Abstract
Background
To analyze the application value of apparent diffusion coefficient (ADC) and exponent apparent diffusion coefficient (EADC) in evaluating the efficacy of radiation and chemotherapy in cervical cancer using pre- and posttreatment diffusion-weighted magnetic resonance imaging (DW-MRI) scans.
Methods
52 patients with cervical cancer were administered radiation and chemotherapy. Both MRI and DW-MRI were obtained at different stages. The ADC and EADC values, as well as the maximum tumor diameter, were measured and analyzed.
Results
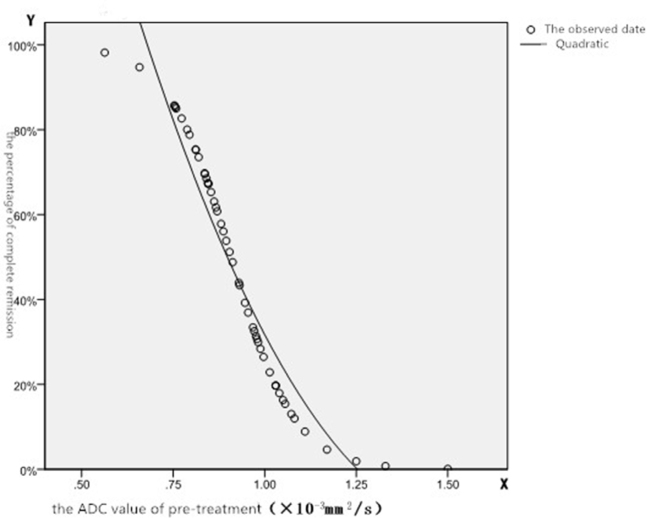
We found that the ADC value increased after treatment, and the EADC value decreased. Changes in the calculated ADC occurred earlier than the morphologic changes of the tumors. A negative correlation was detected between reduced rates in the maximum tumor diameter two months after treatment and pretreatment ADC value (r = –0.658, P < 0.05). An ROC curve and nonlinear regression analysis showed that the formula, y = (1525500.122x2 – 4689.962x + 3.482) × 100%, can be used to calculate the percentage of complete remission after treatment according to the pretreatment ADC value.
Conclusion
Our data suggest that pretreatment ADC and EADC values are predictive of the efficacy of radiation and chemotherapy. Both ADC and EADC values during treatment were instrumental in early monitoring and dynamic observation.
Keywords: Cervical Cancer, Diffusion-Weighted Imaging (DWI), Radiation, Chemotherapy
1. Introduction
Cervical cancer is one of the most common malignancies of the genital tract in women. With the implementation of cervical screening programs, such as the Pap test, and HPV DNA test, the incidence of cervical cancer in developed countries has decreased; however, it remains the third most common cancer in developing countries [1]. Persistent, recurrent, and metastatic cervical cancers have a poor prognosis, so cervical cancer is still a major cause of morbidity and mortality for women [2]. Multiple therapeutic modalities have been used to treat cervical cancers, including surgery, concurrent chemoradiation, targeted therapy, cell-based therapy, combined siRNA, chemotherapy, and immunotherapy [3]. In general, early-stage cervical cancer is mainly treated with surgery; however, concomitant radiochemotherapy is typically the first choice for intermediate-stage and terminal cervical cancers [4, 5].
In fact, radioresistance and drug resistance are major obstacles for patients with cervical cancer. By enhancing sensitivity, it is hoped that lower doses of radiation and drugs, will increase the survival rate and improve the quality of life [4]. A means of forecasting and monitoring the radio- and chemosensitivity for cervical cancers has become an intense area of clinical research. Studies have shown that radiosensitivity of cervical cancer is related to the apoptosis of cervical cancer cells, and mainly depends on the balance between pro- and antiapoptotic proteins. The expression of Bcl2, Bax, and p53 in cancer cells is related to the sensitivity to radiation therapy [4, 6,7,8]. Tumor hypoxia is also associated with radioresistance, suggesting that monitoring and manipulating the level of tumor hypoxia would be helpful for the treatment of cervical cancer and prediction of the prognosis [9,9,10,11]. Temperature also influenced the treatment effect of cancers. Interestingly, hypothermia enhanced human tumor cell radiosensitivity [12].
