Abstract
Anthracyline (ANT) has been demonstrated as a useful treatment for leukemia and solid tumors. However, ANT has previously reported cardiotoxic effects, which can reduce the therapeutic index for cancer treatment. This study aimed to investigate the associations of glycogen phosphorylase isoenzyme BB (GPBB), myoglobin (Mb), and brain natriuretic peptide (BNP) with anthracycline (ANT-induced cardiotoxicity (AIC)) amongst the Chinese population. Patients suffering from leukemia were recruited. Electrocardiogram and echocardiography were used along with chemotherapy to determine left ventricular ejection fraction (LVEF), mitral ratio of peak early to late diastolic filling velocity (E/A), E-wave deceleration time (EDT), and isovolumic relaxation time (IVRT). Double-antibody sandwich enzyme-linked immunosorbent assay (DAS-ELISA) was employed to examine and compare serum GPBB, Mb, and BNP levels. Following chemotherapy, the patients presented higher levels of serum GPBB, Mb, and BNP than before chemotherapy treatment. The levels of LVEF (%), E/A, and IVRT were significantly decreased after chemotherapy, while EDT was markedly increased. The cumulative ANT dose was positively corelated to serum GPBB, Mb, and BNP levels while it was negatively corelated to LVEF levels. In conclusion, serum GPBB, Mb, and BNP levels in combination might provide higher diagnostic accuracy in the early detection of AIC compared with other single indicators.
Keywords: Glycogen phosphorylase isoenzyme BB, Myoglobin, Brain natriuretic peptide, Anthracyclines , Cardiotoxicity, Combined diagnosis, Left ventricular ejection fraction, Enzyme-linked immunosorbent assay
1. Introduction
Anthracyclines (ANT) are one of the most widely-applied and effective chemotherapeutic agents in the treatment of hematologic malignancies and solid tumors [1]. However, a number of studies have indicated that ANT chemotherapy has cardiotoxic effects [2, 3]. Chronic anthracycline-induced cardiotoxicity (AIC), including early-onset AIC (within one year after ANT chemotherapy termination) and late-onset AIC (years or even decades after ANT chemotherapy termination), is known to be dose-dependent and can cause irreversible heart failure [4, 5]. In most cases, chronic AIC presents as asymptotic systolic and/or diastolic left ventricular dysfunction, which might later develop into severe congestive heart failure and eventually cause death if left without immediate intervention at the early stage [6]. Therefore, the early detection of AIC is of great significance in the improvement of the ANT chemotherapy prognosis. Glycogen phosphorylase isoenzyme BB (GPBB) is a glycogenolytic enzyme mainly produced by brain and heart, which serves as a glucose-provider for heart muscle tissue [7]. GPBB has high sensitivity towards myocardial ischemia and will be released into circulation in less than 4 hours after myocardial injuries, making it a promising biomarker in the early detection of ischemic myocardial injury [8]. Patients with suspected acute coronary syndrome are said to have a better prognosis when they have negative expression of GPBB [9]. Indicators with both high sensitivity and specificity are necessary for high diagnostic accuracy in the early detection of AIC. Although the sensitivity of GPBB is high, its specificity is not ideal for a heart-exclusive product; therefore, other early indicators for an early myocardial injury diagnosis are needed to compensate for the low specificity of GPBB [10].
Myoglobin (Mb), belonging to the globin family, is an intracellular oxygen-binding hemoprotein highly concentrated in the heart and striated muscle tissue [11]. It is an O2 reservoir and O2 delivery facilitator, protecting the heart from ischemia and reperfusion damage by regulating nitric oxide homeostasis [11, 12]. Mb is the earliest marker in blood after myocardial injuries and can be detected within 2 to 3 hours after the onset [13].
Brain natriuretic peptide (BNP), a cardiac hormone belonging to the family of natriuretic isopeptides, is mainly secreted by ventricular myocytes together with their amino-terminal fragments in response to volume expansion and pressure load [14]. During ANT chemotherapy, the BNP level in plasma increases, making it a useful indicator in the prediction of the development of congestive heart failure (CHF) [15, 16]. BNP is up-regulated in a number of cardiac pathologies related to hemodynamic overload and elevated cardiac fibrosis [17, 18].
In the present study, the levels of GPBB, Mb, and BNP in patients receiving ANT chemotherapy were examined and the independent and combined diagnostic values of GPBB, Mb, and BNP were calculated, aiming to find better scheme in the early detection of AIC.
