Abstract
Intestinal sulfate-reducing bacteria are often isolated from patients with inflammatory bowel disease, including ulcerative colitis, and can be involved in the development of gut inflammation. A comparison of the metabolism of intestinal sulfate-reducing bacteria isolated from individuals with colitis and healthy controls using statistical analysis has never been studied and described before. The aim of our research was to evaluate the parameters of dissimilatory sulfate reduction in Desulfovibrio species that were isolated from the feces of healthy objects and individuals with colitis. Principal component analysis indicates that the strains that were isolated from individuals with colitis grouped in the same cluster by biomass accumulation and sulfide production, same as the strains isolated from healthy individuals. Sulfate and lactate consumption measured over time showed negative correlation (Pearson correlations, p<0.01), healthy: -0.760; colitis: -0.770; healthy: -0.828; colitis: -0.847, respectively. The calculated linear regression (R2) was lower in biomass accumulation and hydrogen sulfide production, 0.531; 0.625 respectively. Thus, biomass accumulation and sulfide production, together with measured kinetic parameters play an important factor in bowel inflammation, including ulcerative colitis. Additionally, acetate production can also synergize with H2S, while sulfate consumption and lactate oxidation likely represent minor factors in bowel disease.
Keywords: inflammatory bowel disease, ulcerative, colitis, sulfate-reducing bacteria, Desulfovibrio, hydrogen, sulfide, component analysis
1. Introduction
The pathogenesis of ulcerative colitis (UC) is known to be significantly influenced by the gut microbiota [1]. A major risk factor for inflammatory bowel disease in both animals and humans is indicated by increased numbers of sulfate-reducing bacteria (SRB), and intense dissimilatory sulfate reduction (DSR) in the gut [2, 3, 4]. While SRB are present in the normal gut microbiota, an increased frequency of SRB may contribute to colitis development, especially in association with hydrogen sulfide production [5]. Another contributing factor is reduced mucosal thickness in the presence of Desulfovibrio species [6]. Interestingly, SRB have also been associated with rheumatic diseases and ankylosing spondylitis [7]. The production of hydrogen sulfide has been shown to affect the metabolism of intestinal cells and give rise to various inflammatory bowel diseases [8]. The presence of SRB may also be responsible for some forms of rectum cancer. The most frequently detected species of SRB are Desulfovibrio genus in patients with bloody diarrhea, weight loss, anorexia, epithelial hyperplasia and abscesses (both is animals and humans) [9, 10, 11]. Additionally, in the feces of both humans and animals with ulcerative colitis, SRB are often detected with increased frequency [8, 12]. The prevalence of ulcerative colitis (UC) in Western countries is observed at a rate of 12 per 100,000 people, mostly between the ages of 15 and 30 years old [13]. Certainly, the location and severity of UC is influenced by medication and dosage in treatment [4, 8, 14, 15].
The final product of SRB is hydrogen sulfide, which is toxic, and in addition to contributing to IBD development (including UC and Crohn’s disease), it is also associated with a higher risk for neurodegenerative illness, likely through DNA damage and mutation, enzyme inhibition and mitochondrial respiration inhibition [16, 17, 18, 19, 20, 21].
The novelty of our study lies in the fact that the comparison of dissimilatory sulfate reduction (DSR), including sulfate and lactate consumption, the production of hydrogen sulfide and acetate, as well as growth parameters of the SRB strains isolated from healthy and individuals with UC has never been presented before. The aim of our research was to evaluate the parameters of growth (biomass) of Desulfovibrio species that were isolated from the feces of individuals with colitis and healthy controls, as well as to investigate changes in dissimilatory sulfate reduction of these bacterial strains.
