Abstract
Neutrophils are vital to both the inflammatory cascade and tissue repair after an injury. Neutrophil heterogeneity is well established but there is less evidence for significant, different functional roles for neutrophil subsets. OLFM4 (Olfactomedin-4) is expressed by a subset of neutrophils, and high expression of OLFM4 is associated with worse outcomes in patients with sepsis and acute respiratory distress syndrome. We hypothesized that an increased number of OLFM4+ neutrophils would occur in trauma patients with worse clinical outcomes. To test this, we prospectively enrolled patients who suffered a blunt traumatic injury. Blood was collected at the time of admission, Day 3, and Day 7 and analyzed for the percentage of neutrophils expressing OLFM4. We found that a subset of patients who suffered blunt traumatic injury upregulated their percentage of OLFM4+ neutrophils. Those who upregulated their OLFM4 had an increased length of stay, days in the ICU, and ventilator days. A majority of these patients also suffered from hemorrhagic shock. To establish a potential role for OLFM4+ neutrophils, we used a murine model of hemorrhagic shock because mice also express OLFM4 in a subset of neutrophils. These studies demonstrated that wild type mice had higher concentrations of cytokines in the plasma and myeloperoxidase in the lungs compared with OLFM4-null mice. In addition, we used an anti-OLFM4 antibody, which when given to wild type mice led to the reduction of myeloperoxidase in the lungs of mice. These findings suggest that OLFM4+ neutrophils are a unique subset of neutrophils that affect the inflammatory response after tissue injury.
Keywords: Olfactomedin 4, neutrophils, hemorrhagic shock, trauma, blunt traumatic injury
Clinical Relevance
Major gaps persist in our understanding of the heterogeneity in patient immune responses during critical illness. Here, we present evidence for a subset of neutrophils that are upregulated after blunt traumatic injury and hemorrhagic shock.
Despite advances in the care of patients who suffer from blunt traumatic injuries (BTIs), a subset of patients still develops multiorgan dysfunction and a complicated recovery after initially surviving their injuries. Prior studies have found that hemorrhagic shock and ischemia-reperfusion lead to the development of multiorgan dysfunction after a BTI and are predictive of mortality across all age groups (1, 2). After injury and resuscitation, the inflammatory cascade after reperfusion frequently worsens the initial injury. Further work is needed to understand the mechanisms associated with reperfusion inflammatory response as well as developing interventions to combat this response.
The OLFM4 (Olfactomedin-4) gene encodes a glycoprotein that has been identified in various tissues and a subset of neutrophils. OLFM4 is highly expressed in inflammatory disease processes, such as septic shock and acute respiratory distress syndrome (3, 4), and is the most highly upregulated gene in children who die of septic shock (5). The functional role of OLFM4 in neutrophils is less clear. Healthy patients typically express OLFM4 in approximately 25% of circulating neutrophils within the specific granules. During health, the percentage of OLFM4+ neutrophils does not change over time (6). However, in some pediatric patients with septic shock, there were changes in the percentage of OLFM4+ neutrophils over a 2-day period in the ICU (7), and higher percentages of OLFM4+ neutrophils at the time of admission correlated with a higher likelihood of a complicated disease course and mortality (7).
Studies in mice have begun to elucidate the potential functions of OLFM4. There is a 66% protein sequence identity between murine and human OLFM4 (8). The expression between humans and mice is similar in that OLFM4 is expressed in intestinal crypt stem cells and a subset of neutrophils in humans and mice (9, 10). Some murine neutrophil studies suggest it may inhibit the cellular antimicrobial response (11–14). In addition, murine models have shown that OLFM4 knock out (KO) mice are protected against sepsis induced by cecal ligation and puncture, and intestinal ischemia-reperfusion injury (15).
To our knowledge, no studies have assessed the role of OLFM4 in patients with BTI. Our objective was to assess the role of OLFM4+ neutrophils in patients who underwent BTI. Patients with underwent blunt trauma are unique because blood collected at the time of admission provides a baseline OLFM4 percentage before the bone marrow response to injury. Trending blood percentage of OLFM4+ over the days after the injury allows the comparison of changes in OLFM4+ neutrophils and outcomes. We hypothesized that an increased number of OLFM4+ neutrophils would occur in trauma patients with worse clinical outcomes. This confirmation would support that there are functional and meaningful differences between OLFM4+ and OLFM4− neutrophils.
Methods
Enrollment of Study Subjects
The prospective collection and use of residual biological samples and clinical data were approved by the Institutional Review Board of Cincinnati Children’s Hospital Medical Center and the University of Cincinnati Medical Center. All patients 18 years and older who sustained acute BTI necessitating admission to the surgical ICU after BTI occurred were considered for the study. Additional inclusion criteria consisted of systolic blood pressure ≤90 mm Hg in the field or within 60 minutes of arrival to the Emergency Department, and/or a base deficit ≥6 mEq/L, and a requirement for a blood transfusion within the first 12 hours of arrival (this criteria was added after the first few months of enrollment to decrease the number of patients with minor injuries). Exclusion criteria consisted of white blood cell counts less than 1, severe traumatic brain injury with a Glasgow Coma Scale score of less than 8 because of the associated poor prognosis, less than 48 hours of expected survival time, spinal cord injury, concomitant penetrating trauma, prisoners, pregnant patients, delayed presentation, or transfer from another hospital.
