Abstract
Objective
The aim of this study was to evaluate the expression of Angiopoietin-1 (Ang-1), Angiopoietin-2 (Ang-2) and vascular endothelial growth factor (VEGF) in cervical cancer and its clinical significance.
Methods
Immunohistochemical assay was used to examine the expression of Ang-1/2 and VEGF in tumor tissue from 56 cervical squamous cell carcinoma patients treated with operation only (SCC-O group), as well as 51 subjects with cervical squamous cell carcinoma treated with neoadjuvant radiotherapy (SCC-RCO group, n=28) or neoadjuvant chemotherapy (SCC-CO group, n=23). Both microvessel density (MVD) and lymphatic vessel density (LVD) were examined in the three groups through detection of CD34 and D2-40 expression in respective tissue samples.
Results
With the progression of cervical cancer, the positive expression scores of Ang-2 and VEGF were significantly increased (p<0.05). Compared with surgical intervention, neoadjuvant chemoradiotherapy significantly reduced the positive expression scores of Ang-1, Ang-2, and VEGF in cervical cancer tissues (p<0.05). The MVD values of the SCC-CO and SCC-RO groups were significantly reduced as compared to the SCC-O group (p<0.05). Similarly, the LVD values of the SCC-CO and SCC-RO groups were also significantly reduced when compared to those of the SCC-O group (p<0.05). However, LVD values of the SCC-CO and SCC-RO groups were not statistical different (p>0.05).
Conclusion
Ang-1, Ang-2 and VEGF may play an important role in the development of cervical cancer. Mutual synergism of Ang-2 and VEGF demonstrated a close relationship with the generation of cervical blood and lymphatic vessels. Cervical cancer radiotherapy and chemotherapy could significantly inhibit the formation of blood vessels and lymphatic vessels in tumor tissue.
Keywords: angiogenin, vascular endothelial growth factor, cervical cancer, microvessel density
1. Introduction
Carcinoma of uterine cervix is one of the most common malignant carcinomas that seriously threaten women’s lives, and ranks second to breast cancer in terms of morbidity, accounting for half of the malignant tumors occurring in the female reproductive system [1]. The morbidity and mortality of cervical cancer have decreased with the development of long-term and large-scale censuses and women’s health care in China [2]. Surgery, radiotherapy, and chemotherapy are the major treatments for cervical cancer. In recent years, several studies have verified that the occurrence, development, invasion, and metastasis of tumors are closely linked to angiogenesis [3, 4]. In the absence of blood vessel formation, tumor tissue stops growing when its diameter reaches 1 to 2 mm. Therefore, neoplastic angiogenesis is a prerequisite for tumor development. Angiogenesis is the synergistic effect of a series of vascular growth factors and vascular growth inhibitors [5]. The angiogenin and vascular endothelial cell growth factor (VEGF) family is essential to the mutual synergistic development of tumor invasion and metastasis. Moreover, angiogenesis and lymphatic circulation formation are closely related. The effect of the Ang family and VEGF in the angiogenesis in a tumor, especially cervical cancer, has been frequently discussed. In this study, the expression levels of Ang-1/2 and VEGF in the cervical squamous cell carcinoma were measured through the immunohistochemical assay. The influence of radiotherapy and chemotherapy on Ang-1/2 and VEGF expression were also determined, and the relationship between this effect and the formation of new blood vessels and lymphatics in cervical squamous cell carcinoma was analyzed.
2. Materials and methods
2.1. Patients
From March 2012 to February 2016, 107 paraffin embedded specimens of cervical cancer patients from our hospital were selected for this study, and all the specimens were classified as squamous carcinoma of cervix (SCC). Of the 107 specimens, 56 were samples of surgical resection of cervical cancer (SCC-O), 28 were samples of preoperative neoadjuvant chemotherapy (SCC-CO), and 23 were samples of neoadjuvant chemotherapy (SCC-RO).
