Abstract
Context
Although sexuality influences well-being and quality of life (QoL), studies on sexual dysfunction (SD) in adult growth hormone deficiency (AGHD) patients are lacking.
Objective
To investigate the prevalence of SD in AGHD patients grouped according to recombinant human growth hormone (r-hGH) therapy.
Design
Prospective, cross-over, 24 months, monocentric study.
Setting
Real-life clinical setting in a tertiary, endocrinological center.
Patients
83 AGHD patients (31 women, 52 men, mean age 56.3 ± 14.7 years) were enrolled according to stringent criteria.
Intervention(s)
Patients already on long-term r-hGH therapy (Group 1, n = 32) vs untreated (Group 2, n = 51).
Main outcome measure(s)
Serum hormones, QoL Satisfaction in Hypopituitarism (QLS-H) and QoL Assessment of GHD in Adults (QoL-AGHDA) questionnaires for QoL, Index for Erectile Function-15 (IIEF-15) in men, and Female Sexual Function Index (FSFI) in women for SD.
Results
The overall prevalence of SD was 71.2% (60% men, 89% women). All IIEF-15 scores were lower (P = 0.001) and erectile dysfunction was more prevalent in Group 2 (75%) than Group 1 (35%). IGF-1 was correlated to scores of all IIEF-15 domains, particularly with that of erectile function (EF) (R2=0.123, P = 0.019). EF domain score correlated with QLS-H (P < 0.005) and QoL-AGHDA (P = 0.001). Despite the high prevalence of female SD also in untreated AGHD women, FSFI scores did not correlate with IGF-1 levels and QoL scores.
Conclusions
SD is highly prevalent in AGHD patients, especially in those untreated. SD represents an overlooked and neglected issue in AGHD, regardless the contribution of sexual life on QoL. The evaluation of sexual function should be integrated in the global assessment of AGHD patients.
Keywords: sexual dysfunction, adult growth hormone deficiency, quality of life, International Index of Erectile Function-15, Female Sexual Function Index
Growth hormone (GH) exerts many actions on several physiological processes by acting both directly and indirectly through insulin growth factor 1 (IGF-1). In adults, GH deficiency (GHD) is characterized by impaired quality of life (QoL), altered body composition, and impaired lipid and glucose metabolism [1,2]. Treatment with recombinant human GH (r-hGH) is able to significantly improve QoL, serum lipids, and glucose tolerance [3-6]. QoL improvement is one of the major endpoints of r-hGH therapy in adult GHD (AGHD) patients [1,2]. Although a satisfying sexual life is considered one of the major items contributing to a good QoL [7-9], sexual function has not been systematically investigated in AGHD patients by using specific and validated questionnaires for the assessment of patients’ sexuality. Information on sexual life of adult patients with GHD is indirectly available from questionnaires validated for the global assessment of QoL and well-being, where only some general items on sexuality are included [10-12]. At the moment, the relationship between GH and sexual function has been rarely investigated [13-15] and reviewed in men [16-19]. Conversely, no data on GH deficiency and r-hGH on female sexuality are available [16,20], except for a study showing no differences in sexual function between women with AGHD and controls, a study that was, however, limited by the small number of subjects and by the menopausal status of most of them [21].
In males, Brill et al did not find any change of sexual function in a small group of older men without AGHD when physiological doses of r-hGH were administered alone or in combination to physiological doses of exogenous testosterone (T) [13]. Data coming from patients with acromegaly suggest that acromegalic men have an increased risk of developing ED, the latter being mainly related to comorbidities associated to acromegaly (eg, cardiovascular and metabolic diseases) [14] rather than directly to GH excess. However, a direct role of GH excess has been suggested by Chen et al [15]. GH seems to increase in serum and within the penile vessels during arousal and to decrease during penile detumescence [22], but opposite results showing no changes during the phases of erection are also available [16], thus leaving this observation controversial. In a laboratory setting, r-hGH stimulates the production of cyclic guanosine monophosphate by isolated human cavernous tissue [23], while in transgenic male mice with knockout for the GH gene, a reduced copulatory behavior, in terms of number of mounts, was documented [24].
The primary aim of this study was to investigate the prevalence of sexual dysfunction (SD) by using gender-specific questionnaires, validated for the assessment of sexual function, in AGHD patients referring to a single, tertiary, endocrinological center and grouped according to r-hGH therapy. The secondary aim was to assess the correlation between sexual function and QoL scores, r-hGH treatment, clinical and hormonal parameters. Here we report baseline data on QoL and SD.
Subjects and Methods
Study design
In a prospective, cross-over, 24-month, open-label, monocentric study, performed in a real-life clinical setting, we recruited AGHD patients attending the Unit of Endocrinology of Modena. The main goal of this study was to improve the management of AGHD (MAGHD), providing a comprehensive feedback to the clinician as well as a simple feedback to the patient. For achieving this objective, a smartphone app (MAGHD App), able to record patients’ answers to QoL and well-being questionnaires, was developed, and it was linked with a wearable device collecting patients’ daily activities (steps, burnt calories, sleep duration). The study protocol was composed of 2 consecutive phases: (i) the first year, when patients performed their biannual clinical, biochemical, and hormonal analysis coupled with a multidimensional assessment based on filling in a set of self-reported questionnaires, according to our standard clinical practice, and (ii) the second year, when patients, besides biannual routine evaluation, were invited to use both the MAGHD App to answer questionnaires and a wearable device to register their daily activity. The study is currently ongoing and only baseline data, related to the first phase, are available for the all patients.
Subjects
From January to December 2018, 83 AGHD patients were enrolled. Patients were assessed as eligible in the presence of a documented diagnosis of AGHD, according to the latest Endocrine Society clinical practice guidelines [2], age higher than 18 years, good understanding of the Italian language in cases of foreign patients, and well-replaced with hormonal treatments at substitutive dose in cases of other pituitary deficits. Exclusion criteria were the following: unwillingness of patients to continue follow-up at the Endocrinology Unit of Modena, a diagnosis of biochemical AGHD outside the appropriate clinical context of pituitary disease, and major psychiatric diseases or chronic severely invalidating diseases (kidney, heart, respiratory insufficiency, or severe hepatic failure).
According to the clinical history of AGHD treatment, they were grouped in patients on long-term r-hGH therapy (n = 32, Group 1) and not treated with r-hGH (n = 51, Group 2).
Main outcome measures
We created a large database collecting personal data, medical history, clinical and biochemical parameters, and multidimensional assessment results. During the clinical evaluation, we asked for clinical symptoms, previous medical history (obtained by recording date of AGHD diagnosis, cause of AGHD, diagnostic tests performed, and start and duration in months of r-hGH therapy including dosage and withdrawal), the presence of other pituitary deficits and their replacement therapy. We recorded anthropometric parameters [weight, height, body mass index (BMI), waist and hip circumferences] and clinical data (blood pressure, heart rate) obtained at the time of first visit.
Biochemical parameters
Hormonal and biochemical analyses were performed in the morning, in a fasting condition as a part of the clinical investigations routinely performed in patients with hypopituitarism, including luteinizing hormone (LH), follicle-stimulating hormone (FSH), estradiol, serum total T, sex hormone-binding globulin (SHBG), serum-calculated free T, IGF-1, and insulin growth factor binding protein 3 (IGFBP-3), and routine biochemical examinations.