Residual blood was used for analysis at the time of presentation, hospital Day 3, and hospital Day 7 if still inpatient and receiving blood draws. For patients discharged before Day 7, the OLFM4 percentage was imputed as unchanged from the Day 3 percentage based on previously published literature (6).
Flow Cytometry
Residual blood samples underwent flow cytometry using standard protocols. Further information regarding the processing of samples is provided in the data supplement. Figure E1 in the data supplement shows the gating strategy for neutrophil flow cytometry.
Murine Model of Hemorrhagic Shock
Young (3–5 mo) male C57Bl/6 wild type (WT) and OLFM4 KO mice in C57Bl6/ background were used for the experiments. The mice initially underwent hemorrhagic shock (16) (see the data supplement) and a second experiment was conducted using a polyclonal antibody to attempt to block OLFM4. Anti-OLFM4 IgG (0.5 mg) or control IgG (Rabbit IgG isotype control) (SouthernBiotech), were both dialyzed to the same buffer and injected intraperitoneally into WT mice 2 hours before the cannulation for hemorrhagic shock.
Lung Injury
MPO (Myeloperoxidase) activity was assessed using spectrophotometry to evaluate neutrophil accumulation in homogenized lung tissue as described previously (17). Lung injury scoring is described in the data supplement.
Cytokine Assay
Plasma from murine blood was used to evaluate for IL-6, KC, IFN-γ, IL-1β, IL-10, IL-17, MIP-1α, and TNF-α. Cytokine detection and measurement were performed using MILLIPLEX Assays (MilliporeSigma).
Statistical Analysis
Statistical analysis was performed using JMP Pro 14.0.0 (SAS Institute), SAS 9.4 (SAS Institute), and SigmaStat (Systat Software), and results were plotted using GraphPad Prism 8 (GraphPad Software). Human data are represented as the median and interquartile range (IQR). Continuous data between groups were analyzed using the Wilcoxon rank-sum test, whereas categorical variables were analyzed using a chi-squared or Fisher’s exact test. Given the large number of covariates with respect to the sample size, a variable selection approach was taken and a forward selection method based on the Akaike information criterion was used. Multiple linear regression analyses were performed to examine two outcomes, length of stay (LOS) and ICU days, after log transforming these outcomes because of skewness. A negative binomial regression model was deemed to be the best method, among other methods for count data, to examine the ventilator days as an outcome. A logistic regression model was used to examine complications. For murine data, a t test was applied for normally distributed data and the Mann–Whitney rank-sum test was used for abnormally distributed data. P values less than 0.05 were considered significant.
Results
Demographics of Trauma Cohort
From 2018 to 2019, 56 patients presented with a BTI and met the criteria for enrollment (Table 1). The mean age was 41.5 years (IQR, 30–59). The most common mechanisms of injury were motor vehicle crashes (n = 28; 50.0%), falls (n = 7; 12.5%), and motorcycle crashes (n = 6; 10.7%). The median of the Charlson Comorbidity Score was zero (IQR, 0–2.5), suggesting that most patients were in good health before the BTI.
Table 1.
Demographics
| Total patients, N | 56 |
| Age, yr, median (IQR) | 41.5 (30–59) |
| Sex, n (%) | |
| Female | 16 (28.6) |
| Male | 40 (71.4) |
| Race, n (%) | |
| White | 41 (73.2) |
| Black | 11 (19.6) |
| Hispanic | 4 (7.1) |
| Charlson comorbidity score, median (IQR) | 0 (0–2.5) |
| Mechanism of injury, n (%) | |
| Assault | 1 (1.8) |
| Bike accident | 3 (5.4) |
| Fall | 7 (12.5) |
| Machine injury | 1 (1.8) |
| Motorcycle crash | 6 (10.7) |
| Motor vehicle crash | 28 (50.0) |
| Other | 5 (8.9) |
| Pedestrian struck | 5 (8.9) |
Definition of abbreviation: IQR = interquartile range.
OLFM4 in Patients With BTI
The residual blood was collected and analyzed for the percentage of neutrophils expressing OLFM4. The median percentage of the OLFM4+ neutrophils at admission was 22.3% (IQR, 12.7–36.6), 23.2% (IQR, 12.3–35.9) on Day 3, and 37.8% (IQR, 21.6–50.4) on Day 7. Most patients did not change the percentage of OLFM4+ neutrophils on any of the measured days. However, a subgroup of patients was noted to increase the percentage of OLFM4+ neutrophils between the Day 3 and Day 7 measurements. Patients were then categorized into two groups (shifters and nonshifters) based on the change in percentage of OLFM4+ neutrophils from admission to Day 7. The shifters had a change in OLFM4+ neutrophil percentage ≥5%, whereas the nonshifters had a percentage change <5%. A total of 21 patients were shifters with a median change of 13.6% (IQR, 8.2–24.7%), and 35 patients were nonshifters with a median change of 1.1% (IQR, 0–2.4%) (P < 0.001). One patient had a decrease in the percentage of OLFM4+ neutrophils and was excluded from the analysis. However, we also conducted an analysis with this patient included in the shifter group and the results stayed the same (data not shown).
