To the Editor:
Hematopoietic stem cell transplant (HSCT) recipients and patients receiving chimeric antigen receptor T-cell therapy represent a delicate subgroup within the ICU. Amid the ongoing coronavirus disease (COVID-19) pandemic, the clinical course of the disease in these patients who are at high risk and have elevated mortality rates is still poorly understood. Here, we provide comprehensive data on viral dynamics and outcomes in 6 consecutive patients with a history of HSCT or chimeric antigen receptor T-cell therapy (Tx) requiring ICU treatment for COVID-19 compared with 18 patients with COVID-19 without a history of malignant disease as control patients (individual patient characteristics of patients with Tx are presented in Table 1).
Table 1.
Overall and Special Demographic Characteristics of ICU Patients with COVID-19 without Underlying Hematologic Diseases and after Stem Cell Therapy
| Parameter | Patients with Tx (n = 6) | No Hematologic Disease (n = 18) | OR | 95% CI | P Value |
|---|---|---|---|---|---|
| Overall demographic characteristics of the cohort | |||||
| Age, yr | 66.5 (58.3–73.3) | 64 (55–73) | 1.019 | 0.421–1.101 | 0.646 |
| Sex, M | 4 (67) | 16 (89) | 8.000 | 0.911–70.275 | 0.061 |
| BMI, kg/m2 | 23 (20–27) | 27.5 (27.5–32.8) | 0.735 | 0.540–1.001 | 0.051 |
| Severity of COVID-19 and ARDS | |||||
| COVID-19 | — | — | 0.228 | 0.021–1.021 | 0.829 |
| Mild/moderate | 1 (17) | 2 (11) | — | — | — |
| Severe | — | — | — | — | — |
| Critical | 5 (83) | 16 (89) | — | — | — |
| ARDS | — | — | 0.625 | 0.046–8.432 | 0.723 |
| No ARDS | 1 (17) | 2 (11) | — | — | — |
| Mild | — | — | — | — | — |
| Moderate | 2 (33) | 5 (28) | — | — | — |
| Severe | 3 (50) | 11 (61) | — | — | — |
| Disease severity | |||||
| SAPS II, pts. | 46 (40.5–50) | 39.5 (33.3–50.8) | 1.034 | 0.949–1.126 | 0.445 |
| SOFA at admission, pts. | 5.5 (4.3–7.5) | 7.5 (3.3–11.8) | 0.898 | 0.719–1.121 | 0.341 |
| SOFA, max. at 72 h, pts. | 10.5 (4.8–14) | 11 (10.3–14.5) | 0.891 | 0.713–1.114 | 0.312 |
| Procedures during ICU stay | |||||
| Ventilation | |||||
| Mechanical ventilation | 5 (83) | 16 (89) | 0.723 | 0.046–8.432 | 0.723 |
| ECMO | — | 5 (28) | — | — | — |
| Other respiratory support | |||||
| High-flow nasal cannula | 4 (67) | — | 5.200 | 0.714–37.895 | 0.104 |
| Noninvasive ventilation | 1 (17) | 1 (6) | 3.400 | 0.179–64.682 | 0.415 |
| Low/no respiratory support | 1 (17) | — | — | — | — |
| Tracheostomy | 6 (33) | — | — | — | |
| ARDS management | |||||
| Prone positioning | 5 (83) | 12 (67) | 2.500 | 0.236–26.480 | 0.447 |
| Neuromuscular blockade | 1 (17) | 7 (39) | 0.393 | 0.036–4.276 | 0.443 |
| Inhaled vasodilatory treatment | 3 (50) | 7 (39) | 1.571 | 0.245–10.093 | 0.634 |
| Glucocorticoid th. | 4 (67) | 7 (39) | 3.143 | 0.450–21.958 | 0.248 |
| Other ICU th. | |||||
| Vasopressor th. | 5 (83) | 18 (100) | 0.250 | 0.024–2.594 | 0.246 |
| Renal replacement th. | 3 (50) | 11 (61) | 0.636 | 0.099–4.087 | 0.634 |
| Red blood cell transfusion | 12 (10–18) | 3.5 (1.5–5) | 3.200 | 0.296–34.588 | 0.338 |
| Thrombocyte transfusion | 13 (13–14) | — | — | — | — |
| Outcome | |||||
| Overall mortality | 5 (83) | 5 (28) | 13.00 | 1.201–140.34 | 0.035 |
| Special Tx Patient Demographic Characteristic | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 |
|---|---|---|---|---|---|---|
| Sex* | F | M | M | F | M | M |
