Abstract
Objective:
Knee pain from osteoarthritis is frequent in the adult population. Prior trials have had conflicting results concerning vitamin D’s therapeutic effects on knee pain and few trials have investigated marine omega-3 fatty acids (n-3 FA).
Methods:
The double-blind, placebo-controlled VITamin D and OmegA-3 TriaL (VITAL) randomized 25,871 U.S. adults in a two-by-two factorial design to vitamin D and n-3 FA. We identified a subgroup with chronic knee pain prior to randomization and assessed knee pain at baseline and annually during follow-up with the Western Ontario and McMaster Universities Arthritis Index (WOMAC; 0–100, 100 worst). Repeated measures modeling tested the effect of randomized treatment on WOMAC Pain over follow-up after adjustment for age and sex. Analyses were repeated for WOMAC Function and Stiffness.
Results:
We included 1,398 participants who returned at least one knee pain questionnaire. Mean age was 67.7 years, 66% were female, and mean WOMAC Pain was 37 (SD 19). Mean follow-up time was 5.3 years (SD 0.7). WOMAC Pain did not differ between vitamin D or n-3 FA and placebo at any time point during follow-up. Linear time by treatment interactions were not statistically significant for either treatment (vitamin D p= 0.41, n-3 FA p= 0.77). Vitamin D and n-3 FA supplementation did not significantly affect WOMAC Function or Stiffness scores over time.
Conclusion:
Vitamin D and n-3 FA supplementation for a mean of 5.3 years did not reduce knee pain or improve function or stiffness in a large sample of U.S adults with chronic knee pain.
Introduction
Osteoarthritis (OA) is a frequent cause of knee pain in older adults, with symptomatic knee OA affecting an estimated 14 million in the U.S.1,2 An estimated 25% of older adults have knee pain, making knee symptoms one of the most common reasons patients seek outpatient care.3,4 Despite the increasing prevalence of symptomatic knee OA, medical therapies are largely limited to exercise, weight management and medications for pain control that may confer adverse effects.5,6 Identifying safe and inexpensive therapies that reduce pain could vastly improve management of chronic knee pain. Recent interest has focused on the role of vitamin D in OA due to vitamin D’s function in bone resorption and muscle strength, as well as its anti-inflammatory properties.7–9 Marine omega-3 fatty acids (n-3 FA), found in fish oils, have also been touted for their ability to mitigate inflammation and the catabolic environment that promotes cartilage degradation.10,11
While prospective studies have demonstrated an association of lower vitamin D levels with pain and OA progression, several randomized controlled trials (RCT) of vitamin D supplementation in OA have yielded conflicting results.12–17 Sanghi et al conducted a one-year trial of vitamin D versus placebo in patients with symptomatic knee OA and vitamin D insufficiency and reported a small, but statistically significant improvement in pain and function in patients assigned vitamin D supplementation.16 Conversely, a two-year RCT of vitamin D supplementation in participants with symptomatic knee OA did not demonstrate statistically significant differences in pain or cartilage volume15, nor did a subsequent three-year RCT demonstrate benefit of vitamin D for joint space narrowing or pain.17 Fewer RCTs have been conducted using fish oil, although Hill et al conducted a two-year RCT of low versus high-dose fish oil on patients with symptomatic knee OA and demonstrated that participants on low-dose fish oil had statistically significantly lower pain scores at 18 and 24 months in comparison to the high-dose group; however, no placebo group was analyzed.18
The VITamin D and OmegA-3 TriaL (VITAL), a large, nation-wide, population-based trial with five years of randomized intervention, provides a unique opportunity to investigate the potential roles of vitamin D and fish oil on knee pain.19,20 We aimed to test the long-term effects of vitamin D and n-3 FA supplementation upon chronic knee pain, hypothesizing that we would discover prolonged beneficial effects of both vitamin D and n-3 FA supplements on the severity of knee pain.