Injury Scores and Patient Outcomes
The shifters presented to the hospital with a greater injury severity score (29; IQR, 22–41) than the nonshifters (22; IQR, 14–29; P = 0.003) (Table 2). The worst abbreviated injury score for a single system for the majority of nonshifters was ≤3 compared with the majority of shifters with scores of ≥4 (P = 0.02). The shifters also had higher Marshall scores (3 vs. 1, P = 0.004) and higher Denver scores (1 vs. 0; P = 0.002). The median lactate on admission for shifters was 5.3 (IQR, 3.8–6.3), which was higher than that of nonshifters (2.7; IQR, 2–5.1; P = 0.002). A total of 17 (81.0%) of the 21 shifters presented in hemorrhagic shock compared with only 5 of the 35 nonshifters (P < 0.001). In the first 24 hours, there was a significant difference in the required amount of intravenous fluid (3.6 L for shifters vs. 3.1 L in nonshifters, P = 0.05) and a greater number of transfusions of packed red blood cells (3 U vs. 0 U, P < 0.001). The shifters also had higher total transfusion requirements throughout the hospitalization for packed red blood cells (7 vs. 0, P < 0.001) and fresh frozen plasma (3 vs. 0, P = 0.002).
Table 2.
Injury Severity and Outcomes Comparing Shifters and Nonshifters
| Shifters (n = 21) | Nonshifters (n = 35) | P Value | |
|---|---|---|---|
| OLFM4 and WBC | |||
| Admission OLFM4%, median (IQR) | 29.3 (22.1 to 40.4) | 21.6 (10 to 32.9) | 0.03 |
| Day 7 OLFM4%, median (IQR) | 48.8 (37.8 to 63.9) | 21.6 (9.9 to 32.4) | <0.001 |
| Change in OLFM4%, median (IQR) | 13.6 (8.2 to 24.7) | 1.1 (0 to 2.4) | <0.001 |
| Admission WBC × 1,000/mcl, median (IQR) | 19.4 (10.4 to 21.7) | 13.3 (11.1 to 19.5) | 0.161 |
| 48-h WBC × 1,000/mcl, median (IQR) | 10.5 (7.7 to 14.3); n = 13 | 8.9 (7.6 to 11.8); n = 31 | 0.269 |
| Day 7 WBC × 1,000/mcl, median (IQR) | 13.5 (10.3 to 16.8); n = 20 | 10.3 (7.1 to 13.9); n = 15 | 0.067 |
| Injury scores | |||
| ISS, median (IQR) | 29 (22 to 41) | 22 (14 to 29) | 0.003 |
| Highest Marshall score, median (IQR) | 3 (2 to 5) | 1 (0 to 3) | 0.004 |
| Highest Denver score, median (IQR) | 1 (1 to 3) | 0 (0 to 1) | 0.002 |
| Worst AIS, n (%) | |||
| 2 | 0 (0.0) | 2 (5.7) | 0.02 |
| 3 | 6 (28.6) | 21 (60.0) | |
| 4 | 9 (42.9) | 10 (28.6) | |
| 5 | 6 (28.6) | 2 (5.7) | |
| First 24 h | |||
| IVF, L, median (IQR) | 3.6 (2.7 to 5.7) | 3.1 (1.8 to 4.1) | 0.05 |
| pRBC, U, median (IQR) | 3 (1 to 7) | 0 (0 to 1) | <0.001 |
| Admit lactate mg/dl, median (IQR) | 5.3 (3.8 to 6.3) | 2.7 (2 to 5.1) | 0.002 |
| Admit base deficit, mEq/L, median (IQR) | −0.2 (−4.2 to 4.6) | 0.6 (−1.8 to 2.9) | 0.62 |
| Hemorrhagic shock, n (%) | 17 (81.0) | 5 (14.3) | <0.001 |
| Outcomes | |||
| Total pRBC, median (IQR) | 7 (3 to 9) | 0 (0 to 3) | <0.001 |
| Total FFP, median (IQR) | 3 (1 to 5) | 0 (0 to 2) | 0.002 |
| LOS, median (IQR) | 15 (11 to 20) | 8 (4 to 12) | <0.001 |
| ICU days, median (IQR) | 6 (4 to 16) | 3 (2 to 5) | <0.001 |
| Ventilator days, median (IQR) | 3 (1 to 7) | 0 (0 to 0) | <0.001 |
| Any surgery, n (%) | 21 (100) | 22 (62.9) | 0.001 |
| Emergent surgery, n (%) | 15 (71.4) | 12 (34.3) | 0.01 |
| 30-d readmission, n (%) | 4 (19.1) | 5 (14.3) | 0.72 |
| Complication, n (%) | 14 (66.7) | 9 (25.7) | 0.003 |
| Infection, n (%) | 7 (33.3) | 5 (14.3) | 0.11 |
| Disposition, n (%) | |||
| Home | 4 (19.1) | 18 (51.4) | 0.03 |
| Rehab, SNF, LTAC | 16 (76.2) | 17 (48.6) | |
| Death | 1 (4.8) | 0 (0.0) |
Definition of abbreviations: AIS = abbreviated injury score; FFP = fresh frozen plasma; ISS = injury severity score; IVF = intravenous fluid; LOS = length of stay; LTAC = long-term acute care hospital; OLFM4 = Olfactomedin 4; pRBC = packed red blood cells; Rehab = rehabilitation; SNF = skilled nursing facility; WBC = white blood cell count.
Comparisons meeting statistical significance are in bold typeface.
The shifters had a longer LOS, with a median LOS of 15 days (IQR, 11–20) compared with the nonshifters who had a median LOS of 8 days (IQR, 4–12) (P < 0.001). The shifters also required more days in the ICU (6 vs. 3 d) (P < 0.001) and more ventilator days compared with the nonshifters (3 vs., 0 d) (P < 0.001). The shifters were more likely to require emergent surgery at admission (71.4% vs. 34.3%, P = 0.01) and were more likely to require any surgery during the admission (100% vs. 62.9%, P = 0.001). There were no differences in infection rates or the 30-day readmissions. Ultimately, the majority of shifters required inpatient rehabilitation, a skilled nursing facility, or a long-term acute care facility on discharge compared with the majority of the nonshifters who were discharged home (P = 0.03).
