Abstract
Rationale: Although early antimicrobial discontinuation guided by procalcitonin (PCT) has shown decreased antibiotic consumption in lower respiratory tract infections, the outcomes in long-term sepsis sequelae remain unclear.
Objectives: To investigate if PCT guidance may reduce the incidence of long-term infection-associated adverse events in sepsis.
Methods: In this multicenter trial, 266 patients with sepsis (by Sepsis-3 definitions) with lower respiratory tract infections, acute pyelonephritis, or primary bloodstream infection were randomized (1:1) to receive either PCT-guided discontinuation of antimicrobials or standard of care. The discontinuation criterion was ≥80% reduction in PCT levels or any PCT ≤0.5 μg/L at Day 5 or later. The primary outcome was the rate of infection-associated adverse events at Day 180, a composite of the incidence of any new infection by Clostridioides difficile or multidrug-resistant organisms, or any death attributed to baseline C. difficile or multidrug-resistant organism infection. Secondary outcomes included 28-day mortality, length of antibiotic therapy, and cost of hospitalization.
Measurements and Main Results: The rate of infection-associated adverse events was 7.2% (95% confidence interval [CI], 3.8–13.1%; 9/125) versus 15.3% (95% CI, 10.1–22.4%; 20/131) (hazard ratio, 0.45; 95% CI, 0.20–0.98; P = 0.045); 28-day mortality 15.2% (95% CI, 10–22.5%; 19/125) versus 28.2% (95% CI, 21.2–36.5%; 37/131) (hazard ratio, 0.51; 95% CI, 0.29–0.89; P = 0.02); and median length of antibiotic therapy 5 (range, 5–7) versus 10 (range, 7–15) days (P < 0.001) in the PCT and standard-of-care arms, respectively. The cost of hospitalization was also reduced in the PCT arm.
Conclusions: In sepsis, PCT guidance was effective in reducing infection-associated adverse events, 28-day mortality, and cost of hospitalization.
Clinical trial registered with www.clinicaltrials.gov (NCT03333304).
Keywords: procalcitonin, sepsis, multidrug-resistant, mortality
At a Glance Commentary
Scientific Knowledge on the Subject
The procalcitonin (PCT)-guided discontinuation of antibiotic therapy was demonstrated to reduce antibiotic exposure in patients with lower respiratory tract infections and/or sepsis in several randomized trials. However, the effect on the incidence of infections by resistant microorganisms has not been studied.
What This Study Adds to the Field
The PROGRESS (Procalcitonin-guided Antimicrobial Therapy to Reduce Long-Term Sequelae of Infections) trial was designed as a real-world pragmatic trial, enrolling patients with sepsis. The trial demonstrated that PCT-guided antimicrobial treatment in sepsis was effective in reducing infection-associated adverse events like infections by multidrug-resistant organisms and Clostridioides difficile, as well as in-hospital and 28-day mortality. Generated evidence implicates that PCT guidance in sepsis is a safe strategy with long-term benefits that may have a substantial impact on public health, particularly for countries with high baseline antimicrobial consumption.
The U.S. CDC and the European Centre for Disease Prevention and Control have identified antimicrobial resistance as an alarming global public health threat (1). Antimicrobial resistance is associated with substantial morbidity, mortality, prolonged hospitalization, and increased medical costs owing to infections caused by multidrug-resistant organisms (MDROs) and Clostridioides difficile (2, 3). Prudent use of antimicrobials integrated with antibiotic stewardship programs is vital in overcoming emergence of antimicrobial resistance. A predictive biomarker indicating the risk of bacterial infections could guide the clinical assessment and aid in the decision-making process for judicious use of antimicrobials. In this regard, the use of a host-response marker, procalcitonin (PCT), has received ample scientific attention recently as an adjunct to clinical judgment (4).
The PCT-guided discontinuation of antibiotic therapy was demonstrated to reduce antibiotic exposure and the risk for adverse outcomes in patients with lower respiratory tract infections (LRTIs) compared with the standard of care (SOC) in several randomized trials (5−10). Additionally, data from meta-analyses of randomized clinical trials have confirmed the survival benefit of PCT-guided discontinuation of antibiotics (11−13). However, the mechanisms underlying this survival benefit are yet to be clarified.
Long-term use of antibiotics causes substantial damage to the gut flora, increasing the risk of infections caused by C. difficile and MDROs in critically ill patients, which are associated with poor clinical outcomes (14, 15). We conducted the PROGRESS (Procalcitonin-guided Antimicrobial Therapy to Reduce Long-Term Sequelae of Infections) trial to investigate whether the PCT-guided early discontinuation of antibiotic therapy would reduce the incidence of adverse events associated with these long-term infection sequelae in patients with sepsis that are driven by the prolonged use of antibiotics. These include death by MDROs and acquisition of infections by C. difficile and MDROs.
Some of the results have been previously reported in the form of an abstract (https://www.escmid.org/escmid_publications/eccmid_abstract_book/).
Methods
Participants
Enrolled patients were adults hospitalized with LRTIs (community, hospital-acquired, or ventilator-associated), acute pyelonephritis, or primary bloodstream infection and meeting the Sepsis-3 definitions (16) (see online supplement). PROGRESS was conducted in seven departments of Internal Medicine (National Ethics Committee approval 62/17; National Organization for Medicines approval IS-62/17). Exclusion criteria were need of prolonged treatment, viral or parasite infections, tuberculosis, cystic fibrosis, neutropenia, infection by HIV with low CD4 count, and pregnancy or lactation. Written informed consent was provided by the patient or legal representative before enrollment.
Procedures
The first 24 hours from the start of antibiotics, patients were 1:1 randomized into the PCT-guidance arm or the SOC arm by a generated list per site kept in a sealed envelope until randomization. Patients and investigators were aware of treatment assignment.