Methods
Study Population
VITAL is a completed randomized, double-blind, placebo-controlled clinical trial performed to assess the effect of vitamin D and n-3 FA supplementation in the primary prevention of cancer and cardiovascular disease.21 VITAL included 25,871 U.S. adults without cancer or cardiovascular disease at the time of enrollment. Men aged ≥50 and women aged ≥55 were enrolled beginning in March 2011 from the community through brochures, targeted mailings, media reports, and advertisements. Black participants were oversampled (n=5,106, 20%).21 Participants were randomized to vitamin D3 (cholecalciferol; 2000 IU/day), marine omega-3 fatty acids (Omacor® 1g/d, 840 mg eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) in a 1.3 to 1 ratio), or placebo in a two-by-two factorial design.21 Participants were required to limit personal supplements of vitamin D to 800 IU/day and calcium to 1200mg/day, and avoid fish oil supplements. Randomization was computer generated in blocks of eight stratified by sex, race, and 5-year age groups.21 Participants in the parent trial were mailed follow-up questionnaires at 6-months and then annually. The median follow-up time was 5.3 years with a range of 3.8 to 6.1 years. The details of this trial have been previously published.19–22 Within a subcohort of VITAL providing blood samples, the mean 25-hydroxyvitamin D level at baseline was 30.8 ng/ml (SD 10) and the mean n-3 FA index 2.7 % (SD 0.9).19,20 In participants randomized to Vitamin D and providing baseline and 1-year blood samples, vitamin D levels increased by 12ng/ml.19 Adherence was defined based on participant report of taking at least two thirds of the study medication. For vitamin D the adherence was 82.0% on treatment, and 80.3% in the placebo group.19 For n-3FA the adherence was 81.6% on treatment and 81.5% on placebo.20
We identified a “Knee Pain Cohort” consisting of VITAL participants who self-reported frequent and chronic knee pain at enrollment prior to randomization and who were highly likely to have knee OA based on an affirmative response to all of the following: a) self-reported knee pain symptoms in walking 2–3 blocks; b) knee pain > 1 day/week c) knee pain for ≥ 1 year; and d) a physician’s diagnosis of knee OA. These questions were chosen based on the development of a knee OA screening instrument with ‘enhanced specificity’ by LaValley et al which included pain in the past month in walking 2–3 blocks and report of every having a physician diagnose knee OA.23 This instrument proved particularly specific for knee OA, 94% specific and 46% sensitive.23 We additionally queried about duration and frequency of knee symptoms as we felt it important to investigate a group with active and persistent knee symptoms. We performed a medical record review on a subset of knee pain participants to validate osteoarthritis as is outlined below. Patients with previous bilateral total knee replacement (TKR) were excluded. For participants with prior unilateral TKR, the native contralateral knee was eligible for inclusion. Assuming a WOMAC Pain standard deviation of 18 points we had 93% power to detect an effect size of 0.2.
During the VITAL trial run-in period (baseline), 1,430 (6%) trial participants were eligible for the Knee Pain Cohort and were mailed supplementary baseline knee pain questionnaires. Of the participants eligible for the Knee Pain Cohort, 1,221(85%) returned a baseline questionnaire. In year two of the trial, an additional 186 participants were identified as eligible for the Knee Pain Cohort (due to delayed baseline questionnaire return) and were sent questionnaires; 177 of these participants returned the questionnaire providing additional post-randomization data. There were no differences in age, race or geographic location between the final 1,398 in the Knee Pain Cohort and the 218 participants eligible at enrollment who did not return any knee pain questionnaires. Participants not returning questionnaires had higher BMI (34 vs. 32) and were more likely to be female (74% vs. 66%). Eighty-six participants completed the baseline knee pain questionnaire, but none of the follow up questionnaires.
The VITAL trial is registered at clinicaltrials.gov (NCT01169259 for the parent trial and NCT01351805 for the knee pain ancillary study). The Partners’ Human Research Committee approved this study.
Data Elements at Baseline
Participants’ demographic and socioeconomic characteristics, including age, sex, race/ethnicity, geographic location of residence, education level, and income, were collected on the baseline survey. Data on body mass index (BMI in kg/m2, calculated from self-reported weight and height), and self-reported physical activity (MET-hours per week) were also recorded.
