Abstract
The essential oil of Mosla chinensis Maxim cv. Jiangxiangru is known for its antibacterial ability. This study aimed to investigate the chemical composition of Jiangxiangru essential oil and its inhibitory effect on Staphylococcus aureus biofilm formation. Gas chromatography/mass spectrometry (GC–MS) was used to determine the chemical composition of Jiangxiangru essential oil. Subsequently, the eight major chemical components were quantitatively analyzed using GC– MS, and their minimum inhibitory concentration (MIC) values against S. aureus were tested. Biofilm formation was detected by crystal violet semi-quantitative method and silver staining. Of the 59 peaks detected, 29 were identified by GC–MS. Of these peaks, thymol, carvacrol, p-cymene, γ-terpinene, thymol acetate, α-caryophyllene, 3-carene, and carvacryl acetate were present at a relatively higher concentration. The results of the quantitative test showed that thymol, carvacrol, p-cymene, and γ-terpinene were the major components of the essential oil. Among the eight reference substances, only thymol, carvacrol, and thymol acetate had lower MICs compared with the essential oil. Essential oil, carvacrol, carvacryl acetate, α-caryophyllene, and 3-carene showed the better inhibition of S. aureus biofilm formation. When one fourth of the MIC concentrations were used for these substances (0.0625 mg/mL for essential oil, 0.0305 mg/mL for carvacrol, 1.458 mg/mL for carvacryl acetate, 0.1268 mg/mL for α-caryophyllene, and 2.5975 mg/mL for 3-carene), the inhibition rates were over 80%. However, thymol, γ-terpinene, thymol acetate, and p-cymene showed a relatively poor inhibition of S. aureus biofilm formation. When 1× MIC concentrations of these substances were used, the inhibition rates were less than 50%. In conclusion, Jiangxiangru essential oil and its major components, carvacrol, carvacryl acetate, α-caryophyllene, and 3-carene, strongly inhibited biofilm formation in S. aureus.
Keywords: Mosla chinensis Maxim cv. Jiangxiangru, essential oil; GC–MS analysis, Staphylococcus aureus, biofilms, antibacterial ability
1. Introduction
Staphylococcus aureus is an important foodborne pathogen and one of the most common species responsible for nosocomial infection [1,2,3]. The incidence of nosocomial infections caused by S. aureus has increased in recent years. Multiple S. aureus mutants obtained as clinical isolates have been found to be resistant to a majority of antibacterial agents, including macrolides, quinolones, and β-lactams [4,5,6,7]. Also, the resistance to methicillin and β-lactam has shown an annual increasing trend [8, 9]. S. aureus employs various mechanisms to resist the killing and inhibitory effects of antibiotics, such as enzymatic inactivation of the antibiotic (penicillinase and aminoglycoside-modifying enzymes), altering the target by reducing its decreased affinity to the antibiotic (e.g., penicillin-binding protein 2a of methicillin-resistant S. aureus, and d-Ala-d-Lac of peptidoglycan precursors of vancomycin-resistant strains), trapping antibiotics within the cell (vancomycin) [10, 11], and using bacterial biofilms (BBFs).
BBFs are defined as an aggregation of bacteria adherent to an inert contact surface embedded within a self-produced slimy extracellular matrix composed of polysaccharides, proteins, and other polymers. Biofilms are involved in multiple infections and often contribute significantly to the difficulties encountered in treatment [3]. Staphylococci are commensals on the skin, therefore mucous surfaces are most likely to infect medical devices that penetrate the skin or these surfaces. The presence of S. aureus biofilms pose difficulties in eradicating S. aureus infection in patients because the biofilms protect bacteria from the effects of both the host immune system and antibacterial drugs [12]. A previous study demonstrated that staphylococci residing in biofilms exhibit increased mutability, accelerating the emergence of heritable antibiotic resistance through spontaneous mutations [13]. Another study demonstrated that S. aureus biofilms dramatically increased the frequency of plasmid transfer events by both conjugation and mobilization, thereby promoting horizontal spread of antibiotic resistance [14]. Therefore, identifying drugs against BBFs has become an important focus of current pharmaceutical drug development research.
Effective alternative modalities against biofilm-producing microorganisms have gained interest. Of note, natural substances offer a wide repository of compounds with potential efficacy against bacterial pathogens, including antibiotic-resistant strains. A number of studies have explored the therapeutic and antimicrobial use of plant materials. The antibiotic effects of many plants are already known and applied in many forms of traditional medicine [15]. Mosla chinensis Maxim cv. Jiangxiangru is an annual herb belonging to the Lamiaceae family. It is recorded in Chinese Pharmacopoeia [16] and distributed mainly in Fenyi and Xinyu, Jiangxi province, China. As a medicinal and edible herb, it exerts a good therapeutic effect [17]. It can remove dampness. It is also used to treat stomach ailments and relieve external symptoms by diaphoresis. Moreover, it can alleviate symptoms such as summer heat and dampness, chills, and fever, no sweat and chest tightness, abdominal pain, vomiting, and diarrhea [18]. M. chinensis polysaccharide extract has also been shown to have a preventive action against oxidative damage in the immune system of mice [19]. Apart from polysaccharides, the essential oil is one of the major components in M. chinensis Maxim cv. Jiangxiangru having antibacterial, immunity-improving, and antioxidant effects [18]. Several studies have shown the antimicrobial and antioxidant effects of M. chinensis essential oil extract against a variety of pathogens, including S. aureus [20]. However, whether the M. chinensis Maxim cv. Jiangxiangru essential oil or its major chemical components can inhibit the growth and development of S. aureus biofilm has not been reported.
2. Materials and Methods
2.1. Preparation of the essential oil in M. chinensis Maxim cv. Jiangxiangru
The traditional Chinese medicine of Jiangxiangru prepared in ready-to-use forms was provided by Jiangxi Zhangshu Tianqitang Chinese Medicine Co. Ltd., China (lot number 1508005) and identified by the Department of Biological Medicine in Jiangxi Science and Technology Normal University School of Pharmacology as the dried leaves of M. chinensis Maxim cv. Jiangxiangru. The essential oil was prepared according to the Appendix XD of Essential Oil Determination Method A in the “Chinese Pharmacopoeia” 2015 edition. Briefly, 100 g of Jiangxiangru Chinese medicine was placed in a round-bottomed flask and subjected to steam distillation for 5 h using an essential oil extractor. The resulting faint yellow oily liquid was collected and dried using anhydrous sodium sulfate, transferred to a brown bottle, and stored at 2–8°C for future use.