2. Materials and Methods
2.1. Bacterial culture and cultivation
In total, feces from 48 patients were analyzed: 24 of them were with UC (12 female + 12 male) and 24 healthy UC (12 female + 12 male). All patients were 20 to 30 years old. SRB in all patients were calculated and identified, and their physiological and biochemical properties were studied as described previously [22]. Bacterial isolates have been preserved in the collection of microorganisms at the Department of Experimental Biology, Faculty of Science at the Masaryk University (Brno, Czech Republic). Bacteria were grown for 36 hours at 37°C under anaerobic conditions in nutrition modified Postgate’s liquid medium [23]. Before bacterial passage, 0.05 ml.L-1 of sterile solution of Na2S×9H2O (1%) was added. The sterile 10 mol.L-1 solution of NaOH (0.9 ml.L-1) was used to adjust the medium to pH 7.2. The medium was heated in boiling water for 30 min in order to obtain an oxygen-free medium and cooled to 35°C. Tubes were brim-filled with medium and closed to provide anaerobic conditions.
2.2. Assay of bacterial biomass
About 1 mL of liquid medium without Mohr’s salt was transferred into a plastic cuvette and taken to a biophotometer (Eppendorf®) for taring. Subsequently, 1 mL of bacterial suspension was transferred into another cuvette and taken again to the biophotometer for measuring at OD λ=340. Before SRB were used for the experiments, optical density (OD340) was measured to insure approximately the same number of bacteria was used in each experiment [4].
2.3. Assay of sulfate, lactate, hydrogen sulfide and acetate in cultivation medium
The sulfate ion concentration in the medium was determined by the turbidimetric method after it had been precipitated by barium chloride. To stabilize the suspension, glycerol was added [24]. Lactate concentration was measured through the dehydrogenation reaction using a Lactate Assay Kit (Sigma-Aldrich, Catalog Number MAK064). Sulfide concentration in the culture medium was assayed by the spectrophotometric method as described [25]. Accumulation of acetate ions during bacterial growth was determined using an Acetate Assay Kit (Colorimetric, Catalog Number KA3764).
2.4. Statistical analysis
Overall differences between bacterial growth (biomass) and DSR parameters in the Desulfovibrio strains isolated from individuals with UC and healthy controls were determined by principal component analysis (PCA). According to an Eigen value of greater than 1, two factors described total variability. The relationship between time, bacterial growth (biomass) and DSR parameters were confirmed by linear regression. The correlation between bacterial biomass and DSR parameters was conducted by Pearson’s correlation analysis. SPSS 20 statistical software (IBM Corporation, Armonk, USA) was used. The research results were also analyzed using software package Origin 7.0 (www.origin-lab.com) Using the experimental data, the basic statistical parameters (mean: M, standard error: m, M ± m) were calculated. The accurate approximation was when P≤ 0.05 [26].
3. Results
Principal component analysis (PCA) of the bacterial growth (biomass) and dissimilatory sulfate reduction (DSR) parameters (sulfate consumption, hydrogen sulfide production as well as lactate oxidation and acetate accumulation) by Desulfovibrio strains isolated from individuals with colitis and healthy controls is shown in Fig. 1. PCA analysis revealed four groups: I) strains isolated from individuals with colitis were grouped in the same cluster by biomass accumulation and sulfide production, II) strains isolated from healthy individuals were grouped in the cluster according to sulfide production and biomass accumulation, III) strains isolated from individuals with colitis and healthy controls formed a distinct cluster by acetate accumulation, IV) strains isolated from individuals with colitis and healthy controls were positioned separately in another cluster by lactate (healthy individuals) and sulfate (individuals with colitis). Between these groups, statistical significant differences (p<0.05) were observed, thus supporting the importance of PCA analysis of the collected data.
Fig. 1.