Studies have shown that the diffusion properties of water vary in areas of necrosis, high cellularity, inflammation, and fibrosis. Based on this mechanism, quantitative analysis of water diffusion in diseased tissues would be helpful to diagnose and predict the prognosis of diseases. Diffusion-weighted imaging (DWI) is an MRI technique widely used to quantify the movement of water molecules at a cellular level [13, 14]. Recent studies have shown that DWI could be used for the detection cancers, staging and follow-up after treatment [15, 16]. DWI can also be used to differentiate true progression and pseudoprogression of tumors after chemoradiotherapy [17, 18]. In this study, we explored the predictive value of DWI on the efficacy of radiation and chemotherapy in patients with cervical cancer.
2. Materials and Methods
2.1. Study patients
Patients with cervical cancer (n = 52) were administered concomitant radiochemotherapy in the Affiliated Hospital of Qingdao University from January 2012 to November 2014. Cervical cancers included squamous carcinoma (n = 48) and adenocarcinoma (n = 4). The average patient age was 53.3 ± 2.4 years (range, 37-75 years). The average maximum diameter was 47.2 ± 3.8 mm (range, 23-89 mm). Patients who underwent treatment prior to admission were excluded from this study.
Informed consent: Informed consent has been obtained from all individuals included in this study.
Ethical approval: The research related to human use has been complied with all the relevant national regulations, institutional policies and in accordance the tenets of the Helsinki Declaration, and has been approved by the authors’ institutional review board or equivalent committee.
2.2. Radiotherapy planning and chemotherapy regimen
All patients received concurrent chemoradiotherapy. The chemotherapy regimen was a TP (Taxol+ Cisplatin) scheme based on platinum. In detail, Cisplatin (50-70mg/m2) and Taxol aclitaxel (135-175mg/m2) were administered through intravenous drip for one to two courses. The treatment course was 4 weeks. The radiation regimen (5 times per week and 2 Gy per dosing) lasted for two weeks and the total dosage was 20 Gy.
2.3. MRI and DWI
Imaging patients was performed using a GE Signa 3.0T MR. Scanning and DWI examinations were performed at different stages, including pretreatment, 15 days after treatment, and at 1 and 2 months following treatment. Patients fasted 8–12 h before the examination. Scanning areas included the superior border of the ilium to the lower margin of the pubic bone. Imaging parameters were as follows: axial T1W TR, 300–600 ms; TE, 2–20 ms; T2W TR, 2000–8000 ms; TE, 80–150 ms; coronal T2W TR, 2000–8000 ms; TE, 80–150 ms; axial DWI TR, 2000–8000 ms; TE, 80–150 ms; depth, 6 mm; interlayer spacing, 2 mm; matrix, 230 × 256; FOV, 40 × 40; NEX, 4 times; and b value, 0.800 s/mm2.
2.4. Imaging post-processing and measurement
The original data were uploaded to an AW 4.3 workstation and Functool software was used for further analysis. According to T2WI and DWI imaging, signal homogeneity for the ADC was selected as the region of interest (ROI), and the ADC and EADC values were measured. Based on the response evaluation criteria in solid tumors (RECIST), cases were divided into complete remission (CR), partial remission (PR), and stable disease (SD) [19].
2.5. Statistical analyses
Two independent sample t-test, random analysis of variance, and a Pearson correlation analysis between the 2 variables were used to analyze the data using SPSS 19.0. A p value of <0.05 was regarded as significant.