SCC-O group: This group did not receive preoperative radiotherapy, chemotherapy, or immunotherapy. The age in this group ranged from 31 years to 68 years, and the median age was 47 years. 22 patients were less than 45 years old, and 34 patients were greater than 45 years old. According to the FIGO staging system [6], 27 cases were stage I and 29 cases were stage II. Fourteen cases of pelvic lymph node metastases, and 42 cases of no-metastases were also included.
SCC-CO group: The preoperative examination of patients all showed local cervical tumors with diameters of ≥4 cm. A course of DSA intubation chemotherapy was performed before surgery, which was performed 3–4 weeks after chemotherapy. The age range was 32–65 years, and the median age was 45 years. Eleven patients were less than 45 years old, and 18 were greater than 45 years old. FIGO staging showed 7 cases of stage I and 22 cases of stage II.
SCC-CO group: The preoperative examination of patients all showed local cervical tumors with diameters of ≥4 cm. All patients received radiotherapy 3–4 weeks before surgery. The age range was 29–66 years old, and the median age was 44 years old. 10 cases were less than 45 years old and 13 cases were greater than 45 years old. FIGO staging demonstrated 8 cases of staging I, 17 cases of stage II.
Informed consent: Informed consent has been obtained from all individuals included in this study.
Ethical approval: The research related to human use has been complied with all the relevant national regulations, institutional policies and in accordance the tenets of the Helsinki Declaration, and has been approved by the ethics committee of the Lishui People’s Hospital review board and the clinical study committee.
2.2. Reagent
Sheep anti-human Ang 1-2 polyclonal antibody was purchased from SANTA CRUZ Biotechnology Co., Ltd. (United States) and used at a working concentration of 1:100. Mouse anti-human VEGF monoclonal antibodies, mouse anti-human CD34 monoclonal antibodies, and mouse anti-human d2-40 monoclonal antibodies were purchased from Beijing Zhongshan Jinqiao Biotechnology Co., LTD, China, and used at a working concentration of 1:100. The ready-to-use Polink-2 Plus goat immunohistochemical staining kit with a hypersensitivity two-step method (Cat. No: PV-9003) and ready-to-use Immuno-Bridge + two-step method rat immunohistochemical kit (Cat. No: PV-9000) used in this study are products of GBI Biotechnology Co., Ltd (United States).
2.3. Immunohistochemical assay
The original paraffin sections of the above cases were re-examined and the paraffin specimens containing continuous cervical epithelium and cancer foci were selected. In each case, the paraffin tissues were successively sliced to 4 μm × 6 μm sections. One slice was stained with HE to determine the slice quality, which was further confirmed by a pathologist. Immunohistochemical staining was performed on the remaining sections according to the manufacturer’s instructions
2.4. Immunohistochemistry results evaluation
Instead of a monoclonal antibody, PBS was used as a negative control, and a known positive was used as the positive control. Immunohistochemical staining showed that the expression of Ang-2 or VEGF was positive when the cytoplasm or cell membrane was stained brown or brown yellow. For each section 5 high power fields (×400) were randomly selected, and the frequency of positive cells in 500 tumor cells was counted continuously. Determination of staining 1) 0-3 grades was divided according to the number of staining cells in the total number of visual field cells. Stage 0: 0, Stage 1: <25%, stage 2: 26%–50%, stage 3: >50%. 2) We divided 0–3 stages according to the staining strength of the stained cells: Stage 0: Negative, stage 1: Weak, faint yellow, stage 2: Medium, claybank, stage 3: Strong, sepia. Take 0-2 as the negative(-), >3 is the positive (+). Image Pro Plus (Media Cybemetics Inc USA) 8000 image analysis was used to analyze the expression level of each immunohistochemical slice, and input the slice images by the PC-Image analysis device through the image acquisition system with a ×20 objective lens. The corresponding part of each image was randomly selected, and the average optical (OD) of its positive positions was selected. The average value was used as the specimen result. MVD counting: CD34 positive reactant was indicated by the presence of light brown to dark brown particles, which localize in the vascular endothelial cell. Most of the stained capillaries and microvessels in tumor tissues were calculated using the Gasparini method [7]. The number of single endothelial cells or clusters of endothelial cells that were dyed brownish yellow in tumor areas were counted as single blood vessels. A lumen diameter of 50 Ixm was judged to be a microvessel, while vessels with >50 Ixm in diameter, or a lumen diameter of more than 8 red blood cells wide, or the wall of the tube containing the muscular layer was not counted as microvessels. Individual staining cells or groups of cells without lumina were counted as independent microvascular counts. Cases exhibiting tumor areas with the highest MVD under low power (×100) were selected. Then, five microvessels within the fields of vision under the high power (×400) were randomly counted, and the average value of the MVD was calculated. The method used for D2-40 markers and microlymphatic counting was similar to the above method.
