Abstract
Background: Migraine is a neurologic disorder. Although, based on previous evidence, migraine is related with inflammation and oxidative stress, its relationship with the inflammatory potential of the diet is still unknown. Thus, the aim of this study was to show the correlation between Dietary Inflammatory Index (DII) and severity and duration of migraine headache.
Methods: In this cross-sectional study, 266 women who suffered from migraine, were included. Demographic and anthropometric data were collected form all participants. 147-item semiquantitative food frequency questionnaire (FFQ) was collected to assess dietary intake and consequently, DII scores were calculated. Migraine Disability Assessment (MIDAS) questionnaire, Visual Analog Scale (VAS), and a 30-day headache diary were also completed by each participant.
Results: The DII score ranged between -4.22 and 5.19 and its median [interquartile range (IQR)] was 0.003 (-1.48-1.55). There was no meaningful association between age, occupation, physical activity (PA), weight, height, Body Mass Index (BMI), waist circumference (WC), hip circumference, waist-to-hip ratio (WHR) and DII score classifications (P > 0.050). Subjects with more than 20 days of headache had higher DII score compared to those with less than 10 days per month [odds ratio (OR) = 1.60, 95% confidence interval (CI) = 1.12-2.08, P = 0.001]. There was no association between DII and migraine severity (VAS and MIDAS) in the crude and adjusted model of logistic regression. Although there was a significant association between headache duration and DII (P = 0.020), this relationship was not meaningful after adjusting for age, PA, BMI, and job status (OR = 0.53, 95% CI = 0.28-1.00, P = 0.052).
Conclusion: The present study showed a direct association between headache frequency and DII. Nevertheless, any relationship was not found between headache duration or migraine severity and DII score. Future large and prospective studies are needed to explore the effect of inflammatory potential of diet in migraine characteristics.
Key Words: Migraine Disorders, Inflammation, Dietary
Introduction
Migraine is a neurologic disorder that is characterized by recurrent episodes of headache and usually lasts about 4 to 72 hours.1 This disorder is responsible for 5.6% of disablilities in whole population in the world.2 The severe nature of migraine causes a lower quality of life with high individual and society burden.3 This condition is listed as the first highest cause of disability in under 50 years old.2 Because of mentioned complications, World Health Organization (WHO) describes the primary headache, including migraine, as a major public health problem.4 Based on attack frequency, migraine has been divided into chronic and episodic subtypes. Although chronic migraineurs experience severe headache-related disability, episodic migraine is more prevalent and could be developed to chronic migraine over time.5-7
The exact pathophysiology of migraine is unknown. However, previous research showed that the causes of migraine could be a combination of environmental and genetic factors.8 So far, many underlying mechanisms, including vascular inflammation, neurogenic and trigeminovascular system activation, and changes in immunologic system functions have been proposed.9 There is some evidence regarding the association between migraine and systemic inflammation and oxidative stress.9-11 “Neurogenic inflammation theory” has gained much attention recently, which involves the release of several vasoactive neuropeptides that activate and sensitize the cerebral nociceptors.12 Calcitonin gene-related peptide (CGRP) is a principal vasoactive neuropeptide that is widely distributed in cerebral vascular nerve fibers. This neuropeptide involves in pathology of migraine by several mechanisms such as vasodilation in cerebral blood vessels, activating nociceptive trigeminovascular process, and increase in mast cell degranulation.13 Moreover, it has been shown that the relase of other neuropeptides such as Substance-P (SP), neurokinin A (NKA), and neurokinin B (NKB) in response to environmental stimuli may also be related to vacular inflammation and trigering migraine headache.12
Certain dietary patterns have been reported to have a pro-inflammatory potential.14 Recently, the Dietary Inflammatory Index (DII) has been developed and validated based on six inflammatory markers including interleukin (IL)-1b, IL-4, IL-6, IL-10, tumor necrosis factor-α (TNF-α), and C-reactive protein (CRP) to estimate the inflammatory factor of the diet. This index estimates the inflammatory potential of the diet, according to the intake of 45 dietary items that have the most inflammatory or anti-inflammatory effect.15 The association between the upper DII score with several inflammatory diseases, including cardiovascular disease (CVD), cancer, and metabolic syndrome has been shown.16-18 Although the studies have not directly surveyed the association between migraine and DII score so far, it has been reported that anti-inflammatory diet may reduce pain through the establishment of the oxidant/antioxidant balance.19 Therefore, regarding the association between migraine and inflammation, it has been hypothesized that there might be a link between pro-inflammatory potential of diet and migraine.
Migraine is a genetic disorder that can be affected by lifestyle such as sleep, physical activity (PA), diet, and stress.20 According to the recent studies on single food items or nutrients, red wine, caffeine, chocolate, and monosodium glutamate (MSG) were among the most consistent food triggers of migraine.21-24 Moreover, the effect of different diets, including gluten-free diets,25 immunoglobulin G (IgG) elimination diets,26 Dietary Approaches to Stop Hypertension (DASH) diet,27 low-fat diets,28 low-glycemic diets,29 diets containing high omega-3 and low omega-6 fatty acids,30 and ketogenic diets31 have been studied on migraine headache. Despite some promising results, currently there is no specific “migraine diet” due to inconsistency between studies.20 Although previous studies showed that inflammation and oxidtive stress triggered migraine, the effect of diet inflammatory capacity was not evaluated.32 Since the migraine is approximately two times more prevalent in women compared to men33 and considering the fact that the inflammatory potential of the diet has not been evaluated in migraineurs, the aim of the this study was to survey the correlation between DII scores and migraine characteristics including severity and duration of headache attacks among women.