3. Results
3.1. Prediction of ADC for treatment efficacy in cervical cancer
Two months after treatment, MR scans showed that tumors had completely disappeared in 9 patients (CR group); partial remission was observed in 42 patients (PR group, at least a 30% decrease in the maximum tumor diameter). Less than a 30% decrease was observed in 1 patient (SD group). We did not analyze the SD group due to the small number of samples. The ADC and EADC values for the different time periods (pretreatment, 15 days after treatment, 1 month after treatment, and 2 months after treatment) are shown in Table 1. There were significant differences observed in the ADC and EADC values between the CR and PR groups before treatment (p < 0.05). However, no differences in ADC and EADC values were seen between CR and PR groups during and posttreatment scan. In both groups, the ADC value increased after treatment, but the EADC value decreased. The maximum ADC value in the CR group before treatment was 0.895 × 10−3 mm2/s. The minimum EADC value in the CR group before treatment was 0.489. The minimum ADC value in the PR group before treatment was 0.754 × 10−3 mm2/s. The maximum EADC value in the PR group before treatment was 0.547.
Table 1.
ADC value and EADC value in different stages of treatment
| Stages | CR Group | PR Group | ||
|---|---|---|---|---|
| ADC value (×10-3mm2/s) | EADC value | ADC value (×10-3mm2/s) | EADC value | |
| Pretreatment | 0.764±0.073 | 0.546±0.033 | 0.986±0.049 | 0.457±0.018 |
| 15 days after treatment | 1.703±0.044** | 0.258±0.009* | 1.577±0.047** | 0.289±0.012** |
| One month after treatment | 1.730±0.024** | 0.251±0.005* | 1.674±0.025** | 0.261±0.005** |
| Two month after treatment | 1.799±0.035** | 0.237±0.007* | 1.752±0.009** | 0.246±0.002** |
VS before treatment
p<0.01
p<0.05
Figure 1.
Relationship between the probability of CR and pretreatment ADC value
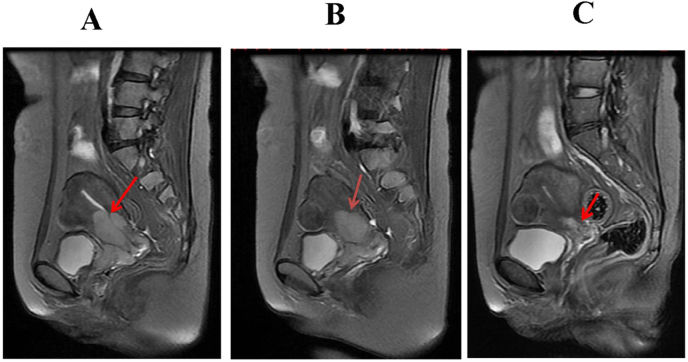
Figure 2.
Representative image of cervical squamous cell carcinoma, 45-year-old female; A, B, and C are T2WI images of the patient in a sagittal position before treatment, 15 days after treatment, and 2 months after treatment, respectively. The tumor was noticeably reduced.
An ROC curve analysis for the ADC is shown in Table 2. The value of the state variable was selected from the PR group. After ROC curve analysis, the maximum point for Youden’s index (YI) was analyzed using the ROC curve and was designated as the diagnosis point. The ADC value at this point was 0.891 × 10−3 mm2/s, and the YI was 0.794. If the ADC value was less than, or equal to the diagnosis point, it implied that the tumor would completely disappear after treatment. If the ADC value was greater than the diagnosis point, it implied that a CR would be difficult to achieve for a patient with this tumor.
Table 2.
ROC curve analysis of ADC
| Parameter | AUC | Standard error | P value | 95% confidence intervals | Threshold (×10-3mm2/s) | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|---|---|
| ADC | 0.902 | 0.046 | 0.000 | 0.812~0.991 | 0.891 | 84.4 | 95.0 |
The significance of the ADC value in the CR and PR groups was assessed in a discriminant analysis, curve estimation, and nonlinear regression analysis to determine the probability that the ADC value can help predict the CR of tumors. The formula, y = (1525500.122x2 – 4689.962x + 3.482) × 100%, can be used to calculate the percentage of CR after treatment according to the pretreatment ADC value. X represents the pretreatment ADC value (mm2/s). Y represents the percentage of CR. The standard error was 0.079, and the probability of PR was equal to 1 – Y.
