Abstract
Objective
The current study aimed to explore the predictive ability of serum uric acid (SUA) in patients suffering from acute ST segment elevation myocardial infarction (STEMI).
Method
PubMed, EMBASE, Cochrane Library, and Medline databases were systematically searched from their respective inceptions to February 2018. Systematic analysis and random-effects meta–analysis of prognostic effects were performed to evaluate STEMI outcomes [i.e., in-hospital mortality, one-year mortality, in-hospital Major Adverse Cardiovascular Events (MACE)] in relation to SUA.
Results
A total of 12 studies (containing 7,735 patients with acute STEMI) were identified (5,562 low SUA patients and 3,173 high SUA patients). Systematic analysis of these studies showed that high SUA patients exhibited a higher incidence of in-hospital MACE (OR, 2.30; P < 0.00001), in-hospital mortality (OR, 3.03; P < 0.0001), and one-year mortality (OR, 2.58; P < 0.00001), compared with low SUA patients.
Conclusions
Acute STEMI patients with high SUA exhibited an elevated incidence rate of in-hospital MACE, in-hospital mortality, and one-year mortality. Further randomized controlled trials will be needed to verify these results.
Keywords: serum uric acid (SUA), ST segment elevation myocardial infarction (STEMI), mortality, Major Adverse Cardiovascular Events (MACE), meta–analysis
1. Introduction
The effects of serum uric acid (SUA) on the in-hospital and one-year follow-up prognosis in patients with acute ST segment elevation myocardial infarction (STEMI) are debatable, and clinical application of its measurement remains uncertain. Uric acid (UA) is the end–point degradation product of purine nucleotides and can be synthesized by several different types of tissue, including those outside the muscles of the cardiovascular system. Within tissues, UA increases rapidly and is then released into the vascular lumen. Once there, a decrease in intracellular pH and a reversal of negative membrane potential occurs. According to studies, synthesis of UA and the activity of xanthine oxidase both increase in cases of myocardial ischemia [1, 2, 3]. The Thrombolysis in Myocardial Infarction (TIMI) scores can be converted to clinical risk scores to develop prognoses for patients suffering from acute coronary syndrome. An increase or decrease in UA/ xanthine oxidase status is used to determine risk factor [4]. A previous study from our lab discovered that the level of SUA is closely related to patients with acute STEMI [5, 6]. Thus, SUA can be used as a predictor of STEMI in patients. Furthermore, studies have shown that the inclusion of SUA in risk scores increases the accuracy risk prediction. The current study conducted a meta–analysis to explore the difference between high and low SUA in STEMI patients.
2. Methods
2.1. Search methods
PubMed, EMBASE, Ovid Medline, and Cochrane Library databases were systematically searched from their respective inceptions through to February 2018. The following MeSH terms and keywords were included in the search strategy to identify articles in English: “uric acid or UA,” “hyperuricemia,” “acute myocardial infarction or AMI,” “ST segment elevation myocardial infarction or STEMI,” and “Acute coronary syndrome or ACS.”
2.2. Study choice
All studies of STEMI patients with high and low SUA were examined. According to the SUA level, SUA-positive and SUA-negative groups were classified. The positive and negative groups had patients with elevated or normal SUA levels, as defined by each study. Our study enrolled only randomized controlled trials (RCTs) of STEMI patients in which follow-up data of SUA levels were measured during hospitalization. We removed non RCTs, studies covering non-ST segment elevation myocardial infarction (NSTEMI), studies covering unstable angina, and studies without follow-up results. No restrictions were placed on the study results. All of the analyses were based on previously published studies; thus no ethical approval or patient consent was required.
2.3. Data extraction and quality assessment
Data from each study regarding research design, lead author, sample size, research location, clinical baseline characteristics, proportion of percutaneous coronary intervention patients, and follow-up duration were abstracted by three independent reviewers (Wang, H.L., Yang, J.J., and Pang, X.H.). In-hospital MACE, in-hospital mortality, and one-year mortality were assessed. In-hospital MACE was defined as the primary endpoint.