2.2. GC–MS analysis of essential oil in M. chinensis Maxim cv. Jiangxiangru
An Agilent 6890N+5975i GC–MS system (Agilent Technologies, USA) was used. An HP-5MS (Agilent Technologies, USA) fused silica capillary column (30m × 0.25mm × 0.25μm) was used along with helium (He) with 99.999% purity as the carrier gas. The following chromatographic conditions were used: injector temperature, 250°C; carrier gas flow rate, 1.0 mL/min; inlet pressure, 7.69 psi; sample volume, 1.0 µL; and injected in the split mode with a split ratio of 50:1. The initial inlet temperature was programmed to increase from 50°C (held for 2 min) to 100°C at 3°C/min, and then held for 10 min at 100°C. The temperature was further increased to 120°C at the rate of 1°C/min (held for 5 min), and then to 150°C at the rate of 2°C/min (held for 3 min). Finally, the temperature was increased to 250°C at the rate of 4°C/min and held for 5 min.
The MS conditions used were as follows: the ionization mode was set to EI, with an ion source temperature of 230°C, quadrupole temperature of 150°C, ionization energy of 69.9 eV, interface temperature of 280°C, solvent delay of 3 min, and scanning mass range of m/z 35–550 amu.
The essential oil extracted from M. chinensis Maxim cv. Jiangxiangru was examined to obtain total ion chromatograms using GC–MS under the aforementioned conditions. A mass spectrogram was obtained after scanning the mass spectra of the peaks in total ion chromatograms and matching them with the spectra in the NIST11 mass spectrum database. The chemical composition of essential oil in M. chinensis Maxim cv. Jiangxiangru was identified according to the retention index of each peak and comparison of mass spectrogram of each peak with the mass spectra in the NIST11 mass spectrum database in combination with manual spectrum interpretation and verification of the mass spectrograms of each peak with the mass spectra in the literature [20]. The relative percentages of each component were determined by the peak area normalization method.
2.3. Determination of the content of each main component in the M. chinensis Maxim cv. Jiangxiangru essential oil
The thymol standard (99.9%) content was obtained from the National Institutes for Food and Drug Control. The carvacrol, 3-carene, γ-terpinene, and p-cymene standards (>95% content) were procured from Dr Ehrenstorfer Company. The thymol acetate standard (≥98% content) was purchased from Shanghai Yihe Biological Technology Co., Ltd., China. The carvacrol acetate standard (RG) was procured from ChromaDex Inc., USA. The α-caryophyllene standard (≥93.0% content) was produced by Tokyo Kasei Kogyo Co. Further, 1616.02, 197.00, 221.70, 172.00, 148.95, 52.81, 378.00, and 1604.80 mg of carvacrol, carvacryl acetate, thymol acetate, p-cymene, 3-carene, γ-terpinene, α-caryophyllene, and thymol standards, respectively, were transferred to 10-mL volumetric flasks and dissolved in ethanol to a constant volume to determine the essential oil components. Hence, 161.60, 19.70, 22.17, 17.20, 14.90, 5.28, 37.80, and 160.480 mg/mL of carvacrol, carvacryl acetate, thymol acetate, p-cymene, 3-carene, γ-terpinene, α-caryophyllene, and thymol standard stock solutions, respectively, were prepared. Each standard stock solution was measured to obtain solutions of five different concentrations, and 1.5 mL of the standard solutions were placed in 2-mL centrifuge tubes. The five different concentrations of the standard solutions were 1/2, 1/4, 1/8, 1/16, and 1/32 of the standard stock solution. Standard solutions with different concentrations were analyzed and examined by GC–MS. The standard curve was drawn with the peak area as the ordinate and the concentration as the abscissa.
The M. chinensis Maxim cv. Jiangxiangru essential oil was examined by the GC–MS analysis. The contents of eight chemical components in the essential oil, namely, carvacrol, carvacrol acetate, thymol acetate, p-cymene, 3-carene, γ-terpinene, α-caryophyllene, and thymol, were calculated.
2.4. GC–MS analysis and verification
Precision test: Standard solutions of carvacrol, carvacrol acetate, thymol acetate, p-cymene, 3-carene, γ-terpinene, α-caryophyllene, and thymol with 1/2 stock concentration were obtained for six replicate sample experiments. The peak area and retention time were measured and recorded.
Stability test: Standard solutions with the same concentration were taken for injection at 0, 2, 4, 6, 8, and 10h. The peak area and retention time were measured and recorded.
Reproducibility test: For each standard, five standard solutions with the same concentration were prepared under the same conditions six times. The peak area and retention time were measured and recorded.
Recovery test: For the recovery test, the same amount of M. chinensis Maxim cv. Jiangxiangru essential oil was injected with the addition of 60%, 80%, 100%, 120%, and 140% standards. The recovery rate was calculated.
2.5. Pretreatment of strains
Lyophilized powder of the S. aureus standard strain ATCC 25923 (National Institutes for Food and Drug Control) was cultured at 37°C for 24 h in tryptic soy broth (TSB) containing 2.5% glucose. An appropriate amount of bacteria was spread on Luria-Bertani (LB) plates and cultured at 37°C for 24 h. Single colonies were selected from the LB plates and inoculated in Congo red medium [21]. After incubation at 37°C for 48 h, positive strains that turned black and grew well were screened for subsequent experiments.
2.6. MIC determination
For the determination of MIC, reagent solutions containing 10.00, 10.00, 19.52, 207.80, 204.00, 233.28, 7.80, 205.68, and 20.28 mg thymol, carvacrol, 3-carene, γ-terpinene, carvacryl acetate, thymol acetate, p-cymene, and α-caryophyllene, respectively, were precisely measured. The reagents to be tested were first fully dissolved in an appropriate amount of dimethyl sulfoxide (final content ≤2%, volume fraction) and then diluted to 0.5 mL using MH broth. After mixing, a 0.22-μm sterile filter was used to filter the reagents for future use.