3.2. Correlation between tumor diameter and the ADC and EADC values
The average value of the maximum tumor diameter at different stages is shown in Table 3. There was no apparent difference between 15 days after treatment and pretreatment. However, after 1 month and 2 months, the maximum tumor diameter had decreased. The ADC value increased after treatment and the EADC value decreased. The difference in the ADC and EADC values was identified 15 days after treatment and morphological changes occurred after the ADC and EADC values had changed. The average ADC value for the 52 patients before treatment was (0.944 ± 0.046) × 10−3 mm2/s. After 2 months, the decreased ratio of the maximum tumor diameter was 85.69 ± 2.38%. A negative correlation was observed between the decreased maximum tumor diameter 2 months after treatment and pretreatment ADC value (r = –0.658, P < 0.05). The increase in ADC values was 40.24 ± 3.24% and 44.03 ± 2.51% at 15 days and 1 month after treatment, respectively. The ADC values were positively correlated with the reduction ratio of the maximum tumor diameter after 2 months (r = 0.783, p = 0.000; r = 0.676, p = 0.001, respectively).
Table 3.
ADC value, EADC value and the maximum tumor diameter
| Stages | ADC value (×10-3mm2/s) | EADC value | Maximum tumor diameter (mm) |
|---|---|---|---|
| Pretreatment | 0.944±0.046 | 0.474±0.017 | 47.21±3.83 |
| 15 days after treatment | 1.601±0.040** | 0.283±0.010** | 46.86±3.77 |
| One month after treatment | 1.685±0.097** | 0.259±0.004** | 21.57±1.50** |
| Two month after treatment | 1.761±0.047** | 0.244±0.002** | 6.48±1.09** |
VS before treatment p<0.01
4. Discussion
Radiological imaging techniques are widely used to evaluate the posttreatment tumor response; however, this evaluation is dependent on the reduction in tumor size after treatment [20].
Interestingly, the increased use of targeted therapies in advanced cancer, which typically induces necrosis and cavitation, does not consistently reduce tumor size. Thus, traditional anatomical methods are not efficient to evaluate treatment efficacy [21]. DWI is a newly developed MRI technique that provides functional information regarding the behavior of water molecules in tissues [22]. Studies have shown that DWI holds promise in cancer diagnosis and outcome prediction [23,24,25]. In cervical cancer, DWI improved the prediction of disease progression after concurrent chemoradiotherapy (CCRT), along with T2WI [26]. As a promising tool for functional imaging, studies of cell and animal models also showed that DWI has the potential to help predict the effect of different treatments on tumors [27,28,29,30].
In this study, we found that the ADC value in cervical cancer was increased 15 days after treatment and that the EADC value was decreased. However, the maximum tumor diameter had no apparent changes, indicating that the ADC and EADC values changed before the tumor volume changed. After 15 days, there was a negative correlation between the decreased maximum tumor diameter and ADC value, suggesting that the ADC value is also an indicator of tumor diameter. In our study, the results also showed there were significant differences in the ADC and EADC values between the CR and PR groups before treatment. In fact, CR and PR groups were grouped according to the extent of tumor shrinkage, but not histopathology. There were no differences of ADC and EADC values between CR and PR groups during and posttreatment scan. This inconsistency may be related to the histopathology.
