Abstract
Objective.
The aim of this multicenter study was to evaluated whether cold or warm cardioplegia are associated with postoperative atrial fibrillation (POAF) and the prognostic role of the latter on early stroke and neurological mortality.
Methods.
This was a retrospective analysis of prospective collected data from 9 cardiac centers in Italy and the United States including patients undergoing surgery between 2010 and 2018. From the 9 institutional databases, 17,231 patients underwent isolated CABG on-pump, using either warm cardioplegia (n = 7,730) or cold cardioplegia (n = 9,501); among the latter group blood and crystalloid cardioplegia were used in 691 and 8,810 patients, respectively. After matching, two pairs of 4,162 patients (overall cohort 8,324) were analyzed.
Results.
In matched population, the rate of POAF was 18% (1472 cases), 15% (608) in warm group versus 21% (864) in cold group (p<0.001). Multivariable analysis confirmed that cold cardioplegia was associated with higher rate of POAF, along with age, hypercholesterolemia, LVEF, reoperation, preoperative IABP, previous stroke, cardiopulmonary and cross-clamp. Moreover, cold cardioplegia as well as POAF increased the rate of postoperative stroke as well as early mortality and neurological mortality Propensity-weighted cohort included 11830 (70%) patients out of 17231. After adjustment, both cold blood and cold crystalloid cardioplegia negatively influenced POAF, stroke and neurological mortality.
Conclusions.
Warm cardioplegia may reduce the rate of POAF in CABG patients with respect to cold cardioplegia, either blood or crystalloid. This has a prognostic impact on postoperative stroke and neurological mortality.
Keywords: Atrial fibrillation, coronary artery bypass, stroke, blood cardioplegia, crystalloid cardioplegia, cold cardioplegia, warm cardioplegia
Graphical Abstract
Introduction
The prevalence of postoperative atrial fibrillation (POAF) ranges between 20% and 50%; this wide range is due to differences in definitions of postoperative atrial fibrillation and type of surgery (coronary surgery, valvular, combined coronary and valvular surgery). In fact, POAF occurred in 20-30% of patients undergoing coronary artery bypass grafting (CABG) and in 40-50% after valvular surgery, particularly mitral valve surgery [1–5].
Several risk factors predisposing to atrial fibrillation (AF) have been identified so far: 1) preoperative: age, left ventricular dysfunction, left atrial enlargement, obesity, hypertension, chronic heart failure, chronic pulmonary disease, history of AF; 2) intra- and postoperative: cross-clamp and cardiopulmonary bypass duration, intra-aortic balloon pump, bicaval cannulation, venting via pulmonary vein, inotropic and diuretic use, pericardial fluid [3–9].
There are several evidences that POAF is associated with inflammatory response, and cardiopulmonary bypass (CPB) might contribute to the systemic inflammatory state [10–12]. In addition to CPB, other factors including hypothermia, hemodilution, electrolyte imbalance, pharmacological agents used during surgery have also been implicated in initiating inflammatory responses, being so a possible trigger for POAF [13–15].
The onset of POF has a remarkable impact on the hospital length-of-stay, but it is also linked to severe complication. Indeed, POAF has been repetitively shown to play an important role in the genesis of cerebrovascular events in the postoperative phase.
Hence, the aim of this multicenter study was to evaluated whether cold or warm cardioplegia are associated with POAF and the prognostic role of the latter on early stroke and neurological mortality.
Materials and Methods
This was a retrospective analysis of prospective collected data from 9 cardiac centers in Italy and the United States including patients undergoing CABG between 2010 and 2018. All patients consented to surgery. The need for retrospective consent to data collection was waived by local ethical committees. A common dataset, with agreed definitions and variables, was used for this study. Only in-hospital events and outcomes were assessed. Inclusion criteria were isolated, elective, on-pump, with cardioplegia (cold crystalloid, cold blood, or warm blood cardioplegia)-induced cardiac arrest, CABG in adult patients.
Each data frame from each center was saved in .csv format and double-checked for consistency and possible errors (eg, extreme outliers/wrong coding). Inconsistencies were resolved after consultation with the local principal investigator. The nine data frames were then merged and uploaded in a common dataset for the analysis. The study was approved by the ethical committee of the Community Hospital, Brescia, Italy (nr. 1434, 04/03/2014) and further released to each participating center for local approval.
End-points
The primary outcome was POAF, defined as new-onset AF in the immediate period after surgery. [1]. The main aim of the analysis was to assess whether the use of cold (Cold group) versus warm (Warm group) cardioplegia was associated with the primary outcome. The secondary end-point was to evaluate the impact of POAF on postoperative stroke rate and early mortality, mainly due to neurological causes.
Statistical Analysis
Continuous variables were tested for normality with Shapiro– Wilk test and were reported as mean and SD or median and interquartile range; t test or Wilcoxon–Mann–Whitney test were used to compare continuous variables. Categorical variables were reported as counts and percentages and compared with chi-square test.