2.4. Statistical analysis
The individual risk of bias for each study was evaluated via Cochrane’s risk assessment tool. Data collation was conducted based upon the PRISMA Statement and guidelines set forth by the Cochrane Collaboration. Meta–analysis was performed using Review Manager 5.1 (RevMan). Heterogeneity between studies was calculated by a chi-square tests of heterogeneity and the I2 statistics of inconsistency. I2 values were 75%, 50%, and 25%, specific to the definition of high, moderate, and low heterogeneity, respectively. The random effect risk ratios (RR) were calculated with 95% confidence intervals (95% CI) for the convenience of individual comparison. A P < 0.05 was set for statistical significance. Test values were two-tailed. Begg’s funnel plot analyses, Begg’s log-rank tests, and Egger’s tests were conducted to evaluate publication bias and small research effects.
3. Results
3.1. Study selection
A total of 1,421 articles were identified involving both UA and acute STEMI from the PubMed, Medline, EMBASE, and Cochrane databases (through February 2018). Of these, 524 repeated articles were excluded, and 852 articles were found to be inappropriate given the goals of the current study. The full texts of 45 studies were carefully reviewed. A total of 12 studies, with a total of 7,357 patients, were analyzed (Figure 1) [7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18]. Table 1 shows baseline characteristics of the analyzed studies and Table 2 presents the population characteristics for each study.
Figure 1.
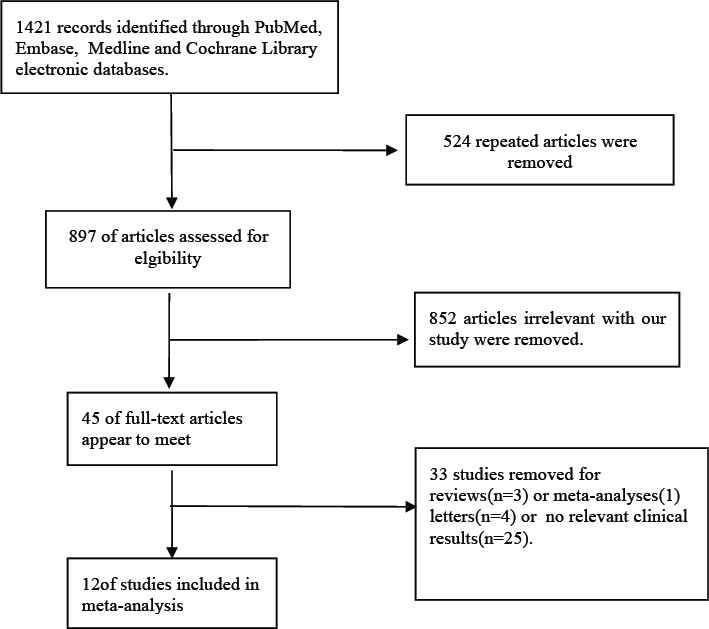
Search strategy conducted for all included trials. Abbreviations: MeSH, medical subject headings.
Table 1.
Baseline characteristics of randomized studies.