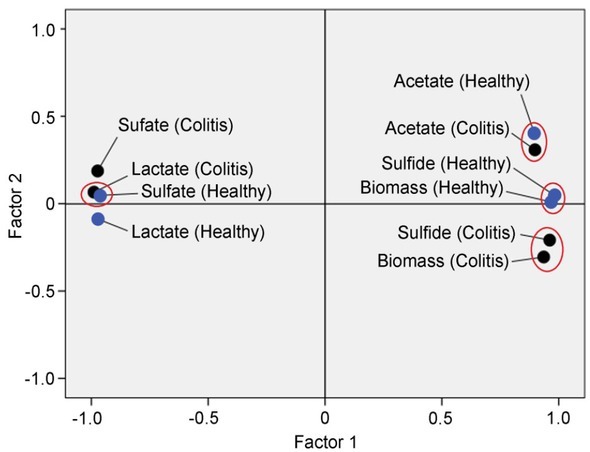
Principal component analysis (PCA) of the bacterial growth (biomass) and DSR parameters in the Desulfovibrio strains isolated from healthy and individuals with UC
Correlations between time (range from 0 to 10 days) and sulfate reduction parameters in Desulfovibrio isolates from healthy objects and people with colitis are shown in Table 1. Negative correlations were observed between time and sulfate and lactate consumption, while positive correlations were observed between the remaining measured parameters. Strains isolated from individuals with colitis produced a lower correlation with time only in biomass and sulfide accumulation as compared to the strains from healthy individuals. In other cases, they showed a higher correlation.
Table 1.
Pearson correlations between sulfate reduction parameters in Desulfovibrio isolates from healthy subjects and individuals with colitis
| Correlations | Time |
|---|---|
| (n=48) | Range: 0 – 10 days |
| Biomass (healthy) | 0.783** |
| Biomass (colitis) | 0.729** |
| Sulfate (healthy) | -0.760** |
| Sulfate (colitis) | -0.770** |
| Sulfide (healthy) | 0.838** |
| Sulfide (colitis) | 0.790** |
| Lactate (healthy) | -0.828** |
| Lactate (colitis) | -0.847** |
| Acetate (healthy) | 0.875** |
| Acetate (colitis) | 0.941** |
Comments: Correlation is significant at the 0.01 level (2-tailed)
Linear regression plots of growth (biomass accumulation, sulfate consumption, hydrogen sulfide production, and lactate oxidation and acetate production) with time of Desulfovibrio species isolated from individuals with colitis and healthy controls are shown in Fig. 2–3. R2 factors (p<0.01) were higher for strains isolated from colitis than healthy persons, though in comparison with healthy individuals, lower R2 were observed in biomass accumulation and hydrogen sulfide production.
Fig.2.
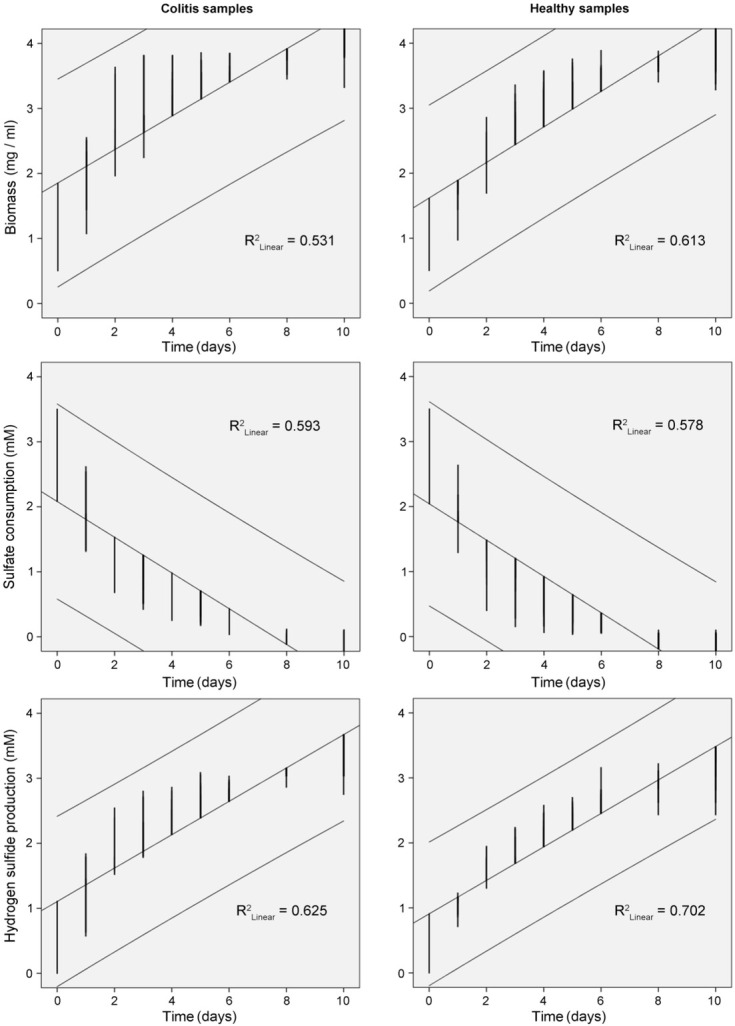
Linear regression plots of growth (biomass) of Desulfovibrio species, sulfate consumption and hydrogen sulfide production with time by bacterial strains isolated from healthy and individuals with colitis
Fig.3.