Briefly, 10 strains of S. aureus were inoculated on LB agar plates using a sterile inoculation loop and incubated in a 37°C incubator for 24 h. Then, the colonies were picked up, dispersed in MH broth, and diluted to A600 of 0.10, which was measured in a UV-visible light spectrophotometer (UV-2550; Shimadzu, Japan). The bacterial count in the suspension was approximately 1 × 107 CFU/mL. The MIC of M. chinensis Maxim cv. Jiangxiangru against S. aureus was measured using the broth macrodilution antimicrobial susceptibility test [22]. The MIC value obtained by this method was used to determine the concentration of drug solution in the S. aureus biofilm test.
2.7. Inhibition of S. aureus biofilm formation by reference substances using the crystal violet semi-quantitative method
The crystal violet semi-quantitative method was performed as described previously [23]. An S. aureus bacterial suspension was prepared in TSB containing 2.5% glucose at an absorbance (A600) of 0.05. The bacterial suspension was then used to prepare drug solutions of 2, 1, 0.5, 0.25, 0.125, and 0.0625× MIC. Briefly, 200-μL aliquots of bacterial suspension containing each concentration of drug were transferred into the wells of a 96-well plate. Six wells were used for each drug concentration, in addition to six blank wells (medium only) and six negative control wells (biofilm without drugs). After incubation at 37°C for 48 h, the supernatant was discarded, and the cells were washed thrice with phosphate-buffered saline (PBS; pH 7.4). The cells were then stained with 0.1% crystal violet for 30 min. The crystal violet dye was discarded, and the cells were washed with distilled water until the water ran clear. Finally, 200 μL of anhydrous ethanol was added for dissolution, and each well was measured at an absorbance of 570 nm using a microplate reader (MK3; Thermo Fisher Scientific Co., Ltd., Shanghai, China).
The inhibition rate of drugs on S. aureus biofilm (A570) was calculated as follows:
where A0 is the average value of A570 in the control group, A1 is the average value of A570 in the drug effect group, and A2 is the average value of A570 in the negative control group.
2.8. Morphological observation of S. aureus biofilm using silver staining
An S. aureus bacterial suspension was prepared in TSB containing 2.5% glucose at an absorbance (A600) of 0.05, as described previously [24]. The bacterial suspension was then used to prepare drug solutions of 2, 1, 0.5, and 0.25× MIC. Briefly, 10 mL of bacterial suspension containing different concentrations of drugs was measured and transferred into Petri dishes. A separate negative control with 10 mL of bacterial suspension without drugs was prepared. Subsequently, four coverslips soaked in anhydrous ethanol for 30 min were placed in each dish. After incubation at 37°C for 48 h, nonadherent bacteria were removed from the coverslips by rinsing them thoroughly with saline. The coverslip with a biofilm was soaked in 2.5% glutaraldehyde in PBS for 1 h, washed with distilled water for 1 min, soaked in saturated calcium chloride solution for 15 min and washed with distilled water for 1 min, soaked in 5% silver nitrate solution for 15 min, stained with 1% hydroquinone for 2 min, and washed with distilled water for 1 min. Finally, the cells were fixed using 5% sodium thiosulfate and rinsed with distilled water for 1 min. After air-drying, S. aureus BF morphology was observed under an optical biological microscope (BH300-OPTEC; Chongqing Ott Optical Instruments Co., Ltd).
3. Results
3.1. Reliability of GC–MS analysis of essential oil in M. chinensis Maxim cv. Jiangxiangru
Precision test: The relative standard deviation (RSD) values were all less than 2.0% for the area of GC chromatographic peaks measured for each standard. The RSD values of retention time were all less than 1.0%, indicating that the instrument had good precision.
Stability test: The RSD values of peak areas for injection at 0, 2, 4, 6, 8, and 10 h for all the eight standards were <1.5%, and the RSD values of retention time were all less than 1.5%, indicating that the reference standard solution was stable within 10 h.
Reproducibility test: The RSD values of peak areas of the prepared solutions were all less than 1.6%, and the RSD values of retention time were all less than 1.1%, indicating that the reaction conditions had good reproducibility and repeatability.
Recovery test: The experimentally measured recovery rates ranged from 99.0% to 101.5%. The average recovery rates of carvacrol, carvacryl acetate, thymol acetate, p-cymene, 3-carene, γ-terpinene, α-caryophyllene, and thymol were 99.97%, 99.50%, 100.11%, 100.32%, 99.83%, 99.45%, 101.21%, and 99.87%, respectively, indicating that the standard curve had good accuracy.
3.2. GC–MS analysis of the chemical composition of M. chinensis Maxim cv. Jiangxiangru essential oil
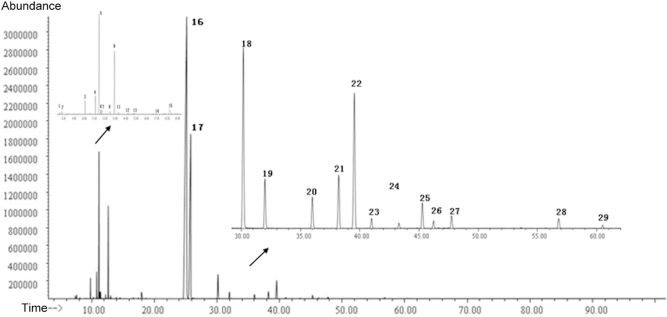
The GC–MS analysis of the essential oil extracted from M. chinensis Maxim cv. Jiangxiangru was performed. Figure 1 shows the total ion chromatography of the essential oil.
Figure 1.
Total ion chromatography of the essential oil from Mosla chinensis Maxim cv. Jiangxiangru.