| Randomized studies | Year | Sample size | Inclusion criteria | Exclusion criteria | Endpoints | Follow-up period | |
|---|---|---|---|---|---|---|---|
| Low UA | High UA | ||||||
| Basar et al | 2011 | 140 | 45 | All patients with the diagnosis of STEMI within 12 hours from the onset of symptoms,cardiogenic shock within 24 hours. | Patients with culprit lesion in the left main coronary artery, previous CABG, end-stage renal disease, hepatic or hemolytic disorders, concomitant inflammatory diseases, neoplastic diseases, recent major surgical procedures, trauma, and any systemic disorders. | All-cause mortality,Major Adverse Cardiovascular Events. | During hospitalization or one year |
| Wang et al | 2012 | 178 | 98 | Patients with the diagnosis of STEMI within 12 hours from the onset of symptoms undergoing primary PCI. | Patients with hrombolysis treatment within 24 hours, oncomitant inflammatory diseases, autoimmune disorders, neoplastic diseases,liver or kidney failure. | Major Adverse Cardiovascular Events. | During hospitalization |
| Mehmet et al | 2012 | 1643 | 606 | STEMI patients with underwent primary PCI. | PCI was not performed, UA values were missing or unavailable, or no follow-up was documented after primary PCI. | All-cause mortality, Major Adverse Cardiovascular Events. | During hospitalization |
| Li et al | 2012 | 383 | 119 | Consecutive patients with STEMI, given standard treatment. | The patients who had liver and kindey diseases, gout, alcoholism and violent exercise. | All-cause mortality. | During hospitalization |
| Bita et al | 2012 | 127 | 57 | The patients with acute STEMI. | Not receive thrombolytic therapy during the first six hours after the onset of chest pain; cardiogenic shock; previous pacemaker implantation; a recent myocardial infarction (<3 months); severe valvular disease; renal function impaired(serum creatinine level >1.5 mg/dl); cases of hypothyroidism, malignancy, gout or other inflammatory diseases and using corticosteroid or cytotoxic drugs. | All-cause mortality. | During hospitalization |
| Chiara et al | 2012 | 436 | 207 | Consecutive patients with STEMI (within 12 h from symptoms onset)after primary percutaneous coronary intervention (PCI). | no exclusion criteria. | All-cause mortality. | During hospitalization |
| Ozgur et al | 2014 | 291 | 143 | Patients with STEMI, > 30 minutes of continuous typical chest pain, ST-segment elevation / 2 mm in two contiguous electrocardiography leads within 12 hours of symptom onset, or evidence of continuing ischemia or hemodynamic instability for up to 18 hours. | Patients with no indication of PCI , not suitable for PCI, missing or unavailable data about uric acid level upon admission. | All-cause mortality,Major Adverse Cardiovascular Events. | During hospitalization |
| Emine et al | 2014 | 479 | 107 | Patients with STEMI. | patients who had no UA measurements and who had to be sent to another cardiology center for rescue percutaneous transluminal coronary angioplasty (PTCA). | All-cause mortality. | During hospitalization |
| Chiara et al | 2015 | 220 | 109 | Patients with STEMI (within 12 h from symptoms onset), submitted to primary PCI, and eGFR below 60 ml/min/1.73m2. | no exclusion criteria. | All-cause mortality | During hospitalization or one year |
| Reza et al | 2016 | 518 | 90 | Patients with STEMI. | Patients with liver disease, progressive kidney disorders (creatinine >1.8), gout, alcoholism or taking antihyperuricemic drugs. Patients with previous history of diuretic and losartan use, also patients with previous history of MI. | All-cause mortality | During hospitalization |
| Mora-Ramirez et al | 2017 | 504 | 291 | Patients with STEMI,SUA measurement on admission; underwent myocardial reperfusion therapy(thrombolytic therapy or primary percutaneous coronary intervention) within 12 hours of onset. | Patients with current use of uric acid-lowering drugs(e.g. allopurinol, probenecid, benzbromarone) or thiazides, active neoplastic disease,end-stage renal disease with dialyss,history of gouty arthritis or urolithiasis; and missing values in the data registry. | All-cause mortality,Major Adverse Cardiovascular Events. | During hospitalization |
| Cheng-Wei et al | 2017 | 643 | 301 | The STEMI patients who presented to our Emergency Department directly. | patients without definite door-to-balloon time, mainly those who were transferred from another hospital, those who were transferred from our outpatient department, and those who had in-hospital STEMI. | All-cause mortality. | one year |
Table 2.