2.5. Statistical analysis
Data were analyzed with SPSS 18.0 statistical software package (IBM, Armonk, NY, USA). Measurement data are expressed as mean ± standard deviation. Comparison of multiple groups was performed by one-way ANOVA, and the LSD method was used for comparison between groups. Enumeration data were expressed with a relative number, and the comparison between groups was made based on the c2 test. P<0.05 was considered to be statistically different.
3. Results
3.1. Ang-1, Ang-2 and VEGF expression
Ang-1 and Ang-2 showed expression in the cytoplasm or cell membrane as indicated by sepia or brown particles with uniform distribution. VEGF was mainly expressed in the cytoplasm, with some expression in the cell membrane, displaying brown particles (Figure 1).
Figure 1.
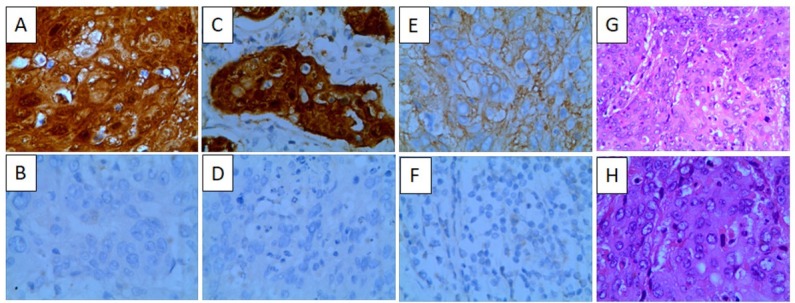
Ang-1, Ang-2 and VEGF expression in cervical cancer (A: Ang-1 positive 400×; B: Ang-1 negative 400×; C: Ang-2 positive 400×; D: Ang-2 negative 400×; E: VEGF positive 400×; F: VEGF negative 400×; G: HE stain 200×; H: HE stain 400×)
3.2. Ang-1, Ang-2 and VEGF expression score of different groups
With the progression of cervical cancer, the positive scores of Ang-2 and VEGF were significantly increased (p<0.05; Table 1). Compared with surgery, we also observed that preoperative neoadjuvant chemoradiotherapy can significantly reduce the positive scores of Ang-1, Ang-2, and VEGF in cervical cancer tissues (p<0.05; Figure 2).
Table 1.
Ang-1, Ang-2 and VEGF expression in SCC-O'SCC-CO'SCC-RO groups
| Group | N | Ang-1 | Ang-2 | VEGF |
|---|---|---|---|---|
| SCC-O | - | - | - | - |
| I | 27 | 34.23±15.66 | 38.64±16.21 | 45.21±21.12 |
| II | 29 | 32.05±16.22 | 58.66±18.56 | 68.42±29.21 |
| SCC-CO | 28 | 26.23±12.24 | 28.12±18.65 | 26.41±17.36 |
| SCC-RO | 23 | 25.23±13.04 | 24.35±16.54 | 25.12±19.33 |
Figure 2.
