From Chlamydia (1) to malaria (2) to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (3), it is increasingly recognized that individuals without recognizable symptoms play an important role in the transmission of infectious pathogens. To date, however, this paradigm has largely not been applied to Mycobacterium tuberculosis (Mtb) even though many people with prevalent tuberculosis (TB) do not experience symptoms. The World Health Organization has adopted ambitious targets to end the TB epidemic, including a 90% reduction in incidence from 2015 to 2035. The corresponding End TB Strategy focuses on the early diagnosis and treatment of individuals with recognizable symptoms and treatment of latent TB infection in people at high risk (4). However, these targets may not be achievable without increased attention to averting transmission from the millions of people who have subclinical TB.
Historically, TB has been conceptualized by some as consisting of latent infection (asymptomatic and noninfectious) and active disease (symptomatic and infectious). Recently, others have articulated this as a false dichotomy that masks a broader spectrum of disease, including incipient and subclinical stages (Figure 1) (5, 6). Yet subclinical TB is still frequently conceptualized as both noninfectious and as part of a “progression of disease activity” (5) that ultimately leads to recognizable symptoms (7). Under this paradigm, early diagnosis and treatment of people with symptomatic TB is capable of averting the vast majority of transmission events. If, however, a large fraction of Mtb transmission originates from people who do not have recognizable symptoms, then finding and treating these individuals (for example, by screening for TB with algorithms that do not rely on symptom screening) may be necessary to achieve major reductions in TB incidence by 2035.
Figure 1.
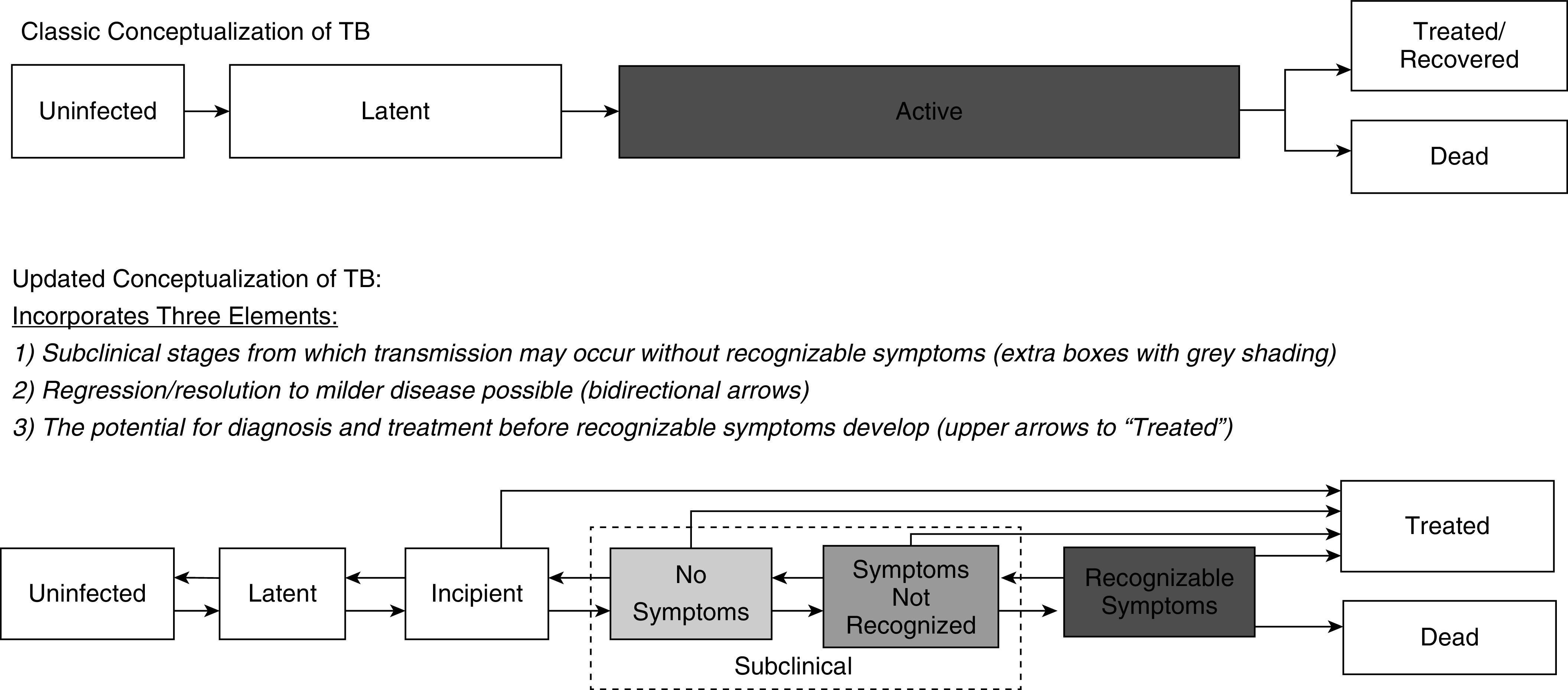
The spectrum of tuberculosis (TB) disease. The top panel shows a “classic” conceptualization of TB as a two-stage entity with inevitable forward progression, transmission (denoted by gray shading) occurring only from those with active TB, and diagnosis and treatment implicitly focused on people with symptomatic (“active”) TB. Others have demonstrated that this conceptualization is an oversimplification, namely, that the development of active TB represents distinct incipient and subclinical stages (5, 6). In the bottom panel, we further expand on this understanding, presenting an updated conceptualization of the spectrum of TB disease, illustrating that individuals with subclinical TB may be truly asymptomatic or have symptoms that are not recognized, both progression and regression across stages occur without inevitable development of recognizable symptoms, and individuals with milder forms of TB disease can be effectively diagnosed and treated. Shading intensity indicates that both subclinical and active TB states may be infectious and that infectiousness is likely to increase with more advanced disease, although the degree of correlation is uncertain.
