To the Editor:
Pulmonary vascular disease, including pulmonary hypertension (PH), is a heterogeneous group of disorders that are associated with a high risk of death. Regardless of etiology, this condition is characterized histologically by the narrowing and loss of the small distal pulmonary vessels, thereby contributing to higher pulmonary vascular resistance and poor outcomes (1).
Remodeling and loss of the small pulmonary vessels, referred to as vascular “pruning,” can be evaluated noninvasively using image analysis of computed tomographic (CT) imaging (2, 3). Prior work has shown that CT-based pruning is associated with clinical indicators of pulmonary vascular disease. Among heavy smokers with chronic obstructive pulmonary disease (COPD), a population at risk for PH, radiographic pruning is associated with histologic vascular remodeling, higher pulmonary artery pressures, right ventricular dysfunction, and greater mortality (4–6). However, it is unknown whether radiographic pruning can predict poor clinical outcomes, including death, in general populations without high burdens of heart or lung disease. Using data from the Framingham Heart Study, we investigated the association of pulmonary vascular pruning on CT scan with all-cause mortality in a population not selected on the basis of any disease-related factors.
Methods
The study population consists of 2,470 participants of the Framingham Heart Study who underwent inspiratory noncontrast chest CT examination between 2008 and 2011. Using software based on the Chest Imaging Platform, three-dimensional vascular reconstructions were generated (Figure 1), from which the total volume of all intraparenchymal vessels (TBV) and of the small vessels (cross-sectional area of less than 5 mm2; BV5) were calculated (3). The small vessel fraction (BV5/TBV) represents the relative volume within the smallest, most peripheral vessels detectable by CT imaging and is an imaging surrogate of pulmonary vascular pruning; lower values indicate more severe pruning.
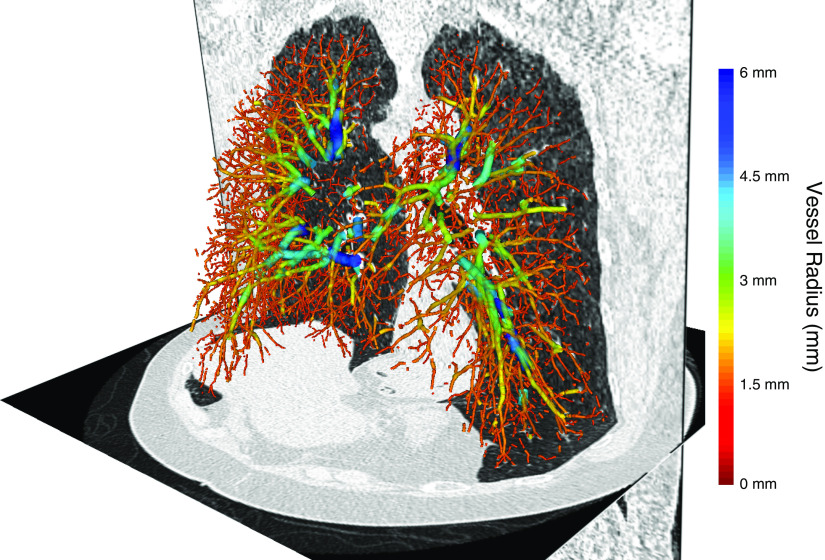
Figure 1.
Three-dimensional volumetric reconstruction of the pulmonary vascular tree from a Framingham Heart Study participant overlaid onto axial and coronal computed tomographic sections. Vessels are color coded by cross-sectional size.
Deaths were confirmed through review of death certificates, medical records, and/or information provided by family members. Participants were followed from the date of their CT scan through December 31, 2017.
We used multivariable Cox proportional hazards models to examine associations of CT-based pruning BV5/TBV (as a continuous exposure and by quartile) with all-cause mortality on follow-up. We adjusted for covariates selected a priori based on known or suspected associations with abnormalities of pulmonary vessels and/or risk of death. These included age, sex, height, weight, smoking status, pack-years of cigarette exposure, education, occupation, median neighborhood income, study cohort, any history of cardiovascular disease (including congestive heart failure, myocardial infarction, angina pectoris, and/or cerebrovascular accident), systolic/diastolic blood pressure, antihypertensive medication use, high-density lipoprotein concentration, low-density lipoprotein concentration, triglyceride concentration, statin use, glycated Hb concentration, antidiabetic medication use, FEV1, FVC, diffusing capacity for carbon monoxide, and visually evident emphysema or interstitial lung abnormality on CT scan.