Some of the nonshifters were discharged before the Day 7 blood collection, necessitating imputation for the Day 7 result. For these patients, the Day 7 result was imputed from the Day 3 measure of OLFM4 percentage. This was based on previous reports that healthy individuals do not change the percentage of OLFM4, and these patients were discharged home because they no longer required hospital care. This is further supported by those patients who stayed in the hospital for 7 days with minor injuries who did not show an increase in the percentage of OLFM4. It is possible that this imputation may have biased our results, thus we conducted the same analysis without imputed data (Table E1). This analysis decreased the number of nonshifters and thus weakened some of the findings. However, the data continues to support an increased illness severity in patients who increase OLFM4 percentage relative to those who did not.
Multivariable Analysis
Next, we performed multivariable analyses associating the shifter versus the nonshifters and also the change in OLFM4 percentage with each of the four outcomes (LOS, ICU days, ventilator days, and complications) (Table 3). Even after controlling for race, complications, and infection, the ICU days increased by 56% in shifters compared with nonshifters (P = 0.02). The shifters also saw a 52% increase in LOS (P = 0.04) and a 1.8% increase in ventilator days (P = 0.02). When examining each percentage increase in OLFM4, it trended to be associated with longer ICU days and ventilator days, though not statistically significant. However, each percentage increase in OLFM4 from the day of admission to Day 7 was associated with a 1.8% increase in LOS (P = 0.01).
Table 3.
Results of Multivariable Analyses Examining Change in OLFM4% and Shifters versus Nonshifters in Relation to Various Outcomes
| Study Outcomes | Change in OLFM4%* | P Value | Shifters vs. Nonshifters | P Value |
|---|---|---|---|---|
| ICU days,† relative change (LCL–UCL) | 1.011 (0.996–1.028) | 0.15 | 1.560 (1.068–2.777) | 0.02 |
| LOS,‡ relative change (LCL–UCL) | 1.018 (1.004–1.032) | 0.01 | 1.518 (1.015–2.270) | 0.04 |
| Ventilator days,§ estimate ± SE | 0.017 ± 0.019 | 0.37 | 1.018 ± 0.443 | 0.02 |
| Complications,‖ odds ratio (LCL–UCL) | 1.01 (0.93–1.09) | 0.84 | 1.64 (0.71–8.50) | 0.55 |
Definition of abbreviations: LCL = lower confidence limit; UCL = upper confidence limit.
Per 1-U percentage change.
Adjusted for race, complications, and infection.
Adjusted for age, race, complications, infections, and hemorrhagic shock.
Adjusted for complications and Marshall scores.
Adjusted for hemorrhagic shock, Marshall scores, and total FFP.
This is the first publication to associate the change in OLFM4 percentage with clinical outcome. We a priori selected a change of 5% in OLFM4 expression, based on previous reports that OLFM4 does not change over time. It is possible that 5% is not the ideal cutoff to group a physiological meaningful change in the percentage of OLFM4 expressing neutrophils. To address this, we repeated the analysis using cutoffs of over 3% change and over 10% change (Table E2). With both of these cutoffs, LOS and complications remained significant for those patients who increased OLFM4 by over 3% or over 10%. ICU days were no longer significant when using the cutoff of over 10%.
Effect of Hemorrhage-induced Hypotension in OLFM4-Null Mice
The majority of patients who increased the percentage of OLFM4 neutrophils suffered from hemorrhagic shock. To start to understand the role of OLFM4 and why these patients increased this subset of neutrophils, we set out to corroborate our findings in a murine model of hemorrhagic shock comparing WT and OLFM4-null mice. The mice underwent hemorrhagic shock to a mean arterial pressure (MAP) of 30 ± 5 mm Hg for 60 minutes followed by resuscitation and observation. There were no differences in the heart rate (HR) throughout the experiment (Figure E2), however, WT mice had a significantly lower MAP throughout the final two time-points of the experiment (KO, 63.3 vs. WT, 51.2 at 210 min, P = 0.03; KO, 61.4 vs. WT, 49.1 at 240 min, P = 0.01).
Effect on Plasma Cytokines
Three hours after hemorrhagic shock, plasma samples and tissue samples were collected for analysis, and a panel of cytokines was measured. At baseline, there was no difference between cytokines when comparing WT and OLFM4-null animals (data not shown). After the hemorrhagic shock, plasma concentrations of IL-6, TNF-α, and MIP1α were increased significantly in the WT mice than in the OLFM4-null mice (Figure 1).
Figure 1.
