Abstract
Rationale: Weight loss is recommended to treat obstructive sleep apnea (OSA).
Objectives: To determine whether the initial benefit of intensive lifestyle intervention (ILI) for weight loss on OSA severity is maintained at 10 years.
Methods: Ten-year follow-up polysomnograms of 134 of 264 adults in Sleep AHEAD (Action for Health in Diabetes) with overweight/obesity, type 2 diabetes mellitus, and OSA were randomized to ILI for weight loss or diabetes support and education (DSE).
Measurements and Main Results: Change in apnea–hypopnea index (AHI) was measured. Mean ± SE weight losses of ILI participants of 10.7 ± 0.7, 7.4 ± 0.7, 5.1 ± 0.7, and 7.1 ± 0.8 kg at 1, 2, 4, and 10 years, respectively, were significantly greater than the 1-kg weight loss at 1, 2, and 4 years and 3.5 ± 0.8 kg weight loss at 10 years for the DSE group (P values ≤ 0.0001). AHI was lower with ILI than DSE by 9.7, 8.0, and 7.9 events/h at 1, 2, and 4 years, respectively (P values ≤ 0.0004), and 4.0 events/h at 10 years (P = 0.109). Change in AHI over time was related to amount of weight loss, baseline AHI, visit year (P values < 0.0001), and intervention independent of weight change (P = 0.01). OSA remission at 10 years was more common with ILI (34.4%) than DSE (22.2%).
Conclusions: Participants with OSA and type 2 diabetes mellitus receiving ILI for weight loss had reduced OSA severity at 10 years. No difference in OSA severity was present between ILI and DSE groups at 10 years. Improvement in OSA severity over the 10-year period with ILI was related to change in body weight, baseline AHI, and intervention independent of weight change.
Keywords: polysomnogram, apnea–hypopnea index; obstructive sleep apnea, lifestyle modification
At a Glance Commentary
Scientific Knowledge on the Subject
Obesity is a major risk factor for obstructive sleep apnea, and weight loss reduces the severity of obstructive sleep apnea.
What This Study Adds to the Field
The 10-year Sleep AHEAD results represent the longest longitudinal study examining the effects of an intensive lifestyle intervention with weight loss on obstructive sleep apnea severity.
Obesity is a major risk factor for obstructive sleep apnea (OSA) (1, 2). Short-term randomized controlled trials have reported reductions in OSA severity following weight loss, and two studies have assessed the effects of weight loss over a 4-year follow-up (3–15). Tuomilehto and colleagues (15) evaluated the effects on OSA severity of a 12-week, very low-calorie diet and 1-year lifestyle intervention focused on weight reduction, healthy diet, and physical activity in obese adults with mild OSA. Compared with the control group that received several healthy lifestyle counseling sessions, participants receiving the active intervention had greater weight loss and greater improvement in apnea–hypopnea index (AHI), a measure of OSA severity, at 1 year and 4 years.
We previously reported the effect of weight loss on OSA severity at 4 years in adults with overweight/obesity and type 2 diabetes enrolled in the Sleep AHEAD (Action for Health in Diabetes) study (11). Sleep AHEAD was an ancillary study of Look AHEAD, a randomized controlled trial in adults with type 2 diabetes and overweight/obesity. Look AHEAD compared the long-term health effects of an intensive lifestyle intervention (ILI) focused on weight loss versus diabetes support and education (DSE) (16–18). Sleep AHEAD enrolled Look AHEAD participants at 4 of the 16 Look AHEAD sites to compare the effect of weight loss on OSA severity in ILI and DSE participants (9, 10). At baseline, 87% of the 305 participants enrolled in Sleep AHEAD had OSA (AHI ≥ 5 events/h) (9). In those participants with OSA at baseline, weight and AHI were significantly reduced in the ILI group versus the DSE group at 1 year (10). Similar to the results of Tuomilehto and colleagues (15), the improvement in OSA severity in the ILI group was still present at 4 years, despite an almost 50% weight regain (11). Change in AHI over the 4-year follow-up was related to the amount of weight loss and intervention, independent of weight loss.
The primary objective of the current study was to determine, among Sleep AHEAD participants with baseline OSA, whether the Look AHEAD intervention resulted in improvements in AHI at 10 years and over the 10-year follow-up period; secondary objectives were to determine whether this effect was independent of weight change and whether the intervention had an effect on changing OSA category. To our knowledge, this is the longest randomized trial assessing the effects of weight loss on OSA severity. OSA is a chronic disease, and improvement in OSA severity with weight loss, if sustained long term, would increase the advocacy and acceptance of this treatment option.
Methods
Participants
Details of the Look AHEAD and Sleep AHEAD study designs, participant characteristics at baseline, intervention, and outcomes have been previously published (9–11, 16–21). Additional information is provided in the online supplement. Primary inclusion criteria of the Look AHEAD study were age 45–76 years, body mass index ≥25 kg/m2 (or ≥27 kg/m2 if taking insulin), physician-verified type 2 diabetes, and HbA1C <11%. Look AHEAD participants were randomly assigned to the ILI or DSE intervention using a web-based data management system. Randomization was stratified by clinical center and blocked with random block sizes. Sleep AHEAD excluded Look AHEAD participants with previous surgical or current medical treatment for OSA. The institutional review board at each site approved the protocol, and all participants signed a written informed consent. The study was registered at www.clinicaltrials.gov (registration, NCT00194259).
Intensive Lifestyle Intervention
ILI participants received a group behavioral weight loss program developed for individuals with obesity and type 2 diabetes with the goal of at least a 10% weight loss in Year 1 (see online supplement) (17). In Year 1, participants were prescribed portion-controlled diets with liquid meal replacements, frozen food entrees, and snack bars for the first 4 months (with reduced use from months 5 through 12). In Years 2–4, intervention consisted of at least one on-site visit per month and a second contact by telephone, mail, or e-mail. In Years 2–10, all sites offered a monthly group meeting at which members weighed in, reviewed diet and activity records, and participated in a lifestyle modification session (21). Each year, sites also offered at least one 6- to 8-week Refresher Group and one National campaign, as used in the Diabetes Prevention Program (22). The intervention was stopped after a median 9.6 years owing to futility for the Look AHEAD primary outcome.