We used multiple imputation by chained equations to impute data for covariates with missing information, using results from 15 imputed datasets to generate estimates for the hazard ratios (HRs). As a sensitivity analysis, we performed a complete case analysis for the 1,947 participants (78.8%) with complete data for every covariate.
Results
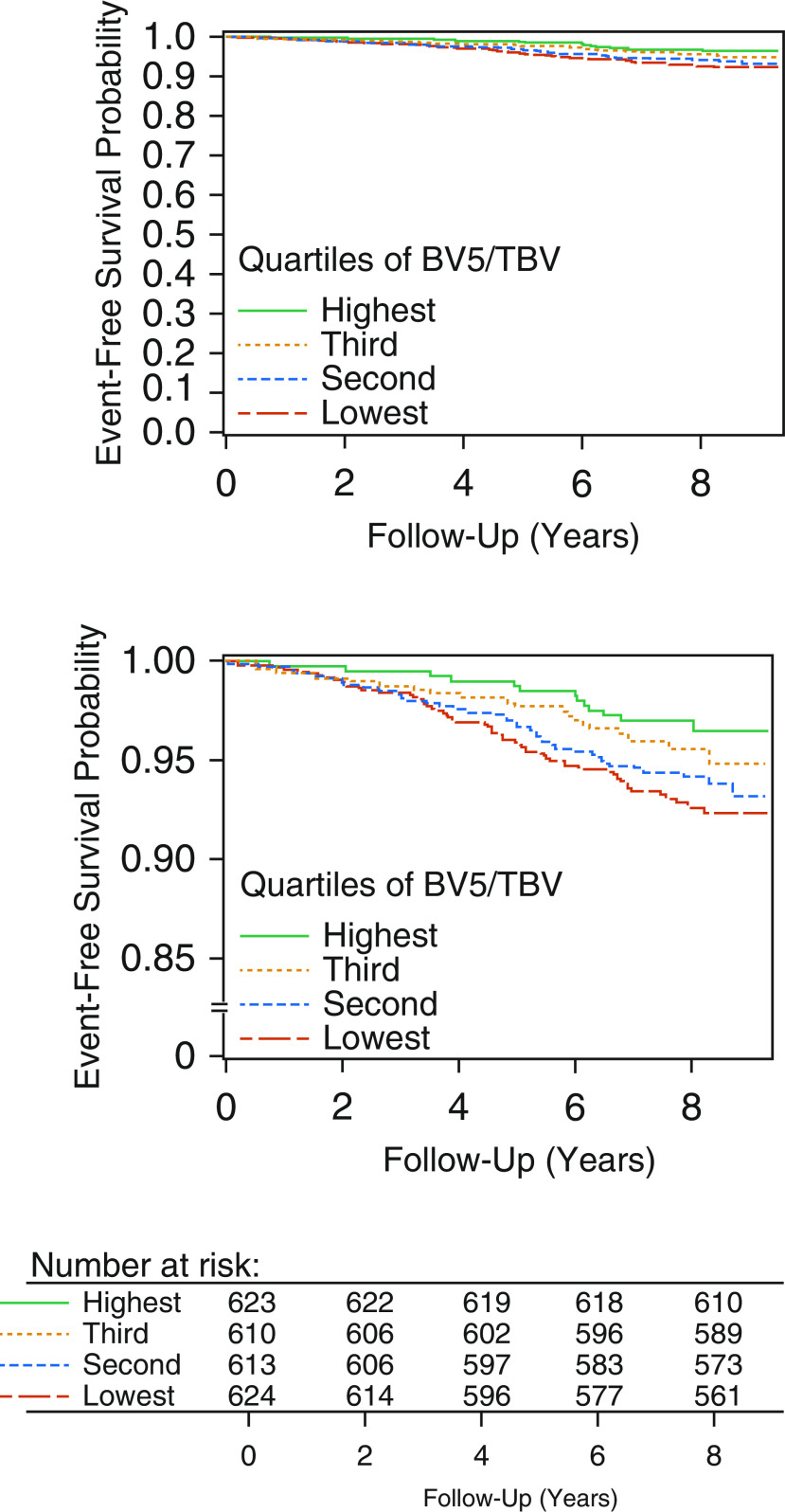
Roughly half of our cohort was female (51.1%), with a mean age of 59.3 ± 11.7 years. Nearly half the participants (48.2%) were never-smokers. There was a low prevalence of cardiovascular disease (6.8%) and COPD/emphysema (13.0%). Over 7.8 ± 1.2 years of follow-up, there were 137 deaths in the cohort, for an overall survival of 94.5%. In the adjusted models, we found that more severe CT pruning was associated with a higher rate of death. Each SD lower BV5/TBV (i.e., more severe pruning) was associated with a 35% greater rate of death (HR, 1.35; 95% confidence interval [CI], 1.09–1.68; P = 0.006). Similar results were seen when examining quartiles; the quartile of individuals with the most severe pruning had a 2.64-fold higher rate of death (95% CI, 1.30–5.39; P = 0.008) compared with those with the least amount of pruning (Figure 2), with a 4.3 percentage-point higher absolute adjusted mortality (7.9% vs. 3.6%) at the end of follow-up. The HR for death increased linearly across quartiles (P trend = 0.005). In those with complete data, CT pruning remained associated with greater mortality (HR, 1.54; 95% CI, 1.19–1.99; P = 0.001).
Figure 2.
Top shows adjusted survival curves by quartiles of pruning on computed tomographic imaging. Bottom is with truncated y-axis. Results are shown of multivariable Cox proportional hazards models. Imputation of missing data was performed using multiple imputation by chained equations with a total of 15 imputations. BV5 = total volume of small (cross-sectional area <5 mm2) vessels; TBV = total volume of all intraparenchymal vessels.
Discussion
In this large cohort of adults who were not selected on the basis of any disease and who had a low overall risk of death, we found that a quantitative CT-based measure of pulmonary vascular pruning was associated with greater all-cause mortality. This association was robust to adjustment for demographic factors and measures of cardiovascular and pulmonary health. These findings suggest that CT-based pruning may be a noninvasive marker of pulmonary vascular abnormalities that influence clinical outcomes, including death.