Serum LH and FSH were assayed by immuno-chemiluminescence (ADVIA Centaur, Siemens). The inter- and intra-assay coefficients of variation were of 3.6% and 2.4% for LH and 5.2% and 2.6% for FSH, respectively. Serum estradiol was also measured by Chemiluminescent Microparticle Immunoassay on the ARCHITECT platform (Abbott Laboratories). The sensitivity was 0.6 pg/mL (2.2 pmol/L) with the lowest standard at 1.5 pg/mL, linearity to 150 pg/mL, and an ED50 of 20 pg/mL. Serum total T was assayed by immuno-chemiluminescence (Architect 2nd Generation T, Abbott, USA), with an intra-assay coefficient of variation <10%. SHBG was assessed by chemiluminescent immunoassay (Architect, Abbott GmbH & Co, Germany) with both inter- and intra-assay coefficients of variation of 10.0%. Serum calculated free T was obtained by using the Vermeulen equation [25]. Serum IGF-1 was assessed by chemiluminescence immuno-assay (CLIA, Diasorin), with intra-assay coefficient of variation of 8%. Given the strong dependency of serum IGF-1 on age and gender, the IGF-1 standard deviation score (SDS) was also calculated [26]. Serum IGFBP-3 was measured by enzyme immuno-assay (Mediagnost), with intra-assay coefficient of variation of 10%.
Evaluation of QoL
Questions on Life Satisfaction in Hypopituitarism (QLS-H) and Quality of Life Assessment of Growth Hormone Deficiency in Adults (QoL-AGHDA) are 2 standardized and validated disorder-related questionnaires developed specifically to evaluate QoL of patients with hypopituitarism and AGHD, respectively [27]. Through QLS-H, patients indicate how important a particular dimension of life is for them and how satisfied they are with it. To each item a score from 1 (“not important” or “dissatisfied”) to 5 (“extremely important” or “very satisfied”) is assigned. The weighted score for the degree of satisfaction with a certain dimension of QoL is then calculated by the following formula: weighted satisfaction = (importance − 1) × (2 × satisfaction − 5). The absolute QLS-H score is calculated adding all the fields previously obtained and can range from −108 (very low satisfaction) to +180 (high satisfaction). However, because QLS-H scores vary between countries and are dependent on sex and age, algorithms to calculate Z scores adjusted for these variables were developed as a measure for the deviation of patients’ scores from those of the general population [3,28]. Even though the score calculation is complex and usually requires a calculation program, this tool has the advantage of weighting each item by the individual patient [27,28] and demonstrates its effectiveness in assessing impaired life satisfaction in AGHD and its usefulness in monitoring the QoL improvement related to r-hGH therapy [28].
On the other hand, QoL-AGHDA is a short and easy to complete questionnaire including 25 questions with a “yes” or “no” answer. A score of 1 is assigned to each “yes” answer and the sum of the number of “yes” answers (range 0-25) is used as a measure of QoL, with a high QoL-AGHDA score indicating a poor QoL [29].
The interpretation of these questionnaires is not based on a defined cut-off score, but relies on the comparison with mean values scored in the normal population. For QLS-H, the application of Z-scores already assesses the deviation from the QLS-H reference ranges obtained from general population, while for QoL-AGHDA, mean values registered in the normal population in various international studies range between 4 and 7 [30]. Moreover, at the UK National Institute for Health and Clinical Excellence, the QoL-AGHDA score is included among the 3 criteria used to determine if r-hGH treatment should be given, in particular a score of 11 or more meets the requirements for GH replacement therapy [31].
Evaluation of sexual function
Sexual function was assessed in male and female patients through the validated International Index for Erectile Function-15 (IIEF-15) questionnaire and Female Sexual Function Index (FSFI), respectively.
IIEF-15 is a self-administered questionnaire consisting of 15 questions addressing erectile function (EF), sexual desire (SD), orgasmic function, intercourse satisfaction, and overall satisfaction (OS) [32,33]. Highest scores suggest better EF; in particular, a cut-off score of 25 for the EF domain is currently used to diagnose ED in the clinic [34]. According to the classification criteria of this questionnaire, the severity of ED is divided into 4 categories: (i) no ED (EF score = 26-30); (ii) mild ED (EF score = 17-25); (iii) moderate ED (EF score = 11-16), and (iv) severe ED (EF score = 6-10) [33,34].
Similarly, FSFI is a brief multidimensional scale for assessing sexual function in women, including 19 questions divided into 6 domains: desire, arousal, lubrication, orgasm, satisfaction, and pain. Domain scores are derived by summing scores within the domain and multiplying the sum by a factor weight (each domain has a specific factor). A total punctuation was obtained by summing the results from all of the domains. The overall score ranged between 2 and 36 points, and FSFI overall score lower than 26.55 was defined as suggestive of female SD (FSD) as described by Wiegel et al and in various studies [35-37].
Ethics
The Institutional review board of Modena approved the protocol study (protocol n. 346/2017). This trial was registered in ClinicalTrials.gov (Identifier: NCT03525587). Written informed consent has been obtained from each participant.
Statistical analysis
Data are reported as the mean ± SD, median with minimum and maximum for continuous variables, and number and percentages for categorical variables.
Statistical analysis was carried out to compare the outcomes of patients divided in 3 groups according to r-hGH therapy. The nonparametric Mann-Whitney test was used for comparisons of continuous variables not normally distributed at the Kolmogorov-Smirnov test. Categorical variables were compared by Chi-square test.
Linear regression was used to examine the association between continuous variables; results were expressed through β and R2 coefficients and were used to decide what variables should be included in the stepwise regression analysis. Stepwise, linear, and multiple regression analyses were performed to identify factors able to influence and predict the selected dependent variable.
Statistical analysis was performed using the Statistical Package for the Social Sciences software for Windows (version 26.0; SPSS Inc., Chicago, IL, USA). For all comparisons, P-values < 0.05 were considered statistically significant.
Results
Patients characteristics
Eighty-three patients (52 males, 31 females) with a mean age of 56.3 ± 14.7 years were enrolled (Table 1). The diagnosis of AGHD lasted from less than <1 to 40 years (mean duration 157 ± 122.8 months) with a mean duration of hypopituitarism of 180.3 ± 149.8 months (Table 1). Characteristics related to the diagnosis of AGHD are summarized in Table 1. The most common cause of AGHD was pituitary surgery (56.6%), and the most common disease was pituitary adenoma. Considering pituitary function, only 7 patients (8.4%) had isolated AGHD. Anthropometric data and questionnaires’ scores are summarized in Table 1.
Table 1.
Clinical, anthropometric, and questionnaire data of the entire cohort of AGHD patients at baseline
| Patient’s characteristic | Data |
|---|---|
| AGHD patients, n | 83 |
| Females | 31 (37.4%) |
| Males | 52 (62.6%) |
| AGHD type | |
| Isolated AGHD | 7 (8.4%) |
| AGHD plus multiple pituitary deficiencies | 76 (91.6%) |
| Adult-onset AGHD | 72 (86.8%) |
| Childhood-onset AGHD | 11 (13.2%) |
| Congenital AGHD | 5 (6%) |
| Acquired AGHD | 78 (94%) |
| AGHD timing | |
| Age (years) | 56.3 ± 14.7 (58; 19 to 86) |
| Age at AGHD diagnosis (years) | 42.9 ± 17.6 (44.5; 0 to 73) |
| AGHD duration (months) | 157 ± 122.8 (139; 3 to 489) |
| Hypopituitarism duration (months) | 180.3 ± 149.8 (150; 2 to 489) |
| Duration r-hGH treatment (months) | 142.4 ± 108.1 (131.5; 2 to 489) |
| Anthropometric parameters | |
| Weight (kg) | 87.6 ± 19.3 (85.5; 49 to 137) |
| Height (cm) | 168.2 ± 10.2 (169; 147 to 190) |
| BMI (kg/m2) | 31 ± 7 (29.8; 17.6 to 50) |
| Waist circumference (cm) | 102.4 ± 15.9 (102.5; 68 to 136) |
| Hip circumference (cm) | 108.6 ± 14.6 (108; 65 to 150) |
| Waist-Hip ratio | 0.9 ± 0.08 (0.9; 0.76 to 1.28) |
| Questionnaire scores | |
| QLS-H score | 32.3 ± 41.2 (36; −81 to +127) |
| QLS-H Z-score | −0.89 ± 1.3 (−0.93; −4.5 to- +2.35) |
| QoL-AGHDA score | 7.6 ± 6.3 (6; 0 to 23) |
| IIEF-15 score | 16.3 ± 12.2 (1 to 30) |
| FSFI score | 10.5 ± 10.8 (3.6; 1.2 to 34.8) |
Data are expressed as number (percentage) or mean ± SD (median; min to max).