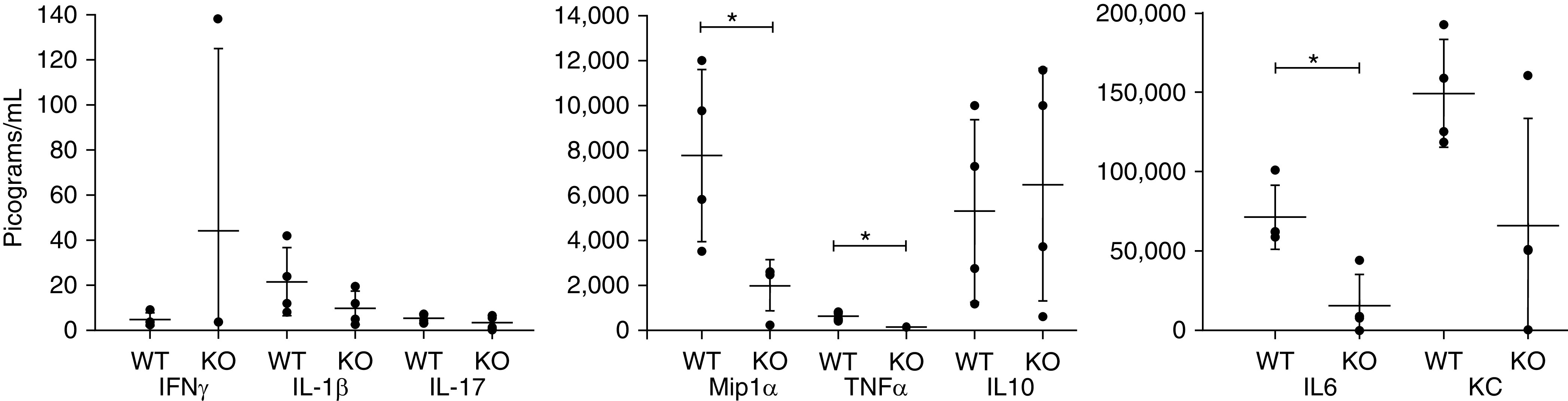
Cytokine concentrations after hemorrhagic shock. The WT mice were found to have higher concentrations of Mip1α (P = 0.027), TNFα (P = 0.003), and IL6 (P = 0.029) than the KO mice after undergoing hemorrhagic shock. Each circle represents a measurement from an individual animal with the central bar marking the mean and error bars showing the SD. * = statistically significant; KC = keratinocyte-derived chemokine; KO = knockout; Mip = macrophage inflammatory peptide; WT = wild type.
Neutrophil Migration after Hemorrhagic Shock
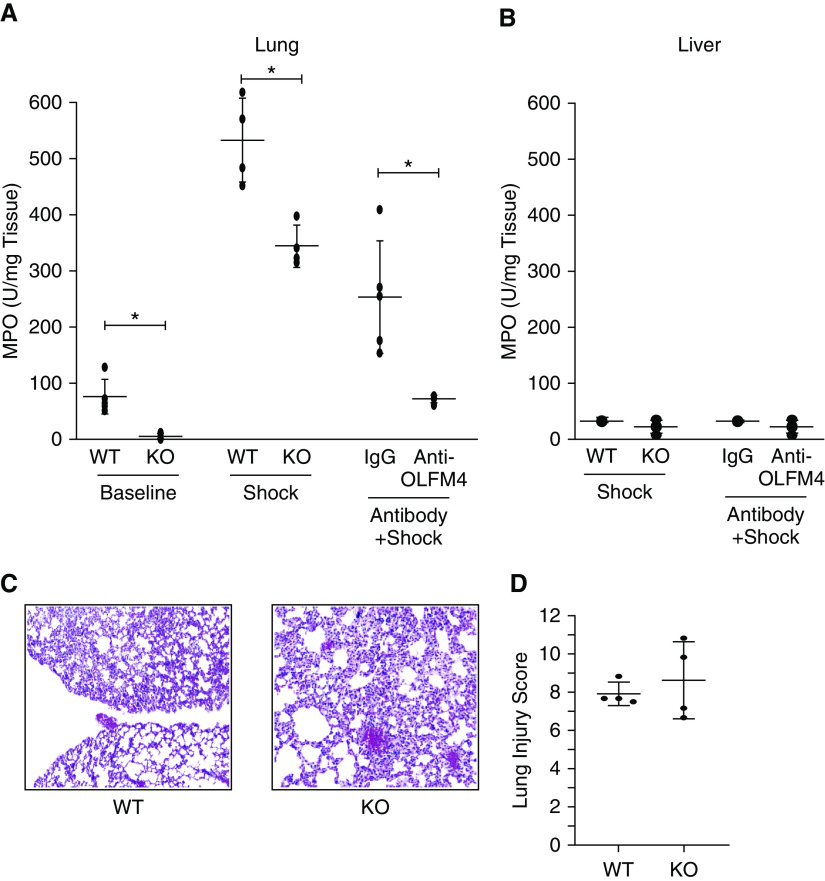
We used amounts of MPO as a surrogate measurement for neutrophils present in the lungs. We tested the control WT and OLFM4-null mice for baseline MPO amounts. Interestingly, the OLFM4-null mice had less MPO at baseline when compared with WT mice in the lungs (47.1 vs. 3.4 U MPO/100 mg, P < 0.01) (Figure 2A). We also tested the liver, intestine, and kidney and found no difference (Figure 2B) (only the liver data is shown). After the hemorrhagic shock, the WT mice still had higher MPO activity in the lungs than the-null (530.5 vs. 342.6 U MPO/100 mg, P < 0.01) (Figures E2A and E2B) with no difference in MPO in the liver. At the three-hour time-point, histopathological analysis revealed that lung injury in both groups was severe (Figure 2C). No difference was seen in injury score in the lung, however, a greater than 50% increase in injury score was seen in both groups (Figures 2C and 2D).
Figure 2.
