Abstract
BACKGROUND:
The causal biology underlying schizophrenia is not well understood, but it is likely to involve a malfunction in how neurons adjust synaptic connections in response to patterns of activity in networks. We examined statistical dependencies between neural signals at the cell, local circuit, and distributed network levels in the prefrontal and parietal cortex of monkeys performing a variant of the AX-CPT paradigm. We then quantified changes in the pattern of neural interactions across levels of scale following NMDAR blockade and related these changes to a pattern of cognitive control errors closely matching the performance of patients with schizophrenia.
METHODS:
We recorded the spiking activity of 1762 neurons along with local field potentials at multiple electrode sites in prefrontal and parietal cortex concurrently, generated binary time series indicating the presence or absence of spikes in single neurons, or LFP power above or below a threshold. We then applied causal discovery analysis to the time series to detect statistical dependencies between the signals (causal interactions) and compared the pattern of these interactions before and after NMDAR blockade.
RESULTS:
Global blockade of NMDAR produced distinctive, and frequently opposite changes in neural interactions at the cell, local circuit and network levels in prefrontal and parietal cortex. Cognitive control errors were associated with decreased interactions at the cell level and opposite changes at the network level in prefrontal and parietal cortex.
CONCLUSIONS:
NMDAR synaptic deficits change causal interactions between neural signals at different levels of scale that correlate with schizophrenia-like deficits in cognitive control.
Keywords: AX-CPT, cognitive control, NMDA, primate, schizophrenia, causal modeling, neural dynamics
MAIN TEXT
Patients with schizophrenia (1–3) and other neuropsychiatric disorders (4–8) exhibit deficits in cognitive control, defined as the ability to use contextual information, such as goals or rules, stored in working memory, to modify behavioral responses to environmental stimuli (9). This deficit has been measured using variants of the AX continuous performance task (AX-CPT)(3,7,10) in which a contextual cue (designated A or B) stored in working memory modifies the subsequent response to a probe stimulus (designated X or Y). Patients with schizophrenia (1,3), including those at first episode (11,12), as well as their first-degree relatives (13), exhibit robust deficits on the AX-CPT, suggesting this task measures a specific deficit in cognition. Patients exhibit the most profound deficit when a B-cue stored in working memory countermands the habitual response to a subsequent X-probe (‘BX’ errors), a pattern of deficit observed to a lesser degree in other neuropsychiatric disorders, including depression (7,8), and bipolar disorder (4–6). To better understand the underlying defect in neural circuit operation that may contribute to this specific defect in cognitive control, we translated a dot-pattern variant (DPX) of the AX-CPT to nonhuman primates and conducted neural recording in prefrontal and posterior parietal cortex. We have shown previously that monkeys treated with an NMDAR antagonist exhibit the same BX-selective error pattern (14) as patients with schizophrenia performing the DPX task (15). Here we contrast neural dynamics in the prefrontal-parietal network under baseline conditions and following NMDAR blockade to understand how reduction of this synaptic mechanism disrupts neural circuit dynamics at the cellular, local circuit, and distributed network levels.
Cognitive control is thought to depend on goal or rule information that is represented in working memory by the persistent activation of subsets of prefrontal neurons selective for the items of stored information (9,16–20). The persistent activation of prefrontal neurons in turn is thought to depend on recurrent excitation in axon collateral networks that are particularly prominent in layer III (16,21,22) and particularly dependent on NMDAR synaptic currents. Pharmacological blockade of NMDAR but not AMPAR synaptic currents reduces persistent activity in prefrontal neurons of monkeys performing working memory tasks (23,24), and the sustained activity of neurons in artificial neural networks modeled on prefrontal cortex depends on NMDAR currents (25,26).
The above synaptic and circuit mechanisms of working memory in prefrontal cortex are selectively degraded in schizophrenia. Functional neuroimaging studies show reduced delay period prefrontal activation in patients performing working memory tasks (27), specifically on B-cue trials of the AX-CPT (1,11). NMDAR antagonists administered to healthy subjects replicate both hypofrontality and working memory deficits seen in schizophrenia (28,29), and a prominent cluster of schizophrenia risk mutations occur near genes with functional roles at NMDAR synapses (30–33). NMDAR are found on dendritic spines (34), which are reduced in density in schizophrenia, particularly in prefrontal cortex and particularly in layer III (21,35,36) where recurrent excitation in axon collateral networks is thought to contribute to persistent activity. These facts identify candidate neural circuit and synaptic mechanisms in prefrontal cortex the disruption of which could contribute to working memory and cognitive control deficits in schizophrenia.
However, it is not known how biological events at the cell, synaptic, local circuit and distributed network levels mutually influence each other to drive pathogenesis in schizophrenia or any other neuropsychiatric disease. There is as yet no ready way to measure the operation or functional state of individual neurons in the human brain of patients with neuropsychiatric disorders. To enable cell level analysis of brain network failure during cognitive control deficits like those seen in schizophrenia, we have used a nonhuman primate model (14,18,37). Here we apply causal discovery analysis to time series of neural activity recorded at different levels of scale in prefrontal and parietal cortex to understand how physiological signals at the cellular, local circuit and distributed network levels interact during cognitive control, and how these interactions are disrupted by NMDAR synaptic malfunction.
METHODS AND MATERIALS
Behavioral task
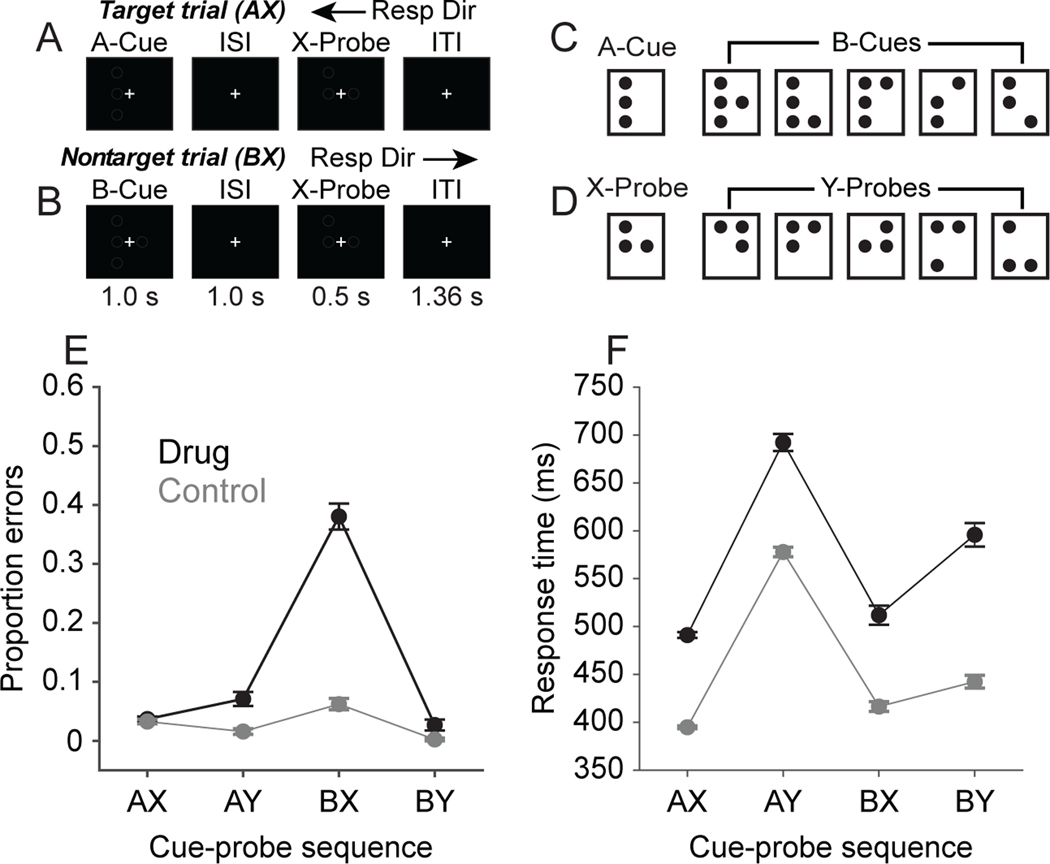
The behavioral and neural data we analyze in the present report were collected as part of our prior studies (18,37). Additional detail regarding experimental methods can be found in those reports, and in the Supplemental Information. Two male rhesus macaque monkeys performed the dot-pattern expectancy (DPX) task (Fig. 1A-D). The DPX task is identical to the AX continuous performance task except that dot patterns replace letters as stimuli. Gaze angle was monitored using a video eye tracking system (ISCAN, Inc.), and monkeys maintained gaze fixated on a central target throughout each trial. Following 500 ms of initial fixation, a cue stimulus (1 s) was presented, followed by a delay period (1 s), and then a probe stimulus (0.5 s). One dot pattern constituted the A-cue, and 5 dot patterns collectively constituted B-cues. (Fig. 1C). Similarly, one dot pattern constituted the X-probe, and 5 dot patterns collectively constituted Y-probes (Fig. 1D). Monkeys moved a joystick to the left or right using their right hand following the onset of the probe. The rewarded joystick direction was a function of the cue-probe sequence. The AX cue-probe sequence was the target sequence and required a leftward movement (Fig. 1A). All other cue-probe sequences were nontarget requiring a rightward movement (Fig. 1B; BX sequence shown). On the majority of trials (69%), the AX sequence was presented, establishing a prepotent tendency to produce the target (leftward) response to the X-probe. On the remaining 31% of trials, nontarget sequences were presented (12.5% AY, 12.5% BX, 6% BY). Successful trials were rewarded with a drop of sweetened water. All animal care and experimental procedures conformed to National Institutes of Health guidelines and were approved by the Animal Care and Use Committee at the Minneapolis Veterans Administration Medical Center.