Propensity score matching was used to balance the distributions of measured confounding baseline covariates between the Cold and Warm groups. The propensity score was obtained using with Machine Learning- Random Forest; Overlapping was tested with common support plots; 1:1 matching with different calipers from 0.5 to 0.65 was tested, choosing the best one (0.65). [16]. Variables included into the propensity model were age, gender, diabetes, hypertension, hypercholesterolemia, chronic pulmonary disease (COPD), chronic renal failure (CRF) previous stroke, carotid disease, pluri-vascular disease (PVD), left ventricular ejection fraction (LVEF), left main disease (LMS), redo, pulmonary hypertension (PH), preoperative intra-aortic balloon pump (IABP). Balance of the two matched groups was tested with standardized mean difference (SMD), considered as optimal below 0.20. Then, propensity score weighting was used to adjust casual inference with multiple treatment (warm blood cardioplegia vs cold blood cardioplegia vs crystalloid blood cardioplegia) [17].
Logistic regression was used to identify risk factors for primary and secondary endpoints. Bootstapping in 1000 samples was used to correct both estimators and 95% confidence limits. Model discrimination was evaluated by the area under the receiver operating characteristic curve. R-studio version 1.1.463 (2009-2018) was used for all statistical analyses and P value significance was set at 0.05.
Results
From the 9 institutional databases, 17,231 patients underwent isolated CABG on-pump, using either warm cardioplegia (n = 7,730) or cold cardioplegia (n = 9,501); among the latter group blood and crystalloid cardioplegia were used in 691 and 8,810 patients, respectively. Three centers used only cold cardioplegia, two centers used only warm cardioplegia, two centers used mainly cold cardioplegia, two centers mainly warm cardioplegia. After matching two pairs of 4,162 patients (overall cohort 8,324) were analyzed. Table 1 reports the baseline characteristics of matched groups. Among 4162 patients in group cold. After matching patients in cold group still showed lower prevalence of hypercholesterolemia and lower CPB and cross-clamp duration.
Table 1.
Pre- and operative data according to warm or cold cardioplegia.
| Warm (n=4162) | Cold (n=4162) | SMD before matching | SMD after matching | |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Age, y | 67±9 | 66±10 | 0.029 | −0.069 |
| Men | 2660 (64) | 2388 (57) | −1.105 | −0.163 |
| LVEF, % | 52±10 | 52±12 | 0.335 | −0.001 |
| Systemic hypertension | 1742 (42) | 1528 (37) | −0.255 | −0.102 |
| Hypercholesterolemia | 1347 (32) | 669 (16) | −0.353 | −0.381 |
| Diabetes | 887 (21) | 695 (16) | 0.199 | −0.115 |
| COPD | 134 (3) | 101 (2) | 0.209 | −0.030 |
| Renal dysfunction* | 96 (2) | 98 (2) | 0.372 | 0.001 |
| Stroke history | 22 (0.5) | 9 (0.2) | 0.041 | −0.040 |
| Carotid stenosis >50% | 92 (2) | 49 (1) | −0.105 | −0.138 |
| Peripheral vascular disease | 425 (10) | 211 (5) | −0.164 | −0.193 |
| Preoperative IABP | 112 (3) | 71 (2) | −0.001 | −0.079 |
| Pulmonary hypertension | 121 (3) | 169 (4) | 0.039 | 0.059 |
| Left main disease | 907 (22) | 657 (16) | −0.145 | −0.153 |
| Redo surgery | 79 (2) | 121 (3) | 0.0554 | 0.0752 |
| CPB duration | 75±26 | 72±28 | −0.897 | −0.577 |
| Cross-clamp duration | 52±18 | 50±20 | −0.754 | −0.458 |
| Crystalloid | 3041 (73%) | |||
| Blood | 1121 (27%) | |||
Values are expressed as mean±SD or number (percentage). COPD, chronic obstructive pulmonary disease; CPB, cardiopulmonary bypass; IABP, intra-aortic balloon pump; LVEF, left ventricular ejection fraction. SMD, standardized mean difference.
Creatinine >2 mg/dL.
Warm vs Cold
In matched population, the rate of POAF was 18% (1472 cases), 15% (608) in warm group versus 21% (864) in cold group (p<0.001) (Figure 1).
Figure 1.
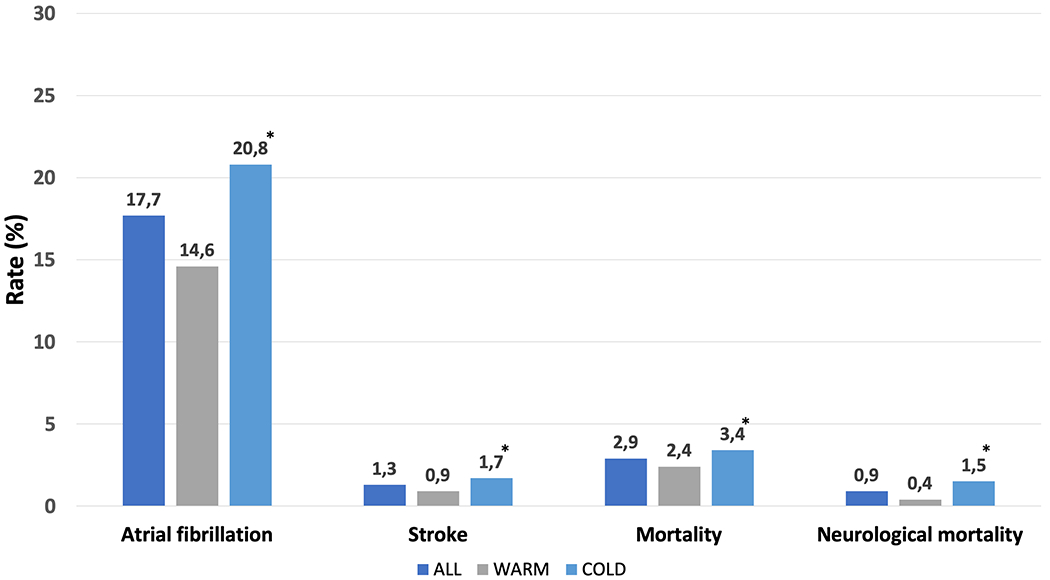
Incidence of postoperative atrial fibrillation, stroke, mortality and neurological mortality in patients having warm vs cold cardioplegia; All (dark blue), Warm (gray), Cold (light blue) cardioplegia. *p-value <0.01.