Markers of lung injury after hemorrhagic shock. (A) WT mice had significantly higher amounts of myeloperoxidase in the lungs at baseline (n = 9/group, P = 0.002), after hemorrhagic shock (n = 4/group, P = 0.005), and after receiving control IgG than KO animals (n = 5/group, P = 0.008). * = statistically significant. (B) No difference was seen in myeloperoxidase in the liver. (C) Hematoxylin and eosin staining of both WT and KO lungs after hemorrhagic shock revealed the significant injury in both groups. (D) Lung injury scores from WT and OLFM4 (Olfactomedin-4)–KO animals showing greater than 50% lung injury in both groups. In all graphs, black circles represent measurements from a single animal with the bar marking the mean and error bars of the SD.
We next attempted to block the effect of secreted OLFM4 by intraperitoneal injection of polyclonal antibodies against OLFM4 or control rabbit IgG. The MPO activity in the lungs of the mice that received the control IgG was significantly higher than mice that received anti-OLFM4 IgG (252.1 vs. 70.9 U MPO/100 mg, P < 0.01) (Figure 2A). We also measured cytokines in these animals after the administration of anti-OLFM4 IgG and control IgG, however, the antiinflammatory effects of IgG or its interference with the cytokine measurement assay lowered cytokines dramatically in all animals, precluding meaningful comparison of the two groups (data not shown).
Discussion
In this study, we analyzed the percentage of OLFM4+ neutrophils in patients who suffered BTIs. We found that some patients had an increase in their percentage of OLFM4+ neutrophils between admission and hospital Day 7, which correlated with higher injury scores, increased resuscitative needs, and worse outcomes. These patients had prolonged ICU days, increased LOS, and increased ventilator days compared with patients who did not shift their percentage of OLFM4+ neutrophils. Because the majority of these patients also suffered from hemorrhagic shock, we further corroborated our findings in a murine model of hemorrhagic shock and found that OLFM4 KO mice had less hypotension and lower degrees of inflammatory markers, including cytokines and myeloperoxidase than WT mice.
Higher percentages of OLFM4+ neutrophils have previously been shown to be associated with worse outcomes. Clemmensen and colleagues first found that OLFM4 was expressed by approximately 20%–25% of neutrophils in humans (6, 18) and that the percentage of OLFM4+ neutrophils did not change during health. High mRNA expression of OLFM4 in whole blood has also been correlated with worse outcomes in patients with sepsis, respiratory syncytial virus, and acute respiratory distress syndrome (3, 4, 7). The percentage of OLFM4+ neutrophils has only been evaluated in pediatric sepsis, with the patients with a higher percentage of OLFM4+ neutrophils having worse outcomes (7). In this study, we collected admission blood shortly after injury and followed this percentage over the following week to evaluate the bone marrow response. The patients who had a greater than 5% increase in their baseline percentage of OLFM4+ neutrophils had a higher illness severity by almost all measures than those who had less than a 5% shift. The cutoff of over 5% was selected before and may not be the ideal cutoff to represent a clinically meaningful change in OLFM4 percentage. Futures studies with larger patient cohorts may be able to more accurately address what percentage change in OLFM4 is meaningful.
The timing of the change in OLFM4+ neutrophils suggests bone marrow programming rather than simply de novo protein translation. Of the patients who had an increased percentage of OLFM4+ neutrophils, all but one did not increase until sometime between Days 3 and 7. This suggests that whatever the signal is to increase the percentage of OLFM4+ neutrophils, it takes between 2 and 6 days before these neutrophils are detectable in the peripheral circulation. If the signal to increase OLFM4 expression activated de novo OLFM4 translation in neutrophils, this would only take minutes to hours to be detectable by flow cytometry, and we would have detected it at the Day 3 sampling. The finding that it takes several days for this to occur suggests that bone marrow reprogramming is needed to generate OLFM4+ neutrophils and that this occurs early in myeloid development. Data from mice also support this finding. OLFM4+ and OLFM4− neutrophils can be differentiated by flow cytometry early in the promyelocyte and myelocyte stages of development in mice (10). Interestingly, those patients who increased the percentage of OLFM4+ neutrophils had a higher percentage of OLFM4+ neutrophils at the time of admission. This may reflect the rapid mobilization of bone marrow neutrophils from patients with a significant injury. Taken together, this provides further evidence that OLFM4+ and OLFM4− neutrophils are different subsets that are determined early in myeloid development.
The clinical significance of an increased percentage of OLFM4+ neutrophils is more difficult to elucidate. Patients with an increased percentage of OLFM4+ neutrophils had greater injury resuscitation, mechanical ventilation, and longer ICU days. There are two potential interpretations for the change in the percentage of OLFM4+ neutrophils. First, it is possible that OLFM4+ neutrophils are generated after tissue injury. Perhaps they are important for repair and the signal to generate these cells is greater in those patients with a greater initial injury. Because these patients also had a greater injury initially, they also needed more resuscitation, more mechanical ventilation, and a longer ICU stay. Second, it is possible that OLFM4+ neutrophils are a more pathogenic subset of neutrophils that are prone to host damage. In this case, part of these patients’ prolonged stay and longer mechanical ventilation may be owed to “collateral damage” caused by a higher number of OLFM4+ neutrophils. The latter hypothesis is supported by murine data, in which the OLFM4-null mice were protected from challenges with acute inflammation by cecal ligation and puncture induced sepsis, intraperitoneal injection with bacteria, and small bowel ischemia-reperfusion (10, 12, 15). To start to understand which, or both, of these explanations is possible, we used a model of hemorrhagic shock in mice.