Patient characteristics in each randomized trial.
| Study | Groups (SUA) | Age mean | Male sex (n) | Smoking history (n) | Hypertension (n) | Diabetes mellitus (n) | Previous aspirin(n) |
|---|---|---|---|---|---|---|---|
| Basar et al | Low | 58.2 ±9.7 | 112 | 85 | 40 | 29 | 33 |
| High | 60.4 ± 9.8 | 36 | 30 | 21 | 10 | 12 | |
| Wang et al | Low | 56 ±11 | 139 | 106 | 89 | 45 | 17 |
| High | 57±11 | 82 | 66 | 50 | 26 | 10 | |
| Mehmet et al | Low | 55.9±11.6 | 1393 | 960 | 585 | 370 | NA |
| High | 60.5±12.6 | 460 | 306 | 308 | 172 | NA | |
| Li et al | Low | 61.19±14.06 | 335 | NA | 197 | 110 | NA |
| High | 61.51±14.01 | 82 | NA | 60 | 41 | NA | |
| Bita et al | Low | NA | 99 | 69 | 40 | 42 | NA |
| High | NA | 28 | 16 | 28 | 21 | NA | |
| Chiara et al | Low | NA | NA | NA | NA | NA | NA |
| High | NA | NA | NA | NA | NA | NA | |
| Ozgur et al | Low | 54.8±11.6 | 70 | 223 | 97 | 61 | NA |
| High | 56.8±13.9 | 23 | 98 | 54 | 28 | NA | |
| Emine et al | Low | 60 | 81 | 176 | 142 | 185 | 496 |
| High | 66 | 38 | 34 | 28 | 45 | 102 | |
| Chiara et al | Low | NA | 111 | 76 | 160 | 70 | NA |
| High | NA | 66 | 55 | 72 | 25 | NA | |
| Reza et al | Low | 61.8±13.4 | 378 | NA | 216 | 96 | NA |
| High | 67.5±12.4 | 58 | NA | 51 | 21 | NA | |
| Mora-Ramirez | Low | 57.6±11.3 | 448 | 304 | 206 | 195 | 501 |
| et al | High | 61.2±11.9 | 220 | 156 | 158 | 115 | 288 |
| Cheng-Wei et al | Low | 56 | 571 | 428 | 366 | 165 | 635 |
| High | 58 | 263 | 188 | 183 | 70 | 290 |
NA: not available
3.2. Begg’s funnel plot analysis and quality assessment
A Begg’s funnel plot showed no obvious asymmetry in the prognostic value of SUA in acute STEMI. Furthermore, all of the studies were evaluated as demonstrating a low risk of bias. Therefore, it is concluded that the meta–analysis exhibits no obvious publication bias. The quality assessment of RCTs included in this meta–analysis is shown in Figure 2.
Figure 2.
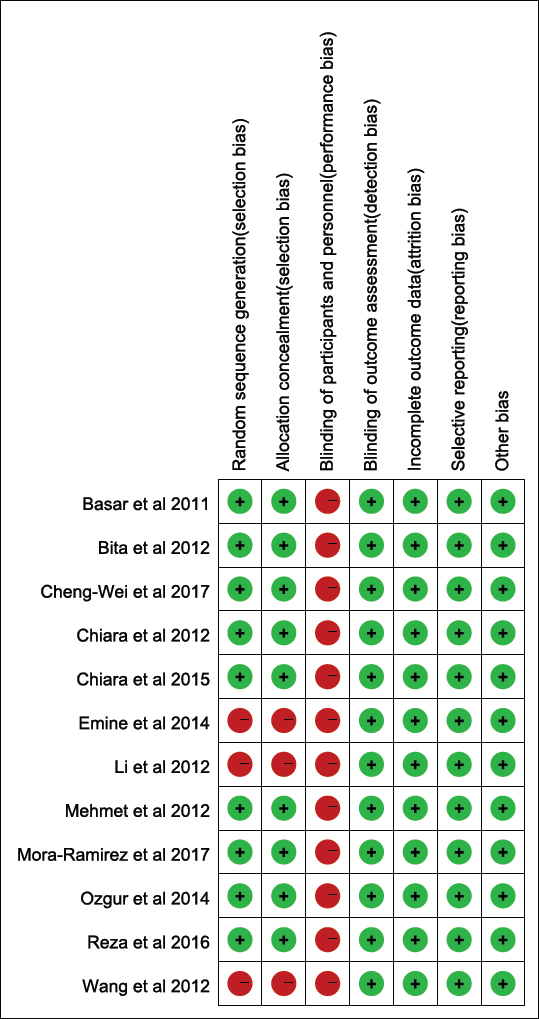
Assessment of the quality of selected RCTs. Low risk of bias (green circles), unclear risk of bias (yellow circles) and high risk of bias (red circles).