Subclinical TB: Conceptual Framework
Subclinical TB is defined as “disease due to viable M. tuberculosis bacteria that does not cause clinical TB-related symptoms but causes other abnormalities that can be detected using existing radiologic or microbiologic assays” (5). This contrasts with latent TB (which is not expected to progress to disease “in the near future”) and incipient TB (which is likely to progress but does not cause detectable abnormalities) (5). In aligning this definition with available epidemiological data and interventions (which generally focus on pulmonary TB), it is helpful to conceptualize subclinical pulmonary TB as a disease state that is detectable by sputum culture or chest radiography but during which patients would respond “no” if asked whether they are currently experiencing any TB symptom (cough, fever, night sweats, or weight loss). Subclinical TB can, however, involve mild or intermittent symptoms (including cough due to unrelated respiratory conditions) that are not recognized in the context of a clinical interview. For example, patients may not reliably identify when their cough exceeds a normal frequency (8), or a cough may be attributed to unrelated medical (e.g., chronic lung disease, viral respiratory infection, or air pollution) (9) or nonmedical (10) factors. Furthermore, subclinical TB need not indicate an inevitable progression of disease severity; although most people with symptomatic (“active”) TB disease progressed first through a subclinical state, many people with subclinical TB may develop recognizable symptoms only after years, if at all.
This conceptualization highlights that individuals with subclinical TB may be infectious (Figure 1, gray shading) and remain in the community for years without developing symptoms that would lead to diagnosis. Indeed, spontaneous resolution of TB disease occurs with known regularity (11), such that many people with sub‐clinical TB will never be diagnosed at all (Figure 1, bidirectional arrows). To date, very few interventions have attempted to identify and treat such individuals. More attention has been paid to reducing patient delays in care seeking after symptoms develop (12), employing higher-sensitivity tests for symptomatic diagnosis (13), and screening individuals for TB symptoms upon presentation to healthcare facilities (14). The result of these efforts has been an impressive >70% increase in case notifications and 38% decline in the TB mortality rate from 2000 to 2018 (15). Unfortunately, TB incidence has fallen much more slowly than mortality, indicating that these measures have been less effective in halting transmission. One explanation for this finding is that individuals with subclinical TB may be the source of a large fraction of ongoing Mtb transmission. Here, we critically evaluate the evidence for and against this hypothesis and discuss the implications for both disease control strategy and future data collection efforts.
The Large Burden of Subclinical TB
More than two dozen high-burden countries have conducted population-representative TB prevalence surveys in the past 15 years. Because mass sputum screening is both logistically challenging and expensive (16), most estimates of TB prevalence rely on initial screening with symptoms and chest radiography (17) followed by bacteriologic testing (sputum smear microscopy, Xpert MTB/RIF, and/or culture) for those who screen positive based on either symptoms or radiographic findings.
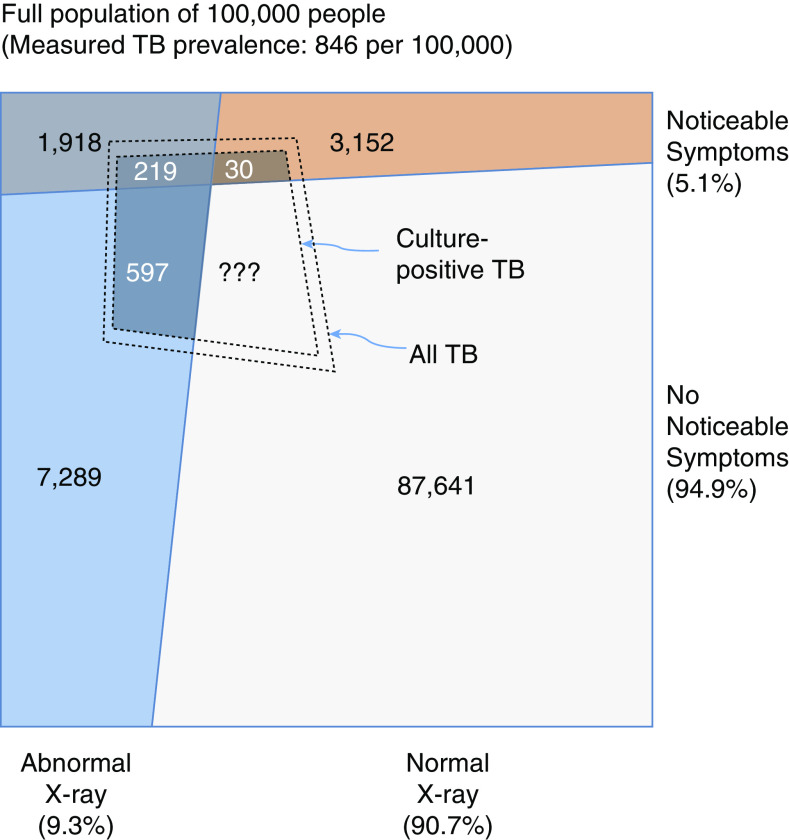
In nearly every such survey, the estimated prevalence has exceeded the annual number of TB notifications—by at least a factor of two in most surveys, and often by more in surveys that use culture rather than smear for confirmatory testing (15). A second consistent result is illustrated in Figure 2; a large proportion of all people with prevalent, bacteriologically positive, and radiographically abnormal TB screen negative for TB symptoms. In a recent review of 23 national and five subnational prevalence surveys (18), median 50% (interquartile range, 40–62%) of the identified TB cases screened positive based on radiography alone, whereas 7% (range 0.7–22%) screened positive based only on symptoms, and the remainder had both symptoms and radiographic abnormalities suggestive of TB (19). Even among the 30–68% of prevalent TB that is smear positive (typically indicating high sputum bacillary loads of ≥10,000 viable organisms/ml [20] and high potential infectivity [21]), a substantial proportion (34–68%) is associated with a negative symptom screen (17). These data underscore the large burden of subclinical TB worldwide; with 7 million people notified to the World Health Organization as having TB each year, an average prevalence:notification ratio of 2:1 would indicate a TB prevalence of approximately 14 million. If half of those prevalent cases lack recognizable symptoms, then an estimated 7 million people are currently living with subclinical TB. Furthermore, the sensitivity of chest radiography depends on the skill of the radiologist and is imperfect even for people with symptomatic TB (19); if chest X-ray sensitivity is no better for subclinical TB than for symptomatic TB (and indeed, it is likely to be worse), then the burden of subclinical TB will be underestimated in prevalence surveys by at least 10% (Figure 2). This fraction may be higher in populations with endemic HIV (22). Thus, it is reasonable to believe that at least 7 million to 10 million people are currently living with TB that is not detectable by symptom screen.
