Abstract
Emodin, a major component of rhubarb, has anti-tumor effects in a variety of cancers, influencing multiple steps of tumor development through modulating several signaling pathways. The aim of this study is to examine the effect of emodin on cell apoptosis and explore the underlying mechanisms in human endometrial cancer cells. Here we report that emodin can inhibit KLE cell proliferation and induce apoptosis in a time- and dose-dependent manner. Western blot assay found that emodin was involved in MAPK and PI3K/Akt signaling pathways. Specifically, emodin significantly suppressed the phosphorylation of AKT, and enhanced the phosphorylation of MAPK pathways. Furthermore, the generation of reactive oxygen species (ROS) was up-regulated in KLE cells upon treatment with emodin, while the anti-oxidant agent N-acetyl cysteine (NAC) can inhibit emodin-induced apoptosis and promote the activation of AKT and Bcl-2. Taken together, we revealed that emodin may induce apoptosis in KLE cells through regulating the PI3K/AKT and MAPK signaling pathways, indicating the importance of emodin as an anti-tumor agent.
Keywords: Endometrial cancer, emodin, apoptosis; MAPK, PI3K/AKT
1. Introduction
Endometrial cancer is the fourth most frequent malignancy and the sixth most common cause of cancer-related deaths among women in the USA [1]. In the last 20 years, despite the rapid development in diagnosis and treatment of endometrial cancer, both incidence and five-year survival rate have remained roughly unchanged [2, 3], which means that the survival rate of these patients still needs to be improved.
Chinese herbs have a long history with confirmed benefits and few side effects in treating various cancers. Emodin (1,3,8-trihydroxy-6 -methylanthraquinone, see as Fig. 1) is derived from the root and rhizome of a herb called Rheum palmatum L. Previous researches showed emodin may exert inhibitory effectiveness against different cancers, such as breast cancer, bladder cancer, hepatic cancer, cervical cancer, and ovarian cancer [4, 5, 6, 7, 8, 9, 10, 11, 12]. This anti-tumor effect appears due mainly to its being a ROS generator, which can stimulate apoptosis of cancer cells. Ding et al. [13] reported that emodin may enhance the chemosensitivity of endometrial cancer, indicating emodin may play a role in inhibiting tumor proliferation. To the best of our knowledge, no study has explored emodin’s role in regulating apoptosis in endometrial cancer. Here we report the inhibitory effect of emodin in the proliferation of KLE cells.
Figure 1.
The molecular structure of emodin.
2. Materials and Methods
2.1. Cell Culture and Reagents
KLE cells (which were kindly provided by Prof. Wang, Peking University People’s Hospital) were cultured in Dulbecco’s modified Eagle’s medium (DMEM, hyclone, SH30022.01B) supplemented with 10% Fetal Bovine Serum (GIBCO, 10099-141). All cultures were hatched in an incubator with 37°C and constant atmosphere comprising 95% air and 5% CO2. Emodin, dimethylsulfoxide (DMSO) and NAC were purchased from Sigma-Aldrich (Merck Millipore, Darmstadt, Germany). Emodin was stocked in a concentration of 100 mM with 100% DMSO at -20˚C. The following concentrations were prepared: 1.25, 2.5 and 5 μM. DMSO (0.1%) was used as control for all the experiments. The Annexin V-FITC apoptosis detection kit (cat. no. KGA106) and rhodamine123 (DHM123, cat. no. KGA217) were purchased from Key Gen Bio-Tech Co., Ltd. (Nanjing, China). Annexin V/PI apoptosis detection kit was obtained from Becton Dickinson (CA, USA). Cell Counting Kit-8 (CCK-8, cat. no. CK-04) was purchased from (Dojindo, JAPAN). Rabbit phosphorylated AKT (p-AKT) and total AKT (t-AKT) polyclonal antibody, rabbit p- ERK1/2 and t-ERK1/2 monoclonal antibody, rabbit p-p38 and t-p38 monoclonal antibody and mouse β-Actin monoclonal antibody were all purchased from Cell Signaling Technology, Inc (Boston, MA, USA). Caspase-3, Bax and Bcl-2 polyclonal antibody were purchased from Protein Tech Group, Inc (Chicago, IL, USA).