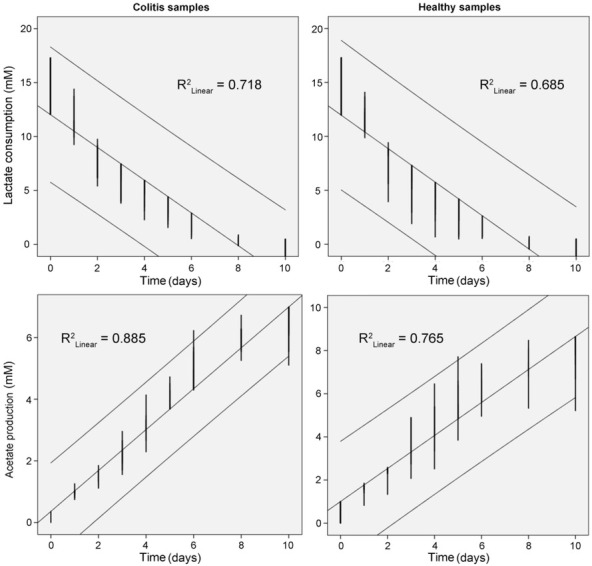
Linear regression plots of growth of lactate oxidation and acetate production with time by Desulfovibrio bacterial strains isolated from healthy and individuals with colitis
Based on our data, kinetic parameters of Desulfovibrio growth and their sulfate reduction isolated from individuals with colitis and healthy controls were calculated (Table 2). It can also be seen that the duration of the exponential growth phase extended for a longer period (142.50 hours) in the samples from healthy individuals. The stationary phase was achieved faster in samples that were collected from individuals with colitis (119.47 hours). These results correspond with a measured lag phase, and generation time (G), which was similar to the maximum growth rate (μmax) (Table 2). The rate of hydrogen sulfide production was also higher in samples that were collected from individuals with colitis.
Table 2.
Kinetic parameters of Desulfovibrio growth and their sulfate reduction isolated from healthy people and colitis patients
| Subject: healthy/colitis | Kinetic parameters |
|||||
|---|---|---|---|---|---|---|
| te | Lag-phase | G | Absolute rate | Specific rate | ||
| (h) | (h) | (h) | (number of cells/hour) | (number of cells/hour) | ||
| Average | μmax | |||||
| Bacterial growth (Biomass) | ||||||
| healthy | 142.50 | 0.38 | 0.63 | 1.59 | 0.022 | 0.046 |
| colitis | 119.47 | 0.27 | 0.59 | 1.70 | 0.024 | 0.057 |
|
Sulfate consumption (mM/hour) | ||||||
| healthy | – | – | – | 1.82 | 0.0260 | 0.0293 |
| colitis | – | – | – | 1.81 | 0.0230 | 0.0267 |
|
Hydrogen sulfide production (mM/hour) | ||||||
| healthy | – | – | – | 0.0100 | 0.007 | 0.012 |
| colitis | – | – | – | 0.0133 | 0.009 | 0.014 |
|
Lactate consumption (mM/hour) | ||||||
| healthy | – | – | – | 4.082 | 0.019 | 0.023 |
| colitis | – | – | – | 4.080 | 0.018 | 0.021 |
|
Acetate production (mM/hour) | ||||||
| healthy | – | – | – | 0.0208 | 0.014 | 0.018 |
| colitis | – | – | – | 0.0126 | 0.009 | 0.012 |
Comment: te is duration of exponential growth phase (hours), G is generation time (hours), μmax is maximum growth rate
4. Discussion
Part of human and animal intestinal microbiota is composed of sulfate-reducing bacteria; among them, the Desulfovibrio genus is the most often detected in individuals with IBD, including ulcer active colitis [4, 18, 19, 20]. This genus is comprised of anaerobic microorganisms that utilize sulfate ions as an electron acceptor in the process of “dissimilatory sulfate reduction” (also known as “dissimilatory anaerobic sulfate respiration”). For this process, SRB also require an exogenous electron donor. The electron donor can be an organic compound (lactate, propionate, butyrate, acetate, ethanol, etc.), though they may also serve as a carbon source for growth [27, 28]. Lactate is universal electron donor and a carbon source [7]. These compounds can be oxidized incompletely to acetate or completely to carbon (IV) oxide and water, depending on the SRB genera present in the bowel.