| Hematologic disease | PMF† | sAML‡ | HD‡ | tAML§ | DLBCL§ | CML‡ |
| Reason for hospital admission | HSCT (first) | HSCT (second) | GVHD | Second salvage th. | CAR-T th. LD th. | DM2 |
| Prior hematologic th. | Ruxo | Salvage th. | HSCT | HSCT, refract. salvage th. | Mult. Cth., aSCT twice | HSCT |
| Comorbidities | DM2 | AHT, DM2, TAA | AHT, CKD OSAS, obesity | AHT, Parkinson’s disease, breast CA (hist.) | RLS, PNP | AHT, prostate CA |
| Last hematologic th. to VD, d | 18 | 45 | 358 | 23 | 14 | 4,279 |
| VD to ICU admission | 18 | 8 | 18 | 9 | 21 | 7 |
| VD to death | 25 | 37 | 33 | 41 | 44 | — |
| Immunosuppression | ||||||
| At SARS-CoV-2 detection | CSA, MMF | CSA | MMF, Ruxo. | None | None | Steroids |
| Before death | CSA, steroids | CSA, steroids | TAC, steroids | None | Steroids | — |
| COVID-19 symptoms (at diagnosis) | Subfebrile temperature | Subfebrile temperature, weakness, cough | None (screening result) | None | Fever | Fever, cough, weakness |
| COVID-19 th. (specific) | Igs | TOCI | Igs, TOCI | None | Igs | None |
Definition of abbreviations: AHT = arterial hypertension; ARDS = acute respiratory distress syndrome; aSCT = autologous stem cell transplantation; BMI = body mass index; CA = cancer; CAR-T = chimeric antigen receptor T cell; CI = confidence interval; CKD = chronic kidney disease; CML = chronic myelogenous leukemia; COVID-19 = coronavirus disease; CSA = cyclosporine A; DLBCL = diffuse large B-cell lymphoma; DM2 = diabetes mellitus type 2; ECMO = extracorporeal membrane oxygenation; GVHD = graft-versus-host disease; HD = Hodgkin’s disease; hist. = history; Igs = immunglobulins; HSCT = hematopoietic stem cell transplant; LD = lymphocyte-depleting; max. = maximum; MMF = mycophenolate mofetil; Mult. Cth = multiple chemotherapies; OR = odds ratio; OSAS = obstructive sleep apnea syndrome; PMF = primary myelofibrosis; PNP = polyneuropathy; pts. = points; refract. = refractory; RLS = restless leg syndrome; Ruxo = ruxolitinib; sAML = secondary acute myeloid leukemia; SAPS = Simplified Acute Physiology Score; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2; SOFA = Sequential Organ Failure Assessment; TAA = tachyarrhyrthmia absoluta; TAC = tacrolimus; tAML = th.-associated acute myeloid leukemia; th. = therapy; TOCI = tocilizumab; Tx = hist. of HSCT or CAR-T th.; VD = viral diagnosis of SARS-CoV-2.
Data are expressed as n (%) or median (interquartile range). Statistically significant values are bold.
Comment: The median age of the patients was 66.5 (interquartile range, 58.3–73.3) years. The exact age is not provided in order to protect confidentiality.
Active disease.
Complete remission.
Relapse.
BAL samples and plasma samples were assessed. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA was detected and quantified by using real-time RT-PCR, as previously described (1, 2). Antibody titers were determined with the Elecsys Anti–SARS-CoV-2 Ig assay (N-protein) (Elecsys Corp.). Viral isolation was performed using native bronchoalveolar samples as described previously (3). After 48–72 hours, infected Vero cells (CCL81; American Type Culture Collection) were monitored for cytopathic effect, and viral replication was confirmed via RT-PCR (1). For statistical analysis, chi-square analysis, the Fisher exact and Mann-Whitney U tests (SPSS 24.0 [IBM Corp.]), and Kaplan-Maier analysis were used (GraphPad Prism 8.4.1 [GraphPad Software]).