The results of GC–MS revealed a total of 59 peaks in the essential oil. A total of 29 compounds were identified through a search of the NIST11 MS library. The components accounted for 99.316% of the total essential oil according to the MS ion peak area normalization method. Table 1 presents the results of the GC–MS analysis. As shown in Table 1, the M. chinensis Maxim cv. Jiangxiangru essential oil was composed primarily of phenols, esters, olefins, terpenes, aromatics, and alcohols. Thymol accounted for 58.514% of the essential oil, followed by carvacrol, which accounted for 22.195%. Additionally, 3-carene accounted for 0.937% of the essential oil; p-cymene, 5.601%; γ-terpinene, 3.545%; thymol acetate, 2.223%; carvacrol acetate, 0.611%; and α-caryophyllene, 1.848%. Together, these eight components accounted for 95.774% of the total essential oil.
Table 1.
Gas chromatography–mass spectrometry analysis of the M. chinensis Maxim cv. Jiangxiangru essential oil
| Peak number | Retention time (min) | Compound name | Molecular formula | Relative molecular mass | Retention Index | Relative content (%) |
|---|---|---|---|---|---|---|
| 1 | 7.375 | β-Thujene | C10H16 | 136 | 1068 | 0.078 |
| 2 | 7.592 | α-Pinene | C10H16 | 136 | 1031 | 0.111 |
| 3 | 9.810 | β-Myrcene | C10H16 | 136 | 979 | 0.674 |
| 4 | 10.286 | α-Phellandrene | C10H16 | 136 | 997 | 0.064 |
| 5 | 10.804 | 3-Carene | C10H16 | 136 | 1078 | 0.937 |
| 6 | 11.163 | p-Cimene | C10H16 | 136 | 1025.4 | 5.601 |
| 7 | 11.310 | Sylvestrene | C10H16 | 136 | 1016 | 0.217 |
| 8 | 11.421 | Eucalyptol | C10H18O | 154 | 1023 | 0.254 |
| 9 | 12.216 | cis-β-Ocimene | C10H16 | 136 | 1024 | 0.147 |
| 10 | 12.645 | γ-Terpinene | C10H16 | 136 | 1047 | 3.545 |
| 11 | 13.021 | trans-4-Thujanol | C10H18O | 154 | 1051 | 0.151 |
| 12 | 13.921 | Terpinolene | C10H16 | 136 | 1078 | 0.062 |
| 13 | 14.533 | cis-Sabinene hydrate | C10H18O | 154 | 1059 | 0.061 |
| 14 | 16.668 | Sabinol | C10H16O | 152 | 1107 | 0.060 |
| 15 | 17.962 | Terpinen-4-ol | C10H18O | 154 | 1161 | 0.610 |
| 16 | 25.179 | Thymol | C10H14O | 150 | 1266 | 58.476 |
| 17 | 25.803 | Carvacrol | C10H14O | 150 | 1278 | 22.239 |
| 18 | 30.179 | Thymyl acetate | C12H16O2 | 192 | 1338 | 2.235 |
| 19 | 31.997 | Carvacryl acetate | C12H16O2 | 192 | 1354 | 0.611 |
| 20 | 36.008 | β-Caryophyllene | C15H24 | 204 | 1424 | 0.418 |
| 21 | 38.220 | α-Bergamotene | 15H24 | 204 | 1407 | 0.691 |
| 22 | 39.555 | α-Caryophyllene | 15H24 | 204 | 1456 | 1.848 |
| 23 | 40.996 | (Z)-β-Farnesene | 15H24 | 204 | 1449 | 0.114 |
| 24 | 43.302 | cis-β-Farnesene | 15H24 | 204 | 1449 | 0.066 |
| 25 | 45.278 | (Z,E)-α-Farnesene | 15H24 | 204 | 1486 | 0.321 |
| 26 | 46.237 | γ-Muurolene | 15H24 | 204 | 1480 | 0.085 |
| 27 | 47.754 | β-Sesquiphellandrene | 15H24 | 204 | 1516 | 0.160 |
| 28 | 56.795 | Humulene-1,2-epoxide | C15H24O | 220 | 1601 | 0.137 |
| 29 | 60.510 | Eudesma-3,7(11)-diene | 15H24 | 204 | 1440 | 0.015 |
3.3. Content of each major component in the M. chinensis Maxim cv. Jiangxiangru essential oil
A standard solution was prepared by double dilution. The measured peak area was plotted against the concentration of the standard solution. The resulting linear relationship, using concentration as the abscissa and peak area as the ordinate, is shown in Supplementary Table 1. The GC peak of every standard was integrated using three different integration methods. Different R2 values and linear equations were calculated using different integration methods. Therefore, the linear equation with the best linear relationship, which had the maximum R2 value, was chosen to calculate the percentages of the eight essential oil components.
As shown in Table 2, the thymol, carvacrol, thymol acetate, and carvacryl acetate contents calculated via the standard curve (Supplementary Table 1) were all greater than their corresponding relative contents measured by the peak area normalization method. However, the calculated contents of 3-carene, p-cymene, γ-terpinene, and α-caryophyllene were all lower than their corresponding contents measured by the peak area normalization method. On the whole, the contents of carvacrol and thymol were higher than the content of the remaining six compounds among the relative contents and the contents measured according to the standard curve.
Table 2.
Content of the eight main components of the M. chinensis Maxim cv. Jiangxiangru essential oil
| Sample | 3-Carene | p-Cymene | γ-Terpinene | Thymol | Carvacrol | Thymyl acetate | Carvacryl acetate | α-Caryophyllene |
|---|---|---|---|---|---|---|---|---|
| Relative content(%) | 0.937 | 5.601 | 3.545 | 58.476 | 22.239 | 2.235 | 0.611 | 1.848 |
| Content(%) | 0.118 | 1.025 | 1.303 | 63.370 | 25.258 | 3.575 | 0.791 | 0.694 |
3.4. Antibacterial effect of the major essential oil components and essential oil on S. aureus
Table 3 shows the MICs of essential oil and its eight major components against S. aureus. As shown in Table 3, only thymol, carvacrol, and thymol acetate had smaller MICs compared with the essential oil among the eight reference substances. The MICs of the other reference substances were all higher compared with the essential oil; in particular, the MICs of 3-carene, γ-terpinene, and p-cymene were all >10 mg/mL. This suggested that thymol, carvacrol, and thymol acetate were the major components of the M. chinensis Maxim cv. Jiangxiangru essential oil contributing to its excellent antibacterial effect.
