Abstract
Objective:
To determine at which phase in the recruitment process for participation in clinical research studies do health literacy and other patient characteristics influence recruitment outcomes.
Patients and Methods:
Using a sample of 5,872 patients hospitalized with cardiovascular disease approached for participation in the Vanderbilt Inpatient Cohort Study (VICS) from October 2011 through December 2015, we examined the independent association of patients’ health literacy with two steps in their research participation decision-making process: 1) research interest - willingness to hear more about a research study, and 2) research participation - the decision to enroll after an informed consent discussion. Best practices for effective health communication were implemented in recruitment approaches and informed consent processes. Using logistic regression models, we determined patient characteristics independently associated with patients’ willingness to hear about and participate in the study.
Results:
In unadjusted analyses, participants with higher health literacy, and those who were younger, female, or had more education had higher levels of both research interest and research participation. Health literacy remained independently associated with both outcomes in multivariable models, after adjustment for sociodemographic factors.
Conclusion:
Since identical variables predicted both research interest and eventual consent, efforts to recruit broad populations must include acceptable methods of approaching potential participants as well as explaining study materials.
Clinical Trial Registration:
N/A
INTRODUCTION
Recruiting diverse populations to medical research is essential to ensure that findings are generalizable and new interventions are acceptable to real world communities. Understanding the patient factors that influence interest in research participation may inform the design of recruitment protocols, thereby increasing enrollment likelihood. While research indicates potential interest may vary depending on study details, such as time commitment or invasiveness of research procedures,1–3 differences in patient characteristics may also impact participation likelihood. For example, patients of minority race, lower educational attainment, and older age are less likely to participate in clinical research studies.4–6
In addition to sociodemographic factors, health literacy may also influence research participation likelihood. Health literacy can be defined as “the degree to which individuals have the capacity to obtain, process, and understand basic health information and services needed to make appropriate health decisions.”7 Approximately one-third of American adults have limited health literacy.8 Recent research has found that health literacy independently predicted interest in research participation.9 This study, however, asked about hypothetical participation in various types of research studies, and additional information is needed on patient behavior in real world enrollment scenarios.
Further, researchers would benefit from gaining a more granular understanding of when in the research recruitment process do health literacy and other personal characteristics exert an effect. Prior work has demonstrated that health literacy influences how much of the consent process patients understand10 as well as subsequent study follow up.11 Before informed consent can be given, however, patients are often asked to give either explicit or implicit agreement for the recruiting staff to begin providing study information. It remains unclear if patient characteristics and health literacy begin to exert an influence from the very start of the recruitment interaction.
Understanding the factors that influence patient continuation at different phases of research recruitment (from initial interest through enrollment) may provide insight into how recruitment protocols may be modified to enhance diverse recruitment. For example, if potential participants are declining at the outset of an interaction, then referral from a trusted provider may increase interest. Alternatively, if patients are declining after hearing study details, this suggests that modifying information content and delivery may be the most effective. Obtaining insight into this, however, is difficult as most studies do not have access to data on patients who decline participation. In this study, we examine the independent association of health literacy and sociodemographic characteristics with two steps in patients’ research participation decision-making process: 1) research interest - willingness to hear more about a research study, and 2) research participation - the decision to enroll after an informed consent discussion.
METHODS
Study setting and design
The Vanderbilt Inpatient Cohort Study (VICS) is a prospective cohort study of patients with acute coronary syndrome (ACS) and/or acute decompensated heart failure (ADHF) admitted to Vanderbilt University Hospital, a tertiary referral center in Nashville, Tennessee. VICS was designed to investigate the impact of educational, social, behavioral, and functional factors on post-discharge health outcomes such as quality of life, unplanned hospital utilization, and mortality. Details of VICS, including a conceptual framework and rationale for the selection of measures, are described elsewhere.12 The study was approved by the Vanderbilt University Institutional Review Board with waiver of consent for the present analysis.