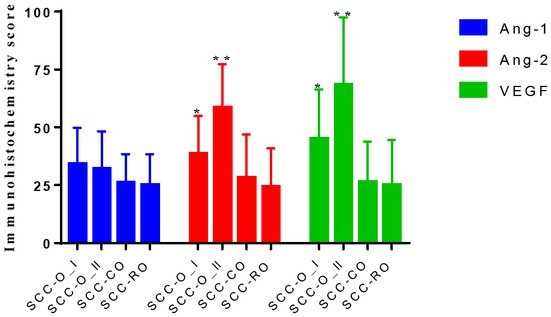
Bar plot of Ang-1, Ang-2 and VEGF expression score of different groups (*p<0.05; **p<0.01)
3.3. MVD and LVD scores in different groups
The MVD value of the SCC-CO and SCC-RO groups was significantly reduced as compared to the SCC-O group (p<0.05). Within this comparison, the SCC-RO group showed a greater reduction compared with the SCC-CO group, (p<0.05). The LVD values of the SCC-CO and SCC-RO groups were significantly reduced compared to that of the SCC-O group (p<0.05). However, the LVD values of the SCC-CO group and SCC-RO group were not statistically different (p>0.05), (Figure 3).
Figure 3.
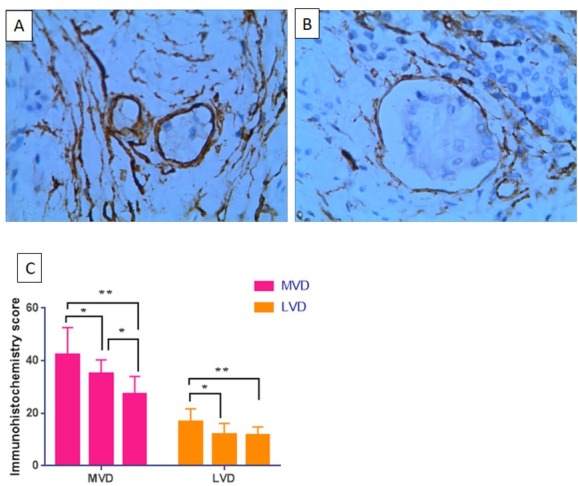
CD34 and D2-40 expression score of different groups (A: CD34 positive expression 400×; D2-40 positive expression 400×) (*p<0.05; **p<0.01)
4. Discussion
The important effects of Ang-1 and Ang-2 on tumor progression have been verified in many solid malignant carcinomas [8, 9, 10]. High Ang-2 expression in tumors have been regarded as having a close relationship to angiogenesis, invasion, metastasis, and poor prognosis [11]. Previously, the mechanism of Ang-1 mediated promotion of vessel maturation and maintenance of vessel structure was conferred primarily through improving the viability of the vascular endothelial cell by inhibiting apoptosis and reducing vascular permeability and degeneration [12]. Ang-2, on the other hand, competitively inhibits the formation of unstable leaky blood vessels by specifically binding to Tie-1/2 receptors and preventing their phosphorylation. Published studies
have demonstrated that Ang-2 exhibited up-regulated expression at the protein and mRNA levels in prostate cancer, hepatocellular cancer, stomach cancer, and oral squamous cell carcinoma, without the expression decline of Ang-1 in the protein level and mRNA level [13, 14]
We found that Ang-1 and Ang-2 both have high expression levels in cervical cancer but showed different characteristics. With the progress of cervical cancer, the expression level of Ang-2 protein significantly increased from stage I to stage II, although the expression was considerably reduced by radiochemotherapy, and to a greater extent, by radiotherapy. However, with the progression of cervical cancer, Ang-1 displayed a reduction in protein expression level stage I to stage II, and radiochemotherapy also reduced expression, although the reduction was non-significant. The expression characteristics of VEGF are similar to those of Ang-2. The growth, invasion, and metastasis of malignant carcinomas was closely related to the generation of tumor vasculature, which was mainly dependent on the strict regulation of the promoting factor of vasculature generation and the inhibiting factor [15, 16]. Among these regulatory factors, VEGF and the Ang family are considered to be two independent and mutually affected vasculature systems. In the early stage of tumor development, tumor cells and the host select and form a vascular area with sufficient blood supply. Given that tumor tissues compete with healthy tissues for the same blood supply and tumor cells proliferate rapidly, the expression levels of VEGF in the body do not increase, whereas those of Ang-1 and Ang-2 increase significantly [17, 18]. Ang-2 showed antagonism to Ang-1 in the absence of VEGF, which produced damaged tumor blood vessels, loosened vasculature, eliminated the restriction of angiogenesis by the vascular basement membrane and