Materials and Methods
Study population: The present study was performed using a cross-sectional design on 266 women referred to neurology clinics of Sina and Khatam Al-Anbia Hospitals and a professional headache clinic in Tehran, Iran. Included patients were aged from 18 to 45 years old who suffer from migraine based on expert neurologic diagnosis, according to the International Classification of Headache Disorders (ICHD–III) criteria (beta version) (3-15 migraine days per month for at least three months). The sample size was calculated using the following formula:
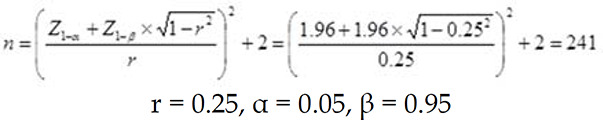 |
Considering 10% for any possible exclusion, totally 266 subjects were included for the study. This study protocol was approved by the Iranian Center of Neurological Research, Neuroscience Institute, Tehran University of Medical Sciences (Ethical code: IR.TUMS.NI.REC.1398.010 and Research number: 97-03-54-39314). The patients were participated in the study if they met the following inclusion criteria: Body Mass Index (BMI) between 18.5-30 kg/m2, visiting the headache clinic for the first time, and diagnosis of migraine by a neurologist. Patients with a history of chronic disease (such as CVD, cancer, diabetes, hepatic or renal diseaes, neurological disorders) or taking medication that would affect serum lipoprotein concentrations (such as Atorvastatin and Lovastatin), blood pressure, and carbohydrate metabolism were excluded from the study. Also, subjects that their daily energy intakes were lower than 500 kcal or higher than 3500 kcal were excluded in the analysis.34 After the description of the methodology and purpose of the research, the consent forms were completed by all participants.
Demographic characteristics and PA: General information including participants’ age, education level, job, marital status, medical history, familial history of migraine, medication and supplement use, and adhering to specific diets was collected using a demographic questionnaire. International Physical Activity Questionnaire (IPAQ) was used to acquire PA, which was shown as metabolic equivalent (MET) hours per week (MET-h/week). Activity levels were classified into low, moderate, and high groups, as described by the IPAQ scoring protocol.35
Anthropometric measurements: Weight, height, waist circumference (WC), and hip circumference were assessed in all subjects. Weight was measured to the nearest 0.1 kg, while wearing one layer of clothing and not wearing shoes by a digital scale (SECA, Hamburg, Germany). Height was recorded to the nearest 0.5 cm by a wall-mounted stadiometer while the shoulders were relaxed and shoes were removed. BMI was calculated based on following equation “weight (kg)/height2 (m2)”. The WC at the midpoint of the lowest rib and the iliac crest hip bone and hip circumference at the broadest area were measured using a flexible tape, to the nearest 0.5 cm.
Assessment of dietary intake: Usual dietary intake was evaluated by using a 147-item semiquantitative food frequency questionnaire (FFQ) which its reliability and validity had been approved in Iran.36 Participants were asked by dietitians during a face-to-face interview to report their consumption frequency for each food item over the past year on a daily, weekly, or monthly basis. Then, each food item was entered to Nutritionist IV software (1997, First DataBank Inc., San Bruno, CA) and mean daily intake of each food item weight in grams per day and nutrients content were estimated.
DII: The DII score was designed to evaluate the effect of diet on six well-established inflammatory biomarkers including IL-1β, IL-4, IL-6, IL-10, TNF-α, and CRP. The development and calculation of the DII were described in detail elsewhere.15 Briefly, The DII consists of 45 foods, spices, nutrients, and bioactive compounds with anti- or pro-inflammatory potential. Each of these 45 dietary components receives an inflammatory effect score. Positive values represent a pro-inflammatory effect of the component, whereas negative values represent an anti-inflammatory effect. In the present study, 28 dietary components including protein, carbohydrate, fat, energy, cholesterol, saturated fatty acids, mono-unsaturated fatty acids, poly-unsaturated fatty acids, β-carotene, vitamin C, iron, vitamin D, vitamin E, thiamin, riboflavin, niacin, vitamin B6, folic acid, vitamin B12, vitamin A, magnesium, zinc, selenium, fiber, omega-3 fatty acids, omega-6 fatty acids, onion, and garlic were considered to calculate DII. The scores of each dietary component are provided in the table 1. First, the dietary intake was linked to world database to obtain a robust mean and standard deviation (SD) for food parameters.15 Individual’s exposure relative to “standard global mean” was expressed as Z-score. Then, these values were changed to a centered percentile score to minimize the effect of “right skewing”. Eventually, centered percentile score was multiplied by the respective food parameter inflammatory effect on table S1. The parameter-specific inflammatory score of each parameter was computed and then all scores were summed to estimate the overall DII score of participants. DII scores range from 7.98 (most pro-inflammatory effect) to −8.87 (most anti-inflammatory effect).
Table 1.