Subjects
Patients in the present analysis were eligible for enrollment in VICS. Shortly after admission, research staff and physicians reviewed patient medical records and identified patients with ADHF and/or an intermediate or high likelihood of ACS. Exclusion criteria included: age <18 years, non-English speaker, unstable psychiatric condition, delirium, low likelihood of follow-up (e.g., no reliable telephone number), on hospice, previously enrolled in VICS or in a conflicting study, or otherwise too ill to complete an interview. Patients who were temporarily too ill to participate (e.g., delirium) were re-assessed for up to 7 days following admission for potential eligibility. This sample includes all consecutive patients eligible to participate from the beginning of enrollment (October 2011) through just before the end of enrollment (December 2015).
Recruitment and materials
Study protocols, recruitment scripts, and consent documents followed best practices for research recruitment and effective communication across health literacy levels.10,13,14 All research staff were trained to use effective health communication techniques when recruiting and consenting patients, with a focus on plain language, teach-back techniques, and open-ended questions.15 (Training materials available upon request.)
Eligible patients were identified via chart review and approached in-hospital by research staff who briefly described the study and offered to explain the study in more detail. Patients either agreed or declined to hear more about VICS. If patients agreed to hear more, research staff confirmed patient eligibility (also checking for logistical limitations that may impact ability to complete the study such as poor vision/hearing, impaired cognition, or no phone number for follow-up phone calls). If patients failed this screen due to a potentially temporary issue (e.g., delirium), staff circled back the next day to re-administer the screen.
Next, research staff engaged patients who were interested in a formal informed consent discussion, during which staff provided a written consent document, verbal description of the study procedures, and answered all patient questions. Patient understanding of the study and its procedures were confirmed through a thorough teach-back procedure which covered key elements such as the study purpose, its voluntary nature, requirements for participants, and compensation. Patients then either consented to participate in VICS or declined. Participants received $10 upon completion of the enrollment interview and an additional $20 upon completion of follow-up.
Measures
After each approach attempt, research staff logged 1) patient willingness to hear about the research study, and 2) patient decisions to participate (consent). Only the result of the first encounter with the research staff was included in this analysis. All subsequent encounters with the research team were censored. That is, if a patient declined to hear about the study during one hospitalization and was readmitted within the recruitment time frame, all subsequent encounters with the team were not counted in this analysis.
Since November 2010 at the study hospital, nursing staff have regularly administered the Brief Health Literacy Screen (BHLS) to all patients admitted to the hospital.16–18 Additionally, patients who visit Vanderbilt primary care clinics complete the BHLS as a part of their standard outpatient clinic intake. The BHLS is a 3-item measure that asks patients to report their level of confidence filling out medical forms, need for assistance in reading hospital materials, and their understanding of written medical information, each on a 5-point response scale. Scores for this measure can range from 3 to 15, with higher scores representing higher subjective health literacy.
These data, which are stored in the electronic health record, were extracted and merged into the study’s screening database, along with age, gender, race/ethnicity, and educational attainment. Thus, the result was a dataset that included basic demographic information, health literacy screening, and the results from the recruitment approach conducted by the research team.
Statistical analyses
We conducted bivariate analyses to determine the unadjusted effects of patient characteristics on recruitment outcomes. Additionally, we used logistic regression models to determine which patient characteristics were independently associated with 1) patients willing to hear about the study versus those who were not, and 2) eligible patients who consent and enroll versus those who decline to participate after hearing about the study. Results are reported as odds ratios (OR). For continuous variables, the values of the OR indicate the increased odds of the outcome per one unit increase in the variable. All statistical analysis was performed with IBM SPSS Statistics for Windows, version 24.19
RESULTS
Of 5,872 eligible patients approached for recruitment, 3,568 (60.8%) were willing to hear more about the study. Among those, 2,892 (81.1%) were consented and enrolled (Figure 1). For those patients included in this analysis, the mean age was 60 years, 59% were male, and 84% were white. Average educational attainment was about 14 years (Table 1). 63% of patients were hospitalized with ACS, 30% with ADHF, and 7% with both diagnoses. Health literacy scores followed a non-parametric distribution with BHLS median=13.7 (interquartile range 11 to 15) and mean=12.5 (SD=2.9). There was no evidence of collinearity between education and BLHS.