3.3. Outcome of In-hospital MACE
The incidence of in-hospital MACE during the in-hospital period was 13.5% in high SUA patients vs. 6.6% in low SUA patients. According to the five studies which provided data for in-hospital MACE, no heterogeneity was observed amongst the results (P = 0.41, I2 =0%). The incidence of in-hospital MACE in the high SUA group was significantly higher than that in the low SUA group (OR, 2.30; 95% CI, 1.83–2.88; P < 0.00001; Figure 3)
Figure 3.
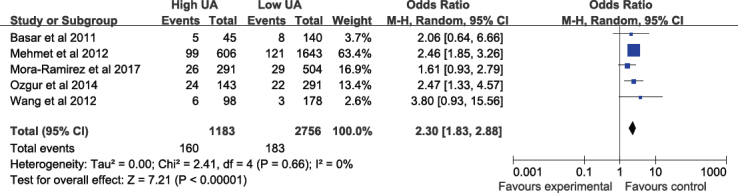
Fixed-effect meta–analysis for In-hospital MACE. The figure presents the number of events, the number of patients in the treatment and control groups, the odds ratio (OR) and 95% confidence interval (CI) for each trial, the overall OR estimate with 95% CI and the P value for the association test, the P value for the heterogeneity test, and between-trial inconsistency (I2) measures.
3.4. Outcomes of In-hospital mortality
The incidence of in-hospital mortality during in-hospital period was 9.2% in high SUA patients vs. 3.4% in low SUA patients, which was a statistically significant difference (I2 = 0%; OR: 3.03, 95% CI: 1.78–5.13; P < 0.0001; Figure 4).
Figure 4.
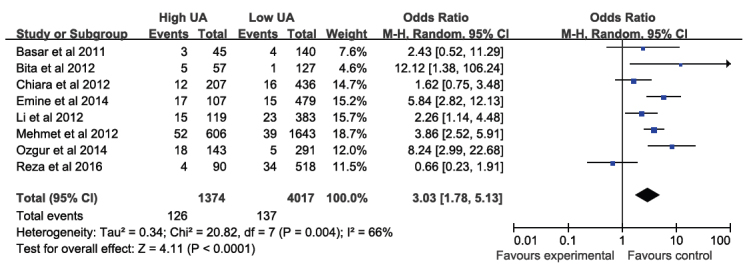
Fixed-effect meta–analysis for In-hospital mortality.
3.5. Outcome of one-year mortality
The incidence of one-year mortality during the follow-up period was 14.4% in high SUA patients vs. 6.1% in low SUA patients, which represented a significant difference (I2 = 0%; OR: 2.5, 95%CI: 1.75–3.80; P < 0.00001; Figure 5).
Figure 5.
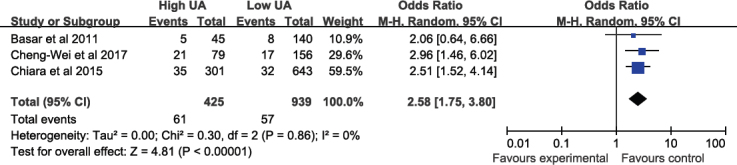
Fixed-effect meta–analysis for one-year hospital.
4. Discussion
There was no significant difference between Egger’s test results and those patients studied. Based on the funnel plot analysis, a consistency was observed between the symmetry and publication bias. UA is the final degradation product of purine metabolism. The value of SUA was shown in our meta–analysis as an effective prognostic biomarker of future adverse events in acute STEMI patients. To the best of our knowledge, previous analyses have not elucidated the potential clinical value of SUA in this way [19].