Multivariable analysis confirmed that cold cardioplegia was associated with higher likelihood to develop POAF, along with age, hypercholesterolemia, LVEF, reoperation, preoperative IABP, previous stroke, cardiopulmonary and cross-clamp duration (Table 2).
Table 2.
Risk factors for higher rate of postoperative atrial fibrillation.
| OR | 95% CL | p-value | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Age, y | 1.037 | 1.030 | 1.044 | <0.001 |
| Hypercholesterolemia, mg/dL | 1.197 | 1.044 | 1.372 | 0.010 |
| LVEF, % | 1.005 | 1.001 | 1.010 | 0.045 |
| REDO | 1.973 | 1.449 | 2.688 | <0.001 |
| Preoperative IABP | 1.525 | 1.059 | 2.198 | 0.024 |
| Stroke history | 2.820 | 1.339 | 5.939 | 0.006 |
| CPB duration, minutes | 1.003 | 1.001 | 1.006 | 0.012 |
| Cross-clamp duration, minutes | 1.003 | 1.001 | 1.007 | 0.040 |
| Cold cardioplegia | 1.618 | 1.438 | 1.821 | <0.001 |
CL, confidence limits; CPB, cardiopulmonary bypass; IABP, intra-aortic balloon pump; LVEF, left ventricular ejection fraction. OR, odds ratio; AUC 0.81; OR, 95CL and p-value are corrected in 1000 bootstrap samples.
The overall rate of stroke, mortality and neurological mortality were 1.3% (108 patients), 2.9% (240) and 78 (0.9%), respectively. Patients undergoing CABG with cold cardioplegia showed higher incidence of stroke, mortality and neurological mortality than patients having warm cardioplegia (Figure 1);
Moreover, cold cardioplegia increased the rate of postoperative stroke as well as early mortality and neurological mortality (Table 3). Patients experiencing POAF showed significantly higher rate of stroke and neurological mortality (Figure 2). Multivariable analysis confirmed that POAF was associated with worse outcome (Table 3).
Table 3.
Risk factors for higher rate of stroke and mortality.
| OR | 95% CL | p-value | ||
|---|---|---|---|---|
| Target: Stroke | Lower | Upper | ||
| Age, y | 1.082 | 1.082 | 1.056 | <0.001 |
| Renal dysfunction* | 2.313 | 2.313 | 1.042 | 0.039 |
| Preoperative IABP | 2.957 | 2.957 | 1.325 | 0.008 |
| CPB duration, minutes | 1.013 | 1.008 | 1.019 | <0.001 |
| Cold cardioplegia | 1.752 | 1.186 | 2.711 | 0.006 |
| POAF | 1.990 | 1.990 | 1.321 | 0.006 |
| Target: Mortality | ||||
| Age, y | 1.035 | 1.019 | 1.051 | <0.001 |
| Diabetes | 1.565 | 1.153 | 2.126 | 0.004 |
| Renal dysfunction* | 3.205 | 1.939 | 5.296 | <0.001 |
| Preoperative IABP | 5.576 | 3.486 | 8.920 | <0.001 |
| CPB duration, minutes | 1.024 | 1.020 | 1.028 | <0.001 |
| Cold cardioplegia | 1.473 | 1.089 | 1.991 | 0.002 |
| POAF | 1.577 | 1.169 | 2.128 | 0.012 |
| Target: Neurological Mortality | ||||
| Age, y | 1.042 | 1.015 | 1.071 | 0.002 |
| Diabetes | 1.670 | 1.000 | 2.789 | 0.049 |
| LVEF | 1.046 | 1.024 | 1.069 | <0.001 |
| Preoperative IABP | 9.495 | 4.202 | 21.458 | <0.001 |
| CPB duration, minutes | 1.026 | 1.021 | 1.030 | <0.001 |
| Cold cardioplegia | 3.943 | 2.251 | 6.906 | <0.001 |
| POAF | 2.403 | 1.496 | 3.859 | 0.004 |
CL, confidence limits; CPB, cardiopulmonary bypass; IABP, intra-aortic balloon pump; LVEF, left ventricular ejection fraction. OR, odds ratio; POAF, postoperative atrial fibrillation; AUC1 0.79; AUC2 0.81; AUC3 0.78; OR, 95CL and p-value are corrected in 1000 bootstrap samples.