Figure 1. DPX task and behavioral performance.
A. AX trial event sequence. The A-cue is followed by the X-probe, requiring a target response (left joystick movement). B. BX trial event sequence. The B-cue is followed by the X-probe, requiring a nontarget response (right joystick movement). C. Cue stimuli. D. Probe stimuli. E. Proportion of errors as a function of cue-probe sequence in the Control condition (gray) and Drug condition (black). F. Response time as a function of cue-probe sequence in the Control condition (gray) and the Drug condition (black). Error bars in (E) and (F) represent twice the standard error of the mean.
Neural recording, NMDAR antagonist regimen, and neural database
We recorded 34 neuronal ensembles in the Control condition (either with or without an injection of saline, before first exposure to NMDAR antagonist) and 34 neuronal ensembles in the Drug condition (following injection of the NMDAR antagonist phencyclidine, 0.25–0.30 mg/kg i.m.). We restricted analyses to the subsets of neurons in prefrontal and parietal cortex that significantly modulated their firing in relation to task events (see Supplementary Information). In total, we analyzed the spiking activity of 1,762 cortical neurons. The 34 neuronal ensembles in the Control condition included 289 task-related parietal neurons (average 11 per ensemble) and 468 task-related prefrontal neurons (average 15 per ensemble). The 34 neuronal ensembles in the Drug condition included 434 task-related parietal neurons (average 13 per ensemble) and 571 task-related prefrontal neurons (average 14 per ensemble).
Format of data for Causal Discovery Analysis
We performed a causal discovery analysis separately on each simultaneously-recorded neuronal ensemble in the Control and Drug conditions. The data for each analysis consisted of time series of both neural and task data, concatenated over trials (average of 217 trials per set for the Control condition and 235 trials per set for the Drug condition). Each trial was represented by a set of binary time series (all values coded as 0 or 1) that represented the spiking of single neurons, modulations in LFP oscillatory power, and task state. Single neuron spiking data (between 2 and 32 neurons across ensembles) indicated whether or not an individual neuron generated an action potential in each 1 ms time bin. LFP data indicated whether oscillatory power fell above (1) or below (0) a threshold value in delta, theta, alpha, beta, and gamma bands (5 LFP variables per cortical area). The threshold was the 75th percentile of the distribution of values in each frequency band. We computed the time-varying power of LFP signals (from 1–100 Hz) using a Morlet wavelet analysis, implemented using the ft_freqanalysis function in the FieldTrip Matlab toolbox (38). Time-frequency LFP data were calculated at a 1000 Hz resolution, and then averaged across frequencies within frequency bands (delta: 1–4 Hz, theta: 4.1–8 Hz, alpha: 8.1–15 Hz, beta: 15.1–35 Hz, gamma: 35.1–100 Hz). LFP data were restricted to one channel selected at random in each cortical area to limit the total number of variables entered. Five task state variables were non-overlapping step functions indicating whether (1) or not (0) the fixation, cue, delay, probe and intertrial epoch was currently active at each 1 ms time step. All data (single neuron, LFP, and task) were represented at 1000 Hz, beginning 499 ms before the onset of the cue stimulus and ending 1500 ms after the onset of the probe stimulus, for a total of 4000 data points per trial.
Causal Discovery Analysis
We used causal discovery (39,40) to estimate causal interactions between neural signals recorded at different levels of scale in the prefrontal-parietal network. We provide a conceptual description of the analysis here and additional detail in Supplemental Information. Each experiment is represented as a set of nodes in a graph, representing task state or neural signals recorded on a single electrode in prefrontal or parietal cortex. We used Fast Greedy Equivalence Search (FGES)(41), a causal discovery algorithm that finds the pattern of causal interactions between nodes (directed edges; Fig. 3), represented as a Bayesian network, that simultaneously (1) maximizes the probability of the data given the model, and (2) minimizes the number of edges. The probability of the data given the model is given by
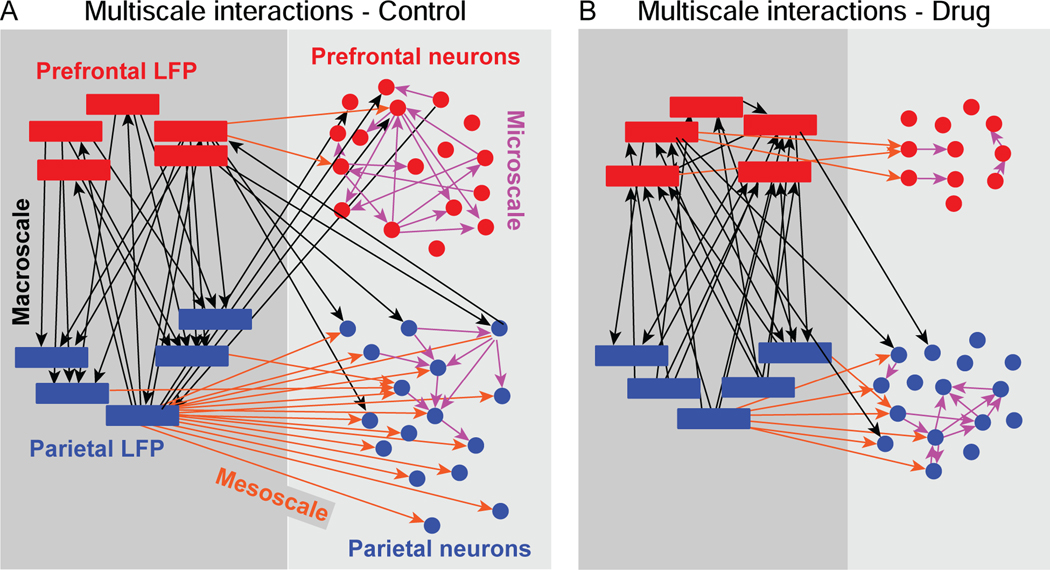
Figure 3. Example graphs illustrating the pattern of multiscale interactions detected in simultaneously recorded neural data.