Figure 2.
Relationship between symptoms, chest X-ray findings, and culture-confirmed tuberculosis (TB). The outer box represents a population of 100,000, based on the extrapolation of crude results from the 2011 Cambodia National TB Prevalence Survey (55). The inner dashed quadrilateral denotes the number of people with TB detected by sputum culture, and the dashed quadrilateral just outside it indicates all TB disease that would be identified by an optimally sensitive reference standard. In this survey—like in most TB prevalence surveys—only individuals with symptoms or abnormal chest X-ray findings submitted sputum for evaluation. Thus, the TB status of those with normal chest X-ray findings and no reported symptoms is unknown; however, it can be inferred. Specifically, the sensitivity of chest X-ray for symptomatic individuals with culture-positive TB is 219/249 = 88% in this survey. The sensitivity of chest X-ray for culture-positive TB that is asymptomatic would be expected to be lower than for symptomatic TB, but even if it were 88%, this would imply at least 597/0.88 − 597 = 81 individuals with culture-positive TB who would be missed by a combination of chest X-ray and symptom screening. After accounting for correlation between symptoms and X-ray, the likely burden of missed subclinical TB is substantially higher. In other settings and surveys, this burden of radiographically undetectable but culture-positive prevalent TB will vary, and it may be higher in settings of high HIV prevalence.
Subclinical TB and Transmission
To be transmitted, Mtb must be expelled from the lung into the environment. Coughing is generally believed to be the main mechanism by which this occurs (23). More severe symptoms, including cough frequency, have been shown to positively correlate with bacillary burden (24, 25), disease severity (26), and higher rates of infection among household contacts (27). However, respiratory droplets can also be expelled without cough, for example, during routine activities including singing (28), talking (23), and tidal breathing. In more recent studies, Mtb DNA has been recovered from face masks worn by people with TB, with no association seen between the quantity of exhaled Mtb and cough frequency, sputum grade, or severity of chest X-ray abnormalities (29). People with no symptoms can still have the high bacillary loads typically associated with transmission (21); in Asian prevalence surveys, symptom screens were negative in 35–65% of patients with prevalent TB even when surveys or analyses were limited to smear-positive prevalent cases (17). In addition, timed transmission trees reconstructed from genomic and clinical data suggest that some transmission events occur well before symptom onset (30). Taken together, these findings suggest that although symptoms facilitate transmission, Mtb transmission can occur in the absence of noticeable symptoms (31).
Even if cough increases the efficiency of Mtb transmission, the contribution of subclinical TB to overall transmission on a population level could still be substantial. First, people spend far more time breathing (and talking) than coughing. Second, as noted by other investigators (32), people with asymptomatic TB may still cough because of either intermittent causes (e.g., viral upper respiratory infections) (32) or chronic respiratory conditions (e.g., indoor or outdoor air pollution) in which mild cough is not identified as unusual (33, 34). Third, individuals who aerosolize even small numbers of viable bacilli may transmit disease effectively. Infection with Mtb can occur after minimal exposure (i.e., with even a single bacillus) (35), and many forms of contact, especially within households and in workplaces, occur repeatedly over longer periods of time, such that people with mild or periodic infectiousness may still have many opportunities to infect their contacts. Finally, because activities other than coughing (e.g., singing) can produce particularly small droplet nuclei that remain suspended in air for extended periods (28), people with subclinical TB in the right high-risk setting (e.g., choir rehearsal) (36) can generate a large number of secondary infections.
In summary, although classic TB symptoms—particularly cough—are associated with higher infectiousness, cough is not necessary for transmission, and subclinical TB can potentially drive a substantial fraction of transmission on a population level because of its high prevalence and long duration.
Natural History of Subclinical TB
As summarized earlier, prevalence surveys indicate that for every individual notified with TB, at least 2 person-years of prevalent culture-positive TB are experienced in the community. These person-years may not be experienced by the same person (for example, this could represent four different people, with each experiencing 6 mo of culture-positive TB), but this person-time is presumably infectious, and at least half of this person-time is not associated with recognizable symptoms. Some of this person-time represents prediagnostic delay in individuals who are eventually notified, but much of this burden may be experienced by people who are never diagnosed as having TB. Speaking to the latter possibility, patients with TB self-report a median of only 1 month of symptoms before they seek care (37, 38), and some individuals, especially those with additional risk factors, progress rapidly from Mtb exposure to severe TB (39). Thus, the simplest way to reconcile prevalence and notification data is to conceptualize the natural history of TB as both bidirectional and heterogeneous, such that the populations who account for most infectious person-time may differ from those who comprise most TB notifications (Figure 3).
Figure 3.