Evidence from current epidemiological studies have shown that elevated SUA levels are an important risk factor for cardiovascular disease, with oxidative stress playing an important pathophysiological role. In addition, xanthine oxidoreductase inhibitor, which reduces levels of SUA, exerts protective effects in the context of oxidative stress (e.g., ischemia-reperfusion injury and cardiovascular disease) [20, 21, 22]. UA can be detected before other cardiac markers, such as cardiac troponins. Thus, SUA is suitable to be an early marker of myocardial ischemia, making it an effective method for predicting the combination of myocardial infarction and troponins.
Previous studies have examined the prognostic features of suitable biomarkers for atherosclerotic cardiovascular disease [23, 24, 25]. Although SUA appears to assist in the clinical evaluation of patients, the level of impact of SUA can have in medical treatment or in improving prognosis remains unclear [26]. According to the current meta–analysis, mortality and MACE were elevated in the high SUA group during the in-hospital period than that observed in the low SUA group. Regardless of the low heterogeneity found, the MACE and mortality of high SUA patients with acute STEMI were significantly different. The data indicated that the number of acute STEMI patients suffering from MACE in the high SUA group during the in-hospital period was approximately 2 times larger than individuals with low SUA. In addition, the mortality rate of patients with high SUA during hospitalization was approximately 2.7 times larger in high SUA, when compared to low SUA individuals. Moreover, the incidences of one-year mortality presented a statistically significant difference between high and low SUA patients, with high SUA patients being approximately 2.36 times more likely to die within one year than low SUA patients. It was also found that an increase in SUA level was associated to an increase in risk of coronary ischemia. However, there is not enough evidence to suggest myocyte necrosis. Therefore, the current results suggest that a high level of SUA will lead to an increase in MACE, in-hospital mortality, and one-year mortality in acute STEMI patients.
There are two caveats with the current study which should be considered carefully. Firstly, due to the lack of professional RCTs focusing on this research, we only extracted data from observational studies, which can certainly lead to a risk of related bias. Secondly, all 12 articles involved in this meta–analysis came from different study groups within different countries. Thus the diagnostic criteria for SUA cutoff may also have differed.
5. Conclusions
Acute STEMI patients suffering from high SUA exhibit higher incidences of in-hospital MACE and in-hospital mortality. Further, the mortality rate was also significantly higher in this group within one year. While SUA might facilitate the advancement of atherosclerosis, it might also serve as a new prognostic marker for short- and long-term follow-up in patients with acute STEMI [21]. Additionally, this measure may become pivotal in clinical prognosis, possibly improving the accuracy of current risk stratification methods. This may be able to assist in the development of more effective medical treatment, the reduction in health care cost, and an improvement in the quality of life of patients by reducing re-hospitalization and medical expenses. All analyses were based on previous published studies, thus no ethical approval and patient consent are required.
Footnotes
Conflict of interest: Authors state no conflict of interest.