Age, duration of hypopituitarism, duration of AGHD, IGF-1, and IGFBP-3 were significantly different between the 2 groups of patients divided according to AGHD treatment status (Table 2). In particular, patients treated with r-hGH (Group 1) were younger than those untreated (Group 2) (P = 0.001), while the mean duration of hypopituitarism (P = 0.035) and of AGHD (P = 0.044) were lower in untreated (Group 2) than treated patients (Group 1). As expected, IGF-1, IGFBP-3, and IGF-1 SDS (P < 0.001) were significantly higher in patients treated with r-hGH (Group 1) than in untreated patients (Group 2) (Table 2).
Table 2.
Clinical, hormonal, QoL, and sexual function outcomes of the entire cohort of AGHD patients grouped according to r-hGH treatment
| Group 1 (AGHD patients on r-hGH treatment) | Group 2 (untreated AGHD patients) | P value | |
|---|---|---|---|
| Patients, n | 32 | 51 | na |
| Age (years) | 49.5 ± 14 (52.5; 19 to 69) | 60.6 ± 13.5 (62; 24 to 86) | 0.001 |
| Hypopituitarism duration (months) | 216.6 ± 139.4 (190.5; 10 to 489) | 159.2 ± 153 (102.5; 2 to 488) | 0.035 |
| AGHD duration (months) | 179.3 ± 111.7 (167; 12 to 489) | 142.9 ± 128.4 (104.5; 3 to 488) | 0.044 |
| Rate of answering to QLS-H | 30/32 (93.75%) | 45/51 (88.2%) | 0.407 |
| QLS-H score | 45.3 ± 43.4 (49.5; −60 to +127) | 23.7 ± 37.7 (20; −81 to +114) | 0.016 |
| QLS-H Z-score | -0.6 ± 1.3 (-0.4; −4.3 to +2.35) | −1.1 ± 1.2 (−1.2; −4.5 to +1.8) | 0.033 |
| Rate of answering to QoL-AGHDA | 30/32 (93.75%) | 47/51 (92.1%) | 0.785 |
| QoL-AGHDA score | 7 ± 6.1 (6; 0 to 22) | 8 ± 6.5 (7; 0 to 23) | 0.457 |
| IGF-1 | 167.4 ± 59.3 (162.9; 75.7 to 373.2) | 78.8 ± 41.1 (73.2; 14.9 to 182.6) | <0.001 |
| IGF-1 SDS | −0.3 ± 1.5 (−0.3; −3.7 to +1.9) | −2.8 ± 1.7 (−2.8; −9.3 to +1.5) | <0.001 |
| IGFBP-3 | 3178.3 ± 878.7 (3210; 304 to 4415) | 2342.4 ± 947 (2364; 220 to 4628) | <0.001 |
Data are expressed as number (percentage) or mean ± SD (median; min to max).
QoL and sexual function questionnaires
QLS-H was filled by 75 (90.4%) and QoL-AGHDA by 77 (92.8%) out of 83 AGHD patients. The rate of answering to QLS-H was significantly different comparing males (50/52, 96.1%) and females (25/31, 80.6%) (P = 0.021). No differences were found between sexes concerning the rate of answering of QoL-AGHDA (males 94.2% vs females 90.3%; P = 0.506) and of questionnaires of male and FSD: the IIEF-15 (45/52 males, 86.5%) compared to the FSFI (28/31 females 90.3%; P = 0.608). Similarly, the rate of answering did not differ when comparing each other sexual (IIEF and FSFI) with QoL (QLS-H and QoL AGHDA) questionnaires.
QoL
The rate of questionnaires’ answering for both QLS-H (93.75% vs 88.2%) and QoL-AGHDA (93.75% vs 92.1%) was not significantly different between the 2 groups (P = 0.407; P = 0.785), although Group 2 showed slightly lower percentages than Group 1 (Table 2). Analyzing the entire cohort, both QLS-H score (P = 0.016) and QLS-H Z score (P = 0.033) were significantly higher in r-hGH treated patients (Group 1), while no differences between groups were found in QoL-AGHDA scores (Table 2). Similarly, considering only men, QLS-H score (P = 0.012) and QLS-H Z score (P = 0.036) were higher in r-hGH treated (Group 1) than in untreated patients, while no differences were documented in QoL-AGHDA scores (Table 3). Instead, in females we did not document any significant difference in QoL questionnaires results comparing patients according to r-hGH treatment status (Table 4).
Table 3.
Clinical, hormonal, QoL, and sexual function outcomes and ED prevalence in AGHD male patients grouped according to r-hGH treatment
| Group 1 (AGHD patients on r-hGH treatment) | Group 2 (untreated AGHD patients) | P-value | |
|---|---|---|---|
| Men, n | 18 | 34 | na |
| Age (years) | 48.1 ± 13.4 (47.5; 22 to 65) | 61 ± 12.6 (62; 24 to 86) | 0.002 |
| Hypopituitarism duration (months) | 174.1 ± 126.7 (167; 10 to 466) | 169.1 ± 159.1 (105; 3 to 488) | 0.594 |
| AGHD duration (months) | 152.9 ± 98.7 (143; 12 to 354) | 140.6 ± 129.4 (105; 3 to 488) | 0.359 |
| Rate of answering to QLS-H | 17/18 (94.4%) | 33/34 (97%) | 0.641 |
| QLS-H score | 53.6 ± 45.5 (69; −60 to +114) | 28.6 ± 34.3 (29; −33 to +114) | 0.012 |
| QLS-H Z-score | −0.5 ± 1.5 (−0.2; −4.3 to +1.35) | −1.1 ± 1.1 (−1.2; −3 to +1.7) | 0.036 |
| Rate of answering to QoL-AGHDA | 16/18 (88.8%) | 33/34 (97%) | 0.229 |
| QoL-AGHDA score | 6.4 ± 7 (4; 0 to 22) | 6.9 ± 6.3 (5; 0 to 21) | 0.622 |
| IIEF-15 | |||
| Rate of answering to IIEF-15 | 17/18 (94.4%) | 28/34 (82.3%) | 0.224 |
| Prevalence of ED | 6/17 (35%) | 21/28 (75%) | 0.008 |
| Erectile function | 23.9 ± 8.7 (1 to 30) | 11.6 ± 11.8 (1 to 30) | 0.001 |
| Orgasm function | 7.7 ± 3.4 (9; 0 to 10) | 3 ± 4.1 (0; 0 to 10) | 0.001 |
| Sexual desire | 7.5 ± 2 (2 to 10) | 5 ± 2.5 (5.5; 2 to 9) | 0.001 |
| Intercourse satisfaction | 8.8 ± 3.5 (10; 0 to 12) | 3.6 ± 4.6 (0; 0 to 12) | 0.001 |
| Overall satisfaction | 7.8 ± 2 (2 to 10) | 4.5 ± 2.9 (2.5; 2 to 10) | 0.001 |
| Total T (ng/mL) | 5 ± 1.8 (5; 1.6 to 8.2) | 4.5 ± 3.5 (3.3; 0.1 to 15) | 0.237 |
| Calculated Free T (ng/dL) | 11.5 ± 5 (11.4; 3.2 to 19) | 10.1 ± 8.4 (7.2; 1.3 to 30) | 0.131 |
| SHBG (nmol/L) | 28 ± 15.6 (22.5; 4.6 to 67.9) | 37 ± 20.4 (34; 6 to 100.5) | 0.066 |
| IGF-1 (ng/mL) | 184.4 ± 62.5 (168.2; 75.7 to 373.2) | 80.9 ± 39.5 (74.4; 14.9 to 166.9) | <0.001 |
| IGF-1 SDS | −0.1 ± 1.1 (−0.2; −3.4 to +1.9) | −2.5 ± 1.3 (−2.7; −4.8 to +0.3) | <0.001 |
| IGFBP-3 (ng/mL) | 3012.5 ± 834.1 (3139; 304 to 4171) | 2297.6 ± 966 (2328; 220 to 4076) | 0.003 |
Data are expressed as number (percentage) or mean ± SD (median; min to max). Normal range for serum total T, calculated free T, and SHBG were 2.2-8.7 ng/ml, >6.5 ng/dL, and 13.5-71.4 mmol/L, respectively.