Characteristics of the study population according to Dietary Inflammatory Index (DII) score classifications
| Variable |
DII
|
P | ||
|---|---|---|---|---|
| Low | High | |||
| Quantitative variables | ||||
| Age (year) | 34.75 ± 7.76 | 33.88 ± 7.96 | 0.872 | |
| Height (m) | 1.61 ± 0.05 | 1.62 ± 0.04 | 0.322 | |
| Weight (kg) | 69.96 ± 12.07 | 68.87 ± 14.03 | 0.500 | |
| BMI (kg/m2) | 26.82 ± 4.59 | 26.18 ± 5.19 | 0.287 | |
| WC (cm) | 86.38 ± 12.32 | 85.79 ± 15.10 | 0.732 | |
| Hip circumference (cm) | 102.28 ± 14.89 | 104.46 ± 13.88 | 0.223 | |
| WHR | 0.89 ± 0.61 | 0.86 ± 0.57 | 0.686 | |
| PA (MET-minute/week) | 417.68 ± 419.29 | 394.81 ± 605.15 | 0.720 | |
| Qualitative variables | ||||
| Education | Undergraduate | 53 (39.8) | 51 (38.6) | 0.332 |
| Bachelor | 66 (49.6) | 59 (44.7) | ||
| Master or higher | 14 (10.5) | 22 (16.7) | ||
| Job | Housekeeper | 65 (48.9) | 77 (57.9) | 0.088 |
| Employed | 68 (51.1) | 56 (42.1) | ||
| Marital status | Single or divorced | 38 (28.6) | 40 (30.1) | 0.446 |
| Married | 95 (71.4) | 93 (69.9) | ||
Data are presented as mean ± standard deviation (SD) or number and percentage
*Calculated by independent t-test for quantitative variables and chi-square test for qualitative variables
BMI: Body mass index; WC: Waist circumference; WHR: Waist-to-hip ratio; DII: Dietary Inflammatory Index; PA: Physical activity; MET: Metabolic equivalent
Migraine Disability Assessment (MIDAS), Visual Analog Scale (VAS), headach duration, and headache frequency: The MIDAS questionnaire was used to evaluate the impact of headache on work, home, and social and leisure activities in the past 3 months.37 This questionnaire was validated in Iranian population previously.38 MIDAS includes 7 items that evaluate performance decrease due to migraine with the use of a total score grading scale, so that the participants were classified into one of the following groups: Grade I (score 0-5, little or no disability), Grade II (score 6-10, mild disability), Grade III (score 11-20, moderate disability), and Grade IV (score > 21, severe disability). Moreover, participants were asked to rate their pain using a 10-cm VAS questionnaire.39 Patients marked the line on the VAS to indicate their perception of headache. The severity of pain that patient experienced was estimated by measuring the specified length and then participants were divided into these 3 groups: mild pain (score 1-3), moderate pain (score 4-7), and severe pain (score 8-10). Also, a 30-day headache diary, including the time of migraine attack onest, duration, and frequency of the headache was completed by each participant.
Quantitative and qualitative data were presented as mean ± SD and frequency and percentage, respectively. In order to investigate the relationship between DII (that was calculated according to the consumption of anti- and pro-inflammatory food components) and severity of migraine, individuals were divided into two groups based on DII score median: high DII and low DII. The chi-square test was used to evaluate the association between DII and qualitative variables and its association with quantitative variables was assessed using independent samples t-test. Confounding variables were identified according to the previous studies, a best-fit model and the change in -2 log-likelihood ratio test. The relationship between DII and migraine severity (MIDAS and VAS), headache duration (categorized into three groups: less than 3 hours, between 3-10 hours, more than 10 hours), and headache frequency (categorized into three groups: less than 10 days, between 11-20 days, more than 20 days) was determined using multinomial logistic regression. In the first analysis (crude model), MIDAS, VAS, and headache duration or frequency were entered into the model as dependent variables, and DII classifications were entered as independent variables. Finally, the effects of all confounding variables were adjusted. In this regard, age, PA, BMI, and job were entered into the adjusted regression model. Data were analyzed using SPSS software (version 22, IBM Corporation, Armonk, NY, USA). P-values < 0.050 were considered as statistically significant.
Results
Study population characteristics: Totally, 266 women with mean age of 34.32 ± 7.86 years were recruited in the study. The mean ± SD of height, weight, BMI, and PA were 161.00 ± 15.14 cm, 69.41 ± 13.08 kg, 26.50 ± 4.90 kg/m2, and 406.24 ± 519.73 MET-minute/week, respectively. The distributions of disability (using MIDAS) in the participants were as follows: 13.2% without a disability, 24.8% mild disability, 17.3% moderate disability, and 44.7% severe disability. Also, the percentages of mild, moderate, and severe headache (using VAS) were 13.6%, 42.9%, and 43.6%, respectively. The mean duration of each headache in last month was 10.44 hours and headache lasted more than 10 hours in 32.7%, 3-10 hours in 42.1%, and less than 3 hours in 25.2% of the participants. The DII score ranged between -4.22 and 5.19 and its median [interquartile range (IQR)] was 0.003 (-1.48-1.55).
Association between baseline characteristics and DII: Baseline characteristics of the participants by DII score classification are provided in table 1. According to the results, there was no significant correlation between age, marital status, education level, occupation, PA, weight, height, BMI, WC, hip circumference, waist-to-hip ratio (WHR) and DII score classifications (P > 0.050).
Association between migraine severity and DII: Table 2 provides the association between DII and VAS, MIDAS, headache duration, and headache frequency. No correlation was found between DII and migraine severity and headache frequency (P > 0.050). However, there was a significant association between headache duration and DII (P = 0.020).
Table 2.
Migraine severity according to the Dietary Inflammatory Index (DII) score classification
| Variable |
DII
|
P * | ||||
|---|---|---|---|---|---|---|
| Low | High | |||||
| VAS | Mild | 19 (14.3) | 17 (12.8) | 0.930 | ||
| Moderate | 56 (42.1) | 58 (43.6) | ||||
| Severe | 58 (43.6) | 58 (43.6) | ||||
| MIDAS | Grade I | 19 (14.3) | 16 (12.0) | 0.374 | ||
| Grade II | 27 (20.3) | 39 (29.3) | ||||
| Grade III | 23 (17.3) | 23 (17.3) | ||||
| Grade IV | 64 (48.1) | 55 (41.4) | ||||
| Headache duration (hour) | < 3 | 28 (21.1) | 39 (29.3) | 0.023 | ||
| 3-10 | 67 (50.4) | 45 (33.8) | ||||
| > 10 | 38 (28.6) | 49 (36.8) | ||||
| Headache frequency (day) | < 10 | 52 (39.1) | 68 (51.1) | 0.139 | ||
| 10-19 | 32 (24.1) | 27 (20.3) | ||||
| > 20 | 49 (36.8) | 38 (28.6) | ||||
Data are presented as number and percentage.