surrounding cells, and activated endothelial cells. Under the stimulation of hypoxia, peripheral residual tumor cells produced an abundance of VEGF, concordant with activated endothelial cells becoming extremely sensitive to the effects of VEGF, thereby leading to rapid proliferation, invasion, and migration. Simultaneously, VEGF upregulated Ang-2 in the tumor adjacent to the vascular endothelial tissue through the VEGFR2/KER pathway. The overexpression of Ang-2 altered the balance between Ang-2 and Ang-1 and disrupted the interaction between Ang-1 and Tie-2 formation, leading to the loss of peripheral cells of the capillaries and loss of stability of the vascular walls [19, 20]. Meanwhile, as the continuous high expression of Ang-2 antagonizes the action of Ang-1, the vascular wall of new tumor is incomplete and its permeability is high. Continued production of VEGF can also induce lymph angiogenesis through the vegfr-3 signaling pathway [21].
Locally advanced cervical cancer is a group of high-risk cervical cancers with adverse prognostic factors. This type of cancer contains a large volume of local tumors and exhibits a high rate of lymphatic metastasis. Thus, direct surgical treatment is not suitable for this group. Radiotherapy is the standard treatment for cervical cancer in addition to surgery and can be applied at any stage, especially to patients with diagnosed advanced-stage cervical cancer [22, 23]. The ionizing radiation produced by radiotherapy exerts it’s effect on the DNA, RNA and protein of the tumor cells by inducing chromosomal aberrations, ultimately disrupting the cell cycle, and damaging the vessels in the lesion. After the radiotherapy, we observed that the expression rates of Ang-2 and VEGF in the tumor tissues were considerably reduced relative to those of the group that did not undergo radiochemotherapy. The protein expression of Ang-1 was reduced as well, but changes were not significant. The microvessel density and lymphatic vessel density after radiochemotherapy were reduced as well, suggesting that radiochemotherapy is an effective treatment for carcinoma of uterine cervix. Radiotherapy damages and kills tumor cells and therefore reduces Ang-1, Ang-2, and VEGF expression levels. Vascular and lymphatic vessel development in tumor tissues was decreased under the influence of relatively high Ang-1 and low Ang-2 and VEGF levels. Combined with the effect of radiotherapy on vascular lymphatic vessels, MVD and LVD also decreased. However, since radiotherapy only targets local lesions, it cannot control subclinical lesions or potential systemic subclinical metastases in patients with advanced cervical cancer.
Tumor vascularization is currently a popular area of investigation in antitumor therapy. By blocking tumor angiogenesis, tumor growth and metastasis can be effectively inhibited [24]. Under pathological situations, Ang-2 mainly is expressed in the relevant tissues of the tumor, and Ang-2 antibody treatment has good tumor targeting capability and few systemic side effects. Currently, Ang/Tie-2 receptor interaction is heavily studied and a biological agent of Ang/Tie-2 receptor has been successfully used for blocking angiogenesis. Given our improved understanding of the mechanisms of the gene expression regulation and signal transduction pathway of angiogen-promoting factors, such as VEGF, novel targets for angiopoietin may provide a promising new approach for the clinical treatment of tumors.