DSE
DSE consisted of three group sessions annually (16). These sessions provided information on diet, physical activity, and social support. Behavioral strategies for weight loss were not presented, and participants were not weighed at the sessions.
Polysomnography
Unattended, home polysomnograms (PSGs) were performed at baseline and at 1, 2, 4, and 10 years using standard techniques and were manually scored with the aid of computer software at a centralized reading laboratory using 2012 American Academy of Sleep Medicine scoring guidelines (see online supplement for more details) (23, 24). AHI was the average number of apneas and hypopneas per hour of sleep and was used to classify OSA severity as mild (5 ≤ AHI <15 events/h), moderate (15 ≤ AHI <30 events/h), and severe (AHI ≥ 30 events/h). The PSG scorers demonstrated a high level of interscorer and intrascorer reliability (see online supplement for more details).
To further address the possible effect of interscorer variability on the results, all PSGs, including those obtained in the first 4 years, were rescored for conventional sleep variables by an automated PSG scoring software program (YRT Limited). The automated scoring used the same scoring criteria used for the manually edited scoring (23). This automated PSG scoring program was validated for sleep stage and respiratory event scoring in an independent multicenter study (25).
Statistical Analysis
Analysis of group differences were based on the intention-to-treat principle. The 41 participants who did not have OSA (AHI < 5 events/h) at baseline were excluded from the analysis, resulting in a final sample of 264 participants. The primary analysis was a mixed-effects model of the nonimputed data to estimate change in AHI over time using all available data and adjusting for clinical site, baseline AHI, and visit year (model 1). A first-order autoregressive structure was used to model correlations over time. An interaction term was used to test for possible changes in the intervention effect over time. In secondary analyses, change in weight (model 2) and change in waist circumference (model 3) were analyzed using the same model. Estimates of the change in AHI within each intervention arm and change in weight also used the mixed-effects model.
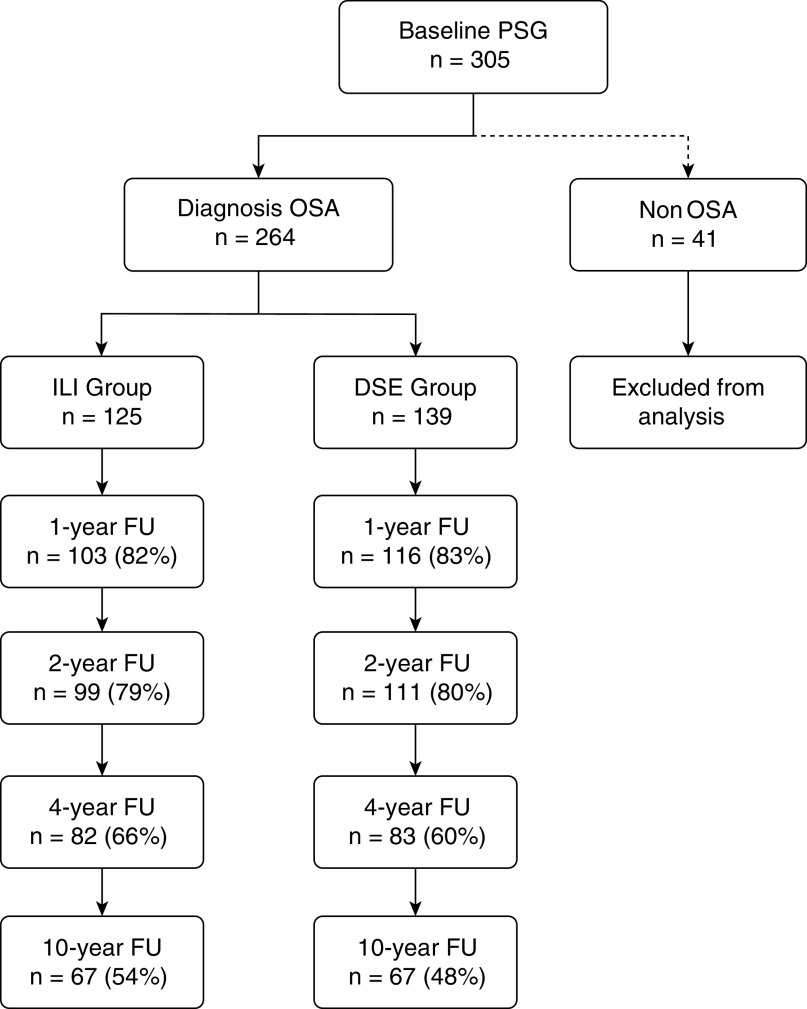
A substantial number of PSGs were not obtained during follow-up (17.0% at 1 yr, 20.5% at 2 yr, 37.5% at 4 yr, and 49.2% at 10 yr; Figure 1). We addressed the impact of these missing data using multiple imputation and weighting for the probability of missingness. The multiple imputation analysis created five data sets with imputed values for missing AHI measurements assuming a multivariate normal distribution for baseline AHI, baseline weight, change in AHI, and change in weight. Because weight measurements from Look AHEAD study visits were rarely missing (3% at 1 yr, 5% at 2 yr, 7% at 4 yr, and 1% at 10 yr) among Sleep AHEAD participants, it was possible to include baseline weight and weight change to improve the imputations. To assess the potential impact of missing data by weighting for the probability of missingness, we used a generalized estimating equation model, limited to participants whose missing data was monotone, that is, with no observed data from visits following the first visit with missing data. Baseline AHI, intervention group, and follow-up weight measurements were not predictive of a missing PSG, although, as expected, study site and follow-up year were. These analyses supported our decision to treat the AHI measurements as missing-at-random, so that the mixed-effects analysis of covariance models used to obtain adjusted mean changes for AHI and weight were not biased by the missing PSG data.