Figure 2.
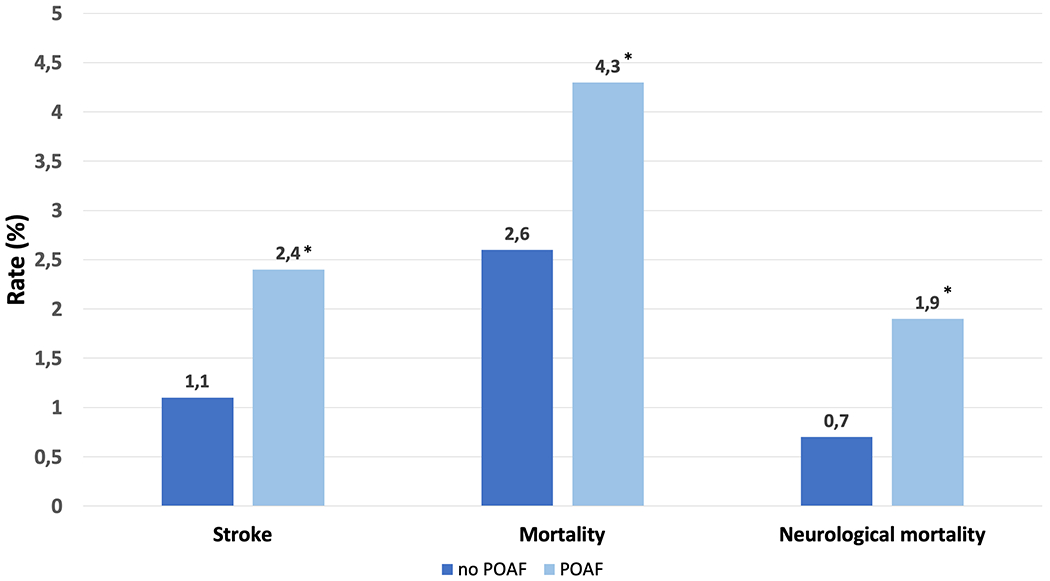
Incidence of stroke, mortality and neurological mortality in patients having POAF (light blue) or not (dark blue). *p-value <0.01
Warm Blood vs Cold Blood vs Cold Crystalloid
Propensity-weighted cohort included 11830 (70%) patients out of 17231. A good balance was reached for following variables: age, gender, hypertension, hypercholesterolemia, carotid disease, pluri-vascular disease (PVD), left ventricular ejection fraction (LVEF), left main disease (LMS). Hence, chronic pulmonary disease (COPD), chronic renal failure (CRF) previous stroke, redo, pulmonary hypertension (PH), preoperative intra-aortic balloon pump (IABP) were included into logistic regression to adjust final outcome models. After adjustment, both cold blood and cold crystalloid cardioplegia negatively influenced POAF, stroke and neurological mortality, having as reference warm blood cardioplegia (figure 3). No statistical difference was found between the two types of cold cardioplegias.
Figure 3.
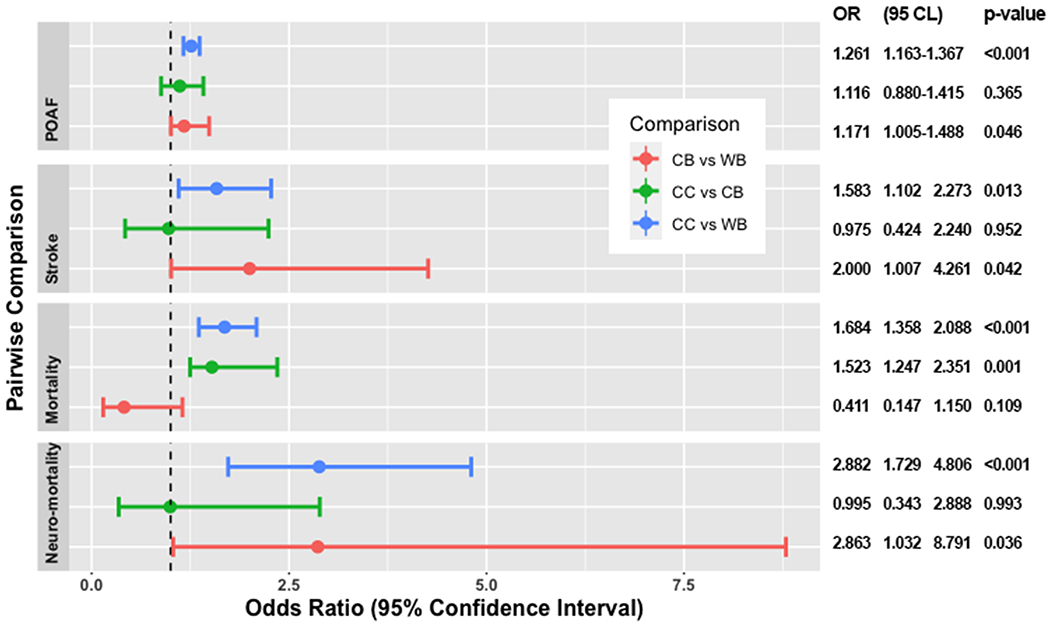
Impact of types of cardioplegia on different outcomes. CB = cold blood; CC = cold crystalloid; CL, confidence limits; OR, odds ratio; POAF, postoperative atrial fibrillation; WB = warm blood
Discussion
The overall rate of POAF reported in this multicenter study was 18%, slightly below the range reported in the literature [1–5]; in particular, patients having warm cardioplegia showed an incidence of 15%, very low compared to others [1–5]. Conversely, patients having cold cardioplegia were more prone to develop POAF (21%).
