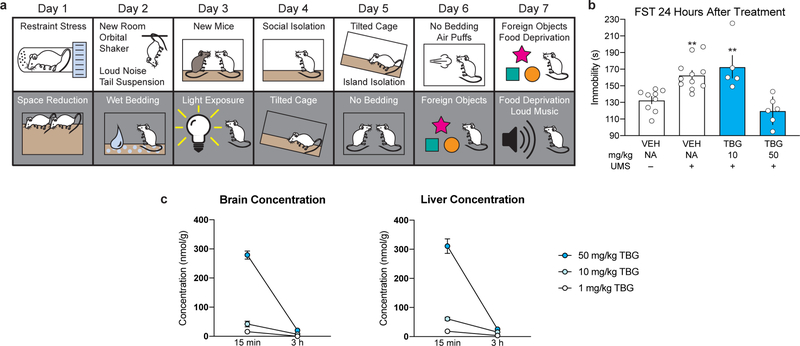
As increased structural plasticity in the anterior parts of the brain (e.g. prefrontal cortex, PFC) have been shown to mediate the sustained (>24 h) antidepressant-like effects of ketamine27 in rodent models and are hypothesized to play a key role in the therapeutic effects of 5-HT2A agonists,14,28 we next evaluated the impact of TBG on forced swim test (FST) behavior following 7 days of unpredictable mild stress (UMS) (Extended Data Fig. 7a–b) in mice. Immobility time was significantly increased after UMS. This effect was rescued by a 50 mg/kg, but not 10 mg/kg, dose of TBG. Preliminary pharmacokinetic studies revealed that a 50 mg/kg dose of TBG produced significantly higher brain concentrations than did a 10 mg/kg dose (Extended Data Fig. 7c). Therefore, the 50 mg/kg dose was used in subsequent mouse behavioral experiments.
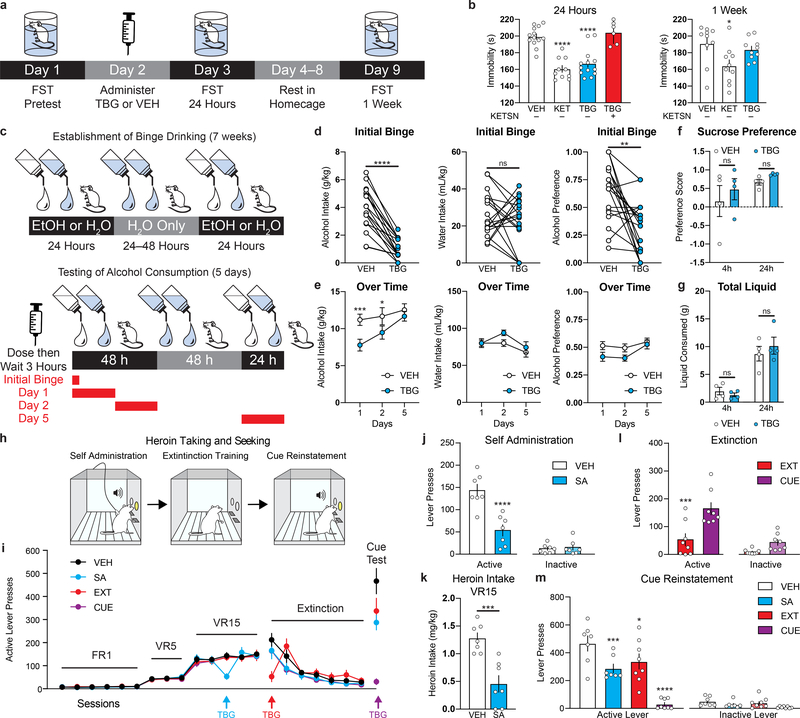
As ketamine produces antidepressant-like effects in the FST even in the absence of UMS, we performed a head-to-head comparison with TBG in this experiment (Fig. 4a–b). Drugs were administered 24 h after a pre-test, and the FST was performed 24 h and 7 d post drug administration. Both ketamine and TBG significantly reduced immobility in mice 24 h following drug administration, however, ketamine’s effects appeared to be more durable. Importantly, TBG had no effect on locomotion 24 h following administration (Extended Data Fig. 6c). As anticipated, treatment with a 5-HT2A antagonist (ketanserin) blocked the antidepressant-like effect of TBG (Fig. 4b). The efficacy exhibited by TBG in the FST is consistent with the fact that other 5-HT2A agonists have demonstrated potential for treating depression.13,28 However, future studies should evaluate the effects of TBG on other behaviors relevant to depression, particularly those measuring anhedonia.
Figure 4. Effects of TBG on animal behaviors relevant to depression, AUD, and SUD.
(a) Schematic illustrating the design of FST experiments conducted without UMS. (b) The antidepressant-like effects of TBG are blocked by ketanserin. (c) Timeline of alcohol binge-drinking experiment. White and blue droplets represent 20% EtOH and H20, respectively. (d) TBG acutely reduced EtOH consumption and preference during a binge drinking session without impacting H20 intake. (e) Acute TBG administration decreased EtOH consumption for at least 48 h. (f–g) TBG did not decrease sucrose preference (f) or reduce total liquid consumption (g) in a two-bottle choice experiment. (h) Schematic illustrating the design of the heroin self-administration experiments. (i) Heroin seeking over time is shown. Colored arrows indicate when each group received TBG. VEH was administered at all other time points to each group. (j–k) TBG acutely reduced heroin self-administration—both lever pressing (j) and heroin intake (k). (l) TBG acutely reduced heroin-seeking when administered immediately before the first extinction session. The CUE (injection 1 = VEH, injection 2 = VEH) and EXT (injection 1 = VEH, injection 2 = TBG) groups were compared, as they were matched for the number of withdrawal days between the last self-administration and first extinction session. (m) Acute TBG completely blocked cued reinstatement (purple bar, CUE). A single prior (12–14 d) administration of TBG during heroin self-administration or the first day of extinction (blue and red bars, respectively) inhibited cued reinstatement. Exact N numbers for each experimental condition are reported in the Source Data and Supplementary Table 1. Specific statistical tests, information on reproducibility, and exact p values are reported in the Methods and Supplementary Table 1.
Effect of TBG on Alcohol- and Heroin-Seeking Behavior
To assess the effect of TBG on alcohol (EtOH) intake, we employed an intermittent access 2-bottle choice (20% EtOH (v/v) vs. H2O) experiment that models binge drinking behavior in humans.29 Mice were subjected to repeated cycles of binge drinking and withdrawal over the course of 7 weeks (Fig. 4c), which resulted in high EtOH consumption (11.44 ± 0.76 g/kg/24h), binge drinking-like behavior (3.89 ± 0.33 g/kg/4h), and generated blood alcohol content equivalent to that of human subjects suffering from alcohol use disorder (AUD). Systemic injections of TBG 3 h prior to a drinking session reduced binge drinking during the first 4 h without affecting water intake (Fig. 4d). Consumption of alcohol was lower for at least two days following TBG administration (Fig. 4e). Similar results were observed previously for ibogaine.3 Importantly, administration of TBG prior to giving mice access to both water and a 5% sucrose solution did not decrease sucrose preference (Fig. 4f) or total fluid consumption (Fig. 4g), indicating that TBG selectively reduced alcohol intake.
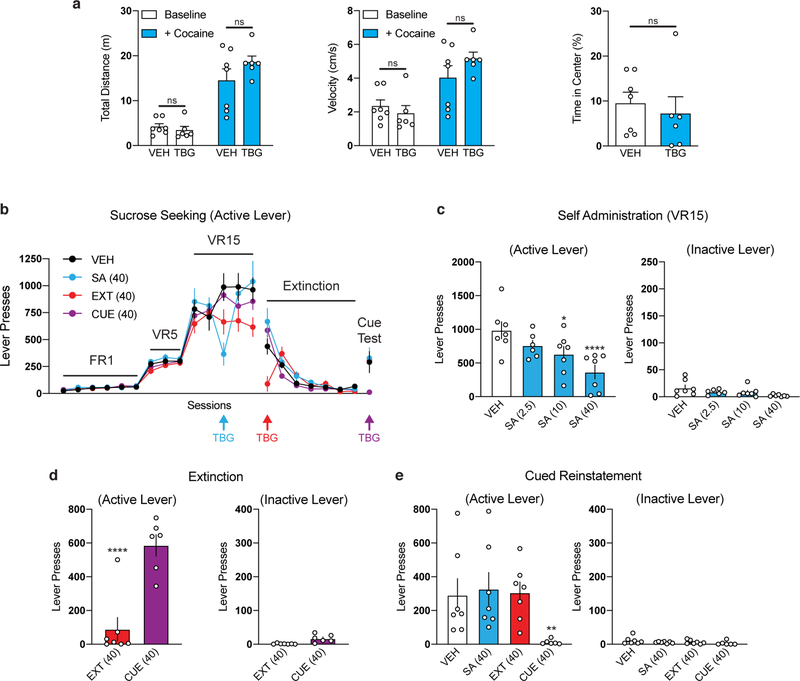
As with alcohol consumption, anecdotal reports suggest that ibogaine can reduce opioid use in humans.1,6 In rodent models of opioid self-administration, ibogaine produces the greatest decrease when administered at a dose of 40 mg/kg.30 Here, we used a rat model of heroin self-administration (Fig. 4h) to assess the effects of TBG (40 mg/kg) administered during three distinct epochs—during self-administration (SA), prior to the first day of extinction (EXT), and immediately before cued reinstatement (CUE). Vehicle was administered at these same time points whenever TBG was not (Fig. 4i). Thus, each group received a total of 3 injections. When administered during self-administration (Fig. 4j–k), immediately prior to extinction (EXT, Fig. 4l) or before cued reinstatement (CUE, Fig. 4m), TBG acutely reduced heroin-seeking behavior. While TBG did not induce any acute locomotor deficits (Extended Data Fig. 8a), the acute effects of TBG should be interpreted with caution. Similar to heroin, acute TBG administration strongly reduced sucrose self-administration at all three timepoints (Extended Data Fig. 8b–e), indicating that the acute effects of TBG may be due to non-selective disruption of operant responding.