Results
On admission, the Sequential Organ Failure Assessment Score and Simplified Acute Physiology Score II did not differ significantly in either study group (see Table 1 for basic patient characteristics). Within the course at the ICU, the need for mechanical ventilation, vasopressor therapy, and renal replacement therapy was high in both study groups (88%, 96%, and 58%, respectively), but mortality was significantly higher for patients with Tx (83% vs. 28%; P < 0.05). SARS-CoV-2 RNA could be detected in respiratory samples of all 24 patients without significant differences between the two study groups (100%; mean virus load, 6.25 × 105 copies/ml; range, 4.05 × 102 to 4.55 × 109), whereas viremia was present in 16 of 21 patients with ethylenediaminetetraacetic acid–plasma samples available on admission to the ICU (6 of 6 patients with Tx and 10 of 15 control patients; 76.19% viremia; mean, 2.21 × 103 copies/ml; range, 0 to 1.30 × 105). Notably, viral RNA loads in plasma were not significantly different between patients with Tx and control patients on admission (mean, 2.04 × 104 vs. 2.01 × 104 copies/ml; P = 0.9875). Over the course of the ICU treatment, viral loads remained constantly high in respiratory samples (mean, 4.21 × 109, 5.58 × 107, and 6.49 × 108 copies SARS-CoV-2 RNA/ml at Days 14, 21, and 28, respectively), and viral RNA counts in plasma increased in five of six patients with Tx (mean, 1.92 × 103, 3.55 × 103, and 1.0 × 106 copies SARS-CoV-2 RNA/ml at Days 14, 21, and 28 respectively). In contrast, 18 of 18 patients without hematologic disease could effectively control the infection, which was reflected by decreasing viral RNA loads in both compartments (Figures 1A and 1B). SARS-CoV-2 RNA was below the limit of detection after 21 and 11 days in respiratory and plasma samples, with a median time to 50% clearance of 7 and 11 days (P = 0.0006 and P = 0.006) (Figures 1C and 1D). Seroconversion with detection of high titers of antibodies against spike and nucleocapsid was detected in only one of the patients with Tx (patient 6) but was detected in all 18 patients in the control group (median antispike IgG at 4 wk, 290 AU/ml). To analyze infectivity of respiratory samples, viral isolation in cell culture was attempted from bronchoalveolar fluids. Five samples from four patients with Tx (range of RNA load, 3.62 × 105 to 5.14 × 109 copies/ml, 4 to 28 d after admission to the ICU) and four control patients (range of RNA load, 1.02 × 104 to 1.56 × 107 copies/ml; <7 d after admission to the ICU) were used. Although time after admission to the ICU was higher in the group with Tx, infectious virus was successfully rescued from five of five samples from patients with Tx, whereas isolation failed in four of four control patients.