Whether cold or warm cardioplegia can be associated with POAF has not been established yet [17,18]. Franke et al [18] reported 41% of POAF in warm group versus 31% in cold group, but, given the very small sample size, the statistical significance was not reached. Similarly, a recent meta-analysis [19] pooled the results of eight studies, failing to evidence any difference in terms of POAF between warm (610 cases) and cold (532 cases) relative risk = 1.07 (0.84—1.36), p = 0.57. However, all these studies were very small, ranging from 48 to 137 cases in warm group and from 21 to 144 cases in cold group. The present is the first large multicenter, propensity-matched study demonstrating the superiority of warm over cold cardioplegia to reduce POAF in CABG, confirmed also by a multivariate analysis.
Postoperative atrial fibrillation POAF occurs when some postoperative stimuli trigger ectopic firing due to either triggered activity or re—entry. However, so that happens, a vulnerable atrial substrate needs to be induced by preoperative (i.e. age, hypertension, hypercholesterolemia, etc), surgical (cardiopulmonary bypass, atriotomy) or postoperative (inflammation, oxidative stress, myocardial damage, augmented activity of autonomic nervous system, pericardial fluid) factors [6,20,21].
Among the above-mentioned factors, inflammatory response, oxidative stress and myocardial damage can explain the pathophysiological link between cold cardioplegia and higher incidence of POAF, herein reported. Several studies have shown an association between some markers of inflammatory response (IL-2, IL-6, C-reactive protein, white blood cells) and POAF [10–13,15,22–24]. Postoperative white blood cell count was higher in patients with POAF than in controls, and the timing of peak counts coincides with the onset of POAF [12,14,23]. Similarly, postoperative IL-2 and IL-6 levels are associated with POAF in some studies [10,12,24]. Postoperative activation of C-reactive protein has also been associated with POAF occurrence [22,24].
Other upstream factors that may influence both the re-entry-promoting substrate and ectopic firing, include reactive oxygen species (ROS). Levels of ROS are increased in the atria of patients who develop POAF [25]. Likewise, levels of NADPH oxidase-derived and monoamine oxidase derived superoxide anion (O2 −) and peroxynitrite (ONOO−) are both increased in the right atria of patients with POAF [25].
Cardiopulmonary bypass per se is able to produce a substrate for POAF, since it is associated with an awesome inflammatory response, along with other factors such as hypothermia, hemodilution, electrolyte imbalance, pharmacological agents [10–15]. Moreover, myocardiocytes synthesize cytokine mRNAs as the myocardium is infiltrated by inflammatory cells; thus, an intrinsic tissue inflammatory condition is triggered by CPB and it is auto-sustained. In addition, cardioplegic arrest is at basis of myocardial oxidative stress due to ischemia-reperfusion injury [26]. In fact, cardiomyocytes are a major source of ROS in the myocardium with reperfusion injury [27]. Ischemia can lead to the accumulation of activated neutrophils in the myocardium resulting in the release ROS and other proteolytic enzymes [27]. Further myocardial damage can occur during reperfusion as a consequence of mitochondrial dysfunction driven by ROS production [15], and activation of NF-KB, NRF2 and MAPK leading to inflammation, apoptosis or necrosis [28].
Despite reducing metabolic activity, hypothermia induces many detrimental effects on inflammatory response, and myocardial injury has been documented in this setting [18,19,29]. Fan et al [18] reported the pooled results of 41 trials, including 5879 patients, where the warm group showed significantly higher postoperative cardiac index (weighted mean difference, WMD = 0.28, 95% CI: 0.26—0.31, p < 0.00001), as well as lower peak cardiac troponin concentrations (WMD= −1.45, 95% CI: −2.47 to −0.42, P = 0.006).
Cold cardioplegia reduces mitochondrial respiration, decreases the production of myocardial high energy phosphates, and also affects various enzymatic systems, such as sodium, potassium, and calcium adenyl-pyrophosphatase, altering the ionic composition of the cell and water homeostasis [30]. In addition to generating high levels of inflammatory cytokines, hypothermia seems to be associated with reduced NO generation at least up to 24 hours after the end of CPB, causing vasoconstriction in addition to an increase in the adhesion and proliferation activity of inflammatory cells, which are normally inhibited by NO [31,32]. Mezzetti et al [33] demonstrated that cold cardioplegia, when compared to the warm one, causes ischemia-reperfusion injury with increased tissue oxidant burden.