Strikingly, cue-induced relapse was reduced in the groups that had received TBG treatment long before cued reinstatement (SA and EXT, Fig. 4m). In sharp contrast, TBG had no impact on cued reinstatement of sucrose-seeking behavior when administered 12–14 days prior (Extended Data Fig. 8e). Thus, a single administration of TBG elicited anti-addictive effects lasting up to 12–14 days. Long-lasting protections against relapse after a single treatment have rarely been reported, but they are reminiscent of results in a cocaine self-administration model following direct injection of BDNF into the PFC.31
Discussion
Compounds capable of modifying neural circuits controlling motivation, anxiety, and drug-seeking behavior have been hypothesized to be effective treatments for a diverse range of neuropsychiatric disorders including depression, post-traumatic stress disorder, and SUD.13 In principle, such psychoplastogenic medicines could produce sustained therapeutic effects by rectifying underlying pathological changes in circuitry rather than masking disease symptoms. Psychedelic compounds may prove useful in this regard because they promote structural and functional neural plasticity in the PFC of rodents.14 While their putative therapeutic mechanisms of action are unknown, anecdotal reports and small clinical trials suggest that they might produce sustained therapeutic responses in multiple neuropsychiatric disorders following a single administration.
While psychedelics and ketamine have been hypothesized to share a common antidepressant mechanism of action related to cortical neuron growth in the PFC, a causal link between psychedelic-induced neuronal growth and behavior has yet to be established in either humans or rodents. In contrast, Liston and co-workers recently used a synapse targeting photoactivatable Rac1 to demonstrate that the sustained antidepressant-like effects of ketamine in mice are mediated in part by spinogenesis in the PFC.27
Here, we used the principles of FOS to identify the indole-fused tetrahydroazepine as the key psychoplastogenic pharmacophore of ibogaine. This information enabled us to develop a 1-step synthesis of ibogaine analogs capable of promoting structural neural plasticity both in cell culture and in vivo. Simplification of ibogaine’s architecture to produce TBG not only enhanced synthetic tractability; it also improved physicochemical properties and safety. Compounds lacking the isoquinuclidine were significantly less potent inhibitors of hERG channels and did not induce bradycardia or show signs of toxicity in zebrafish. With the exception of 18-methoxycoronaridine (18-MC), which is currently in phase II clinical trials, very few ibogaine analogs have demonstrated this level of safety while also producing therapeutic effects.32 It is unknown if 18-MC is psychoplastogenic, but unlike ibogaine, it does not increase GDNF expression in SH-SY5Y cells or reduce alcohol self-administration following direct infusion into the ventral tegmental area, suggesting their mechanisms of action are likely distinct.33 While the synthesis of 18-MC is 13 steps,34 TBG can be synthesized in a single step.
Not only does TBG potently promote neuronal growth, it also produces antidepressant-like behavioral responses and reduces alcohol (but not sucrose) consumption in mice. In rats, acute administration of TBG strongly inhibits both heroin- and sucrose-seeking behavior, likely by disrupting operant responding. However, when administered days in advance, TBG only prevents cued reinstatement of heroin-seeking behavior (but not sucrose). Future work is required to further characterize the optimal dosing regimen and time course for producing these behavioral effects in rodents, and to determine whether structural plasticity plays a causal role.
METHODS
Data Analysis and Statistics.
Treatments were randomized, and data were analyzed by experimenters blinded to treatment conditions. Statistical analyses were performed using GraphPad Prism (version 8.1.2) unless noted otherwise. All comparisons were planned prior to performing each experiment. Data are represented as mean ± SEM, unless otherwise noted, with asterisks indicating *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001. Boxplots depict the three quartile values of the distribution with whiskers extending to points that lie within 1.5 IQRs (interquartile range) of the lower and upper quartile. Observations falling outside this range are displayed independently. For Figs. 2a, 3a, 3b, 3d, 3g, 4b, Extended Data Fig. 2b, and Extended Data Fig. 7b, compound treatments were compared to the VEH control using a one-way ANOVA with Dunnett’s post hoc test. For Extended Data 3a and Fig. 2c, time 0 h and time 1 h (before and after drug administration, respectively) data were compared using a paired t-test. For Extended Data 3f, compound treatments were compared using Fisher’s exact test and p-values are indicated in the text. For Fig. 4d and Extended Data Fig. 6b, data were analyzed using a two-tailed paired t-test. Data in Fig. 4e–g and Extended Data Fig. 8a (distance and velocity) were analyzed using a two-way ANOVA with Sidak’s post hoc test. For Fig. 4j, Fig. 4l–m, and Extended Data Fig. 8c–e, two-way repeated measures (RM) ANOVAs with treatment group as the between-subject factor and lever type (active versus inactive) as the within-subject factor were used. Sidak post-hoc tests were conducted as appropriate. For Extended Data Fig. 6c, a one-way ANOVA with Tukey’s post hoc test was used. For Fig. 4k and Extended Data Fig. 8a (thigmotaxis) two-tailed unpaired t-tests were used.
Drugs.
The NIDA Drug Supply Program provided ibogaine hydrochloride (IBO), noribogaine (NOR), heroin (diamorphine hydrochloride), and cocaine hydrochloride. Other chemicals were purchased from commercial sources such as ketamine hydrochloride (KET, Fagron), ketanserin (KETSN, ApexBio), eugenol (Tokyo Chemical Industries), and 5-hydroxytryptamine (Sigma-Aldrich). The fumarate salt of 5-methoxy-N,N-dimethyltryptamine (2:1, 5-MeO-DMT:fumaric acid) was synthesized in house as described previously20 and judged to be analytically pure based on NMR and LC-MS data. For cell culture experiments, VEH = 0.1% (agonist studies) or 0.2% (antagonist studies) molecular biology grade dimethyl sulfoxide (Sigma-Aldrich). For in vivo experiments, VEH = USP grade saline (0.9%). Free bases were used for all cellular experiments while the fumarate salts of ibogainalog and tabernanthalog were used for the in vivo studies.
Animals.
All experimental procedures involving animals were approved by either the UCD, UCSF, UCSC or CU Anschutz Institutional Animal Care and Use Committee (IACUC) and adhered to principles described in the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Power analyses were conducted to ensure appropriate sample size for all experiments involving animals. The University of California, Davis (UCD), the University of California, San Francisco (UCSF), the University of California, Santa Cruz (UCSC), and the University of Colorado Denver, Anschutz Medical Campus (CU Anschutz) are accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC).
Calculation of CNS MPO Score.
CNS MPO scores were calculated using a previously published method.18 Predicted pKa values were determined using Marvin Sketch (19.25.0). LogP and total polar surface area were predicted using Molinspiration (https://www.molinspiration.com/). LogD was calculated using the following equation LogD = LogP - LOG10(1+10(pka−7.4)).
Dendritogenesis Experiments.
For the dendritogenesis experiments conducted using cultured cortical neurons, timed pregnant Sprague Dawley rats were obtained from Charles River Laboratories (Wilmington, MA). Full culturing, staining, and analysis details were performed as previously described.20 Dendrites were visualized using a chicken anti-MAP2 antibody (1:10,000; EnCor, CPCA-MAP2) as previously reported.20
Head-Twitch Response (HTR).
The head-twitch response assay was performed as described previously20 using both male and female C57BL/6J mice (2 per treatment). The mice were obtained from Jackson Laboratory (Sacramento, C.A.) and were approximately 8 weeks old at the time of the experiments. Compounds were administered via intraperitoneal injection (5 mL/kg) using 0.9% saline as the vehicle. As a positive control, we utilized 5-MeO-DMT fumarate (2:1 amine/acid), which was synthesized as described previously.20 Behavior was videotaped, later scored by two blinded observers, and the results were averaged (Pearson correlation coefficient = 0.93).
hERG Inhibition Studies.
All experiments were conducted manually using a HEKA EPC-10 amplifier at room temperature in the whole-cell mode of the patch-clamp technique. HEK293 cells stably expressing hKv11.1 (hERG) under G418 selection were a gift from Craig January (University of Wisconsin, Madison). Cells were cultured in DMEM containing 10% fetal bovine serum, 2 mM glutamine, 1 mM sodium pyruvate, 100 U/mL penicillin, 100 μg/mL streptomycin, and 500 mg/ml G418. The cell line was not authenticated or tested for mycoplasma contamination. Before experiments, cells were grown to 60–80% confluency, lifted using TrypLE, and plated onto poly-L-lysine-coated coverslips. Patch pipettes were pulled from soda lime glass (micro-hematocrit tubes) and had resistances of 2–4 MΩ. For the external solution, normal sodium Ringer was used (160 mM NaCl, 4.5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM HEPES, pH 7.4 and 290–310 mOsm). The internal solution used was potassium fluoride with ATP (160 mM KF, 2 mM MgCl2, 10 mM EGTA, 10 mM HEPES, 4 mM NaATP, pH = 7.2 and 300–320 mOsm). A 2-step pulse (applied every 10 sec) from −80 mV first to 40 mV for 2 sec and then to −60 mV for 4 sec, was used to elicit hERG currents. The percent reduction of tail current amplitude by the drugs was determined and data are shown as mean +/− SD (n = 3–4 per data point). For all experiments, solutions of the drugs were prepared fresh from 10 mM stocks in DMSO. The final DMSO concentration never exceeded 1%.