2. Materials and Methods
2.1. Study population
A total of 129 patients (87 males and 42 females with mean age of 44.72 ± 15.98) diagnosed with leukemia with the use of bone marrow aspiration and flow cytometry in Huzhou Central Hospital (Huzhou, Zhejiang, China) from January 2013 to December 2016 were selected for the study. The exclusion criterias included patients with myocardial ischemic injury, arrhythmia, congenital heart disease, cardiomyopathy, heart failure, acute myocardial infarction, pericarditis and myocarditis; patients with combined diabetes mellitus, hypertension, chronic respiratory disease, chronic systemic disease, anemia, acute infection, and electrolyte imbalance and acid-base balance disorder; patients with combined hepatic and renal injury, cardiopulmonary insufficiency, late-stage cancer cachexia, secondary tumor; or pregnant or lactating female patients.
Informed consent Informed consent has been obtained from all individuals included in this study
Ethical approval The research related to human use has been complied with all the relevant national regulations, institutional policies and in accordance the tenets of the Helsinki Declaration [19], and has been approved by the Ethics Committee of Huzhou Central Hospital.
2.2. Chemotherapy and dose conversion
Patients with acute lymphocytic leukemia (ALL) were first treated with induction chemotherapy with DVCL [daunorubicin (DNR) + vincristine (VCR) + cyclophosphamide (CTX) + L-asparaginase (LASP) + prednisone (Pred)], VDCP (VCR+ DNR + CTX + Pred), and VDP [VCR + DNR + DNR (DNR + Pred)], then received consolidation therapy with etoposide (VP16) + cytarabine (Arac) and early reinforcement and maintenance therapy with high-dose methotrexate (HD-MTX). Patients with acute myeloid leukemia (AML) went through remission induction chemotherapy and consolidation chemotherapy alternatively with DA (DNR + Arac), AA [aclarubicin (ACR) + Arac], TA [pirarubicin (THP) + Arac], and MA [mitoxantrone (MIT) + Arac) and then received reinforcement therapy with high dose Arac (HDA). All drugs were purchased from Sigma (Saint Louis, MO, USA). Afterwards, the cumulative ANT dose was converted based on the cardiotoxicity and on conversion coefficients. Based on the cumulative ANT dose, the patients were divided into four groups: group A included patients with a cumulative dose below 150 mg/m2, group B had patients with a cumulative dose between 150 and 300 mg/m2, group C had patients with a cumulative dose between 300 and 450 mg/m2, and group D had patients with a cumulative dose over 450 mg/m2 [20] (Table 1).
Table 1.
Dose conversion of ANTs with the threshold dose of 5% cardiotoxicity occurrence and the conversion coefficients for calculating cumulative ANT dose
| Drugs | Conversion coefficients | Dose inducing 5% cardiotoxicity (mg/m2) |
|---|---|---|
| DOX | 1 | 450 |
| DAU | 0.5 | 900 |
| EPI | 0.5 | 935 |
| IDA | 2 | 225 |
| MTX | 2.2 | 200 |
| THP | 0.5 | 900 |
Note: ANT, anthracycline; DOX, doxorubicin; DAU, Daunorubicin; EPI, epirubicin; IDA, idarubicin; MTX, Mitoxantrone; THP, Pirarubicin.
2.3. Electrocardiogram
A 12-lead electrocardiogram (Michael Weiss electrocardiograph, CN110) was used to monitor and record sinus cycle length, loss of R wave voltage, flat T waves, ST segment shifts, and QT interval of all patients 1 d before chemotherapy and 24 h after chemotherapy, respectively [21]. The electrocardiographic diagnosis was performed by well-experienced operators without any knowledge of each patient’s clinical history.
2.4. Echocardiography
Echocardiography (left decubitus position) was performed on patients 1 d before chemotherapy and 24 h after chemotherapy, respectively [21]. Ultrasound cardiotachography (Philip Sonos, America) was used to obtain a cardiogram for each patient in strict accordance with the recommendations provided by the American Society of Echocardiography’s Guidelines and Standards Committee. An apical four chamber view was adopted for M-mode ultrasonography, two-dimensional ultrasonography and Continuous-wave Doppler echocardiographic detection. Left ventricular ejection fraction (LVEF) was calculated according to modified Simpson’s rule. Mitral ratio of peak early to late diastolic filling velocity (E/A), E-wave deceleration time (EDT), and isovolumic relaxation time (IVRT) were also recorded. The final measurement was obtained using the average value from the three cardiac cycles. Echocardiographic diagnosis was also performed by well-experienced operators without any information on each patient’s clinical history.
2.5. Serum biochemical parameters
Fasting blood (4 mL) was collected from the peripheral vein 1 d before chemotherapy and the day after chemotherapy, while the patients were in a decubitus position with an empty stomach. The samples were then preserved in a tube with the addition of anticoagulants (heparin lithium) and blended with 40 μL aprotinin. Following a 10-minute centrifugation at 4°C and at a speed of 2000 r/min, 1.5 mL serum was stored in a tube and refrigerated at –80°C. Subsequently, serum GPBB, Mb, and BNP levels were detected by the Double-antibody sandwich enzyme-linked immunosorbent assay (DAS-ELISA) using a kit from Roche Diagnostics GmbH and analyzed by the ES300 fully Automated microplate ELISA analyzer from Boehringer Mannheim GmbH (Germany).