Figure 1. Study Flow Diagram.
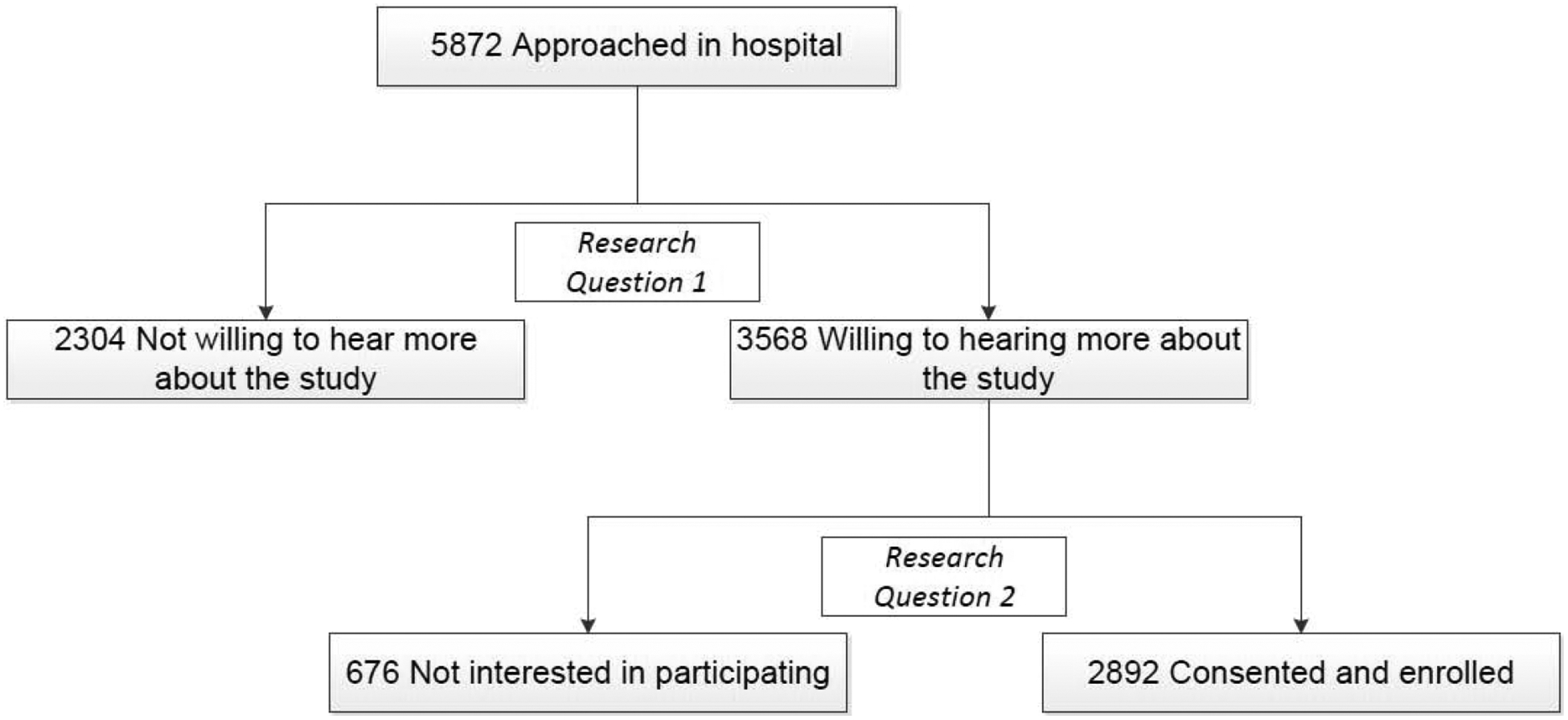
Research Question 1: Which patient characteristics are associated with willingness to hear more about the research study?