Other risk factors
Besides cold cardioplegia, we found other risk factors for POAF such as age, LVEF, IABP, hypercholesterolemia, redo, previous stroke and CPB and cross-clamp duration. All the reported factors have been already described by the literature [34,35]. The probability of developing POAF increases exponentially by age, with elderly showing a rate of POAF between 50% and 60% [36]. Similarly, left ventricular dysfunction and IABP were also found to be risk factors for POAF[15,34–37]. High preoperative levels of cholesterol may mirror a higher grade of chronic inflammation that can predispose patients to develop POAF [38]. Previous stroke has not been identified as risk factor for POAF, so far, whereas, prior episodes of AF are strictly correlated with POAF [39]. However, we were not able to collect this data, so we can just speculate that patients with history of stroke can mirror a cohort with cardio-embolic stroke due to AF occurrence. Finally, CPB and aortic cross-clamp duration are confirmed to be consistently associated with POAF [39].
Prognostic impact of POAF
In the present study, either cold cardioplegia or POAF are associated with worse early outcome, especially with the incidence of postoperative stroke and neurological mortality. A very recent meta-analysis [40], including 35 studies and 2,458,010 patients, showed that POAF was associated with increased risks of early stroke (odds ratio, 1.62; 95% CI, 1.47–1.80) and early mortality (odds ratios, 1.44; 95% CI, 1.11–1.88). The onset of acute AF occurs mainly between days 2 and 4 after surgery with frequent recurrences, especially within the first postoperative week, when, in most of cases, low molecular weight heparin administration is very likely to be reduced or even discontinued, since the patient is starting to move from the bed. Stroke is a devasting complication leading to higher likelihood to die; in our series, out of 108 patients experienced a postoperative stroke, 27% died (versus 0.6%, p<0.001); This finding is also confirmed by others [3,4,35,40].
Blood and crystalloid cardioplegia
Interestingly, in a sub-group analysis of the present study, either cold crystalloid and cold blood cardioplegia seems to not show any difference in terms of POAF, stroke and neurological mortality, after a propensity score weighting. Even blood cardioplegia, when hypothermic, may cause higher rate of POAF and very likely consequent stroke than warm blood cardioplegia. Hence, the detrimental key-role in terms of inflammatory response and oxidative stress is played by the hypothermia regardless of type of cardioplegia, blood or crystalloid. However, an interesting finding of our study is the higher all-causes mortality in crystalloid group compared to either warm blood (OR 1.6, 1.3-2.1) or cold blood (OR 1.5, 1.2-2.3) groups. A previous meta-analysis comparing blood with crystalloid cardioplegia indicated that the former was superior in terms of low output syndrome and myocardial protection [41]. Barra et al. [42] reported a 4-fold increased risk of postoperative myocardial infarction with the antegrade cold crystalloid cardioplegia. In fact, despite its efficacy to cause electromechanical arrest, hyperkalemic crystalloid cardioplegia is only partially cardioprotective [43], with higher likelihood to cause postoperative cardiomyocyte apoptosis [43,44]
Limitations of the study
There are some limitations of the present study that are to be disclaimed; firstly, the retrospective observational nature of this multicenter cohort prevented us to collect some data that could be interesting such as parameters related to postoperative inflammation, anamnestic data such as the presence of previous episodes of AF, or postoperative data such as low output syndrome. Moreover, we miss some important surgical details. Finally, the bias due to unbalance between the two compared groups which was partially overcome with propensity matching.
Conclusions.
Warm cardioplegia may reduce the rate of postoperative atrial fibrillation in CABG patients with respect to cold cardioplegia, either blood or crystalloid. This has a prognostic impact on postoperative stroke and neurological mortality. A further randomized trial is deemed to be necessary to confirm the results of the present study.
Acknowledgments
Funding: no fund was used for this study
Footnotes
Disclosures: There are no relationships with industry.
References
- 1.Lubitz SA, Yin X, Rienstra M, Schnabel RB et al. Long-term outcomes of secondary atrial fibrillation in the community: the Framingham Heart Study. Circulation 2015;131: 1648–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greenberg JW, Lancaster TS, Schuessler RB, Melby S J. Postoperative atrial fibrillation following cardiac surgery: a persistent complication. Eur. J. Cardiothorac. Surg 2017;52:665–72. [DOI] [PubMed] [Google Scholar]