2.2. Xenograft Tumor Models
Four- to five-week old female BALB/c nude mice, weighing between 16-18 g were purchased from Shanghai Laboratory Animal Company (SLAC, Shanghai, China). Mice were maintained in the animal facility at Zhejiang Chinese Medical University, China. The animal experiments were approved by Animal Care and Use Committee. KLE cells (5×106) resuspended in 100 μL medium were implanted subcutaneously in the right flanks of the nude mice, 10 days later, mice were intraperitoneally injected with 40 mg/kg emodin or vehicle (DMSO) once every 2 days for 1 month. Tumor size and body weight were measured with calipers every five days until 45 days after tumor cell implantation. Tumor volume was calculated using the following formulae: V = (length × width2/2).
Ethical approval The research related to animal use has complied with all the relevant national regulations and institutional policies for the care and use of animals.
2.3. CCK-8 Assay for Cell Viability Detection
Cell viability was determined by CCK-8 assay. Briefly, cells were seeded into a 96-well plate at a density of 1×104 cells/ well. After 24h, cells were treated with serial doses of Emodin for 12, 24 and 48h respectively. Then, 10μl CCK-8 solution was added to each well and incubated for an additional 4h at 37 degrees C. The absorbance of medium was detected at 450 nm using a multiskan spectrum microplate reader (Tendometrical cancern, Infinite F50).
2.4. 4’, 6-Diamidino-2-phenylindole dihydrochloride (DAPI) Staining
A total of 1×105 KLE cells were seeded into each well of the 12-well plates 24h before emodin treatment. KLE cells were cultured for 24 h in 10% serum medium with control or different concentrations of emodin. For DAPI, cells were fixed with 3.7% formaldehyde for 15 min, permeabilized with 0.1% Triton X-100 and then stained with 1 mg/ml DAPI for 5 min at 37 degrees C. The cells were washed with PBS twice and finally examined by fluorescence microscopy (Olympus IX 70).
2.5. Flow Cytometric Analysis of Apoptosis
Apoptosis of KLE was detected using the Annexin V-FITC apoptosis detection kit according to the manufacturer’s instructions. Cells were seeded at a density of 3×105 cells/well into a 12-well plate. Different concentrations (1.25, 2.5 and 5 μM) of emodin or control were added to the wells after 24h incubation. Cells of each sample were harvested after an additional 24 or 48 h and suspended in 500 μl of Annexin V binding buffer (1×). 5μl of Annexin V-FITC and propidium iodide (PI) were added and incubated for 15 min in dark conditions. The stained cells were analyzed by flow cytometry using a FACS Calibur (BD Accuri C6, Biosciences, San Jose, CA, USA).
2.6. Quantitative Real-time RT-PCR (qRT-PCR)
The expression of mRNAs of Bax, Bcl-2 and Caspase-3 was examined by qRT-PCR. After treatment with the agents, the total RNA was extracted from the cells using Trizol reagent (Invitrogen, Carlsbad, CA, USA). Then, the RNAs were reverse transcribed into cDNAs with the AMV reverse transcription system (Promega, USA). The SYBR Green PCR master mix (Applied Biosystems, USA) carried out the qRT-PCR reactions using 7300 Real-Time PCR System (Applied Biosystems, USA). GAPDH gene acted as the internal control gene. The change in expression levels of each gene was calculated using the standard curve method.
2.7. Western Blot Analysis
Cells were plated in a 6-well plate at a density of 2×105 cells/well. After treatment with different concentrations of emodin and control for 24 h, the cells were harvested and then washed twice using ice-cold PBS (pH 7.4) and lysed with RIPA buffer containing 1mM PMSF on ice. The protein were centrifuged from the cell lysates at 12,000× g at 4°C for 15 min and the concentration was measured in the collected supernatant. The protein from each sample was separated by 10% SDS-PAGE gel, then electro-transferred to a polyvinylidene difluoride (PVDF) membrane. After blocking with Tris-buffered saline and Tween 20 for 1h at room temperature, the membranes were incubated with appropriate concentrations of primary antibodies, including anti AKT, anti p-AKT, anti ERK, anti p-ERK, anti JNK, anti p-JNK, anti p38, anti p-p38, anti Bax, anti Bcl-2, anti Caspase-3 and β-Actin at 4°C overnight. After washing the membrane with TBST three times for 15 min, the membrane was incubated with horse radish peroxidase-conjugated goat anti-rabbit secondary antibody for 2 h at room temperature. Following three washes with TBST for 15min, the immune-reactive bands were detected by FluorChem E System (Protein Simple, Santa Clara, CA).