Table 3.
MIC of the M. chinensis Maxim cv. Jiangxiangru essential oil and its main components against S. aureus
| Sample | Essential oil | Thymol | Carvacrol | 3-Carene | γ-Terpinene | Carvacryl acetate | p-Cymene | Thymyl acetate | α-Caryophyllene |
|---|---|---|---|---|---|---|---|---|---|
| MIC (mg/mL) | 0.25 | 0.245 | 0.122 | 10.39 | 10.2 | 5.832 | 10.284 | 0.195 | 0.507 |
3.5. Morphological observation of the inhibition of S. aureus biofilm formation by the major essential oil components and essential oil
Figure 2 illustrates the morphological analysis of the S. aureus biofilm using silver staining. The microscopic observation of the stained biofilm revealed an evenly distributed biofilm in the negative control group. In comparison, biofilm formation in the group containing less than 1/4× MIC of the test substance was found to be significantly reduced and sparsely distributed.
Figure 2.
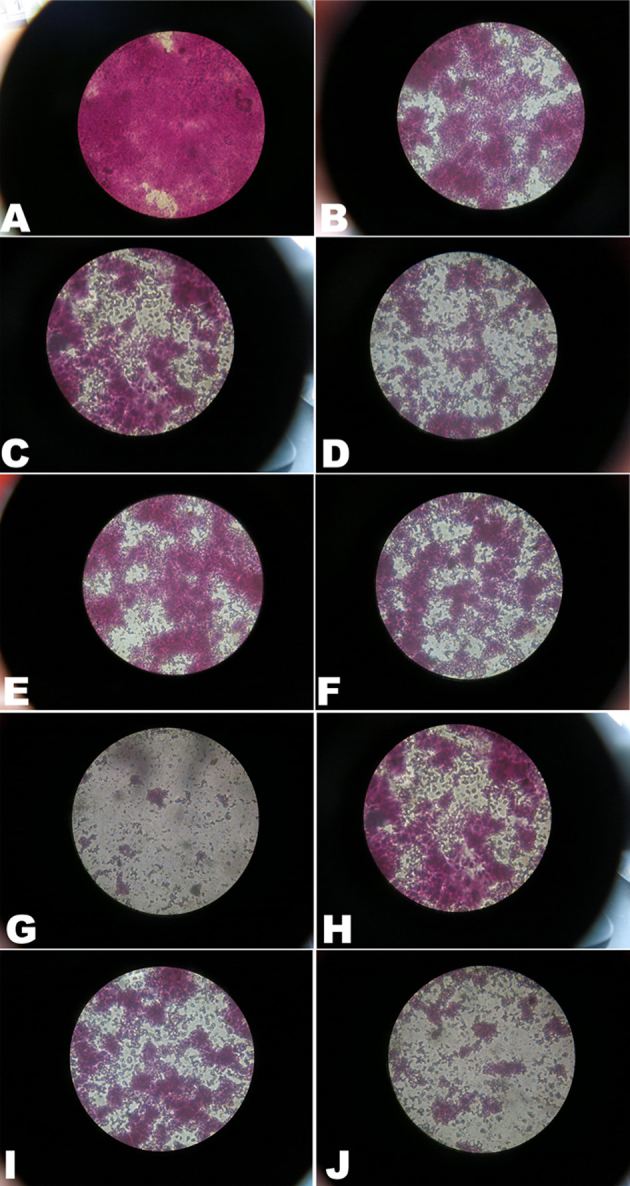
Effect of essential oil from M. chinensis Maxim cv. Jiangxiangru on S. aureus biofilm morphology using silver staining. (A) Negative control; (B) thymol 1/16 MIC; (C) thymol 1/4 MIC; (D) thymol 2 MIC; (E) carvacrol 1/16 MIC; (F) carvacrol 1/4 MIC; (G) carvacrol 2 MIC; (H) carvacrol acetate 1/16 MIC; (I) carvacrol acetate 1/4 MIC; (J) carvacrol acetate 2 MIC. Magnification: ×1000.
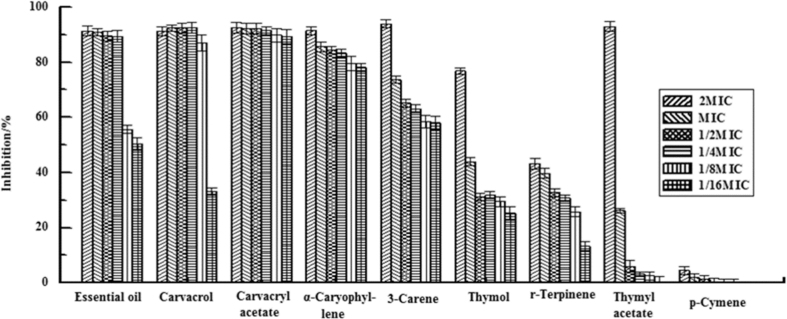
3.6. Inhibition of S. aureus biofilm formation by the major essential oil components and essential oil using the crystal violet semi-quantitative method
Figure 3 illustrates the morphological analysis of the S. aureus biofilm using crystal violet staining. Figure 4 illustrates the rate of inhibition of the essential oil and its eight components on the formation of S. aureus biofilm, and half maximal inhibitory concentration (IC50) is shown in Table 4. Of all the tested substances, M. chinensis Maxim cv. Jiangxiangru essential oil, thymol, carvacrol, carvacryl acetate, α-caryophyllene, and 3-carene showed better inhibition, and IC50 values were 0.018 ± 0.005, 0.007 ± 0.005, 0.008 ± 0.007, 0.343 ± 0.012, 0.019 ± 0.025 and 0.356 ± 0.019 mg/mL, respectively (Table 4). When concentrations of 1/4× MIC were used for these substances, the inhibition rates were >80% (Fig. 2). However, thymol, γ-terpinene, thymol acetate, and p-cymene showed a relatively poor inhibition of S. aureus biofilm formation. When 1× MIC concentrations of these substances were used, the inhibition rates were less than 50% (Fig. 2), indicating that carvacrol, carvacryl acetate, α-caryophyllene, and 3-carene were the main components in the M. chinensis Maxim cv. Jiangxiangru essential oil that inhibited S. aureus biofilm formation.