Reference
- [1].Kroll K, Bukowski TR, Schwartz LM, Knoepfler D, Bassingthwaighte JB. Capillary endothelial transport of uric acid in guinea pig heart. American Journal of Physiology. 1992;262:420. doi: 10.1152/ajpheart.1992.262.2.H420. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Castelli P, Condemi AM, Brambillasca C, Fundaro` P, Botta M, Lemma M. Improvement of cardiac function by allopurinol in patients undergoing cardiac surgery. Journal of Cardiovascular Pharmacology. 1995;25:119. doi: 10.1097/00005344-199501000-00019. et al. –. [DOI] [PubMed] [Google Scholar]
- [3].Kogure K, Ishizaki M, Nemoto M, Kuwano H, Tatemoto K, Maruyama Y. Evaluation of serum uric acid changes in different forms of hepatic vascular inflow occlusion in human liver surgeries. Life Sciences. 1999;64:305. doi: 10.1016/s0024-3205(98)00566-9. et al. –. [DOI] [PubMed] [Google Scholar]
- [4].D’Ascenzo F, Biondi-Zoccai G, Moretti C. TIMI, GRACE and alternative risk scores in acute coronary syndromes: a metaanalysis of 40 derivation studies on 216,552 patients and of 42 validation studies on 31,625 patients. Contemp Clin Trials. 2012;33:507. doi: 10.1016/j.cct.2012.01.001. –. [DOI] [PubMed] [Google Scholar]
- [5].Kojima S, Sakamoto T, Ishihara M, Kimura K, Miyazaki S, Yamagishi M. Japanese acute coronary syndrome study (JACSS) investigators. Prognostic usefulness of serum uric acid after acute myocardial infarction. Am J Cardiol. 2005;96:489. doi: 10.1016/j.amjcard.2005.04.007. et al. –. [DOI] [PubMed] [Google Scholar]
- [6].Car S, Trkulja V. Higher serum uric acid on admission is associated with higher short-term mortality and poorer long-term survival after myocardial infarction: retrospective prognostic study. Croat Med J. 2009;50:559. doi: 10.3325/cmj.2009.50.559. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Basar N, Sen N, Ozcan F, Erden G, Kanat S, Sokmen E. Elevated serum uric acid predicts angiographic impaired reperfusion and 1-year mortality in ST-segment elevation myocardial infarction patients undergoing percutaneous coronary intervention. Journal of Investigative Medicine. 2011;59(6):931. doi: 10.2310/JIM.0b013e318214ebaf. et al. –. [DOI] [PubMed] [Google Scholar]
- [8].Wang J-W, Chen Y-D, Wang C-H, Zhu X-L. Correlation of serum uric acid levels with coronary flow in patients with ST-segment elevation myocardial infarction undergoing primary coronary intervention. Nat Med J China. 2012;92(44):3100. –. [PubMed] [Google Scholar]
- [9].Kaya MG, Uyarel H, Akpek M, Kalay N, Ergelen M, Ayhan E. Prognostic value of uric acid in patients with ST-elevated myocardial infarction undergoing primary coronary intervention. Am J Cardiol. 2012;109(4):486. doi: 10.1016/j.amjcard.2011.09.042. et al. –. [DOI] [PubMed] [Google Scholar]
- [10].Chen L, Li X-L, Qiao W, Ying Z, Qin Y, Wang Y. Serum uric acid in patients with acute ST–elevation myocardial infarction. World J Emerg Med. 2012;3(1):35. doi: 10.5847/wjem.j.issn.1920-8642.2012.01.006. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Omidvar B, Ayatollahi F, Alasti M. The prognostic role of serum uric acid level in patients with acute ST elevation myocardial infarction. J Saudi Heart Assoc. 2012;24(2):73. doi: 10.1016/j.jsha.2012.01.005. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lazzeri C, Valente S, Chiostri M, Picariello C, Gensini GF. Uric acid in the early risk stratification of ST–elevation myocardial infarction. Intern Emerg Med. 2012;7(1):33. doi: 10.1007/s11739-011-0515-9. –. [DOI] [PubMed] [Google Scholar]
- [13].Akgul O, Uyarel H, Pusuroglu H, Gul M, Isiksacan N, Turen S. Predictive Value of Elevated Uric Acid in Turkish Patients Undergoing Primary Angioplasty for ST Elevation Myocardial Infarction. Acta Cardiol Sin. 2014;30(2):119. et al. –. [PMC free article] [PubMed] [Google Scholar]