DII: Dietary Inflammatory Index; VAS: Visual Analog Scale; MIDAS: Migraine Disability Assessment
Calculated by chi-square test
Pearson correlations (Table 3) between DII and VAS (r = 0.01, P = 0.842), MIDAS (r = 0.01, P = 0.841), headache duration (r = 0.06, P = 0.293), and headache frequency (r = 0.12, P = 0.055) were not statistically significant. Multinomial logistic regression was used to adjust the effect of age, PA, BMI, and job status in the relation between DII and migraine severity and headache duration (Table 4). There was no association between DII and migraine severity (VAS and MIDAS) in the crude and adjusted models of logistic regression.
Table 3.
Pearson correlation between Dietary Inflammatory Index (DII) and migraine severity, headache duration, and headache frequency
| Variable | Coefficient (r) | P |
|---|---|---|
| VAS | 0.01 | 0.842 |
| MIDAS | 0.01 | 0.841 |
| Headache duration | 0.06 | 0.293 |
| Headache frequency | 0.12 | 0.055 |
VAS: Visual Analog Scale; MIDAS: Migraine Disability Assessment
Table 4.
The relationship between Dietary Inflammatory Index (DII) score classifications and migraine severity using multinomial logistic regression
| Variable |
Crude
|
Adjusted
*
|
|||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | ||
| VAS | Mild | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Moderate | 1.15 | 0.54-2.45 | 0.702 | 1.43 | 0.63-3.26 | 0.390 | |
| Severe | 1.11 | 0.52-2.36 | 0.770 | 1.71 | 0.71-4.12 | 0.231 | |
| MIDAS | Grade I | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Grade II | 1.71 | 0.75-3.92 | 0.201 | 2.13 | 0.87-5.22 | 0.096 | |
| Grade III | 1.18 | 0.49-2.86 | 0.702 | 1.84 | 0.68-5.01 | 0.229 | |
| Grade IV | 1.02 | 0.47-2.17 | 0.958 | 1.56 | 0.62-3.91 | 0.337 | |
| Headache duration (hour) | < 3 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| 3-10 | 0.48 | 0.26-0.89 | 0.020 | 0.53 | 0.28-1.00 | 0.052 | |
| > 10 | 0.92 | 0.48-1.76 | 0.815 | 1.09 | 0.55-2.19 | 0.792 | |
| Headache frequency (day) | < 10 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| 10-19 | 1.02 | 0.73-1.42 | 0.891 | 0.98 | 0.65-1.32 | 0.902 | |
| > 20 | 1.60 | 1.12-2.08 | 0.001 | 1.49 | 1.10-1.88 | 0.021 | |
VAS: Visual Analog Scale; MIDAS: Migraine Disability Assessment; OR: Odds ratio; CI: Confidence interval
Adjusted for age, physical activity (PA), Body Mass Index (BMI), and job
Multinomial logistic regression showed a lower odd of 3-10 hours compared to 3 hours of headache duration in participants with high DII score [odds ratio (OR) = 0.48, 95% confidence interval (CI) = 0.26-0.89, P = 0.020]. However, this relationship was not significant after adjusting for the effect of age, PA, BMI, and job status (OR = 0.53, 95% CI = 0.28-1.00, P = 0.052). In the crude model of logistic regression, subjects with more than 20 days of headache had higher DII score compared to those with less than 10 days per month (OR = 1.60, 95% CI = 1.12-2.08, P = 0.001). Also, this relationship remained statistically significant after adjusting for the effect of age, PA, BMI, and job status (P = 0.021).
Discussion
The present study investigated the association between DII score and migraine severity, headache duration, and headache frequency. Although more than half of the participants followed a dietary pattern with high inflammatory potential, the results did not reveal a significant association between headache duration or migraine severity and DII after adjusting for confounding variables. Nevertheless, more DII score was associated with more frequency of migraine headaches.
This study is the first study to address the correlation between DII and migraine severity. Recent studies found some promising results regarding the relationship between whole dietary patterns and migraine severity.40 It has been proposed that some healthy diets, including gluten-free diets,25 DASH diet,27 and diets containing high omega-3 and low omega-6 fatty acids30,41 may attenuate unpleasant symptoms of migraine. However, inconsistencies in the findings of the studies have prevented a complete and definitive food guide for patients with migraine.20 Diet may result in headache through its vasodilator and vasoconstrictor components and also its effect on nervous system function.42,43 So far, most of the studies have focused on the food items that have been linked to migraine headache frequency and severity. For instance, processed foods, fermented products, pickles, salty food, caffeine, artificial sweeteners, seafood, dairies, and tyramine-containing foods are known as migraine drivers.44 In contrast, the intensity and frequency of migraine headaches may decrease by certain nutrients such as vitamin D, magnesium, coenzyme Q10, alpha-lipoic acid (ALA), and eicosapentaenoic acid (EPA).45,46 Previous studies did not directly investigate the association between migraine and DII score. However, primary evidence showed a significant association between DII and other neurological disorders and brain function.47-51 At the other extreme, no relationship was observed between DII and neurological disorders in a more acute condition such as multiple sclerosis (MS).52 Thus, according to equivocal results in the previous studies, investigating the correlation between DII and migraine severity as a neurological disorder does not seem to be unreasonable. On the other hand, previous findings suggest a role for neuroinflammation in migraine pathogenesis. There has been some reports regarding the higher DII scores in some inflammatory disorders including CVD, cancer, and metabolic syndrome.16-18 With this in mind, investigating the association between migraine and DII scores to establish a role of diet in migraine characteristics through its inflammatory potential can be of great value.