Figure 3.
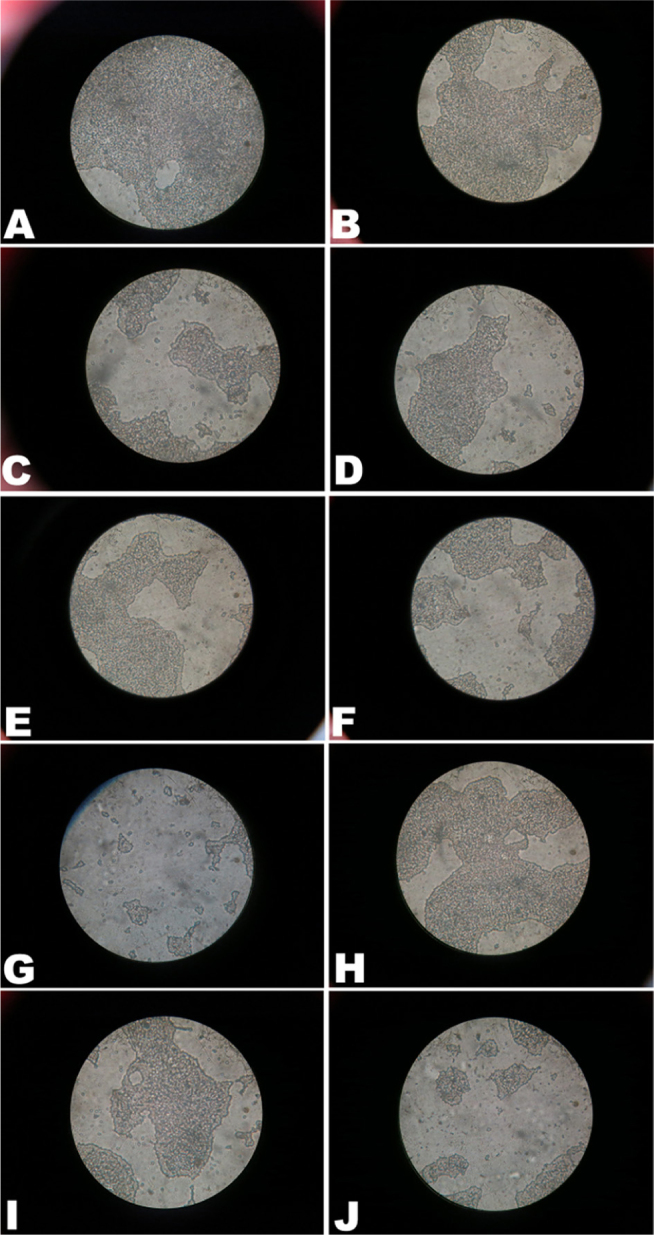
Effect of essential oil from M. chinensis Maxim cv. Jiangxiangru on S. aureus biofilm morphology using crystal violet staining. (A) Negative control; (B) thymol 1/16 MIC; (C) thymol 1/4 MIC; (D) thymol 2 MIC; (E) carvacrol 1/16 MIC; (F) carvacrol 1/4 MIC; (G) carvacrol 2 MIC; (H) carvacrol acetate 1/16 MIC; (I) carvacrol acetate 1/4 MIC; (J) carvacrol acetate 2 MIC. Magnification: ×1000.
Figure 4.
Inhibition of S. aureus biofilm formation by essential oil and eight reference substances. Data are shown as means ± standard deviation from six independent experiments.
Table 4.
Inhibition of S. aureus biofilm formation by essential oil and its eight main components (IC50 values)
| Sample | Essential oil | Thymyl acetate | Carvacrol | Carvacrol acetate | α-Caryophyllene | 3-Carene | Thymol | γ-Terpinene | p-Cymene |
|---|---|---|---|---|---|---|---|---|---|
| IC50 (mg/mL) | 0.018 ± 0.005 | 0.278 ± 0.025 | 0.008 ± 0.007 | 0.343 ± 0.012 | 0.019 ± 0.025 | 0.356 ± 0.019 | 0.259 ± 0.149 | 22.55 ± 0.044 | 237.6 ± 0.183 |
Data are shown as means ± standard deviation from six independent experiments.
4. Discussion
Plants have a long evolutionary history with respect to developing resistance against viruses and are increasingly drawing attention as potential sources for development of antiviral drugs [25]. M. chinensis Maxim cv. Jiangxiangru has a good therapeutic effect as a medicinal and edible herb, especially its essential oil and polysaccharides [19, 20]. In this study, the chemical composition of essential oil in M. chinensis Maxim cv. Jiangxiangru was first analyzed using GC–MS. Of the 59 peaks detected, 29 were identified, accounting for 99.316% of the total essential oil. The thymol, carvacrol, p-cymene, carvacryl acetate, thymol acetate, and α-caryophyllene contents in the essential oil were relatively high, consistent with that previously reported [21]. Additionally, nine of the essential oil components, namely, 3-carene, sylvestrene, eucalyptol, trans-4-thujanol, (Z)-β-farnesene, cis-β-farnesene, (Z,E)-α-farnesene, γ-muurolene, and eudesma-3,7(11)-diene, were newly identified in the present study. Another 30 compounds were not identified. This might be because fresh M. chinensis Maxim cv. Jiangxiangru specimens were used in previous studies, but in the present study, the ready-to-use preparation of this plant according to traditional Chinese medicine was used. The M. chinensis Maxim cv. Jiangxiangru essential oil was altered during the preparation and storage of herb drugs. The quantitative analysis of the essential oil components showed a good linear relationship within the measured concentration range, indicating a highly significant linear relationship between the concentration of each component and the corresponding chromatographic peak area. An established regression equation could be used to calculate the concentration of tested compounds in the essential oil. The concentration results of each component showed higher concentrations of thymol and carvacrol in the essential oil; these results were consistent with the quantification results from the area normalization method.