2.6. Statistical analysis
Data analysis was performed using SPSS 18.0 (IBM Corp. Armonk, NY, USA). Measurement data were presented as mean ± standard deviation and compared using t-test of analysis of variance. Enumeration data were expressed as percentage or rate and compared using Chi-square test. The correlations of serum GPBB, Mb, BNP, and LVEF levels to cumulative ANT dose were analyzed by Spearman’s correlation analysis, with p < 0.05 indicating a statistically significant difference.
3. Results
3.1. Comparisons of baseline characteristics amongst the 129 patients
Prior to chemotherapy, all 129 patients were found without any obvious electrocardiographic abnormalities. Following chemotherapy, 31 patients (cardiotoxicity group) exhibited the following electrocardiographic abnormalities [20]: sinus tachycardia (n=5), sinus bradycardia (n=3), loss of R wave voltage (n=5), flat T wave (n=7), ST segment depression (n=5), or QT interval prolongation (n=6), while 98 patients (normal heart group) presented without any abnormalities on electrocardiography. There were no significant differences in sex, age, body mass index (BMI) and smoking status between the cardiotoxicity group and the normal heart group (all p > 0.05) (Table 2).
Table 2.
The clinical characteristics of 129 patients
| characteristics | normal heart group (n = 98) | cardiotoxicity group (n = 31) | p value |
|---|---|---|---|
| Average age (year) | 44.16 ± 16.37 | 46.48 ± 14.79 | 0.483 |
| Sex (male/female) | 63/35 | 24/7 | 0.174 |
| BMI (kg/m2) | 24.75 ± 6.16 | 26.30 ± 7.02 | 0.273 |
| Smoking (Yes/No) | 16/82 | 6/25 | 0.696 |
Notes: BMI, body mass index.
3.2. Patients with cardiotoxicity present with higher levels of serum GPBB, Mb, BNP, EDT but lower LVEF, E/A, and IVRT after chemotherapy
DAS-ELISA was employed in order to detect whether serum GPBB, Mb, BNP played an influential role in the AIC. The results demonstrated that, before chemotherapy, the cardiotoxicity group and the normal heart group showed no significant difference in GPBB, Mb, and BNP levels (all p > 0.05). After chemotherapy, GPBB, Mb, and BNP levels increased significantly in both groups, with significantly higher GPBB, Mb, and BNP levels in the cardiotoxicity group than that in the normal heart group (all p < 0.05) (Table 3). Electrocardiograms and Echocardiography were performed in order to determine the values of LVEF, E/A, EDT, and IVRT, and the results obtained revealed no significant differences in these values between the two groups before chemotherapy (all p > 0.05). After chemotherapy, while the values of LVEF, E/A, and IVRT markedly decreased, the value of EDT increased in both groups (all p < 0.05). Compared with the normal heart group, LVEF, E/A, and IVRT were apparently lower in the cardiotoxicity group, while EDT was higher (all p < 0.05) (Table 4). These findings revealed that patients with cardiotoxicity presented with high levels of serum GPBB, Mb, BNP, EDT but lower LVEF, E/A, and IVRT after chemotherapy.
Table 3.
Comparisons of serum GPBB, Mb and BNP levels in study population before chemotherapy and those after chemotherapy respectively
| Before chemotherapy | p | After chemotherapy | p | |
|---|---|---|---|---|
| GPBB (pg/mL) | ||||
| Normal heart group | 22.19 ± 7.16 | 0.343 | 30.09 ± 6.46* | < 0.001 |
| Cardiotoxicity group | 23.46 ± 3.49 | 39.89 ± 10.22* | ||
| Mb (pg/mL) | ||||
| Normal heart group | 31.71 ± 12.58 | 44.75 ± 16.49* | ||
| Cardiotoxicity group | 31.55 ± 13.22 | 0.952 | 51.59 ± 16.43* | 0.030 |
| BNP (pg/mL) | ||||
| Normal heart group | 29.80 ± 14.53 | 0.276 | 65.28 ± 21.96* | < 0.001 |
| Cardiotoxicity group | 33.15 ± 15.93 | 86.01 ± 36.84* |
Note: GPBB, glycogen phosphorylase isoenzyme BB; Mb, myoglobin; BNP, brain natriuretic peptide. * refers to p < 0.05 vs the serum GPBB, Mb and BNP levels in study population before chemotherapy.
Table 4.