The presence of sulfate and lactate in the human intestine contributes to intensive bacterial growth and the accumulation of their metabolites: acetate and hydrogen sulfide. Hydrogen sulfide is toxic, mutagenic and carcinogenic in epithelial intestinal cells [12, 13]. There is also a hypothesis that sulfate-reducing bacteria can cause some forms of cancer of the rectum through the formation of hydrogen sulfide.
One of the main factors that drives disease pathogenesis is increased intestinal SRB and their production of hydrogen sulfide. This is in agreement with the results of our research, where hydrogen sulfide production, biomass accumulation in individuals with colitis and healthy controls clustered in two separate groups. This was also confirmed by our Pearson correlation and linear regression results (Fig. 2–3, Table 1). Acetate production formed a single cluster with healthy and colitis affected individuals, thus suggesting that acetate production cannot be the main contributing factor in bowel disease development. On the other hand, acetate is thought to synergize with hydrogen sulfide increase the risk of IBD development. From our results, it is unlikely that sulfate consumption and lactate oxidation are responsible factors for illness development. Interestingly, in an assay in which DNA repair is inhibited, Attene-Ramos et al. (2006) showed that Na2S above of 500 μmol/L is able to induce genomic DNA damage in HT-29-Cl.16E colonic cells [16].
It should be noted that previous work has indicated that the physiological properties of intestinal sulfate-reducing bacteria (kinetic characteristics) isolated from mice with colitis are higher in comparison with healthy samples [21, 29]. Our results are clearly in agreement with this same observation (Table 2)
Positive or negative attributes of sulfide are described by following parameters [29]: I) sulfide concentration inside the colonic lumen and thus substrate availability from exogenous (alimentary) and endogenous origins and metabolic capacity for the microbiota to produce H2S, II) percentage of sulfide in free and bound forms, III) capacity of colonic epithelial cells to detoxify and to use sulfide as an energy source, as well as the availability of anaerobic metabolic pathways (i.e., glycolysis) for energy production when mitochondrial oxygen consumption is impaired, IV) capacity of colonic epithelial cells to rapidly adapt to excessive luminal sulfide production. The metabolic pathway of sulfate reduction in SRB is depicted in Fig. 4, as published by Kushkevych et al. (2016) [30]. However, it is clear that a better understanding of the pathways involved in H2S metabolism in colonocytes is still needed. According to previous work it is likely that imbalances between the concentration of free sulfide in the large intestinal mucosa and the metabolic Achaea capacity of epithelial cells results in a loss of normal oxidative cell capacity and epithelial cell renewal, with possible consequences for the process of mucosal inflammation and/or relapse risk. Recent works have also emphasized that endogenously produced H2S plays a role in the neuromodulation of chloride secretion, influencing intestinal contractility and large intestine nociception. It has yet to be shown whether H2S acts as a pro- or antinociceptive agent in the large intestine. It is possible that mammalian cells use the very simple sulfur-containing gas molecule due to are function as indicated by metabolic studies with bacteria and marine animals living in sulfur-containing rich environments [31].
