Abstract
Aim:
To examine associations between the deep medullary vein white matter injury global severity scoring system and neurodevelopmental impairment.
Methods:
This is a prospective observational cohort study of infants born at ≥32 weeks, diagnosed with deep medullary vein thrombosis and infarction on neuroimaging in the first month of life. Developmental testing was performed using validated measures for early, preschool and school age follow-up.
Results:
Nineteen (37%) patients had major neurodevelopmental impairment. Global severity score was higher among patients with neurodevelopmental impairment (21.6 vs 13.4, p = .04). 78% of patients with epilepsy had neurodevelopmental impairment. A greater degree of asymmetry with right-sided injury predominance was associated with lower Bayley-III cognitive scores and presence of neurodevelopmental impairment (p < .01).
Conclusions:
Results suggest a need for targeted clinical surveillance for patients with a high global severity score and/or asymmetric, predominantly right cerebral white matter injury and for those who develop epilepsy.
INTRODUCTION
Cerebral sinovenous thrombosis is an important cause of morbidity and mortality in children, with nearly half of cases occurring in neonates.1,2 Refined neonatal neuroimaging protocols, improved MR image quality, and advanced imaging techniques such as susceptibility weighted imaging (SWI) allow earlier and more accurate detection of previously under-recognized cerebral deep vein thrombosis and infarction.3 Linear, radially oriented, fan-shaped lesions in the periventricular white matter are indicative of deep medullary vein (DMV) involvement.4 The characteristic imaging pattern of DMV thrombosis and infarction, explained by the anatomic distribution of the white matter venous drainage,5,6 has been of increasing concern to clinicians caring for infants and children over the past decade.4,5,7 We first developed a DMV white matter injury severity scoring system to comprehensively and objectively define brain injury after DMV thrombosis and infarction in neonates, to facilitate clinician response in the neonatal period.8 The DMV white matter injury global severity score allows stratification of patients by severity of radiologic findings at the time of diagnosis and is independent of gestational age and other antenatal risk factors.8
Published literature describing outcomes after neonatal cerebral sinovenous thrombosis is limited and outcome reports vary widely: 23–79% of patients have adverse neurologic or developmental sequelae depending on the study.1,2,9–13 Specific developmental impairment is typically related to the location and extent of injury; cognitive, language, neurosensory or behavioral problems, cerebral palsy or epilepsy have all been reported.2,9,10,14,15 Given this variability in outcomes and populations, many reports publish aggregate neurodevelopmental data to communicate outcomes (i.e. normal vs. abnormal neurologic or developmental outcomes, or mild, moderate or severe impairment).2,9,13,16 At this time, clinical outcome data after DMV pathology in infants are limited.4,7 Evaluating DMV characteristics associated with impairments throughout childhood may help direct early clinical surveillance following DMV thrombosis and infarction in the neonatal period.
The aim of this study was therefore to examine associations between the published DMV white matter injury global severity scoring system in a retrospectively identified cohort, to predict prospectively measured neurodevelopmental impairments. We hypothesized that infants with higher white matter injury global injury severity scores would have higher rates of neurodevelopmental impairment (NDI), even when controlling for gestational age and socioeconomic status (SES).
METHODS
Study design and participants
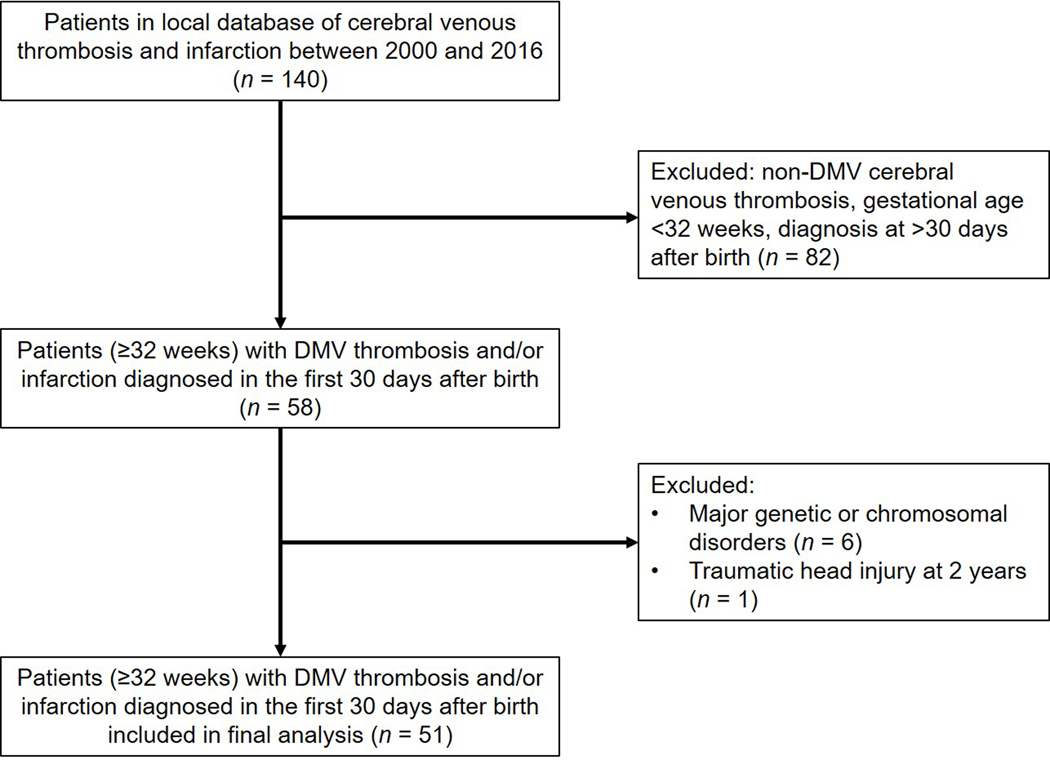
This was a prospective observational cohort design. We retrospectively identified patients with a diagnosis of DMV thrombosis and infarction in a pediatric neuroradiology database. All subjects were admitted to the neonatal intensive care unit or other hospital unit at our institution between 2000 and 2016. Included in the review were infants born at ≥32 weeks of gestation, diagnosed with DMV thrombosis and infarction on neuroimaging during the first 30 days after birth. We excluded infants with congenital central nervous system anomalies or major genetic or chromosomal disorders (Figure 1). The electronic medical record was reviewed and all pertinent clinical, laboratory, imaging and follow-up data was collected. Qualifying patients were invited for prospective neuropsychological testing; the specific tests selected were based on the child’s age at the time of testing and to provide the most consistent standardized approach. This study was approved by the Nationwide Children’s Hospital institutional review board for human research (IRB16–00644). Written informed consent was obtained from a parent or guardian of the participant and verbal assent was obtained from the participant when appropriate. Study data were managed using REDCap electronic data capture tools (https://www.project-redcap.org) hosted at our institution.17
Figure 1.