The theoretical basis of DWI in distinguishing between normal and tumor tissues lies within the difference between water diffusion in the different tissues [28]. The density of cells is also related to DWI signals and ADC values [31]. Naganawa et al. showed that the average ADC value for cervical lesions and normal cervical tissues was 1.09 ± 0.20 × 10−3 and 1.79 ± 0.24 × 10−3 mm2/s, respectively [32]. Malignant cells proliferate robustly, and the water content inside and outside cells is increased. Protein adsorption of water is enhanced, and the volume of cells is consequently increased, and the effective movement of water in tumors is limited. Thus, the DWI signal increases, and the ADC value decreases [33]. After effective treatment, cells die or undergo apoptosis. As the integrity of the cell membrane disappears, extracellular space is increased and the density of cells decreases. The DWI signal decreased, and the ADC value increased [34]. Killing of malignant cells and protection of normal cells is the purpose of treatment. Certainly, functional changes precede morphologic changes [35], and early assessment is feasible with DWI before changes are apparent in the tumor size. Since the ADC value of tumors is linked to their growth rates and remission after treatment, it can be used to predict the sensitivity of tumors to treatment [36]. Following treatment, the ADC value of the cervical tumors returns to normal[32]. DWI can be easily assessed and is more rapid. Combined with other methods, the ADC value certainly has a significant clinical value for the early monitoring of cervical cancers [37, 38]
In this study, we also analyzed the maximum point of YI using an ROC curve that was defined as the diagnosis point. The ADC value at this point was 0.891 × 10−3 mm2/s and the YI was 0.794. If the ADC value was less than or equal to the diagnosis point, it implied that the tumor would completely disappear after treatment. If the ADC value was greater than the diagnosis point, it implied that CR would be difficult to achieve. To further analyze the probability that the ADC value could predict the ability of a tumor to achieve a CR, the significant ADC value in the CR and PR groups was assessed using a discriminant analysis, curve estimation and nonlinear regression. The derived formula, y = (1525500.122x2- 4689.962x + 3.482) × 100%, could be used to calculate the likelihood of CR after treatment according to the pretreatment ADC value. A standard error of 0.079 was calculated. According to the formula, the probability of CR could be determined. It holds great value in predicting the sensitivity of cervical cancers to radiation and chemotherapy. In fact, Meng J et al reported that pre- and mid-treatment whole-lesion ADC histogram and texture analysis hold great potential in predicting tumor recurrence of advanced cervical cancer treated with concurrent chemo-radiotherapy [39]. Certainly, our results and other studies suggest ADC value and EADC values are useful for the prediction of cervical cancer treatment efficacy and recurrence.
Although there were limitations in this study, including the small number of samples, and subjectivity in measuring the diameter of tumors, our data warrant further study using a larger number of samples and optimized measuring criteria to explore a new grouping standard. Indeed, our data provide strong evidence for the quantitative prognostic value of ADC and EADC during cervical cancer treatment.
5. Conclusion
In this study, we observed that changes in ADC values preceded changes that occurred in tumors after treatment. We also found that pretreatment ADC and EADC values could be used to predict the efficacy of radiation and chemotherapy. The results from our study further underscore the instrumental use of ADC and EADC values during treatment during early monitoring and dynamic observation.
Footnotes
Conflict of interest: Authors state no conflict of interest.