Comparisons of values of echocardiogram indexes in study population before chemotherapy and those after chemotherapy respectively
| Before chemotherapy | p | After chemotherapy | p | |
|---|---|---|---|---|
| LVEF(%) | ||||
| Normal heart group | 56.29 ± 5.14 | 0.844 | 53.99 ± 6.85* | 0.009 |
| Cardiotoxicity group | 56.51 ± 6.27 | 50.63 ± 4.77* | ||
| E/A | ||||
| Normal heart group | 1.27 ± 0.34 | 0.891 | 1.14 ± 0.03* | < 0.001 |
| Cardiotoxicity group | 1.26 ± 0.27 | 1.03 ± 0.04* | ||
| EDT/ms | ||||
| Normal heart group | 174.23 ± 35.15 | 0.509 | 187.65 ± 22.90* | 0.001 |
| Cardiotoxicity group | 169.37 ± 37.38 | 204.39 ± 29.84* | ||
| IVRT/ms | ||||
| Normal heart group | 82.17 ± 10.23 | 0.774 | 70.46 ± 16.34* | 0.011 |
| Cardiotoxicity group | 81.53 ± 12.56 | 61.86 ± 17.86* |
Note: LVEF, left ventricular ejection fraction; E/A, mitral ratio of peak early to late diastolic filling velocity; IVRT, isovolumic relaxation time; EDT, E-wave deceleration time. * refers to p < 0.05 vs values of echocardiogram indexes in study population before chemotherapy.
3.3. Serum GPBB, Mb, and BNP levels are responsible for diagnosis of AIC
In diagnosing AIC, the area under the Receiver Operating Characteristic Curve (AUC) of GPBB was 0.814 (95% confidence interval: 0.694 ~ 0.934) with 80.60% sensitivity and 91.80% specificity, respectively, at the optimal diagnostic threshold of 38.085 pg/mL. For Mb, the AUC was 0.810 (95% confidence interval: 0.719 ~ 0.901), and the sensitivity and specificity were 77.40% and 72.40%, respectively, at the optimal diagnostic threshold of 48.76 pg/mL. For BNP, AUC was 0.897 (95% confidence interval: 0.826 ~ 0.968), with sensitivity and specificity of 74.20% and 94.90%, respectively, at the optimal diagnostic threshold of 89.02 pg/mL. For combined GPBB, Mb, and BNP, AUC was 0.930 (95% confidence interval: 0.880 ~ 0.980) and the sensitivity and specificity were 90.30% and 85.70%, respectively (Fig. 1). These results highly indicated that GPBB, Mb, and BNP had high sensitivity and specificity in the diagnosis of AIC individually or combined.
Fig 1.
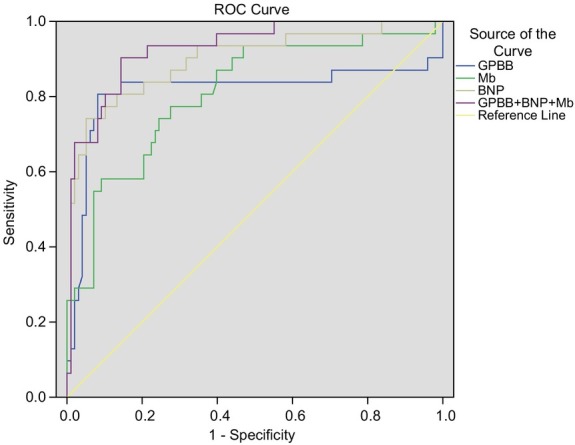
Individual ROC curves of serum GPBB, Mb, and BNP as well as ROC curves of GPBB, Mb and BNP in combination in the diagnosis of AIC, AUC increase with the diagnostic accuracy. As shown in the figure, the area under the ROC curve of GPBB + Mb + BNP is the largest, suggesting that the combination of the three parameters has the highest diagnostic accuracy. ROC curve, Receiver Operating Characteristic curve; GPBB, lycogen phosphorylase isoenzyme BB; Mb, myoglobin; BNP, brain natriuretic peptide; AIC, anthracycline-induced cardiotoxicity; AUC, area under the curve.
3.4. Patients exhibit higher GPBB, Mb, and BNP after chemotherapy
The cumulative ANT dose was calculated within the range of 50 to 699 mg/m2, with a mean dose of 316.53 ± 177.93 mg/m2 in the end of the chemotherapy courses. Afterwards, the cumulative ANT dose was converted based on the cardiotoxicity and on conversion coefficients, and then the patients were divided into four groups: group A included patients with a cumulative dose below 150 mg/m2 (n=39), group B included patients with a cumulative dose between 150 and 300 mg/m2 (n=23), group C had patients with a cumulative dose between 300 and 450 mg/m2 (n=29), and group D had patients with a cumulative dose over 450 mg/m2 (n=38). Before chemotherapy, there was no significant difference in the GPBB, Mb and BNP levels (p > 0.05). After chemotherapy, the GPBB, Mb, and BNP levels increased significantly in all four groups (p < 0.05). After chemotherapy, the results of echocardiography in the four groups showed significant differences compared with the results of echocardiography before chemotherapy
(all p > 0.05). After chemotherapy, there was no significant difference in the values of E/A, EDT and IVRT (all p > 0.05), while LVEF levels showed significant difference among four groups (p < 0.05) (Table 5). These findings revealed that patients presented higher GPBB, Mb, and BNP levels after chemotherapy.