Flow diagram summarizing patients excluded and those included in the final analysis.
MR imaging acquisition
All brain MR imaging was obtained at either 1.5T or 3T (Excite HDXT 1.5T and GE Signa MR750 3T; GE Healthcare, Milwaukee, Wisconsin; and Magnetom Espree 1.5T and Magnetom Skyra 3T; Siemens, Erlangen Germany). Due to the retrospective nature of the cohort identification, imaging protocols, sequences, and parameters varied. Scans included a combination of T1, T2, T1 FLAIR, DWI, and SWI sequences.
MR imaging assessment
While all scans for all subjects were reviewed, the MRI obtained at the time of initial radiologic diagnosis of DMV thrombosis and infarction was used for scoring purposes. A previously published standardized scoring system was used to describe the severity of cerebral white matter injury after DMV thrombosis and infarction.6
The DMV white matter injury-severity scoring system assigns points for the degree of injury for focal lesions within the right and left frontal, parietal, occipital, and temporal lobes, for focal infarction within the corpus callosum and for diffuse white matter cerebral edema.8 All scores were added to yield a global severity score (0–102 points) with an asymmetry score comparing injury severity between right and left hemispheres. Degree of asymmetry was calculated as the absolute value of the difference between left and right scores, while the predominant side was defined as left if (L-R) value was positive, as right if (L-R) value was negative, and as equal if (L-R) value was zero. Two pediatric radiologists independently scored the scans and then agreed on the final consensus scoring after review of any discrepancies. On a random testing set of the entire sample, initial interrater reliability had an intra class coefficient for average measures of 0.802 (two-way mixed effects model). After review and consensus of the testing set, with improved specificity of definitions, intra class coefficient was >0.990.
Neurodevelopmental outcome assessment measures
The choice of instruments and testing were dictated by two priorities: (1) use of validated assessments using standardized scores with a mean of 100 and standard deviation of 15, that could be performed in a highly reliable manner by trained examiners (2) continuity of constructs throughout infancy and childhood. For these reasons, we chose the Bayley Scales of Infant and Toddler Development, Third Edition (Bayley-III) to assess cognitive, motor, and language development in children under 30 months. Composite scores for all three domains could be obtained from our high-risk infant follow-up clinic, where testers were tested for reliability and adherence to standard administration as part of the Neonatal Research Network.18 Scores derived included cognitive, motor and language composite scores (standardized population mean 100, standard deviation 15).19
Cognitive function in children ≥30 months was assessed via the Wechsler Preschool and Primary Scale of Intelligence, Fourth Edition (WPPSI-IV) (ages 2:6 to 6:11)20 or the Wechsler Intelligence Scale for Children, Fifth Edition (WISC-V) (ages 7:0 to 16:11)21 depending on the child’s age, as these two assessments were designed to have continuity.22–24 Scores derived included verbal IQ, performance IQ and age-standardized full-scale IQ (standardized population mean 100, standard deviation 15). Cerebral palsy diagnosis was confirmed through a review of neurological exams and clinic notes, and classified according to the Gross Motor Function Classification System25 on the basis of the motor function documented at the last visit.
Behavioral and emotional problems were assessed using the Child Behavior Checklist (CBCL) completed by the child’s primary caregiver. The CBCL (1 ½−5 year and 6–18 year form) is a validated questionnaire, designed to provide continuity in assessment from 18 months to 18 years.26,27 The CBCL allows screening for internalizing (anxious/depressed, withdrawn/depressed, somatic complaints) and externalizing (rule-breaking behavior, aggressive behavior) problems corresponding to Diagnostic and Statistical Manual of Mental Disorders classifications. Scoring results in calculation of internalizing, externalizing and total problem T scores with higher scores indicating a higher level of behavioral problems. A T score ≥64 is classified as clinically abnormal, corresponding to a score above the 90th percentile for age and gender. Socioeconomic status was represented by the Hollingshead Four-Factor Index score, based on report of maternal and paternal occupation, education and marital status.28 Primary outcome was the presence of major NDI, defined as any of the following: a Bayley-III, WPPSI-IV or WISC-V score more than 2 standard deviations below the mean, major hearing impairment (bilateral hearing loss requiring amplification), major vision impairment (cortical vision impairment, bilateral visual field loss, bilateral surgical correction of visual defect), major language impairment or a diagnosis of cerebral palsy.
Statistical Analysis
Data were summarized using frequencies and percentages for categorical variables and means with standard deviations for continuous variables. Two-sample t-tests were used to compare differences by NDI status, and Pearson correlations were used to assess associations among all continuous study variables. Univariate and multivariable logistic regression examined whether global severity score was associated with development of NDI. Area under the ROC curve (AUC) values described discriminatory ability of each logistic regression model. AUC values range from 0.5 to 1, with values near 0.5 suggesting the model is no better random chance at predicting which patients will develop NDI, and values near 1.0 suggesting perfect prediction; typically, values above 0.8 are considered to have good discriminatory ability. An optimum threshold value for using global severity score to predict NDI was calculated using Youden’s J statistic, which simultaneously maximizes the sensitivity and specificity of the threshold. Univariate and multivariable linear regression studied whether global severity score was associated with each of the other neurodevelopmental outcomes with continuous distributions. For all linear regression models, R-squared values were used as a measure of goodness of fit and explanatory ability of the model; R-squared values range from 0 to 1, with higher values suggesting better model fit, and represent the proportion of variability in the outcome that is explained by the model. For all multivariable models, gestational age and Hollingshead Score (to represent SES) were included as covariates. Evaluation of categorical outcomes other than NDI was not performed due to insufficient effective sample size. All analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC) with two-sided p-values <.05 considered statistically significant.