Table 4.
Clinical, hormonal, QoL, and sexual function outcomes and FSD prevalence in AGHD female patients grouped according to r-hGH treatment
| Group 1 (AGHD patients on r-hGH treatment) | Group 2 (untreated AGHD patients) | P-value | |
|---|---|---|---|
| Women | 14 | 17 | na |
| Age (years) | 51.4 ± 15.1 (57.5; 19 to 69) | 59.5 ± 15.5 (62; 29 to 84) | 0.161 |
| Hypopituitarism duration (months) | 273.2 ± 140.2 (263.5; 50 to 489) | 137.5 ± 141.5 (74; 5 to 410) | 0.009 |
| AGHD duration (months) | 213.3 ± 121.6 (224; 50 to 489) | 147.2 ± 130.3 (104; 5 to 410) | 0.071 |
| Rate of answering to QLS-H | 13/14 (92.9%) | 12/17 (70.6%) | 0.118 |
| QLS-H score | 34.5 ± 39.5 (39; −20 to +127) | 10.2 ± 44.5 (12; −81 to +89) | 0.205 |
| QLS-H Z-score | −0.65 ± 1.17 (−0.69; −2.2 to +2.35) | −1.1 ± 1.5 (−1; −4.5 to +1.6) | 0.437 |
| Rate of answering to QoL-AGHDA | 14/14 (100%) | 14/17 (82.3%) | 0.098 |
| QoL-AGHDA score | 7.6 ± 5.1 (6.5; 0 to 18) | 10.8 ± 6.3 (11.5; 2 to 23) | 0.178 |
| FSFI | |||
| Rate of answering to FSFI | 13/14 (92.9%) | 15/17 (88.2%) | 0.665 |
| Prevalence of FSD | 10/13 (77%) | 15/15 (100%) | 0.049 |
| Desire | 3 ± 1.2 (3; 1.2 to 6) | 1.6 ± 0.9 (1.2; 1.2 to 3.6) | 0.002 |
| Arousal | 2.7 ± 2.3 (2.4; 0 to 6) | 0.7 ± 1 (0; 0 to 3.3) | 0.029 |
| Lubrification | 3.1 ± 2.3 (3.6; 0 to 6) | 0.8 ± 1.4 (0; 0 to 4.2) | 0.005 |
| Orgasm | 2.9 ± 2.2 (3.2; 0 to 6) | 1 ± 1.8 (0; 0 to 5.2) | 0.041 |
| Satisfaction | 2.4 ± 2.5 (1.2; 0 to 6) | 1 ± 1.8 (0; 0 to 4.8) | 0.097 |
| Pain | 2.7 ± 2.7 (3.2; 0 to 6) | 0.7 ± 1.3 (0; 0 to 3.6) | 0.072 |
| Overall | 16 ± 12 (15.4; 1.2 to 34.8) | 5.8 ± 7 (1.8; 1.2 to 21) | 0.013 |
| Estradiol (pg/mL) | 30 ± 33.6 (10; 10 to 107) | 18 ± 18.6 (10; 10 to 72) | 0.421 |
| IGF-1 (ng/mL) | 146.6 ± 49.7 (152.3; 78 to 212) | 74.6 ± 45.2 (62.5; 15 to 182.6) | <0.001 |
| IGF-1 SDS | −0.65 ± 1.8 (−0.75; −3.7 to +1.8) | −3.3 ± 2.3 (−3.1; −9.3 to +1.5) | 0.001 |
| IGFBP-3 (ng/mL) | 3379.8 ± 919.7 (3707.5; 1756 to 4415) | 2429.5 ± 931.5 (2400; 1086 to 4628) | 0.013 |
Data are expressed as number (percentage) or mean ± SD (median; min-max). normal range for serum estradiol in pre-menopausal women was 20-250 pg/mL in follicular phase, 38-650 pg/mL in ovulatory phase, and 21-312 pg/mL in luteal phase, and in menopausal women, it was <40 pg/mL.
No predictive model was found at stepwise, linear, and multiple regression analyses by using age, AGHD duration, BMI, IGF-1, and IGFBP-3 as independent variables and QoL-AGHDA, QLS-H total score, or QLS-H z-score as the dependent variable, respectively. Even adjusting for age, no significant model predictive for QoL in AGHD patients was found.
Sexual function
Sexual function was assessed with properly validated and gender-specific questionnaires, IIEF-15 for males and FSFI for females, and analyzed separately. All the results were analyzed considering both the whole male and female cohort and dividing male and female patients according to AGHD treatment status (Groups 1 and 2). Overall 71.2% of patients with AGHD (25/28 women and 27/45 men) had scores of sexual questionnaires consisting with a condition of overt SD.
Male sexual function
The number of AGHD male patients (n = 52) was 18 in Group 1 (r-hGH-treated) and 34 in Group 2 (untreated), according to r-hGH treatment (Table 3). The IIEF-15 rate of answering was not different between the 2 groups (P = 0.224) (Table 3).