2.8. Detection of Reactive Oxygen Species (ROS)
Cells were loaded with 25 μM DHR123 (Sigma-Aldrich, Poole, UK) for 20 min. Excess DHR123 was removed (centrifugation and washing with PBS) then, and fluorescence intensity measurement was done using a fluorimeter (Cary Eclipse, Varian, UK) (λex=488 nm and λem=520 nm, respectively). Cell viability was determined by the MTS assay (Promega, UK). Absorbance was measured at 490 nm with a 96-well plate reader (SpectraMax 250, Molecular Devices) using SOFTmax pro 3.1.1.
2.9. Statistical Analysis
Data were reported as mean ± SEM of at least three independent experiments. For statistical analysis, one-way ANOVA was used for comparison of one variance among groups and two-way ANOVA was used for comparison of two independent variances among groups followed by the Tukey post hoc test by using SPSS 15.0. A P value less than 0.05 was considered to be significant.
3. Results
3.1. Emodin Inhibits the Proliferation of KLE
In order to investigate whether emodin has an anti-proliferation effect on endometrial cancer, we used a CCK-8 assay to quantify the effect on KLE cells treated with emodin at different concentrations ranging from 0 to 5 μM (0, 1.25, 2.5 and 5 μM) for various time points (12, 24, 48 and 72 h), as we had previously determined that the IC50 value was higher than 5 μM in KLE cell line (24H, IC50=6.002). As shown in Fig. 2A with increasing emodin concentration, cell viability decreases significantly in a dose- and time-dependent manner. Compared to the control, emodin treatment significantly inhibited KLE proliferation, even with 1.25μM for the first 12h, indicating the remarkable inhibitory effect of emodin. Furthermore, to evaluate whether emodin can inhibit the tumorigenicity of endometrial cancer in vivo, nude mice were implanted with KLE cells subcutaneously for 10 days followed by intraperitoneal injection of 40 mg/kg emodin or vehicle (DMSO) once every 2 days for 1 month. As seen in Fig. 2B the tumor volume was significantly larger in the control group than that in the emodin-treated group. Consistently, the body weight of the emodin-treated group decreased more slowly than that of the control (Fig. 2C) These results showed that emodin can effectively inhibit endometrial cancer development both in vitro and in vivo.
Figure 2.
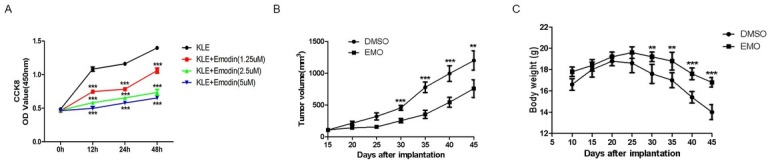
Emodin inhibits the proliferation of endometrial cancer cells both in vivo and in vitro. (A) CCK-8 assay was performed to detect KLE growth curve treated or not treated with emodin at various time points. (B) and (C) Tumor xenografts’ volume and host body weight were measured in nude mice, individually (n=10/group). *p< 0.05, **p< 0.01, ***p< 0.001. Here shows one representative result.
3.2. Emodin Induces Apoptosis of KLE Cells
Emodin was reported to promote apoptosis of many cancer cells. To detect whether the inhibition of KLE cells was achieved through emodin’s promotion of apoptosis, we performed DAPI staining and flow cytometry. Treating KLE cells with serial doses of emodin for 24h resulted in changes in cell number and morphology (Fig. 3A) KLE cells reduced as the amount of emodin increased, and cells were separated from the surrounding cells with loss of connection, and decreased volume. Furthermore, DAPI staining showed there was a gradual increase in the proportion of nuclear condensation in KLE cells accompanied by an increase in emodin concentration. Some of the nuclei split into pieces as an indication of apoptotic body formation (Fig. 3B) Flow cytometry was used to assess the cell apoptosis rate to prove the effect of emodin on apoptosis induction in KLE cells. After treatment with various concentrations of emodin (1.25, 2.5 and 5 μM for the experimental groups) for 24 h, KLE cells were stained with FITC-Annexin V/PI. As shown in Fig. 3C and Fig. 3D in the control group, only a small number of apoptotic cells was detected, however the rate went up to 4 times as much as control at 5 μM emodin for 48 h. The apoptotic proportion gradually increases with higher concentration of emodin. These results suggested that emodin was able to significantly induce apoptosis in KLE cells in a dose-dependent manner.