Attending physicians prescribed antimicrobials according to European and national guidelines (17). Blood samples were collected at baseline and on Day 5 for procalcitonin measurements using the VIDAS assay (lower detection limit 0.05 μg/L; bioMérieux). Antibiotics were discontinued if PCT was reduced by at least 80% or if it was <0.5 μg/L. When the rule did not apply, blood sampling was repeated daily and antibiotics were discontinued when the rule was met. Exceptions were allowed for medically unstable patients defined as febrile and/or requiring vasopressors. For patients in the SOC arm, the investigators were unaware of PCT kinetics and the duration of antimicrobial treatment was decided according to international guidelines (18).
Stool samples of 0.5 g were collected at baseline and on follow-up Days 7, 28, and 180 to detect C. difficile and MDRO colonization (see online supplement).
Outcomes
The rate of infection-associated adverse events until Day 180 was the primary outcome. This was an endpoint composed of any of the following: new case of C. difficile infection; new case of MDRO infection; and death associated with either MDROs or C. difficile baseline infection (see online supplement).
The time until the incidence of the first infection episode by MDROs or C. difficile during follow-up was recorded. Secondary endpoints were the time until the first new infection, the length of antibiotic therapy (LOT), 28-day and 180-day mortality, and cost of hospitalization.
Data were captured by investigators blinded to the allocation group. Discharged patients were followed up monthly by phone calls; if their health status had changed, outpatient clinical assessment was performed. Study was monitored for serious and nonserious adverse events (see online supplement).
Statistical Analysis
The sample size was calculated assuming the primary outcome would decrease from 30% in the SOC to 15% in the PCT arm. To achieve so with 80% power at the 5% level of significance, 133 patients were calculated in each arm. Predefined analysis was done among the intention-to-treat population using the Fisher’s exact test and confirmatory forward stepwise Cox analysis (IBM SPSS Statistics v. 25.0). Sensitivity analyses were conducted for the effect of early death, protocol compliance, and extreme LOT. Any two-sided P value <0.05 was statistically significant. Adjustment for multiple comparisons was not performed because the endpoints were predefined (19).
Results
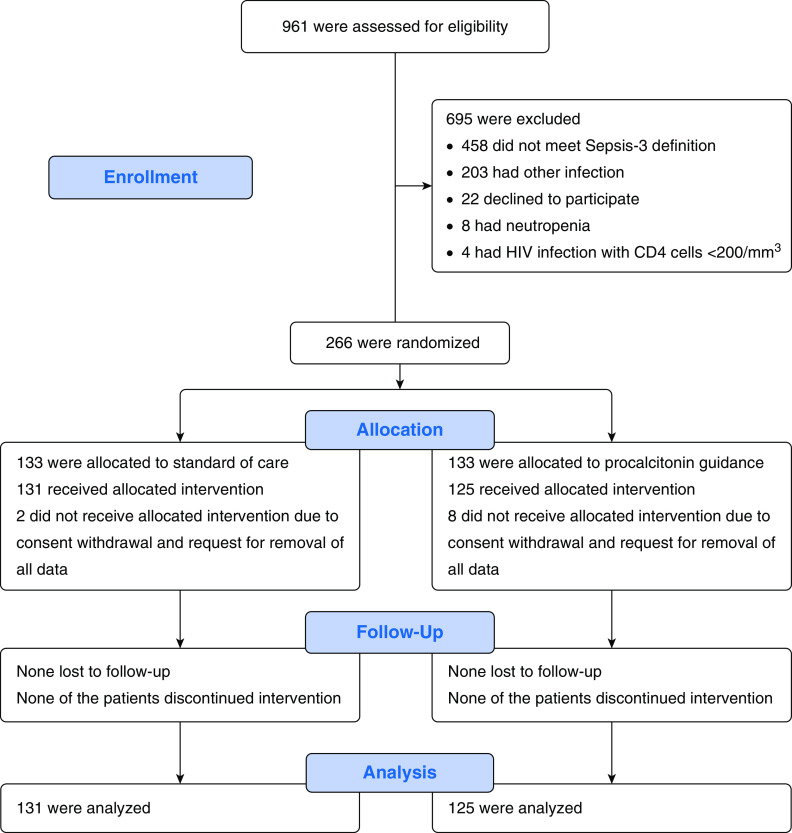
From November 2017 through January 2019, 266 patients were enrolled and randomized; 10 patients withdrew consent before Day 5 and requested removal of all data, leaving a final intention-to-treat analysis cohort of 256 patients. No patient was reported as lost to follow-up (Figure 1). Baseline characteristics were similar between the two arms (Tables 1 and E1 in the online supplement). Τhe serum levels of C-reactive protein and PCT were not significantly different on Day 5 when intervention was started (Table E2). In total, 109 patients in the PCT arm met the predefined criteria for antimicrobial discontinuation: 89 patients on Day 5 and another 20 patients in the next days. However, antimicrobials were discontinued in 96 patients (76.8%) because 13 (10.4%) were considered medically unstable. Bacterial pathogens were isolated at baseline among 105 patients: 56 in the SOC arm and 49 in the PCT arm; 98.2% and 98.0%, respectively, were treated with an antibiotic active against the pathogens. These percentages signify the antimicrobial activity of the administered antibiotics and not their appropriateness from the antimicrobial policy point of view.
Figure 1.
Trial profile.
Table 1.