As mentioned earlier, DII score is an estimation of dietary inflammatory potential based on 6 inflammatory biomarkers including IL-1β, IL-6, IL-4, TNF-α, IL-10, and CRP. It has been established that headache attacks are affected by cytokines fluctuations53 and cytokines induce headache in patients with migraine.54 Although there are controversial results in this regard, current evidence suggests that TNF-α, IL-1β, and IL-10 are possibly involved in the pathogenesis of migraine.54 An increase in these cytokines could activate the trigeminal nerves and release of the vasoactive peptides. Further, these cytokines may act as pain mediators in neurovascular inflammation and induce sterile inflammation in blood vessels of meninges.54 Also, some studies found an increase in inflammatory markers during the inter-attack of migraine.55-58 However, some other studies observed no association between inflammatory markers and migraine headache severity and duration.59
The present study found an association between headache frequency and DII. However, no relationship was observed between migraine severity and attack duration and dietary inflammatory potential. Some points should be considered in this regard. Despite all assumptions, it seems that inflammation is not the only underlying mechanism in migraine, but it is a main factor in the etiology of migraine. Also, there is a complex relationship between migraine attack severity and diet. As mentioned earlier, 28 dietary components were used to compute the DII score. It has been reported previously that some of these components, such as vitamin D, riboflavin, magnesium, omega-3 fatty acids,45 vitamin A,59 vitamin E,60 thiamin,61 niacin,62 vitamin B6, folic acid,63 and zinc64 may alleviate migraine severity. These nutrients receive a negative value (anti-inflammatory effect) in the calculation of the DII score. Also, there are other dietary components, such as protein,65 fat,28 cholesterol, and saturated fatty acids66 that may induce migraine attack and are considered as pro-inflammatory factors in the DII. However, there are some controversies in the components of DII in relation to the migraine. For instance, caffeine, citrus fruits (source of vitamin C and octopamine),42 and omega-6 fatty acids67 that have been reported as migraine triggers, are considered as anti-inflammatory factors in the DII calculation. In contrast, energy, carbohydrate, and iron intake receive a positive score (pro-inflammatory effect), while skipping meals, fasting,42 and iron deficiency anemia68 may exacerbate the severity of migraine. Moreover, higher intakes of spices (such as ginger, turmeric, thyme/oregano, rosemary, and pepper), onion, and garlic that receive negative score may result in migraine attack due to odor sensitivity in these patients. In addition, dietary sources of flavones and anthocyanidins may also be rich in tyramine and phenylethylamine (PEA) (such as chocolate) that is a trigger for migraine.69 Also, there is not enough evidence for the effect of monounsaturated fatty acids (MUFA), β-carotene, selenium, and fiber that have anti-inflammatory effects on the migraine severity. Therefore, it seems that in patients with migraine, a more specific index by eliminating known factors in triggering migraine headaches is more useful.
The strength of this study is that it is the first study to investigate the correlation between DII and migraine intensity and headache duration and also the migraine diagnosis was based on expert neurologist diagnosis. The main limitations of our study are the cross-sectional design, relatively small sample size, lack of control healthy group, as well as limitation of the subjects to the women which compromises the generalizability of the study. Also, using a questionnaire for collecting information may result in bias due to its dependence on participants’ ability to recall and education level. Moreover, assessing serum levels of inflammatory biomarkers along with DII evaluation could increase the reliability of the results and help explain the mechanism more accurately.
Conclusions
The present study showed a direct association between headache frequency and DII. Nevertheless, no relationship was found between headache duration or migraine severity and DII score. However, about the relationship between migraine and inflammatory potential of diet, we are at the beginning of the way. Also, the present study was conducted only in female patients and it is not possible to generalize the results to all patients. Further precise and large studies are needed to establish the role of whole diet in migraine pathogenesis or treatment.
Acknowledgments
The authors thank the study participants, those involved in nutritional evaluation and database management, as well as the neurology clinics of Sina and Khatam Al-Anbia Hospitals.
This study was supported by Tehran University of Medical Sciences and by grants from Tehran University of Medical Sciences (Grants ID: 95-01-103-31348) and also was supported by the Student’s Scientific Research Center, Tehran.
Notes:
How to cite this article: Khorsha F, Mirzababaei A, Ghodoosi N, Togha M, Yekaninejad MS, Askarpour M, et al. Association between diet and migraine characteristics: The role of dietary inflammatory index. Curr J Neurol 2020; 19(2): 67-75.
Conflict of Interests
The authors declare no conflict of interest in this study.