Figure 3.
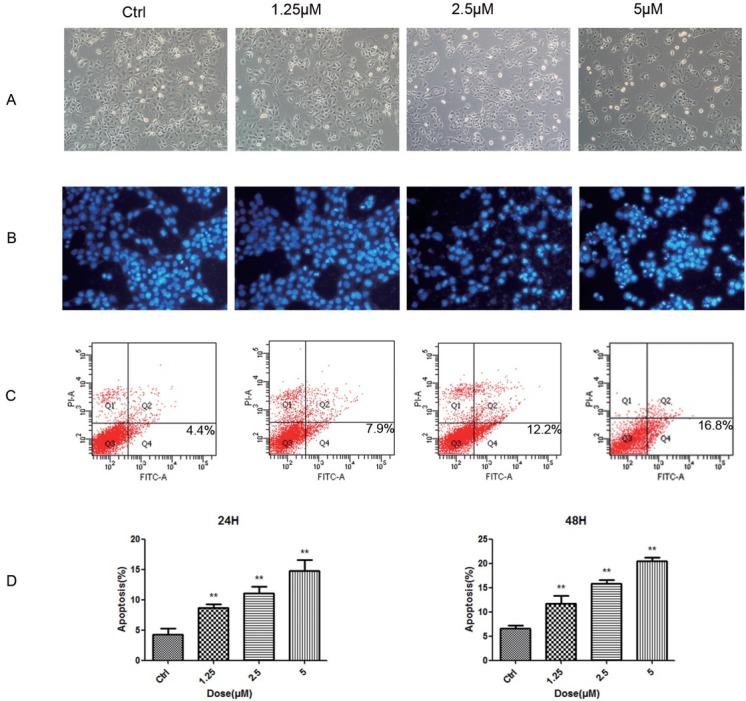
Emodin promotes apoptosis in KLE Cells. (A) The morphology of KLE cells was photographed by phase-contrast microscopy after treatment with serial doses of emodin. (B) Representative images of DAPI staining results of KLE cells treated with serial doses of emodin. (C) Cell flow cytometry was performed to detect the apoptosis of KLE cells double-stained with Annexin V-FITC and PI-A. (D) The apoptotic KLE cells was quantified. *p< 0.05, **p< 0.01, ***p< 0.001. Here shows one representative result.
3.3. Emodin Regulates the Activity of Bax, Bcl-2 and Caspase-3 in KLE Cells
The caspase-dependent pathway is one of the most important mechanisms for induction of cell apoptosis. We examined whether emodin induces apoptosis in KLE cells through a caspase-dependent pathway. KLE cells were exposed to serial concentrations of emodin (1.25, 2.5, 5 μM) for 24 hours and the expression of Bax, Bcl-2 and Caspase-3 was determined by WB and qRT-PCR, respectively. Interestingly, as the concentration of emodin increased, protein expression of Bcl-2 was significantly inhibited, while the pro-apoptosis proteins, Bax and Caspase-3 were up-regulated (Fig. 4A) Consistent with the WB results, the RNA transcription of Bax and Caspase-3 increased as much as twofold, while Bcl-2 was down-regulated by 50% compared to the control at a concentration of 5 μM (Fig. 4B) These results demonstrated that emodin promoted the expression of caspase-3 and inverted the ratio of Bcl-2/Bax in a dose-dependent way.
Figure 4.