Knee Symptom Assessment
Participants in the Knee Pain Cohort completed a ‘Knee Symptom Questionnaire’ at baseline and annually thereafter on VITAL follow-up questionnaires based on ‘their worst knee during the last week’, or both knees if both were equally painful. Participants were randomized from November 2011 through March 2014. The trial intervention ended December 31, 2017. The median follow-up time for the Knee Pain Cohort was 5.3 years (SD 0.7) and on average each participant completed 3.8 (SD 1.3) out of a possible 6 questionnaires. We assessed patient-reported knee symptoms using a modified Western Ontario and McMaster’s Universities Osteoarthritis Index (WOMAC) for Pain, Stiffness and Function subscales. 24,25 The modified WOMAC Function scale reduces redundancy by compressing the Function scale from 17 to 7 items (ascending stairs, rising from sitting, walking on a flat surface, getting in/out of car, putting on socks/stockings, rising from bed, sitting) yet has a Cronbach’s α of 0.87–0.93 and retains responsiveness.25 The WOMAC Pain and Stiffness scales were used in their original format. WOMAC subscale scores were individually summed and scaled (0–100, 100 worst). A WOMAC end-user license was obtained for this study. Knee pain frequency was assessed as follows: never, less than 1 day/week, 1–2 days/week, 3–6 days/week, and daily. Frequency of use of the following medications was collected at baseline and during follow up: 1) acetaminophen, 2) nonsteroidal anti-inflammatory drugs (NSAID): naproxen, cox-2 inhibitors, indomethacin, etodolac, ibuprofen, nabumetone, diclofenac, salsalate, and piroxicam, 3) analgesics including opioids: morphine, propoxyphene, oxycodone, hydrocodone, tramadol, amitriptyline, butalbital, and gabapentin. Prevalent TKR was assessed at baseline and incident TKR was ascertained during follow up.
Validation
Validation of osteoarthritis as the primary cause of knee pain as well as a subset of patient-reported TKR has been reported previously.26 Briefly, of the 1,398 participants in the Knee Pain Cohort, medical records from 200 men and 200 women (29%, randomly selected) were requested for validation of OA. Of these 400 participants, we were able to obtain 226 (57%) medical records for review. Of those received, 207 (92% of reviewed) were confirmed to have knee OA based on physician’s diagnosis and/or radiographic imaging. For the 174 participants in whom we were unable to obtain medical records the majority were due to inability to contact either the participant or treating physician (103 records). Additionally, 32 participants did not consent, 15 could not provide records for miscellaneous reasons including destruction of older records. Only 24 participants denied being treated or evaluated for knee OA.26
Outcomes and Statistical Analysis
Baseline characteristics were assessed using means and percentages. We used an intention to treat analysis and included eligible participants who returned at least one of the knee pain questionnaires over the trial period. We first examined the relationship between change in WOMAC Pain over the study duration (dependent variable) and treatment arm (independent variable). For the primary analysis we used a repeated measures model with unstructured variance to assess the effect of treatment arm on measures of WOMAC Pain over follow-up after adjustment for age, sex, and the other treatment arm. The linear time by treatment interaction term was used to assess change in WOMAC Pain over time between participants randomized to treatment versus placebo. While the primary analysis examined the trend in the treatment effect over time, we also considered the treatment difference at each time point. In this factorial trial we assessed the main effects of the treatment arms comparing all those randomized to vitamin D versus vitamin D placebo, regardless of n-3 FA status; and similarly for n-3 FAs. Because receipt of TKR during the trial period likely would have influenced self-reported knee symptoms, participants were censored at TKR. WOMAC Pain was the prespecified primary outcome, the above analyses were also repeated for the WOMAC Function and WOMAC Stiffness subscales.
We evaluated the effect of vitamin D and n-3 FA supplementation on WOMAC Pain over time in several subgroups after stratifying by sex, race (white vs. non-white), BMI (<27 vs. ≥ 27), baseline fish consumption (< 1.5 servings/week vs ≥ 1.5 servings/week) and other treatment (i.e. randomized to n-3 FA and stratified on active versus placebo vitamin D). In the subset of participants with measured baseline vitamin D levels (n=854) and n-3 FA index (n=840), we assessed effect modification based on vitamin D <20 ng/ml and ≥20 ng/ml and n-3 FA index <2.7% and ≥2.7%. We again used a repeated measures model with censoring for TKR. We adjusted for age, sex and other treatment arm in analyses in which covariates were not regarded as potential modifiers.