Figure 1.
Diagram showing the number of Sleep AHEAD (Action for Health in Diabetes) participants in the ILI and DSE groups who completed assessments over time. DSE = diabetes support and education; FU = follow-up; ILI = intensive lifestyle intervention; OSA = obstructive sleep apnea; PSG = polysomnogram.
The mixed-effects maximum likelihood and generalized estimating equation analyses of repeated outcomes were performed in Proc Mixed software (SAS, version 9; SAS Institute Inc) using an α level of 0.05.
Results
Participants
As previously reported, 38.7% of the 264 participants had mild OSA, 35.2% had moderate OSA, and 26.1% had severe OSA at baseline (9). No differences in ILI and DSE participant characteristics at baseline were noted (Table 1). Figure 1 shows the number of ILI and DSE participants completing assessments over the 10 years. The 10-year PSGs were obtained in 54% of ILI participants and 48% of DSE participants. Eleven participants (9%) in the ILI group and 20 participants (14%) in the DSE group were deceased or had withdrawn from Look AHEAD. We were unable to contact 15% of the remaining participants in each group, and 22% of participants in each group who were contacted declined to have a 10-year PSG. The PSGs were unsuccessful or could not be scheduled in 2–3% of participants in the two groups. At the 10-year assessment, 15.7% of participants (8 ILI and 13 DSE participants) reported obtaining treatment with positive airway pressure compared with 5.9%, 9.0%, and 11.5% at the 1-, 2-, and 4-year visits, respectively.
Table 1.
Baseline Characteristics of the Study Participants
| Overall (n = 264) |
Diabetes Support and Education* (n = 139) |
Intensive Lifestyle Intervention* (n = 125) |
||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Age. yr | 61.3 | 6.5 | 61.3 | 6.4 | 61.2 | 6.6 |
| Sex, F, % | 59 | — | 57 | — | 62 | — |
| Race/ethnicity, % | ||||||
| African American | 18.6 | — | 18.8 | — | 18.4 | — |
| Hispanic | 3.8 | — | 3.6 | — | 4.0 | — |
| White | 73.4 | — | 73.9 | — | 72.8 | — |
| BMI, kg/m2 | 36.6 | 5.7 | 36.4 | 5.5 | 36.8 | 5.8 |
| Weight, kg | 102.3 | 18.2 | 101.9 | 16.9 | 102.8 | 19.5 |
| Height, cm | 167.1 | 9.5 | 167.4 | 9.3 | 166.8 | 9.7 |
| Waist circumference, cm | 115.8 | 13.2 | 115.7 | 12.1 | 115.8 | 14.4 |
| Neck circumference, cm | 41.3 | 4.2 | 41.6 | 4.1 | 41.1 | 4.3 |
| Apnea–hypopnea index† | 23.3 | 16.5 | 23.7 | 15.0 | 22.9 | 18.0 |
| Obstructive apnea index† | 12.8 | 13.1 | 12.5 | 11.9 | 13.0 | 14.4 |
| Central apnea index† | 0.4 | 1.0 | 0.5 | 1.1 | 0.4 | 1.0 |
| Hypopnea index† | 10.1 | 8.2 | 10.7 | 8.4 | 9.4 | 7.9 |
| Oxygen desaturation index >4%† | 19.5 | 14.9 | 20.3 | 13.7 | 18.6 | 16.1 |
| Fasting glucose, mg/dl | 151.0 | 41.6 | 152.9 | 44.0 | 148.9 | 38.8 |
| HbA1c, % | 7.2 | 1.0 | 7.4 | 1.1 | 7.0 | 0.9 |
| Self-reported duration of diabetes, yr | 7.3 | 7.1 | 7.5 | 6.3 | 7.2 | 7.9 |
Definition of abbreviation: BMI = body mass index.
None of the variables were statistically different between groups.
Events per hour of sleep.
Weight and AHI
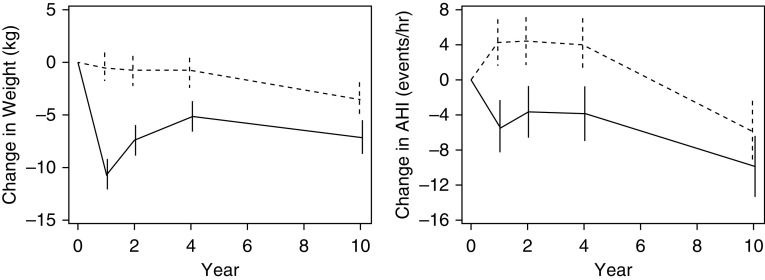
Changes in AHI and weight from baseline are shown in Figure 2. Based on the mixed-effects model of the nonimputed data, the previously reported greater weight loss in the ILI than in the DSE Sleep AHEAD participants at 1, 2, and 4 years was attenuated but still present at 10 years (Table 2). Difference in body weight between all ILI and DSE participants was 10.2 ± 1.0, 6.6 ± 1.0, 4.3 ± 1.0, and 3.6 ± 1.1 kg at 1, 2, 4, and 10 years, respectively (P values ≤ 0.001). Changes in waist circumference showed a similar between-group pattern (Table 2). The difference in AHI between the groups was 9.7 ± 2.0, 8.0 ± 2.0, and 7.9 ± 2.2 events/h at 1, 2, and 4 years, respectively (Table 2, P ≤ 0.0004).
Figure 2.
Estimated mean (SE) changes in body weight (kg) and AHI at Years 1, 2, 4, and 10. The dashed line indicates the diabetes support and education group; the solid line indicates the intensive lifestyle intervention group. AHI = apnea–hypopnea index.
Table 2.