Research Question 2: Which patient characteristics are associated with consenting to participate in the research study?
Table 1.
Unadjusted differences in patient characteristics by participation status.
| Not willing to hear more about study | Not interested in participating in study | Consented, enrolled | P value | |
|---|---|---|---|---|
| N=2304 | N=676 | N=2892 | ||
| Agea | 65.8 ± 13.4 | 64.5 ± 12.6 | 60.4 ± 12.5 | <.001e |
| Gender, Maleb | 1433 (62.2) | 417 (61.7) | 1719 (59.4) | .11f |
| Race, Whiteb | 1944 (84.4) | 542 (80.2) | 2419 (83.6) | .32f |
| Diagnosisb | ||||
| ACS onlyc | 1379 (59.9) | 412 (60.9) | 1829 (63.2) | .09f |
| ADHF onlyd | 769 (33.4) | 226 (33.4) | 874 (30.2) | |
| Both | 156 (6.8) | 38 (5.6) | 189 (6.5) | |
| Years of Education | 12.7 ± 3.0 | 13.0 ± 2.9 | 13.6 ± 2.9 | <.001e |
| Health Literacy(BHLS)a | 11.9 ± 3.2 | 12.3 ± 2.9 | 12.9 ± 2.6 | <.001e |
P-values represent a comparison across the 3 columns.
M ± SD
N (%)
Acute coronary syndrome (ACS)
Acute decompensated heart failure (ADHF)
Kruskal-Wallis test
Chi-Square test
In bivariate analyses, patients who consented to enroll in the study were younger and had more education than patients who were not willing to hear about the study at all and those who were not interested in participating in the study after hearing about it (P<.001) (Table 1). We also found that patients who consented to the study had significantly higher health literacy scores (P<.001). No other patient characteristics varied between the groups who were not willing to hear about the study, declined enrollment, or consented.
In multivariable models, characteristics associated with willingness to hear about the study included health literacy score (OR 1.07 per point change in BHLS, 95% CI 1.05–1.09), years of education (OR 1.08 per year, 95% CI 1.06–1.11), female gender (OR 1.19, 95% CI 1.05–1.36), and age (OR 0.98 per year, 95% CI 0.97–0.98) (Table 2). Diagnosis and race were not significantly associated with willingness to hear about the study.
Table 2.
Adjusted predictors of recruitment outcomes.
| Outcome 1: Predictors of willingness to hear more about the study (N=5872) | Outcome 2: Predictors of consenting to the study (N=3568) | |
|---|---|---|
| Patient characteristic | OR (95% CI) | OR (95% CI) |
| Age | 0.98 (0.97–0.98)d | 0.97 (0.96–0.98)d |
| Gender, Female | 1.19 (1.05–1.36)c | 1.25 (1.02–1.53)c |
| Race, Black/Other | 0.99 (0.82–1.19) | 0.81 (0.61–1.07) |
| Diagnosis, ADHFa only | 1.00 (0.87–1.15) | 0.95 (0.76–1.17) |
| Diagnosis, ACSb and ADHFa | 1.01 (0.78–1.29) | 1.41 (0.92–2.16) |
| Diagnosis, ACS only | (Reference) | (Reference) |
| Education | 1.08 (1.06–1.11)d | 1.07 (1.04–1.12)d |
| Health Literacy (BHLS) | 1.07 (1.05–1.09)d | 1.04 (1.00–1.08)c |
For continuous variables, values of the OR indicate the increased odds of the outcome per one unit increase in the variable.