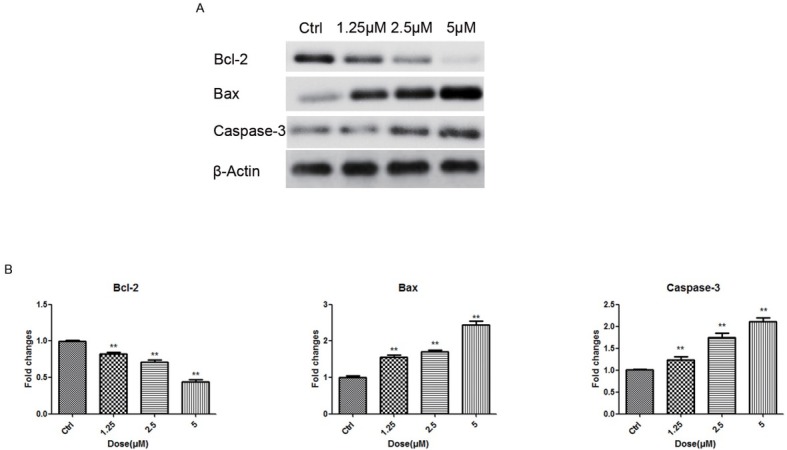
Emodin promotes the expression of Bax, Bcl-2 and Caspase-3 in KLE cells. (A) After 24h, total cellular protein was extracted for western blotting to detect the Bcl-2, Bax and Caspase-3 expression. (B) After 24h, qRT-PCR was performed to detected the expression of Bax, Bcl-2 and Caspase-3 in RNA level. *p< 0.05, **p< 0.01, ***p< 0.001. Here shows one representative result.
3.4. Emodin Induces Activation of the MAPK pathway
Upon cell apoptosis, the MAPK signaling pathway and PI3K/AKT signaling pathway are usually involved. In order to examine whether the MAPK and PI3K/AKT pathways are regulated by emodin in the process of KLE cell apoptosis, WB assay was performed to detect the phosphorylation of key proteins in these pathways such as p38, ERK, JNK and AKT. As shown in Fig 5, emodin treatment promoted the phosphorylation of p38, ERK and JNK, while it inhibited the phosphorylation of AKT. This effect gradually increased as concentration increased and as the timeframe expanded between 6 h to 24 h, meaning that emodin regulates these pathways in a dose- and time-dependent manner in KLE cells.
Figrue 5.
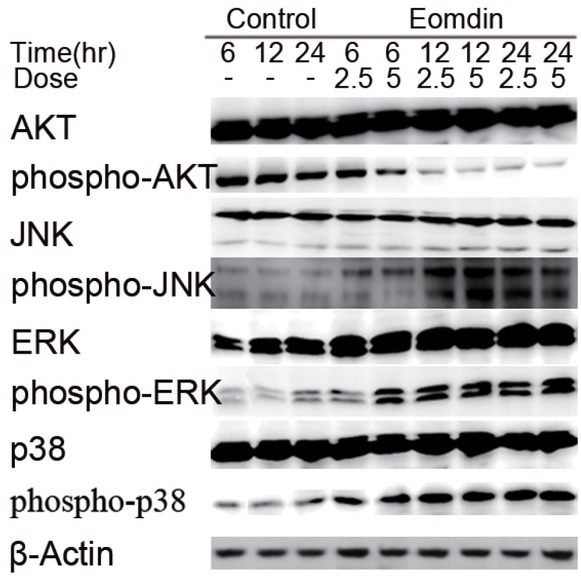
Emodin inhibits the phosphorylation of AKT and promtes MAPK pathway of the ERK, JNK and p38. Total cellular protein was extracted and 30μg proteins were added for electrophoresis. Time points: 3 h, 6 h, 12 h, 24 h.
3.5. Emodin-promoted KLE Apoptosis is Dependent on ROS Production
As already known, ROS may trigger cell apoptosis, especially in cancer cells. Emodin was reported to promote ROS generation in many cancers [5, 8, 9, 12]. To determine whether ROS is involved in emodin-induced apoptosis in KLE cells, cells were exposed to different concentrations of emodin and the level of DHR fluorescence was monitored at 24h and 48h after emodin treatment via flow cytometry. ROS generation increased significantly in a dose dependent manner (Fig. 6A, B) as the percentage increased from 17.6% at 1.25 μM to as high as 42.1% at 5 μM. To further confirm whether the ROS is essential for induction of apoptosis by emodin, NAC was used to block the effect of ROS. As shown in Figure 6C NAC significantly reduced emodin-induced ROS generation, and blocked emodin-triggered apoptosis in KLE cells. Meanwhile, NAC restoredthe ratio of Bcl-2/Bax and phosphorylation of AKT upon emodin treatment (Fig. 6D) These results establish an association between emodin’s anti-cancer effect and ROS activity, indicating that emodin-induced apoptosis in KLE cells is dependent on ROS production.
Figure 6.