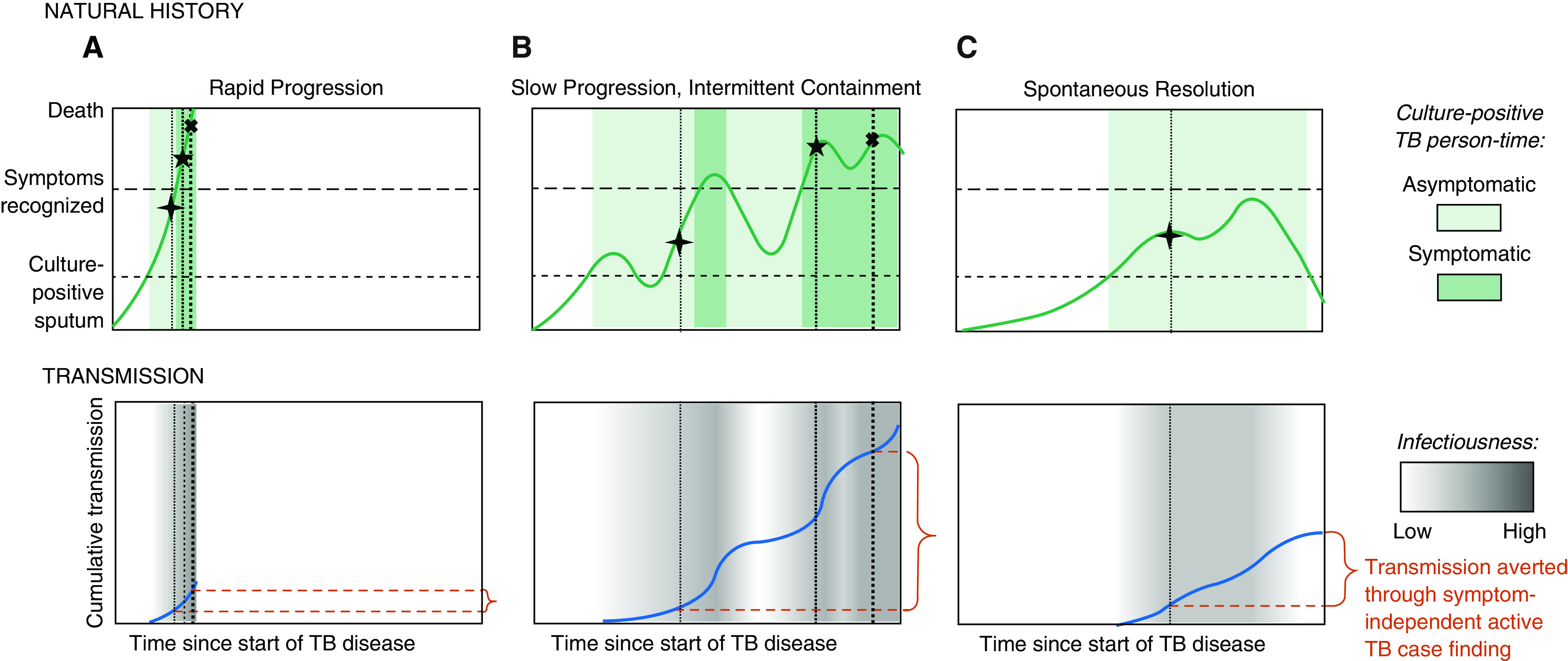
Three paradigmatic patient trajectories of tuberculosis (TB) natural history, transmission, and diagnosis. The top row shows three possible trajectories of TB natural history in the absence of treatment, ordered from the most aggressive (e.g., in those with the weakest immune response) to the most indolent (e.g., those with robust immune responses). The y axis of this top row corresponds with the patient’s burden of disease (abstractly conceptualized), with horizontal lines denoting thresholds above which viable Mycobacterium tuberculosis might be detected in sputum (lower dashed line corresponding with active TB), individuals would confirm TB symptoms if asked during screening (upper dashed line), and death occurs (upper boundary). Note that individuals with TB may have symptoms below the “symptoms recognized” line, but those symptoms (e.g., mild cough) may not be sufficiently recognizable to warrant care seeking or a positive response to a standard symptom screen. Shaded areas denote periods of time during which TB is microbiologically confirmable, with dark shading corresponding with periods of time during which symptoms are recognizable, illustrating that people whose symptoms progress more slowly spend more time in a microbiologically confirmable state. The bottom row presents the amount of M. tuberculosis transmission (in arbitrary units) that might occur during each of these paradigmatic trajectories, with intensity of transmission (per unit person-time) denoted by the darkness of gray shading and cumulative transmission over time denoted by the height of the dark blue line. Vertical dotted lines with corresponding symbols illustrate points of time during which diagnosis might occur, as follows: the first (cross) through active case finding that does not rely on symptom screening, the second (star) through symptom-triggered active case finding, and the third (X) through patient-triggered symptomatic care seeking. The degree to which detecting subclinical cases might avert M. tuberculosis transmission on a population level (shown in red braces) therefore depends on the relative frequency of each trajectory and the shape of each transmission-versus-time curve. A shows rapid progression, B slow progression with intermittent containment, and C spontaneous resolution.
Heterogeneity in the natural history of TB would suggest that the duration of TB disease can be very long in some individuals but progress rapidly in others. As illustrated in Figure 3, those most likely to develop severe TB, including those who are immunocompromised or at extremes of age (40, 41), may spend relatively little time in subclinical stages (Figure 3A). By contrast, people whose TB advances more slowly, such as the individual with mild and intermittently symptomatic disease depicted in Figure 3B, may be diagnosed and notified only after years of infectiousness. This heterogeneity in disease progression (and in the resulting duration of prevalent TB) is reflected, for example, in differential HIV coprevalence between prevalent and notified TB cases; because HIV-positive people progress more rapidly from infection to disease, HIV-positive individuals comprise a smaller proportion of undiagnosed prevalent cases than of routinely notified cases. When HIV-negative individuals develop TB, they experience a longer average duration of subclinical disease, resulting in lower levels of HIV among prevalent cases (36, 37). Individuals with milder and more slowly progressive disease may have greater opportunity to generate Mtb transmission, providing epidemiologic justification to expand case-finding efforts beyond the populations (e.g., HIV-positive) who are at highest risk for rapid disease progression.