Baseline Characteristics of Enrolled Patients
| Standard of Care (n = 131) | PCT Guidance (n = 125) | All Patients (N = 256) | |
|---|---|---|---|
| Age, mean (SD), yr | 78.0 (13.1) | 79.6 (9.8) | 78.6 (11.6) |
| Sex, M, n (%) | 62 (45.8) | 52 (40.8) | 114 (44.5) |
| Charlson’s comorbidity index, mean (SD) | 6.0 (2.4) | 5.6 (1.9) | 5.8 (2.2) |
| Septic shock, n (%) | 9 (6.9) | 9 (7.2) | 18 (7.0) |
| ΑPACHE II score, mean (SD) | 13.3 (4.7) | 13.0 (4.6) | 13.2 (4.7) |
| SOFA score, mean (SD) | 4.1 (2.2) | 4.1 (2.1) | 4.1 (2.2) |
| Procalcitonin, median (Q1–Q3), μg/L | 0.53 (0.15–5.03) | 0.86 (0.17–5.95) | 0.65 (0.17–5.77) |
| Meeting stopping rule on Day 5, n (%) | |||
| ≥80% decrease of initial serum PCT | 32 (24.4) | 35 (28.0) | 67 (26.2) |
| Serum PCT <0.5 μg/L | 74 (56.5) | 71 (56.8) | 145 (56.6) |
| Combined criteria | 92 (70.2) | 89 (71.2) | 181 (70.7) |
| Meeting stopping rule after Day 5*, n (%) | N/A | 20 (16.0) | N/A |
| Noncompliance with PCT stopping rule because of medical instability, n (%) | N/A | 13 (10.4) | N/A |
| Overall compliance with PCT stopping rule, n (%) | N/A | 96 (76.8) | N/A |
| Type of infection, n (%) | |||
| Community-acquired pneumonia | 57 (43.5) | 55 (44.0) | 112 (43.8) |
| Healthcare-associated pneumonia | 27 (20.6) | 16 (12.8) | 43 (16.8) |
| Acute pyelonephritis | 44 (33.6) | 51 (40.8) | 95 (37.1) |
| Primary bloodstream infection | 1 (0.8) | 2 (1.6) | 3 (1.2) |
| Hospital-acquired pneumonia | 2 (1.5) | 1 (0.8) | 3 (1.2) |
| Microbiological documentation, n (%) | |||
| E. coli | 22 (16.7) | 24 (19.2) | 46 (18.0) |
| K. pneumoniae | 7 (5.3) | 5 (4.0) | 12 (4.7) |
| P. aeruginosa | 2 (1.5) | 1 (0.8) | 3 (1.2) |
| S. pneumoniae | 8 (6.1) | 4 (3.2) | 12 (4.7) |
| H. influenzae | 8 (6.7) | 6 (5.0) | 14 (5.5) |
| S. aureus | 3 (2.3) | 3 (2.4) | 6 (2.3) |
| C. difficile | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Other | 6 (4.6) | 9 (7.2) | 15 (5.9) |
| Multidrug-resistant pathogen | 6 (4.6) | 4 (3.2) | 10 (3.9) |
| Extensively drug-resistant | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Pandrug-resistant | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Positive blood culture, n (%) | 23 (17.6) | 19 (15.2) | 42 (16.4) |
| Empiric treatment according to ESCMID guidelines, n (%)† | 112 (85.5) | 103 (82.4) | 215 (84.0) |
Definition of abbreviations: APACHE = Acute Physiology and Chronic Health Evaluation; C. difficile = Clostridioides difficile; E. coli = Escherichia coli; ESCMID = European Society of Clinical Microbiology and Infectious Diseases; H. influenzae = Haemophilus influenzae; K. pneumoniae = Klebsiella pneumoniae; N/A = not applicable; P. aeruginosa = Pseudomonas aeruginosa; PCT = procalcitonin; Q = quartile; S. aureus = Staphylococcus aureus; SOFA = Sequential Organ Failure Assessment; S. pneumoniae = Streptococcus pneumoniae.
Days 6 and 7.
Attending physicians and/or primary investigators were infectious diseases specialists, members of the ESCMID following the ESCMID guidelines for antimicrobial treatment. In addition, the Hellenic Society for Chemotherapy, incorporating European guidelines, has published in cooperation with the National Organization for Medicines an issues-tool, namely, “Guide of antimicrobial stewardship for the hospitalized patient and for primary care,” handed out in all Greek hospitals and also available online (17).
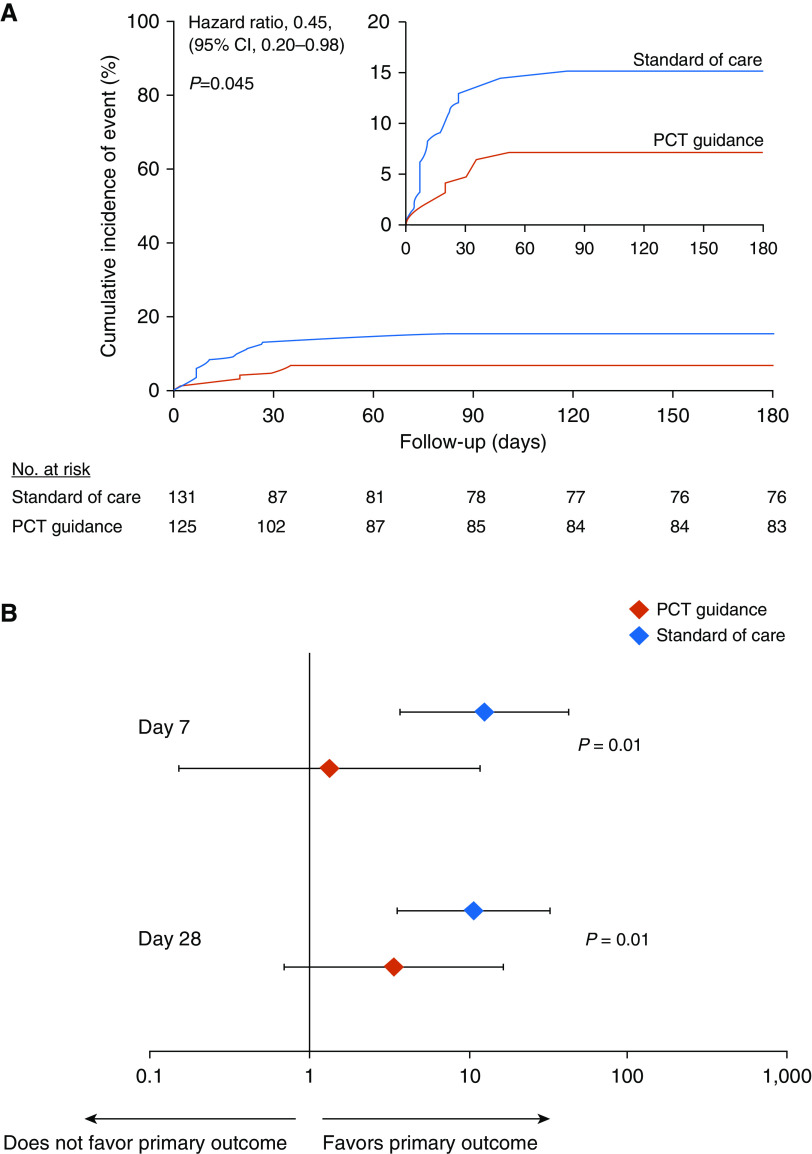
In the intention-to-treat population, the primary outcome of infection-associated adverse events at Day 180 developed in 9 of 125 patients (7.2%; 95% confidence interval [CI], 3.8–13.1%) in the PCT-guidance group compared with 20 of 131 patients (15.3%; 95% CI, 10.1–22.4%) in the SOC group (hazard ratio, 0.45; 95% CI, 0.20–0.98; P = 0.045) (Figure 2A and Table 2). Stepwise Cox regression analysis showed that PCT guidance was an independent protective factor for infection-associated adverse events at Day 180 (hazard ratio, 0.38; 95% CI, 0.17–0.85; P = 0.01) (Tables 3 and E3). Three sensitivity analyses were done among patients who survived at least 5 days to receive the allocated intervention; among patients who were completely compliant to the allocated treatment; and among patients without extremely prolonged LOT. All three analyses confirmed the findings (Table E4).