Our murine experiments suggest that OLFM4 may affect neutrophil migration to the lungs. Patients who increased their percentage of OLFM4+ neutrophils in our study had more ventilator days than the nonshifters. In our murine model, the WT mice had higher amounts of MPO in the lungs than the KO mice but no significant differences in the liver, kidney, or intestine. Injection of an anti-OLFM4 antibody also decreased the MPO signal in the lung, suggesting that secreted OLFM4 may play a role indirectly in the inflammatory cascade or directly by facilitating neutrophil entry into the lungs. Notably, the control IgG injection also decreased the MPO signal, we speculate this may come from the antiinflammatory effect of IgG, however, anti-OLFM4 IgG reduced MPO more than the IgG isotype control. In addition, there is no assay to measure OLFM4 activity, so the ability of our polyclonal antibody to block OLFM4 activity cannot be conclusively proven at this time. Previous work has shown that when MPO is released from azurophilic granules of neutrophils, it indirectly influences cytokine production in the lungs (18, 19). The decreased amount of MPO in the lung in KO mice may serve to be protective by reducing cytokine production and decreasing overall damage by neutrophil exocytosis of reactive species and proteases. Histopathologic analysis in this study revealed over 50% damage in the lungs of both KO and WT animals, suggesting a severe mechanism of injury. However, further investigation using a less severe mechanism of injury to induce lung damage may be beneficial to provide further information on the role of OLFM4 in the lung. Future studies will need to address if the decrease in MPO signal from KO and OLFM4 antibody injected mice arises from decreased neutrophils within the alveoli, lung parenchyma, or the microvascular or the lungs, which have been shown to house a large number of neutrophils.
Further work will need to reconcile the differences between clinical observations and our murine model. Our clinical data suggest that patients with hemorrhagic shock and greater organ injury upregulate OLFM4+ neutrophils. Mice without OLFM4 have less MPO in the lungs and injecting anti-OLFM4 reduced MPO in the lungs. Although this data is suggestive of a role for OLFM4, more data is needed to understand the mechanism of OLFM4. Futures studies will need to more closely recapitulate the clinical scenario of hemorrhagic shock and then follow the murine regulation of OLFM4 expression. Humans also have greater variability of OLFM4 baseline expression relative to inbreed mice, thus it is unclear how baseline OLFM4 expression may affect outcomes as well.
Limitations
Our study has several limitations. First, we imputed OLFM4 percentages for healthy patients who were discharged home before Day 7. However, prior studies have shown that healthy patients do not have significant changes in their OLFM4 percentage, and those patients with a minor injury and a Day 7 sample also did not increase the percentage of OLFM4+ neutrophils. Second, although MPO amounts varied between animals, there was no significant difference in histopathologic injury severity. This might have suggested there was no clinical significance based on the presence or absence of OLFM4; however, our hemorrhagic shock model produces profound hypotension and likely led to the injury being too severe to differentiate clinically meaningful differences between the groups. Next, the hemorrhagic shock study is a nonsurvival model, and survival studies were not able to be performed. We were also unable to test if mice, like humans, increase the percentage of OLFM4+ neutrophils after hemorrhagic shock. Finally, we used a whole animal KO for OLFM4, as a floxed OLFM4 locus is not currently available. The use of a floxed mouse would provide a clearer understanding of whether OLFM4 expression in other cell types contributed to these findings.
In summary, we found that a subset of adult patients who suffered BTIs upregulated their percentage of OLFM4 positive neutrophils. Those who upregulated their OLFM4 had increased LOS, ICU days, ventilator days, and resuscitative needs. This was complemented by our successful development of a murine model of hemorrhagic shock, which demonstrated that WT mice with OLFM4 had worse inflammation compared with OLFM4 KO mice. Although these mice had systemic findings, such as elevated cytokine concentrations, further analysis suggests a lung mediated mechanism given the significant differences in lung neutrophil activity. In addition, we produced an anti-OLFM4 antibody, which when given to WT mice, led to the reduction of MPO. These findings suggest that OLFM4+ neutrophils are a unique subset of neutrophils that affect the inflammatory response after tissue injury.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank the Cincinnati Children’s Hospital Medical Center Imaging and Flow Cytometry Core facilities and the University of Cincinnati Trauma Registry for assistance with this work. They also thank Dr. Basilia Zingarelli and the Zingarelli laboratory for training in the murine hemorrhagic shock model.
Footnotes
Supported by U.S. National Institutes of Health–National Institute of General Medical Sciences grants R35 GM126943 (H.R.W.), K08 GM124298 (M.N.A.), and T32 GM008478-25 (N.C.L.).