Footnotes
Conflict of interest: Authors state no conflict of interest.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018. 68:7. doi: 10.3322/caac.21442. –. [DOI] [PubMed] [Google Scholar]
- [2].Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F. Cancer statistics in China, 2015. CA Cancer J Clin 2016. 66:115. doi: 10.3322/caac.21338. et al. –. [DOI] [PubMed] [Google Scholar]
- [3].Tomao F, Papa A, Rossi L, Zaccarelli E, Caruso D, Zoratto F. Angiogenesis and antiangiogenic agents in cervical cancer. Onco Targets Ther. 2014;7:2237. doi: 10.2147/OTT.S68286. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Minion LE, Tewari KS. Cervical cancer - State of the science: From angiogenesis blockade to checkpoint inhibition. Gynecol Oncol. 2018;148:609. doi: 10.1016/j.ygyno.2018.01.009. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Yu JQ, Zhou Q, Zhu H, Zheng FY, Chen ZW. Overexpression of astrocyte elevated gene-1 (AEG-1) in cervical cancer and its correlation with angiogenesis. Asian Pac J Cancer Prev. 2015;16:2277. doi: 10.7314/apjcp.2015.16.6.2277. –. [DOI] [PubMed] [Google Scholar]
- [6].Horn LC, Schierle K, Schmidt D, Ulrich U, Liebmann A, Wittekind C. [Current TNM/FIGO classification for cervical and endometrial cancer as well as malignant mixed müllerian tumors. Facts and background]. Pathologe. 2011;32:239. doi: 10.1007/s00292-010-1273-6. –. [DOI] [PubMed] [Google Scholar]
- [7].Gasparini G, Harris AL. Clinical importance of the determination of tumor angiogenesis in breast carcinoma: much more than a new prognostic tool. J Clin Oncol. 1995;13:765. doi: 10.1200/JCO.1995.13.3.765. –. [DOI] [PubMed] [Google Scholar]
- [8].Ramanathan R, Olex AL, Dozmorov M, Bear HD, Fernandez LJ, Takabe K. Angiopoietin pathway gene expression associated with poor breast cancer survival. Breast Cancer Res Treat. 2017;162:191. doi: 10.1007/s10549-017-4102-2. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chen JW, Luo YJ, Yang ZF, Wen LQ, Huang L. Knockdown of angiopoietin-like 4 inhibits the development of human gastric cancer. Oncol Rep. 2018;39:1739. doi: 10.3892/or.2018.6253. –. [DOI] [PubMed] [Google Scholar]
- [10].Carbone C, Piro G, Merz V, Simionato F, Santoro R, Zecchetto C. Angiopoietin-Like Proteins in Angiogenesis, Inflammation and Cancer. Int J Mol Sci. 2018;19 doi: 10.3390/ijms19020431. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chen JX, Stinnett A. Disruption of Ang-1/Tie-2 signaling contributes to the impaired myocardial vascular maturation and angiogenesis in type II diabetic mice. Arterioscler Thromb Vasc Biol. 2008;28:1606. doi: 10.1161/ATVBAHA.108.169235. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Oka N, Yamamoto Y, Takahashi M, Nishitani M, Kanayama HO, Kagawa S. Expression of angiopoietin-1 and -2. and its clinical significance in human bladder cancer. BJU Int. 2005;95:660. doi: 10.1111/j.1464-410X.2005.05358.x. –. [DOI] [PubMed] [Google Scholar]
- [13].Mitsuhashi N, Shimizu H, Ohtsuka M, Wakabayashi Y, Ito H, Kimura F. Angiopoietins and Tie-2 expression in angiogenesis and proliferation of human hepatocellular carcinoma. Hepatology. 2003;37:1105. doi: 10.1053/jhep.2003.50204. et al. –. [DOI] [PubMed] [Google Scholar]