Figure 2.
Study primary outcome and the effect of fecal colonization in the intention-to-treat population. (A) Kaplan-Meier curve for primary outcome. The study primary outcome was the rate of infection-associated adverse events for patients allocated to the PCT-guidance group compared with the standard of care after 180 days. Infection-associated adverse events were a composite outcome comprising the advent of any of the following: new case of Clostridioides difficile infection; new case of infection by multidrug-resistant organisms (MDROs); and death due to baseline infection by MDROs or C. difficile. The inset shows the same data on an enlarged y-axis. (B) Odds ratios of reaching or not the primary outcome in dependence of presence or absence of fecal colonization by C. difficile or MDROs by Days 7 and 28. P values of the interaction effect of the arm of treatment by colonization on primary outcome are provided. CI = confidence interval; PCT = procalcitonin.
Table 2.
Primary and Secondary Study Outcomes
| Standard of Care (n = 131) | PCT Guidance (n = 125) | Odds Ratio (95% CI) | P Value | |
|---|---|---|---|---|
| Infection-associated adverse events until Day 180, n (%) | 20 (15.3) | 9 (7.2) | 0.43 (0.19–0.99) | 0.045 |
| New infection by MDROs until Day 180, n (%) | 8 (6.1) | 5 (4.0) | 0.64 (0.20–2.01) | 0.57 |
| New infection by C. difficile until Day 180, n (%) | 12 (9.2) | 6 (4.8) | 0.50 (0.18–1.38) | 0.22 |
| Mortality associated with baseline infection by MDROs, n (%) | 5 (3.8) | 1 (0.8) | 0.20 (0.02–1.76) | 0.21 |
| In-hospital mortality, n (%) | 33 (25.2) | 17 (13.6) | 0.47 (0.25–0.89) | 0.03 |
| 28-d mortality, n (%) | 37 (28.2) | 19 (15.2) | 0.46 (0.26–0.85) | 0.02 |
| 180-d mortality, n (%) | 50 (38.2) | 38 (30.4) | 0.71 (0.42–1.19) | 0.24 |
| Antimicrobial treatment duration, median (Q1–Q3), d | 10 (7–15) | 5 (5–7) | N/A | <0.001 |
| Cost of hospitalization, median (Q1–Q3), € | 1,183.49 (718.98–2,011.57) | 956.99 (725.02–1,355.90) | N/A | 0.05 |
| Fecal colonization by Day 180, n (%) | ||||
| C. difficile | 13 (9.9) | 14 (11.2) | 1.15 (0.52–2.54) | 0.84 |
| MDROs | 13 (9.9) | 15 (13.3) | 1.22 (0.55–2.70) | 0.69 |
Definition of abbreviations: C. difficile = Clostridioides difficile; CI = confidence interval; MDROs = multidrug-resistant organisms; N/A = not applicable; PCT = procalcitonin; Q = quartile.
Bold indicates any P < 0.05.
Table 3.
PCT Guidance as an Independent Protective Factor from Development of IAAEs by Day 180
| Parameters | IAAE (−) (n = 227) | IAAE (+) (n = 29) | Univariate Analysis |
Multivariate Analysis |
||
|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |||
| PCT guidance | 116 (51.1) | 9 (31.0) | 0.45 (0.20–0.98) | 0.045 | 0.38 (0.17–0.85) | 0.01 |
| Dementia | 54 (23.8) | 16 (55.2) | 3.53 (1.70–7.34) | 0.001 | 4.30 (2.02–9.14) | <0.001 |
| Residency in healthcare facilities | 14 (6.2) | 6 (20.7) | 3.56 (1.45–8.74) | 0.006 | — | — |
| Hospitalization in last 3 mo | 39 (17.2) | 10 (34.5) | 2.47 (1.15–5.31) | 0.02 | — | — |
| SOFA score >4* | 74 (32.6) | 15 (51.7) | 2.09 (1.01–4.34) | 0.05 | — | — |
| Community-acquired pneumonia | 105 (46.3) | 7 (24.1) | 0.39 (0.17–0.90) | 0.03 | — | — |
| Primary or secondary bacteremia | 31 (13.7) | 11 (37.9) | 3.52 (1.66–7.45) | 0.001 | 2.93 (1.34–6.43) | 0.01 |
| Septic shock | 13 (5.7) | 5 (17.2) | 3.17 (1.21–8.32) | 0.02 | — | — |
| Empiric treatment according to the ESCMID guidelines | 86 (37.9) | 17 (58.6) | 2.25 (1.07–4.70) | 0.001 | — | — |
| Intake of cefepime | 3 (1.3) | 3 (10.3) | 5.58 (1.68–18.51) | 0.005 | — | — |
| Intake of piperacillin/tazobactam | 122 (53.7) | 24 (82.8) | 3.81 (1.45–9.98) | 0.007 | — | — |
| Intake of a carbapenem | 27 (11.9) | 10 (34.5) | 3.37 (1.57–7.26) | 0.002 | 2.91 (1.29–6.52) | 0.01 |
| Intake of a glycopeptide | 35 (15.4) | 10 (34.5) | 2.66 (1.24–5.72) | 0.01 | — | — |
| Intake of fluconazole | 8 (3.5) | 5 (17.2) | 4.46 (1.70–11.70) | 0.002 | — | — |
Definition of abbreviations: CI = confidence interval; ESCMID = European Society of Clinical Microbiology and Infectious Diseases; HR = hazard ratio; IAAEs = infection-associated adverse events; PCT = procalcitonin; SOFA = Sequential Organ Failure Assessment.