RESULTS
Our cohort included 51 patients, 63% were male. Mean gestational age at birth was 37 weeks (range, 32–41 weeks) with mean birth weight 3182 ± 720g. Neonatal comorbidities included encephalopathy (20%), neonatal infection (18%), hypoglycemia (35%) complex congenital heart disease (8%), meningitis (8%) and prothrombotic disorders (8%). Infants presented on day of life 4.5 ± 5.3 with seizures (55%), apnea (53%), lethargy (49%) and poor feeding (35%). The average age at diagnosis of DMV thrombosis or infarction via MR imaging was postnatal day 10.1 ± 6.1.
Group level neurodevelopmental outcomes are summarized in Table 1. Current age of cohort patients ranged from 2–17 years. Nineteen (37%) patients had major NDI, 8 (16%) had language impairment, 2 (4%) had major hearing impairment, 10 (20%) had major vision impairment and 10 (20%) had cerebral palsy. Eight (24%) patients had a CBCL total problems T score above the risk cutoff (≥64). Similarly, 10 (30%) and 8 (24%) patients had CBCL T scores above the risk cutoff on the internalizing and externalizing problem scales respectively.
Table 1:
Cohort neurodevelopmental outcomes
| Variable | Test n (total cohort = 51) | |
|---|---|---|
| Cognitive | ||
| FSIQ composite score a, mean ± SD | 26 | 82.6 ± 24.2 |
| FSIQ composite score a <70, n (%) | 26 | 6 (23) |
| Bayley-III cognitive composite, mean ± SD | 28 | 91.3 ± 24.9 |
| Bayley-III cognitive composite <70, n (%) | 28 | 3 (11) |
| Behavioral | ||
| CBCL total problems T score b ≥64, n (%) | 33 | 8 (24) |
| CBCL internalizing T score b ≥64, n (%) | 33 | 11 (22) |
| CBCL externalizing T score b ≥64, n (%) | 33 | 8 (24) |
| Major behavioral diagnosis c, n (%) | 51 | 11 (22) |
| Motor | ||
| Bayley-III motor composite, mean ± SD | 29 | 89.8 ± 24.3 |
| Bayley-III motor composite <70, n (%) | 29 | 4 (14) |
| Cerebral palsy, n (%) | 51 | 10 (20) |
| Language | ||
| Bayley-III language composite, mean ± SD | 25 | 92 ± 14.9 |
| Bayley-III language composite <70, n (%) | 25 | 2 (8) |
| Major language impairment | 51 | 8 (16) |
| Other impairments | ||
| Epilepsy, n (%) | 51 | 9 (18) |
| Major hearing impairment, n (%) | 51 | 2 (4) |
| Major vision impairment, n (%) | 51 | 10 (20) |
| NDI d, n (%) | 51 | 19 (37) |
Full Scale IQ assessed by WPPSI-IV or WISC-V depending on child’s age, score <70 corresponds to more than 2 SD below the mean
CBCL total problems, internalizing and externalizing T scores ≥64 considered clinically abnormal (above 90th percentile)
Major behavioral diagnosis defined as ADHD, ASD, PDD, mood disorders, other
NDI defined as any of the following: Bayley-III, WPPSI-IV or WISC-V score >2 SD below the mean (i.e. score <70 on any domain), major hearing impairment, major vision impairment, major language impairment or a diagnosis of cerebral palsy
ADHD, attention-deficit hyperactivity disorder; ASD, autism spectrum disorder; Bayley-III, Bayley Scales of Infant and Toddler Development, Third Edition; CBCL, Child Behavior Checklist; FSIQ, full-scale IQ; NDI, neurodevelopmental impairment; PDD, pervasive developmental disorder; SD, standard deviation; WISC-V, Wechsler Intelligence Scale for Children, Fifth Edition; WPPSI-IV, Wechsler Preschool and Primary Scale of Intelligence, Fourth Edition
Gestational age, birth weight, SES, neurologic comorbidities, and neuroimaging findings were compared between patients with and without neurodevelopmental impairment in Table 2. Gestational age, birth weight and Hollingshead score (representing SES) were not different between groups. Patients who developed epilepsy were more likely to have neurodevelopmental impairment (78% of patients with epilepsy had NDI). DMV white matter injury global severity scores ranged from 1–53 and were divided into quartiles (1st quartile score 1–5; 2nd quartile score 6–11; 3rd quartile score 12–25; 4th quartile score ≥26). Representative MR images with corresponding diagrammatic scoring from a patient without neurodevelopmental impairment (global severity score 11, 2nd quartile) and a patient with neurodevelopmental impairment (global severity score 25, 3rd quartile) are shown in Figures 2 and 3 respectively. The global severity score was significantly higher among patients with NDI (mean global severity score = 21.6 in patients with NDI vs. 13.4 in patients without NDI, p = .04, Table 2). Based on ROC curve analysis, the optimal cut-point of the global severity score for differentiating patients who developed NDI versus those who did not was a score >16. However, sensitivity, specificity and AUC were 66%, 58% and 0.63 respectively.
Table 2.