The results of the GC–MS analysis showed that the main essential oil components were thymol, carvacrol, 3-carene, p-cymene, γ-terpinene, thymol acetate, carvacryl acetate, and α-caryophyllene. These eight chemical components accounted for 95.774% of the total essential oil. Then antibacterial effect was further studied. Among the eight reference substances, only thymol, carvacrol, and thymol acetate had smaller MICs compared with the essential oil. In a previous study on the effects of carvacrol and thymol on S. aureus and S. aureus biofilms, the biofilm inhibitory concentration ranged from 0.031% to 0.125% v/v for carvacrol and thymol, which were two to four times greater than the concentration required to inhibit the planktonic growth [26]. These data supported the antibacterial effect of the essential oil components found in the present study. This study also indicated that a greater concentration of the antimicrobial agent was required to have the same inhibitory effect on sessile bacteria compared with planktonic bacteria, suggesting the protective effect of BBFs. Many previous studies have reported that essential oils extracted from plants exhibit antibiotic and antimicrobial activities in vitro against many bacteria, including antibiotic-resistant strains [27, 28]. For instance, Adukwu et al. tested the MICs of lemongrass, grapefruit, bergamot, and lime against S. aureus strains. They found that although all strains were susceptible to these essential oils, lemongrass oil exhibited the most effective antimicrobial and anti-biofilm activities against the S. aureus strains [29].
Therefore, as a follow-up, this study then examined the effects of M. chinensis Maxim cv. Jiangxiangru essential oil and its eight components on S. aureus biofilms at concentrations of 2, 1, 0.5, 0.25, 0.125, and 0.0625× MIC using the semi-quantitative method. After co-incubation of test substances at a concentration of 2× MIC and S. aureus for 48 h, the essential oil, carvacrol, 3-carene, carvacryl acetate, thymol acetate, and α-caryophyllene all significantly inhibited S. aureus biofilm formation, with inhibition rates of >90%. This result indicated that the aforementioned tested substances were capable of killing a large number of floating S.aureus at 2× MIC, thereby inhibiting biofilm formation. However, at a concentration of 0.25× MIC, only carvacrol, carvacryl acetate, α-caryophyllene, and 3-carene showed an inhibition rate of >80%. The morphological observation in the biofilm formation experiment revealed that at 0.25× MIC, the biofilm distribution significantly decreased in the test drug group than in the negative control and showed a sparse distribution. This indicated that 0.25× MIC could significantly inhibit the formation of S. aureus biofilm. Meanwhile, thymol, γ-terpinene, thymol acetate, and p-cymene had a relatively weak inhibitory effect on S. aureus biofilm formation at all concentrations. Moreover, No biofilm formation occurred when these compounds were used at 2× MIC. All these results showed that carvacrol, carvacryl acetate, α-caryophyllene, and 3-carene were the major components inhibiting biofilm formation in the essential oil. They might exert their effect by reducing the secretion of extracellular DNA, inhibiting the expression of polysaccharide intercellular adhesion, or regulating genes associated with biofilm formation, but not by killing planktonic bacteria. Further studies are required to determine the specific mechanism by which the essential oil and its components can inhibit biofilm formation.
5. Conclusions
In summary, the essential oil in M. chinensis Maxim cv. Jiangxiangru is a potential drug against S. aureus biofilm. Low doses of this essential oil and its major components, carvacrol, carvacryl acetate, α-caryophyllene, and 3-carene, can significantly inhibit the formation of S. aureus biofilm.
Footnotes
Conflicts of interest: Authors state no conflict of interest.
References
- [1].Archer G.L.. Staphylococcus aureus: a well-armed pathogen. Clin Infect Dis. 1998;26:1179–1181. doi: 10.1086/520289. [DOI] [PubMed] [Google Scholar]
- [2].Lowy F.D.. Staphylococcus aureus infections. N Engl J Med. 1998;339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- [3].Otto M.. Staphylococcal biofilms. Curr Top Microbiol Immunol. 2008;322:207–228. doi: 10.1007/978-3-540-75418-3_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Alekshun M.N., Levy S.B.. Molecular mechanisms of antibacterial multidrug resistance. Cell. 2007;128:1037–1050. doi: 10.1016/j.cell.2007.03.004. [DOI] [PubMed] [Google Scholar]
- [5].Lowy F.D.. Antimicrobial resistance: the example of Staphylococcus aureus. J Clin Invest. 2003;111:1265–1273. doi: 10.1172/JCI18535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Shalit I., Berger S.A., Gorea A., Frimerman H.. Widespread quinolone resistance among methicillin-resistant Staphylococcus aureus isolates in a general hospital. Antimicrob Agents Chemother. 1989;33:593–594. doi: 10.1128/aac.33.4.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ito H., Yoshida H., Bogaki-Shonai M., Niga T., Hattori H., Nakamura S.. Quinolone resistance mutations in the DNA gyrase gyrA and gyrB genes of Staphylococcus aureus. Antimicrob Agents Chemother. 1994;38:2014–2023. doi: 10.1128/aac.38.9.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Seal J.B., Moreira B., Bethel C.D., Daum R.S.. Antimicrobial resistance in Staphylococcus aureus at the University of Chicago Hospitals: a 15-year longitudinal assessment in a large university-based hospital. Infect Control Hosp Epidemiol. 2003;24:403–408. doi: 10.1086/502222. [DOI] [PubMed] [Google Scholar]