References
- [1].Tsikouras P, Zervoudis S, Manav B, Tomara E, Iatrakis G, Romanidis C. Cervical cancer: screening, diagnosis and staging. J BUON. 2016;21(2):320. et al. –. [PubMed] [Google Scholar]
- [2].Bizzarri N, Ghirardi V, Alessandri F, Venturini PL, Valenzano MM. Bevacizumab for the treatment of cervical cancer. Expert Opin Biol Ther. 2016;16(3):407. doi: 10.1517/14712598.2016.1145208. –. [DOI] [PubMed] [Google Scholar]
- [3].Yee GP, de Souza P, Khachigian LM.. Current and potential treatments for cervical cancer. Curr Cancer Drug Targets. 2013;13(2):205. doi: 10.2174/1568009611313020009. –. [DOI] [PubMed] [Google Scholar]
- [4].Qin C, Chen X, Bai Q, Davis MR, Fang Y. Factors associated with radiosensitivity of cervical cancer. Anticancer Res. 2014;34(9):4649. –. [PubMed] [Google Scholar]
- [5].Atahan IL, Onal C, Ozyar E, Yiliz F, Selek U, Kose F. Long-term outcome and prognostic factors in patients with cervical carcinoma: a retrospective study. Int J Gynecol Cancer. 2007;17(4):833. doi: 10.1111/j.1525-1438.2007.00895.x. –. [DOI] [PubMed] [Google Scholar]
- [6].Kitahara O, Katagiri T, Tsunoda T, Harima Y, Nakamura Y. Classification of sensitivity or resistance of cervical cancers to ionizing radiation according to expression profiles of 62 genes selected by cDNA microarray analysis. Neoplasia. 2002;4(4):295. doi: 10.1038/sj.neo.7900251. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ohno T., Nakano T, Niibe Y, Tsujii H, Oka K. Bax protein expression correlates with radiation-induced apoptosis in radiation therapy for cervical carcinoma. Cancer. 1998;83(1):103. –. [PubMed] [Google Scholar]
- [8].Mukherjee G, Freeman A, Moore R, Kumaraswamy Devi KU, Morris LS. Biologic factors and response to radiotherapy in carcinoma of the cervix. Int J Gynecol Cancer. 2001;11(3):187. doi: 10.1046/j.1525-1438.2001.01014.x. et al. –. [DOI] [PubMed] [Google Scholar]
- [9].Harrison L, Blackwell K. Hypoxia and anemia: factors in decreased sensitivity to radiation therapy and chemotherapy? Oncologist. 2004;9(5):31. doi: 10.1634/theoncologist.9-90005-31. –. [DOI] [PubMed] [Google Scholar]
- [10].Suzuki Y, Nakano T, Ohno T, Kato S, Niibe Y, Morita S. Oxygenated and reoxygenated tumors show better local control in radiation therapy for cervical cancer. Int J Gynecol Cancer. 2006;16(1):306. doi: 10.1111/j.1525-1438.2006.00341.x. et al. –. [DOI] [PubMed] [Google Scholar]
- [11].Dehdashti F, Grigsby PW, Mintun MA, Lewis JS, Siegel BA, Welch MJ. Assessing tumor hypoxia in cervical cancer by positron emission tomography with 60Cu-ATSM: relationship to therapeutic response-a preliminary report. Int J Radiat Oncol Biol Phys. 2003;55(5):1233. doi: 10.1016/s0360-3016(02)04477-2. –. [DOI] [PubMed] [Google Scholar]
- [12].Burton SA, Paljug WR, Kalnicki S. Hypothermia-enhanced human tumor cell radiosensitivity. Cryobiology. 1997;35(1):70. doi: 10.1006/cryo.1997.2027. Werts ED. –. [DOI] [PubMed] [Google Scholar]
- [13].Boone D, Taylor SA, Halligan S.. Diffusion weighted MRI: overview and implications for rectal cancer management. Colorectal Dis. 2013;15(6):655. doi: 10.1111/codi.12241. –. [DOI] [PubMed] [Google Scholar]
- [14].Wilhelm T, Stieltjes B. Whole-body-MR-diffusion weighted imaging in oncology. Rofo. 2013;184(10):950. doi: 10.1055/s-0033-1335428. Schlemmer HP. –. [DOI] [PubMed] [Google Scholar]