Causal discovery analysis was applied to time series of neural signals recorded simultaneously in prefrontal (red) and parietal (blue) cortex. Data are from examples of individual neural recording sessions. Thresholded LFP time series at each of five frequency bands are represented by boxes. Time series of spiking activity in individual neurons are represented by circles. Edges indicate detected interactions between neural signals, color indicates the level of scale of the interaction: microscale (between spiking neurons; pink arrows), mesoscale (between spiking neurons and LFP signals in the same cortical area, orange arrows), or macroscale (between cortical areas, either spiking neurons or LFP signals: black arrows). A. Example directed graph derived from an individual neural recording session under the Control condition. B. Example directed graph derived from a different individual neural recording session under the Drug condition.
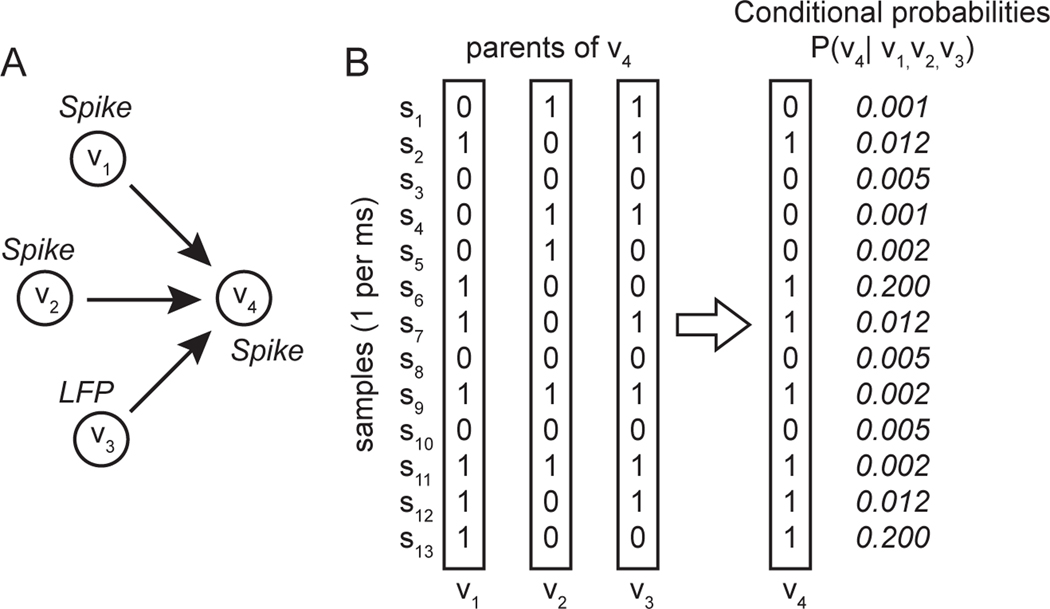
Where X is the data, M is the model, and S and V are the samples and variables. PM is a multinomial probability distribution computed from the data and stored as conditional probability tables in model M, Xs,ν is the value of variable v in sample s (0 or 1 in our case), Mpa(ν) is the set of variables that are parents of v (e.g. send directed edges to v) in model M, and is the corresponding set of values in those parent variables in sample s. The conditional probabilities between nodes are represented by directed edges in the graphs (Fig. 3). This expression states that the conditional probability of the data X given model M is the product across connected nodes and samples of the conditional probabilities of the sample values. The conditional probabilities between the sample values defined by the pattern of connected nodes are computed directly from the data. In the example shown (Fig. 2A), three nodes (two spike trains in individual neurons and one LFP power time series) are ‘parents’ providing input to a fourth, ‘child’ node (neuron). The directed edges imply that the state of the parents at each time step (sample), represented as combinations of 0s and 1s (Fig. 2B, left; [0,1,1; 1,0,1...]) influences the state of the child (Fig. 2B, right; [0 or 1]). This influence is expressed as the conditional probability that the child (V4) takes on a given value given the concurrent values of its three parents (V1, V2,, V3). At the first time step (S1), the parents have values [0,1,1] and the child has value [0]. The conditional probability associated with this concurrent set of values in parents and child is P(V4 = 0 | V1, = 0, V2,=1, V3=1 ) (0.001 in this hypothetical example). That conditional probability is determined by counting the number of instances that this particular combination of values in parents and child occurs, divided by the total number of instances when the parents took on the values [0, 1, 1] regardless of the state of the child. The edges can be interpreted as ‘causal’ in the sense that they indicate which nodes influence other nodes, in terms of providing conditional probabilities included in the model. We evaluated conditional probabilities at 0-lag to measure synchronous neural interactions, analogous to the 0-lag spike correlation in prefrontal cortex we investigated previously (37). Consequently, the interactions we detected are likely mediated by reciprocal dynamics in polysynaptic networks that modulate synchrony at multiple levels of scale. We define microscale interactions as conditional probabilities between the spike trains (action potential time series) of individual neurons. We define mesoscale interactions as statistical dependencies between the spike trains of individual neurons and modulations in LFP power recorded in the same cortical area. We define macroscale interactions as statistical dependencies in neural signals (either spikes or LFPs) between cortical areas.
Figure 2. Conceptual schematic illustrating application of causal discovery analysis to neural time series data.
A. Graph showing directed edges from three parent nodes V1, V2, V3, (two spiking neuron and one LFP power) to a child node, V4 (spiking neuron). B. Sequence of activity states (samples) each represented as a 0 or a 1 at each time step, in the three parent nodes and the one child node, along with the conditional probabilities associated with each combination of states at each time step. FGES finds the pattern of edges that maximizes the product of the conditional probabilities over all samples and nodes in the network, while minimizing the number of edges.
Quantification of neural interactions
We expressed the number of directed edges obtained in Bayesian networks as a proportion of the total possible number of edges given the number of variables entered, and bias corrected these proportions by subtracting the number of edges that we could expect either at chance or to reflect entrainment of neural signals to external events (stimuli and responses), rather than real-time physiological interactions between neurons in circuits. For this purpose, we generated a permutation distribution of 100 Bayesian graphs for each neuronal ensemble after applying causal discovery analysis to trial-shuffled neural data (keeping the time series for each trial within each variable intact). This retained the entrainment of neural signals to external events but broke the simultaneity of the neural signals, precluding physiological interactions between neurons in circuits from contributing to the detected interactions. We then subtracted the mean of the number of directed edges in the permutation distribution from the number of directed edges in the original data. (See Supplemental Figure 1 for an example of this correction procedure.) Differences in the proportion of bias-corrected directed edges in the Bayesian networks between Control and Drug conditions in the original data were deemed significant if they exceeded the 95th percentile of 100 condition differences generated from the trial-shuffled data
RESULTS
Behavioral performance
In the baseline condition, the monkeys’ performance on the DPX task (Fig. 1A, B) was 97% correct, and the BX error rate was 8% (Fig. 1E, gray). In the drug condition, the BX error rate increased to 38% (Fig. 1E, black), and the proportion of errors was significantly greater on BX trials in comparison to the other trial types (X2 1 = 2218, n = 12,676 trials, p < 0.001). BX trials impose high proactive cognitive control demand (12,42) as the B-cue stored in working memory must countermand the habitual target response to the subsequent X-probe. AY trials impose high reactive cognitive control demand because presentation of the Y-probe must countermand the habitual target response associated with the A-cue stored in working memory. AY trials were associated with elevated response time (RT) in both the Drug (Fig. 1F, black) and Control Fig. 1F, gray) conditions, perhaps reflecting elevated reactive control demand. In the Drug condition, RTs significantly differed as a function of trial type (Fig. 1F; Kruskal-Wallis test; 2 = 2292, d.f. = 3, 11488, p < 0.001), with RTs on AY trials being significantly greater than RTs on BY trials (Tukey HSD, p < 0.05). The similarity in RTs across trial types in the Drug and Control conditions (Fig. 2F) suggests that monkeys applied similar cognitive strategies to task stimuli with and without NMDAR blockade. The difference in RT on AY versus BY trials in the Drug condition provides evidence that monkeys continued to treat the task as a conditional response task following NMDAR blockade, as the cue (A vs. B-cue) continued to influence processing time to respond to the probe (Y-probe).