Acute decompensated heart failure (ADHF)
Acute coronary syndrome (ACS)
P<.05,
P<.001
The same characteristics were associated with consenting to enroll relative to those who declined after hearing more about the study: health literacy score (OR 1.04 per point change in BHLS, 95% CI 1.00–1.08), years of education (OR 1.07 per year, 95% CI 1.04–1.12), female gender (OR 1.25, 95% CI 1.02–1.53), and age (OR 0.97 per year, 95% CI 0.96–0.98) (Table 2). Diagnosis and race were not significantly associated with research participation.
CONCLUSIONS
We found that, in spite of following best practices for effective health communication during recruitment and informed consent, not all patient groups were equally likely to express interest in hearing more about the research study or consent to participate. Patients with higher research interest were more likely to be younger, female, more educated, or have higher health literacy. Among eligible patients who agreed to hear about the study, patients with these characteristics were also more likely to participate. Health literacy was independently associated with both research interest and participation, controlling for patient demographic factors. For each 3-point decrease in health literacy score (equal to 1 SD, or a change of one response value in each question on a 5-point Likert scale) patients had 21% lower odds of research interest and 12% lower odds of consenting to participate. Notably, race was not significantly associated with research interest or participation.
Results of this large analysis of hospitalized adults are consistent with our recent outpatient study in which patients with lower health literacy were less likely to express interest in participating in a variety of types of hypothetical research studies, particularly those that might involve higher literacy-related demands such as completing surveys.9 Here we see that patient behavior is influenced early in the recruitment process in different ways. First, those with lower health literacy skills may be avoiding a situation that is optional and would require the use of these skills, such as interacting with research staff to learn more about a potential study. Second, health literacy may also influence patients to decline enrollment after study details are shared. In this scenario, a patient’s challenges in understanding study procedures and informed consent may influence a patient to view research participation as confusing, and therefore potentially risky and unpleasant. Studies have found that even when informed consent is conducted orally, using various effective health communication aids such as visual presentations and teach-back,10,20 patients with lower health literacy continue to understand less information. This challenge may explain why disparities in research interest and participation persisted despite the use of known practices to foster recruitment and informed consent.
In this study, health literacy, gender, age, and education had a consistent impact across phases of research recruitment. This suggests that health literacy may exert an early role in patient disinterest in research, and that efforts to engage patients should begin in the initial patient approach, though this step has received far less attention than the explanation of informed consent.21 Patients who explicitly decline opportunities to hear about research (or have already mentally checked out of research study descriptions) are unlikely candidates for eventual consent and participation.
One recent theory-based study found that perceived behavioral control and ability of the research to contribute to collective health were related to individuals’ intention to participate.22 Another found that even small changes in wording may affect recruitment.23 For example, our recruitment script used plain language but included the phrases “research coordinator” and “research project.” If preferred by patients and acceptable to IRBs, alternate wording such as “project” or “survey” might be more effective for early engagement, while still making clear during the consent process that the project is a research study and participation is voluntary. Additional research is needed among patients with low health literacy, as well as other disadvantaged groups, to further explore factors that could influence interest levels,24 and to test approaches in practice.
We did not find a significant relationship between minority race and research interest and participation, in contrast to many4–6 but not all25 prior research studies. It is possible that the inclusion of health literacy in our models better accounted for the relationship between minority status and research interest and participation. Alternatively, the communication strategies in this study, which also focused on showing respect and effectively building rapport with patients, may have been particularly effective at reducing disparities in enrollment between minority groups while being less effective at addressing disparities driven by gender, age, education, or health literacy.