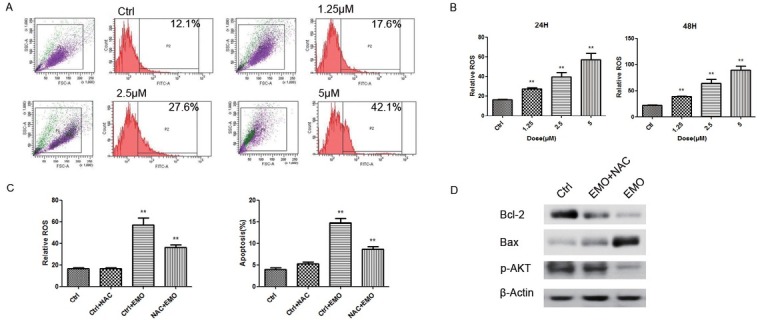
Emodin promotes KLE apoptosis in dependent of ROS production. (A) The ROS production of KLE cells was measured by flow cytometery. (B) The ROS of KLE cells was quantified at 24h and 48h. (C) The ROS production and apoptosis percentage were detected in KLE cells treated with 5 μM emodin and/or NAC (1 mM). (D) Total cellular protein was extracted for western blot to detect the expression of Bcl-2, Bax and p-AKT treated with emodin and/or NAC for 24h. *p< 0.05, **p< 0.01, ***p< 0.001. Here shows one representative result.
4. Discussion
Endometrial cancer is one of the most frequent malignancies with high metastasis and mortality rates. In the past decades, there has been great progress achieved in diagnosis and treatment of endometrial cancer, as well as dramatic advances in the field of formation and development of endometrial tumors, including the pharmacological study of Chinese traditional herbs. Promoting apoptosis of cancer cells through drugs or molecular antibodies to reduce tumor volume has great potential. Persistent oxidative stresses universally exist in cancer cells [14, 15]. ROS-induced apoptosis is a key mechanism of programmed cell death, especially in cancer cells. When ROS reaches an intolerably high level, it may trigger downstream cellular and molecular events such as altering mitochondrial function and signaling transduction, promoting cell death or apoptosis [5, 11, 16]. Here we report that emodin, a major component of rhubarb, exerts its apoptosis effect in KLE cells. We found that a low dose of emodin (5 μM) is adequate to cause this effect, contrasting with other cancers like ovarian cancer, cervical cancer, hepatocellular cancer and breast cancer [4 5 6, 10], indicating endometrial cancer may be sensitive to emodin and emodin is therefore a powerful agent for induction of apoptosis in endometrial cancer. Using NAC to block the effect of ROS, we then found emodin’s promotion of apoptosis is dependent on ROS generation.
AKT is found to be over-activated in the majority of endometrial cancers [17, 18, 19]. Recent sequencing analysis for the Cancer Genome Atlas has reported that more than 90% of endometrial tumors have some genetic aberration in the PI3K pathway, leading to increased AKT activity, which then promotes the growth of cancer cells by inhibiting apoptosis [20]. Furthermore Qiong et al. proved that the growth of endometrial cancer KLE cells can be suppressed through inhibiting the PI3K/AKT pathway [21]. Previous studies have shown that AKT expression can be activated when ROS is inhibited, resulting in the reverse of the Bcl-2/Bax ratio [12, 22], just as observed in the present experiment. Our research indicates that emodin may inhibit the phosphorylation of AKT in the KLE cell line in a time- and dose-dependent manner, thus restraining KLE cell proliferation.
It has also been well-established that MAPK signaling is involved in cell apoptosis. In mammals, MAPKs include three key factors, namely ERK, p38, and JNK [6, 7, 23 24 25]. Studies reported that sustained activation of ERK and JNK promotes cell differentiation and apoptosis [26, 27]. Furthermore, p38 MAPK also mediates cell apoptosis and growth resistance [28, 29]. In the present study, we observed that emodin affects the signaling pathway of MAPKs in a time- and dose-dependent manner in KLE cells. Emodin significantly induced the phosphorylation of ERK, p38 and JNK, without changing the basal expression level of these proteins.
The current study observed that emodin can induce apoptosis (by significantly upregulating ROS production) and suppress the proliferation of KLE cells, an endometrial cancer cell line with high invasion potential. Partially at least, emodin exerts this function through inhibiting the PI3K/Akt pathways while activating MAPK signaling. These results not only deepened our understanding of emodin’s anti-cancer activity, but also augmented solid evidence for future clinical application of emodin in human endometrial cancer treatment.