Causal interactions between neural signals
The results of the causal discovery analysis applied to our neural data described interactions at three levels of scale in the brain. Microscale interactions (Fig. 3; pink arrows) were detected between the spike trains of simultaneously recorded neurons within the same cortical area, indicating the influence of spiking in one neuron on spiking in another neuron. Mesoscale interactions were detected between spike trains of neurons and simultaneously recorded LFP signals within the same cortical area (Fig. 3; orange arrows). Mesoscale interactions captured the extent to which power fluctuations in oscillatory LFP components influenced single neuron spiking in local circuits. Macroscale interactions were detected between LFP signals (or LFP signals and neuronal spike trains) recorded simultaneously in different cortical areas (Fig. 3; black arrows). Macroscale interactions captured neural dynamics across cortical areas communicating in distributed corticocortical networks.
Microscale (cell-cell) interactions
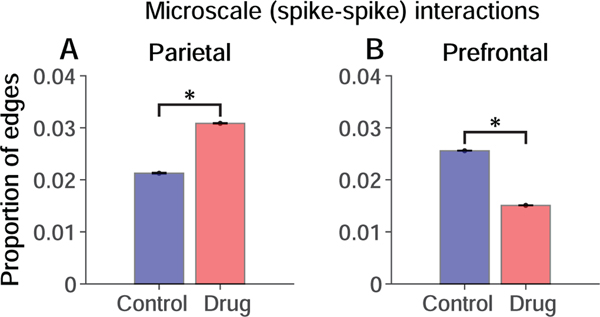
We quantified microscale (cell-cell) interactions between the spike trains of neurons at the population level by counting the number of directed edges between neurons detected by causal discovery analysis in relation to the total number of edges possible in the data (constrained by the number of pairs of simultaneously recorded neurons). We found that systemically blocking NMDAR produced opposite effects on microscale (cell-cell) interactions in parietal and prefrontal local circuits, significantly enhancing these interactions in parietal cortex (Fig. 4A; p < 0.01, permutation test, Methods), and suppressing them in prefrontal cortex (Fig. 4B; p < 0.01). The prefrontal finding is consistent with our prior report based on a different statistical analysis (37).
Figure 4. Influence of NMDAR blockade on microscale interactions.
Permutation-corrected proportion of significant interactions between neurons relative to the total number of possible interactions given the numbers of neurons recorded. Significant differences are indicated by * (p < 0.01; permutation test). Blue, Control condition. Red, Drug condition. A. Parietal neurons. B. Prefrontal neurons. Error bars represent the standard deviation of the permutation distributions.
Mesoscale (local circuit-cell) interactions
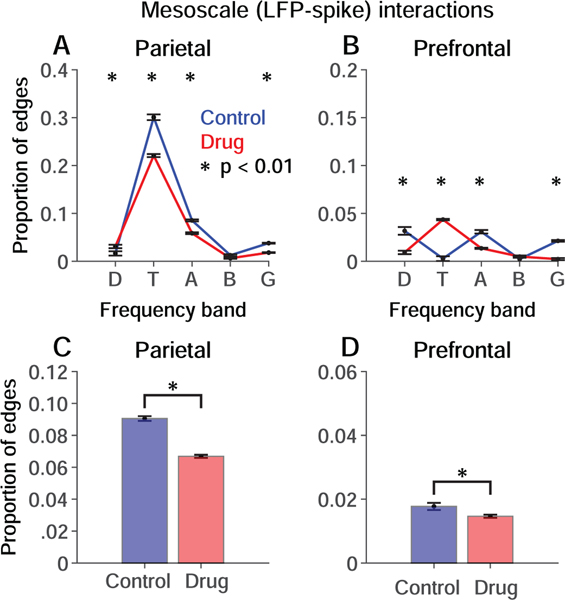
In parietal cortex, mesoscale interactions between LFP and neuronal spike trains were dominated by LFP oscillations in the theta band (4–8 Hz) relative to other frequencies (Fig. 5A). In prefrontal cortex, the prominent peak of mesoscale interactions at theta frequencies was not evident (Fig. 5B). NMDAR blockade significantly weakened theta band mesoscale interactions in parietal cortex, and significantly enhanced them in prefrontal cortex (Fig. 5A, B, red vs. blue; p < 0.01). Collapsing across frequencies, mesoscale interactions were significantly weakened in both cortical areas, although modestly in prefrontal cortex (Fig. 5C, D).
Figure 5. Influence of NMDAR blockade on mesoscale interactions.
Permutation-corrected proportion of significant interactions between LFP signals and spike trains within the same cortical area relative to the total number possible given the number of paired LFP recordings, the number of frequency bands analyzed, and the number of recorded neurons. Blue, Control condition. Red, Drug condition. Significant differences are indicated by * (p < 0.01; permutation test). A, B. Mesoscale interactions within (A) parietal and (B) prefrontal cortex separated by LFP frequency band. Delta, theta, alpha, beta and gamma bands are represented by D, T, A, B and G respectively. C, D. Mesoscale interactions in (C) parietal and (D) prefrontal cortex collapsed across frequency bands. Error bars represent the standard deviation of the permutation distributions.
Macroscale (area-area) interactions
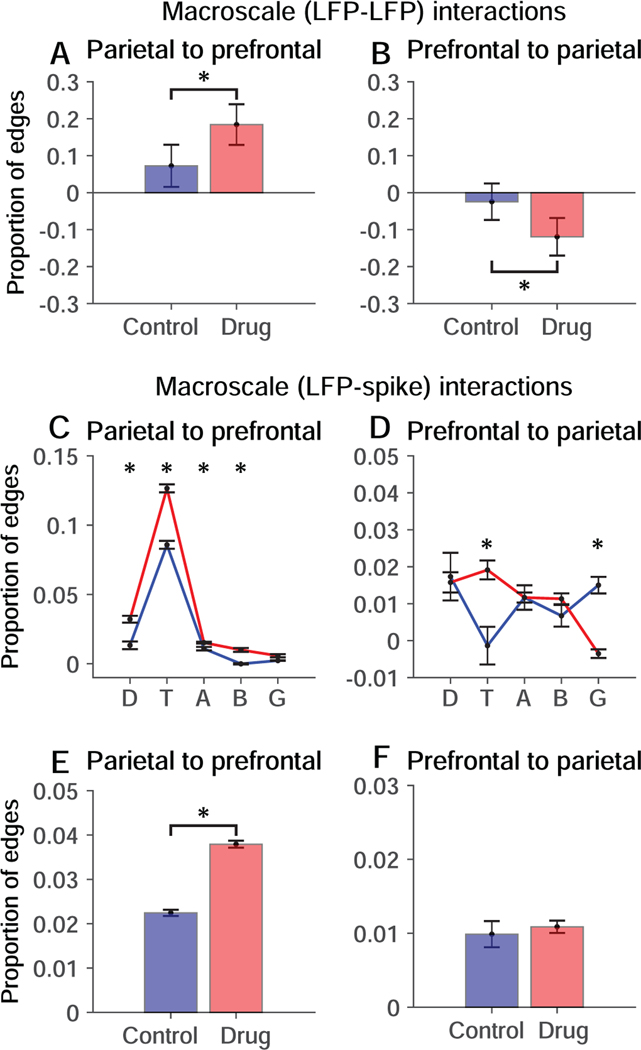
Blocking NMDAR significantly increased bottom-up (Fig. 6A) and decreased top-down (Fig. 6B) macroscale interactions between LFP signals in prefrontal and parietal cortex. Blocking NMDAR increased bottom-up (Fig. 6C) and also top-down (Fig. 6D) macroscale interactions between LFP signals in the theta band in one cortical area and spikes in the other. Collapsing across frequency bands, bottom-up macroscale interactions between LFP signals in parietal cortex and spikes in prefrontal cortex were significantly enhanced (Fig. 6E).