Several limitations were present. First, the study was conducted at a single hospital, which limits generalizability. Second, because potential research participants who decline enrollment also do not provider further data, this study relied on electronic health records (EHR) which limits our ability to examine the relationship of other important factors related to research participation, like mistrust, which has been documented as a barrier to research participation, particularly among minority groups.4,26 Interestingly, among participants enrolled in the present study, levels of trust in physicians and in the healthcare system were not significantly associated with race and other demographic characteristics,27 but this leaves open the question of whether patients with lower levels of trust chose not to participate. Third, while the demographic characteristics of the approached patients generally reflect the population of the Mid-South, certain groups were underrepresented including Latinos (due to low prevalence among hospitalized cardiac patients) or individuals who do not speak English fluently (due to ineligibility). Future studies are needed on a broader population. Lastly, to simplify the study design and treat all patients similarly, we only analyzed the first time patients were approached by research staff; some patient groups might have agreed to hear about the study or enroll on subsequent contact attempts.
Controlling for education level, gender, and age, individuals with lower health literacy were less interested in hearing about the research study, and less likely to consent to participate after completing the informed consent process. This disparity was present despite following best practices for effective health communication in the approaches to recruitment and informed consent. Identical factors predicted outcomes in research interest and enrollment, suggesting that best practices must also attend to how to initially approach potential participants. Researchers should be aware of the effect of low health literacy on research interest and participation and should test additional strategies to close this gap.
Acknowledgments
We acknowledge the outstanding work of the recruitment staff and project coordinators throughout this study: Courtney Cawthon, Catherine Couey, Monika Rizk, Hannah Rosenberg, Daniel Lewis, Blake Hendrickson, Olivia Dozier, Vanessa Fuentes, Cardella Leak, Mary Lou Jacobsen, Catherine Evans, Joanna Lee, Emily Lucianno, and Erin Acord. We also acknowledge the following additional co-investigators in the VICS study group: Susan P. Bell, MD, MSCI; Frank E. Harrell, Jr., PhD; Amanda S. Mixon, MD, MS, MSPH; Russell L. Rothman, MD, MPP; and Jonathan S. Schildcrout, PhD.
Funding: This study was supported by grant R01 HL109388 from the National Heart, Lung, and Blood Institute and in part by grant 2 UL1 TR000445-06. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funding agency was not involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Abbreviations and Acronyms
- ACS
Acute Coronary Syndrome
- ADHF
Acute Decompensated Heart Failure
- BHLS
Brief Health Literacy Screen
- VICS
Vanderbilt Inpatient Cohort Study
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest disclosures: No relevant conflicts to disclose.
References
- 1.Bonevski B, Randell M, Paul C, et al. Reaching the hard-to-reach: a systematic review of strategies for improving health and medical research with socially disadvantaged groups. BMC Med Res Methodol. 2014;14:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taylor RG, Hounchell M, Ho M, Grupp-Phelan J. Factors associated with participation in research conducted in a pediatric emergency department. Pediatr Emerg Care. 2015;31(5):348–352. [DOI] [PubMed] [Google Scholar]
- 3.Voss R, Gravenstein S, Baier R, et al. Recruiting hospitalized patients for research: how do participants differ from eligible nonparticipants? J Hosp Med. 2013;8(4):208–214. [DOI] [PubMed] [Google Scholar]
- 4.George S, Duran N, Norris K. A systematic review of barriers and facilitators to minority research participation among African Americans, Latinos, Asian Americans, and Pacific Islanders. Am J Public Health. 2014;104(2):e16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scharff DP, Mathews KJ, Jackson P, Hoffsuemmer J, Martin E, Edwards D. More than Tuskegee: understanding mistrust about research participation. J Health Care Poor Underserved. 2010;21(3):879–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stewart JH, Bertoni AG, Staten JL, Levine EA, Gross CP. Participation in surgical oncology clinical trials: gender-, race/ethnicity-, and age-based disparities. Ann Surg Oncol. 2007;14(12):3328–3334. [DOI] [PubMed] [Google Scholar]