Acknowledgement
This research was supported by grants from the Foundation of Zhejiang Chinese Medical University (2013ZY02) and Science Technology Department of Zhejiang Province (2016C33129).
Footnotes
Conflict of interest: Authors state no conflict of interest
References
- [1].Siegel RL, Miller KD, Jemal A.. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- [2].Brasseur K, Gevry N, Asselin E. Chemoresistance and targeted therapies in ovarian and endometrial cancers. Oncotarget. 2017;8:4008–4042. doi: 10.18632/oncotarget.14021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Vale CL, Tierney J, Bull SJ, Symonds PR. Chemotherapy for advanced, recurrent or metastatic endometrial carcinoma. Cochrane Database Syst Rev. 2012. [DOI] [PMC free article] [PubMed]
- [4].Yaoxian W, Hui Y, Yunyan Z, Yanqin L, Xin G, Xiaoke W. Emodin induces apoptosis of human cervical cancer hela cells via intrinsic mitochondrial and extrinsic death receptor pathway. Cancer Cell Int. 2013;13(71) doi: 10.1186/1475-2867-13-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ma J, Yang J, Wang C, Zhang N, Dong Y, Wang Y. Emodin augments cisplatin cytotoxicity in platinum-resistant ovarian cancer cells via ROS-dependent MRP1 downregulation. Biomed Res Int. 2014;2014:107671. doi: 10.1155/2014/107671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lin W, Zhong M, Yin H, Chen Y, Cao Q, Wang C. Emodin induces hepatocellular carcinoma cell apoptosis through MAPK and PI3K/AKT signaling pathways in vitro and in vivo. Oncol Rep. 2016;36:961–967. doi: 10.3892/or.2016.4861. [DOI] [PubMed] [Google Scholar]
- [7].Cui Y, Lu P, Song G, Liu Q, Zhu D, Liu X. Involvement of PI3K/Akt, ERK and p38 signaling pathways in emodin-mediated extrinsic and intrinsic human hepatoblastoma cell apoptosis. Food Chem Toxicol. 2016;92:26–37. doi: 10.1016/j.fct.2016.03.013. [DOI] [PubMed] [Google Scholar]
- [8].Kolitsida P, Abeliovich H. Selective emodin toxicity in cancer cells. Oncotarget. 2017;8:36932–36933. doi: 10.18632/oncotarget.16611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Li X, Wang H, Wang J, Chen Y, Yin X, Shi G. Emodin enhances cisplatin-induced cytotoxicity in human bladder cancer cells through ROS elevation and MRP1 downregulation. BMC Cancer. 2016;16:578. doi: 10.1186/s12885-016-2640-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Iwanowycz S, Wang J, Hodge J, Wang Y, Yu F, Fan D. Emodin Inhibits Breast Cancer Growth by Blocking the Tumor-Promoting Feedforward Loop between Cancer Cells and Macrophages. Mol Cancer Ther. 2016;15:1931–1942. doi: 10.1158/1535-7163.MCT-15-0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Huang XZ, Wang J, Huang C, Chen YY, Shi GY, Hu QS. Emodin enhances cytotoxicity of chemotherapeutic drugs in prostate cancer cells: the mechanisms involve ROS-mediated suppression of multidrug resistance and hypoxia inducible factor-1. Cancer Biol Ther. 2008;7:468–475. doi: 10.4161/cbt.7.3.5457. [DOI] [PubMed] [Google Scholar]
- [12].Shrimali D, Shanmugam MK, Kumar AP, Zhang J, Tan BK, Ahn KS. Targeted abrogation of diverse signal transduction cascades by emodin for the treatment of inflammatory disorders and cancer. Cancer Lett. 2013;341:139–149. doi: 10.1016/j.canlet.2013.08.023. [DOI] [PubMed] [Google Scholar]
- [13].Ding N, Zhang H, Su S, Ding Y, Yu X, Tang Y. Emodin enhances the chemosensitivity of endometrial cancer by inhibiting ROS-mediated Cisplatin-resistance. Anticancer Agents Med Chem. Forthcoming. 2017. [DOI] [PubMed]
- [14].Toyokuni S, Okamoto K, Yodoi J, Hiai H. Persistent oxidative stress in cancer. FEBS Lett. 1995;358:1–3. doi: 10.1016/0014-5793(94)01368-b. [DOI] [PubMed] [Google Scholar]