Univariate and multivariate (Cox Forward Conditional) models for the intention-to-treat population (N = 256) are presented. Only parameters reaching statistical significance are provided. All baseline parameters that may act as confounders for the primary outcome were tested in univariate models (shown in Table E3). All 14 parameters reaching statistical significance in univariate models were subsequently entered in a multivariate model. Statistical probability threshold for entering the model was set at 0.05 and for removal at 0.10. Data are shown as n (%).
Cutoff value was calculated by the Youden’s Index of the respective receiver operator characteristics curve of the score in relation to primary outcome.
In the SOC arm, the patients’ risk to reach the primary outcome was higher among those colonized by C. difficile or MDROs compared with noncolonized patients. This was shown by the results of the fecal colonization tests of both Days 7 and 28 (odds ratio, 12.6; 95% CI, 3.7–42.8; P < 0.001 for Day 7; and 10.8; 95% CI, 3.6–32.5; P = 0.003 for Day 28). However, in the PCT-guidance arm, the risk to reach the primary outcome was not different by the presence or absence of colonization on Days 7 and 28 (odds ratio, 1.3; 95% CI, 0.1–11.6; P = 0.59; and 3.4; 95% CI, 0.7–16.4; P = 0.14, respectively) (Figure 2B).
Major benefit from PCT guidance was observed in three main secondary endpoints: 28-day mortality, LOT, and cost of hospitalization (Table 2). More precisely, 28-day mortality was lower in the PCT-guidance arm compared with the SOC arm (15.2% [19/125 patients] vs. 28.2% [37/131 patients]; hazard ratio, 0.51; 95% CI, 0.29–0.89; P = 0.02). PCT guidance was also an independent protective factor from death after 28 days (hazard ratio, 0.51; 95% CI, 0.29–0.89; P = 0.02) (Figure 3 and Tables E5 and E6). A trend for decreased 180-day mortality was shown in the PCT-guidance arm (30.4%) compared with SOC (38.2%), but it did not reach statistical significance (hazard ratio, 0.71; 95% CI, 0.42–1.19; P = 0.24). Median LOT was 10 days in the SOC arm compared with 5 days in the PCT-guidance arm (P < 0.001); this reduction in the LOT was observed irrespective of the type of underlying infection (Figures E1 and E2). Furthermore, the median hospital stay was shorter in the PCT-guidance arm (Figure E3).
Figure 3.
Kaplan-Meier curve for 28-day survival in the intention-to-treat population. The inset shows the same data on an enlarged y-axis. CI = confidence interval; PCT = procalcitonin.
The median cost of hospitalization was estimated to be €1,183.49 per patient in the SOC arm and €956.99 in the PCT-guidance arm (P = 0.05) (Table 2). This difference was mainly due to the decrease in the consumption of drugs (Figure E4).
Exploratory analysis showed that treatment with at least two broad-spectrum antibiotics was associated with the highest colonization rate. Analyzed antibiotics were piperacillin/tazobactam, ceftolozane/tazobactam, carbapenems, tigecycline, and amikacin. Treatment with any two was associated with higher colonization by MDROs on Days 7 and 28 in the SOC arm but not in the PCT-guidance arm (Figure E5).
The incidence of antimicrobial-associated adverse events, particularly diarrhea and acute kidney injury, was lower in the PCT-guidance arm (Table 4 and Figure E6A). These adverse events developed earlier in the SOC arm (Figures E6B and E6C). Moreover, the incidence of serious adverse events did not differ and none of the serious adverse events were related to the study.
Table 4.
Adverse Events
| Standard of Care (n = 131) | PCT Guidance (n = 125) | Odds Ratio (95% CI) | P Value | |
|---|---|---|---|---|
| At least one serious adverse event by Day 180, n (%) | 71 (54.2) | 68 (54.4) | 1.01 (0.62–1.65) | >0.99 |
| More than two serious adverse events, n (%) | 8 (6.1) | 1 (0.8) | 0.12 (0.02–0.99) | 0.04 |
| Type of serious adverse event, n (%)* | ||||
| Death by Day 28 | 37 (28.2) | 19 (15.2) | 0.46 (0.26–0.85) | 0.02 |
| Death by Day 180 | 50 (38.2) | 38 (30.4) | 0.71 (0.42–1.19) | 0.24 |
| Rehospitalization by Day 28, n/total n of discharged patients | 9/98 (9.2) | 9/108 (8.3) | 1.02 (0.38–2.76) | >0.99 |
| Rehospitalization by Day 180, n/total n of discharged patients | 23/98 (23.5) | 34/108 (31.5) | 1.50 (0.81–2.78) | 0.22 |
| Rehospitalization due to infection by Day 180, n/total n of discharged patients | 16/98 (16.3) | 20/108 (18.5) | 1.17 (0.57–2.40) | 0.72 |
| Extended hospitalization, n (%) | 17 (13.0) | 11 (8.8) | 0.65 (0.29–1.44) | 0.32 |
| Life-threatening event, n (%) | 8 (6.1) | 4 (3.2) | 0.51 (0.15–1.73) | 0.38 |
| At least one adverse event, n (%) | 83 (63.4) | 64 (51.2) | 0.61 (0.37–0.99) | 0.05 |
| More than two adverse events, n (%) | 20 (15.3) | 6 (4.8) | 0.28 (0.11–0.72) | 0.007 |
| Type of adverse event, n (%) | ||||
| Diarrhea | 48 (36.6) | 24 (19.2) | 0.41 (0.23–0.73) | 0.002 |
| Acute kidney injury | 23 (17.6) | 9 (7.2) | 0.36 (0.16–0.82) | 0.01 |
| Nonserious organ-threatening adverse event (electrolyte disorder, elevated liver function tests, arrhythmia) | 47 (35.9) | 20 (16.0) | 0.36 (0.20–0.66) | <0.001 |
Definition of abbreviations: CI = confidence interval; PCT = procalcitonin.