Comparison of patients with and without neurodevelopmental impairment
| Total (n=51) | No NDI (n=32) | NDI (n=19) | ||
|---|---|---|---|---|
| Mean ± SD or N (%) | Mean ± SD or N (%) | Mean ± SD or N (%) | P value | |
| Gestational age, weeks | 37.3 ± 2.2 | 37.5 ± 2.2 | 37 ± 2 | .37 |
| Birth weight, g | 3182 ± 720 | 3279 ± 643 | 3029 ± 822 | .24 |
| Hollingshead total score | 40.9 ± 13.9 | 42.7 ± 14.5 | 38.2 ± 13 | .37 |
| Neurologic comorbidity a | 14 (27) | 9 (28) | 5 (26) | .89 |
| Epilepsy | 9 (18) | 2 (6) | 7 (37) | .009 |
| GSS | 16.5 ± 14 | 13.4 ± 12.1 | 21.6 ± 15.6 | .04 |
| GSS 3rd & 4th quartile b | 24 (47) | 12 (38) | 12 (63) | .08 |
| Predominant side of injury c | .10 | |||
| Left | 17 (33) | 10 (31) | 7 (37) | |
| Right | 17 (33) | 8 (25) | 9 (47) | |
| Equal | 17 (33) | 14 (44) | 3 (16) | |
| Degree of asymmetry d | 2.2 ± 2.6 | 1.6 ± 1.9 | 3.1 ± 3.4 | .09 |
Neurologic comorbidity at diagnosis of DMV thrombosis/infarction defined as HIE and/or meningitis
3rd & 4th quartile corresponds to GSS 12–25 and ≥26 respectively
Predominant side of injury was defined as left if (L-R) value was positive, as right if (L-R) value was negative, and as equal if (L-R) value was zero.
Degree of asymmetry was calculated as the absolute value of the difference between left and right scores
DMV, deep medullary veins; g, grams; GSS, global severity score; HIE, hypoxic-ischemic encephalopathy; L, left; NDI, neurodevelopmental impairment; R, right; SD, standard deviation
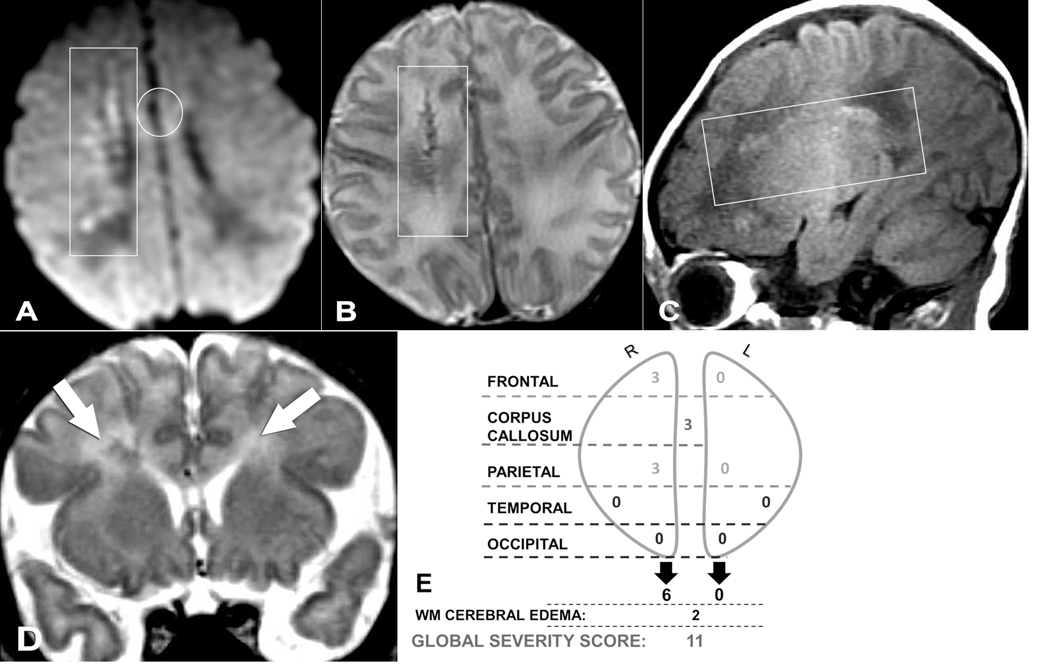
Figure 2.
(A) Axial DWI, (B) axial T2-weighted, (C) sagittal T1-weighted, and (D) coronal T2-weighted magnetic resonance images (MRIs) of a 7-day-old male neonate who presented with seizures. (E) Global severity score = 11 (second quartile). There are severe right frontal (3 points) and severe right parietal (3 points) hemorrhagic linear lesions (T1 bright and T2 dark) with restricted diffusion (+DWI) (A-C, boxes). There is restricted diffusion throughout the corpus callosum (genu shown, A, circle) (3 points) and asymmetric, bilateral white matter edema, R > L (D, arrows) (2 points). Neuropsychological testing was normal at 8 years of age, and the patient had no major neurodevelopmental or neurosensory impairment. DWI, diffusion-weighted imaging; R, right; L, left; WM, white matter.
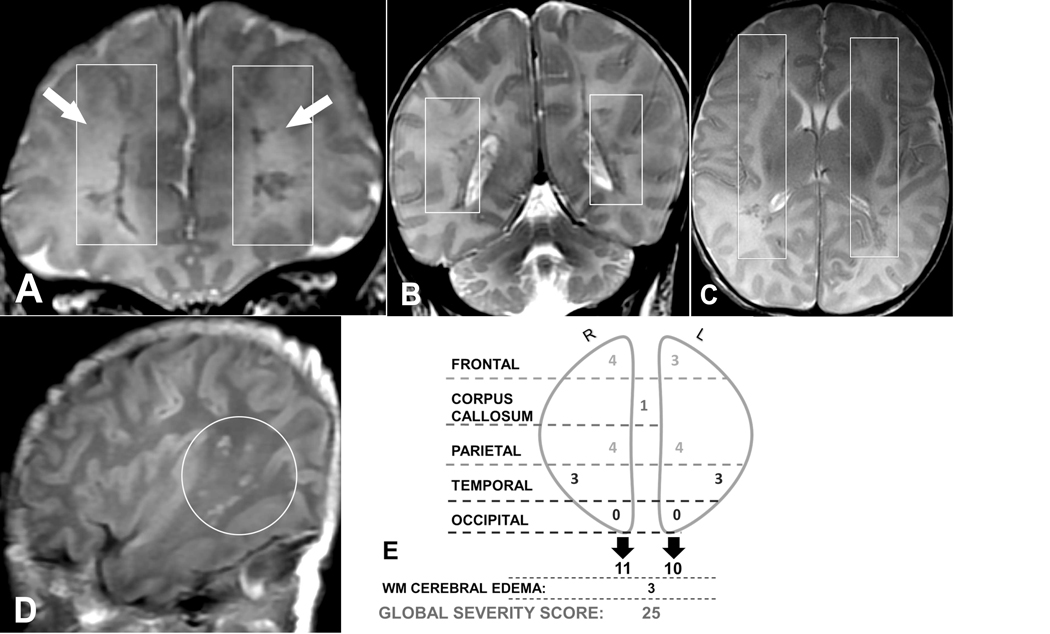
Figure 3.