Estimated Mean (SE) Changes from Baseline in Body Weight, AHI, and Waist Circumference at 1, 2, 4, and 10 Years
| Measure | Year | Intensive Lifestyle Intervention Group | P Value* | Diabetes Support and Education Group | P Value* | Between Groups | P Value† |
|---|---|---|---|---|---|---|---|
| AHI, events/h | 1 | −5.5 ± 1.4 | 0.0001 | 4.3 ± 1.4 | 0.0017 | −9.7 ± 2.0 | <0.0001 |
| 2 | −3.6 ± 1.5 | 0.0133 | 4.4 ± 1.4 | 0.0014 | −8.0 ± 2.0 | <0.0001 | |
| 4 | −3.8 ± 1.6 | 0.0149 | 4.0 ± 1.5 | 0.0093 | −7.9 ± 2.2 | 0.0004 | |
| 10 | −9.9 ± 1.8 | <0.0001 | −5.9 ± 1.8 | 0.0011 | −4.0 ± 2.5 | 0.1088 | |
| Weight, kg | 1 | −10.7 ± 0.7 | <0.0001 | −0.5 ± 0.7 | 0.514 | −10.2 ± 1.0 | <0.0001 |
| 2 | −7.4 ± 0.7 | <0.0001 | −0.8 ± 0.7 | 0.255 | −6.6 ± 1.0 | <0.0001 | |
| 4 | −5.1 ± 0.7 | <0.0001 | −0.9 ± 0.7 | 0.225 | −4.3 ± 1.0 | <0.0001 | |
| 10 | −7.1 ± 0.8 | <0.0001 | −3.5 ± 0.8 | <0.0001 | −3.6 ± 1.1 | 0.001 | |
| Waist circumference, cm | 1 | −9.0 ± 0.7 | <0.0001 | −0.6 ± 0.7 | 0.376 | −8.4 ± 1.0 | <0.0001 |
| 2 | −5.9 ± 0.7 | <0.0001 | −0.7 ± 0.7 | 0.306 | −5.2 ± 1.0 | <0.0001 | |
| 4 | −3.4 ± 0.7 | <0.0001 | 0.2 ± 0.7 | 0.731 | −3.6 ± 1.0 | 0.000 | |
| 10 | −2.1 ± 0.8 | 0.010 | 1.6 ± 0.8 | 0.047 | −3.7 ± 1.1 | 0.001 |
Definition of abbreviation: AHI = apnea–hypopnea index.
Compared with baseline value.
Between-group comparison.
At 10 years, 60.9% of the ILI participants had an improvement in their OSA category from baseline compared with the 47.6% improvement of DSE participants (Figure 3). At 10 years, only 6.3% of ILI compared with 17.5% of DSE participants had a worsening of OSA category from baseline (Figure 3). Improvement in AHI from year 4 to year 10 occurred in 38 of 52 (73%) ILI participants and 38 of 51 (74%) DSE participants.
Figure 3.
Percentage of participants in ILI (solid bars, n = 90) and DSE (open bars, n = 97) whose OSA category improved (≥1 category change), worsened (≥1 category change), or was unchanged from baseline to Year 10. Data are from the participants who had baseline and Year-10 data. DSE = diabetes support and education; ILI = intensive lifestyle intervention; OSA = obstructive sleep apnea.
The difference in AHI of 4.0 ± 2.5 events/h between the two arms at 10 years was not statistically significant (P = 0.109). The estimated effect of weight change on change in AHI was 0.68 in the ILI group (P < 0.0001) and 0.54 in the DSE group (P ≤ 0.0001), that is, a 0.68 and 0.54 improvement in AHI, respectively, for every kilogram of weight loss. Considering the overall intervention effect on AHI, averaged over all of the follow-up visits, ILI participants had 7.40 ± 1.39 events/h (P < 0.0001) greater reduction than DSE participants.
At 10 years, 34.4% of ILI participants and 22.2% of DSE participants had remission of their OSA. In both groups, remission at 10 years was most likely to occur in participants who had mild and moderate OSA at baseline (Figure 4). At 10 years, 9.4% in the ILI group and 17.4% in the DSE group had severe OSA. Comparing ILI and DSE participants who had severe OSA at baseline, more than twice as many DSE participants continued to have severe OSA at 10 years (Figure 4).
Figure 4.

Percentage of ILI (solid bars) and DSE (open bars) participants without OSA and with mild, moderate, and severe OSA at 10 years compared with their OSA severity at baseline. For definition of abbreviations, see Figure 3.
Treatment Effect Factors
The change in AHI from baseline over time was related to the intervention arm, adjusting for clinical site, baseline AHI, and visit year (Table 3; P < 0.0001). Follow-up year had an effect on the change in AHI over time (P < 0.0001), indicating that the change in AHI at Year 1 did not remain constant over time. Furthermore, when change in weight was added to the model, the intervention arm remained significant (P = 0.006), indicating an effect of ILI on AHI over time independent of weight change. We found a similar result at the 4-year follow-up. Change in AHI over time was strongly related to baseline AHI (P < 0.0001), with greater changes in AHI occurring in individuals with higher AHI at baseline. When change in waist circumference was added to the model, no relationship was present between change in waist circumference and change in AHI over time, and the previously noted relationships with the other covariates remained significant (Table 3).
Table 3.
Mixed-Effects Models Estimating Change in AHI over Time
| Effect | Model 1 (Primary) |
Model 2 |
Model 3 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate | SE | P Value | Estimate | SE | P Value | Estimate | SE | P Value | |
| Intervention arm (ILI vs. DSE) | 4.01 | 2.50 | <0.0001 | 2.03 | 2.42 | 0.006 | 1.17 | 2.72 | 0.01 |
| Study site | — | — | 0.20 | — | — | 0.35 | — | — | 0.28 |
| Baseline AHI, events/h | −0.40 | 0.04 | <0.0001 | −0.38 | 0.04 | <0.0001 | −0.38 | 0.04 | <0.0001 |
| Visit year | — | — | <0.0001 | — | — | <0.0001 | — | — | <0.0001 |
| Weight change, kg | — | — | — | 0.55 | 0.07 | <0.0001 | 0.64 | 0.12 | <0.0001 |
| Waist change, cm | — | — | — | — | — | — | −0.12 | 0.12 | 0.32 |
Definition of abbreviations: AHI = apnea–hypopnea index; DSE = diabetes support and education; ILI = intensive lifestyle intervention.