- [15].Dharmaraja AT. Role of Reactive Oxygen Species (ROS) in Therapeutics and Drug Resistance in Cancer and Bacteria. J Med Chem. 2017;60:3221–3240. doi: 10.1021/acs.jmedchem.6b01243. [DOI] [PubMed] [Google Scholar]
- [16].Buttke TM, Sandstrom PA. Redox regulation of programmed cell death in lymphocytes. Free Radic Res. 1995;22:389–397. doi: 10.3109/10715769509147548. [DOI] [PubMed] [Google Scholar]
- [17].Mjos S, Werner HMJ, Birkeland E, Holst F, Berg A, Halle MK. PIK3CA exon9 mutations associate with reduced survival, and are highly concordant between matching primary tumors and metastases in endometrial cancer. Sci Rep. 2017;7:10240. doi: 10.1038/s41598-017-10717-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lee II, Maniar K, Lydon JP, Kim JJ. Akt regulates progesterone receptor B-dependent transcription and angiogenesis in endometrial cancer cells. Oncogene. 2016;35:5191–5201. doi: 10.1038/onc.2016.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chen HX, Xu XX, Tan BZ, Zhang Z, Zhou XD. MicroRNA-29b Inhibits Angiogenesis by Targeting VEGFA through the MAPK/ERK and PI3K/Akt Signaling Pathways in Endometrial Carcinoma. Cell Physiol Biochem. 2017;41:933–946. doi: 10.1159/000460510. [DOI] [PubMed] [Google Scholar]
- [20].Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, Shen H. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67–73. doi: 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Meng Q, Sun X, Wang J, Wang Y, Wang L. The important application of thioridazine in the endometrial cancer. Am J Transl Res. 2016;8:2767–2775. [PMC free article] [PubMed] [Google Scholar]
- [22].Franke TF, Hornik CP, Segev L, Shostak GA, Sugimoto C. PI3K/Akt and apoptosis: size matters. Oncogene. 2003;22:8983–8998. doi: 10.1038/sj.onc.1207115. [DOI] [PubMed] [Google Scholar]
- [23].Shen HM, Liu ZG. JNK signaling pathway is a key modulator in cell death mediated by reactive oxygen and nitrogen species. Free Radic Biol Med. 2006;40:928–939. doi: 10.1016/j.freeradbiomed.2005.10.056. [DOI] [PubMed] [Google Scholar]
- [24].Boutros T, Chevet E, Metrakos P. Mitogen-activated protein (MAP) kinase/MAP kinase phosphatase regulation: roles in cell growth, death, and cancer. Pharmacol Rev. 2008;60:261–310. doi: 10.1124/pr.107.00106. [DOI] [PubMed] [Google Scholar]
- [25].Kim EK, Choi EJ. Compromised MAPK signaling in human diseases: an update. Arch Toxicol. 2015;89:867–882. doi: 10.1007/s00204-015-1472-2. [DOI] [PubMed] [Google Scholar]
- [26].Kim JY, Lee SG, Chung JY, Kim YJ, Park JE, Koh H. Ellipticine induces apoptosis in human endometrial cancer cells: the potential involvement of reactive oxygen species and mitogen-activated protein kinases. Toxicology. 2011;289:91–102. doi: 10.1016/j.tox.2011.07.014. [DOI] [PubMed] [Google Scholar]
- [27].Thongsom S, Suginta W, Lee KJ, Choe H, Talabnin C. Piperlongumine induces G2/M phase arrest and apoptosis in cholangiocarcinoma cells through the ROS-JNK-ERK signaling pathway. Apoptosis. 2017;22:1473–1484. doi: 10.1007/s10495-017-1422-y. [DOI] [PubMed] [Google Scholar]
- [28].Olson JM, Hallahan AR. p38 MAP kinase: a convergence point in cancer therapy. Trends Mol Med. 2004;10:125–129. doi: 10.1016/j.molmed.2004.01.007. [DOI] [PubMed] [Google Scholar]
- [29].Manohar M, Fatima I, Saxena R, Chandra V, Sankhwar PL, Dwivedi A. (-)-Epigallocatechin-3-gallate induces apoptosis in human endometrial adenocarcinoma cells via ROS generation and p38 MAP kinase activation. J Nutr Biochem. 2013;24:940–947. doi: 10.1016/j.jnutbio.2012.06.013. [DOI] [PubMed] [Google Scholar]