(A, B) Coronal T2-weighted, (C) axial T2-weighted, and (D) sagittal T1-weighted magnetic resonance images (MRIs) of a 9-day-old male neonate who presented with fever, lethargy, poor feeding, and respiratory failure. The global severity score was 25 (third quartile) (E). There are right frontal (severe punctate, 3 points, and mild linear, 1 point), right parietal (severe punctate, 3 points, and mild linear, 1 point), left frontal (severe punctate, 3 points), and left parietal (severe punctate, 3 points, and mild linear, 1 point) hemorrhagic T2 dark lesions (A-C, boxes). There are bilateral, symmetric, hemorrhagic T1 bright temporal lobe (severe punctate, 3 points each) lesions (right shown, D, circle). There is restriction diffusion in the splenium of the corpus callosum (not shown, 1 point). There is bilateral diffuse increased cerebral white matter T2 signal of edema (A, arrows) (3 points). Neuropsychological testing at 4.5 years revealed a full-scale IQ of 69 (>2 SDs below the mean) corresponding to neurodevelopmental impairment. R, right; L, left; WM, white matter.
Global severity score and degree of asymmetry were positively correlated (r = 0.41, p < .01) and patients with any asymmetry had a significantly higher global severity score compared to those without asymmetry (median [IQR] = 4 [3–7] vs 18 [10–28], p = .001). White matter injury was more commonly asymmetric in patients with NDI (84% asymmetric in patients with NDI versus 56% asymmetric in patients without NDI, p = .04). A greater degree of asymmetry with right-sided injury predominance (right sided lesions larger and/or more severe than left sided lesions) was associated with significantly lower Bayley-III cognitive composite scores and presence of NDI (p < .01). On its own, the degree of asymmetry accounted for 30% of the variability in cognitive composite scores. After controlling for global severity score, gestational age and SES, right side injury predominance was the only variable to remain significantly associated with cognitive composite scores (p = .04).
Table 3 shows unadjusted and adjusted odds of developing NDI. When the DMV white matter injury global severity score, SES, gestational age and predominant side of injury were considered together in a multivariable model, their combined ability to discriminate NDI from non-NDI was good (AUC = 0.82).
Table 3.
Unadjusted and adjusted odds of neurodevelopmental impairment and multivariable model
| Univariate | Multivariable Model a | |||||
|---|---|---|---|---|---|---|
| Unadjusted OR (95% CI) | P value | Variable AUC | Adjusted OR (95% CI) | P value | Model AUC | |
| GSS | 1.04 (1.0–1.1) | .048 | 0.66 | 1.03 (1.0–1.1) | .33 | 0.82 |
| 4th quartile GSS (≥26) | 2 (0.5–0.7) | .30 | 0.56 | |||
| Degree asymmetry | 1.3 (1.0–1.6) | .07 | 0.65 | |||
| Predominant side of injury b | ||||||
| Left | 3.3 (0.7–15.8) | .14 | 2.6 (0.3–23.4) | .40 | ||
| Equal | 0.67 | |||||
| Right | 5.3 (1.1–25.2) | .04 | 7.3 (0–7.3) | .09 | ||
| Hollingshead score | 1.0 (0.9–1.0) | .36 | 0.60 | 1.0 (0.9–1.1) | .59 | |
| Gestational age | 0.9 (0.7–1.2) | .35 | 0.61 | 0.9 (0.7–1.3) | .71 | |
| Birth weight | 1.0 (0.9–1.0) | .24 | 0.61 | |||
Model was adjusted for GSS, SES, GA and predominant side of injury
Predominant side of injury was defined as left if (L-R) value was positive, as right if (L-R) value was negative, and as equal if (L-R) value was zero.
AUC, area under the curve; GA, gestational age; GSS, global severity score; OR, odds ratio; SES, socioeconomic status
DISCUSSION
This is the first study to evaluate long-term neurodevelopmental outcomes in patients with neonatal DMV thrombosis and infarction. While lesions attributed to DMV pathology have been described in children and neonates4,8,29–31, neurodevelopmental outcome data related to DMV pathology exist only in isolated case reports of motor impairment.7 In our cohort of patients with neonatal DMV thrombosis and infarction, a higher DMV white matter injury global severity score8 was associated with neurodevelopmental impairment. Additionally, global severity score and degree of asymmetry were positively correlated, and white matter injury was more commonly asymmetric in patients with neurodevelopmental impairment.
Although, the potential max global severity score from the DMV white matter injury-severity scoring system is 102 points, the max score in our cohort was 53 (median 11, interquartile range [25th-75th percentile], 5–25).8 In our previous work, we compared regional white matter injury scores and determined that the occipital white matter and white matter cerebral edema scores were not significantly different among quartiles and therefore do not meaningfully impact the global severity score.8 Scores for all other regions (corpus callosum, right and left frontal, parietal and temporal regions) were significantly different among quartiles.8 This lesional distribution concentrated in the frontoparietal area may explain the narrow range of scores in our cohort and a modified, simpler score that excludes occipital and white matter cerebral edema scores might be considered in future studies.
In our cohort, a higher global severity score, indicating more severe brain injury, was associated with neurodevelopmental impairment. This is consistent with other forms of neonatal brain injury (hypoxic-ischemic encephalopathy, intraventricular hemorrhage) in which severity of injury on MRI corresponds with likelihood of NDI.32–37 This report serves to validate the white matter injury global severity scoring system for DMV thrombosis and infarction. Not only does a higher global severity score indicate more severe injury as visualized on MRI in the neonatal period but it predicts later outcomes with a higher global severity score associated with neurodevelopmental impairment. Furthermore, analyses indicate that a global severity score of 16 is an optimal cut point to identify infants at higher risk for long-term neurodevelopmental impairment. Infants with a global severity score higher than 16 should be most closely monitored for neurodevelopmental delays and referred for early intervention if indicated. However, due to moderate sensitivity and specificity of the model, as well as variability among this population, heighted surveillance for developmental delays of infants with a global severity score below 16 is still recommended.