- [15].Malayeri AA, El Khouli RH, Zaheer A, Jacobs MA, Corona-Villalobos CP, Kamel IR. Principles and applications of diffusion-weighted imaging in cancer detection, staging, and treatment follow-up. Radiographics. 2011;31(6):1773. doi: 10.1148/rg.316115515. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Cao K, Gao M, Sun YS, Li YL, Sun Y, Gao YN. Apparent diffusion coefficient of diffusion weighted MRI in endometrial carcinoma-Relationship with local invasiveness. Eur J Radiol. 2012;81(8):1926. doi: 10.1016/j.ejrad.2011.04.019. et al. –. [DOI] [PubMed] [Google Scholar]
- [17].Lee WJ, Choi SH, Park CK, Yi KS, Kim TM, Lee SH. Diffusion-weighted MR imaging for the differentiation of true progression from pseudoprogression following concomitant radiotherapy with temozolomide in patients with newly diagnosed high-grade gliomas. Acad Radiol. 2012;19(11):1353. doi: 10.1016/j.acra.2012.06.011. et al. –. [DOI] [PubMed] [Google Scholar]
- [18].Yoo RE, Choi SH, Kim TM, Lee SH, Park CK, Park SH. Independent Poor Prognostic Factors for True Progression after Radiation Therapy and Concomitant Temozolomide in Patients with Glioblastoma: Subependymal Enhancement and Low ADC Value. AJNR Am J Neuroradiol. 2015;36(10):1846. doi: 10.3174/ajnr.A4401. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228. doi: 10.1016/j.ejca.2008.10.026. et al. –. [DOI] [PubMed] [Google Scholar]
- [20].Marcus CD, Ladam-Marcus V, Cucu C, Bouché O, Lucas L, Hoeffel C. Imaging techniques to evaluate the response to treatment in oncology: current standards and perspectives. Crit Rev Oncol Hematol. 2009;72(3):217. doi: 10.1016/j.critrevonc.2008.07.012. –. [DOI] [PubMed] [Google Scholar]
- [21].Heijmen L, Verstappen MC, Ter Voert EE, Punt CJ, Oyen WJ, de Geus-Oei LF. Tumour response prediction by diffusion-weighted MR imaging: ready for clinical use? Crit Rev Oncol Hematol. 2012;83(2):194. doi: 10.1016/j.critrevonc.2011.12.008. et al. –. [DOI] [PubMed] [Google Scholar]
- [22].Ibrahiem EI, Mohsen T, Nabeeh AM, Osman Y, Hekal IA, Abou El-Ghar M. DWI-MRI: single, informative, and noninvasive technique for prostate cancer diagnosis. Scientific World Journal. 2012 doi: 10.1100/2012/973450. 2012:973450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hauser T, Essig M, Jensen A, Laun FB, Münter M, Maier-Hein KH. Prediction of treatment response in head and neck carcinomas using IVIM-DWI: Evaluation of lymph node metastasis. Eur J Radiol. 2014;83(5):783. doi: 10.1016/j.ejrad.2014.02.013. et al. –. [DOI] [PubMed] [Google Scholar]
- [24].Lei J, Tian Y, Zhu SC, Han Q, Wei Y, Yang S. Preliminary study of IVIM-DWI and DCE-MRI in early diagnosis of esophageal cancer. Eur Rev Med Pharmacol Sci. 2015;19(18):3345. et al. –. [PubMed] [Google Scholar]
- [25].Joye I, Deroose CM, Vandecaveye V. The role of diffusion-weighted MRI and (18)F-FDG PET/CT in the prediction of pathologic complete response after radiochemotherapy for rectal cancer: a systematic review. Radiother Oncol. 2014;113(2):158. doi: 10.1016/j.radonc.2014.11.026. Haustermans K. –. [DOI] [PubMed] [Google Scholar]
- [26].Park JJ, Kim CK, Park BK. Prediction of disease progression following concurrent chemoradiotherapy for uterine cervical cancer: value of post-treatment diffusion-weighted imaging. Eur Radiol. 2016;26(9):3272. doi: 10.1007/s00330-015-4156-7. –. [DOI] [PubMed] [Google Scholar]