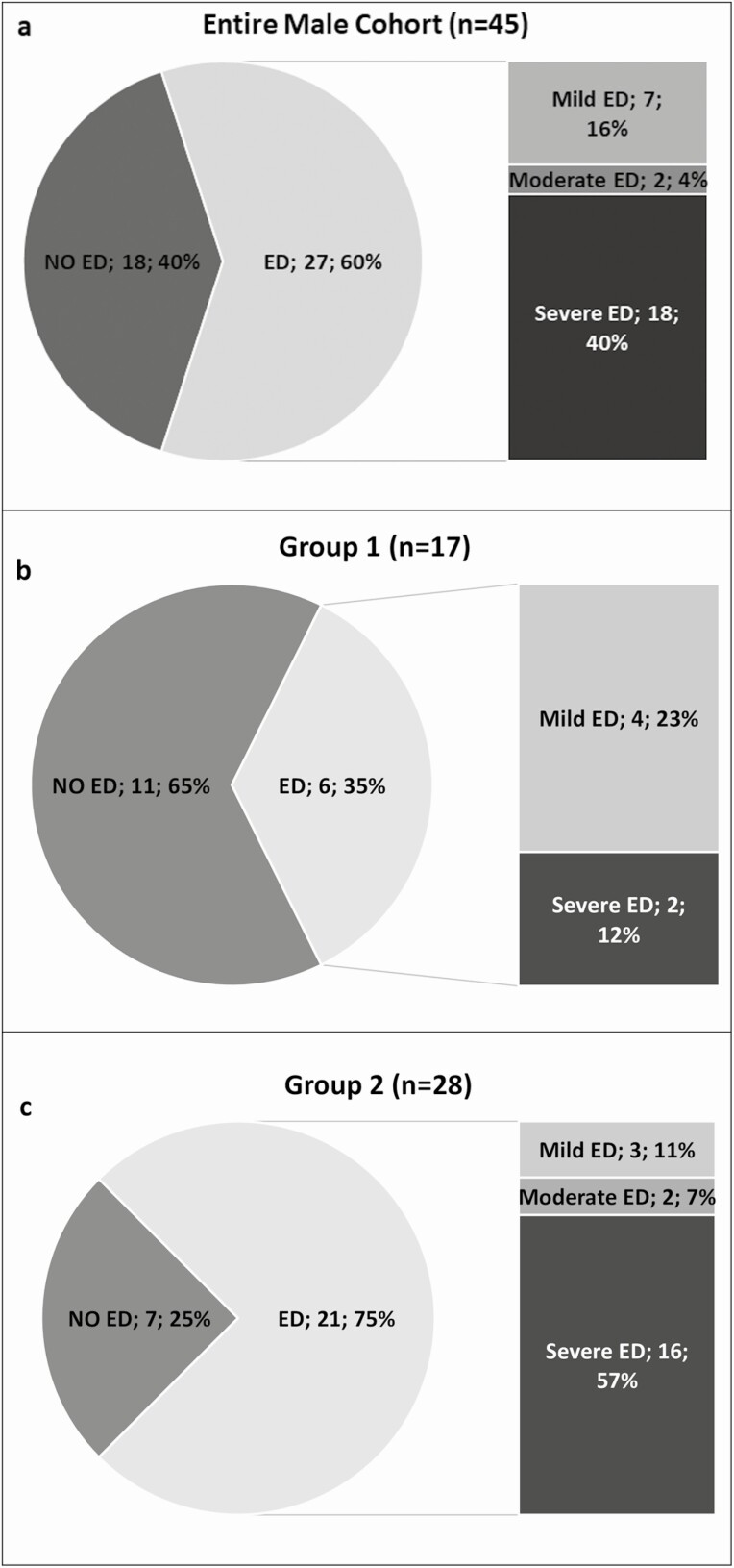
According to the EF score of the IIEF-15 questionnaire, the prevalence of overt ED in the entire male cohort was of 60% (27 patients), classified in mild (n = 7; 16%), moderate (n = 2; 4%), and, in most of the cases, severe (n = 18, 40%) ED (Figure 1A). Comparing r-hGH treated (Group 1) and untreated (Group 2) males, a significantly higher ED prevalence was found in Group 2 (21/28 patients, 75%) than in Group 1 (6/17 patients, 35%; P = 0.008) (Table 3), as shown in Figure 1C and 1B, respectively. All IIEF-15 domains’ scores were significantly higher in Group 1 than in Group 2 (P = 0.001) (Table 3). Moreover, patients of Group 1 were significantly younger than patients of Group 2 (P = 0.002). Mean serum T (P = 0.273), calculated free T (P = 0.131), and SHBG (P = 0.066) levels did not differ between the 2 groups (Table 3).
Figure 1.
Overall prevalence of erectile dysfunction (ED) and prevalence split according to mild, moderate, and severe ED as assessed by IIEF-15 in the entire male cohort (A), in AGHD men treated with r-hGH (Group 1) (B), and untreated AGHD men (Group 2) (C). ED was defined an EF domain score ≤25.
By excluding patients older than 65 years, the prevalence of ED remained significantly lower in Group 1 (6/17 patients; 35%) than in Group 2 (14/18 patients; 78%) (P = 0.011). In this subanalysis, no age difference was documented between the 2 groups (P = 0.183), but significant differences in IIEF-15 domains scores were confirmed as showed in Supplemental Table 2 [38]. Moreover, to avoid the influence of serum T levels on sexual function, we calculated the prevalence of ED in the 2 groups after excluding patients with serum T lower than 2 ng/mL, which represent a threshold for serum T below which EF is impaired [39,40]; even in this case, we documented a significantly lower prevalence of ED in Group 1 (6/16 patients; 37.5%) than in Group 2 (15/21 patients; 71%) (P = 0.039). In a subanalysis, IIEF-15 scores were significantly different even when patients with an integrity of pituitary-gonadal axis were excluded (Supplemental Table 3B) [38].
At linear regression analysis, patient’s age was inversely correlated with all IIEF-15 domain scores, especially with EF (R2 = 0.130, P = 0.015) and SD domain scores (R2 = 0.139, P = 0.012). Conversely, serum IGF-1 levels were directly correlated to all IIEF-15 domain scores, in particular EF (R2 = 0.156, P = 0.008) and SD domains (R2 = 0.159, P = 0.007).
IGF-1 SDS was directly correlated to all IIEF-15 domain scores, in particular EF (R2 = 0.123, P = 0.019) and SD domains (R2 = 0.109, P = 0.029). No significant correlation was documented between both serum total T and calculated free T and IIEF-15 domains.
Stepwise, linear, and multiple regression analyses using age, AGHD duration, BMI, serum IGF-1, and total T as independent variables documented that IGF-1 (R2 = 0.156, P = 0.008) in model 1 and IGF-1 (R2 = 0.275, P = 0.003) and AGHD duration (R2 = 0.275, P = 0.013) in model 2 were the most significant predictive factors for EF domain score (Supplemental Table 1A) [38]. Conversely, only IGF-1 (R2 = 0.159, P = 0.007) was the most significant predictive factor for SD domain score, when SD domain score was the dependent variable. Stepwise, linear, and multiple regression analyses adjusted for age documented that AGHD duration (R2 = 0.221, P = 0.028) in model 1 and AGHD duration (R2 = 0.298, P = 0.015) and IGF-1(R2 = 0.298, P = 0.042) in model 2 were the most significant predictive factor for EF domain score (Supplemental Table 1B) [38]. No predictive model was found adjusting for age when SD domain score was the dependent variable.
At linear regression analysis, QLS-H score (both total and Z-scores) was directly related to the EF (R2 = 0.533, P < 0.005), SD (R2 = 0.535, P < 0.005), and OS domain scores (R2 = 0.563, P < 0.005) of IIEF-15; the same results were obtained using the Z-score. The QoL-AGHDA score was inversely related to the EF (R2 = 0.221, P = 0.001), SD (R2 = 0.181, P = 0.004), and OS domains scores (R2 = 0.180, P = 0.005).
Females
The number of AGHD female patients (n = 31) was 14 in Group 1 (r-hGH-treated) and 17 in Group 2 (untreated) according to r-hGH treatment (Table 4). The rate of answering to FSFI was not different between the 2 groups (Table 4).
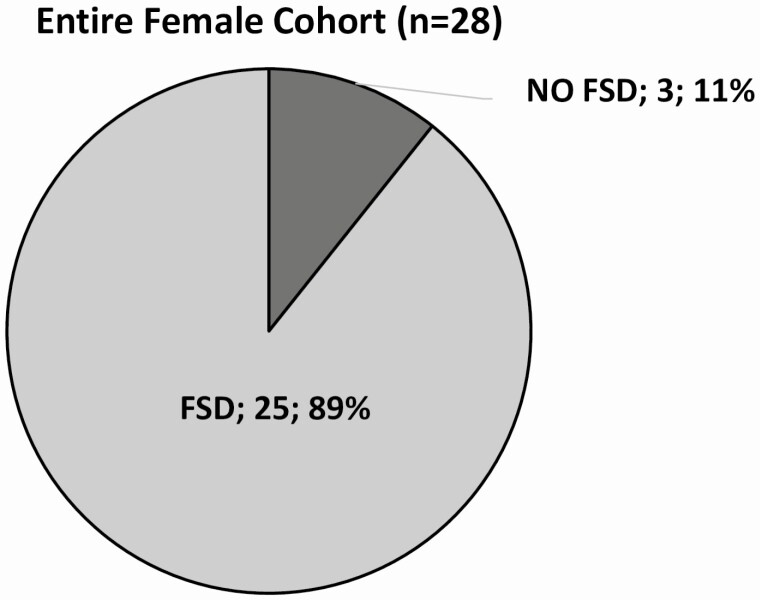
According to FSFI overall score, the prevalence of FSD in the entire female cohort was 89% (25/28 patients) (Fig. 2). Comparing r-hGH treated (Group 1) and untreated (Group 2) females, a significantly higher FSD prevalence was found in Group 2 (15/15 patients; 100%) than in Group 1 (10/13 patients; 77%; P = 0.049) (Table 4). Only 3 patients (11%) reported a score suggestive for the absence of FSD, and all of them belonged to Group 1.