Figure 6. Influence of NMDAR blockade on macroscale interactions.
Permutation-corrected proportion of interactions between LFP signals or between LFP signals and neuronal spike trains in prefrontal and parietal cortex relative to the total number possible given the number of LFP frequency bands and simultaneously recorded neurons. Blue, Control condition. Red, Drug condition. Significant differences are indicated by * (p < 0.01; permutation test). A, B. Bottom-up (A) and top-down (B) macroscale (LFP-LFP) interactions. C, D. Bottom-up (C) and top-down (D) macroscale (LFP-spike) interactions separated by frequency band. Delta, theta, alpha, beta and gamma bands are represented by D, T, A, B and G respectively. E, F. Bottom-up (E) and top-down (F) macroscale (LFP-spike) interactions collapsed across frequency bands. Error bars represent the standard deviation of the permutation distributions.
Multiscale interactions that predict failure in cognitive control
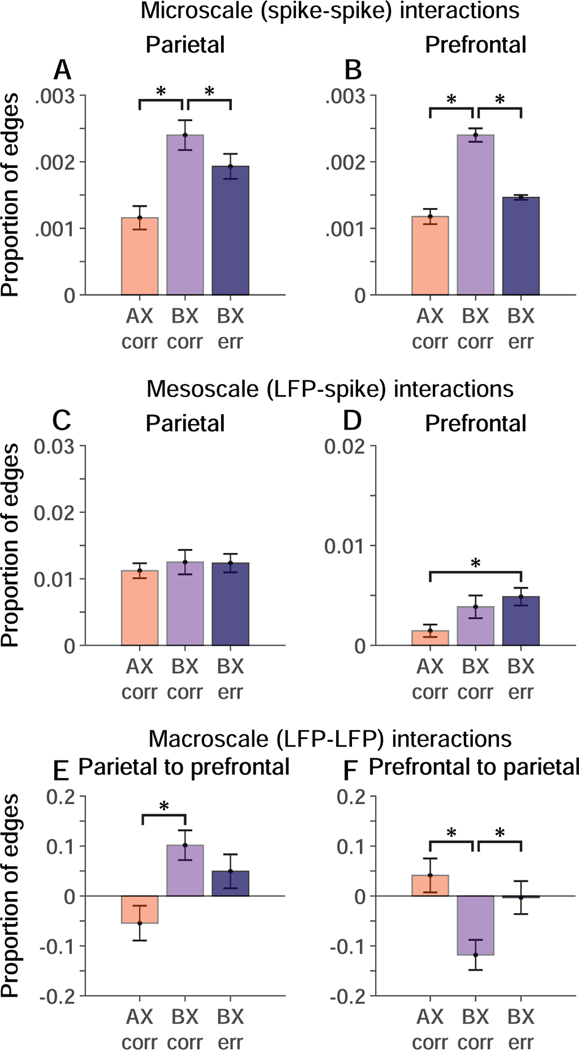
Patients with schizophrenia (11,12), and monkeys administered NMDAR antagonists (14) (Fig. 1E) performing the AX-CPT and DPX tasks both exhibit a selective increase in errors on BX trials, when a B-cue stored in working memory must countermand a habitual response to the X-probe. To isolate changes in network dynamics associated with the commission of BX errors, we stratified trials by cue-probe sequence and trial outcome and repeated the causal discovery analysis on the resulting subsets of trials (Fig. 7). To understand how network dynamics change in response to increased cognitive control demand associated with the B-cue, we compared causal interactions on correct BX trials to interactions on AX trials. To understand how failure of network interactions leads to BX errors, we compared causal interactions on BX correct trials to interactions on BX error trials. We restricted this analysis to trials performed in the Drug condition, when there were enough errors to analyze (Fig. 1E). We found that in the Drug Condition, microscale (spike-spike) interactions between neurons increased in both parietal cortex (Fig. 7A) and prefrontal cortex (Fig. 7B) on correct BX trials (light purple) relative to correct AX trials (orange), and further that these changes reversed when monkeys made BX errors (dark purple). We also found that in the Drug Condition, macroscale (LFP-LFP) parietal-to-prefrontal interactions increased (Fig. 7A), whereas prefrontal-to-parietal interactions decreased (Fig. 7B) on correct BX trials (light purple) relative to correct AX trials (orange). These changes in macroscale interactions on correct BX relative to AX trials (Fig. 7E, F) were in the same direction as changes seen in the Drug relative to the Control conditions overall (Fig. 6A, B; collapsing across trial types). That suggests that perturbations in macroscale interactions in the Drug relative to the Control condition may have been pronounced on BX trials in the Drug condition. The decrease in prefrontal-to-parietal macroscale interactions evident on BX correct trials reversed on BX error trials (Fig. 7F). Mesoscale interactions exhibited a weaker relation to trial type and outcome (Fig. 7C, D). These data provide a dynamical signature of cognitive control failure in the form of altered neural interactions in the prefrontal-parietal network when monkeys made BX errors.
Figure 7. Relation of multiscale interactions to behavioral performance in the Drug condition.
Causal interactions stratified by trial type (cue-probe sequence) and trial outcome (correct = corr, error = err). Data are permutation-corrected proportion of significant interactions between neural signals at different levels of scale relative to the total number possible on AX correct (orange), BX correct (light purple), and BX error (dark purple) trials. Significant differences are indicated by * (p < 0.01; permutation test). A, B. Microscale (spike-spike) causal interactions in parietal (A) and prefrontal (B) cortex as a function of trial type and outcome. C, D. Mesoscale (LFP-spike) interactions within parietal (C) and prefrontal (D) cortex as a function of trial type and outcome. E, F. Macroscale (LFP-LFP) interactions in parietal (E) and prefrontal (F) cortex as a function of trial type and outcome. Error bars represent the standard deviation of the permutation distributions.
DISCUSSION
Schizophrenia is a complex disorder resulting from interactions among biological variables operating at different levels of scale in the brain. For example, risk mutations have been identified that alter cell-level variables, such as the molecular mechanisms of synaptic transmission, synaptic plasticity and electrical excitability in neurons (31–33,43). Other risk mutations alter circuit-level variables, such as the pattern and density of axonal projections linking cells into neural circuits (44–46). These changes in neural function and circuit connectivity are likely to distort the physiological dynamics of neural circuits. Schizophrenia risk mutations engineered into animal models have been shown to distort attractor dynamics in visual cortex (47) and hippocampus (48), as well as oscillatory synchrony between the prefrontal cortex and hippocampus (49). Since schizophrenia is likely to involve both cell and circuit level changes, it is important to understand how cell and circuit level variables interact during pathogenesis. Neurons adjust their electrical excitability and synaptic connectivity to other neurons in response to the spatial and temporal pattern of synaptic inputs they experience (50). Those changes in synaptic connectivity in turn modify patterns of network activity, and therefore feedback to further modify synaptic inputs to neurons. A complex interplay between cell state and circuit activity therefore tunes neural networks as neurons adjust their intrinsic properties in response to electrical activity patterns. We recently proposed that schizophrenia may involve a distortion of this feedback process, by which reduction in synchronous spiking between neurons at the circuit level and disconnection of synapses at the cell level may accelerate each other in a positive feedback loop driving a downward spiral that ultimately disconnects prefrontal networks via an activity-dependent process (37). This theory has much in common with other theories of schizophrenia pathogenesis that also involve feedback interactions between cell and circuit level variables as elaborated by other groups (51–53). To further explore the relationship between cell and circuit level variables, and learn how their interaction might be distorted in schizophrenia, we applied causal discovery analysis to time series of neural activity recorded at the cell, local circuit, and distributed network level in prefrontal and posterior parietal cortex of monkeys performing a cognitive control task that measures deficits in schizophrenia (7,14,18). This allowed us to investigate how interactions between neural events across levels of scale were distorted by NMDAR synaptic malfunction, a schizophrenia-relevant manipulation (30,31,54).