Bold indicates any P < 0.05.
None of the serious adverse events were judged by the site principal investigator as study related.
Discussion
In this multicenter randomized trial, we found that early discontinuation of antimicrobial therapy guided by a PCT measurement below <0.5 μg/L or a reduction of at least 80% from the baseline at Day 5 or later significantly reduced the rate of infection-associated adverse events. Following the PCT-guidance approach, the length of antibiotic therapy was reduced and there were survival benefits in terms of reduction in both in-hospital and 28-day mortality reflecting a direct impact on all baseline infections.
In the PROGRESS trial, we demonstrate for the first time that PCT-guided early discontinuation of antimicrobials in patients with sepsis prevents infection caused by MDROs and/or C. difficile. Two important findings of this trial could be attributed to observed clinical benefits. First, the rate of gut colonization by MDROs and C. difficile did not differ between the two groups on Days 7 and 28, and second, the risk to develop an infection-associated adverse event was significantly higher in colonized patients in the SOC arm but not in the PCT-guidance arm. These results indicate that despite initial colonization after exposure to antimicrobials, early discontinuation of antimicrobials in the PCT-guidance arm did not allow development of clinical infection. However, long-term antibiotic exposure to gut microbiota in the SOC arm could either affect the integrity of the mucosal barrier or modulate the composition of the gut microbiota and explain the increased incidence of infections by MDROs and C. difficile.
Our study reveals that the use of PCT-guided early discontinuation of antimicrobials led to reduction in 28-day mortality, early discharge from hospital, and lower hospitalization cost. The results of this study are consistent with those reported by de Jong and colleagues (9). Although PCT was measured daily in the SAPS trial, the stopping rule of antibiotics was similar to our study. It needs to be emphasized that the compliance to the stopping rule in the PROGRESS trial was the highest (76.8%) among all trials conducted so far studying the PCT-guided early stop of antibiotics. Participants of the SAPS trial were hospitalized in an ICU. This did not happen in our trial, where patients, although septic, received treatment in the wards under advanced supportive care. This is happening because of the shortage of ICU beds in our country. The results of the SAPS (Stop Antibiotics on Guidance of Procalcitonin Study) trial showed that 28-day mortality was 25.0% in the SOC arm and 20.0% in the PCT arm; however, the authors did not provide any explanation for their findings. PROGRESS study analysis provides a clear-cut explanation for the 28-day survival benefit related to the PCT algorithm. The inappropriate or irrational use of antibiotics predisposes patients with sepsis to organ dysfunction (20, 21). In particular, diarrhea, often leads to electrolyte disturbances, dehydration, cardiovascular instability, and acute kidney injury; all these consequences can be severe and life-threatening, especially in cases of C. difficile infection (22, 23). The results of the PROGRESS trial are consistent with these observations showing a higher incidence of diarrhea, acute kidney injury, electrolyte disorders, elevated liver enzymes, and arrythmia in the SOC arm versus the PCT-guidance arm. From these findings, we postulate that survival benefits offered by PCT guidance could be due to reduction in these antibiotic-associated events. Although the overall incidence of the adverse events was reduced, a similar decrease was not found for the incidence of at least one serious adverse event probably because not all adverse events met the definition of seriousness.
Our findings are in agreement with those of previous studies conducted in patients with LRTIs with or without sepsis that demonstrated that the use of PCT guidance may effectively shorten the duration of antibiotic treatment (5−10, 24−31). Based on this evidence, the U.S. Food and Drug Administration approved the use of PCT guidance for early discontinuation of antibiotics in LRTIs (32). The ProACT (Procalcitonin Antibiotic Consensus Trial) trial failed to replicate these results (33). In this trial, 834 patients were allocated to the SOC arm and 830 patients to the arm of early stop of antibiotics guided by PCT. It should, however, be emphasized that the median length of therapy in the SOC arm was only 4.4 days, raising considerations that this was already too short to allow for the PCT guidance to further shorten this. Importantly, the cohort of the PROGRESS trial was not limited to patients with LRTIs but also enrolled patients with acute pyelonephritis. This indicates that the use of PCT as a surrogate tool for early discontinuation of antimicrobials could be broadened to critically ill patients, particularly for those who are treated with broad-spectrum antibiotics and exert a strong selection pressure, as this was the case for the majority of patients enrolled in our trial. Moreover, the decrease in hospitalization cost is another benefit, which strongly argues in favor of implementing PCT guidance.
The main strengths of the PROGRESS trial are the inclusion of patients meeting the Sepsis-3 definitions, the study of infections other than LRTIs, and the study of infection-associated adverse events as an endpoint that has never been studied so far. The main limitations are the limited stool sampling and the generalizability of the results. More precisely, a large window of stool sampling between Days 28 and 180 was left, making the changes in microbiota difficult to interpret. The effect of this large window can barely be covered even with stool microbiome analysis. PROGRESS is an open-label trial that was conducted in a country with high antimicrobial consumption and high antimicrobial resistance. This should be interpreted in the light of emerging infections by MDROs and C. difficile as a worldwide calamity.