In children and neonates with cerebral sinovenous thrombosis, morbidity and mortality often depend on the extent and localization of thrombosis and associated cerebral parenchymal lesions.1,2,9 Published reports of NDI after sinovenous thrombosis range from 23–79% of patients having adverse neurologic or developmental sequale.1,2,9–11 Overall, 37% of our cohort with neonatal DMV thrombosis and infarction had NDI, consistent with these previous reports.
Predictors of worse neurologic outcome in other studies of perinatal stroke include concurrent neurological comorbidity (hypoxic-ischemic encephalopathy, meningitis) at diagnosis and subsequent development of epilepsy.2,38–41 In our cohort, 27% of patients had a concurrent neurological comorbidity at diagnosis however, rate of NDI was not increased in these patients. In our group however, all cases of meningitis were viral (herpes simplex virus, enterovirus) rather than bacterial, perhaps changing its predictive value. Published reports estimate the risk of epilepsy after neonatal cerebral sinovenous thrombosis to be between 20 and 40%.2,38–41 Eighteen percent of our cohort developed epilepsy and these patients had a significantly higher risk of NDI with 78% of the patients diagnosed with epilepsy having NDI. In conjunction with others, our results highlight the importance of considering neurologic comorbidities to aid in prediction of neurodevelopmental outcome to guide appropriate follow-up.
The predominant side of injury in our cohort with DMV thrombosis and infarction, was evenly split between left, right and equal (each group with 33% of the cohort). However, right cerebral white matter injury predominance was associated with significantly lower cognitive performance. This is consistent with some reports of the differential effects of left and right brain injury on intelligence, particularly that seen after adult unilateral stroke.42–44 In comparison, perinatal stroke in term infants has a left-side predominance.14 Yet, reported lateralized cognitive deficits after perinatal or childhood stroke are inconsistent, as the outcomes of perinatal stroke are largely dependent on the specific location (cortical, subcortical or combined) affected structures and lesion severity.15,43,45–48 Interestingly, degree of asymmetry accounted for approximately 30% of the variance in cognitive ability observed among study participants. This suggests the relationship between asymmetry of DMV thrombosis and infarction and cognitive ability needs further explored.
Although this study represents the largest of its kind to date, it is limited by small size and retrospective identification of patients for inclusion in the cohort. DMV thrombosis and infarction is relatively uncommon but has been recognized with increasing frequency in the past decade.4,7,8,30,31 For many of our analyses, lack of statistical significance might be due to low statistical power as a result of small effective sample size. This study was limited by the retrospective, nonharmonized imaging data. However, all scans were performed at a large academic pediatric radiology institution where the MRI physicist works closely with the vendors and pediatric neuroradiologists to continually optimize MR protocols on each platform. Additionally, the scoring system is relatively simple and can be applied to routine MR neuroimaging sequences. Another limitation is the varied age at time of neuropsychological assessment. We attempted to control for this variation by choosing validated assessments with age-specific standardized scores that could provide continuity throughout infancy and childhood. Additionally, having a cohort that included school-age children allowed for assessment of late outcomes beyond the 2-year Bayley-III that is standard in many high-risk infant follow-up programs. Finally, follow-up rates varied between specific outcome measures, mostly due to current age of cohort patients that determined the most appropriate testing modality. Despite this, we were able to obtain neurodevelopmental testing for 78% of the cohort.
CONCLUSIONS
The current study supports the white matter injury global severity scoring system for prediction of childhood neurodevelopmental outcomes after neonatal DMV thrombosis and infarction. A higher white matter injury global severity score after neonatal DMV thrombosis and infarction was associated with childhood neurodevelopmental impairment. This tool can be used in future research and clinical practice, enabling identification of infants at risk of adverse neurodevelopmental outcomes. Given the variability in impacted developmental domains, interventions targeted to specific developmental deficits is recommended. These results suggest a need for targeted clinical surveillance with neurodevelopmental testing and early intervention for patients with a high global severity score and/or asymmetric, predominantly right cerebral white matter injury and for those who develop epilepsy.
Supplementary Material
Acknowledgments
FUNDING
This work was supported by 1R01HD081120-01A1 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development to N.L.M. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations:
- (AUC)
Area under the curve
- (Bayley-III)
Bayley Scales of Infant and Toddler Development, Third Edition
- (CBCL)
Child Behavior Checklist
- (DMV)
deep medullary vein
- (MRI)
magnetic resonance imaging
- (NDI)
neurodevelopmental impairment
- (SES)
socioeconomic status
- (WPPSI-IV)
Wechsler Preschool and Primary Scale of Intelligence, Fourth Edition
- (WISC-V)
Wechsler Intelligence Scale for Children, Fifth Edition
Footnotes
The Authors declare that there is no conflict of interest.