- [9].Nimmo G.R., Bell J.M., Mitchell D., Gosbell I.B., Pearman J.W., Turnidge J.D.. Agar, Antimicrobial resistance in Staphylococcus aureus in Australian teaching hospitals, 1989-1999. Microb Drug Resist. 2003;9:155–160. doi: 10.1089/107662903765826741. [DOI] [PubMed] [Google Scholar]
- [10].Hiramatsu K.. Vancomycin-resistant Staphylococcus aureus: a new model of antibiotic resistance. Lancet Infect Dis. 2001;1:147–155. doi: 10.1016/S1473-3099(01)00091-3. [DOI] [PubMed] [Google Scholar]
- [11].Pantosti A., Sanchini A., Monaco M.. Mechanisms of antibiotic resistance in Staphylococcus aureus. Future Microbiology. 1974;12:323–334. doi: 10.2217/17460913.2.3.323. [DOI] [PubMed] [Google Scholar]
- [12].Davies D.. Understanding biofilm resistance to antibacterial agents. Nat Rev Drug Discov. 2003;2:114–122. doi: 10.1038/nrd1008. [DOI] [PubMed] [Google Scholar]
- [13].Ryder V.J., Chopra I., O’Neill A.J.. Increased mutability of Staphylococci in biofilms as a consequence of oxidative stress. PLoS One. 2012;7:e47695. doi: 10.1371/journal.pone.0047695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Savage V.J., Chopra I., O’Neill A.J.. Staphylococcus aureus biofilms promote horizontal transfer of antibiotic resistance. Antimicrob Agents Chemother. 2013;57:1968–1970. doi: 10.1128/AAC.02008-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Aumeeruddy-Elalfi Z., Gurib-Fakim A., Mahomoodally M.F.. Chemical composition, antimicrobial and antibiotic potentiating activity of essential oils from 10 tropical medicinal plants from Mauritius. Journal of Herbal Medicine. 2016 [Google Scholar]
- [16].Stewart P.S.. A review of experimental measurements of effective diffusive permeabilities and effective diffusion coefficients in biofilms. Biotechnol Bioeng. 1998;59:261–272. doi: 10.1002/(sici)1097-0290(19980805)59:3<261::aid-bit1>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- [17].Brown M.R., Allison D.G., Gilbert P.. Resistance of bacterial biofilms to antibiotics: a growth-rate related effect? J Antimicrob Chemother. 1988;22:777–780. doi: 10.1093/jac/22.6.777. [DOI] [PubMed] [Google Scholar]
- [18].Cao L., Si J.Y., Liu Y., Sun H., Jin W., Li Z., Zhao X.H., Le Pan R.. Essential oil composition, antimicrobial and antioxidant properties of Mosla chinensis Maxim. Food Chemistry. 2009;115:801–805. [Google Scholar]
- [19].Li J.E., Nie S.P., Xie M.Y., Huang D.F., Wang Y.T., Li C.. Chemical composition and antioxidant activities in immumosuppressed mice of polysaccharides isolated from Mosla chinensis Maxim cv. jiangxiangru. Int Immunopharmacol. 2013;17:267–274. doi: 10.1016/j.intimp.2013.05.033. [DOI] [PubMed] [Google Scholar]
- [20].Li J.E., Nie S.P., Qiu Z.H., Che M.J., Li C., Xie M.Y.. Antimicrobial and antioxidant activities of the essential oil from Herba Moslae. J Sci Food Agric. 2010;90:1347–1352. doi: 10.1002/jsfa.3941. [DOI] [PubMed] [Google Scholar]
- [21].Yang S., Ouyang H., Su X., Gao H., Kong W., Wang M., Shu Q., Fu Z.. Dual-recognition detection of Staphylococcus aureus using vancomycin-functionalized magnetic beads as concentration carriers. Biosens Bioelectron. 2016;78:174–180. doi: 10.1016/j.bios.2015.11.041. [DOI] [PubMed] [Google Scholar]
- [22].CLS I.. Performance standards for antimicrobial susceptibility testing; eighteenth informational supplement M100-S18. Wayne, PA: Clinical and Laboratory Standards Institute. 2008 [Google Scholar]
- [23].Karaolis D.K., Rashid M.H., Chythanya R., Luo W., Hyodo M., Hayakawa Y.. c-di-GMP (3’-5’-cyclic diguanylic acid) inhibits Staphylococcus aureus cell-cell interactions and biofilm formation. Antimicrob Agents Chemother. 2005;49:1029–1038. doi: 10.1128/AAC.49.3.1029-1038.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wang Y., Wang T., Hu J., Ren C., Lei H., Hou Y., Brantner A.H.. Anti-biofilm activity of TanReQing, a Traditional Chinese Medicine used for the treatment of acute pneumonia. J Ethnopharmacol. 2011;134:165–170. doi: 10.1016/j.jep.2010.11.066. [DOI] [PubMed] [Google Scholar]
- [25].Sawai R., Kuroda K., Shibata T., Gomyou R., Osawa K., Shimizu K.. Anti-influenza virus activity of Chaenomeles sinensis. J Ethnopharmacol. 2008;118:108–112. doi: 10.1016/j.jep.2008.03.013. [DOI] [PubMed] [Google Scholar]
- [26].Nostro A., Sudano Roccaro A., Bisignano G., Marino A., Cannatelli M.A., Pizzimenti F.C.. et al. Effects of oregano, carvacrol and thymol on Staphylococcus aureus and Staphylococcus epidermidis biofilms. J Med Microbiol. 2007;56:519–523. doi: 10.1099/jmm.0.46804-0. [DOI] [PubMed] [Google Scholar]
- [27].Fisher K., Phillips C.A.. The effect of lemon, orange and bergamot essential oils and their components on the survival of Campylobacter jejuni, Escherichia coli O157, Listeria monocytogenes, Bacillus cereus and Staphylococcus aureus in vitro and in food systems. J Appl Microbiol. 2006;101:1232–1240. doi: 10.1111/j.1365-2672.2006.03035.x. [DOI] [PubMed] [Google Scholar]
- [28].Warnke P.H., Becker S.T., Podschun R., Sivananthan S., Springer I.N., Russo P.A.. et al. The battle against multi-resistant strains: Renaissance of antimicrobial essential oils as a promising force to fight hospital-acquired infections. J Craniomaxillofac Surg. 2009;37:392–397. doi: 10.1016/j.jcms.2009.03.017. [DOI] [PubMed] [Google Scholar]
- [29].Adukwu E.C., Allen S.C., Phillips C.A.. The anti-biofilm activity of lemongrass (Cymbopogon flexuosus) and grapefruit (Citrus paradisi) essential oils against five strains of Staphylococcus aureus. J Appl Microbiol. 2012;113:1217–1227. doi: 10.1111/j.1365-2672.2012.05418.x. [DOI] [PubMed] [Google Scholar]