Figure 2.
Prevalence of female sexual dysfunction (FSD) in the entire female cohort as assessed by FSFI. FSD was defined when the overall score was <26.55.
The FSFI overall score was significantly higher in treated than in untreated female patients (P = 0.013); in detail the desire (P = 0.002), arousal (P = 0.029), lubrification (P = 0.005), and orgasm (P = 0.041) domain scores were significantly higher (better scores) in female patients treated with r-hGH (Group 1) than in untreated patients (Group 2) (Table 4). No differences were documented between the 2 groups in terms of age and mean serum estradiol levels (Table 4). Only 9 women, however, had detectable levels of estradiol (3 thanks to an integrity of the gonadotropin-ovaries axis and 6 for the assumption of replacement hormonal therapy). These data were influenced by the high mean age of female patients and the high prevalence of menopause.
At linear regression analysis, the desire domain score (β = −0.539, R2 = 0.290, P = 0.003) was inversely correlated to patients’ age, while both desire domain (β = +0.522, R2 = 0.273, P = 0.004) and overall score (β = +0.379, R2 = 0.144, P = .047) were directly related to serum estradiol. No correlation was documented between FSFI domain scores and serum IGF-1 and IGF-1 SDS.
Stepwise, linear, and multiple regression analyses using age, AGHD duration, BMI, serum IGF-1, and estradiol as independent variables indicated that age (R2 = 0.290, P = 0.003) was the most significant predictive factor for desire domain score, when desire domain score was the dependent variable. Conversely, serum estradiol was the most significant predictive factor for arousal (R2 = 0.238, P = 0.008), lubrification (β = 0.411, R2 = 0.169, P = 0.030) and overall score (R2 = 0.144, P = 0.047), when each of them was considered as dependent variable, respectively.
No correlation was documented between FSFI domain scores and both QLS-H and QoL-AGHDA scores, different from males.
Discussion
The results of this study show for the first time that SD is highly prevalent in patients with AGHD, being present in 71.2% of them. Accordingly, in AGHD patients, ED was present in 60% of men and SD in 89% of women. Hitherto no data are available in the literature on SD in patients with AGHD, although QoL is significantly impaired in these patients [1,2,41] and sexuality, a main component of QoL, impacts considerably the well-being [7-9,42]. At present, only indirect information on sexual life in AGHD patients come from a single item included in the QLS-H questionnaire, concerning the ability to become sexually aroused; this item contributes only in part to the final QoL score of QLS-H [28]. Besides, a study in which QoL was evaluated by the patient’s spouse/partner, rather than by self-completed questionnaires, indirectly provided information about sexual life in the context of QoL, even though sexual function and activity were not directly assessed [43].
In men, ED was more prevalent in untreated (Group 2) than in r-hGH treated AGHD patients (Group 1), and the EF score indicated a severe impairment of EF in the majority of AGHD men. All the IIEF-15 scores were higher in r-hGH treated patients (Group 1) than in untreated patients (Group 2), and they resulted directly correlated to serum IGF-1 and, as it happens in the general population, inversely correlated to age and serum T. Moreover, the evidence of better EF scores in r-hGH treated patients, even after the exclusion of men with serum T below 2 ng/mL or men older than 65 years, suggested that concomitant hypogonadism or aging did not impact the prevalence of ED. Furthermore, serum IGF-1 and the duration of AGHD, but not age and serum T, were identified as significant predictors of IIEF-15 scores at logistic regression analysis, thus suggesting that AGHD and low serum IGF-1 are directly associated to worse IIEF-15 scores, having aging and T a minor role. All together these results suggest that GHD may play a role in the occurrence of ED in AGHD patients who are adequately replaced with exogenous T or have normal gonadal function and that r-hGH therapy may have a beneficial effect also on male sexual function. The results of this study do not allow ascertaining if ED is a direct consequence of serum GH and/or IGF-1 below the normal range or of the cardiovascular changes accumulating as an effect of prolonged GHD. The beneficial effect of r-hGH on EF may indirectly result from the mitigation of the increased risk of developing cardiovascular diseases, metabolic syndrome and diabetes mellitus associated to AGHD [6,41]. Indeed, ED is highly prevalent in men with cardiovascular diseases and also predicts major cardiovascular events [44,45]. Similarly, an increased prevalence of ED has been recently demonstrated in men with acromegaly, probably as a consequence of cardiovascular abnormalities related to GH excess, rather than to a direct effect of GH overproduction on EF [14].
In women, the prevalence of SD was slightly, but significantly different between the 2 groups. Even though median overall FSFI scores were under the cut-off for FSD diagnosis (26.55) in both groups, a statistically significant difference between r-hGH treated (Group 1) and untreated (Group 2) patients was documented for the majority of FSFI domains, with better results in r-hGH treated AGHD women (Group1). Nevertheless, it should be remarked that the only 3 women without SD were on r-hGH treatment (Group 1). In addition, data coming from the female cohort suffer from the menopausal or hypogonadal status of almost all women. Estrogen deficiency is a great confounder for the interpretation of female sexual function data since SD is highly prevalent in postmenopausal women or in women with estrogen deficiency [46]. Besides, in the female AGHD cohort, results about sexual outcomes were negatively influenced by both the age of patients (mean age 55.8 + 15.6 years; median age 59; range 19-84) and the small sample size in each group. Even though these results suggest an overall better sexuality also in AGHD females treated with r-hGH, the previously mentioned issues do not allow strengthening this concept as for the male counterpart. The assessment of SD in females is largely overlooked notwithstanding a very high prevalence (41%) of SD in premenopausal women [47]. This unmet need in clinical practice also reflects the coexistence of a research gap on FSD [48], accounting for gender inequalities between sexes concerning the diagnosis and management of SD, [49] and explains why male sexual function is currently investigated in patients with several comorbidities and/or chronic diseases [50], whereas this does not happen in women [49]. In the case of AGHD, the assessment of sexual function is not included in the clinical work-up in both sexes [1,2,31] notwithstanding the strong evidence of QoL impairment in AGHD is evidence-based [3,28].
The finding of better sexual function scores in patients treated with r-hGH in both sexes supports a positive effect of r-hGH therapy on sexual well-being. It remains to be ascertained whether r-hGH therapy exerts itself a direct effect on sexual function and behavior or whether AGHD patients with ongoing therapy are in a better general health status and consequently experience a better sexual life. Accordingly, the direct correlation between better QoL and better sexual function in AGHD patients may be the result of a good healthy condition. Questionnaires validated for assessing QoL in AGHD patients indirectly provide information also on well-being related to satisfactory sexual life; however, in our study, these data were confirmed only for male cohort since QLS-H and AGHDA results were correlated to IIEF-15 scores but not to FSFI scores. Even though QLS-H and AGHDA questionnaires seem to consider also sexual health in the final calculation of QoL, they do not provide any information about the presence/absence of SD both in men and women with AGHD. The high prevalence of SD in AGHD patients found in this study points out that the investigation of sexual function in these patients is always overlooked. The lack of interest in sexual function of these patients probably represents an unmet scientific and clinical need for the management of AGHD.
In clinical practice the evaluation of sexual well-being by means of validated questionnaires, such as IIEF-15 and FSFI, should be considered as part of the global QoL assessment in AGHD patients since it is a simple tool that is not time-consuming that provides information on sexual life, useful to establish its impact on QoL.
This study has some strengths and some limitations. The investigation of sexual function of AGHD patients by specific, validated tools represents a novelty and the real-life setting of a single center cohort warrants a homogeneity of data collected, representing another strength of the study. The main limits of the study are the small sample size, especially for the women subgroup; the absence of a control group; the high prevalence of postmenopausal women; and the outcome mainly based on questionnaires that, by definition, may be biased by patients’ factors (as well as by adherence to fill questionnaires). Besides, the cross-sectional design does not allow establishing a cause-effect relationship between SD and AGHD.