REFERENCES
- 1.deVeber G, Andrew M, Adams C, et al. Cerebral Sinovenous Thrombosis in Children. N Engl J Med. 2001;345(6):417–423. doi: 10.1056/NEJM200108093450604 [DOI] [PubMed] [Google Scholar]
- 2.Moharir MD, Shroff M, Pontigon A-MM, et al. A Prospective Outcome Study of Neonatal Cerebral Sinovenous Thrombosis. J Child Neurol. 2011;26(9):1137–1144. doi: 10.1177/0883073811408094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee S, Mirsky DM, Beslow LA, et al. Pathways for Neuroimaging of Neonatal Stroke. Pediatr Neurol. 2017. doi: 10.1016/j.pediatrneurol.2016.12.008 [DOI] [PubMed] [Google Scholar]
- 4.Arrigoni F, Parazzini C, Righini A, et al. Deep medullary vein involvement in neonates with brain damage: An MR imaging study. Am J Neuroradiol. 2011;32(11):2030–2036. doi: 10.3174/ajnr.A2687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taoka T, Fukusumi A, Miyasaka T, et al. Structure of the Medullary Veins of the Cerebral Hemisphere and Related Disorders. RadioGraphics. 2017;37(1):281–297. doi: 10.1148/rg.2017160061 [DOI] [PubMed] [Google Scholar]
- 6.Okudera T, Huang YP, Fukusumi A, Nakamura Y, Hatazawa J, Uemura K. Micro-angiographical studies of the medullary venous system of the cerebral hemisphere. Neuropathology. 1999;19(1):93–111. doi: 10.1046/j.1440-1789.1999.00215.x [DOI] [PubMed] [Google Scholar]
- 7.Vilan A, Ribeiro JM, Reis C, Sampaio L. Deep Medullary Veins and Brain Injury. J Pediatr. 2018;200:290–290.e1. doi: 10.1016/j.jpeds.2018.03.051 [DOI] [PubMed] [Google Scholar]
- 8.Benninger KL, Maitre NL, Ruess L, Rusin JA. MR imaging scoring system for white matter injury after deep medullary vein thrombosis and infarction in neonates. Am J Neuroradiol. 2019;40(2):347–352. doi: 10.3174/ajnr.A5940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berfelo FJ, Kersbergen KJ, Van Ommen CH, et al. Neonatal cerebral sinovenous thrombosis from symptom to outcome. Stroke. 2010;41(7):1382–1388. doi: 10.1161/STROKEAHA.110.583542 [DOI] [PubMed] [Google Scholar]
- 10.Golomb MR. Outcomes of perinatal arterial ischemic stroke and cerebral sinovenous thrombosis. Semin Fetal Neonatal Med. 2009;14(5):318–322. doi: 10.1016/j.siny.2009.07.003 [DOI] [PubMed] [Google Scholar]
- 11.Fitzgerald KC, Williams LS, Garg BP, Carvalho KS, Golomb MR. Cerebral sinovenous thrombosis in the neonate. Arch Neurol. 2006;63(3):405–409. doi: 10.1001/archneur.63.3.405 [DOI] [PubMed] [Google Scholar]
- 12.Ramenghi LA, Fumagalli M, Bassi L, Mosca F. Neonatal cerebral sinovenous thrombosis. Semin Fetal Neonatal Med. 2009;14(5):278–283. doi: 10.1016/J.SINY.2009.07.010 [DOI] [PubMed] [Google Scholar]
- 13.Fluss J, Dinomais M, Chabrier S. Perinatal stroke syndromes: Similarities and diversities in aetiology, outcome and management. Eur J Paediatr Neurol. 2019;23(3):368–383. doi: 10.1016/j.ejpn.2019.02.013 [DOI] [PubMed] [Google Scholar]
- 14.Hielkema T, Hadders-Algra M. Motor and cognitive outcome after specific early lesions of the brain - a systematic review. Dev Med Child Neurol. 2016;58:46–52. doi: 10.1111/dmcn.13047 [DOI] [PubMed] [Google Scholar]
- 15.Van Buuren L, Van Der AA N, Dekker H, et al. Cognitive outcome in childhood after unilateral perinatal brain injury. Dev Med Child Neurol. 2013;55(10):934–940. doi: 10.1111/dmcn.12187 [DOI] [PubMed] [Google Scholar]
- 16.Kersbergen KJ, Groenendaal F, Benders MJNL, De Vries LS Neonatal cerebral sinovenous thrombosis: Neuroimaging and long-term follow-up. J Child Neurol. 2011;26(9):1111–1120. doi: 10.1177/0883073811408090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)-A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Neonatal Research Network. Follow-up Study Manual of Operations.; 2019. https://neonatal.rti.org/pdf/FUPublic/Public_FU_Manual.pdf. Accessed October 16, 2019.
- 19.Bayley N. Bayley Scales of Infant and Toddler Development. 3rd Edition San Antonio, TX: Harcort Assessment Inc; 2006. [Google Scholar]
- 20.Wechsler D. Wechsler Preschool and Primary Scale of Intelligence. 4th Edition Bloomington, MN: Pearson, Psychological Corporation; 2012. [Google Scholar]
- 21.Wechsler D. Wechsler Intelligence Scale for Children. 5th Edition San Antonio, TX: Pearson, Psychological Corporation; 2014. [Google Scholar]
- 22.Kaplan C. Predictive validity of the WPPSI-R: A four year follow-up study. Psychol Sch. 1996;33(3):211–220. doi: [DOI] [Google Scholar]
- 23.Lowe JD, Anderson HN, Williams A, Currie BB. Long-term predictive validity of the WPPSI and the WISC-R with black school children. Pers Individ Dif. 1987;8(4):551–559. doi: 10.1016/0191-8869(87)90218-2 [DOI] [Google Scholar]
- 24.Yule W, Gold RD, Busch C. Long-term predictive validity of the WPPSI: An 11-year follow-up study. Pers Individ Dif. 1982;3(1):65–71. doi: 10.1016/0191-8869(82)90075-7 [DOI] [Google Scholar]
- 25.Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39(4):214–223. doi: 10.1111/j.1469-8749.1997.tb07414.x [DOI] [PubMed] [Google Scholar]
- 26.Achenbach T. Manual for the Child Behavior Checklist/4–18 and 1991 Profile. Burlington, VT: University of Vermont, Department of Psychiatry; 1991. https://ci.nii.ac.jp/naid/20001666977/. Accessed October 18, 2019. [Google Scholar]
- 27.Achenbach TM, Rescorla LA. Manual for the ASEBA Preschool Forms and Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families; 2000. [Google Scholar]
- 28.Hollingshead A. Four Factor Index of Social Status. New Haven, CT: Yale University; 1975. http://elsinore.cis.yale.edu/sociology/yjs/yjs_fall_2011.pdf#page=21. [Google Scholar]