Author Contributions: A.-F.K., N.C.L., and M.N.A. designed and performed the research, performed the statistical analysis, and prepared the manuscript. J.P.M., K.A., A.O., and P.L. performed research and reviewed the manuscript. R.D.S. and L.F. performed the statistical analysis. V.N. and H.R.W. designed the research and critically reviewed the manuscript.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2020-0276OC on November 30, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.McGhan LJ, Jaroszewski DE. The role of toll-like receptor-4 in the development of multi-organ failure following traumatic haemorrhagic shock and resuscitation. Injury. 2012;43:129–136. doi: 10.1016/j.injury.2011.05.032. [DOI] [PubMed] [Google Scholar]
- 2.Hwabejire JO, Nembhard CE, Oyetunji TA, Seyoum T, Abiodun MP, Siram SM, et al. Age-related mortality in blunt traumatic hemorrhagic shock: the killers and the life savers. J Surg Res. 2017;213:199–206. doi: 10.1016/j.jss.2015.04.056. [DOI] [PubMed] [Google Scholar]
- 3.Kangelaris KN, Prakash A, Liu KD, Aouizerat B, Woodruff PG, Erle DJ, et al. Increased expression of neutrophil-related genes in patients with early sepsis-induced ARDS. Am J Physiol Lung Cell Mol Physiol. 2015;308:L1102–L1113. doi: 10.1152/ajplung.00380.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brand HK, Ahout IM, de Ridder D, van Diepen A, Li Y, Zaalberg M, et al. Olfactomedin 4 serves as a marker for disease severity in pediatric respiratory syncytial virus (RSV) infection. PLoS One. 2015;10:e0131927. doi: 10.1371/journal.pone.0131927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong HR, Cvijanovich N, Allen GL, Lin R, Anas N, Meyer K, et al. Genomics of Pediatric SIRS/Septic Shock Investigators. Genomic expression profiling across the pediatric systemic inflammatory response syndrome, sepsis, and septic shock spectrum. Crit Care Med. 2009;37:1558–1566. doi: 10.1097/CCM.0b013e31819fcc08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clemmensen SN, Bohr CT, Rørvig S, Glenthøj A, Mora-Jensen H, Cramer EP, et al. Olfactomedin 4 defines a subset of human neutrophils. J Leukoc Biol. 2012;91:495–500. doi: 10.1189/jlb.0811417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alder MN, Opoka AM, Lahni P, Hildeman DA, Wong HR. Olfactomedin-4 is a candidate marker for a pathogenic neutrophil subset in septic shock. Crit Care Med. 2017;45:e426–e432. doi: 10.1097/CCM.0000000000002102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tomarev SI, Nakaya N. Olfactomedin domain-containing proteins: possible mechanisms of action and functions in normal development and pathology. Mol Neurobiol. 2009;40:122–138. doi: 10.1007/s12035-009-8076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schuijers J, van der Flier LG, van Es J, Clevers H. Robust cre-mediated recombination in small intestinal stem cells utilizing the olfm4 locus. Stem Cell Reports. 2014;3:234–241. doi: 10.1016/j.stemcr.2014.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alder MN, Mallela J, Opoka AM, Lahni P, Hildeman DA, Wong HR. Olfactomedin 4 marks a subset of neutrophils in mice. Innate Immun. 2019;25:22–33. doi: 10.1177/1753425918817611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu W, Yan M, Liu Y, Coleman WG, Rodgers GP. Olfactomedin 4 down-regulates neutrophil killing of gram-positive and gram-negative bacteria. Blood. 2010;116:3777. [Google Scholar]
- 12.Liu W, Yan M, Liu Y, McLeish KR, Coleman WG, Jr, Rodgers GP. Olfactomedin 4 inhibits cathepsin C-mediated protease activities, thereby modulating neutrophil killing of Staphylococcus aureus and Escherichia coli in mice. J Immunol. 2012;189:2460–2467. doi: 10.4049/jimmunol.1103179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu W, Yan M, Liu Y, Wang R, Li C, Deng C, et al. Olfactomedin 4 down-regulates innate immunity against Helicobacter pylori infection. Proc Natl Acad Sci USA. 2010;107:11056–11061. doi: 10.1073/pnas.1001269107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu W, Yan M, Sugui JA, Li H, Xu C, Joo J, et al. Olfm4 deletion enhances defense against Staphylococcus aureus in chronic granulomatous disease. J Clin Invest. 2013;123:3751–3755. doi: 10.1172/JCI68453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levinsky NC, Mallela J, Opoka AM, Harmon K, Lewis HV, Zingarelli B, et al. The olfactomedin-4 positive neutrophil has a role in murine intestinal ischemia/reperfusion injury. FASEB J. 2019;33:13660–13668. doi: 10.1096/fj.201901231R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klingbeil LR, Kim P, Piraino G, O’Connor M, Hake PW, Wolfe V, et al. Age-dependent changes in AMPK metabolic pathways in the lung in a mouse model of hemorrhagic shock. Am J Respir Cell Mol Biol. 2017;56:585–596. doi: 10.1165/rcmb.2016-0118OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zingarelli B, Sheehan M, Hake PW, O’Connor M, Denenberg A, Cook JA. Peroxisome proliferator activator receptor-γ ligands, 15-deoxy-Δ(12,14)-prostaglandin J2 and ciglitazone, reduce systemic inflammation in polymicrobial sepsis by modulation of signal transduction pathways. J Immunol. 2003;171:6827–6837. doi: 10.4049/jimmunol.171.12.6827. [DOI] [PubMed] [Google Scholar]
- 18.Welin A, Amirbeagi F, Christenson K, Björkman L, Björnsdottir H, Forsman H, et al. The human neutrophil subsets defined by the presence or absence of OLFM4 both transmigrate into tissue in vivo and give rise to distinct NETs in vitro. PLoS One. 2013;8:e69575. doi: 10.1371/journal.pone.0069575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haegens A, Heeringa P, van Suylen RJ, Steele C, Aratani Y, O’Donoghue RJ, et al. Myeloperoxidase deficiency attenuates lipopolysaccharide-induced acute lung inflammation and subsequent cytokine and chemokine production. J Immunol. 2009;182:7990–7996. doi: 10.4049/jimmunol.0800377. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.