Larval Zebrafish Heart Rate Experiments.
Zebrafish express Zerg, an orthologue of hERG, and many hERG inhibitors induce bradycardia an arrhythmia in zebrafish.35 Heart rate was recorded and calculated as reported previously36 with slight modifications (n = 3–9). Briefly, 7 dpf zebrafish larvae were anesthetized with tricaine (Acros Organics) and immobilized in a lateral orientation using 1% low melt agarose (LMA, Gene Mate) dissolved in egg water.37 Tricaine was washed out and drug was added to 4 mL embryo media in a 6-well plate (final concentration = 50 μM). Videos were collected at 30 frames per second (fps) using a Leica M80 scope with an ACHRO 1x nosepiece attachment and a Leica IC80 HD camera. Regions of interest (ROIs) were drawn around the atrium and ventricle of individual zebrafish and average pixel dynamics were calculated using the ImageJ plugin Time Series Analyzer V3. This pixel change oscillation was graphically smoothed using the Savgol filter in SciPy. Peaks were detected using the SciPy package “find_peaks”. Peak time interval and BPM were calculated using custom code. The arrythmia score was calculated as the ratio of atrium BPM to ventricle BPM (n = 6–18).
Larval Zebrafish Behavioral Experiments.
Behavioral data were collected and analyzed as described previously,20,22 with slight modifications. Locomotion of 7 dpf zebrafish was recorded at 100 fps under a 17-minute battery of acoustic and light-based stimuli 1 h post-treatment. Data were collected in concentration–response format on eight 96-well plates with 8 zebrafish per well. Each plate contained all 10 compounds at 8 concentrations plus 8 DMSO (vehicle) and 8 eugenol (lethal) control wells. Treatments and well positions were scrambled according to 2 randomized plate layouts. Motion in Extended Data 3a was smoothed using the mean over a 10-frame sliding window. Classifiers were trained as described.22 For Extended Data 3d, 6 repeat vs-solvent and 6 repeat vs-lethal classifiers (3,000 trees) were trained per comparison, each to classify 8 treatment wells and 8 randomly subsampled control wells. Responses were calculated from the out-of-bag (oob) accuracy values as r = (a−0.4)/(1−0.4). 95% confidence intervals were calculated over 1,000 bootstrap samples per comparison. For Extended Data 3b, a single classifier (10,000 trees) was trained on all data for the treatments shown, and the oob predictions were used.
Larval Zebrafish Seizure Experiments.
At 6 dpf, transgenic zebrafish larvae (Tg(elavl3:GCaMP5G)a4598)38 were anesthetized with tricaine and immobilized in a dorsal orientation using 1% LMA dissolved in egg water. Tricaine was washed out and zebrafish were treated for 1 h with compounds (50 μM for IBO and TBG; 15 mM for PTZ). Videos were acquired using a Zeiss Axiozoom.V16, and GCaMP5G fluorescence was induced using a Lumencor sola light engine. Zen software V2 blue edition controlled an Axiocam 506 mono camera set to 33 fps. Short videos (1–3 min) were acquired per condition. Change in fluorescence intensity was calculated using ImageJ from an ROI drawn in the cerebellar region, and ΔF/F was calculated and visualized using custom functions.
Larval Zebrafish Toxicity.
Tropical 5D wild-type larval zebrafish were obtained from the Sinnhuber Aquatic Research Laboratory (SARL) at Oregon State University (Corvallis, Oregon), and subsequent generations were raised at UC Davis. Zebrafish husbandry, spawning, dechorionation of embryos, and exposures were performed as described previously.24 Chemical stocks were prepared at 100 mM in DMSO and diluted to 200 μM with embryo media. This solution was diluted 2-fold into individual wells of 96-well plates housing larval zebrafish. The final compound and DMSO concentrations were 100 μM and 0.1% (v/v), respectively. Wells were covered with Parafilm M (Bemis, North America, Neenah, Wisconsin) then covered with the plate lid. Plates were maintained in an incubator at 28.5 °C with a 14 h light (~300 lux)/10 h dark cycle. Fish were statically exposed to compounds 6 h post-fertilization (hpf) through 5 dpf. All compounds were tested for mortality/teratology in triplicate experiments (three experiments conducted on independent days using fish from independent spawns). For each experiment, 16 fish were tested per concentration per compound (n = 48 fish/condition). At 1, 2, 3, 4, and 5 dpf, fish were examined for mortality and developmental malformations using a Leica Stereo Microscope Model S6D (Leica, Germany) up to 4.5x magnification.
Serotonin and Opioid Receptor Functional Assays.
Functional assay screens at 5-HT and opioid receptors were performed in parallel using the same compound dilutions and 384-well format high-throughput assay platforms. Assays assessed activity at all human isoforms of the receptors, except where noted for the mouse 5-HT2A receptor. Receptor constructs in pcDNA vectors were generated from the Presto-Tango GPCR library39 with minor modifications. All compounds were serially diluted in drug buffer (HBSS, 20 mM HEPES, pH 7.4 supplemented with 0.1% bovine serum albumin and 0.01% ascorbic acid) and dispensed into 384-well assay plates using a FLIPRTETRA (Molecular Devices). Every plate included a positive control such as 5-HT (for all 5-HT receptors), DADLE (DOR), salvinorin A (KOR), and DAMGO (MOR). For measurements of 5-HT2A, 5-HT2B, and 5-HT2C Gq-mediated calcium flux function, HEK Flp-In 293 T-Rex stable cell lines (Invitrogen) were loaded with Fluo-4 dye for one hour, stimulated with compounds and read for baseline (0–10 seconds) and peak fold-over-basal fluorescence (5 min) at 25°C on the FLIPRTETRA. For measurement of 5-HT6 and 5-HT7a functional assays, Gs-mediated cAMP accumulation was detected using the split-luciferase GloSensor assay in HEKT cells measuring luminescence on a Microbeta Trilux (Perkin Elmer) with a 15 min drug incubation at 25°C. For 5-HT1A, 5-HT1B, 5-HT1F, MOR, KOR, and DOR functional assays, Gi/o-mediated cAMP inhibition was measured using the split-luciferase GloSensor assay in HEKT cells, conducted similarly as above, but in combination with either 0.3 μM isoproterenol (5-HT1A, 5-HT1B, 5-HT1F) or 1 μM forskolin (MOR, KOR, and DOR) to stimulate endogenous cAMP accumulation. For measurement of 5-HT1D, 5-HT1E, 5-HT4, and 5-HT5A functional assays, β-arrestin2 recruitment was measured by the Tango assay utilizing HTLA cells expressing TEV fused-β-arrestin2, as described previously39 with minor modifications. Cell lines were not authenticated or tested for mycoplasma contamination. Data for all assays were plotted and non-linear regression was performed using “log(agonist) vs. response” in Graphpad Prism to yield Emax and EC50 parameter estimates.
Safety Pharmacology Profiling Panel.
Eurofins Discovery (Taipei, Taiwan) screened TBG (10 μM) against their SafetyScreen87™ Panel and in their VMAT (Non-Selective) Human Vesicular Monoamine Transporter Binding Assay.
Conditioned Place Preference (CPP).
The CPP apparatus consisted of two chambers (18 cm L x 20 cm W x 35 cm H) connected by a corridor (10 cm L x 20 cm W x 35 cm H). One chamber had a smooth floor and black walls while the second chamber had a mesh floor and patterned walls. A block was placed in the corridor to restrict mice to a particular chamber. On Day 1 (pre-conditioning), male C57/BL6J mice (9–10 weeks old) were allowed to explore the entire apparatus for 30 min. Mice were randomly sorted into treatment groups (TBG at 50 mg/kg, 10 mg/kg and 1 mg/kg), ensuring that their initial preferences for what would become the TBG-paired side were approximately equal. Next, the mice were administered an intraperitoneal injection of either VEH (saline) or TBG (counterbalanced) immediately before being confined to one of the two chambers for 30 min. The following day, the other treatment was administered, and the mice were confined to the opposite chamber for 30 min. This sequence was repeated twice, such that all mice received 3 VEH-side pairings and 3 TBG-side pairings. The mice were returned to their home cages in between treatment-side pairings. On Day 8 (post conditioning), the mice were allowed to explore the entire apparatus for 30 min, and the time spent on the VEH- and TBG-paired sides was quantified using ANYmaze software (version 6.2). The apparatus was cleaned with 70% ethanol between trials. Drug solutions were prepared fresh daily.
Pharmacokinetic Studies.