Another consequential component of TB natural history is the potential for improvement and resolution in the absence of treatment (illustrated by backward-pointing arrows in Figure 1 and downward-sloping trajectories in Figure 3). These processes are difficult to measure because diagnosis typically occurs only after symptoms deteriorate and because diagnosed patients cannot be left untreated to see whether symptoms spontaneously resolve. Historical data show, however, that TB resolution is not rare. In a systematic review of cohorts enrolled at sanatoria in the preantibiotic era, approximately 50% of patients sufficiently ill to require admission (yet able to survive to the point of enrollment) remained alive years later with apparent cure (42). Given that such highly symptomatic TB could resolve without treatment, spontaneous resolution of subclinical TB is not only possible but likely to be a common occurrence. The result, as depicted in Figure 3C, is that individuals who never develop noticeable symptoms may account for much of the observed prevalence of culturable TB (and correspondingly, a large fraction of Mtb transmission), but in the absence of active case finding with non–symptom-based algorithms, these individuals will never be diagnosed and treated.
This understanding of subclinical TB as heterogeneous, prevalent, and often nonprogressive has important implications for Mtb transmission at the population level; individuals with milder disease forms and slower progression could account for a minority of TB notifications but the majority of infectious person-time. It also has implications for how other epidemiological data are interpreted. For example, if TB can resolve after an unrecognized infectious period, then TB cases infected by household members with such indolent disease courses might be misattributed to transmission outside the household (43). As another example, ostensible discordance between highly sensitive diagnostic assays such as Xpert MTB/RIF Ultra and TB culture (44) might not represent assay error but rather residual nucleic acid from prior (spontaneously-resolved) TB or waxing-and-waning subclinical TB.
A key outstanding question is the extent to which individuals with subclinical TB should be prioritized for detection and treatment. The epidemiological impact and cost-effectiveness of such efforts depend on the magnitude of these individuals’ cumulative contribution to transmission, on the ability to diagnose and treat them using existing tools, and the ability to identify those with the greatest potential for transmission or clinical progression.
Should We Prioritize Detection of Subclinical TB?
If most Mtb transmission originates from people with recognizable symptoms, then emphasis should be placed on strengthening existing diagnostic processes. On the other hand, if substantial interruption of transmission would require detecting and treating people with subclinical TB, then active case finding with non–symptom-based algorithms must be prioritized. If this latter hypothesis is true, then important research activities might include refining assays (e.g., transcriptomic assays) (45) to detect subclinical TB, incorporating point-of-care, non–symptom-based assays (e.g., C-reactive protein) (46) into screening algorithms, and addressing potential implementation barriers to non–symptom-based active case finding (e.g., lower treatment completion [47] and management of false-positives [48]). If it is likely that a substantial proportion of transmission will occur despite these improvements, then attention must shift more completely to prevention of disease. In choosing this preventive focus, it will also be important to understand the role that interventions such as vaccination and preventive therapy might play in aiding the resolution of incipient or subclinical TB (49).
The depiction of potential diagnosis events in Figure 3 (vertical dotted lines) illustrates the interplay between trajectories of natural history and symptom-based diagnosis. In each panel, the first, thinnest vertical dotted line corresponds with a time at which individuals might be diagnosed through active case finding with a highly sensitive assay without relying on symptoms. Such an approach would be very resource intensive and justifiable only if it would avert a large fraction of transmission. The second line corresponds with symptom-based active case finding (which might require fewer resources), and the third corresponds with “passive” symptom-driven diagnosis (the current standard of care). In the most immu-nologically vulnerable patients (for example, trajectory A), disease progression may occur so rapidly that active case finding of any sort is unlikely to avert much transmission. In patients with a more indolent course but occasional periods of noticeable symptoms (for example, trajectory B), early symptom-based active case finding could avert some transmission, but active diagnosis without relying on symptoms might avert much more. And in patients who ultimately experience resolution of subclinical TB without ever developing noticeable symptoms (for example, trajectory C), symptom-based screening has no ability to avert transmission. The relative epidemiological importance of efforts to find, diagnose, and treat individuals with subclinical disease, therefore, depends on the relative frequency with which such patient-level trajectories are experienced on a population level and the corresponding relationships between symptoms, transmission, and diagnosis in each.
Looking Forward
Existing data are increasingly clear that subclinical TB comprises a large fraction of prevalent disease at the population level, has meaningful infectious potential, follows a heterogeneous clinical trajectory, and is difficult to diagnose using passive systems. This implies that subclinical TB has greater epidemiological significance than it has traditionally been assigned and suggests that a large fraction of Mtb transmission at the population level may originate from individuals with subclinical TB. If this is true, then active case finding with a sufficiently sensitive, symptom-independent algorithm could be required to substantially avert Mtb transmission and reduce TB incidence. To appropriately prioritize such a strategy, more precise estimates are needed of the transmission potential of subclinical TB, the future clinical trajectories of people with prevalent subclinical TB, and the resources required to diagnose and treat people with subclinical TB.
To date, at least four major barriers have hindered studies that might address these data gaps. First, identification of subclinical cases requires high-sensitivity testing, and most available assays have not been sufficiently sensitive, rapid, and affordable for large-scale active case finding. Second, ethical constraints have precluded studies of the natural history of untreated subclinical TB. Third, the ideal studies would test closed populations comprehensively and repeatedly over time, but such studies (e.g., Reference 50) are expensive and logistically complex and are therefore rare. Finally, identifying transmission events requires inference of transmission links and their directionality, a task made particularly challenging by the long serial interval, latency, and airborne nature of Mtb transmission.