Table 5.
Changes in serum GPBB, Mb and BNP levels and in the values of echocardiogram indexes among four groups after chemotherapy
| < 150mg/m2 (n = 39) | 150 ~ 300mg/m2 (n = 23) | 300 ~ 450mg/m2 (n = 29) | > 450mg/m2 (n = 38) | F | p | |
|---|---|---|---|---|---|---|
| GPBB | ||||||
| Before chemotherapy | 22.34 ± 7.29 | 22.74 ± 5.60 | 23.06 ± 8.31 | 22.08 ± 4.39 | 0.139 | 0.936 |
| After chemotherapy | 23.68 ± 4.12 | 29.86 ± 4.03*@ | 33.68 ± 4.79*&@ | 42.06 ± 5.44*&#@ | 101.8 | < 0.001 |
| Mb | ||||||
| Before chemotherapy | 29.56 ± 10.07 | 31.46 ± 12.74 | 33.78 ± 15.65 | 32.35 ± 12.72 | 0.660 | 0.578 |
| After chemotherapy | 31.93 ± 12.64 | 44.66 ± 15.30*@ | 59.82 ± 9.80*&@ | 52.05 ± 13.76*@ | 29.22 | <0.001 |
| BNP | ||||||
| Before chemotherapy | 31.20 ± 14.41 | 29.61 ± 16.91 | 28.77 ± 13.22 | 31.98 ± 15.65 | 0.306 | 0.821 |
| After chemotherapy | 51.74 ± 15.10@ | 70.02 ± 25.06*@ | 88.45 ± 23.71*&@ | 75.53 ± 30.92*@ | 13.6 | <0.001 |
| LVEF | ||||||
| Before chemotherapy | 55.27 ± 5.66 | 57.03 ± 5.71 | 55.91 ± 5.33 | 57.36 ± 4.97 | 1.147 | 0.333 |
| After chemotherapy | 59.20 ± 3.97@ | 54.50 ± 4.09* | 51.07 ± 3.61*&@ | 47.81 ± 5.39*&#@ | 46.33 | < 0.001 |
| E/A | ||||||
| Before chemotherapy | 1.28 ± 0.33 | 1.30 ± 0.33 | 1.22 ± 0.36 | 1.27 ± 0.29 | 0.347 | 0.792 |
| After chemotherapy | 1.13 ± 0.05@ | 1.13 ± 0.04@ | 1.12 ± 0.06 | 1.10 ± 0.08@ | 1.948 | 0.125 |
| EDT/ms | ||||||
| Before chemotherapy | 173.64 ± 36.53 | 172.26 ± 42.26 | 171.94 ± 29.29 | 173.81 ± 36.10 | 0.022 | 0.996 |
| After chemotherapy | 189.83 ± 23.03@ | 197.72 ± 28.55@ | 189.28 ± 22.07@ | 191.74 ± 29.09@ | 0.572 | 0.634 |
| IVRT/ms | ||||||
| Before chemotherapy | 81.76 ± 12.35 | 82.49 ± 6.90 | 81.91 ± 10.36 | 82.07 ± 11.66 | 0.023 | 0.995 |
| After chemotherapy | 68.24 ± 20.40@ | 68.71 ± 14.01@ | 68.57 ± 17.97@ | 68.23 ± 14.74@ | 0.006 | 0.999 |
Note: GPBB, glycogen phosphorylase isoenzyme BB; Mb, myoglobin; BNP, brain natriuretic peptide; LVEF, left ventricular ejection fraction; E/A, mitral ratio of peak early to late diastolic filling velocity; IVRT, isovolumic relaxation time; EDT, E-wave deceleration time. *, p < 0.05 vs group of < 150mg/m2; &, p < 0.05 vs group of 150 ~ 300mg/m2; #, p < 0.05 vs group of 300 ~ 450mg/m2; @, p < 0.05 vs levels or values before chemotherapy.
3.5. Serum GPBB, Mb, and BNP levels have positive correlations with the cumulative ANT dose, while LVEF has a negative correlation with the cumulative ANT dose
The results from the correlation analysis revealed that serum GPBB, Mb, and BNP levels had positive correlations with the cumulative ANT dose (GPBB: r = 0.784, p < 0.001; Mb: r = 0.729, p < 0.001; BNP: r = 0.741, p < 0.001), while LVEF was negatively correlated with the cumulative ANT dose (r = -0.686, P < 0.001). E/A, EDT, and IVRT had no significant correlations with the cumulative ANT dose (all p < 0.05).