We conducted two sensitivity analyses. First, we included only the participants who completed baseline pre-randomization knee pain questionnaires (n=1221). Censoring at TKR may introduce bias as those receiving TKR typically do so in response to increased pain. Thus, in a second sensitivity analysis we assumed a ‘worse case scenario’ and added 10 points to the last reported WOMAC Pain score prior to receipt of TKR and imputed this value as the next score after TKR, with subsequent scores kept as missing. Prior work by Collins et al demonstrates that pain trajectories are relatively stable over a 6-year period (i.e. patients with high pain stay in high pain) supporting this assumption.27
We examined two secondary outcomes. The risk of incident TKR in each treatment arm was evaluated using Cox proportional hazard regression after adjustment for age and sex. We also assessed change in use of analgesics including opioids, as defined above, over the study duration. Use of analgesics including opioids was dichotomized as occasional or daily versus no use. General estimating equations with adjustment for age, sex, and censoring for TKR, were created to examine whether use of vitamin D or n-3 FA modified analgesics including opioids use over time.
A two-tailed p value of <0.05 was considered statistically significant, we did not adjust for multiple comparisons. All analyses were performed using SAS 9.4 statistical software (SAS Institute Inc., Cary, NC).
Results
Baseline Characteristics
In the Knee Pain Cohort 674 (48%) were randomized to active vitamin D and 695 (50%) to active n-3 FA. In Table 1, we present the baseline demographic characteristics of the Knee Pain Cohort overall and stratified by treatment group. Further knee-pain-related baseline characteristics collected on the Knee Symptom Questionnaire are displayed in Table 2 by treatment groups. A small percentage (5.4%) of patients who reported at least daily knee pain on the screening questionnaire subsequently reported no or less than daily symptoms once enrolled in the Knee Pain cohort but were included in the analysis. Baseline characteristics were well balanced between those randomized to vitamin D versus placebo. For n-3 FA, those randomized to placebo reported greater physical activity levels (11 MET hrs/wk vs 7 MET hr/wk) and differences in frequency of acetaminophen usage. There were no differences in baseline WOMAC subscales between either of the treatment groups and placebo.
Table 1:
Baseline Characteristics of Knee Pain Cohort within the VITAL Trial
| Knee Pain Cohort (n=1398) | Vitamin D | Omega-3 Fish Oil | |||
|---|---|---|---|---|---|
| Active (n=674) | Placebo (n=724) | Active (n=695) | Placebo (n=703) | ||
| Age, mean years (SD) | 67.7 (6.9) | 67.5 (6.7) | 68.0 (7.13) | 67.9 (7.0) | 67.6 (6.8) |
| BMI, mean (SD) | 31.8 (7.5) | 31.8 (7.0) | 31.8 (7.9) | 32.0 (7.1) | 31.5 (7.8) |
| Physical activity, total MET-hours/week, median (IQR) | 8.5 (1.9–23.2) | 7.6 (2.0–21.8) | 10.0 (1.9–24.5) | 7.2 (1.7–21.5) | 10.6 (2.1–25.0) |
| Female, n (%) | 920 (66) | 439 (65) | 481 (66) | 464 (67) | 456 (65) |
| Race, n (%) | |||||