- [14].Gazi E, Temiz A, Altun B, Barutgu A, Barutcu A, Bekler A. The association between serum uric acid level and heart failure and mortality in the early period of ST–elevation acute myocardial infarction. Turk Kardiyol Dernegi Ars. 2014;42(6):501. doi: 10.5543/tkda.2014.65507. et al. –. [DOI] [PubMed] [Google Scholar]
- [15].Lazzeri C, Valente S, Chiostri M, Gensini GF. Long-term prognostic role of uric acid in patients with ST–elevation myocardial infarction and renal dysfunction. J Cardiovasc Med. 2015;16(11):790. doi: 10.2459/JCM.0000000000000238. –. [DOI] [PubMed] [Google Scholar]
- [16].Hajizadeh R, Ghaffari S, Salehi R, Mazani S, Aghavali S. Association of serum uric acid level with mortality and morbidity of patients with acute ST–elevation myocardial infarction. J Cardiovasc Thorac Res. 2016;8(2):56. doi: 10.15171/jcvtr.2016.11. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mora Ramirez M, Estevez Garcia IO, Irigoyen Camacho ME, Bojalil R, Gonzalez Pacheco H, Amezcua Guerra LM. Hyperuricemia on Admission Predicts Short-Term Mortality due to Myocardial Infarction in a Population with High Prevalence of Cardiovascular Risk Factors. Rev Invest Clin. 2017;69(5):247. doi: 10.24875/ric.17002167. –. [DOI] [PubMed] [Google Scholar]
- [18].Liu CW, Liao PC, Chen KC, Chiu YW, Liu YH, Ke SR. Relationship of serum uric acid and Killip class on mortality after acute ST–segment elevation myocardial infarction and primary percutaneous coronary intervention. Int J Cardiol. 2017;226:26. doi: 10.1016/j.ijcard.2016.10.025. et al. –. [DOI] [PubMed] [Google Scholar]
- [19].Lazzeri C, Valente S, Chiostri M, Picariello C, Gensini GF. Uric acid in the early risk stratification of STelevation myocardial infarction. Internal and Emergency Medicine. 2011;15(7):33. doi: 10.1007/s11739-011-0515-9. –. [DOI] [PubMed] [Google Scholar]
- [20].Glantzounis GK, Tsimoyiannis EC, Kappas AM Gelaris DA. Uric acid and oxidative stress. Current Pharmaceutical Design. 2005;11:4145. doi: 10.2174/138161205774913255. –. [DOI] [PubMed] [Google Scholar]
- [21].Zweier JL, Kuppusamy P, Lutty GA. Measurement of endothelial cell free radical generation: evidence for a central mechanisms of free radical injury in post ischemic tissue. Proceedings of the National Academy of Sciences. 1998;85:4046. doi: 10.1073/pnas.85.11.4046. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hare JM, Johnson RJ. Uric acid predicts clinical outcomes in heart failure: insights regarding the role of xanthine oxidase and uric acid in disease pathophysiology. Circulation. 2003;107:1951. doi: 10.1161/01.CIR.0000066420.36123.35. –. [DOI] [PubMed] [Google Scholar]
- [23].Bickel C, Rupprecht HJ, Blankenberg S, Rippin G, Hafner G, Daunhauer A. Serum uric acid as an independent predictor of mortality in patients with angiographically proven coronary artery disease. American Journal of Cardiology. 2002;89:12. doi: 10.1016/s0002-9149(01)02155-5. et al. –. [DOI] [PubMed] [Google Scholar]
- [24].Bos MJ, Koudstaal PJ, Hofman A, Witteman JC, Breteler MM. Uric acid is a risk factor for myocardial infarction and stroke: the Rotterdam study. Stroke. 2006;37:1503. doi: 10.1161/01.STR.0000221716.55088.d4. –. [DOI] [PubMed] [Google Scholar]
- [25].Festa A, Haffner SM. Inflammation and cardiovascular disease in patients with diabetes: Lessons from the diabetes control and complications trial. Circulation. 2005;11:2414. doi: 10.1161/01.CIR.0000167558.77793.E8. –. [DOI] [PubMed] [Google Scholar]
- [26].Niskanen LK, Laaksonen DE, Nyyssonen K, Alfthan G, Lakka HM, Lakka TA. Uric acid level as a risk factor for cardiovascular and all-cause mortality in middle-aged men: A prospective cohort study. Archives of Internal Medicine. 2004;164:1546. doi: 10.1001/archinte.164.14.1546. et al. –. [DOI] [PubMed] [Google Scholar]