4. Discussion
Although ANT chemotherapy has high efficacy in the treatment of several kinds of malignancies and tumors, the adverse cardiac effects resulting from it have been widely recognized [6]. It has been previously reported that up-regulated BNP served as a diagnostic marker for the risk of cardiotoxicity and could promote the diagnostic performance of AIC [22]. Therefore, the early detection of AIC will benefit the therapeutic effect and the prognosis of ANT chemotherapy because immediate treatment changes can be made. In the present study, patients were assigned into the cardiotoxicity and normal heart groups using an electrocardiogram and were then further divided into four groups based on the ANT dose in order to investigate the serum GPBB, Mb, and BNP levels in patients under ANT treatment and to evaluate the diagnostic values of GPBB, Mb, and BNP individually and combined, in order to identify the values of combining serum GPBB, Mb, and BNP in the early diagnosis of AIC.
LVEF estimation is the most common way to monitor the cardiac function for dose adaption of chemotherapeutic agents [23]. It is well known that the major mechanism of AIC development involves the iron-dependent generation of oxygen species and subsequent wide oxidative damage to cardiac myocytes [24]. According to the observations from the echocardiography, both the cardiotoxicity group and the normal heart group presented with decreased values of LVEF after chemotherapy, suggesting that all recruited patients suffered from cardiac injuries which were not detectable by electrocardiography. In addition, AIC is dose-dependent, creating injuries to myocytes already at the first exposure to ANT [25]. A decrease in the value of LVEF was observed in all four groups, based on cumulative dose and the dose-response relationship after chemotherapy. Correlation analysis also showed that LVEF was negatively correlated with the cumulative dose.
Based on echocardiography, comparisons of the diagnostic accuracy were made on three parameters separately and combined. First, we observed the changes of serum GPBB, Mb, and BNP in patients after chemotherapy. Both the cardiotoxicity group and normal heart group had increased levels of serum GPBB, Mb, and BNP. In the groups that were based on the cumulative ANT dose, the levels of GPBB, Mb, and BNP increased with ANT accumulation. Correlation analysis demonstrated that GPBB, Mb, and BNP were positively correlated with the cumulative ANT dose. These results indicated that serum GPBB, Mb, and BNP are valuable parameters in the early detection of AIC. The activation of GPBB in myocardial ischemia is a result of the promotion in glycogen degradation, which releases GPBB into the circulation [26]. According to recent studies, it has been reported that GPBB is a prospective marker in the early detection of myocardial ischemia and necrosis [9, 27]. BNP, as a biomarker of cardiac myocytes injury, could suggest early RT-related myocardial damage, which affected indirect myocyte toxicity followed by microvascular injury and ischemia [28]. Zidan A et al. reported that NT-proBNP was potentially effective in early detection of myocardial injury afer anti-cancer treatment and also served as an early marker of subclinical late cardiotoxicity after doxorubicin therapy and mediastinal irradiation [29]. Deoxygenated Mb is also known to lower the concentration of nitrite in order to protect the heart from ischemia and reperfusion injury during hypoxia from myocardial damage [11]. Additionally, GPBB was revealed to be a useful marker for detecting the acute cardiotoxicity related to conventional CT containing ANT and HD-CT followed by HCT [30]. It is also closely related with cardiac geometry and mass, and its amino-terminal fragment is a powerful predictor of hypertension mortality [14].
Furthermore, the values of the diagnostic accuracy of GPBB, Mb, and BNP were calculated separately as well as in combination. Based on these calculations, it was found that Mb and GPBB have a relatively higher sensitivity than BNP, with an AUC of 0.791 and 0.845, respectively. A possible explanation for this pattern is that Mb and GPBB respond to myocardial injuries very quickly, Mb within 2 – 3 hours and GPBB within 4 hours [8, 13]. Compared to GPBB and Mb, BNP had a relatively higher specificity with an AUC of 0.789. Combining these three parameters, it was observed that AUC surprisingly reached 0.916 with a specificity of 98.6%, suggesting that using GPBB, Mb, and BNP in combination is superior and more accurate in the early detection of AIC than any single parameter.
5. Conclusion
The present study revealed that the combination of GPBB, Mb, and BNP levels provided a higher diagnostic accuracy, making it the most effective method for the early detection of AIC. However, as the sample size was not large enough, the analysis of the study may have been. Therefore, more large-scale studies should be conducted in order to confirm the diagnostic accuracy of GPBB, Mb, and BNP in combination during the early detection of AIC.