Model 1 (primary) adjusted for clinical site, baseline AHI, and visit year. A first-order autoregressive structure was used to model correlations over time. Change in weight was added in model 2, and changes in waist circumference were added in model 3.
Over the 10-year period, three ILI and five DSE participants received bariatric surgery. The results were qualitatively similar when these participants were removed from the analysis (Table E2 in the online supplement). To assess whether age was a moderator of the ILI effect, we split the cohort based on median age at randomization and evaluated the interaction with ILI. We found that age had no effect (P = 0.54).
Missing data analyses suggested that the missing AHI data did not distort the results. Probability of missingness was found to vary by clinic and visit but not by baseline AHI or weight. Estimates from the multiple imputation analysis did not differ meaningfully from the analysis ignoring missing data. For the analysis using probability of missingness as a weight, participants with nonmonotone patterns of missingness were excluded. Of 264 participants, 96 had complete data, and another 97 had monotone missing data. Seventy-one had nonmonotone missing data patterns, including 24 with no follow-up data. Using a generalized estimating equation model, inferences on intervention effects at each visit were not affected when the analysis was weighted to account for missingness.
Automated Scoring
The results using the automated software program to score the PSGs at baseline and the follow-up visits were similar to those of the manual scoring (Figure E2). The change in AHI from baseline was significantly different between the ILI and DSE groups at 1 and 4 years (P ≤ 0.038) and nominally significant at 2 years (P = 0.062, Table E1). In addition, the change in AHI from baseline at 10 years was not different between the two groups (P = 0.77). In contrast to the manually scored results, however, AHI on the automated scoring in the ILI group increased from year 4 to year 10 (15.87 ± 1.63 vs. 19.25 ± 1.77 events/h, respectively), and the AHI in the DSE group was not significantly different between year 4 and year 10 (23.17 ± 1.53 vs. 22.15 ± 1.73 events/h, respectively). Based on automated scoring results, 8% of ILI participants and 15% of DSE participants had remission of their OSA at 10 years.
Discussion
The 10-year Sleep AHEAD results represent the longest longitudinal study examining the effects of an intensive lifestyle intervention with weight loss on OSA severity. Extending the results of our previously reported findings of this cohort of adults with overweight/obesity, type 2 diabetes, and OSA (9–11), the current results indicate that the improvement in OSA severity, as measured by AHI, in ILI versus DSE participants in the initial 4 years was no longer present at the 10-year follow-up. However, the overall reduction in AHI averaged over all follow-up visits over 10 years was greater with ILI than DSE. The overall reduction in OSA severity over the 10-year period in the ILI versus DSE groups was related to change in body weight, baseline AHI, and intervention arm, independent of weight change.
Improvement in OSA severity resulted in remission of the disorder (AHI < 5 events/h) in some of the participants. Remission at 1 year was three times more common in the ILI participants (13.6%) than in the DSE participants (3.5%) (10). Remission at 4 years was five times more common in ILI participants (20.7%) than in DSE participants (3.6%) (11). In contrast, at 10 years, 34.4% of ILI participants and 22.2% of DSE participants had remission of their OSA. The increased remission from Year 4 to Year 10 in DSE participants may be related to the weight loss that occurred in this group over that time period. Remission of OSA at 10 years was most likely to occur in ILI and DSE participants who had mild and moderate OSA at baseline.
Similar to our 4-year follow-up results, the change in AHI over time at 10 years was associated with intervention group independent of weight change and baseline AHI. This suggests that factors associated with ILI other than weight loss may have influenced the improvement in OSA severity. Changes in healthy lifestyle behavior that were not monitored may have affected the AHI independent of weight. As suggested by previous studies, diet and physical activity may alter the symptoms and possibly the severity of OSA (26–31). Exercise training in sedentary adults who were overweight and obese improved AHI independent of weight loss (32). However, Kline and colleagues (33) reported that changes in cardiorespiratory fitness in the Sleep AHEAD cohort over the course of the 4-year intervention did not influence OSA severity before and following adjustment for weight change. It is possible that ILI could have resulted in fat redistribution (34, 35). Neck circumference and waist circumference were not predictors of AHI change at the 4-year follow-up after weight change was included in the models (11). Neck circumference was not measured at the 10-year follow-up. However, change in waist circumference at 10 years was not an independent predictor after weight change was included in models.
Although OSA severity of the ILI and DSE participants did not differ at the 10-year follow-up, the greater improvement in the ILI group over the decade may have resulted in symptomatic improvement and a possible decrease in cardiovascular risk. OSA is an independent risk factor for hypertension (36), and numerous randomized controlled trials have reported an improvement in systemic blood pressure in adults with OSA treated with continuous positive airway pressure, especially those with resistant hypertension (37, 38). The Look AHEAD study (in a sample of more than 5,000) reported that ILI did not reduce cardiovascular events over a median follow-up of 9.6 years, but the ILI group, compared with the DSE group, had greater reductions in HbA1c and many cardiovascular risk factors (39). The number of participants in the Sleep AHEAD cohort was insufficient to compare cardiovascular mortality and event rate in the two groups.
The known weight loss with aging in older adults may have contributed to weight reduction in all participants unrelated to the intervention. The 16-site Look AHEAD study reported weight loss in its DSE group at 8–10 years, particularly in older participants (22, 39). In addition, the Diabetes Prevention Program, which recruited older adults at risk of diabetes, also reported a decrease in weight at the 10-year assessment in the control group (40). However, the decrease in AHI in the ILI and DSE groups from 4 to 10 years seemed greater than what might be expected from the reduction in weight in both groups over this time period (Table 2 and Figure 2). We speculate that this may have been related to an aging effect on OSA severity. Epidemiological studies reported that the severity of OSA decreases with aging, independent of weight (41, 42), and the prevalence of OSA, which increases with aging in young and middle-aged adults, remains relatively constant after 55 years of age (43).