Although pulmonary vascular disease encompasses a heterogeneous set of disorders, certain shared histopathologic features of the small pulmonary vessels are observed regardless of etiology. These changes, which include vasoconstriction and intimal/medial hypertrophy (eventually resulting in luminal narrowing and vascular loss) may be represented and quantified as pruning on CT imaging. Prior studies in heavy smokers with COPD have shown that radiographic pruning is linked to structural histologic changes in the pulmonary vessels and to clinical measures, including higher pulmonary arterial pressures, right ventricular remodeling, and mortality (5–8).
Our results suggest that CT pruning may be an indicator of clinically significant pulmonary vasculopathy not just in those with lung disease who are at high risk for PH but also in more general populations who are at lower risk. These findings may be of relevance to screening protocols. Recent studies have found that even mild hemodynamic perturbations are associated with poor prognosis (9), but effective methods of screening for these early pulmonary vascular abnormalities remain lacking. Doppler echocardiography is widely used for noninvasive assessment of PH, but echocardiographic pressure estimation is operator dependent, requires the presence and appropriate measurement of a tricuspid regurgitation jet, and has limited diagnostic accuracy, particularly when hemodynamic disturbances are less severe (10). Furthermore, elevations in pulmonary artery pressures are a downstream consequence of vascular loss and may occur only after much of the pulmonary circulation has been occluded (10). Although our study is not designed to elucidate the underlying mechanisms of this association, our finding that pruning predicts mortality suggests that CT imaging may provide an opportunity to capture clinically meaningful changes in the small pulmonary vessels directly, perhaps before hemodynamically significant pulmonary vascular disease has developed. Additional research is necessary to clarify the precise underlying mechanisms of this association and to determine whether CT imaging may have a role in screening algorithms for pulmonary vascular disease.
In this study of generally healthy, community-dwelling adults, more severe pruning on CT imaging was associated with an increased risk of death. Further research is necessary to determine whether CT imaging—a widely used tool in current clinical practice—may have a role in screening algorithms for pulmonary vascular pathology in at-risk individuals.