Despite these challenges, our expanding research armamentarium can substantially aid in addressing these questions. In-creasingly sensitive molecular diagnostic assays, such as Xpert MTB/RIF Ultra (51), and radiographic tools, such as portable X-ray with computer-aided interpretation (52), now make it possible to screen large numbers of people for subclinical TB with high sensitivity in community-based settings. Additional insight into the natural history of TB might be gained by prospectively identifying and following asymptomatic individuals with negative sputum cultures but positive markers for incipient disease (45), using close observation to understand what characteristics are associated with high risks of clinical or bacteriological progression. Regarding study populations, examples now exist of populations being tested repeatedly with high-sensitivity assays and followed longitudinally with high coverage; these populations include cohorts of households (53) and high-risk populations, such as prisoners (54), and now also entire communities (50). So far, these studies have demonstrated the ability of such an intervention to reduce the prevalence of TB, reinforcing the idea that subclinical TB is prevalent and typically lasts for a long duration. Measuring its impact on transmission may require longer-term follow-up after such large-scale interventions, with measurements of subsequent TB incidence and of the extent of recent transmission. Cluster-randomized trials comparing symptom-based and symptom-neutral approaches to active case finding could also aid in both developing effective interventions to reach the subclinical TB population and better understanding their role in sustaining local epidemics. Finally, whole-genome sequencing and epidemiological data from such population-based studies can now be paired with increasingly sophisticated bioinformatic methods to better infer directionality and timing of transmission (30), with the corresponding potential to confirm or refute high amounts of transmission from subclinical cases.
In summary, available evidence—although still circumstantial—increasingly suggests that a substantial fraction of Mtb transmission originates from people with subclinical TB and that these individuals experience a heterogeneous disease course characterized by frequent resolution or prolonged persistence without diagnosis. Given these realities and the challenges of performing non–symptom-based active TB case finding, ambitious studies are warranted to better quantify the contribution of subclinical TB to transmission at the population level. We now have the diagnostic assays, markers for incipient TB, population-based studies, and tools for inferring transmission that are needed to answer these questions. In the meantime, it is evident that exclusive focus on symptom-driven diagnosis is insufficient to achieve targets for reducing TB incidence, and that people with prevalent subclinical TB in communities warrant greater attention. Detecting and treating such individuals may represent the key to interrupting Mtb transmission; we ignore the potential epidemiological importance of subclinical TB at our own peril.
Supplementary Material
Footnotes
Supported by NIH awards R01HL138728 (D.W.D.), R01AI147681 (D.W.D.), and K08AI127908 (E.A.K.).
Author Contributions: All authors contributed to the conception, design, writing, figure development, and revision of this manuscript. All authors gave final approval of the version to be published and agree to be accountable for all aspects of the work.
Originally Published in Press as DOI: 10.1164/rccm.202006-2394PP on November 16, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Farley TA, Cohen DA, Elkins W. Asymptomatic sexually transmitted diseases: the case for screening. Prev Med. 2003;36:502–509. doi: 10.1016/s0091-7435(02)00058-0. [DOI] [PubMed] [Google Scholar]
- 2.Bousema T, Okell L, Felger I, Drakeley C. Asymptomatic malaria infections: detectability, transmissibility and public health relevance. Nat Rev Microbiol. 2014;12:833–840. doi: 10.1038/nrmicro3364. [DOI] [PubMed] [Google Scholar]
- 3.Bai Y, Yao L, Wei T, Tian F, Jin D-Y, Chen L, et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020;323:1406–1407. doi: 10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. Geneva, Switzerland: World Health Organization; 2015. The END TB strategy. [accessed 2020 Jun 15]. Available from: https://www.who.int/tb/End_TB_brochure.pdf?ua=1. [Google Scholar]
- 5.Drain PK, Bajema KL, Dowdy D, Dheda K, Naidoo K, Schumacher SG, et al. Incipient and subclinical tuberculosis: a clinical review of early stages and progression of infection. Clin Microbiol Rev. 2018;31:e00021–e00018. doi: 10.1128/CMR.00021-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Esmail H, Barry CE, III, Young DB, Wilkinson RJ. The ongoing challenge of latent tuberculosis. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130437. doi: 10.1098/rstb.2013.0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lawn SD, Kerkhoff AD, Wood R. Progression of subclinical culture-positive tuberculosis to symptomatic disease in HIV-infected individuals. AIDS. 2011;25:2190–2191. doi: 10.1097/QAD.0b013e32834cda4e. [DOI] [PubMed] [Google Scholar]
- 8.Yousaf N, Monteiro W, Matos S, Birring SS, Pavord ID. Cough frequency in health and disease. Eur Respir J. 2013;41:241–243. doi: 10.1183/09031936.00089312. [DOI] [PubMed] [Google Scholar]
- 9.Field SK, Escalante P, Fisher DA, Ireland B, Irwin RS CHEST Expert Cough Panel. Cough due to TB and other chronic infections: CHEST guideline and expert panel report. Chest. 2018;153:467–497. doi: 10.1016/j.chest.2017.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ngang PN, Ntaganira J, Kalk A, Wolter S, Ecks S. Perceptions and beliefs about cough and tuberculosis and implications for TB control in rural Rwanda. Int J Tuberc Lung Dis. 2007;11:1108–1113. [PubMed] [Google Scholar]