NMDAR are broadly distributed throughout the cerebral cortex (34,55). One might predict that brain-wide blockade of NMDAR via systemic administration of antagonist would have a globally suppressive effect on neuronal excitability, producing comparable effects in prefrontal and parietal cortex. Instead, we found marked divergence in how prefrontal and parietal neurons, circuits and networks responded to NMDAR blockade. Generally, communication between prefrontal neurons and top-down prefrontal output were suppressed, whereas communication between parietal neurons and bottom-up parietal output were increased following NMDAR blockade (Figs. 4 and 6). This provides evidence that prefrontal and parietal local circuits are differentially dependent on NMDAR synaptic mechanisms, and that global insult to NMDAR can produce circuit-specific effects on neural activity. NMDAR subunits (NR1, NR2A, and NR2B) are expressed by most prefrontal and parietal neurons but their concentration is higher in prefrontal cortex (56,57). Layer 3 pyramidal neurons in prefrontal and parietal cortex differ substantially in morphology, physiological properties and patterns of gene expression (58). In prefrontal cortex, layer 3 pyramidal neurons are more likely to exhibit a bursting response to input, and have more highly branched basilar dendrites that exhibit a higher density of spines in comparison to layer 3 pyramidal neurons in parietal cortex (58). Cross-correlation analysis has indicated that coincident spiking is more prevalent between parietal than prefrontal neurons (59). These data suggest that there may be differences in synaptic and local circuit mechanisms within prefrontal and parietal cortex that contribute to the differences in NMDAR sensitivity we observed.
One important question of particular relevance to schizophrenia is the nature of the communication failure that occurs in prefrontal circuits and networks when monkeys make BX errors in the task, as this is the dominant pattern seen in the performance of patients. We identified specific changes in both microscale (Fig. 7A, B) and macroscale (Fig. 7E, F) neural interactions associated with successful engagement of cognitive control on correctly performed BX trials that reversed on BX errors. These data provide a dynamical signature of neural interactions that can predict cognitive control failure following NMDAR synaptic malfunction on a trial-by-trial basis.
Systemic administration of NMDAR antagonists to monkeys weakens LFP beta oscillations that encode trial outcome (60), as well as patterns of neuronal activity (24) and spike-field coherence (61) that encode cognitive rules. Iontophoretic application or systemic administration of NMDAR antagonists weakens the persistent activity of prefrontal neurons (23), but spares their post-saccadic responses (23), suggesting circuit-specific effects. Administration of NMDAR antagonist to healthy subjects reduces functional connectivity between prefrontal and posterior parietal cortex during working memory tasks (28), but increase functional connectivity in the resting state (62,63), suggesting state-specific effects. In rat prefrontal cortex, NMDAR blockade increases neuronal firing rate but decreases 0-lag spike synchronization (64), suggesting that NMDAR influence spike timing independently of spike rate, as we have observed (37)(Fig. 4B).
Applying a combination of computational modeling and dynamic causal analysis to MEG data, Shaw and colleagues (65) report that persons with schizophrenia performing a visual discrimination task exhibit reductions in gamma power that are consistent with reduced inhibitory tone in cortical circuits. Applying dynamic causal modeling to fMRI data, Zhou and colleagues (66) report reduced top-down drive from prefrontal to parietal cortex, as well as increased bottom-up drive from posterior cingulate to prefrontal cortex, a pattern of results broadly consistent with our own data (Fig. 6A, B). Roche and colleagues (67) demonstrated that administration NMDAR antagonist to healthy subjects disrupted causal interactions within prefrontal cortex, as we observed (Fig. 4B).
Our data document distinctive and in some cases opposite changes in network dynamics within prefrontal and parietal cortex in response to systemic administration of NMDAR antagonists. These findings suggest that NMDAR synaptic mechanisms play circuit-specific roles in the two cortical areas. We found that causal interactions linked neural signals across the cellular, local circuit and distributed network levels of scale, potentially making it possible to infer the state of neurons from the state of networks. We also found that the pattern of causal interactions across scales varied with cognitive control load and predicted cognitive control failure on a trial-by-trial basis following NMDAR blockade. These data open a cell and circuit level window into how prefrontal networks fail following NMDAR synaptic malfunction that could provide mechanistic insight into how prefrontal networks fail in schizophrenia.
Supplementary Material
Acknowledgements and Disclosures
We thank Dean Evans for lab and project management as well as his assistance with surgeries, animal care, and neural recordings; Dale Boeff for his assistance with neurophysiological recording system design and construction, as well as computer programming for signal processing and data analysis; Sofia Sakellaridi for her assistance with neural recordings. The authors would also like to thank the Center for Causal Discovery for developing and supporting the open source Tetrad software package. Support for this work was provided by the National Institute of Mental Health R01MH107491 to MC, R01MH080318 to ADR, R01MH112688 to ADR, NCRR 1UL1TR002494-01 to EK, Willfred Wetzel Graduate Fellowship, T32 GM008244, T32 HD007151 to RKB, F31MH109238 to ALD, R01MH081051, 1R03MH117254-01A1, R21MH110208-01A1 to SV.
Sophia Vinogradov is on the Scientific Advisory Boards of Mindstrong, Inc, Verily, Inc., and Alkermes. She has served as a site Principal Investigator on an NIMH SBIR grant to PositScience, Inc.