Male and female C57/BL6J mice (12 weeks old) were administered TBG via intraperitoneal injection at doses of either 50 mg/kg, 10 mg/kg or 1 mg/kg. Mice were sacrificed 15 min or 3 h post-injection via cervical dislocation. Two males and two females were used per dose/timepoint. Brain and liver were harvested, flash frozen in liquid nitrogen, and stored at −80°C until metabolomic processing. Metabolites were extracted from tissue as described previously.40 Briefly, whole brain and liver sections were lyophilized overnight to complete dryness, then homogenized with 3.2 mm diameter stainless steel beads using a GenoGrinder for 50 seconds at 1500 rpm. Ground tissue was then extracted using 225 μL cold methanol, 190 μL water, 750 μL methyl tert-butyl ether (MTBE). Seven method blanks and seven quality control (QC) samples (pooled human serum, BioIVT) were extracted at the same time as the samples. The nonpolar fraction of MTBE was dried under vacuum and reconstituted in 60 μL of 90:10 (v/v) methanol: toluene containing 1-cyclohexyl-dodecanoic acid urea (CUDA) as an internal standard. Samples were then vortexed, sonicated and centrifuged prior to analysis. For analysis of TBG in liver and brain, samples were randomized prior to injection with method blanks and QC samples analyzed between every ten study samples. A six-point calibration curve was analyzed after column equilibration using blank injections, and then after all study samples. Blanks were injected following the calibration curve to ensure no tabernanthalog was retained on the column and carried over to samples. Reconstituted sample (5 μL) was injected onto a Waters Acquity UPLC CSH C18 column (100 mm x 2.1 mm, 1.7 μm particle size) with an Acquity UPLC CSH C18 VanGuard precolumn (Waters, Milford, MA) using a Vanquish UHPLC coupled to a TSQ Altis triple quadrupole mass spectrometer (ThermoFisher Scientific, San Jose, CA). Mobile phase A consisted of 60:40 v/v acetonitrile/water with 10 mM ammonium formate and 0.1% formic acid. Mobile phase B was 90:10 v/v isopropanol/acetonitrile with 10 mM ammonium formate and 0.1% formic acid. Gradients were run from 0–2 minutes at 15% B; 2–2.5 minutes 30% B; 2.5–4.5 minutes 48% B; 4.5–7.3 minutes 99% B; 7.3–10 minutes 15% B. The flow rate was 0.600 mL/min and the column was heated to 65°C. Mass spectrometer conditions were optimized for TBG by direct infusion. Selected reaction monitoring for the top five ions, with collision energy, source fragmentation, and radio frequency optimized for TBG. Data were processed with TraceFinder 4.1 (ThermoFisher Scientific, San Jose, CA). Organ weights were recorded. The concentration in the brain was calculated using the experimentally determined number of mols of TBG in the whole organ divided by the weight of the organ.
Spinogenesis Experiments.
Spinogenesis experiments were performed as previously described14 with the exception that cells were treated on DIV19 and fixed 24 h after treatment on DIV20. The images were taken on a Nikon HCA Confocal microscope a with a 100x/NA 1.45 oil objective. DMSO and ketamine (10 μM) were used as vehicle and positive controls, respectively.
In Vivo Spine Dynamics.
Male and female Thy1-GFP-M line mice41 (n = 5 per condition) were purchased from The Jackson Laboratory (JAX #007788) and maintained in UCSC animal facilities according to an IACUC approved protocol. In vivo transcranial two-photon imaging and data analysis were performed as previously described.42 Briefly, mice were anesthetized with an intraperitoneal (i.p.) injection of a mixture of ketamine (87 mg/kg) and xylazine (8.7 mg/kg). A small region of the exposed skull was manually thinned down to 20–30 μm for optical access. Spines on apical dendrites in mouse primary sensory cortices were imaged using a Bruker Ultima IV two-photon microscope equipped with an Olympus water-immersion objective (40x, NA = 0.8) and a Ti:Sapphire laser (Spectra-Physics Mai Tai, excitation wavelength 920 nm). Images were taken at a zoom of 4.0 (pixel size 0.143 × 0.143 μm) and Z-step size of 0.7 μm. The mice received an i.p. injection of DOI (10 mg/kg) or TBG (50 mg/kg) immediately after they recovered from the anesthesia of the first imaging session. The mice were re-imaged 24 h after drug administration. Dendritic spine dynamics were analyzed using ImageJ. Spine formation and elimination were quantified as percentages of spine numbers on day 0.
Antidepressant-Like Response Following Unpredictable Mild Stress (UMS).
Male and female mice (8 weeks old) were subjected to 7 d of UMS, as described previously.43 Briefly, the following stressors were utilized: Day 1: Light Phase = 30 min of restraint stress x 2; Dark Phase = home cage space reduction. Day 2: Light Phase = exposure to a new room + 30 min on the orbital shaker, sudden loud noise x 5, tail suspension for 6 min; Dark Phase = wet bedding. Day 3: Light Phase = exposure to new mice; Dark Phase = exposure to light. Day 4: Light Phase = social isolation; Dark Phase = tilted cage. Day 5: Light Phase = tilted cage, island isolation; Dark Phase = no bedding. Day 6: Light Phase = no bedding, random puff of air x 5–10; Dark Phase = foreign objects. Day 7: Light Phase = foreign objects, food deprivation; Dark Phase = food deprivation, continual exposure to loud music. Immediately following UMS, TBG or VEH were administered via intraperitoneal injection, and 24 h later the mice were subjected to a FST using the same procedure as described below.
Forced Swim Test (FST) in the Absence of UMS.
Male C57/BL6J mice (9–10 weeks old at time of experiment) were obtained from the Jackson Lab and housed 4–5 mice/cage in a UCD vivarium following an IACUC approved protocol. After 1 week in the vivarium each mouse was handled for approximately 1 minute by a male experimenter for 3 consecutive days leading up to the first FST. All experiments were carried out by the same male experimenter who performed handling. During the FST, mice underwent a 6 min swim session in a clear Plexiglas cylinder 40 cm tall, 20 cm in diameter, and filled with 30 cm of 24 ± 1°C water. Fresh water was used for every mouse. After handling and habituation to the experimenter, drug-naïve mice first underwent a pretest swim to more reliably induce a depressive phenotype in the subsequent FST sessions. Immobility scores for all mice were determined after the pre-test and mice were randomly assigned to treatment groups to generate groups with similar average immobility scores to be used for the following two FST sessions. The next day, the mice received intraperitoneal injections of TBG (50 mg/kg), a positive control (ketamine, 3 mg/kg), or vehicle (saline). One additional group received ketanserin (4 mg/kg i.p.) 10 min prior to intraperitoneal administration of TBG (50 mg/kg). The following day, the mice were subjected to the FST and then returned to their home cages. One week later, the FST was performed again to assess the sustained effects of the drugs. All FSTs were performed between the hours of 8 am and 1 pm. Experiments were video-recorded and manually scored offline. Immobility time—defined as passive floating or remaining motionless with no activity other than that needed to keep the mouse’s head above water—was scored for the last 4 min of the 6 min trial.
Alcohol Consumption.
Male C57/BL6J mice (6–8 weeks old) were obtained from The Jackson Laboratory (Bar Harbor, ME) and were individually housed in a reverse light/dark cycle room (lights on 10:00pm–10:00am). Temperature was kept constant at 22 ± 2°C, and relative humidity was maintained at 50 ± 5%. Mice were given access to food and tap water ad libitum. After one week of habituation to the vivarium, the two-bottle choice alcohol-drinking experiment was conducted as described previously.29 For 7 weeks, mice were given intermittent access in their home cage to alcohol. On Mondays, Wednesdays, and Fridays, two bottles were made available for 24 h—one containing 20% ethanol and another containing only water. On Tuesdays, Thursdays, Saturdays, and Sundays, the mice were only given access to water. After 7 weeks, mice were administered TBG (50 mg/kg) or vehicle (saline) via intraperitoneal injection 3 h before the beginning of a drinking session. Ethanol (g/kg) and water (ml/kg) intake were monitored during the first 4 h (initial binge), the first 24 h, and the second 24 h. Next, the mice were only given water for 48 h before the start of another drinking session when ethanol and water consumption was monitored. The placement (right or left) of the bottles was altered in each session to control for side preference. Spillage was monitored using an additional bottle in a nearby unused cage. Alcohol preference was calculated as the ratio between alcohol/(water + alcohol). Mice were tested using a counterbalanced, within subject design with one week of drug-free alcohol drinking regimen between treatments. One mouse was excluded because the bottle was leaking.
Sucrose Preference.
Male C57/BL6J mice were individually housed and subjected to a two-bottle choice experiment. First, mice were administered TBG (50 mg/kg) or vehicle (saline) via intraperitoneal injection 3 h before the beginning of a two-bottle choice session. During this 3 h period, mice were not given access to water in an attempt to increase their thirst. At the start of the experiment, mice were given one bottle of water and one bottle of water containing 5% sucrose. Sucrose solution and water intake were monitored during the first 4 h and the first 24 h. Sucrose preference was calculated was calculated as the amount of sucrose solution consumed minus the amount of water consumed, divided by the total amount of liquid consumed.
Heroin Self-Administration Behavior.
Subjects were age-matched male (n = 16) and female (n = 16) Wistar rats (Charles River). Rats were single housed in a temperature and humidity-controlled room with a 12 h light/dark cycle (7:00 A.M. lights on) with free access to standard laboratory chow and water. Two rats (one male and one female) were excluded from the final dataset due to defective catheters for a final n = 30 rats. Rats were surgically implanted with an intravenous catheter as previously described.44 Heroin self-administration training began at least one week after surgery on a fixed ratio 1 (FR1) schedule of reinforcement. Operant chambers were equipped with both an active (heroin-delivering) and inactive lever, and each heroin infusion (0.04 mg, 50 μl, 2.85 s) was coupled with delivery of a light cue located above the active lever and a 3.5 kHz tone (5 s). Both levers retracted upon initiation of a heroin infusion and remained retracted during the tone + light heroin cue presentation. After six self-administration sessions (2.5 h) on FR1, rats progressed to a variable ratio 5 (VR5) for three sessions and continued to the final variable ratio 15 (VR15) for five sessions. Rats then began extinction training. Extinction training sessions (1 h) were conducted in the same operant chambers (context) where rats previously self-administered heroin, but in the absence of heroin and its tone + light cue. Both levers remained extended throughout the session, and responding was recorded, but produced no consequence. After completing a total of 7 extinction sessions, rats underwent a cued reinstatement test (1 h, withdrawal day 10–12). During the cue test, the heroin tone + light cues were available, but heroin was not. The first active lever press resulted in presentation of the heroin cues, and then cues were available on a VR5 schedule (active lever only) for the remainder of the test. Lever retraction occurred during cue presentation (as during self-administration). Injections of TBG (40 mg/kg i.p.) or vehicle (VEH) were administered on the third VR15 session, the first extinction session, and the cued reinstatement test. For each of these timepoints, TBG or VEH was injected 30 min prior to placement in the chamber. Treatment groups were balanced based on response rates, heroin intake, and sex. Behavioral sessions were conducted daily (weekdays only). Catheters were flushed after each self-administration session with cefazolin and taurolidine citrate solution to prevent infection and/or catheter occlusion. Statistical tests were performed in Prism (GraphPad Prism, RRID:SCR_002798; V8.0) software.