We found good agreements in interscorer and intrascorer reliability of the PSG scoring over this 10-year study. To further assure uniform PSG scoring over the 10-year period, we also analyzed the PSGs using an automated software program. Given the many uncalibrated signals on PSG and the reliance on pattern recognition to manually score PSGs, it is not surprising that the results of the automated scoring did not exactly match those of the manual scoring. However, the results of the automated analysis were qualitatively similar to those of the manual scoring. Of particular note, the change in AHI from baseline at 10 years was not different between the two groups.
Although awareness of OSA and its consequences is steadily increasing (44), only 15% of participants were receiving treatment for OSA at the 10-year follow-up. Throughout the 10-year study, all Sleep AHEAD participants and their primary care providers were sent letters reporting the results of every sleep study. The Sleep Heart Health Study, which used a similar disclosure strategy, reported similar findings (45). Interventions are needed to improve the uptake of OSA treatment.
Our study has several limitations. The results may have been influenced by the 41 participants who were withdrawn from the study following the baseline PSG because they did not have OSA (AHI < 5 events/h). Participants with mild OSA are more likely to change disease category (11). As a result, individuals who had an AHI < 5 events/h at baseline may have had an AHI ≥ 5 events/h on follow-up PSGs. Improvements or worsening in OSA severity category at Year 10 may also have been influenced by misclassification owing to the known night-to-night variability in AHI and the arbitrary threshold set to diagnose OSA. Although the extent of misclassification in this randomized controlled trial is unknown, it would have been present in both the ILI and DSE groups and therefore unlikely to have affected the results.
The missing PSG data in 49.2% of participants at 10 years is another limitation of our study. To address this limitation, we evaluated the relationship between the probability of missing PSG results and available covariates. We also assessed whether the patterns of missing PSG results were related to change in AHI. No such associations were found. However, because these analyses were performed post hoc, we cannot be completely confident that our results are unrelated to participant dropout. Finally, our study was performed in older adults with type 2 diabetes. Therefore, the results may not apply to younger adults and adults who do not have type 2 diabetes mellitus.
Conclusions
Sleep AHEAD is the longest randomized controlled study to date evaluating the effect of ILI for weight management on the severity of OSA. Participants with OSA and type 2 diabetes mellitus receiving ILI for weight loss had reduced OSA severity at 10 years. The greater improvement in OSA severity with lifestyle intervention in the first 4 years was no longer present at the 10-year follow-up, and the greater weight loss in the lifestyle intervention group than in the control group observed in the first 4 years was attenuated at 10 years. However, overall OSA severity across the 10-year period was reduced with ILI, and this improvement was related to change in body weight, greater severity of OSA at baseline, and lifestyle intervention independent of weight change.
Supplementary Material
Acknowledgments
Members of the Sleep AHEAD research group: St. Luke’s-Roosevelt Hospital/Clinilabs: Jon Freeman and Jennifer Patricio; University of Pennsylvania: Andrea Sifferman, Brian McGuckin, Stephanie Krauthamer-Ewing, Mary Jones-Parker, Matthew Anastasi, Beth Staley, and Liz Roben; Brown University: Marie Kearns and Caitlin Egan; Temple University: Alexis Wojtanowski, Nida Cassim, Valerie Darcey, Sakhena Hin, and Stephanie Vander Veur. A detailed list of the Look AHEAD Research Group is provided in Reference 11.
Members of the Observational Safety and Management Board: Kingman P. Strohl, Donald L. Bliwise, and Helaine E. Resnick.
Footnotes
Supported by the NIH NHLBI grant HL070301 and National Institute of Diabetes and Digestive and Kidney Diseases grants DK60426, DK56992, and DK057135.
Author Contributions: All authors were involved in the conception and design of the study, data collection, and data analysis and interpretation. All authors provided critical revision of the manuscript and approved the final version. Conception and design: S.T.K., D.M.R., R.P.M., G.Z., and G.D.F. Data collection: S.T.K., E.S.S., D.M.R., R.P.M., G.Z., R.R.W., F.X.P.-S., T.A.W., and G.D.F. Analysis and interpretation: S.T.K., D.M.R., M.P.W., and G.D.F. Drafting the manuscript for important intellectual content: S.T.K., G.D.F., D.M.R., M.P.W., T.A.W., and A.P.S.