All other authors report no biomedical financial interests or potential conflicts of interest. This material is the result of work supported with resources and the use of facilities at the Minneapolis VA Health Care System. The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Perlstein WM, Dixit NK, Carter CS, Noll DC, Cohen JD (2003): Prefrontal cortex dysfunction mediates deficits in working memory and prepotent responding in schizophrenia. Biol Psychiatry 53: 25–38. [DOI] [PubMed] [Google Scholar]
- 2.Ray KL, Lesh TA, Howell AM, Salo TP, Ragland JD, MacDonald AW, et al. (2017): Functional network changes and cognitive control in schizophrenia. Neuroimage Clin 15: 161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barch DM, Carter CS, MacDonald AW III, Braver TS, Cohen JD (2003): Context-processing deficits in schizophrenia: Diagnostic specificity, 4-week course, and relationships to clinical symptoms. J Abnorm Psychol 112: 132–143. [PubMed] [Google Scholar]
- 4.Smucny J, Lesh TA, Iosif A-M, Niendam TA, Tully LM, Carter CS (2018): Longitudinal stability of cognitive control in early psychosis: Nondegenerative deficits across diagnoses. J Abnorm Psychol 127: 781–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smucny J, Lesh TA, Newton K, Niendam TA, Ragland JD, Carter CS (2018): Levels of Cognitive Control: A Functional Magnetic Resonance Imaging-Based Test of an RDoC Domain Across Bipolar Disorder and Schizophrenia. Neuropsychopharmacology 43: 598–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robinson LJ, Thompson JM, Gallagher P, Gray JM, Young AH, Ferrier IN (2013): Performance monitoring and executive control of attention in euthymic bipolar disorder: employing the CPT-AX paradigm. Psychiatry Res 210: 457–464. [DOI] [PubMed] [Google Scholar]
- 7.MacDonald AW 3rd (2008): Building a clinically relevant cognitive task: case study of the AX paradigm. Schizophr Bull 34: 619–28. doi: 10.1093/schbul/sbn038. Epub 2008 May 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Msetfi RM, Murphy RA, Kornbrot DE, Simpson J (2009): Impaired context maintenance in mild to moderately depressed students. Q J Exp Psychol 62: 653–662. [DOI] [PubMed] [Google Scholar]
- 9.Miller EK, Cohen JD (2001): An integrative theory of prefrontal cortex function. Annu Rev Neurosci 24: 167–202. [DOI] [PubMed] [Google Scholar]
- 10.Carter CS, Minzenberg M, West R, Macdonald A 3rd (2012): CNTRICS imaging biomarker selections: Executive control paradigms. Schizophr Bull 38: 34–42. doi: 10.1093/schbul/sbr114. Epub 2011 Nov 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MacDonald AW 3rd, Carter CS, Kerns JG, Ursu S, Barch DM, Holmes AJ, et al. (2005): Specificity of prefrontal dysfunction and context processing deficits to schizophrenia in never-medicated patients with first-episode psychosis. Am J Psychiatry 162: 475–484. [DOI] [PubMed] [Google Scholar]
- 12.Lesh TA, Westphal AJ, Niendam TA, Yoon JH, Minzenberg MJ, Ragland JD, et al. (2013): Proactive and reactive cognitive control and dorsolateral prefrontal cortex dysfunction in first episode schizophrenia. Neuroimage Clin 2: 590–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Snitz BE, Macdonald AW 3rd, Carter CS (2006): Cognitive deficits in unaffected first-degree relatives of schizophrenia patients: a meta-analytic review of putative endophenotypes. Schizophr Bull 32: 179–94. Epub 2005 Sep 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blackman RK, Macdonald AW 3rd, Chafee MV (2013): Effects of ketamine on context-processing performance in monkeys: a new animal model of cognitive deficits in schizophrenia. Neuropsychopharmacology 38: 2090–100. doi: 10.1038/npp.2013.118. Epub 2013 May 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones JA, Sponheim SR, MacDonald AW 3rd (2010): The dot pattern expectancy task: reliability and replication of deficits in schizophrenia. Psychol Assess 22: 131–41. doi: 10.1037/a0017828. [DOI] [PubMed] [Google Scholar]
- 16.Goldman-Rakic PS (1995): Cellular basis of working memory. Neuron 14: 477–485. [DOI] [PubMed] [Google Scholar]
- 17.Goldman-Rakic PS (1990): Cellular and circuit basis of working memory in prefrontal cortex of nonhuman primates. Prog Brain Res 85: 325–35; discussion 335–6. [DOI] [PubMed] [Google Scholar]
- 18.Blackman RK, Crowe DA, DeNicola AL, Sakellaridi S, MacDonald AW 3rd, Chafee MV (2016): Monkey Prefrontal Neurons Reflect Logical Operations for Cognitive Control in a Variant of the AX Continuous Performance Task (AX-CPT). J Neurosci 36: 4067–79. doi: 10.1523/JNEUROSCI.3578-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crowe DA, Goodwin SJ, Blackman RK, Sakellaridi S, Sponheim SR, MacDonald AW 3rd, Chafee MV (2013): Prefrontal neurons transmit signals to parietal neurons that reflect executive control of cognition. Nat Neurosci 16: 1484–91. doi: 10.1038/nn.3509. Epub 2013 Sep 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chafee MV, Goldman-Rakic PS (1998): Matching patterns of activity in primate prefrontal area 8a and parietal area 7ip neurons during a spatial working memory task. J Neurophysiol 79: 2919–2940. [DOI] [PubMed] [Google Scholar]
- 21.Hoftman GD, Datta D, Lewis DA (2017): Layer 3 Excitatory and Inhibitory Circuitry in the Prefrontal Cortex: Developmental Trajectories and Alterations in Schizophrenia. Biol Psychiatry 81: 862–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.González-Burgos G, Barrionuevo G, Lewis DA (2000): Horizontal synaptic connections in monkey prefrontal cortex: an in vitro electrophysiological study. Cereb Cortex 10: 82–92. [DOI] [PubMed] [Google Scholar]
- 23.Wang M, Yang Y, Wang C-J, Gamo NJ, Jin LE, Mazer JA, et al. (2013): NMDA receptors subserve persistent neuronal firing during working memory in dorsolateral prefrontal cortex. Neuron 77: 736–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma L, Skoblenick K, Seamans JK, Everling S (2015): Ketamine-Induced Changes in the Signal and Noise of Rule Representation in Working Memory by Lateral Prefrontal Neurons. J Neurosci 35: 11612–11622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Compte A, Brunel N, Goldman-Rakic PS, Wang XJ (2000): Synaptic mechanisms and network dynamics underlying spatial working memory in a cortical network model. Cereb Cortex 10: 910–923. [DOI] [PubMed] [Google Scholar]
- 26.Wang XJ (1999): Synaptic basis of cortical persistent activity: the importance of NMD Areceptors to working memory. J Neurosci 19: 9587–9603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Driesen NR, Leung H-C, Calhoun VD, Constable RT, Gueorguieva R, Hoffman R, et al. (2008): Impairment of working memory maintenance and response in schizophrenia: functional magnetic resonance imaging evidence. Biol Psychiatry 64: 1026–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Driesen NR, McCarthy G, Bhagwagar Z, Bloch MH, Calhoun VD, D’Souza DC, et al. (2013): The impact of NMDA receptor blockade on human working memory-related prefrontal function and connectivity. Neuropsychopharmacology 38: 2613–2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Umbricht D, Schmid L, Koller R, Vollenweider FX, Hell D, Javitt DC (2000): Ketamine-induced deficits in auditory and visual context-dependent processing in healthy volunteers: implications for models of cognitive deficits in schizophrenia. Arch Gen Psychiatry 57: 1139–47. [DOI] [PubMed] [Google Scholar]
- 30.Timms AE, Dorschner MO, Wechsler J, Choi KY, Kirkwood R, Girirajan S, et al. (2013): Support for the N-methyl-D-aspartate receptor hypofunction hypothesis of schizophrenia from exome sequencing in multiplex families. JAMA Psychiatry 70: 582–90. doi: 10.1001/jamapsychiatry.2013.1195. [DOI] [PubMed] [Google Scholar]
- 31.Schizophrenia_Working_Group_of_the_Psychiatric_Genomics_Consortium (2014): Biological insights from 108 schizophrenia-associated genetic loci. Nature 511: 421–7. doi: 10.1038/nature13595. Epub 2014 Jul 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kirov G, Pocklington AJ, Holmans P, Ivanov D, Ikeda M, Ruderfer D, et al. (2012): De novo CNV analysis implicates specific abnormalities of postsynaptic signalling complexes in the pathogenesis of schizophrenia. Mol Psychiatry 17: 142–53. doi: 10.1038/mp.2011.154. Epub 2011 Nov 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fromer M, Pocklington AJ, Kavanagh DH, Williams HJ, Dwyer S, Gormley P, et al. (2014): De novo mutations in schizophrenia implicate synaptic networks. Nature 506: 179–84. doi: 10.1038/nature12929. Epub 2014 Jan 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Conti F, Barbaresi P, Melone M, Ducati A (1999): Neuronal and glial localization of NR1 and NR2A/B subunits of the NMDA receptor in the human cerebral cortex. Cereb Cortex 9: 110–120. [DOI] [PubMed] [Google Scholar]