- 11.Hunter RL. Tuberculosis as a three-act play: a new paradigm for the pathogenesis of pulmonary tuberculosis. Tuberculosis (Edinb) 2016;97:8–17. doi: 10.1016/j.tube.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vesga JF, Hallett TB, Reid MJA, Sachdeva KS, Rao R, Khaparde S, et al. Assessing tuberculosis control priorities in high-burden settings: a modelling approach. Lancet Glob Health. 2019;7:e585–e595. doi: 10.1016/S2214-109X(19)30037-3. [DOI] [PubMed] [Google Scholar]
- 13.Adepoyibi T, Lilis L, Greb H, Boyle D. Which attributes within target product profiles for tuberculosis diagnostics are the most important to focus on? Int J Tuberc Lung Dis. 2018;22:425–428. doi: 10.5588/ijtld.17.0312. [DOI] [PubMed] [Google Scholar]
- 14.Kranzer K, Lawn SD, Meyer-Rath G, Vassall A, Raditlhalo E, Govindasamy D, et al. Feasibility, yield, and cost of active tuberculosis case finding linked to a mobile HIV service in Cape Town, South Africa: a cross-sectional study. PLoS Med. 2012;9:e1001281. doi: 10.1371/journal.pmed.1001281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization. Geneva, Switzerland: World Health Organization; 2019. Global tuberculosis report 2019. [accessed 2019 Dec 10]. Available from: https://www.who.int/tb/publications/global_report/en/ [Google Scholar]
- 16.van’t Hoog AH, Meme HK, Laserson KF, Agaya JA, Muchiri BG, Githui WA, et al. Screening strategies for tuberculosis prevalence surveys: the value of chest radiography and symptoms. PLoS One. 2012;7:e38691. doi: 10.1371/journal.pone.0038691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Onozaki I, Law I, Sismanidis C, Zignol M, Glaziou P, Floyd K. National tuberculosis prevalence surveys in Asia, 1990-2012: an overview of results and lessons learned. Trop Med Int Health. 2015;20:1128–1145. doi: 10.1111/tmi.12534. [DOI] [PubMed] [Google Scholar]
- 18.Frascella B, Richards AS, Sossen B, Emery JC, Odone A, Law I, et al. Subclinical tuberculosis disease - a review and analysis of prevalence surveys to inform definitions, burden, associations and screening methodology. Clin Infect Dis. doi: 10.1093/cid/ciaa1402. [online ahead of print] 16 Sep 2020; DOI: 10.1093/cid/ciaa1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization. Geneva, Switzerland: World Health Organization; 2016. Chest radiography in tuberculosis detection. [accessed 2020 Apr 7]. Available from: http://www.who.int/tb/publications/chest-radiography/en/ [Google Scholar]
- 20.Yeager H, Jr, Lacy J, Smith LR, LeMaistre CA. Quantitative studies of mycobacterial populations in sputum and saliva. Am Rev Respir Dis. 1967;95:998–1004. doi: 10.1164/arrd.1967.95.6.998. [DOI] [PubMed] [Google Scholar]
- 21.Behr MA, Warren SA, Salamon H, Hopewell PC, Ponce de Leon A, Daley CL, et al. Transmission of Mycobacterium tuberculosis from patients smear-negative for acid-fast bacilli. Lancet. 1999;353:444–449. doi: 10.1016/s0140-6736(98)03406-0. [DOI] [PubMed] [Google Scholar]
- 22.Pepper T, Joseph P, Mwenya C, McKee G-S, Haushalter A, Carter A, et al. Normal chest radiography in pulmonary tuberculosis: implications for obtaining respiratory specimen cultures. Int J Tuberc Lung Dis. 2008;12:397–403. [PubMed] [Google Scholar]
- 23.Loudon RG, Roberts RM. Droplet expulsion from the respiratory tract. Am Rev Respir Dis. 1967;95:435–442. doi: 10.1164/arrd.1967.95.3.435. [DOI] [PubMed] [Google Scholar]
- 24.Fennelly KP, Jones-López EC, Ayakaka I, Kim S, Menyha H, Kirenga B, et al. Variability of infectious aerosols produced during coughing by patients with pulmonary tuberculosis. Am J Respir Crit Care Med. 2012;186:450–457. doi: 10.1164/rccm.201203-0444OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Proaño A, Bravard MA, López JW, Lee GO, Bui D, Datta S, et al. Tuberculosis Working Group in Peru. Dynamics of cough frequency in adults undergoing treatment for pulmonary tuberculosis. Clin Infect Dis. 2017;64:1174–1181. doi: 10.1093/cid/cix039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hales CM, Heilig CM, Chaisson R, Leung CC, Chang KC, Goldberg SV, et al. The association between symptoms and microbiologically defined response to tuberculosis treatment. Ann Am Thorac Soc. 2013;10:18–25. doi: 10.1513/AnnalsATS.201207-038OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loudon RG, Spohn SK. Cough frequency and infectivity in patients with pulmonary tuberculosis. Am Rev Respir Dis. 1969;99:109–111. doi: 10.1164/arrd.1969.99.1.109. [DOI] [PubMed] [Google Scholar]
- 28.Loudon RG, Roberts RM. Singing and the dissemination of tuberculosis. Am Rev Respir Dis. 1968;98:297–300. doi: 10.1164/arrd.1968.98.2.297. [DOI] [PubMed] [Google Scholar]
- 29.Williams CM, Abdulwhhab M, Birring SS, De Kock E, Garton NJ, Townsend E, et al. Exhaled Mycobacterium tuberculosis output and detection of subclinical disease by face-mask sampling: prospective observational studies. Lancet Infect Dis. 2020;20:607–617. doi: 10.1016/S1473-3099(19)30707-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu Y, Cancino-Muñoz I, Torres-Puente M, Villamayor LM, Borrás R, Borrás-Máñez M, et al. High-resolution mapping of tuberculosis transmission: whole genome sequencing and phylogenetic modelling of a cohort from Valencia Region, Spain. PLoS Med. 2019;16:e1002961. doi: 10.1371/journal.pmed.1002961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patterson B, Wood R. Is cough really necessary for TB transmission? Tuberculosis (Edinb) 2019;117:31–35. doi: 10.1016/j.tube.2019.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Esmail H, Dodd PJ, Houben RMGJ. Tuberculosis transmission during the subclinical period: could unrelated cough play a part? Lancet Respir Med. 2018;6:244–246. doi: 10.1016/S2213-2600(18)30105-X. [DOI] [PubMed] [Google Scholar]
- 33.GBD 2015 Chronic Respiratory Disease Collaborators. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir Med. 2017;5:691–706. doi: 10.1016/S2213-2600(17)30293-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cohen AJ, Brauer M, Burnett R, Anderson HR, Frostad J, Estep K, et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. Lancet. 2017;389:1907–1918. doi: 10.1016/S0140-6736(17)30505-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Riley RL, Mills CC, Nyka W, Weinstock N, Storey PB, Sultan LU, et al. Aerial dissemination of pulmonary tuberculosis: a two-year study of contagion in a tuberculosis ward. 1959. Am J Epidemiol. 1995;142:3–14. doi: 10.1093/oxfordjournals.aje.a117542. [DOI] [PubMed] [Google Scholar]