A list of the Sleep AHEAD Research Subgroup of the Look AHEAD Research Group members may be found before the beginning of the References.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201912-2511OC on July 28, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
Contributor Information
Collaborators: the Sleep AHEAD Research Subgroup of the Look AHEAD Research Group, Jon Freeman, Jennifer Patricio, Andrea Sifferman, Brian McGuckin, Stephanie Krauthamer-Ewing, Mary Jones-Parker, Matthew Anastasi, Beth Staley, Liz Roben, Marie Kearns, Caitlin Egan, Alexis Wojtanowski, Nida Cassim, Valerie Darcey, Sakhena Hin, and Stephanie Vander Veur
References
- 1.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–1239. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 2.Gottlieb DJ, Punjabi NM. Diagnosis and management of obstructive sleep apnea: a review. JAMA. 2020;323:1389–1400. doi: 10.1001/jama.2020.3514. [DOI] [PubMed] [Google Scholar]
- 3.Johansson K, Neovius M, Lagerros YT, Harlid R, Rössner S, Granath F, et al. Effect of a very low energy diet on moderate and severe obstructive sleep apnoea in obese men: a randomised controlled trial. BMJ. 2009;339:b4609. doi: 10.1136/bmj.b4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johansson K, Hemmingsson E, Harlid R, Trolle Lagerros Y, Granath F, Rössner S, et al. Longer term effects of very low energy diet on obstructive sleep apnoea in cohort derived from randomised controlled trial: prospective observational follow-up study. BMJ. 2011;342:d3017. doi: 10.1136/bmj.d3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ng SSS, Chan RSM, Woo J, Chan TO, Cheung BHK, Sea MMM, et al. A randomized controlled study to examine the effect of a lifestyle modification program in OSA. Chest. 2015;148:1193–1203. doi: 10.1378/chest.14-3016. [DOI] [PubMed] [Google Scholar]
- 6.Joosten SA, Hamilton GS, Naughton MT. Impact of weight loss management in OSA. Chest. 2017;152:194–203. doi: 10.1016/j.chest.2017.01.027. [DOI] [PubMed] [Google Scholar]
- 7.Igelström H, Åsenlöf P, Emtner M, Lindberg E. Improvement in obstructive sleep apnea after a tailored behavioural sleep medicine intervention targeting healthy eating and physical activity: a randomised controlled trial. Sleep Breath. 2018;22:653–661. doi: 10.1007/s11325-017-1597-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tham KW, Lee PC, Lim CH. Weight management in obstructive sleep apnea: medical and surgical options. Sleep Med Clin. 2019;14:143–153. doi: 10.1016/j.jsmc.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Foster GD, Sanders MH, Millman R, Zammit G, Borradaile KE, Newman AB, et al. Sleep AHEAD Research Group. Obstructive sleep apnea among obese patients with type 2 diabetes. Diabetes Care. 2009;32:1017–1019. doi: 10.2337/dc08-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foster GD, Borradaile KE, Sanders MH, Millman R, Zammit G, Newman AB, et al. Sleep AHEAD Research Group of Look AHEAD Research Group. A randomized study on the effect of weight loss on obstructive sleep apnea among obese patients with type 2 diabetes: the Sleep AHEAD study. Arch Intern Med. 2009;169:1619–1626. doi: 10.1001/archinternmed.2009.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuna ST, Reboussin DM, Borradaile KE, Sanders MH, Millman RP, Zammit G, et al. Sleep AHEAD Research Group of the Look AHEAD Research Group. Long-term effect of weight loss on obstructive sleep apnea severity in obese patients with type 2 diabetes. Sleep (Basel) 2013;36:641–649A. doi: 10.5665/sleep.2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tuomilehto HP, Seppä JM, Partinen MM, Peltonen M, Gylling H, Tuomilehto JO, et al. Kuopio Sleep Apnea Group. Lifestyle intervention with weight reduction: first-line treatment in mild obstructive sleep apnea. Am J Respir Crit Care Med. 2009;179:320–327. doi: 10.1164/rccm.200805-669OC. [DOI] [PubMed] [Google Scholar]
- 13.Tuomilehto H, Gylling H, Peltonen M, Martikainen T, Sahlman J, Kokkarinen J, et al. Kuopio Sleep Apnea Group. Sustained improvement in mild obstructive sleep apnea after a diet- and physical activity-based lifestyle intervention: postinterventional follow-up. Am J Clin Nutr. 2010;92:688–696. doi: 10.3945/ajcn.2010.29485. [DOI] [PubMed] [Google Scholar]
- 14.Tuomilehto HP. Initial improvements in apnoea-hypopnoea index after very low calorie diet maintained for 1 year with weight loss maintenance program. Evid Based Med. 2012;17:32–33. doi: 10.1136/ebm.2011.100169. [DOI] [PubMed] [Google Scholar]
- 15.Tuomilehto H, Seppä J, Uusitupa M, Tuomilehto J, Gylling H Kuopio Sleep Apnea Group. Weight reduction and increased physical activity to prevent the progression of obstructive sleep apnea: a 4-year observational postintervention follow-up of a randomized clinical trial [corrected] JAMA Intern Med. 2013;173:929–930. doi: 10.1001/jamainternmed.2013.389. [DOI] [PubMed] [Google Scholar]
- 16.Ryan DH, Espeland MA, Foster GD, Haffner SM, Hubbard VS, Johnson KC, et al. Look AHEAD Research Group. Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials. 2003;24:610–628. doi: 10.1016/s0197-2456(03)00064-3. [DOI] [PubMed] [Google Scholar]
- 17.Wadden TA, West DS, Delahanty L, Jakicic J, Rejeski J, Williamson D, et al. Look AHEAD Research Group. The Look AHEAD study: a description of the lifestyle intervention and the evidence supporting it. Obesity (Silver Spring) 2006;14:737–752. doi: 10.1038/oby.2006.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bray G, Gregg E, Haffner S, Pi-Sunyer XF, WagenKnecht LE, Walkup M, et al. Look AHEAD Research Group. Baseline characteristics of the randomised cohort from the Look AHEAD (Action for Health in Diabetes) study. Diab Vasc Dis Res. 2006;3:202–215. doi: 10.3132/dvdr.2006.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pi-Sunyer X, Blackburn G, Brancati FL, Bray GA, Bright R, Clark JM, et al. Look AHEAD Research Group. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the Look AHEAD trial. Diabetes Care. 2007;30:1374–1383. doi: 10.2337/dc07-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wing RR Look AHEAD Research Group. Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: four-year results of the Look AHEAD trial. Arch Intern Med. 2010;170:1566–1575. doi: 10.1001/archinternmed.2010.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Look AHEAD Research Group. Eight-year weight losses with an intensive lifestyle intervention: the look AHEAD study. Obesity (Silver Spring) 2014;22:5–13. doi: 10.1002/oby.20662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diabetes Prevention Program (DPP) Research Group. The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care. 2002;25:2165–2171. doi: 10.2337/diacare.25.12.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, et al. American Academy of Sleep Medicine; Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events. J Clin Sleep Med. 2012;8:597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kushida CA, Littner MR, Morgenthaler T, Alessi CA, Bailey D, Coleman J, Jr, et al. Practice parameters for the indications for polysomnography and related procedures: an update for 2005. Sleep. 2005;28:499–521. doi: 10.1093/sleep/28.4.499. [DOI] [PubMed] [Google Scholar]