References
- 1.Baldacci F, Lucchesi C, Cafalli M, Poletti M, Ulivi M, Vedovello M, et al. Migraine features in migraineurs with and without anxiety-depression symptoms: A hospital-based study. Clin Neurol Neurosurg. 2015;132:74–8. doi: 10.1016/j.clineuro.2015.02.017. [DOI] [PubMed] [Google Scholar]
- 2.Steiner TJ, Stovner LJ, Vos T, Jensen R, Katsarava Z. Migraine is first cause of disability in under 50s: Will health politicians now take notice? J Headache Pain. 2018;19(1):17. doi: 10.1186/s10194-018-0846-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zheng H, Chen M, Huang D, Li J, Chen Q, Fang J. Interventions for migraine prophylaxis: protocol of an umbrella systematic review and network meta-analysis. BMJ Open. 2015;5(5):e007594. doi: 10.1136/bmjopen-2015-007594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leonardi M, Musicco M, Nappi G. Headache as a major public health problem: Current status. Cephalalgia. 1998;18(Suppl 21):66–9. doi: 10.1177/0333102498018s2116. [DOI] [PubMed] [Google Scholar]
- 5.Buse DC, Manack AN, Fanning KM, Serrano D, Reed ML, Turkel CC, et al. Chronic migraine prevalence, disability, and sociodemographic factors: Results from the American Migraine Prevalence and Prevention Study. Headache. 2012;52(10):1456–70. doi: 10.1111/j.1526-4610.2012.02223.x. [DOI] [PubMed] [Google Scholar]
- 6.Serrano D, Manack AN, Reed ML, Buse DC, Varon SF, Lipton RB. Cost and predictors of lost productive time in chronic migraine and episodic migraine: Results from the American Migraine Prevalence and Prevention (AMPP) Study. Value Health. 2013;16(1):31–8. doi: 10.1016/j.jval.2012.08.2212. [DOI] [PubMed] [Google Scholar]
- 7.Katsarava Z, Schneeweiss S, Kurth T, Kroener U, Fritsche G, Eikermann A, et al. Incidence and predictors for chronicity of headache in patients with episodic migraine. Neurology. 2004;62(5):788–90. doi: 10.1212/01.wnl.0000113747.18760.d2. [DOI] [PubMed] [Google Scholar]
- 8.Andress-Rothrock D, King W, Rothrock J. An analysis of migraine triggers in a clinic-based population. Headache. 2010;50(8):1366–70. doi: 10.1111/j.1526-4610.2010.01753.x. [DOI] [PubMed] [Google Scholar]
- 9.Martami F, Razeghi JS, Togha M, Ghorbani Z, Seifishahpar M, Saidpour A. The serum level of inflammatory markers in chronic and episodic migraine: A case-control study. Neurol Sci. 2018;39(10):1741–9. doi: 10.1007/s10072-018-3493-0. [DOI] [PubMed] [Google Scholar]
- 10.Eren Y, Dirik E, Neselioglu S, Erel O. Oxidative stress and decreased thiol level in patients with migraine: cross-sectional study. Acta Neurol Belg. 2015;115(4):643–9. doi: 10.1007/s13760-015-0427-y. [DOI] [PubMed] [Google Scholar]
- 11.Yigit M, Sogut O, Tataroglu O, Yamanoglu A, Yigit E, Guler EM, et al. Oxidative/antioxidative status, lymphocyte DNA damage, and urotensin-2 receptor level in patients with migraine attacks. Neuropsychiatr Dis Treat. 2018;14:367–74. doi: 10.2147/NDT.S156710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malhotra R. Understanding migraine: Potential role of neurogenic inflammation. Ann Indian Acad Neurol. 2016;19(2):175–82. doi: 10.4103/0972-2327.182302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Assas BM, Pennock JI, Miyan JA. Calcitonin gene-related peptide is a key neurotransmitter in the neuro-immune axis. Front Neurosci. 2014;8:23. doi: 10.3389/fnins.2014.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Minihane AM, Vinoy S, Russell WR, Baka A, Roche HM, Tuohy KM, et al. Low-grade inflammation, diet composition and health: current research evidence and its translation. Br J Nutr. 2015;114(7):999–1012. doi: 10.1017/S0007114515002093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shivappa N, Steck SE, Hurley TG, Hussey JR, Hebert JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014;17(8):1689–96. doi: 10.1017/S1368980013002115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shivappa N, Godos J, Hebert JR, Wirth MD, Piuri G, Speciani AF, et al. Dietary Inflammatory Index and Cardiovascular Risk and Mortality-A Meta-Analysis. Nutrients. 2018;10(2):200. doi: 10.3390/nu10020200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shivappa N, Godos J, Hebert JR, Wirth MD, Piuri G, Speciani AF, et al. Dietary inflammatory index and colorectal cancer risk-a meta-analysis. Nutrients. 2017;9(9):1043. doi: 10.3390/nu9091043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramallal R, Toledo E, Martinez-Gonzalez MA, Hernandez-Hernandez A, Garcia-Arellano A, Shivappa N, et al. Dietary Inflammatory Index and Incidence of Cardiovascular Disease in the SUN Cohort. PLoS One. 2015;10(9):e0135221. doi: 10.1371/journal.pone.0135221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seaman DR. An anti-inflammatory diet for pain patients. Pract Pain Manag. 2012;12(10):36–46. [Google Scholar]
- 20.Robblee J, Starling AJ. SEEDS for success: Lifestyle management in migraine. Cleve Clin J Med. 2019;86(11):741–9. doi: 10.3949/ccjm.86a.19009. [DOI] [PubMed] [Google Scholar]
- 21.O'Brien M. The role of monosodium glutamate in headache [MSc Thesis] Vancouver, BC, Canada: The University of British Columbia; 2016. [Google Scholar]