- 36.Mangura BT, Napolitano EC, Passannante MR, McDonald RJ, Reichman LB. Mycobacterium tuberculosis miniepidemic in a church gospel choir. Chest. 1998;113:234–237. doi: 10.1378/chest.113.1.234. [DOI] [PubMed] [Google Scholar]
- 37.Sreeramareddy CT, Panduru KV, Menten J, Van den Ende J. Time delays in diagnosis of pulmonary tuberculosis: a systematic review of literature. BMC Infect Dis. 2009;9:91. doi: 10.1186/1471-2334-9-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Getnet F, Demissie M, Assefa N, Mengistie B, Worku A. Delay in diagnosis of pulmonary tuberculosis in low-and middle-income settings: systematic review and meta-analysis. BMC Pulm Med. 2017;17:202. doi: 10.1186/s12890-017-0551-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Edlin BR, Tokars JI, Grieco MH, Crawford JT, Williams J, Sordillo EM, et al. An outbreak of multidrug-resistant tuberculosis among hospitalized patients with the acquired immunodeficiency syndrome. N Engl J Med. 1992;326:1514–1521. doi: 10.1056/NEJM199206043262302. [DOI] [PubMed] [Google Scholar]
- 40.Marais BJ, Schaaf HS. Tuberculosis in children. Cold Spring Harb Perspect Med. 2014;4:a017855. doi: 10.1101/cshperspect.a017855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Negin J, Abimbola S, Marais BJ. Tuberculosis among older adults: time to take notice. Int J Infect Dis. 2015;32:135–137. doi: 10.1016/j.ijid.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 42.Tiemersma EW, van der Werf MJ, Borgdorff MW, Williams BG, Nagelkerke NJD. Natural history of tuberculosis: duration and fatality of untreated pulmonary tuberculosis in HIV negative patients: a systematic review. PLoS One. 2011;6:e17601. doi: 10.1371/journal.pone.0017601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martinez L, Lo NC, Cords O, Hill PC, Khan P, Hatherill M, et al. Paediatric tuberculosis transmission outside the household: challenging historical paradigms to inform future public health strategies. Lancet Respir Med. 2019;7:544–552. doi: 10.1016/S2213-2600(19)30137-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dorman SE, Schumacher SG, Alland D, Nabeta P, Armstrong DT, King B, et al. study team. Xpert MTB/RIF Ultra for detection of Mycobacterium tuberculosis and rifampicin resistance: a prospective multicentre diagnostic accuracy study. Lancet Infect Dis. 2018;18:76–84. doi: 10.1016/S1473-3099(17)30691-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gupta RK, Turner CT, Venturini C, Esmail H, Rangaka MX, Copas A, et al. Concise whole blood transcriptional signatures for incipient tuberculosis: a systematic review and patient-level pooled meta-analysis. Lancet Respir Med. 2020;8:395–406. doi: 10.1016/S2213-2600(19)30282-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoon C, Chaisson LH, Patel SM, Allen IE, Drain PK, Wilson D, et al. Diagnostic accuracy of C-reactive protein for active pulmonary tuberculosis: a meta-analysis. Int J Tuberc Lung Dis. 2017;21:1013–1019. doi: 10.5588/ijtld.17.0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Singh M, Sagili KD, Tripathy JP, Kishore S, Bahurupi YA, Kumar A, et al. Are treatment outcomes of patients with tuberculosis detected by active case finding different from those detected by passive case finding? J Glob Infect Dis. 2020;12:28–33. doi: 10.4103/jgid.jgid_66_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Houben RMGJ, Lalli M, Kranzer K, Menzies NA, Schumacher SG, Dowdy DW. What if they don’t have tuberculosis? The consequences and trade-offs involved in false-positive diagnoses of tuberculosis. Clin Infect Dis. 2019;68:150–156. doi: 10.1093/cid/ciy544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Robertson BD, Altmann D, Barry C, Bishai B, Cole S, Dick T, et al. Detection and treatment of subclinical tuberculosis. Tuberculosis (Edinb) 2012;92:447–452. doi: 10.1016/j.tube.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 50.Marks GB, Nguyen NV, Nguyen PTB, Nguyen T-A, Nguyen HB, Tran KH, et al. Community-wide screening for tuberculosis in a high-prevalence setting. N Engl J Med. 2019;381:1347–1357. doi: 10.1056/NEJMoa1902129. [DOI] [PubMed] [Google Scholar]
- 51.Chakravorty S, Simmons AM, Rowneki M, Parmar H, Cao Y, Ryan J, et al. The new Xpert MTB/RIF ultra: improving detection of Mycobacterium tuberculosis and resistance to rifampin in an assay suitable for point-of-care testing. mBio. 2017;8:e00812–e00817. doi: 10.1128/mBio.00812-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.MacPherson P, Webb EL, Lalloo DG, Nliwasa M, Maheswaran H, Joekes E, et al. Design and protocol for a pragmatic randomised study to optimise screening, prevention and care for tuberculosis and HIV in Malawi (PROSPECT Study) Wellcome Open Res. 2018;3:61. doi: 10.12688/wellcomeopenres.14598.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saunders MJ, Tovar MA, Collier D, Baldwin MR, Montoya R, Valencia TR, et al. Active and passive case-finding in tuberculosis-affected households in Peru: a 10-year prospective cohort study. Lancet Infect Dis. 2019;19:519–528. doi: 10.1016/S1473-3099(18)30753-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lima F, Santos AS, Oliveira RD, Silva CCR, Gonçalves CCM, Andrews JR, et al. Oral swab testing by Xpert® MTB/RIF Ultra for mass tuberculosis screening in prisons. J Clin Tuberc Other Mycobact Dis. 2020;19:100148. doi: 10.1016/j.jctube.2020.100148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.National Tuberculosis Control Program. Phnom Penh, Cambodia: National Tuberculosis Control Program; 2012. Second national tuberculosis prevalence survey, Cambodia, 2011. [accessed 2020 May 5]. Available from: https://openjicareport.jica.go.jp/pdf/12120325.pdf. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.