Sucrose Self-Administration Behavior.
Sucrose self-administration procedures were designed to mimic heroin self-administration conditions. Subjects were age-matched male (n = 24) and female (n = 24) Wistar rats (Charles River). Rats were single housed and had free access to standard laboratory chow and water throughout the experiment. Eight rats (seven males and one female) were excluded from the final dataset due to failure to acquire sucrose self-administration for a final n = 40 rats. The final groups consisted of VEH, SA (2.5), SA (10), SA (40), Ext (40), and CUE (40). The number of animals in each group was 7, 6, 7, 7, 7, 6, respectively (40 animals total). At least one week after arrival and acclimation to the animal facility, sucrose self-administration training began on a fixed ratio 1 (FR1) schedule of reinforcement. Operant chambers were equipped with an active (sucrose-delivering) and inactive lever, and each sucrose reward (45 mg pellet; Bio-Serv F0023) was coupled with the same tone + light cues used for the heroin study. Levers retracted upon pellet delivery and remained retracted during cue presentation (5 s). After six self-administration sessions (2 h) on FR1, rats progressed to a variable ratio 5 (VR5) for three sessions and continued to the final variable ratio 15 (VR15) for five sessions. Rats then began extinction training. Extinction training sessions (1 h) were conducted in the same operant chambers (context) where animals previously self-administered sucrose, but neither sucrose nor the sucrose cues were available. Responding on both levers was recorded during each session, but produced no consequence. After completing 7 extinction sessions, rats underwent a cued reinstatement test (1 h). During the cue test, the sucrose tone + light cues were available, but sucrose was not. The first active lever press resulted in presentation of the sucrose cues, and then cues were available on a VR5 schedule (active lever only) for the remainder of the test. Lever retraction occurred during cue presentation (as during self-administration). Injections were administered on the third VR15 session, the first extinction session, and the cued reinstatement test. For each of these tests, TBG (2.5 mg/kg, 10 mg/kg, or 40 mg/kg IP) or vehicle (VEH) was injected 30 min prior to placement in the chamber. The low (2.5 mg/kg) and intermediate (10 mg/kg) doses of TBG were tested only on the third VR15 sucrose self-administration session. The high dose (40 mg/kg) was tested at all three test time points. Statistical tests were performed in Prism (GraphPad Prism, RRID:SCR_002798; V8.0) software.
Open Field Test.
Naïve male (n = 7) and female (n = 6) Wistar rats (Charles River) were allowed to acclimate to the animal facility for at least one week after arrival. Spontaneous locomotion in response to a novel open field (44 cm long x 36 cm wide x 43 cm tall) was assessed 30 min after injection of vehicle or TBG (40 mg/kg, i.p.). Videos were recorded with an overhead camera connected to the tracking software EthoVision XT (Noldus, The Netherlands) for subsequent offline analysis. Rats were allowed to move freely in the open field for 30 min, then they were briefly removed from the apparatus to receive an injection of cocaine (15 mg/kg, i.p.). The rats were immediately returned to the open field for an additional hour to assess cocaine-induced locomotion. Each open field chamber was cleaned with Clidox-S in between sessions. Locomotion was tracked using EthoVision XT to assess the velocity (cm/s) and total distance traveled (m) during the baseline (first 30 min) and cocaine (last 60 min) phases separately. Thigmotaxis was assessed as the percentage of time spent in the center of the apparatus (26 cm long x 18 cm wide; i.e., 9 cm perimeters) was also analyzed during the baseline period to determine if TBG alters anxiety in the open field.
Extended Data
Extended Data Fig. 1. Synthesis of ibogalogs.
(a) Ibogalogs lacking the tetrahydroazepine of ibogaine were synthesized in only a few steps. Briefly, acylation of pyridine 1 under reductive conditions yielded the Cbz-protected dihydropyridine 2, which was immediately subjected to a Diels-Alder reaction with methyl vinyl ketone (3) followed by an in situ epimerization with NaOMe to afford an inseparable 1:1 mixture of exo (4a) and endo (4b) isomers (73% over 3 steps). Reaction of 4a and 4b with tosylhydrazide yielded the hydrazones 5a and 5b, which were separable via a combination of selective crystallization and chromatography (total yield of the two isomers = 75%). Caglioti reduction of the tosylhydrazones yielded 6a or 6b, which were readily converted to a variety of analogs via reaction sequences involving hydrogenolysis of the Cbz group, hydrogenation of the olefin, and C–N bond formation (see Supporting Information for details). (b) Ibogalogs lacking the isoquinuclidine of ibogaine were synthesized in a single step through Fischer indole cyclization. See Supporting Information for details.
Extended Data Fig. 2. The effects of ibogalogs on dendritogenesis.
(a) Representative images of rat embryonic cortical neurons (DIV6) treated with compounds. Scale bar = 10 μm. (b) Maximum numbers of crossings (Nmax) of the Sholl plots demonstrate that tetrahydroazepine-containing ibogalogs are more effective at increasing dendritic arbor complexity than are isoquinuclicine-containing ibogalogs. (c) Sholl analysis (circle radii = 1.34 μm increments) demonstrates that cultured cortical neurons treated with several ibogalogs have more complex dendritic arbors as compared to vehicle control (n = 52–83 neurons per treatment). The shaded area surrounding each line represents 95% confidence intervals. Control compounds, isoquinuclidines, and tetrahydroazepines are shown in blue, purple, and red, respectively. Exact N numbers for each experimental condition are reported in Supplementary Table 1. Specific statistical tests, information on reproducibility, and exact p values are reported in the Methods and Supplementary Table 1.
Extended Data Fig. 3. TBG is safer than ibogaine.
(a) Unlike ibogaine, IBG and TBG do not induce bradycardia in larval zebrafish. Sertindole (SI) was used as a positive control. (b) Heatmaps are shown representing aggregate larval zebrafish locomotor activity per well compared to vehicle controls (pseudo-Z-score). Red and blue indicate higher and lower activity than the mean of vehicle controls, respectively, while white indicates activity within ± 1 SD from control. Stimuli applied over time are indicated under the heatmaps. Colors indicate bright LED light of respective colors, black traces represent the waveforms of acoustic stimuli, and gray vertical lines indicate physical tapping as secondary acoustic stimuli. (c) Confusion matrix for classification of compounds (200 μM) plus VEH and lethal controls. (d) Concentration–response curves are shown for treated zebrafish subjected to the battery of stimuli depicted in Extended Data 3b. Lower percentages indicate treatments that were more often classified as vehicle (blue) or lethal (red). The solid line denotes the median and the shading denotes a 95th percentile confidence interval calculated by bootstrap. N = 8 wells / condition (64 zebrafish / condition). Blue lines indicate that all compounds produce behavioral phenotypes more distinct from vehicle at higher concentrations. Red lines indicate that known toxins (e.g., PTZ, picrotoxin, endosulfan), known hERG inhibitors (sertindole, haloperidol, terfenadine), and iboga alkaloids (IBO, NOR) produce behavioral phenotypes more closely resembling a lethal phenotype as their concentrations are increased. Increasing concentrations of IBG or TBG do not produce lethal-like behavioral phenotypes. (e) Transgenic larval zebrafish expressing GCaMP5G were immobilized in agarose, treated with compounds, and imaged over time. The known seizure-inducing compound PTZ was used as a positive control. Ibogaine and TBG were treated at 50 μM (n = 2 per condition). (f) Proportion of viable and non-viable (malformed + dead) zebrafish following treatment with VEH and TBG (66 μM) for 5 dpf (Fisher’s exact test: p = 0.3864). Representative images of zebrafish treated with VEH and TBG (66 μM) for 2 and 5 dpf are shown. Scale bar = 2 mm. Exact N numbers for each experimental condition are reported in Supplementary Table 1. Specific statistical tests, information on reproducibility, and exact p values are reported in the Methods and Supplementary Table 1.
Extended Data Fig. 4. Concentration-response curves demonstrating the abilities of ibogalogs and related compounds to activate 5-HT and opioid receptors.
All compounds were assayed in parallel using the same drug dilutions. Graphs reflect representative concentration-response curves plotting mean and SEM of data points performed in duplicate or triplicate. Assay details are described in the methods. Exact N numbers for each experimental condition are reported in Supplementary Table 1. Specific statistical tests, information on reproducibility, and exact p values are reported in the Methods and Supplementary Table 1.
Extended Data Fig. 5. Pharmacological profiles of ibogalogs and related compounds.