- 25.Malhotra A, Younes M, Kuna ST, Benca R, Kushida CA, Walsh J, et al. Performance of an automated polysomnography scoring system versus computer-assisted manual scoring. Sleep (Basel) 2013;36:573–582. doi: 10.5665/sleep.2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barnes M, Goldsworthy UR, Cary BA, Hill CJ. A diet and exercise program to improve clinical outcomes in patients with obstructive sleep apnea: a feasibility study. J Clin Sleep Med. 2009;5:409–415. [PMC free article] [PubMed] [Google Scholar]
- 27.Hong S, Dimsdale JE. Physical activity and perception of energy and fatigue in obstructive sleep apnea. Med Sci Sports Exerc. 2003;35:1088–1092. doi: 10.1249/01.MSS.0000074566.94791.24. [DOI] [PubMed] [Google Scholar]
- 28.Norman JF, Von Essen SG, Fuchs RH, McElligott M. Exercise training effect on obstructive sleep apnea syndrome. Sleep Res Online. 2000;3:121–129. [PubMed] [Google Scholar]
- 29.Peppard PE, Young T. Exercise and sleep-disordered breathing: an association independent of body habitus. Sleep. 2004;27:480–484. doi: 10.1093/sleep/27.3.480. [DOI] [PubMed] [Google Scholar]
- 30.Quan SF, O’Connor GT, Quan JS, Redline S, Resnick HE, Shahar E, et al. Association of physical activity with sleep-disordered breathing. Sleep Breath. 2007;11:149–157. doi: 10.1007/s11325-006-0095-5. [DOI] [PubMed] [Google Scholar]
- 31.Vasquez MM, Goodwin JL, Drescher AA, Smith TW, Quan SF. Associations of dietary intake and physical activity with sleep disordered breathing in the Apnea Positive Pressure Long-Term Efficacy Study (APPLES) J Clin Sleep Med. 2008;4:411–418. [PMC free article] [PubMed] [Google Scholar]
- 32.Kline CE, Crowley EP, Ewing GB, Burch JB, Blair SN, Durstine JL, et al. The effect of exercise training on obstructive sleep apnea and sleep quality: a randomized controlled trial. Sleep (Basel) 2011;34:1631–1640. doi: 10.5665/sleep.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kline CE, Reboussin DM, Foster GD, Rice TB, Strotmeyer ES, Jakicic JM, et al. Sleep AHEAD Research Group of the Look AHEAD Research Group. The effect of changes in cardiorespiratory fitness and weight on obstructive sleep apnea severity in overweight adults with Type 2 diabetes. Sleep (Basel) 2016;39:317–325. doi: 10.5665/sleep.5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Irwin ML, Yasui Y, Ulrich CM, Bowen D, Rudolph RE, Schwartz RS, et al. Effect of exercise on total and intra-abdominal body fat in postmenopausal women: a randomized controlled trial. JAMA. 2003;289:323–330. doi: 10.1001/jama.289.3.323. [DOI] [PubMed] [Google Scholar]
- 35.Stewart KJ, Bacher AC, Turner KL, Fleg JL, Hees PS, Shapiro EP, et al. Effect of exercise on blood pressure in older persons: a randomized controlled trial. Arch Intern Med. 2005;165:756–762. doi: 10.1001/archinte.165.7.756. [DOI] [PubMed] [Google Scholar]
- 36.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 37.Iftikhar IH, Valentine CW, Bittencourt LR, Cohen DL, Fedson AC, Gíslason T, et al. Effects of continuous positive airway pressure on blood pressure in patients with resistant hypertension and obstructive sleep apnea: a meta-analysis. J Hypertens. 2014;32:2341–2350. doi: 10.1097/HJH.0000000000000372. [Discussion, p. 2350.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schein AS, Kerkhoff AC, Coronel CC, Plentz RD, Sbruzzi G. Continuous positive airway pressure reduces blood pressure in patients with obstructive sleep apnea; a systematic review and meta-analysis with 1000 patients. J Hypertens. 2014;32:1762–1773. doi: 10.1097/HJH.0000000000000250. [DOI] [PubMed] [Google Scholar]
- 39.Wing RR, Bolin P, Brancati FL, Bray GA, Clark JM, Coday M, et al. Look AHEAD Research Group. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369:145–154. doi: 10.1056/NEJMoa1212914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Knowler WC, Fowler SE, Hamman RF, Christophi CA, Hoffman HJ, Brenneman AT, et al. Diabetes Prevention Program Research Group. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374:1677–1686. doi: 10.1016/S0140-6736(09)61457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bixler EO, Vgontzas AN, Ten Have T, Tyson K, Kales A. Effects of age on sleep apnea in men: I. Prevalence and severity. Am J Respir Crit Care Med. 1998;157:144–148. doi: 10.1164/ajrccm.157.1.9706079. [DOI] [PubMed] [Google Scholar]
- 42.Bixler EO, Vgontzas AN, Lin HM, Ten Have T, Rein J, Vela-Bueno A, et al. Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med. 2001;163:608–613. doi: 10.1164/ajrccm.163.3.9911064. [DOI] [PubMed] [Google Scholar]
- 43.Young T, Shahar E, Nieto FJ, Redline S, Newman AB, Gottlieb DJ, et al. Sleep Heart Health Study Research Group. Predictors of sleep-disordered breathing in community-dwelling adults: the sleep heart health study. Arch Intern Med. 2002;162:893–900. doi: 10.1001/archinte.162.8.893. [DOI] [PubMed] [Google Scholar]
- 44.Morsy NE, Farrag NS, Zaki NFW, Badawy AY, Abdelhafez SA, El-Gilany AH, et al. Obstructive sleep apnea: personal, societal, public health, and legal implications. Rev Environ Health. 2019;34:153–169. doi: 10.1515/reveh-2018-0068. [DOI] [PubMed] [Google Scholar]
- 45.Gottlieb DJ, Yenokyan G, Newman AB, O’Connor GT, Punjabi NM, Quan SF, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation. 2010;122:352–360. doi: 10.1161/CIRCULATIONAHA.109.901801. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.