In conclusion, the novelty of this study is represented by the structured analysis of sexual function in AGHD patients using validated, gender-specific questionnaires for the assessment of sexual impairment. This kind of approach demonstrated to value sexuality as an important component of QoL and the obtained results shed new light on the association between untreated AGHD and SD, mainly ED. For these reasons, future controlled studies on large samples of patients, specifically aiming to investigate the role of GH on sexual function, are needed to better understand the impact of AGHD on sexuality and if r-hGH therapy is able to improve also sexual function.
Acknowledgments
This clinical study is being conducted thanks to the competitive assignment of an Independent Grant for Learning & Change (“IGCL”) Dissemination & Implementation (“D&I”) by Pfizer Inc. Authors are grateful to the Italian Ministry of University and Research for supporting the Department of Biomedical, Metabolic, and Neural Sciences (University of Modena and Reggio Emilia, Italy) in the context of the Departments of Excellence Programme. We thank Dr. Bruno Madeo; Dr. Antonio R. M. Granata; and Dr. Daniele Santi of the Unit of Endocrinology, Department of Biomedical, Metabolic and Neural Sciences, University of Modena and Reggio Emilia, and the Department of Medical Specialties, Azienda Ospedaliero-Universitaria di Modena, Ospedale Civile di Baggiovara, Modena, Italy, for their effective contribution to patients’ enrollment. We thank all the trainees who belong to the School of Specialization in Endocrinology and Metabolism of the University of Modena and Reggio Emilia for their support and involvement in the study (Sara De Vincentis, Andrea Craparo, Carla Greco, Maria Chiara Decaroli, Antonino Russo, Michela Locaso, Daniela Domenici, Giulia Tartaro, Francesca Piccinini, Gianluca Margiotta, Gisella Boselli, Simona Loiacono, Rossella Corleto, Marilina Romeo, and Barbara Rossi).
Financial Support: This clinical study was conducted thanks to the competitive assignment of an Independent Grant for Learning & Change (“IGCL”) Dissemination & Implementation (“D&I”) by Pfizer Incorporated (www.cybergrants.com/pfizer/loi, Identification grant number: 34515061).
Clinical Trial Information: NCT03525587.
Authors’ Contributions: VR and EM designed the study protocol. EM, MLM, and SP collected data and created the database. VR, MLM, and SP performed statistical analysis and wrote the first draft of the manuscript. All authors contributed to patients’ enrollment and clinical management and approved the final version of the manuscript.
Additional Information
Disclosure Summary: The authors declare that there is no conflict of interest regarding the publication of this article.
Data Availability
Some or all data sets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Ho KK; 2007 GH Deficiency Consensus Workshop Participants . Consensus guidelines for the diagnosis and treatment of adults with GH deficiency II: a statement of the GH Research Society in association with the European Society for Pediatric Endocrinology, Lawson Wilkins Society, European Society of Endocrinology, Japan Endocrine Society, and Endocrine Society of Australia. Eur J Endocrinol. 2007;157(6): 695-700. [DOI] [PubMed] [Google Scholar]
- 2. Molitch ME, Clemmons DR, Malozowski S, Merriam GR, Vance ML; Endocrine Society . Evaluation and treatment of adult growth hormone deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(6):1587-1609. [DOI] [PubMed] [Google Scholar]
- 3. Rosilio M, Blum WF, Edwards DJ, et al. Long-term improvement of quality of life during growth hormone (GH) replacement therapy in adults with GH deficiency, as measured by questions on life satisfaction-hypopituitarism (QLS-H). J Clin Endocrinol Metab. 2004;89(4):1684-1693. [DOI] [PubMed] [Google Scholar]
- 4. Bengtsson BA, Abs R, Bennmarker H, et al. KIMS Study Group and the KIMS International Board. The effects of treatment and the individual responsiveness to growth hormone (GH) replacement therapy in 665 GH-deficient adults.. J Clin Endocrinol Metab. 1999;84(11):3929-3935. [DOI] [PubMed] [Google Scholar]
- 5. Attanasio AF, Bates PC, Ho KK, et al. ; Hypoptiuitary Control and Complications Study International Advisory Board . Human growth hormone replacement in adult hypopituitary patients: long-term effects on body composition and lipid status: 3-year results from the HypoCCS database. J Clin Endocrinol Metab. 2002;87(4):1600-1606. [DOI] [PubMed] [Google Scholar]
- 6. Weber MM, Biller BM, Pedersen BT, Pournara E, Christiansen JS, Höybye C. The effect of growth hormone (GH) replacement on blood glucose homeostasis in adult nondiabetic patients with GH deficiency: real-life data from the NordiNet® International Outcome Study. Clin Endocrinol (Oxf). 2017;86(2):192-198. [DOI] [PubMed] [Google Scholar]
- 7. Ventegodt S. Sex and the quality of life in Denmark. Arch Sex Behav. 1998;27(3):295-307. [DOI] [PubMed] [Google Scholar]
- 8. Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA. 1999;281(6):537-544. [DOI] [PubMed] [Google Scholar]
- 9. World Health Organization. Defining sexual health report of a technical consultation on sexual health 28-31 January 2002, Geneva. https://www.who.int/reproductivehealth/publications/sexual_health/defining_sexual_health.pdf2006. Accessed November 10, 2020.
- 10. Rosén T, Wirén L, Wilhelmsen L, Wiklund I, Bengtsson BA. Decreased psychological well-being in adult patients with growth hormone deficiency. Clin Endocrinol (Oxf). 1994;40(1):111-116. [DOI] [PubMed] [Google Scholar]
- 11. Lagrou K, Xhrouet-Heinrichs D, Massa G, et al. Quality of life and retrospective perception of the effect of growth hormone treatment in adult patients with childhood growth hormone deficiency. J Pediatr Endocrinol Metab. 2001;14(Suppl 5):1249-1260. [PubMed] [Google Scholar]
- 12. Attanasio AF, Shavrikova EP, Blum WF, Shalet SM. Quality of life in childhood onset growth hormone-deficient patients in the transition phase from childhood to adulthood. J Clin Endocrinol Metab. 2005;90(8):4525-4529. [DOI] [PubMed] [Google Scholar]
- 13. Brill KT, Weltman AL, Gentili A, et al. Single and combined effects of growth hormone and testosterone administration on measures of body composition, physical performance, mood, sexual function, bone turnover, and muscle gene expression in healthy older men. J Clin Endocrinol Metab. 2002;87(12): 5649-5657. [DOI] [PubMed] [Google Scholar]
- 14. Lotti F, Rochira V, Pivonello R, et al. Erectile dysfunction is common among men with acromegaly and is associated with morbidities related to the disease. J Sex Med. 2015;12(5):1184-1193. [DOI] [PubMed] [Google Scholar]
- 15. Chen Z, Yu Y, He M, et al. Higher growth hormone levels are associated with erectile dysfunction in male patients with acromegaly. Endocr Pract. 2019;25(6):562-571. [DOI] [PubMed] [Google Scholar]
- 16. Galdiero M, Pivonello R, Grasso LF, Cozzolino A, Colao A. Growth hormone, prolactin, and sexuality. J Endocrinol Invest. 2012;35(8):782-794. [DOI] [PubMed] [Google Scholar]
- 17. Sansone A, Romanelli F, Gianfrilli D, Lenzi A. Endocrine evaluation of erectile dysfunction. Endocrine. 2014;46(3):423-430. [DOI] [PubMed] [Google Scholar]