- 22.Lipton RB, Diener HC, Robbins MS, Garas SY, Patel K. Caffeine in the management of patients with headache. J Headache Pain. 2017;18(1):107. doi: 10.1186/s10194-017-0806-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nowaczewska M, Wicinski M, Kazmierczak W, Kazmierczak H. To eat or not to eat: A review of the relationship between chocolate and migraines. Nutrients. 2020;12(3):608. doi: 10.3390/nu12030608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zis P, Julian T, Hadjivassiliou M. Headache associated with coeliac disease: A systematic review and meta-analysis. Nutrients. 2018;10(10):1445. doi: 10.3390/nu10101445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aydinlar EI, Dikmen PY, Tiftikci A, Saruc M, Aksu M, Gunsoy HG, et al. IgG-based elimination diet in migraine plus irritable bowel syndrome. Headache. 2013;53(3):514–25. doi: 10.1111/j.1526-4610.2012.02296.x. [DOI] [PubMed] [Google Scholar]
- 27.Mirzababaei A, Khorsha F, Togha M, Yekaninejad MS, Okhovat AA, Mirzaei K. Associations between adherence to dietary approaches to stop hypertension (DASH) diet and migraine headache severity and duration among women. Nutr Neurosci. 2020;23(5):335–42. doi: 10.1080/1028415X.2018.1503848. [DOI] [PubMed] [Google Scholar]
- 28.Ferrara LA, Pacioni D, Di F V, Russo BF, Speranza E, Carlino V, et al. Low-lipid diet reduces frequency and severity of acute migraine attacks. Nutr Metab Cardiovasc Dis. 2015;25(4):370–5. doi: 10.1016/j.numecd.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 29.Evcili G, Utku U, Ogun MN, Ozdemir G. Early and long period follow-up results of low glycemic index diet for migraine prophylaxis. Agri. 2018;30(1):8–11. doi: 10.5505/agri.2017.62443. [DOI] [PubMed] [Google Scholar]
- 30.Maghsoumi-Norouzabad L, Mansoori A, Abed R, Shishehbor F. Effects of omega-3 fatty acids on the frequency, severity, and duration of migraine attacks: A systematic review and meta-analysis of randomized controlled trials. Nutr Neurosci. 2018;21(9):614–23. doi: 10.1080/1028415X.2017.1344371. [DOI] [PubMed] [Google Scholar]
- 31.Di Lorenzo C, Coppola G, Sirianni G, Di Lor enzoG, Bracaglia M, Di Lenola D, et al. Migraine improvement during short lasting ketogenesis: A proof-of-concept study. Eur J Neurol. 2015;22(1):170–7. doi: 10.1111/ene.12550. [DOI] [PubMed] [Google Scholar]
- 32.Borkum JM. Migraine triggers and oxidative stress: A narrative review and synthesis. Headache. 2016;56(1):12–35. doi: 10.1111/head.12725. [DOI] [PubMed] [Google Scholar]
- 33.GBD 2016 Disease, Injury Incidence, Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):211–59. doi: 10.1016/S0140-6736(17)32154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Willett W. Nutritional epidemiology. Oxford, UK: Oxford University Press; 2012. [Google Scholar]
- 35.Wanner M, Probst-Hensch N, Kriemler S, Meier F, Autenrieth C, Martin BW. Validation of the long international physical activity questionnaire: Influence of age and language region. Prev Med Rep. 2016;3:250–6. doi: 10.1016/j.pmedr.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mirmiran P, Hosseini-Esfahanil F, Jessri M, Mahan LK, Shiva N, Azizis F. Does dietary intake by Tehranian adults align with the 2005 dietary guidelines for Americans? Observations from the Tehran lipid and glucose study. J Health Popul Nutr. 2011;29(1):39–52. doi: 10.3329/jhpn.v29i1.7564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stewart WF, Lipton RB, Dowson AJ, Sawyer J. Development and testing of the Migraine Disability Assessment (MIDAS) Questionnaire to assess headache-related disability. Neurology. 2001;56(6 Suppl 1):S20–S28. doi: 10.1212/wnl.56.suppl_1.s20. [DOI] [PubMed] [Google Scholar]
- 38.Ghorbani A, Chitsaz A. Comparison of validity and reliability of the Migraine disability assessment (MIDAS) versus headache impact test (HIT) in an Iranian population. Iran J Neurol. 2011;10(3-4):39–42. [PMC free article] [PubMed] [Google Scholar]
- 39.Price DD, McGrath PA, Rafii A, Buckingham B. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain. 1983;17(1):45–56. doi: 10.1016/0304-3959(83)90126-4. [DOI] [PubMed] [Google Scholar]
- 40.Razeghi Jahromi S, Ghorbani Z, Martelletti P, Lampl C, Togha M, On behalf of the School of Advanced Studies of the European Headache Federation (EHF-SAS). Association of diet and headache. J Headache Pain. 2019;20(1):106. doi: 10.1186/s10194-019-1057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Togha M, Razeghi JS, Ghorbani Z, Ghaemi A, Rafiee P. An investigation of oxidant/antioxidant balance in patients with migraine: A case-control study. BMC Neurol. 2019;19(1):323. doi: 10.1186/s12883-019-1555-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Finocchi C, Sivori G. Food as trigger and aggravating factor of migraine. Neurol Sci. 2012;33(Suppl 1):S77–S80. doi: 10.1007/s10072-012-1046-5. [DOI] [PubMed] [Google Scholar]
- 43.Millichap JG, Yee MM. The diet factor in pediatric and adolescent migraine. Pediatr Neurol. 2003;28(1):9–15. doi: 10.1016/s0887-8994(02)00466-6. [DOI] [PubMed] [Google Scholar]
- 44.Ozturan A, Sanlier N, Coskun O. The relationship between migraine and nutrition. Turk J Neurol. 2016;22(2):44–50. [Google Scholar]
- 45.Sun-Edelstein C, Mauskop A. Foods and supplements in the management of migraine headaches. Clin J Pain. 2009;25(5):446–52. doi: 10.1097/AJP.0b013e31819a6f65. [DOI] [PubMed] [Google Scholar]
- 46.Ghorbani Z, Togha M, Rafiee P, Ahmadi ZS, Rasekh MR, Haghighi S, et al. Vitamin D in migraine headache: A comprehensive review on literature. Neurol Sci. 2019;40(12):2459–77. doi: 10.1007/s10072-019-04021-z. [DOI] [PubMed] [Google Scholar]