| Non-Hispanic White | 882 (65) | 422 (64) | 460 (65) | 436 (64) | 446 (65) |
| Black | 375 (28) | 182 (28) | 193 (27) | 197 (29) | 178 (26) |
| Hispanic | 50 (4) | 24 (4) | 26 (4) | 16 (2) | 34 (5) |
| Other/unknown | 59 (4) | 29 (4) | 30 (4) | 29 (4) | 30 (4) |
| Geographic Location, n (%) | |||||
| Northeast | 335 (24) | 155 (23) | 180 (25) | 152 (22) | 183 (26) |
| Midwest | 337 (24) | 157 (23) | 180 (25) | 171 (25) | 166 (24) |
| West | 325 (23) | 163 (24) | 162 (22) | 168 (24) | 157 (22) |
| Southeast | 401 (29) | 199 (30) | 202 (28) | 204 (29) | 197 (28) |
| Education, n (%) | |||||
| ≤ High school diploma or GED, n (%) | 274 (20) | 127 (19) | 147 (20) | 140 (20) | 134 (19) |
| > High school | 1118 (80) | 543 (81) | 575 (80) | 551 (80) | 567 (81) |
| Income, n (%) | |||||
| <$50,000 | 619 (49) | 298 (49) | 321 (49) | 321 (51) | 298 (47) |
| ≥50,000 | 646 (51) | 316 (52) | 330 (51) | 308 (49) | 338 (53) |
BMI; body mass index
MET; metabolic equivalent task
GED; general education development
Table 2:
Baseline knee pain characteristics of Knee Pain Cohort
| Knee Pain Cohort (n=1221) | Vitamin D | Omega-3 Fish Oil | |||
|---|---|---|---|---|---|
| Active (n=591) | Placebo (n=630) | Active (n=595) | Placebo (n=626) | ||
| Knee Pain Frequency, n (%)* | |||||
| Never | 5 (0.4) | 2 (0.3) | 3 (0.5) | 2 (0.3) | 3 (0.5) |
| < 1 day/week | 62 (5) | 32 (6) | 40 (7) | 35 (6) | 27 (4) |
| 1–2 days/week | 136 (12) | 75 (13) | 61 (10) | 71 (12) | 65 (11) |
| 3–6 days/week | 240 (20) | 115 (20) | 125 (21) | 115 (20) | 125 (21) |
| Daily | 736 (62) | 350 (61) | 386 (64) | 354 (61) | 382 (63) |
| Prior Total Knee Replacement, n (%) | 176 (15) | 89 (15) | 87 (14) | 83 (14) | 93 (15) |
| Unilateral knee pain, n (%) | 821 (71) | 400 (71) | 421 (71) | 398 (70) | 423 (71) |
| Bilateral knee pain, n (%) | 338 (29) | 164 (29) | 174 (29) | 168 (30) | 170 (29) |
| Acetaminophen use, n (%) | |||||
| Never | 525 (49) | 243 (47) | 282 (51) | 245 (48) | 280 (51) |
| Occasionally | 380 (36) | 186 (36) | 194 (35) | 175 (34) | 205 (37) |
| Daily | 162 (15) | 83 (16) | 79 (14) | 94 (18) | 68 (12) |
| Nonsteroidal Anti-inflammatory medication use, n (%) | |||||
| Never | 269 (24) | 135 (25) | 134 (23) | 127 (23) | 142 (25) |
| Occasionally | 391 (35) | 192 (35) | 199 (35) | 199 (37) | 192 (33) |
| Daily | 462 (41) | 221 (40) | 241 (42) | 217 (40) | 245 (42) |
| Analgesics Including Opioids | |||||
| Never | 762 (69) | 364 (68) | 398 (70) | 371 (69) | 391 (68) |
| Occasionally | 171 (15) | 87 (16) | 84 (15) | 81 (15) | 90 (16) |
| Daily | 172 (16) | 86 (16) | 86 (15) | 82 (15) | 90 (16) |
| WOMAC Pain, mean, SD | 36.7 (18.7) | 36.2 (18.5) | 37.0 (18.8) | 37.2 (18.5) | 36.1 (18.8) |
| WOMAC Function, mean, SD | 36.7 (19.9) | 36.0 (19.8) | 37.5 (20.0) | 37.0 (20.2) | 36.4 (19.6) |
| WOMAC Stiffness, mean, SD | 44.3 (21.8) | 43.5 (22.1) | 45.0 (21.6) | 45.0 (22.5) | 43.7 (21.3) |
WOMAC; Western Ontario and McMaster Osteoarthritis Index. Scored 0–100, 100 worst.
Data from a baseline “knee pain questionnaire’ after eligibility established, thus some included participants now report having no or infrequent knee pain.