Acknowledgments
We would like to give our sincere appreciation to the reviewers for their helpful comments on this article. This study was supported by 2015 Zhejiang Medical and Health Research Program (Class A) (No. 2015KYA210).
Footnotes
Conflict of interest: Authors state no conflict of interest
References
- [1].Cardinale D, Colombo A, Bacchiani G, Tedeschi I, Meroni CA, Veglia F. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation. 2015;131(22):1981. doi: 10.1161/CIRCULATIONAHA.114.013777. et al. –. [DOI] [PubMed] [Google Scholar]
- [2].Menna P, Paz OG, Chello M, Covino E, Salvatorelli E, Minotti G. Anthracycline cardiotoxicity. Expert Opin Drug Saf. 2012;1(11):S21. doi: 10.1517/14740338.2011.589834. –. [DOI] [PubMed] [Google Scholar]
- [3].Trachtenberg BH, Landy DC, Franco VI, Henkel JM, Pearson EJ, Miller TL. Anthracycline-associated cardiotoxicity in survivors of childhood cancer. Pediatr Cardiol. 2011;32(3):342. doi: 10.1007/s00246-010-9878-3. et al. –. [DOI] [PubMed] [Google Scholar]
- [4].Geisberg CA, Sawyer DB. Mechanisms of anthracycline cardiotoxicity and strategies to decrease cardiac damage. Curr Hypertens Rep. 2010;12(6):404. doi: 10.1007/s11906-010-0146-y. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lipshultz SE, Alvarez JA, Scully RE. Anthracycline associated cardiotoxicity in survivors of childhood cancer. Heart. 2008;94(4):525. doi: 10.1136/hrt.2007.136093. –. [DOI] [PubMed] [Google Scholar]
- [6].Patane S. Cardiotoxicity: anthracyclines and long term cancer survivors. Int J Cardiol. 2014;176(3):1326. doi: 10.1016/j.ijcard.2014.07.149. –. [DOI] [PubMed] [Google Scholar]
- [7].Lippi G, Mattiuzzi C, Comelli I, Cervellin G. Glycogen phosphorylase isoenzyme BB in the diagnosis of acute myocardial infarction: a meta-analysis. Biochem Med (Zagreb) 2013;23(1):78. doi: 10.11613/BM.2013.010. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Horacek JM, Vasatova M, Pudil R, Tichy M, Zak P, Jakl M. Biomarkers for the early detection of anthracycline-induced cardiotoxicity: current status. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2014;158(4):511. doi: 10.5507/bp.2014.004. et al. –. [DOI] [PubMed] [Google Scholar]
- [9].Lillpopp L, Tzikas S, Ojeda F, Zeller T, Baldus S, Bickel C. Prognostic information of glycogen phosphorylase isoenzyme BB in patients with suspected acute coronary syndrome. Am J Cardiol. 2012;110(9):1225. doi: 10.1016/j.amjcard.2012.06.020. et al. –. [DOI] [PubMed] [Google Scholar]
- [10].Bozkurt S, Kaya EB, Okutucu S, Aytemir K, Coskun F, Oto A. The diagnostic and prognostic value of first hour glycogen phosphorylase isoenzyme BB level in acute coronary syndrome. Cardiol J. 2011;18(5):496. doi: 10.5603/cj.2011.0004. –. [DOI] [PubMed] [Google Scholar]
- [11].Totzeck M, Hendgen-Cotta UB, Rammos C, Petrescu AM, Meyer C, Balzer J. Assessment of the functional diversity of human myoglobin. Nitric Oxide. 2012;26(4):211. doi: 10.1016/j.niox.2012.03.001. et al. –. [DOI] [PubMed] [Google Scholar]
- [12].Flogel U, Fago A, Rassaf T. Keeping the heart in balance: the functional interactions of myoglobin with nitrogen oxides. J Exp Biol. 2010;213(Pt 16):2726. doi: 10.1242/jeb.041681. –. [DOI] [PubMed] [Google Scholar]
- [13].Dudnyk VM, Zborovskaya OO. [Biochemical Markers of Myocardial Damage in Children after Surgical Correction of Congenital Heart Disease] Lik Sprava. 2015;1-2:127. –. [PubMed] [Google Scholar]
- [14].Paget V, Legedz L, Gaudebout N, Girerd N, Bricca G, Milon H. N-terminal pro-brain natriuretic peptide: a powerful predictor of mortality in hypertension. Hypertension. 2011;57(4):702. doi: 10.1161/HYPERTENSIONAHA.110.163550. et al. –. [DOI] [PubMed] [Google Scholar]
- [15].Feola M, Garrone O, Occelli M, Francini A, Biggi A, Visconti G. Cardiotoxicity after anthracycline chemotherapy in breast carcinoma: effects on left ventricular ejection fraction, troponin I and brain natriuretic peptide. Int J Cardiol. 2011;148(2):194. doi: 10.1016/j.ijcard.2009.09.564. et al. –. [DOI] [PubMed] [Google Scholar]