- 35.Glantz LA, Lewis DA (2000): Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry 57: 65–73. [DOI] [PubMed] [Google Scholar]
- 36.Kolluri N, Sun Z, Sampson AR, Lewis DA (2005): Lamina-specific reductions in dendritic spine density in the prefrontal cortex of subjects with schizophrenia. Am J Psychiatry 162: 1200–2. [DOI] [PubMed] [Google Scholar]
- 37.Zick JL, Blackman RK, Crowe DA, Amirikian B, DeNicola AL, Netoff TI, Chafee MV (2018): Blocking NMDAR Disrupts Spike Timing and Decouples Monkey Prefrontal Circuits: Implications for Activity-Dependent Disconnection in Schizophrenia. Neuron 98: 1243–1255.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oostenveld R, Fries P, Maris E, Schoffelen J-M (2011): FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci 2011: 156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spirtes P, Glymour CN, Scheines R, Heckerman D, Meek C, Cooper G, Richardson T (2000): Causation, Prediction, and Search. MIT Press. [Google Scholar]
- 40.Pearl J (2009): Causality. Cambridge University Press. [Google Scholar]
- 41.Ramsey J, Glymour M, Sanchez-Romero R, Glymour C (2017): A million variables and more: the Fast Greedy Equivalence Search algorithm for learning high-dimensional graphical causal models, with an application to functional magnetic resonance images. Int J Data Sci Anal 3: 121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barch DM, Ceaser A (2012): Cognition in schizophrenia: core psychological and neural mechanisms. Trends Cogn Sci 16: 27–34. doi: 10.1016/j.tics.2011.11.015. Epub 2011 Dec 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fénelon K, Mukai J, Xu B, Hsu P-K, Drew LJ, Karayiorgou M, et al. (2011): Deficiency of Dgcr8, a gene disrupted by the 22q11.2 microdeletion, results in altered short-term plasticity in the prefrontal cortex. Proc Natl Acad Sci U S A 108: 4447–4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sekar A, Bialas AR, de Rivera H, Davis A, Hammond TR, Kamitaki N, et al. (2016): Schizophrenia risk from complex variation of complement component 4. Nature 530: 177–83. doi: 10.1038/nature16549. Epub 2016 Jan 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sellgren CM, Gracias J, Watmuff B, Biag JD, Thanos JM, Whittredge PB, et al. (2019): Increased synapse elimination by microglia in schizophrenia patient-derived models of synaptic pruning. Nat Neurosci 22: 374–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fernandez A, Meechan DW, Karpinski BA, Paronett EM, Bryan CA, Rutz HL, et al. (2019): Mitochondrial Dysfunction Leads to Cortical Under-Connectivity and Cognitive Impairment. Neuron. 10.1016/j.neuron.2019.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hamm JP, Peterka DS, Gogos JA, Yuste R (2017): Altered Cortical Ensembles in Mouse Models of Schizophrenia. Neuron 94: 153–167.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zaremba JD, Diamantopoulou A, Danielson NB, Grosmark AD, Kaifosh PW, Bowler JC, et al. (2017): Impaired hippocampal place cell dynamics in a mouse model of the 22q11.2 deletion. Nat Neurosci 20: 1612–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sigurdsson T, Stark KL, Karayiorgou M, Gogos JA, Gordon JA (2010): Impaired hippocampal-prefrontal synchrony in a genetic mouse model of schizophrenia. Nature 464: 763–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dan Y, Poo MM (2006): Spike timing-dependent plasticity: from synapse to perception. Physiol Rev 86: 1033–48. [DOI] [PubMed] [Google Scholar]
- 51.Krystal JH, Anticevic A, Yang GJ, Dragoi G, Driesen NR, Wang X-J, Murray JD (2017): Impaired Tuning of Neural Ensembles and the Pathophysiology of Schizophrenia: A Translational and Computational Neuroscience Perspective. Biol Psychiatry 81: 874–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Uhlhaas PJ, Singer W (2015): Oscillations and neuronal dynamics in schizophrenia: the search for basic symptoms and translational opportunities. Biol Psychiatry 77: 1001–9. doi: 10.1016/j.biopsych.2014.11.019. Epub 2014 Dec 3. [DOI] [PubMed] [Google Scholar]
- 53.Stephan KE, Friston KJ, Frith CD (2009): Dysconnection in schizophrenia: from abnormal synaptic plasticity to failures of self-monitoring. Schizophr Bull 35: 509–27. doi: 10.1093/schbul/sbn176. Epub 2009 Jan 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Javitt DC, Zukin SR, Heresco-Levy U, Umbricht D (2012): Has an angel shown the way? Etiological and therapeutic implications of the PCP/NMDA model of schizophrenia. Schizophr Bull 38: 958–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huntley GW, Vickers JC, Janssen W, Brose N, Heinemann SF, Morrison JH (1994): Distribution and synaptic localization of immunocytochemically identified NMDA receptor subunit proteins in sensory-motor and visual cortices of monkey and human. J Neurosci 14: 3603–3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scherzer CR, Landwehrmeyer GB, Kerner JA, Counihan TJ, Kosinski CM, Standaert DG, et al. (1998): Expression of N-methyl-D-aspartate receptor subunit mRNAs in the human brain: hippocampus and cortex. J Comp Neurol 390: 75–90. [DOI] [PubMed] [Google Scholar]
- 57.Akbarian S, Sucher NJ, Bradley D, Tafazzoli A, Trinh D, Hetrick WP, et al. (1996): Selective alterations in gene expression for NMDA receptor subunits in prefrontal cortex of schizophrenics. J Neurosci 16: 19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.González-Burgos G, Miyamae T, Krimer Y, Gulchina Y, Pafundo DE, Krimer O, et al. (2019): Distinct Properties of Layer 3 Pyramidal Neurons from Prefrontal and Parietal Areas of the Monkey Neocortex. J Neurosci 39: 7277–7290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Katsuki F, Qi X-L, Meyer T, Kostelic PM, Salinas E, Constantinidis C (2014): Differences in intrinsic functional organization between dorsolateral prefrontal and posterior parietal cortex. Cereb Cortex 24: 2334–2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Skoblenick KJ, Womelsdorf T, Everling S (2016): Ketamine Alters Outcome-Related Local Field Potentials in Monkey Prefrontal Cortex. Cereb Cortex 26: 2743–2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ma L, Skoblenick K, Johnston K, Everling S (2018): Ketamine Alters Lateral PrefrontalOscillations in a Rule-Based Working Memory Task. J Neurosci 38: 2482–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grimm O, Gass N, Weber-Fahr W, Sartorius A, Schenker E, Spedding M, et al. (2015): Acute ketamine challenge increases resting state prefrontal-hippocampal connectivity in both humans and rats. Psychopharmacology 232: 4231–4241. [DOI] [PubMed] [Google Scholar]
- 63.Anticevic A, Corlett PR, Cole MW, Savic A, Gancsos M, Tang Y, et al. (2015): N-methyl-D-aspartate receptor antagonist effects on prefrontal cortical connectivity better model early than chronic schizophrenia. Biol Psychiatry 77: 569–580. [DOI] [PubMed] [Google Scholar]
- 64.Molina LA, Skelin I, Gruber AJ (2014): Acute NMDA receptor antagonism disrupts synchronization of action potential firing in rat prefrontal cortex. PLoS One 9: e85842. doi: 10.1371/journal.pone.0085842. eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shaw AD, Knight L, Freeman TCA, Williams GM, Moran RJ, Friston KJ, et al. (2019): Oscillatory, Computational, and Behavioral Evidence for Impaired GABAergic Inhibition in Schizophrenia. Schizophr Bull. 10.1093/schbul/sbz066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou Y, Zeidman P, Wu S, Razi A, Chen C, Yang L, et al. (2018): Altered intrinsic and extrinsic connectivity in schizophrenia. Neuroimage Clin 17: 704–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rosch RE, Auksztulewicz R, Leung PD, Friston KJ, Baldeweg T (2019): Selective Prefrontal Disinhibition in a Roving Auditory Oddball Paradigm Under N-Methyl-D-Aspartate Receptor Blockade. Biol Psychiatry Cogn Neurosci Neuroimaging 4: 140–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.