- 29.Friedman DP. Abnormalities of the deep medullary white matter veins: MR imaging findings. Am J Roentgenol. 1997;168(4):1103–1108. doi: 10.2214/ajr.168.4.9124123 [DOI] [PubMed] [Google Scholar]
- 30.Konanki R, Varma DR, Ratha C, Lingappa L, Shah N. Teaching neuro images: Fetal deep medullary vein thrombosis presenting as progressive intracerebral hemorrhage. Neurology. 2015;85(1):e5–e6. doi: 10.1212/WNL.0000000000001719 [DOI] [PubMed] [Google Scholar]
- 31.Doneda C, Righini A, Parazzini C, Arrigoni F, Rustico M, Triulzi F. Prenatal MR imaging detection of deep medullary vein involvement in fetal brain damage. Am J Neuroradiol. 2011;32(8):E146–9. doi: 10.3174/ajnr.A2249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rutherford M, Ramenghi LA, Edwards AD, et al. Assessment of brain tissue injury after moderate hypothermia in neonates with hypoxic-ischaemic encephalopathy: a nested substudy of a randomised controlled trial. Lancet Neurol. 2010;9(1):39–45. doi: 10.1016/S1474-4422(09)70295-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shankaran S, Barnes PD, Hintz SR, et al. Brain injury following trial of hypothermia for neonatal hypoxic-ischaemic encephalopathy. Arch Dis Child Fetal Neonatal Ed. 2012;97:F3988-F404. doi: 10.1136/archdischild-2011-301524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chalak LF, Dupont TL, Sánchez PJ, et al. Neurodevelopmental outcomes after hypothermia therapy in the era of Bayley-III. J Perinatol. 2014;34(8):629–633. doi: 10.1038/jp.2014.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patra K, Wilson-Costello D, Taylor HG, Mercuri-Minich N, Hack M. Grades I-II intraventricular hemorrhage in extremely low birth weight infants: Effects on neurodevelopment. J Pediatr. 2006;149(2):169–173. doi: 10.1016/j.jpeds.2006.04.002 [DOI] [PubMed] [Google Scholar]
- 36.Sherlock RL, Anderson PJ, Doyle LW, et al. Neurodevelopmental sequelae of intraventricular haemorrhage at 8 years of age in a regional cohort of ELBW/very preterm infants. Early Hum Dev. 2005;81(11):909–916. doi: 10.1016/j.earlhumdev.2005.07.007 [DOI] [PubMed] [Google Scholar]
- 37.Futagi Y, Toribe Y, Ogawa K, Suzuki Y. Neurodevelopmental outcome in children with intraventricular hemorrhage. Pediatr Neurol. 2006;34(3):219–224. doi: 10.1016/j.pediatrneurol.2005.08.011 [DOI] [PubMed] [Google Scholar]
- 38.Vendrame M, Alexopoulos AV, Boyer K, et al. Longer duration of epilepsy and earlier age at epilepsy onset correlate with impaired cognitive development in infancy. Epilepsy Behav. 2009;16(3):431–435. doi: 10.1016/j.yebeh.2009.08.008 [DOI] [PubMed] [Google Scholar]
- 39.Hirschberger RG, Kuban KCK, O’Shea TM, et al. Co-occurrence and Severity of Neurodevelopmental Burden (Cognitive Impairment, Cerebral Palsy, Autism Spectrum Disorder, and Epilepsy) at Age Ten Years in Children Born Extremely Preterm. Pediatr Neurol. 2018;79:45–52. doi: 10.1016/j.pediatrneurol.2017.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wanigasinghe J, Reid SM, Mackay MT, Reddihough DS, Harvey AS, Freeman JL. Epilepsy in hemiplegic cerebral palsy due to perinatal arterial ischaemic stroke. Dev Med Child Neurol. 2010;52(11):1021–1027. doi: 10.1111/j.1469-8749.2010.03699.x [DOI] [PubMed] [Google Scholar]
- 41.Mineyko A, Kirton A, Billinghurst L, et al. Seizures and Outcome One Year After Neonatal and Childhood Cerebral Sinovenous Thrombosis. Pediatr Neurol. 2020;105:21–26. doi: 10.1016/j.pediatrneurol.2019.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nass R, Peterson H deCoudres, Koch D. Differential effects of congenital left and right brain injury on intelligence. Brain Cogn. 1989;9(2):258–266. doi: 10.1016/0278-2626(89)90035-3 [DOI] [PubMed] [Google Scholar]
- 43.Westmacott R, MacGregor D, Askalan R, deVeber G. Late emergence of cognitive deficits after unilateral neonatal stroke. Stroke. 2009;40(6):2012–2019. doi: 10.1161/STROKEAHA.108.533976 [DOI] [PubMed] [Google Scholar]
- 44.Warrington EK, James M, Maciejewski C. The WAIS as a Lateralizing and Localizing Diagnostic Instrument: A Study of 656 Patients with Unilateral Cerebral Lesions. Neuropsychologia. 1986;24(2):223–239. https://journals.ohiolink.edu/pg_99?312512716892486::NO::P99_ENTITY_ID,P99_ENTITY_TYPE:33493207,MAIN_FILE&cs=3jDAJN8PwZbMy55Mo5gZe0THV81rF0VMm5d_uZVsLyOuoC50-Nl1jROyiYFdet5LZMYUx3wjMOnSFYT0mU_b3CA. Accessed July 19, 2019. [DOI] [PubMed] [Google Scholar]
- 45.Nass RD, Trauner D. Social and Affective Impairments are Important Recovery After Acquired Stroke in Childhood. CNS Spectr. 2004;9(6):420–434. doi: 10.1017/S1092852900009469 [DOI] [PubMed] [Google Scholar]
- 46.Vargha-Khadem F, Isaacs E, Van Der Werf S, Robb S, Wilson J. Development of Intelligence and Memory in Children with Hemiplegic Cerebral Palsy. Brain. 1992;115:315–329. https://academic.oup.com/brain/article-abstract/115/1/315/295787. Accessed July 19, 2019. [DOI] [PubMed] [Google Scholar]
- 47.Murias K, Brooks B, Kirton A, Iaria G. A review of cognitive outcomes in children following perinatal stroke. Dev Neuropsychol. 2014;39(2):131–157. doi: 10.1080/87565641.2013.870178 [DOI] [PubMed] [Google Scholar]
- 48.Montour-Proulx I, Braun CMJ, Daigneault S, Rouleau I, Kuehn S, Bégin J. Predictors of intellectual function after a unilateral cortical lesion: Study of 635 patients from infancy to adulthood. J Child Neurol. 2004;19(12):935–943. doi: 10.1177/088307380401901205. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.