Primary Outcome
Over the course of the trial, 61 (4%) participants in the knee pain cohort died. Twenty-six were in the vitamin D arm and 35 in placebo. Thirty-seven participants died in the n-3 FA arm and 24 in placebo. During the follow-up period for the knee pain cohort, 296 (21%) of participants reported having a TKR. One hundred and forty were receiving active vitamin D and 156 placebo; 146 were receiving active n-3 FA and 150 placebo. No deaths were attributable to the interventions.
Vitamin D
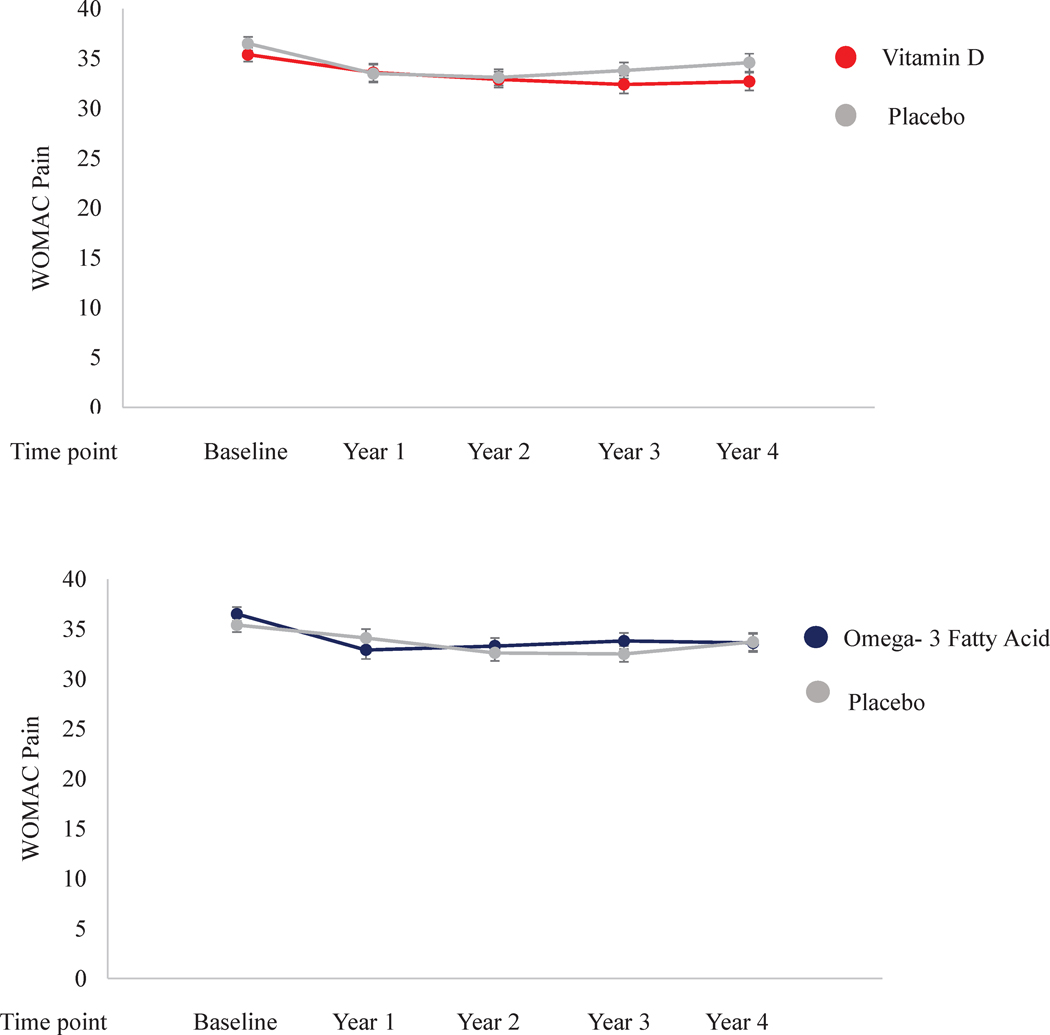
After adjustment for age and sex, the least squared mean WOMAC Pain at baseline was 35.4 (SE 0.7) in the vitamin D arm and 36.5 (SE 0.7) in the placebo arm. At time of last follow-up, the mean WOMAC Pain score was 32.7 (SE 0.9) in the vitamin D arm and 34.6 (SE 0.9) in the placebo arm. Figure 1 (top) There were no statistically significant differences in WOMAC Pain between the vitamin D and placebo arm at any of the time points or as a trend over time (p interaction with time= 0.41). For both WOMAC Function and Stiffness, we found no statistically significant differences between vitamin D and placebo at any of the time points. Neither WOMAC Function or Stiffness changed significantly between vitamin D and placebo over time (all p interaction with time > 0.05). Among men, while WOMAC Pain did not differ between active and placebo vitamin D at baseline (32.3 vs. 33.1), at last follow-up WOMAC Pain was 29.4 (SE 1.5) among the men randomized to active vitamin D versus 34.0 (SE 1.5) in those randomized to placebo (p=0.04). The overall linear time by treatment interaction after adjustment for age and the other treatment arm was statistically significant (p=0.04). However, the 3-way interaction (time by treatment by sex) was not statistically significant indicating that sex did not modify the effect of vitamin D on WOMAC Pain. In none of the other subgroups, did we find modification of the effect of vitamin D on WOMAC Pain. Appendix 1
Figure 1:
Repeated measure analyses of mean WOMAC Pain with standard error over time for Vitamin D and Omega- 3 Fatty acid versus placebo with censoring for total knee replacement adjusted for age, sex, and other treatment group respectively
Omega-3 Fatty Acid