Supplementary Material
Footnotes
Supported in part by the NHLBI (Framingham Heart Study contract N01-HC-25195 and HHSN268201500001I). This work was conducted with support from Harvard Catalyst, The Harvard Clinical and Translational Science Center, National Center for Research Resources and the National Center for Advancing Translational Sciences, NIH (award UL1 TR002541), and financial contributions from Harvard University and its affiliated academic healthcare centers. A.J.S. is supported by grants from the American Lung Association (CA-626548) and the NIH (grant F32 HL143819). R.S.J.E. is supported by the NIH (grants 1R01 HL116931 and R01 HL116473). G.R.W. is supported by the NIH (grants R01 HL122464 and R01 HL116473). M.A.M. reports grants from the NIH and U.S. Environmental Protection Agency during the conduct of the study. M.B.R. is supported by the National Institute for Environmental Health Sciences (grant K23 ES026204). The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, or the NIH.
Originally Published in Press as DOI: 10.1164/rccm.202005-1671LE on September 14, 2020
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Humbert M, Guignabert C, Bonnet S, Dorfmüller P, Klinger JR, Nicolls MR, et al. Pathology and pathobiology of pulmonary hypertension: state of the art and research perspectives. Eur Respir J. 2019;53:1801887. doi: 10.1183/13993003.01887-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Estépar RSJ, Kinney GL, Black-Shinn JL, Bowler RP, Kindlmann GL, Ross JC, et al. COPDGene Study. Computed tomographic measures of pulmonary vascular morphology in smokers and their clinical implications. Am J Respir Crit Care Med. 2013;188:231–239. doi: 10.1164/rccm.201301-0162OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Synn AJ, Li W, San José Estépar R, Zhang C, Washko GR, O’Connor GT, et al. Radiographic pulmonary vessel volume, lung function and airways disease in the Framingham Heart Study. Eur Respir J. 2019;54:1900408. doi: 10.1183/13993003.00408-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wells JM, Iyer AS, Rahaghi FN, Bhatt SP, Gupta H, Denney TS, et al. Pulmonary artery enlargement is associated with right ventricular dysfunction and loss of blood volume in small pulmonary vessels in chronic obstructive pulmonary disease. Circ Cardiovasc Imaging. 2015;8:e002546. doi: 10.1161/CIRCIMAGING.114.002546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Washko GR, Nardelli P, Ash SY, Vegas Sanchez-Ferrero G, Rahaghi FN, Come CE, et al. Arterial vascular pruning, right ventricular size and clinical outcomes in chronic obstructive pulmonary disease: a longitudinal observational study. Am J Respir Crit Care Med. 2019;200:454–461. doi: 10.1164/rccm.201811-2063OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsuoka S, Washko GR, Yamashiro T, Estepar RSJ, Diaz A, Silverman EK, et al. National Emphysema Treatment Trial Research Group. Pulmonary hypertension and computed tomography measurement of small pulmonary vessels in severe emphysema. Am J Respir Crit Care Med. 2010;181:218–225. doi: 10.1164/rccm.200908-1189OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Regan EA, Lynch DA, Curran-Everett D, Curtis JL, Austin JHM, Grenier PA, et al. Genetic Epidemiology of COPD (COPDGene) Investigators. Clinical and radiologic disease in smokers with normal spirometry. JAMA Intern Med. 2015;175:1539–1549. doi: 10.1001/jamainternmed.2015.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rahaghi FN, Argemí G, Nardelli P, Domínguez-Fandos D, Arguis P, Peinado VI, et al. Pulmonary vascular density: comparison of findings on computed tomography imaging with histology. Eur Respir J. 2019;54:1900370. doi: 10.1183/13993003.00370-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Douschan P, Kovacs G, Avian A, Foris V, Gruber F, Olschewski A, et al. Mild elevation of pulmonary arterial pressure as a predictor of mortality. Am J Respir Crit Care Med. 2018;197:509–516. doi: 10.1164/rccm.201706-1215OC. [DOI] [PubMed] [Google Scholar]
- 10.Lau EMT, Humbert M, Celermajer DS. Early detection of pulmonary arterial hypertension. Nat Rev Cardiol. 2015;12:143–155. doi: 10.1038/nrcardio.2014.191. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.