- 3.Ahlsson A, Fengsrud E, Bodin L, Englund A. Postoperative atrial fibrillation in patients undergoing aortocoronary bypass surgery carries an eightfold risk of future atrial fibrillation and a doubled cardiovascular mortality. Eur. J. Cardiothorac. Surg 2010;37:1353–1359. [DOI] [PubMed] [Google Scholar]
- 4.Steinberg BA, Zhao Y, He X et al. Management of postoperative atrial fibrillation and subsequent outcomes in contemporary patients undergoing cardiac surgery: insights from the Society of Thoracic Surgeons CAPS-Care Atrial Fibrillation Registry. Clin. Cardiol 2014;37:7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Melduni RM, Schaff HV, Bailey KR et al. Implications of new-onset atrial fibrillation after cardiac surgery on long-term prognosis: a community-based study. Am. Heart J 2015;170, 659–668. [DOI] [PubMed] [Google Scholar]
- 6.Dobrev D, Aguilar M, Heijman J, Guichard JB, Nattel S. Postoperative atrial fibrillation: mechanisms, manifestations and management. Nature Reviews Cardiology 2019; 16:417–36. [DOI] [PubMed] [Google Scholar]
- 7.Mathew JP, Fontes ML, Tudor IC et al. A multicenter risk index for atrial fibrillation after cardiac surgery. JAMA 2004;291:1720–29. [DOI] [PubMed] [Google Scholar]
- 8.Lee SH, Kang DR, Uhm JS et al. New-onset atrial fibrillation predicts long-term newly developed atrial fibrillation after coronary artery bypass graft. Am. Heart J 2014;167:593–600. [DOI] [PubMed] [Google Scholar]
- 9.Bessissow A, Khan J, Devereaux PJ, Alvarez-Garcia J, Alonso-Coello P. Postoperative atrial fibrillation in non-cardiac and cardiac surgery: an overview. J Thromb Haemost. 2015;13 Suppl 1:S304–12. [DOI] [PubMed] [Google Scholar]
- 10.Gaudino M, Andreotti F, Zamparelli R et al. The −174G/C interleukin-6 polymorphism influences postoperative interleukin-6 levels and postoperative atrial fibrillation. Is atrial fibrillation an inflammatory complication? Circulation 2003;108 (Suppl. 1): II195–II199. [DOI] [PubMed] [Google Scholar]
- 11.Hak Ł, Myśliwska J, Wieckiewicz J, Szyndler K, Siebert J, Rogowski J et al. Interleukin-2 as a predictor of early postoperative atrial fibrillation after cardiopulmonary bypass graft (CABG). J. Interferon Cytokine Res 2009;29: 327–332. [DOI] [PubMed] [Google Scholar]
- 12.Abdelhadi RH, Gurm HS, Van Wagoner DR, Chung MK. Relation of an exaggerated rise in white blood cells after coronary bypass or cardiac valve surgery to development of atrial fibrillation postoperatively. Am. J. Cardiol 2004;93:1176–78. [DOI] [PubMed] [Google Scholar]
- 13.Levy JH, Tanaka KA Inflammatory response to cardiopulmonary bypass. Ann Thorac Surg 2003;75:S715–S720. [DOI] [PubMed] [Google Scholar]
- 14.Rinder C Cellular inflammatory response and clinical outcome in cardiac surgery. Curr Opin Anaesthesiol 2006;19:65–68. [DOI] [PubMed] [Google Scholar]
- 15.Zakkar M, Ascione R, James AF, Angelini GD, Suleiman MS. Inflammation, oxidative stress and postoperative atrial fibrillation in cardiac surgery. Pharmacol Ther. 2015;154:13–20. [DOI] [PubMed] [Google Scholar]
- 16.Zhao P, Su X, Ge T, Fan J. Propensity score and proximity matching using random forest. Contemp Clin Trials. 2016;47:85–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li F, Li F. Propensity score weighting for causal inference with multiple treatments. The Annals of Applied Statistics,2019;13:2389–2415 [Google Scholar]
- 18.Franke UF, Korsch S, Wittwer T et al. Intermittent antegrade warm myocardial protection compared to intermittent cold blood cardioplegia in elective coronary surgery--do we have to change? Eur J Cardiothorac Surg. 2003;23:341–6 [DOI] [PubMed] [Google Scholar]
- 19.Fan Y, Zhang AM, Xiao YB, Weng YG, Hetzer R. Warm versus cold cardioplegia for heart surgery: a meta-analysis. Eur J Cardiothorac Surg. 2010;37:912–9. [DOI] [PubMed] [Google Scholar]
- 20.Andrade J, Khairy P, Dobrev D, Nattel S. The clinical profile and pathophysiology of atrial fibrillation: relationships among clinical features, epidemiology, and mechanisms. Circ. Res 2014;114:1453–1468. [DOI] [PubMed] [Google Scholar]
- 21.Heijman J, Guichard JB, Dobrev D, Nattel S Translational challenges in atrial fibrillation. Circ. Res 2018;122:752–773. [DOI] [PubMed] [Google Scholar]
- 22.Bruins P, te Velthuis H, Yazdanbakhsh AP et al. Activation of the complement system during and after cardiopulmonary bypass surgery: postsurgery activation involves C-reactive protein and is associated with postoperative arrhythmia. Circulation 1997;96:3542–3548. [DOI] [PubMed] [Google Scholar]
- 23.Gibson PH, Cuthbertson BH, Croal BL et al. Usefulness of neutrophil/lymphocyte ratio as predictor of new-onset atrial fibrillation after coronary artery bypass grafting. Am. J. Cardiol 2010;105:186–191. [DOI] [PubMed] [Google Scholar]