EC50 and Emax estimates from at least two independent concentration-response curves performed in duplicate or triplicate. Log(Emax/EC50) activity is included as an estimate of system agonist activity. Inactive = inactive in agonist mode; N.D. = not determined; blue boxes = exhibits antagonist activity; dark grey boxes = inactive in agonist mode but not tested in antagonist mode; orange boxes indicate inverse agonist. Ibogalogs are more selective 5-HT2A agonists than 5-MeO-DMT. Exact N numbers for each experimental condition are reported in Supplementary Table 1. Specific statistical tests, information on reproducibility, and exact p values are reported in the Methods and Supplementary Table 1.
Extended Data Fig. 6. High doses of TBG do not produce a conditioned place preference (CPP).
(a) Schematic illustrating the design of the CPP experiments. On day 1, the amount of time the mice spent in each distinct side of a two-chamber apparatus was recorded. Next, VEH and TBG were administered to mice on alternating days while they were confined to chamber A (white box) or B (gray parallel lines), respectively. Conditioning lasted for a total of 6 days. On day 8, preference for each distinct side of the two-chamber apparatus was assessed. (b) A low dose of TBG (1 mg/kg) did not produce CPP or conditioned place aversion (CPA). Higher doses (10 and 50 mg/kg) produce a modest CPA. (c) TBG does not produce any long-lasting (>24 h) effects on locomotion. There is no statistical difference in locomotion between any pre- or post-conditioning groups (p = 0.9985, one-way ANOVA). White bars indicate groups prior to receiving TBG (i.e, pre-conditioning), while blue bars indicate groups 24 h after the last TBG administration (i.e, post-conditioning). Exact N numbers for each experimental condition are reported in Supplementary Table 1. Specific statistical tests, information on reproducibility, and exact p values are reported in the Methods and Supplementary Table 1.
Extended Data Fig. 7. TBG produces antidepressant effects in mice.
(a) Schematic illustrating the stressors employed as part of the 7-day UMS protocol. White and grey boxes represent the light and dark phases of the light cycle, respectively. (b) TBG rescues the effects of UMS on immobility. (c) TBG (50 mg/kg) reaches high brain concentrations and is rapidly eliminated from the body. Mice were administered 3 different doses of TBG via intraperitoneal injection and sacrificed either 15 min or 3 h later. Whole brains and livers were collected, dried, homogenized, and extracted with methyl tert-butyl ether. Quantification was accomplished using LC-MS and concentrations of TBG in the two organs were calculated. Several samples for the 10 and 1 mg/kg doses at the 3 h time point had TBG at levels below the limit of quantification (~5 nmol/g). In those cases, the values were recorded as 0. Exact N numbers for each experimental condition are reported in Supplementary Table 1. Specific statistical tests, information on reproducibility, and exact p values are reported in the Methods and Supplementary Table 1.
Extended Data Fig. 8. Effects of TBG on locomotion and sucrose-seeking behavior in rats.
(a) Acute administration of TBG does not impair locomotion in the open field. Rats were subjected to novelty-induced locomotion (Baseline) for 30 min. At that time, cocaine was administered and psychostimulant-induced locomotion (+ Cocaine) was assessed for 60 min. There were no differences between the VEH- and TBG-treated groups with respect to total distance traveled or average velocity. Furthermore, there was no difference in thigmotaxis measured during the baseline period (i.e, % time in the center of the open field). (b–e) A sucrose self-administration experiment was conducted in a similar manner to the heroin self-administration experiment in Fig. 4. Doses in mg/kg are shown in parentheses. (b) Sucrose seeking over time is shown. Colored arrows indicate when each group received TBG. VEH was administered at all other time points to each group. (c) TBG acutely reduces sucrose-seeking behavior in a dose-dependent manner when administered during self-administration. (d) TBG acutely reduces sucrose seeking when administered immediately before the first extinction session. The CUE (injection 1 = VEH, injection 2 = VEH) and EXT (injection 1 = VEH, injection 2 = TBG) groups were compared, as they were matched for the number of withdrawal days between the last self-administration and first extinction session. (o) TBG does not have long-lasting effects on sucrose-seeking behavior, as it does not reduce active lever pressing during the cued reinstatement when administered 12–14 days prior during self-administration (SA) or immediately before extinction (EXT). Exact N numbers for each experimental condition are reported in Supplementary Table 1. Specific statistical tests, information on reproducibility, and exact p values are reported in the Methods and Supplementary Table 1.
Extended Data Table 1. TBG and IBG are more soluble than ibogaine.
(a) The fumarate salts of TBG and IBG are readily soluble in saline (0.9%) while ibogaine hydrochloride is not. (b) Ibogaine hydrochloride exhibits limited solubility in various saline-based vehicles. Solutions of saline (0.9%) containing various percentages of co-solvents/additives were added to finely crushed ibogaine hydrochloride. All of our attempts to improve its solubility through pulverizing, sonication, and mild heating (< 50 °C) were unsuccessful. Moreover, the addition of co-solvents (ethanol, dimethyl sulfoxide, glycerol), surfactants (Kolliphor), or hydrotropes (ATP) to the vehicle did not substantially improve its solubility. We confirmed the purity and identity of the ibogaine hydrochloride used in these studies through a combination of NMR, LC-MS, and X-ray crystallography experiments. Exact N numbers for each experimental condition are reported in Supplementary Table 1. Specific statistical tests, information on reproducibility, and exact p values are reported in the Methods and Supplementary Table 1.
| a | Solubility at [Cmpd] (mg/mL) | |||
| Cmpd | 40 | 8 | 4 | |
| IBO | no × |
no × |
no × |
|
| IBG | yes ✓ |
yes ✓ |
yes ✓ |
|
| TBG | yes ✓ |
yes ✓ |
yes ✓ |
|
| b | Vehicle Conditions | Concentration | Soluble? | |
| Saline + 10% EtOH + 10% DMSO | 40 mg/ml | NO |  |
|
| Saline + 10% EtOH | 8 mg/ml | NO |  |
|
| Saline + 10% DMSO | 8 mg/ml | NO |  |
|
| Saline + 10% EtOH + 10% DMSO | 8 mg/ml | NO |  |
|
| Saline + 10% EtOH + 10% Kolliphor | 8 mg/ml | NO |  |
|
| Saline + 10% EtOH + 20% Kolliphor | 8 mg/ml | NO |  |
|
| Saline + 10% EtOH + 25% Kolliphor | 8 mg/ml | NO |  |
|
| Saline + 10% EtOH + 30% Kolliphor | 8 mg/ml | NO |  |
|
| Saline + 10% EtOH + 40% Kolliphor | 8 mg/ml | NO |  |
|
| Saline + 10% DMSO + 10% Kolliphor | 8 mg/ml | NO |  |
|
| Saline + 10% DMSO + 25% Kolliphor | 8 mg/ml | NO |  |
|
| Saline + 10% DMSO + 15% Glycerol | 8 mg/ml | NO |  |
|
| Saline + 10 mM ATP | 8 mg/ml | NO |  |
|
| Saline + 10% DMSO | 4 mg/ml | NO |  |
|
| Saline + 10% DMSO + 25% Kolliphor | 4 mg/ml | NO |  |
|
| Saline + 10% DMSO | 1 mg/ml | YES |  |
|
Extended Data Table 2. Safety Pharmacology Screen.
The effects of TBG (10 μM) on a wide range of targets was assessed by Eurofins Discovery. Assays were conducted in duplicate and the results were averaged. Targets with ≥ 50% inhibition are highlighted in blue. Exact N numbers for each experimental condition are reported in Supplementary Table 1. Specific statistical tests, information on reproducibility, and exact p values are reported in the Methods and Supplementary Table 1.