Figure 1.
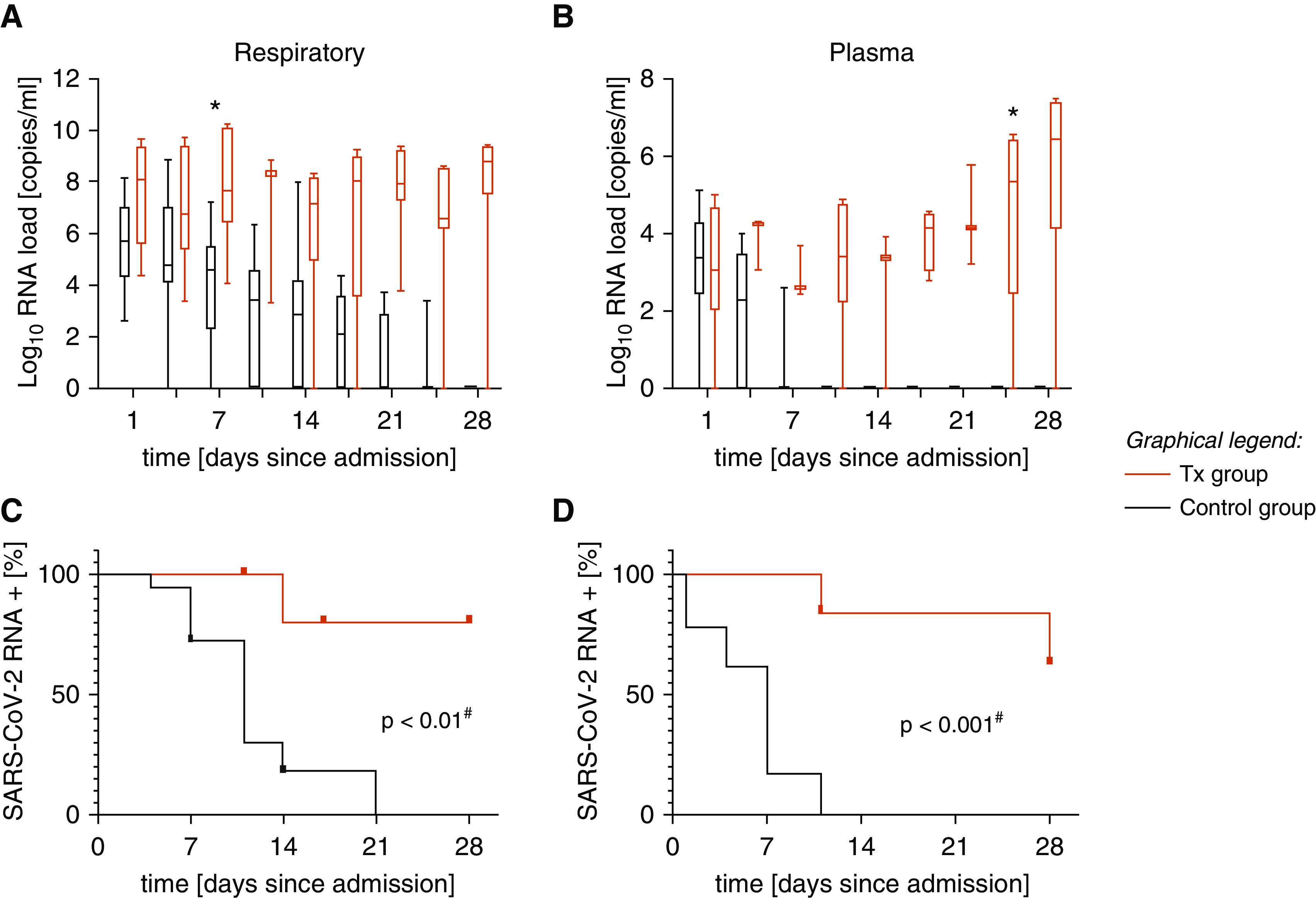
The log10 severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA load in (A) respiratory samples and (B) ethylenediaminetetraacetic acid–plasma samples during the observation period of 4 weeks is displayed. Black plots indicate RNA loads in the control group, and red plots show RNA loads in six patients with a history of hematopoietic stem cell transplant or chimeric antigen receptor T-cell therapy (Tx). Kaplan-Meier curves illustrate the time to viral clearance, defined as a PCR result below the limit of detection in (C) respiratory samples and (D) plasma samples. The red lines correspond to patients with Tx, and the black lines correspond to the control group. *P < 0.001 (two-sided ANOVA); #log-rank test.
Discussion
Overall, our data show that in patients with Tx, a high mortality rate (83%) correlated with failure to control SARS-CoV-2 replication. High mortality (36.8%) in patients with hematologic disease and COVID-19 was also observed in other cohorts analyzing SARS-CoV-2 infection in this special patient cohort (4). All 24 patients had significant levels of SARS-CoV-2 RNA detectable in respiratory samples, but in contrast to previous studies (5, 6), we could not find a correlation between the viral load in the respiratory tract at admission and mortality. Interestingly, the majority (16 of 21) of critically ill patients developed SARS-CoV-2 RNA viremia, indicating systemic spreading of the viral disease. This is in line with postmortem data demonstrating viral RNA in plasma and diverse organs, such as the kidney and heart (7, 8). In four of six of the patients with Tx, constantly high viral RNA loads, or even further increases in viral loads, were observed both in the lung and in the plasma, suggesting an inability to control viral infection. In line with this, in these patients, no virus-specific antibodies could be detected. This is particularly evident in the example of patient 6, an outlier in the group with Tx (HSCT more than 10 yr ago with stable cell counts and mild immunosuppressive therapy with steroids), and 18 of 18 control patients were able to control the infection and to mount a significant and potentially neutralizing antibody response against viral spike domains. To compare the infectivity of viruses detected by using RT-PCR, we performed viral cultures. Interestingly, we successfully isolated infectious virus from five of five samples from patients with Tx (obtained 4–28 d after admission to the ICU), but, in line with data from Wölfel and colleagues (9), we were unable to detect infectious virus after Day 7 in immunocompetent control patients (zero of four patients).