- 24.Kaireviciute D, Blann AD, Balakrishnan B et al. Characterisation and validity of inflammatory biomarkers in the prediction of post-operative atrial fibrillation in coronary artery disease patients. Thromb. Haemost 2010;104:122–127. [DOI] [PubMed] [Google Scholar]
- 25.Anderson EJ, Efird JT, Davies SW et al. Monoamine oxidase is a major determinant of redox balance in human atrial myocardium and is associated with postoperative atrial fibrillation. J. Am. Heart Assoc 2014;3:e000713 doi: 10.1161/JAHA.113.000713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wan S, Yim AP, Wong CK, et al. Expression of FHL2 and cytokine messenger RNAs in human myocardium after cardiopulmonary bypass. Int J Cardiol 2002;86:265–72. [DOI] [PubMed] [Google Scholar]
- 27.Suleiman MS, Zacharowski K, Angelini GD. Inflammatory response and cardioprotection during open-heart surgery: the importance of anaesthetics. Br J Pharmacol 2008;153:21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anedda A, Lopez-Bernardo E, Acosta-Iborra B, Saadeh Suleiman M, Landazuri MO, Cadenas S. The transcription factor Nrf2 promotes survival by enhancing the expression of uncoupling protein 3 under conditions of oxidative stress. Free Radic Biol Med 2013;61, 395–407. [DOI] [PubMed] [Google Scholar]
- 29.Bical OM, Fromes Y, Paumier D, Gaillard D, Foiret JC, Trivin F. Does warm antegrade intermittent blood cardioplegia really protect the heart during coronary surgery?. Cardiovasc Surg 2001;9:188–93. [DOI] [PubMed] [Google Scholar]
- 30.Baikoussis NG, Papakonstantinou NA, Verra C, et al. Mechanisms of oxidative stress and myocardial protection during open-heart surgery. Ann Card Anaesth. 2015;18:555–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Daniel S Review on the multifactorial aspects of bioincompatibility in CPB. Perfusion 1996;11:246–55 [DOI] [PubMed] [Google Scholar]
- 32.Tavares-Murta BM, Cordeiro AO, Murta EF, Cunha Fde Q, Bisinotto FM. Effect of myocardial protection and perfusion temperature on production of cytokines and nitric oxide during cardiopulmonary bypass. Acta Cir Bras 2007;22:243–50 [DOI] [PubMed] [Google Scholar]
- 33.Mezzetti A, Calafiore AM, Lapenna D et al. Intermittent antegrade warm cardioplegia reduces oxidative stress and improves metabolism of the ischemic-reperfused human myocardium. J Thorac Cardiovasc Surg. 1995;109:787–95 [DOI] [PubMed] [Google Scholar]
- 34.Gianetti J, Del Sarto P, Bevilacqua S et al. Supplemental nitric oxide and its effect on myocardial injury and function in patients undergoing cardiac surgery with extracorporeal circulation. J Thorac Cardiovasc Surg 2004;127:44–50 [DOI] [PubMed] [Google Scholar]
- 35.Greenberg JW, Lancaster TS, Schuessler RB, Melby SJ. Postoperative atrial fibrillation following cardiac surgery: a persistent complication. Eur J Cardiothorac Surg. 2017;52:665–672. [DOI] [PubMed] [Google Scholar]
- 36.Akintoye E, Sellke F, Marchioli R, Tavazzi L, Mozaffarian D. Factors associated with postoperative atrial fibrillation and other adverse events after cardiac surgery. J Thorac Cardiovasc Surg. 2018;155:242–251. [DOI] [PubMed] [Google Scholar]
- 37.Shen J, Lall S, Zheng V, Buckley P, Damiano RJ Jr, Schuessler RB. The persistent problem of new-onset postoperative atrial fibrillation: a single institution experience over two decades. J Thorac Cardiovasc Surg 2011;141:559–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anogeianaki A, Angelucci D, Cianchetti E et al. Atherosclerosis: a classic inflammatory disease. Int J Immunopathol Pharmacol 2011;24:817–825. [DOI] [PubMed] [Google Scholar]
- 39.Villareal RP, Hariharan R, Liu BC et al. Postoperative atrial fibrillation and mortality after coronary artery bypass surgery. J Am Coll Cardiol 2004;43:742–8. [DOI] [PubMed] [Google Scholar]
- 40.Lin MH, Kamel H, Singer DE, Wu YL, Lee M, Ovbiagele B. Perioperative/Postoperative Atrial Fibrillation and Risk of Subsequent Stroke and/or Mortality. Stroke. 2019;50:1364–1371 [DOI] [PubMed] [Google Scholar]
- 41.Guru V, Omura J, Alghamdi AA, Weisel R, Fremes SE. Is blood superior to crystalloid cardioplegia? A meta-analysis of randomized clinical trials. Circulation 2006;114(1 Suppl):I331–8. [DOI] [PubMed] [Google Scholar]
- 42.Barra JA, Bezon E, Mondine P et al. Surgical angioplasty with exclusion of atheromatous plaques in case of diffuse disease of the left anterior descending artery: 2 years’ follow-up. Eur J Cardiothorac Surg 2000;17:509–1 [DOI] [PubMed] [Google Scholar]
- 43.Yeh CH, Wang YC, Wu YC, Chu JJ, Lin PJ. Continuous tepid blood cardioplegia can preserve coronary endothelium and ameliorate the occurrence of cardiomyocyte apoptosis. Chest 2003;123:1647–54 [DOI] [PubMed] [Google Scholar]
- 44.López JR, Jahangir R, Jahangir A, Shen WK, Terzic A. Potassium channel openers prevent potassium‑induced calcium loading of cardiac cells: Possible implications in cardioplegia. J Thorac Cardiovasc Surg 1996;112:820–31. [DOI] [PubMed] [Google Scholar]