| Eurofins Catalog # | Assay Name | Species | % Inhibition |
|---|---|---|---|
| 107710 | ATPase, Na+/K+, Heart, Pig | pig | −9 |
| 104010 | Cholinesterase, Acetyl, ACES | human | 13 |
| 116030 | Cyclooxygenase COX-1 | human | 16 |
| 118030 | Cyclooxygenase COX-2 | human | 7 |
| 140010 | Monoamine Oxidase MAO-A | human | 66 |
| 140120 | Monoamine Oxidase MAO-B | human | 16 |
| 107300 | Peptidase, Angiotensin Converting Enzyme | rabbit | 2 |
| 112510 | Peptidase, CTSG (Cathepsin G) | human | −2 |
| 152300 | Phosphodiesterase PDE3A | human | 6 |
| 154420 | Phosphodiesterase PDE4D2 | human | 1 |
| 200510 | Adenosine A1 | human | −4 |
| 200610 | Adenosine A2A | human | −8 |
| 203110 | Adrenergic α1A | human | 15 |
| 203210 | Adrenergic α1B | human | 20 |
| 203400 | Adrenergic α1D | human | 15 |
| 203630 | Adrenergic α2A | human | 81 |
| 203710 | Adrenergic α2B | human | 27 |
| 204010 | Adrenergic β1 | human | 9 |
| 204110 | Adrenergic β2 | human | 9 |
| 206000 | Androgen (Testosterone) | human | 6 |
| 210030 | Angiotensin AT1 | human | 3 |
| 212620 | Bradykinin B2 | human | 9 |
| 214510 | Calcium Channel L-Type, Benzothiazepine | rat | 18 |
| 214600 | Calcium Channel L-Type, Dihydropyridine | rat | 6 |
| 215000 | Calcium Channel L-Type, Phenylalkylamine | rat | 42 |
| 216000 | Calcium Channel N-Type | rat | −3 |
| 217050 | Cannabinoid CB1 | human | 1 |
| 217100 | Cannabinoid CB2 | human | −21 |
| 217510 | Chemokine CCR1 | human | 11 |
| 244500 | Chemokine CXCR2 (IL-8RB) | human | −2 |
| 219500 | Dopamine D1 | human | 3 |
| 219600 | Dopamine D2L | human | 18 |
| 219700 | Dopamine D2S | human | 0 |
| 224010 | Endothelin ETA | human | 8 |
| 226010 | Estrogen ERα | human | 1 |
| 226810 | GABAA, Chloride Channel, TBOB | rat | −1 |
| 226600 | GABAA, Flunitrazepam, Central | rat | −2 |
| 226630 | GABAA, Ro-15-1788, Hippocampus | rat | 10 |
| 228610 | GABAB1A | human | 0 |
| 232030 | Glucocorticoid | human | 12 |
| 232600 | Glutamate, AMPA | rat | 8 |
| 232710 | Glutamate, Kainate | rat | 8 |
| 237000 | Glutamate, Metabotropic, mGlu5 | human | 1 |
| 232810 | Glutamate, NMDA, Agonism | rat | 0 |
| 232910 | Glutamate, NMDA, Glycine | rat | 2 |
| 233000 | Glutamate, NMDA, Phencyclidine | rat | 3 |
| 234000 | Glutamate, NMDA, Polyamine | rat | −2 |
| 239000 | Glycine, Strychnine-Sensitive | rat | 19 |
| 239610 | Histamine H1 | human | 35 |
| 239710 | Histamine H2 | human | −12 |
| 250460 | Leukotriene, Cysteinyl CysLT1 | human | 3 |
| 251100 | Melanocortin MC1 | human | −3 |
| 251350 | Melanocortin MC4 | human | −8 |
| 252610 | Muscarinic M1 | human | 2 |
| 252710 | Muscarinic M2 | human | 13 |
| 252810 | Muscarinic M3 | human | 18 |
| 252910 | Muscarinic M4 | human | 7 |
| 257010 | Neuropeptide Y Y1 | human | 16 |
| 258700 | Nicotinic Acetylcholine α1, Bungarotoxin | human | 19 |
| 258730 | Nicotinic Acetylcholine α3β4 | human | 16 |
| 260130 | Opiate δ1 (OP1, DOP) | human | 14 |
| 260210 | Opiate κ (OP2, KOP) | human | 7 |
| 260410 | Opiate μ (OP3, MOP) | human | 17 |
| 299037 | Platelet Activating Factor (PAF) | human | 7 |
| 265600 | Potassium Channel [KATP] | hamster | 6 |
| 265900 | Potassium Channel hERG | human | 28 |
| 267500 | PPARγ | human | −6 |
| 299005 | Progesterone PR-B | human | 4 |
| 271110 | Serotonin (5-Hydroxytryptamine) 5-HT1A | human | 39 |
| 271230 | Serotonin (5-Hydroxytryptamine) 5-HT1B | human | 66 |
| 271650 | Serotonin (5-Hydroxytryptamine) 5-HT2A | human | 57 |
| 271700 | Serotonin (5-Hydroxytryptamine) 5-HT2B | human | 86 |
| 271800 | Serotonin (5-Hydroxytryptamine) 5-HT2C | human | 99 |
| 271910 | Serotonin (5-Hydroxytryptamine) 5-HT3 | human | 14 |
| 279510 | Sodium Channel, Site 2 | rat | 24 |
| 255520 | Tachykinin NK1 | human | 18 |
| 202000 | Transporter, Adenosine | guinea pig | −9 |
| 226400 | Transporter, GABA | rat | 1 |
| 274030 | Transporter, SERT | human | 88 |
| 287530 | Vasopressin V1A | human | −6 |
| 252030 | Transporter, Vesicular Monoamine (Non-Selective) | human | 10 |
Supplementary Material
Acknowledgement
This work was supported by funds from the National Institutes of Health (NIH) (R01GM128997 to DEO, R37AA01684 to DR, R01AA022583 to DK, R01MH109475 to YZ, R01MH104227 to YZ, R01NS104950 to YZ, R01DA045836 to JP, and U19AG023122 to OF), a Hellman Fellowship (DEO), UC Davis STAIR and STAIR Plus grants (DEO), a Max Planck Fellowship at MPFI (YZ), four NIH training grants (T32GM113770 to RJT, T32MH112507 to LPC, 5T32GM099608 to MV, and 4T32GM6754714 to DMT), two UC Davis Provost’s Undergraduate Fellowships (to JV and AJP), the Paul G. Allen Family Foundation (MM and DK), the Genentech Fellowship Program (DMT), and a Medical College of Wisconsin Research Affairs Counsel Pilot Grant (JDM). BMB was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant number UL1 TR001860 and linked award TL1 TR001861. Delix Therapeutics funded the large receptor screen conducted at Eurofins Discovery. We thank Florence F. Wagner for her help coordinating with Eurofins Discovery. This project used the Biological Analysis Core of the UC Davis MIND Institute Intellectual and Development Disabilities Research Center (U54 HD079125). The Olympus FV1000 confocal used in this study was purchased using NIH Shared Instrumentation Grant 1S10RR019266-01. We thank the MCB Light Microscopy Imaging Facility, which is a UC Davis Campus Core Research Facility, for the use of this microscope. Several of the drugs used in this study were provided by the NIDA Drug Supply Program. We also thank Dennis R. Carty for assistance with larval zebrafish toxicity assays.
Abbreviations.
- VEH
vehicle
- KET
ketamine
- IBO
ibogaine
- NOR
noribogaine
- IBG
ibogainalog
- TBG
tabernanthalog
- KETSN
ketanserin
- SI
sertindole
- BPM
beats per minute
- DOI
2,5-dimethoxy-4-iodoamphetamine
- MDMA
3,4-methylenedioxymethamphetamine
- DMT
N,N-dimethyltryptamine
- FST
forced swim test
- EtOH
ethanol
- DMSO
dimethyl sulfoxide
- ATP
adenosine triphosphate
- dpf
days post fertilization
- PTZ
pentylenetetrazole
Footnotes
Disclosure
DEO is the president and chief scientific officer of Delix Therapeutics, Inc. Delix Therapeutics has licensed TBG-related technology from the University of California, Davis.
Code Availability
Custom written data analysis codes are available upon request.
Data Availability.
Data are available at the following link https://doi.org/10.6084/m9.figshare.11634795.
REFERENCES
- 1.Wasko MJ; Witt-Enderby PA; Surratt CK DARK Classics in Chemical Neuroscience: Ibogaine. ACS Chem. Neurosci, 2018, 9, 2475–2483. [DOI] [PubMed] [Google Scholar]
- 2.Noller GE; Frampton CM; Yazar-Klosinski B Ibogaine Treatment Outcomes for Opioid Dependence from a Twelve-Month Follow-up Observational Study. Am. J. Drug Alcohol Abuse, 2018, 44, 37–46. [DOI] [PubMed] [Google Scholar]
- 3.He DY; McGough NN; Ravindranathan A; Jeanblanc J; Logrip ML; Phamluong K; Janak PH; Ron D Glial cell line-derived neurotrophic factor mediates the desirable actions of the anti-addiction drug ibogaine against alcohol consumption. J. Neurosci, 2005, 25, 619–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marton S; González B; Rodríguez-Bottero S; Miquel E; Martínez-Palma L; Pazos M; Prieto JP; Rodríguez P; Sames D; Seoane G; Scorza C; Cassina P; Carrera I Ibogaine Administration Modifies GDNF and BDNF Expression in Brain Regions Involved in Mesocorticolimbic and Nigral Dopaminergic Circuits. Front. Pharmacol, 2019, 10, 193. doi: 10.3389/fphar.2019.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jenks CW Extraction studies of Tabernanthe iboga and Voacanga africana. Nat. Prod. Lett, 2002, 16, 71–76. [DOI] [PubMed] [Google Scholar]
- 6.Iyer RN; Favela D; Zhang G; Olson DE The Iboga Enigma: The Chemistry and Neuropharmacology of Iboga Alkaloids and Related Analogs. Nat. Prod. Rep, 2020, doi: 10.1039/d0np00033g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hough LB; Pearl SM; Glick SD Tissue Distribution of Ibogaine after Intraperitoneal and Subcutaneous Administration. Life Sci, 1996, 58, 119–122. [DOI] [PubMed] [Google Scholar]
- 8.Koenig X; Kovar M; Boehm S; Sandtner W; Hilber K Anti-Addiction Drug Ibogaine Inhibits HERG Channels: A Cardiac Arrhythmia Risk. Addict. Biol, 2014, 19, 237–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thurner P; Stary-Weinzinger A; Gafar H; Gawali VS; Kudlacek O; Zezula J; Hilber K; Boehm S; Sandtner W; Koenig X Mechanism of HERG Channel Block by the Psychoactive Indole Alkaloid Ibogaine. J. Pharmacol. Exp. Ther, 2014, 348, 346–358. [DOI] [PubMed] [Google Scholar]
- 10.Alper KR; Stajic M; Gill JR Fatalities Temporally Associated with the Ingestion of Ibogaine. J. Forensic Sci, 2012, 57, 398–412. [DOI] [PubMed] [Google Scholar]