Conclusions
The use of PCT guidance for early discontinuation of antimicrobials in medically stable and afebrile patients with sepsis demonstrated significant clinical benefits. The PCT-guidance approach was associated with lower infection-associated adverse events, lower 28-day mortality, shorter LOT, early hospital discharge, and decreased costs of hospitalization. These benefits may have substantial impact on public health, particularly for countries with high antimicrobial consumption.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank the patients, their families, and the clinical, laboratory, and research staff who contributed to the trial as well as CBCC Global Research for editorial support in the development of this manuscript.
Footnotes
Supported by grants from the Hellenic Institute for the Study of Sepsis and by bioMérieux. Procalcitonin assays, material for detection of fecal colonization, and laboratory training were provided by bioMérieux.
Author Contributions: E.J.G.-B. conceptualized the study design, participated in data analysis and drafting the manuscript, and had full access to all of the study data and takes responsibility for their integrity and the accuracy of the analysis. E.K. participated in data analysis, drafted the manuscript, and had full access to all of the study data and takes responsibility for their integrity and the accuracy of the analysis. M. Kyprianou performed data analysis and revised the manuscript for important intellectual content. L.L.-A., G.A., A.P., N.M., E.D., K.M., G.C., A.S., N.A., S.S., Z.A., S.L., V.K., T.G., I.A., G.P., M.L., A.M., E.R., M. Koupetori, V.A., D.P., T.N., and A.A. recruited patients in this study, collected data, and revised the manuscript for important intellectual content. All authors gave final approval for the version to be published.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202004-1201OC on August 6, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.European Centre for Disease Prevention and Control. US CDC report on antibiotic resistance threats in the United States. 2013 ECDC comment. [accessed 2019 Sep 23]. Available from: https://ecdc.europa.eu/en/news-events/us-cdc-report-antibiotic-resistance-threats-united-states-2013.
- 2.Peters L, Olson L, Khu DTK, Linnros S, Le NK, Hanberger H, et al. Multiple antibiotic resistance as a risk factor for mortality and prolonged hospital stay: a cohort study among neonatal intensive care patients with hospital-acquired infections caused by gram-negative bacteria in Vietnam. PLoS One. 2019;14:e0215666. doi: 10.1371/journal.pone.0215666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cosgrove SE. The relationship between antimicrobial resistance and patient outcomes: mortality, length of hospital stay, and health care costs. Clin Infect Dis. 2006;42:S82–S89. doi: 10.1086/499406. [DOI] [PubMed] [Google Scholar]
- 4.Schuetz P, Beishuizen A, Broyles M, Ferrer R, Gavazzi G, Gluck EH, et al. Procalcitonin (PCT)-guided antibiotic stewardship: an international experts consensus on optimized clinical use. Clin Chem Lab Med. 2019;57:1308–1318. doi: 10.1515/cclm-2018-1181. [DOI] [PubMed] [Google Scholar]
- 5.Schuetz P, Christ-Crain M, Thomann R, Falconnier C, Wolbers M, Widmer I, et al. ProHOSP Study Group. Effect of procalcitonin-based guidelines vs standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA. 2009;302:1059–1066. doi: 10.1001/jama.2009.1297. [DOI] [PubMed] [Google Scholar]
- 6.Bouadma L, Luyt CE, Tubach F, Cracco C, Alvarez A, Schwebel C, et al. PRORATA trial group. Use of procalcitonin to reduce patients’ exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet. 2010;375:463–474. doi: 10.1016/S0140-6736(09)61879-1. [DOI] [PubMed] [Google Scholar]
- 7.Christ-Crain M, Jaccard-Stolz D, Bingisser R, Gencay MM, Huber PR, Tamm M, et al. Effect of procalcitonin-guided treatment on antibiotic use and outcome in lower respiratory tract infections: cluster-randomised, single-blinded intervention trial. Lancet. 2004;363:600–607. doi: 10.1016/S0140-6736(04)15591-8. [DOI] [PubMed] [Google Scholar]
- 8.Christ-Crain M, Stolz D, Bingisser R, Müller C, Miedinger D, Huber PR, et al. Procalcitonin guidance of antibiotic therapy in community-acquired pneumonia: a randomized trial. Am J Respir Crit Care Med. 2006;174:84–93. doi: 10.1164/rccm.200512-1922OC. [DOI] [PubMed] [Google Scholar]
- 9.de Jong E, van Oers JA, Beishuizen A, Vos P, Vermeijden WJ, Haas LE, et al. Efficacy and safety of procalcitonin guidance in reducing the duration of antibiotic treatment in critically ill patients: a randomised, controlled, open-label trial. Lancet Infect Dis. 2016;16:819–827. doi: 10.1016/S1473-3099(16)00053-0. [DOI] [PubMed] [Google Scholar]
- 10.Nobre V, Harbarth S, Graf JD, Rohner P, Pugin J. Use of procalcitonin to shorten antibiotic treatment duration in septic patients: a randomized trial. Am J Respir Crit Care Med. 2008;177:498–505. doi: 10.1164/rccm.200708-1238OC. [DOI] [PubMed] [Google Scholar]
- 11.Schuetz P, Wirz Y, Sager R, Christ-Crain M, Stolz D, Tamm M, et al. Effect of procalcitonin-guided antibiotic treatment on mortality in acute respiratory infections: a patient level meta-analysis. Lancet Infect Dis. 2018;18:95–107. doi: 10.1016/S1473-3099(17)30592-3. [DOI] [PubMed] [Google Scholar]
- 12.Schuetz P, Chiappa V, Briel M, Greenwald JL. Procalcitonin algorithms for antibiotic therapy decisions: a systematic review of randomized controlled trials and recommendations for clinical algorithms. Arch Intern Med. 2011;171:1322–1331. doi: 10.1001/archinternmed.2011.318. [DOI] [PubMed] [Google Scholar]