- [16].Skovgaard D, Hasbak P, Kjaer A. BNP predicts chemotherapy-related cardiotoxicity and death: comparison with gated equilibrium radionuclide ventriculography. PLoS One. 2014;9(5):e96736. doi: 10.1371/journal.pone.0096736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Geske JB, McKie PM, Ommen SR, Sorajja P. B-type natriuretic peptide and survival in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2013;61(24):2456. doi: 10.1016/j.jacc.2013.04.004. –. [DOI] [PubMed] [Google Scholar]
- [18].Gerber IL, Stewart RA, Legget ME, West TM, French RL, Sutton TM. Increased plasma natriuretic peptide levels reflect symptom onset in aortic stenosis. Circulation. 2003;107(14):1884. doi: 10.1161/01.CIR.0000060533.79248.0C. et al. –. [DOI] [PubMed] [Google Scholar]
- [19].World M PN.. Medical Association publishes the Revised Declaration of Helsinki. Natl Med J India. 2014;27(1):56. [PubMed] [Google Scholar]
- [20].Keefe DL. Anthracycline-induced cardiomyopathy. Semin Oncol. 2001;28(4):2. Suppl 12. –. [PubMed] [Google Scholar]
- [21].Lenihan DJ, Stevens PL, Massey M, Plana JC, Araujo DM, Fanale MA. The Utility of Point-of-Care Biomarkers to Detect Cardiotoxicity During Anthracycline Chemotherapy: A Feasibility Study. J Card Fail. 2016;22(6):433. doi: 10.1016/j.cardfail.2016.04.003. et al. –. [DOI] [PubMed] [Google Scholar]
- [22].Wang YD, Chen SX, Ren LQ. Serum B-type natriuretic peptide levels as a marker for anthracycline-induced cardiotoxicity. Oncol Lett. 2016;11(5):3483. doi: 10.3892/ol.2016.4424. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Troughton RW, Frampton CM, Brunner-La Rocca HP, Pfisterer M, Eurlings LW, Erntell H. Effect of B-type natriuretic peptide-guided treatment of chronic heart failure on total mortality and hospitalization: an individual patient meta-analysis. Eur Heart J. 2014;35(23):1559. doi: 10.1093/eurheartj/ehu090. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Volkova M, Russell R. Anthracycline cardiotoxicity: prevalence, pathogenesis and treatment. Curr Cardiol Rev. 2011;7(4):214. doi: 10.2174/157340311799960645. 3rd. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Colombo A, Cardinale D. Using cardiac biomarkers and treating cardiotoxicity in cancer. Future Cardiol. 2013;9(1):105. doi: 10.2217/fca.12.73. –. [DOI] [PubMed] [Google Scholar]
- [26].Cubranic Z, Madzar Z, Matijevic S, Dvornik S, Fisic E, Tomulic V. Diagnostic accuracy of heart fatty acid binding protein (H-FABP) and glycogen phosphorylase isoenzyme BB (GPBB) in diagnosis of acute myocardial infarction in patients with acute coronary syndrome. Biochem Med (Zagreb) 2012;22(2):225. doi: 10.11613/bm.2012.025. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Pongprot Y, Sittiwangkul R, Charoenkwan P, Silvilairat S. Use of cardiac markers for monitoring of doxorubixin-induced cardiotoxicity in children with cancer. J Pediatr Hematol Oncol. 2012;34(8):589. doi: 10.1097/MPH.0b013e31826faf44. –. [DOI] [PubMed] [Google Scholar]
- [28].Palumbo I, Palumbo B, Fravolini ML, Marcantonini M, Perrucci E, Latini ME. Brain natriuretic peptide as a cardiac marker of transient radiotherapy-related damage in left-sided breast cancer patients: A prospective study. Breast. 2016;25:45. doi: 10.1016/j.breast.2015.10.004. et al. –. [DOI] [PubMed] [Google Scholar]
- [29].Zidan A, Sherief LM, El-sheikh A, Saleh SH, Shahbah DA, Kamal NM. NT-proBNP as early marker of subclinical late cardiotoxicity after doxorubicin therapy and mediastinal irradiation in childhood cancer survivors. Dis Markers. 2015;2015:513219. doi: 10.1155/2015/513219. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Horacek JM, Vasatova M, Tichy M, Pudil R, Jebavy L, Maly J. The use of cardiac biomarkers in detection of cardiotoxicity associated with conventional and high-dose chemotherapy for acute leukemia. Exp Oncol. 2010;32(2):97. –. [PubMed] [Google Scholar]