- [14].Kuboki S, Shimizu H, Mitsuhashi N, Kusashio K, Kimura F, Yoshidome H. Angiopoietin-2 levels in the hepatic vein as a useful predictor of tumor invasiveness and prognosis in human hepatocellular carcinoma. J Gastroenterol Hepatol. 2008;23:e157. doi: 10.1111/j.1440-1746.2007.05175.x. et al. –. [DOI] [PubMed] [Google Scholar]
- [15].Chen HA, Kuo TC, Tseng CF, Ma JT, Yang ST, Yen CJ. Angiopoietin-like protein 1 antagonizes MET receptor activity to repress sorafenib resistance and cancer stemness in hepatocellular carcinoma. Hepatology. 2016;64:1637. doi: 10.1002/hep.28773. et al. –. [DOI] [PubMed] [Google Scholar]
- [16].Faillaci F, Marzi L, Critelli R, Milosa F, Schepis F, Turola E. Liver Angiopoietin-2 is a key predictor of de novo or recurrent hepatocellular cancer after HCV direct-acting antivirals. Hepatology. 2018. et al. [DOI] [PMC free article] [PubMed]
- [17].Bessho H, Wong B, Huang D, Tan J, Ong CK, Iwamura M. Effect of Ang-2-VEGF-A Bispecific Antibody in Renal Cell Carcinoma. Cancer Invest. 2015;33:378. doi: 10.3109/07357907.2015.1047505. et al. –. [DOI] [PubMed] [Google Scholar]
- [18].Chen ZB, Shen SQ, Ding YM, Wang WX, Tao JP, Liang LJ. The angiogenic and prognostic implications of VEGF. Ang-1, Ang-2, and MMP-9 for hepatocellular carcinoma with background of hepatitis B virus. Med Oncol. 2009;26:365. doi: 10.1007/s12032-008-9130-7. et al. –. [DOI] [PubMed] [Google Scholar]
- [19].Tang KD, Holzapfel BM, Liu J, Lee TK, Ma S, Jovanovic L. Tie-2 regulates the stemness and metastatic properties of prostate cancer cells. Oncotarget. 2016;7:2572. doi: 10.18632/oncotarget.3950. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sun Y, Liu JH, Pan L, Jin L, Yang Y, Sui YX. Modulatory effects of Beclin 1 on expression of angiopoietin and Tie-2 receptor in human cervical cancer cells. Asian Pac J Cancer Prev. 2011;12:2985. et al. –. [PubMed] [Google Scholar]
- [21].Zehnder-Fjällman AH, Marty C, Halin C, Hohn A, Schibli R, Ballmer-Hofer K. Evaluation of anti-VEGFR-3 specific scFv antibodies as potential therapeutic and diagnostic tools for tumor lymph-angiogenesis. Oncol Rep. 2007;18:933. et al. –. [PubMed] [Google Scholar]
- [22].Huang K, Jia M, Li P, Han J, Zhang R, Li Q. Radiotherapy Improves the Survival of Patients With Metastatic Cervical Cancer: A Propensity-Matched Analysis of SEER Database. Int J Gynecol Cancer. 2018. et al. [DOI] [PubMed]
- [23].Cibula D, Pötter R, Planchamp F, Avall-Lundqvist E, Fischerova D, Haie MC. The European Society of Gynaecological Oncology/European Society for Radiotherapy and Oncology/European Society of Pathology guidelines for the management of patients with cervical cancer. Radiother Oncol. 2018;127:404. doi: 10.1016/j.radonc.2018.03.003. et al. –. [DOI] [PubMed] [Google Scholar]
- [24].Alcázar JL, Jurado M, López-García G. Tumor vascularization in cervical cancer by 3-dimensional power Doppler angiography: correlation with tumor characteristics. Int J Gynecol Cancer. 2010;20:393. doi: 10.1111/IGC.0b013e3181d159f9. –. [DOI] [PubMed] [Google Scholar]