Limitations
First, the sample size of our study is limited, but the significant findings with potential major impact on patient management are of great importance. Second, three patients remained under intensive care treatment on the day of data censoring, resulting in an unknown outcome. Third, some cases had incomplete documentation of clinical data, missing laboratory-testing results, or both.
Conclusions
To our knowledge, this is the first comprehensive report on COVID-19 in patients with a history of HSCT that focuses on virological parameters. In these patients, as compared with immunocompetent patients, prolonged shedding of infectious virus, viremia, and high viral loads in respiratory samples highlight the need of the immune system for viral control but also indicate virus-induced mortality and a higher risk for transmission to other patients and medical staff.
Supplementary Material
Footnotes
Supported by the institutional funds of the University Medical Center Hamburg–Eppendorf, Hamburg, Germany.
Reproducible research statement: Study protocol: Available with approval through written agreement with K.R. (e-mail: k.roedl@uke.de). Data set: Not available.
Originally Published in Press as DOI: 10.1164/rccm.202009-3386LE on November 30, 2020
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Pfefferle S, Reucher S, Nörz D, Lütgehetmann M. Evaluation of a quantitative RT-PCR assay for the detection of the emerging coronavirus SARS-CoV-2 using a high throughput system. Euro Surveill. 2020;25:2000152. doi: 10.2807/1560-7917.ES.2020.25.9.2000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nörz D, Frontzek A, Eigner U, Oestereich L, Wichmann D, Kluge S, et al.Pushing beyond specifications: evaluation of linearity and clinical performance of the cobas 6800/8800 SARS-CoV-2 RT-PCR assay for reliable quantification in blood and other materials outside recommendations J Clin Virol 2020132104650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pfefferle S, Huang J, Nörz D, Indenbirken D, Lütgehetmann M, Oestereich L, et al. Complete genome sequence of a SARS-CoV-2 strain isolated in Northern Germany. Microbiol Resour Announc. 2020;9:e00520-20. doi: 10.1128/MRA.00520-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biernat MM, Zińczuk A, Biernat P, Bogucka-Fedorczuk A, Kwiatkowski J, Kalicińska E, et al. Nosocomial outbreak of SARS-CoV-2 infection in a haematological unit: high mortality rate in infected patients with haematologic malignancies. J Clin Virol. 2020;130:104574. doi: 10.1016/j.jcv.2020.104574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pujadas E, Chaudhry F, McBride R, Richter F, Zhao S, Wajnberg A, et al. SARS-CoV-2 viral load predicts COVID-19 mortality. Lancet Respir Med. 2020;8:e70. doi: 10.1016/S2213-2600(20)30354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Magleby R, Westblade LF, Trzebucki A, Simon MS, Rajan M, Park J, et al. Impact of SARS-CoV-2 viral load on risk of intubation and mortality among hospitalized patients with coronavirus disease 2019 Clin Infect Dis[online ahead of print] 30 Jun 202010.1093/cid/ciaa851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Puelles VG, Lütgehetmann M, Lindenmeyer MT, Sperhake JP, Wong MN, Allweiss L, et al. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med. 2020;383:590–592. doi: 10.1056/NEJMc2011400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lindner D, Fitzek A, Bräuninger H, Aleshcheva G, Edler C, Meissner K, et al. Association of cardiac infection with SARS-CoV-2 in confirmed COVID-19 autopsy cases. JAMA Cardiol. 2020;5:1281–1285. doi: 10.1001/jamacardio.2020.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wölfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Müller MA, et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