- 13.Wirz Y, Meier MA, Bouadma L, Luyt CE, Wolff M, Chastre J, et al. Effect of procalcitonin-guided antibiotic treatment on clinical outcomes in intensive care unit patients with infection and sepsis patients: a patient-level meta-analysis of randomized trials. Crit Care. 2018;22:191. doi: 10.1186/s13054-018-2125-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zilahi G, Artigas A, Martin-Loeches I. What’s new in multidrug-resistant pathogens in the ICU? Ann Intensive Care. 2016;6:96. doi: 10.1186/s13613-016-0199-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karanika S, Paudel S, Zervou FN, Grigoras C, Zacharioudakis IM, Mylonakis E. Prevalence and clinical outcomes of Clostridium difficile infection in the intensive care unit: a systematic review and meta-analysis. Open Forum Infect Dis. 2015;3:ofv186. doi: 10.1093/ofid/ofv186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hellenic Society for Chemotherapy. Guide of antimicrobial stewardship for the hospitalized patient and for primary care. [accessed 2020 Jun 15]. Available from: https://eex.org.gr/
- 18.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43:304–377. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 19.Li G, Taljaard M, Van den Heuvel ER, Levine MA, Cook DJ, Wells GA, et al. An introduction to multiplicity issues in clinical trials: the what, why, when and how. Int J Epidemiol. 2017;46:746–755. doi: 10.1093/ije/dyw320. [DOI] [PubMed] [Google Scholar]
- 20.Tamma PD, Avdic E, Li DX, Dzintars K, Cosgrove SE. Association of adverse events with antibiotic use in hospitalized patients. JAMA Intern Med. 2017;177:1308–1315. doi: 10.1001/jamainternmed.2017.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iftikhar S, Sarwar MR, Saqib A, Sarfraz M. Causality and preventability assessment of adverse drug reactions and adverse drug events of antibiotics among hospitalized patients: a multicenter, cross-sectional study in Lahore, Pakistan. PLoS One. 2018;13:e0199456. doi: 10.1371/journal.pone.0199456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silverman MA, Konnikova L, Gerber JS. Impact of antibiotics on necrotizing enterocolitis and antibiotic-associated diarrhea. Gastroenterol Clin North Am. 2017;46:61–76. doi: 10.1016/j.gtc.2016.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jabbar A, Wright RA. Gastroenteritis and antibiotic-associated diarrhea. Prim Care. 2003;30:63–80, vi. doi: 10.1016/s0095-4543(02)00060-x. [DOI] [PubMed] [Google Scholar]
- 24.Hochreiter M, Köhler T, Schweiger AM, Keck FS, Bein B, von Spiegel T, et al. Procalcitonin to guide duration of antibiotic therapy in intensive care patients: a randomized prospective controlled trial. Crit Care. 2009;13:R83. doi: 10.1186/cc7903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Townsend J, Adams V, Galiatsatos P, Pearse D, Pantle H, Masterson M, et al. Procalcitonin-guided antibiotic therapy reduces antibiotic use for lower respiratory tract infections in a United States Medical Center: results of a clinical trial. Open Forum Infect Dis. 2018;5:ofy327. doi: 10.1093/ofid/ofy327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stolz D, Smyrnios N, Eggimann P, Pargger H, Thakkar N, Siegemund M, et al. Procalcitonin for reduced antibiotic exposure in ventilator-associated pneumonia: a randomised study. Eur Respir J. 2009;34:1364–1375. doi: 10.1183/09031936.00053209. [DOI] [PubMed] [Google Scholar]
- 27.Andriolo BN, Andriolo RB, Salomão R, Atallah ÁN. Effectiveness and safety of procalcitonin evaluation for reducing mortality in adults with sepsis, severe sepsis or septic shock. Cochrane Database Syst Rev. 2017;1:CD010959. doi: 10.1002/14651858.CD010959.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shehabi Y, Sterba M, Garrett PM, Rachakonda KS, Stephens D, Harrigan P, et al. ProGUARD Study Investigators; ANZICS Clinical Trials Group. Procalcitonin algorithm in critically ill adults with undifferentiated infection or suspected sepsis: a randomized controlled trial. Am J Respir Crit Care Med. 2014;190:1102–1110. doi: 10.1164/rccm.201408-1483OC. [DOI] [PubMed] [Google Scholar]
- 29.Wirz Y, Branche A, Wolff M, Welte T, Nobre V, Reinhart K, et al. Management of respiratory infections with use of procalcitonin: moving toward more personalized antibiotic treatment decisions. ACS Infect Dis. 2017;3:875–879. doi: 10.1021/acsinfecdis.7b00199. [DOI] [PubMed] [Google Scholar]
- 30.Neeser O, Branche A, Mueller B, Schuetz P. How to: implement procalcitonin testing in my practice. Clin Microbiol Infect. 2019;25:1226–1230. doi: 10.1016/j.cmi.2018.12.028. [DOI] [PubMed] [Google Scholar]
- 31.Odermatt J, Friedli N, Kutz A, Briel M, Bucher HC, Christ-Crain M, et al. Effects of procalcitonin testing on antibiotic use and clinical outcomes in patients with upper respiratory tract infections: an individual patient data meta-analysis. Clin Chem Lab Med. 2017;56:170–177. doi: 10.1515/cclm-2017-0252. [DOI] [PubMed] [Google Scholar]
- 32.US Food and Drug Administration. FDA news release: FDA clears test to help manage antibiotic treatment for lower respiratory tract infections and sepsis. 2017 [accessed 2020 Jun 15]. Available from: https://www.fda.gov/news-events/press-announcements/fda-clears-test-help-manage-antibiotic-treatment-lower-respiratory-tract-infections-and-sepsis.
- 33.Huang DT, Yealy DM, Filbin MR, Brown AM, Chang CH, Doi Y, et al. ProACT Investigators. Procalcitonin-guided use of antibiotics for lower respiratory tract infection. N Engl J Med. 2018;379:236–249. doi: 10.1056/NEJMoa1802670. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.