Fig. 4.
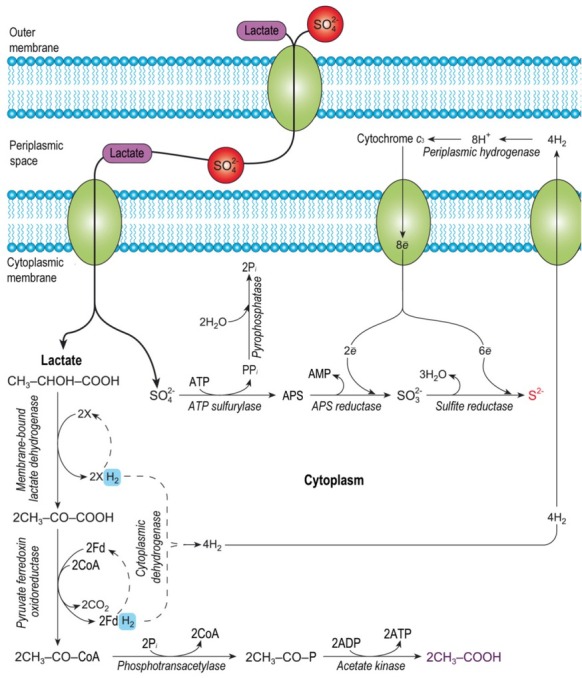
The model of the dissimilatory sulfate reduction in intestinal sulfate-reducing bacteria of Desulfovibrio species (modified from Kushkevych 2016) [29]
Alternatively, many bacterial genera and species are intertwined in the human microbiome, and in healthy individuals they establish metabolic equilibrium. The problem arises when metabolic equilibrium is not formed, and dysbiosis occurs and thus leads to the development of IBD. Intestinal SRB, including the Desulfovibrio sp., are detected not only in feces but also in the samples of colonic biopsies. Studies using Desulfovibrio indonesiensis as a model mono-culture have shown interactions between SRB and the human colon and intestinal epithelial cells, and in co-culture with an E. col isolate, as well as SRB consortia from human biopsy samples. Coutinho et al. (2017) emphasized that the influence of SRB on inflammation formation is higher in the presence of E. coli, and that these interactions are not observed in healthy individuals. Certainly, these findings further highlight the importance of SRB in IBD development [32].
5. Conclusions
Defining the role of intestinal SRB in colonic conditions is important to better understand their ability to inhibit and/or reduce the production of sulfide and acetate. Overall, our findings help describe the factors that influence sulfide production in the human and animal colon.
In summary, our research supports the conclusion that SRB biomass accumulation and hydrogen sulfide production are important factors in IBD development, including UC, and could be utilized as risk indicators of IBD. Although acetate production can synergize with H2S, it was shown to exert a lesser effect. Similarly, sulfate consumption and lactate oxidation also likely represent negligible factors in the development of IBD as well.
Acknowledgements
This study was supported by University of Veterinary and Pharmaceutical Sciences Brno (project IGA VFU 303/2018/FaF).
Footnotes
Conflict of interest: Authors state no conflict of interest.