- 47.Shin D, Kwon SC, Kim MH, Lee KW, Choi SY, Shivappa N, et al. Inflammatory potential of diet is associated with cognitive function in an older adult Korean population. Nutrition. 2018;55-56:56–62. doi: 10.1016/j.nut.2018.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Frith E, Shivappa N, Mann JR, Hebert JR, Wirth MD, Loprinzi PD. Dietary inflammatory index and memory function: Population-based national sample of elderly Americans. Br J Nutr. 2018;119(5):552–8. doi: 10.1017/S0007114517003804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hayden KM, Beavers DP, Steck SE, Hebert JR, Tabung FK, Shivappa N, et al. The association between an inflammatory diet and global cognitive function and incident dementia in older women: The Women's Health Initiative Memory Study. Alzheimers Dement. 2017;13(11):1187–96. doi: 10.1016/j.jalz.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kesse-Guyot E, Assmann KE, Andreeva VA, Touvier M, Neufcourt L, Shivappa N, et al. Long-term association between the dietary inflammatory index and cognitive functioning: findings from the SU. VI. MAX study. Eur J Nutr. 2017;56(4):1647–55. doi: 10.1007/s00394-016-1211-3. [DOI] [PubMed] [Google Scholar]
- 51.Arzani M, Jahromi SR, Ghorbani Z, Vahabizad F, Martelletti P, Ghaemi A, et al. Gut-brain Axis and migraine headache: a comprehensive review. J Headache Pain. 2020;21(1):15. doi: 10.1186/s10194-020-1078-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.da Costa Silva BY, de Carvalho Sampaio HA, Shivappa N, Hebert JR, da Silva AL, Carioca AAF, et al. Dietary Inflammatory Index and clinical course of multiple sclerosis. Eur J Clin Nutr. 2019;73(7):979–88. doi: 10.1038/s41430-018-0294-8. [DOI] [PubMed] [Google Scholar]
- 53.Kemper RH, Meijler WJ, Korf J, Ter Horst GJ. Migraine and function of the immune system: a meta-analysis of clinical literature published between 1966 and 1999. Cephalalgia. 2001;21(5):549–57. doi: 10.1046/j.1468-2982.2001.00196.x. [DOI] [PubMed] [Google Scholar]
- 54.Bruno PP, Carpino F, Carpino G, Zicari A. An overview on immune system and migraine. Eur Rev Med Pharmacol Sci. 2007;11(4):245–8. [PubMed] [Google Scholar]
- 55.Theodoropoulos DS, Katzenberger DR, Jones WM, Morris DL, Her C, Cullen NA, et al. Allergen-specific sublingual immunotherapy in the treatment of migraines: A prospective study. Eur Rev Med Pharmacol Sci. 2011;15(10):1117–21. [PubMed] [Google Scholar]
- 56.Meng WG, Shi L, Wu LY, Lai LY, Song RX, Huang SZ. Clinical research on treatment of migraine with pine needle moxibustion. Zhongguo Zhen Jiu. 2012;32(6):519–22. [In Chinese] [PubMed] [Google Scholar]
- 57.Guzel I, Tasdemir N, Celik Y. Evaluation of serum transforming growth factor beta1 and C-reactive protein levels in migraine patients. Neurol Neurochir Pol. 2013;47(4):357–62. doi: 10.5114/ninp.2013.36760. [DOI] [PubMed] [Google Scholar]
- 58.Welch KM, Brandes AW, Salerno L, Brandes JL. C-reactive protein may be increased in migraine patients who present with complex clinical features. Headache. 2006;46(2):197–9. doi: 10.1111/j.1526-4610.2006.00330.x. [DOI] [PubMed] [Google Scholar]
- 59.Tanik N, Celikbilek A, Metin A, Gocmen AY, Inan LE. Retinol-binding protein-4 and hs-CRP levels in patients with migraine. Neurol Sci. 2015;36(10):1823–7. doi: 10.1007/s10072-015-2262-6. [DOI] [PubMed] [Google Scholar]
- 60.Ziaei S, Kazemnejad A, Sedighi A. The effect of vitamin E on the treatment of menstrual migraine. Med Sci Monit. 2009;15(1):CR16–CR19. [PubMed] [Google Scholar]
- 61.Faraji H, Paknahad Z, Chitsaz A. Dietary intake of thiamine in migraine patients and healthy subjects: A case-control study. Clin Nutr Res. 2018;7(1):40–7. doi: 10.7762/cnr.2018.7.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pizza V, Busillo V, Iannuzzi S, Agresta A, Cassano D, Capasso A. Magnesium, l-tryptophan and niacin in prophylaxis therapy of pediatric migraine. Pharmacologyonline. 2013;1:39–46. [Google Scholar]
- 63.Askari G, Nasiri M, Mozaffari-Khosravi H, Rezaie M, Bagheri-Bidakhavidi M, Sadeghi O. The effects of folic acid and pyridoxine supplementation on characteristics of migraine attacks in migraine patients with aura: A double-blind, randomized placebo-controlled, clinical trial. Nutrition. 2017;38:74–9. doi: 10.1016/j.nut.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 64.Gonullu H, Gonullu E, Karadas S, Arslan M, Kalemci O, Aycan A, et al. The levels of trace elements and heavy metals in patients with acute migraine headache. J Pak Med Assoc. 2015;65(7):694–7. [PubMed] [Google Scholar]
- 65.Unge G, Malmgren R, Olsson P, Theorell H, Tornling G. Effects of dietary protein-tryptophan restriction upon 5-HT uptake by platelets and clinical symptoms in migraine-like headache. Cephalalgia. 1983;3(4):213–8. doi: 10.1046/j.1468-2982.1983.0304213.x. [DOI] [PubMed] [Google Scholar]
- 66.Bic Z, Blix GG, Hopp HP, Leslie FM, Schell MJ. The influence of a low-fat diet on incidence and severity of migraine headaches. J Womens Health Gend Based Med. 1999;8(5):623–30. doi: 10.1089/jwh.1.1999.8.623. [DOI] [PubMed] [Google Scholar]
- 67.Ramsden CE, Faurot KR, Zamora D, Suchindran CM, Macintosh BA, Gaylord S, et al. Targeted alteration of dietary n-3 and n-6 fatty acids for the treatment of chronic headaches: A randomized trial. Pain. 2013;154(11):2441–51. doi: 10.1016/j.pain.2013.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gur-Ozmen S, Karahan-Ozcan R. Iron deficiency anemia is associated with menstrual migraine: A case-control study. Pain Med. 2016;17(3):596–605. doi: 10.1093/pm/pnv029. [DOI] [PubMed] [Google Scholar]
- 69.Wober C, Holzhammer J, Zeitlhofer J, Wessely P, Wober-Bingol C. Trigger factors of migraine and tension-type headache: Experience and knowledge of the patients. J Headache Pain. 2006;7(4):188–95. doi: 10.1007/s10194-006-0305-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