- [27].Heo SH, Shin SS, Kim JW, Lim HS, Jeong YY, Kang WD. Pre-treatment diffusion-weighted MR imaging for predicting tumor recurrence in uterine cervical cancer treated with concurrent chemoradiation: value of histogram analysis of apparent diffusion coefficients. Korean J Radiol. 2013;14(4):616. doi: 10.3348/kjr.2013.14.4.616. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].She D, Yang X, Xing Z, Cao D. Differentiating Hemangioblastomas from Brain Metastases Using Diffusion-Weighted Imaging and Dynamic Susceptibility Contrast-Enhanced Perfusion-Weighted MR Imaging. AJNR Am J Neuroradiol. 2016. [DOI] [PMC free article] [PubMed]
- [29].Makino H, Kato H, Furui T, Morishige K. Predictive value of diffusion-weighted magnetic resonance imaging during chemoradiotherapy for uterine cervical cancer. J Obstet Gynaecol Res. 2014;40(4):1098. doi: 10.1111/jog.12276. Kanematsu M. –. [DOI] [PubMed] [Google Scholar]
- [30].Birlik B, Obuz F, Elibol FD, Celik AO, Sokmen S, Terzi C. Diffusion-weighted MRI and MR- volumetry--in the evaluation of tumor response after preoperative chemoradiotherapy in patients with locally advanced rectal cancer. Magn Reson Imaging. 2015;33(2):201. doi: 10.1016/j.mri.2014.08.041. et al. –. [DOI] [PubMed] [Google Scholar]
- [31].Guo AC, Cummings TJ, Dash RC. Lymphomas and high-grade astrocytomas: comparison of water diffusibility and histologic characteristics. Radiology. 2002;224(1):177. doi: 10.1148/radiol.2241010637. Provenzale JM. –. [DOI] [PubMed] [Google Scholar]
- [32].Naganawa S, Sato C, Kumada H, Ishigaki T, Miura S, Takizawa O.. Apparent diffusion coefficient in cervical cancer of the uterus: comparison with the normal uterine cervix. Eur Radiol. 2005;15(1):71. doi: 10.1007/s00330-004-2529-4. –. [DOI] [PubMed] [Google Scholar]
- [33].Xue HD, Li S, Sun F, Sun HY, Jin ZY, Yang JX. Clinical application of body diffusion weighted MR imaging in the diagnosis and preoperative N staging of cervical cancer. Chin Med Sci J. 2008;23(3):133. doi: 10.1016/s1001-9294(09)60027-4. et al. –. [DOI] [PubMed] [Google Scholar]
- [34].Rizzo S, Summers P, Raimondi S, Belmonte M, Maniglio M, Landoni F. Diffusion-weighted MR imaging in assessing cervical tumour response to nonsurgical therapy. Radiol Med. 2011;116(5):766. doi: 10.1007/s11547-011-0650-4. et al. –. [DOI] [PubMed] [Google Scholar]
- [35].Bae JM, Kim CK, Park JJ, Park BK. Can diffusion-weighted magnetic resonance imaging predict tumor recurrence of uterine cervical cancer after concurrent chemoradiotherapy? Abdom Radiol (NY) 2016. [DOI] [PubMed]
- [36].Harry VN, Semple SI, Gilbert FJ. Diffusion-weighted magnetic resonance imaging in the early detection of response to chemoradiation in cervical cancer. Gynecol Oncol. 2008;111(2):213. doi: 10.1016/j.ygyno.2008.07.048. Parkin DE. –. [DOI] [PubMed] [Google Scholar]
- [37].Mahajan A, Engineer R, Chopra S, Mahanshetty U, Juvekar SL, Shrivastava SK. Role of 3T multiparametric-MRI with BOLD hypoxia imaging for diagnosis and post therapy response evaluation of postoperative recurrent cervical cancers. Eur J Radiol Open. 2016;3:22. doi: 10.1016/j.ejro.2015.11.003. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Xie H, Sun T, Chen M, Wang H, Zhou X, Zhang Y. Effectiveness of the apparent diffusion coefficient for predicting the response to chemoradiation therapy in locally advanced rectal cancer: a systematic review and meta-analysis. Medicine (Baltimore) 2015;94(6):e517. doi: 10.1097/MD.0000000000000517. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Meng J, Zhu L, Zhu L, Xie L, Wang H, Liu S. Whole-lesion ADC histogram and texture analysis in predicting recurrence of cervical cancer treated with CCRT.Oncotarget. 2017;8(54):92442. doi: 10.18632/oncotarget.21374. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]