- 7.Institute of Medicine. Health Literacy. A Prescription to End Confusion. Washington, DC: National Academies Press; 2004. [PubMed] [Google Scholar]
- 8.Kutner M, Greenberg E, Jin Y, Paulsen C. The Health Literacy of America’s Adults: Results from the 2003 National Assessment of Adult Literacy (NCES 2006–483). Washington, DC: U.S. Department of Education, National Center for Education Statistics; 2006. [Google Scholar]
- 9.Kripalani S, Heerman WJ, Patel NJ, et al. Association of health literacy and numeracy with interest in research participation. J Gen Intern Med. 2019;34(4):544–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kripalani S, Bengtzen R, Henderson LE, Jacobson TA. Clinical research in low-literacy populations: using teach-back to assess comprehension of informed consent and privacy information. IRB: Ethics & Human Research. 2008;30(2):13–19. [PubMed] [Google Scholar]
- 11.Leak C, Goggins K, Schildcrout JS, et al. Effect of health literacy on research follow-up. J Health Commun. 2015;20 Suppl 2:83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meyers AG, Salanitro A, Wallston KA, et al. Determinants of health after hospital discharge: rationale and design of the Vanderbilt Inpatient Cohort Study (VICS). BMC Health Serv Res. 2014;14:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kripalani S, Weiss BD. Teaching about health literacy and clear communication. J Gen Intern Med. 2006;21:888–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Winter SS, Page-Reeves JM, Page KA, et al. Inclusion of special populations in clinical research: important considerations and guidelines. J Clin Transl Res. 2018;4(1):56–69. [PMC free article] [PubMed] [Google Scholar]
- 15.Kripalani S, Jacobson KL, Brown S, Manning K, Rask KJ, Jacobson TA. Development and implementation of a health literacy training program for medical residents. Med Educ Online. 2006;11(13):1–8. [DOI] [PubMed] [Google Scholar]
- 16.Cawthon C, Mion LC, Willens DE, Roumie CL, Kripalani S. Implementing routine health literacy assessment in hospital and primary care patients. Jt Comm J Qual Patient Saf. 2014;40(2):68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chew LD, Bradley KA, Boyko EJ. Brief questions to identify patients with inadequate health literacy. Fam Med. 2004;36(8):588–594. [PubMed] [Google Scholar]
- 18.Wallston KA, Cawthon C, McNaughton CD, Rothman RL, Osborn CY, Kripalani S. Psychometric properties of the brief health literacy screen in clinical practice. J Gen Intern Med. 2014;29(1):119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.IBM SPSS Statistics for Windows, Version 24.0. [computer program]. Armonk, NY: IBM Corp.; Released 2016. [Google Scholar]
- 20.Ownby RL, Acevedo A, Goodman K, Caballero J, Waldrop-Valverde D. Health literacy predicts participant understanding of orally-presented informed consent information. Clin Res Trials. 2015;1(1):15–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flory J, Emanuel E. Interventions to improve research participants’ understanding in informed consent for research: a systematic review. JAMA. 2004;292(13):1593–1601. [DOI] [PubMed] [Google Scholar]
- 22.Cote M, Harrison S, Lapointe A, et al. A cross-sectional survey examining motivation and beliefs to participating in a web-based prospective cohort study on nutrition and health among individuals with a low socioeconomic status. BMC Public Health 2020;20(1):348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Godinho A, Schell C, Cunningham JA. How one small text change in a study document can impact recruitment rates and follow-up completions. Internet Interv. 2019;18:100284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phoenix M, Nguyen T, Gentles SJ, VanderKaay S, Cross A, Nguyen L. Using qualitative research perspectives to inform patient engagement in research. Res Involv Engagem. 2018;4:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wendler D, Kington R, Madans J, et al. Are racial and ethnic minorities less willing to participate in health research? PLoS Med. 2006;3(2):e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilkins CH. Effective engagement requires trust and being trustworthy. Med Care. 2018;56 Suppl 10 Suppl 1:S6–S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gupta C, Bell SP, Schildcrout JS, et al. Predictors of health care system and physician distrust in hospitalized cardiac patients. J Health Commun. 2014;19 Suppl 2:44–60. [DOI] [PMC free article] [PubMed] [Google Scholar]