References
- [1].Hirano A, Umeno J, Okamoto Y, Shibata H, Ogura Y, Moriyama T. Comparison of the microbial community structure between inflamed and non-inflamed sites in patients with ulcerative colitis. J Gastroenterol Hepatol. 2018 doi: 10.1111/jgh.14129. Feb 20. [DOI] [PubMed] [Google Scholar]
- [2].Gibson GR, Cummings JH, Macfarlane GT. Growth and activities of sulphate-reducing bacteria in gut contents of health subjects and patients with ulcerative colitis. FEMS Microbiol Ecol. 1991;86:103–112. [Google Scholar]
- [3].Gibson GR, Macfarlane S, Macfarlane GT. Metabolic interactions involving sulphate-reducing and methanogenic bacteria in the human large intestine. FEMS Microbiol Ecol. 1993;12:117–125. [Google Scholar]
- [4].Kushkevych I, Kollar P, Suchy P, Parak K, Pauk K, Imramovsky A. Activity of selected salicylamides against intestinal sulfate-reducing bacteria. Neuroendocrinol Lett. 2015;36:106–113. [PubMed] [Google Scholar]
- [5].Figliuolo VR, Dos Santos LM, Abalo A, Nanini H, Santos A, Brittes NM. Sulfate-reducing bacteria stimulate gut immune responses and contribute to inflammation in experimental colitis. Life Sci. 2017;189:29–38. doi: 10.1016/j.lfs.2017.09.014. [DOI] [PubMed] [Google Scholar]
- [6].Håkansson Å, Tormo-Badia N, Baridi A, Xu J, Molin G, Hagslätt ML. Immunological alteration and changes of gut microbiota after dextran sulfate sodium (DSS) administration in mice. Clin Exp Med. 2015;15:107–120. doi: 10.1007/s10238-013-0270-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Barton LL, Hamilton WA. Sulphate-Reducing Bacteria. Environmental and Engineered Systems. Cambridge University Press; 2010. [Google Scholar]
- [8].Cummings JH, Macfarlane GT, Macfarlane S.. Intestinal Bacteria and Ulcerative Colitis. Curr Issues Intest Microbiol. 2003;4:9–20. [PubMed] [Google Scholar]
- [9].Loubinoux J, Mory F, Pereira IA, Le Faou AE. Bacteremia caused by a strain of Desulfovibrio related to the provisionally named Desulfovibrio fairfieldensis. J Clin Microbiol. 2000;38:931–934. doi: 10.1128/jcm.38.2.931-934.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Loubinoux J, Bronowicji JP, Pereira IA. Sulphate-reducing bacteria in human feces and their association with inflammatory diseases. FEMS Microbiol Ecol. 2002;40:107–112. doi: 10.1111/j.1574-6941.2002.tb00942.x. [DOI] [PubMed] [Google Scholar]
- [11].Loubinoux J, Valente FMA., Pereira IAC. Reclassification of the only species of the genus Desulfomonas Desulfomonaspigra as Desulfovibrio piger comb. nov. Int J of System and Evol Microbiol. 2002;52:1305–1308. doi: 10.1099/00207713-52-4-1305. [DOI] [PubMed] [Google Scholar]
- [12].Pitcher MC, Cummings JH. Hydrogen sulphide: a bacterial toxin in ulcerative colitis? Gut. 1996;39:1–4. doi: 10.1136/gut.39.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Rowan FE, Docherty NG, Coffey JC, O’Connell PR. Sulphate-reducing bacteria and hydrogen sulphide in the aetiology of ulcerative colitis. British J Surgery. 2009;96:151–158. doi: 10.1002/bjs.6454. [DOI] [PubMed] [Google Scholar]
- [14].Kushkevych I, Kollar P., Ferreira AL, Palma D. Antimicrobial effect of salicylamide derivatives against intestinal sulfate-reducing-bacteria. J Appl Biomed. 2016;14:125–130. [Google Scholar]
- [15].Kushkevych I, Vítězová M, Kos J, Kollár P, Jampílek J. Effect of selected 8-hydroxyquinoline-2-carboxanilides on viability and sulfate metabolism of Desulfovibrio piger. J. App Biomed. 2018;16:1–6. [Google Scholar]
- [16].Attene-Ramos MS, Wagner ED, Plewa MJ, Gaskins HR.. Evidence that hydrogen sulfide is a genotoxic agent. Mol Cancer Res. 2006;4:9–14. doi: 10.1158/1541-7786.MCR-05-0126. [DOI] [PubMed] [Google Scholar]
- [17].Kovac J, Kushkevych I. New modification of cultivation medium for isolation and growth of intestinal sulfate-reducing bacteria. ProceedofInternat PhD Stud Conf MendelNet. 2017. p. 702–707.