- 11.Koenig X; Hilber K The Anti-Addiction Drug Ibogaine and the Heart: A Delicate Relation. Molecules, 2015, 20, 2208− 2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baumann MH; Pablo JP; Ali SF; Rothman RB; Mash DC Noribogaine (12-Hydroxyibogamine): A Biologically Active Metabolite of the Antiaddictive Drug Ibogaine. Ann. N. Y. Acad. Sci, 2000, 914, 354–368. [DOI] [PubMed] [Google Scholar]
- 13.Olson DE Psychoplastogens: A Promising Class of Plasticity-Promoting Neurotherapeutics. J. of Exp. Neurosci, 2018, 12, 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ly C; Greb AC; Cameron LP; Wong JM; Barragan EV; Wilson PC; Burbach KF; Soltanzadeh Zarandi S; Sood A; Paddy MR; Duim WC; Dennis MY; McAllister AK; Ori-McKenney KM; Gray JA; Olson DE Psychedelics Promote Structural and Functional Neural Plasticity. Cell Rep, 2018, 23, 3170–3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bogenschutz MP; Johnson MW Classic hallucinogens in the treatment of addictions. Prog. Neuropsychopharmacol. Biol. Psychiatry, 2016, 64, 250–258. [DOI] [PubMed] [Google Scholar]
- 16.Wender PA; Verma VA; Paxton TJ; Pillow TH Function Oriented Synthesis, Step Economy, and Drug Design. Acc. Chem. Res, 2008, 41, 40–49. [DOI] [PubMed] [Google Scholar]
- 17.Gassaway MM; Jacques TL; Kruegel AC; Karpowicz RJ Jr.; Li X; Li S; Myer Y; Sames D Deconstructing the Iboga Alkaloid Skeleton: Potentiation of FGF2-induced Glial Cell Line-Derived Neurotrophic Factor Release by a Novel Compound. ACS Chem. Biol, 2016, 11, 77–87. [DOI] [PubMed] [Google Scholar]
- 18.Wager TT; Hou X; Verhoest PR; Villalobos A Central Nervous System Multiparameter Optimization Desirability: Application in Drug Discovery. ACS Chem. Neurosci, 2016, 6, 767–775. [DOI] [PubMed] [Google Scholar]
- 19.Glennon RA; Young R.l; Jacyno JM; Slusher M; Rosecrans JA DOM-stimulus generalization to LSD and other hallucinogenic indolealkylamines. Eur. J. Pharmacol, 1983, 86, 453–459. [DOI] [PubMed] [Google Scholar]
- 20.Dunlap LE; Azinfar A; Ly C; Cameron LP; Viswanathan J; Tombari RJ; Myers-Turnbull D; Taylor JC; Grodzki AC; Lein PJ; Kokel D; Olson DE Identification of Psychoplastogenic N,N-Dimethylaminoisotryptamine (isoDMT) Analogs Through Structure-Activity Relationship Studies. J. Med. Chem, 2019, 2020, 63, 1142–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Halberstadt AL; Chatha M; Klein AK; Wallach J; Brandt SD Correlation between the potency of hallucinogens in the mouse head-twitch response assay and their behavioral and subjective effects in other species. Neuropharmacology, 2020, 167, 107933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCarroll MN; Gendelev L; Kinser R; Taylor J; Bruni G; Myers-Turnbull D; Helsell C; Carbajal A; Rinaldi C; Kang HJ; Gong JH; Sello JK; Tomita S; Peterson RT; Keiser MJ; Kokel D Zebrafish Behavioural Profiling Identifies GABA and Serotonin Receptor Ligands Related to Sedation and Paradoxical Excitation. Nat. Commun, 2019, 10, 4078. doi: 10.1038/s41467-019-11936-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Breuer L; Kasper BS; Schwarze B; Gschossmann JM; Kornhuber J; Müller HH “Herbal seizures”--atypical symptoms after ibogaine intoxication: a case report. J. Med. Case Rep, 2015, 9, 243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dach K; Yaghoobi B; Schmuck MR; Carty DR; Morales KM; Lein PJ Teratological and Behavioral Screening of the National Toxicology Program 91-Compound Library in Zebrafish (Danio rerio). Toxicol. Sci, 2019, 167, 77–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rothman RB; Baumann MH Serotonergic drugs and valvular heart disease. Expert Opin. Drug Saf, 2009, 8, 317–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Phoumthipphavong V; Barthas F; Hassett S; Kwan AC Longitudinal Effects of Ketamine on Dendritic Architecture In Vivo in the Mouse Medial Frontal Cortex. eNeuro, 2016, 3, pii: ENEURO.0133–15.2016. doi: 10.1523/ENEURO.0133-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moda-Sava RN; Murdock MH; Parekh PK; Fetcho RN; Huang BS; Huynh TN; Witztum J; Shaver DC; Rosenthal DL; Always EJ; Lopez K; Meng Y; Nellissen L; Grosenick L; Milner TA; Deisseroth K; Bito H; Kasai H; Liston C Sustained rescue of prefrontal circuit dysfunction by antidepressant-induced spine formation. Science, 2019, 364, pii: eaat8078. doi: 10.1126/science.aat8078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cameron LP; Olson DE Dark Classics in Chemical Neuroscience: N,N-Dimethyltryptamine (DMT). ACS Chem. Neurosci, 2018, 9, 2344–2357. [DOI] [PubMed] [Google Scholar]
- 29.Warnault V; Darcq E; Levine A; Barak S; Ron D Chromatin remodeling--a novel strategy to control excessive alcohol drinking. Transl. Psychiatry, 2013, 3, e231. doi: 10.1038/tp.2013.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glick S; Kuehne M; Raucci J; Wilson T; Larson D; Keller R; Carlson J Effects of Iboga Alkaloids on Morphine and Cocaine Self-Administration in Rats: Relationship to Tremorigenic Effects and to Effects on Dopamine Release in Nucleus Accumbens and Striatum. Brain Res, 1994, 657, 14–22. [DOI] [PubMed] [Google Scholar]
- 31.Giannotti G; Barry SM; Siemsen BM; Peters J; McGinty JF Divergent Prelimbic Cortical Pathways Interact with BDNF to Regulate Cocaine-seeking. J. Neurosci, 2018, 38, 8956–8966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glick SD; Kuehne ME; Maisonneuve IM; Bandarage UK; Molinari HH 18-Methoxycoronaridine, a Non-Toxic Iboga Alkaloid Congener: Effects on Morphine and Cocaine Self-Administration and on Mesolimbic Dopamine Release in Rats. Brain Res, 1996, 719, 29–35. [DOI] [PubMed] [Google Scholar]
- 33.Carnicella S; He DY; Yowell QV; Glick SD; Ron D Noribogaine, but not 18-MC, exhibits similar actions as ibogaine on GDNF expression and ethanol self-administration. Addict. Biol, 2010, 15, 424–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bandarage UK; Kuehne ME; Glick SD Total Syntheses of Racemic Albifloranine and Its Anti-Addictive Congeners, Including 18-Methoxycoronaridine. Tetrahedron, 1999, 55, 9405–9424. [Google Scholar]
- 35.Langheinrich U1; Vacun G; Wagner T Zebrafish embryos express an orthologue of HERG and are sensitive toward a range of QT-prolonging drugs inducing severe arrhythmia. Toxicol. Appl. Pharmacol, 2003, 193, 370–382. [DOI] [PubMed] [Google Scholar]
- 36.Sampurna BP; Audira G; Juniardi S; Lai Y-H; Hsiao C-D A Simple ImageJ-Based Method to Measure Cardiac Rhythm in Zebrafish Embryos. Inventions, 2018, 3, 21 10.3390/inventions3020021 [DOI] [Google Scholar]
- 37.Westerfield M (2007) THE ZEBRAFISH BOOK, 5th Edition; A guide for the laboratory use of zebrafish (Danio rerio), Eugene, University of Oregon Press; ). [Google Scholar]
- 38.Ahrens MB; Orger MB; Robson DN; Li JM; Keller PJ Whole-brain functional imaging at cellular resolution using light-sheet microscopy. Nat. Methods, 2013, 10, 413–420. [DOI] [PubMed] [Google Scholar]
- 39.Kroeze WK; Sassano MF; Huang XP; Lansu K; McCorvy JD; Giguère PM; Sciaky N; Roth BL PRESTO-Tango as an open-source resource for interrogation of the druggable human GPCRome. Nat. Struct. Mol. Biol, 2015, 22, 362–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barupal DK; Zhang Y; Shen T; Fan S; Roberts BS; Fitzgerald P; Wancewicz B; Valdiviez L; Wohlgemuth G; Byram G; Choy YY; Haffner B; Showalter MR; Vaniya A; Bloszies CS; Folz JS; Kind T; Flenniken AM; McKerlie C; Nutter LMJ; Lloyd KC; Fiehn O A Comprehensive Plasma Metabolomics Dataset for a Cohort of Mouse Knockouts within the International Mouse Phenotyping Consortium. Metabolites, 2019, 9 pii: E101. doi: 10.3390/metabo9050101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feng G; Mellor RH; Bernstein M; Keller-Peck C; Nguyen QT; Wallace M; Nerbonne JM; Lichtman JW; Sanes JR Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron, 2000, 28, 41–51. [DOI] [PubMed] [Google Scholar]
- 42.Xu T; Yu X; Perlik AJ; Tobin WF; Zweig JA; Tennant K; Jones T; Zuo Y Rapid formation and selective stabilization of synapses for enduring motor memories. Nature, 2009, 462, 915–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen CC; Lu J; Yang R; Ding JB; Zuo Y Selective activation of parvalbumin interneurons prevents stress-induced synapse loss and perceptual defects. Mol. Psychiatry, 2018, 23, 1614–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vazquez M; Frazier JH; Reichel CM; Peters J Acute ovarian hormone treatment in freely cycling female rats regulates distinct aspects of heroin seeking. Learn. Mem, 2019, 27, 6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available at the following link https://doi.org/10.6084/m9.figshare.11634795.