- 18. Maggi M, Buvat J, Corona G, Guay A, Torres LO. Hormonal causes of male sexual dysfunctions and their management (hyperprolactinemia, thyroid disorders, GH disorders, and DHEA). J Sex Med. 2013;10(3):661-677. [DOI] [PubMed] [Google Scholar]
- 19. Tenuta M, Carlomagno F, Cangiano B, et al. Somatotropic-testicular axis: a crosstalk between GH/IGF-I and gonadal hormones during development, transition, and adult age. Andrology. 2021;9(1):168-184. [DOI] [PubMed] [Google Scholar]
- 20. Carosa E, Sansone A, Jannini EA. Management of endocrine disease: Female sexual dysfunction for the endocrinologist. Eur J Endocrinol. 2020;182(6):R101. [DOI] [PubMed] [Google Scholar]
- 21. Menezes M, Salvatori R, Oliveira CR, et al. Climacteric in untreated isolated growth hormone deficiency. Menopause. 2008;15(4 Pt 1):743-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Becker AJ, Uckert S, Stief CG, et al. Serum levels of human growth hormone during different penile conditions in the cavernous and systemic blood of healthy men and patients with erectile dysfunction. Urology. 2002;59(4):609-614. [DOI] [PubMed] [Google Scholar]
- 23. Uckert S, Scheller F, Stief CG, et al. Potential mechanism of action of human growth hormone on isolated human penile erectile tissue. Urology. 2010;75(4):968-973. [DOI] [PubMed] [Google Scholar]
- 24. Bartke A, Chandrashekar V, Turyn D, et al. Effects of growth hormone overexpression and growth hormone resistance on neuroendocrine and reproductive functions in transgenic and knock-out mice. Proc Soc Exp Biol Med. 1999;222(2):113-123. [DOI] [PubMed] [Google Scholar]
- 25. Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84(10):3666-3672. [DOI] [PubMed] [Google Scholar]
- 26. Mavromati M, Kuhn E, Agostini H, et al. Classification of patients with GH disorders may vary according to the IGF-I assay. J Clin Endocrinol Metab. 2017;102(8):2844-2852. [DOI] [PubMed] [Google Scholar]
- 27. Webb SM, Crespo I, Santos A, Resmini E, Aulinas A, Valassi E. Management of endocrine disease: quality of life tools for the management of pituitary disease. Eur J Endocrinol. 2017;177(1):R13-R26. [DOI] [PubMed] [Google Scholar]
- 28. Blum WF, Shavrikova EP, Edwards DJ, et al. Decreased quality of life in adult patients with growth hormone deficiency compared with general populations using the new, validated, self-weighted questionnaire, questions on life satisfaction hypopituitarism module. J Clin Endocrinol Metab. 2003;88(9): 4158-4167. [DOI] [PubMed] [Google Scholar]
- 29. McKenna SP, Doward LC, Alonso J, et al. The QoL-AGHDA: an instrument for the assessment of quality of life in adults with growth hormone deficiency. Qual Life Res. 1999;8(4): 373-383. [DOI] [PubMed] [Google Scholar]
- 30. Koltowska-Häggström M, Mattsson AF, Monson JP, et al. Does long-term GH replacement therapy in hypopituitary adults with GH deficiency normalise quality of life? Eur J Endocrinol. 2006;155(1):109-119. [DOI] [PubMed] [Google Scholar]
- 31.National Institute for Health and Clinical Excellence. Human growth homone (somatropin) in adults with growth hormone deficiency. http://guidance.nice.org.uk/TA64/Guidance/Recommendation (2003)2003. Accessed November 10, 2020.
- 32. Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The International Index of Erectile Function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49(6):822-830. [DOI] [PubMed] [Google Scholar]
- 33. Cappelleri JC, Rosen RC, Smith MD, Mishra A, Osterloh IH. Diagnostic evaluation of the erectile function domain of the International Index of Erectile Function. Urology. 1999;54(2):346-351. [DOI] [PubMed] [Google Scholar]
- 34. Rosen RC, Cappelleri JC, Gendrano N 3rd. The International Index of Erectile Function (IIEF): a state-of-the-science review. Int J Impot Res. 2002;14(4):226-244. [DOI] [PubMed] [Google Scholar]
- 35. Wiegel M, Meston C, Rosen R. The female sexual function index (FSFI): cross-validation and development of clinical cutoff scores. J Sex Marital Ther. 2005;31(1):1-20. [DOI] [PubMed] [Google Scholar]
- 36. Rosen R, Brown C, Heiman J, et al. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26(2):191-208. [DOI] [PubMed] [Google Scholar]
- 37. Witting K, Santtila P, Jern P, et al. Evaluation of the female sexual function index in a population based sample from Finland. Arch Sex Behav. 2008;37(6):912-924. [DOI] [PubMed] [Google Scholar]
- 38. Monzani ML, Pederzoli S, Volpi L, Magnani E, Diazzi C, Rochira V. supplemental data: sexual dysfunction: a neglected and overlooked issue in adult growth hormone deficiency (AGHD). results from the management of AGHD (MAGHD) study. 2020. Deposited 31 December 2020. 10.6084/m9.figshare.13507281.v2. Accessed December 31, 2020. [DOI]
- 39. Granata AR, Rochira V, Lerchl A, Marrama P, Carani C. Relationship between sleep-related erections and testosterone levels in men. J Androl. 1997;18(5):522-527. [PubMed] [Google Scholar]
- 40. Zitzmann M, Faber S, Nieschlag E. Association of specific symptoms and metabolic risks with serum testosterone in older men. J Clin Endocrinol Metab. 2006;91(11):4335-4343. [DOI] [PubMed] [Google Scholar]
- 41. Jørgensen JOL, Juul A. Therapy of endocrine disease: growth hormone replacement therapy in adults: 30 years of personal clinical experience. Eur J Endocrinol. 2018;179(1):R47-R56. [DOI] [PubMed] [Google Scholar]
- 42. Flynn KE, Lin L, Bruner DW, et al. Sexual satisfaction and the importance of sexual health to quality of life throughout the life course of U.S. adults. J Sex Med. 2016;13(11):1642-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Burman P, Broman JE, Hetta J, et al. Quality of life in adults with growth hormone (GH) deficiency: response to treatment with recombinant human GH in a placebo-controlled 21-month trial. J Clin Endocrinol Metab. 1995;80(12):3585-3590. [DOI] [PubMed] [Google Scholar]
- 44. Dong JY, Zhang YH, Qin LQ. Erectile dysfunction and risk of cardiovascular disease: meta-analysis of prospective cohort studies. J Am Coll Cardiol. 2011;58(13):1378-1385. [DOI] [PubMed] [Google Scholar]
- 45. Guay A, Jacobson J. The relationship between testosterone levels, the metabolic syndrome (by two criteria), and insulin resistance in a population of men with organic erectile dysfunction. J Sex Med. 2007;4(4 Pt 1):1046-1055. [DOI] [PubMed] [Google Scholar]
- 46. Nappi RE, Cucinella L, Martella S, Rossi M, Tiranini L, Martini E. Female sexual dysfunction (FSD): prevalence and impact on quality of life (QoL). Maturitas. 2016;94:87-91. [DOI] [PubMed] [Google Scholar]
- 47. McCool ME, Zuelke A, Theurich MA, Knuettel H, Ricci C, Apfelbacher C. Prevalence of female sexual dysfunction among premenopausal women: a systematic review and meta-analysis of observational studies. Sex Med Rev. 2016;4(3):197-212. [DOI] [PubMed] [Google Scholar]
- 48. Vignozzi L, Reisman Y. Testosterone in women: are we closing the gender gap? Nat Rev Urol. 2020;17(2):67-68. [DOI] [PubMed] [Google Scholar]
- 49. McCool-Myers M, Theurich M, Zuelke A, Knuettel H, Apfelbacher C. Predictors of female sexual dysfunction: a systematic review and qualitative analysis through gender inequality paradigms. BMC Womens Health. 2018;18(1):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Irwin GM. Erectile dysfunction. Prim Care. 2019;46(2):249-255. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data sets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.