- [18].Kushkevych IV. Kinetic Properties of Pyruvate Ferredoxin Oxidoreductase of Intestinal Sulfate-Reducing Bacteria Desulfovibrio piger Vib-7 and Desulfomicrobiumsp. Rod-9. Polish J Microbiol. 2015;64:107–114. [PubMed] [Google Scholar]
- [19].Kushkevych I, Fafula R, Parak T, Bartos M. Activity of Na+/ K+-activated Mg2+-dependent ATP hydrolase in the cell-free extracts of the sulfate-reducing bacteria Desulfovibrio piger Vib-7 and Desulfomicrobium sp. Rod-9. Acta Vet Brno. 2015;84:3–12. [Google Scholar]
- [20].Kushkevych IV. Activity and kinetic properties of phosphotransacetylase from intestinal sulfate-reducing bacteria. Acta Biochemica Polonica. 2015;62:1037–108. doi: 10.18388/abp.2014_845. [DOI] [PubMed] [Google Scholar]
- [21].Kushkevych I, Vítězová M, Fedrová M, Vochyanová Z, Paráková L, Hošek J.. Kinetic properties of growth of intestinal sulphate-reducing bacteria isolated from healthy mice and mice with ulcerative colitis. Acta Vet Brno. 2017;86:405–411. [Google Scholar]
- [22].Kushkevych IV. Identification of sulfate-reducing bacteria strains of human large intestine. Stud Biol. 2013;7:115–124. [Google Scholar]
- [23].Postgate JR. The sulfate-reducing bacteria. Cambridge University Press; Cambridge: 1984. [Google Scholar]
- [24].Kolmert A, Wikstrom P, Hallberg KB. A fast and simple turbidimetric method for the determination of sulfate in sulfate-reducing bacterial cultures. J Microbiol Methods. 2000;41:179–184. doi: 10.1016/s0167-7012(00)00154-8. [DOI] [PubMed] [Google Scholar]
- [25].Cline JD. Spectrophotometric determination of hydrogen sulfide in natural water. Limnol and Ocean. 1969;14:454–458. [Google Scholar]
- [26].Bailey NTJ. Statistical Methods in Biology. Cambridge University Press; 1995. [Google Scholar]
- [27].Kushkevych I, Vítězová M, Vítěz T, Bartoš M.. Production of biogas: relationship between methanogenic and sulfate-reducing microorganisms. Open Life Sciences. 2017;12:82–91. [Google Scholar]
- [28].Kushkevych I, Kováč J, Vítězová M, Vítěz T, Bartoš M.. The diversity of sulfate-reducing bacteria in the seven bioreactors. Arch Microbiol. 2018;200(6):945–950. doi: 10.1007/s00203-018-1510-6. [DOI] [PubMed] [Google Scholar]
- [29].Kushkevych I, Dordević D, Vítězová M, Kollár P.. Cross-correlation analysis of the Desulfovibrio growth parameters of intestinal species isolated from people with colitis. Biologia. 2018;73(11):1137–1143. [Google Scholar]
- [30].Kushkevych IV. Dissimilatory sulfate reduction in the intestinal sulfate-reducing bacteria. Studia Biologica. 2016;10:197–228. [Google Scholar]
- [31].Griesbeck C, Schutz M, Schodl T, Bathe S, Nausch L, Mederer N. Mechanism of sulfide-quinone reductase investigated using site-directed mutagenesis and sulfur analysis. Biochemistry. 2002;41:11552–11565. doi: 10.1021/bi026032b. [DOI] [PubMed] [Google Scholar]
- [32].Coutinho CMLM., Coutinho-Silva R, Zinkevich V, Pearce CB, Ojcius DM, Beech I. Sulphate-reducing bacteria from ulcerative colitis patients induce apoptosis of gastrointestinal epitheli-alcells. Microbial pathogenesis. 2017;112:126–134. doi: 10.1016/j.micpath.2017.09.054. [DOI] [PubMed] [Google Scholar]