In the n-3 FA arm at baseline, the mean WOMAC Pain at baseline was 36.5 (SE 0.7) versus 35.4 (SE 0.7) in the placebo arm after adjustment for age and sex. At time of last follow-up, the mean WOMAC Pain score was 33.6 (SE 0.9) in the n-3 FA arm and 33.7 (SE 0.9) in the placebo arm. Figure 1 (bottom) There were no statistically significant differences between the n-3 FA arm and the placebo arm at any of the time points or in trend over time between the n-3 FA and placebo treatment arms (p interaction with time= 0.77). For both WOMAC Function and Stiffness, we found no statistically significant differences n-3 FA and placebo at any of the time points. Neither WOMAC Function nor Stiffness changed significantly over time by treatment arm (all p interaction with time > 0.05). In none of the subgroups, did we find modification of the effect of n-3 FA on WOMAC Pain. Appendix 1
Sensitivity Analysis
The results of the sensitivity analyses, including only the 1221 participants with baseline knee pain questionnaires, and imputing last pain score prior to TKR plus 10 points did not change the results of the primary analyses. For both vitamin D and n-3 FA, there were no statistically significant differences in WOMAC Pain at any time point and the overall linear time by treatment interactions were null.
Secondary Outcomes
In analyses for the secondary outcome of incident TKR, over a mean of 4.5 (1.6) person-years, the hazard ratios (HR) for incident TKR in the vitamin D and n-3 FA arms were 0.97 (95% CI 0.78, 1.22) and 0.99 (95% CI 0.79, 1.24), respectively, in comparison to placebo after adjustment for age and sex.
At baseline 173 (32%) of participants randomized to vitamin D reported occasional or daily analgesics including opioids use, while 364 (68%) reported no use. In those randomized to vitamin D placebo, 170 (30%) reported occasional/daily use and 398 (70%) reported no use. Of those in the n-3 FA arm 163 (31%) reported occasional/daily use and 371 (69%) no use. In those on n-3 FA placebo 180 (32%) reported occasional/daily use and 391 (68%) no use. Neither vitamin D nor n-3 FA altered the use of analgesics including opioids over the study period (vitamin D p interaction =0.81, n-3 FA p interaction= 0.25).
Discussion
In this large population-based cohort of patients with chronic, frequent knee pain, we found that randomized supplementation with neither vitamin D nor n-3 FA led to a decrease in patient-reported knee pain over placebo. Over the mean of 5.3 years of follow-up, a small decrease in reported knee pain was observed, but this occurred in both the treatment and placebo groups and may reflect regression to the mean and some loss of participants to TKR. At no time point did pain scores statistically differ between treatment and placebo, and treatment arm did not significantly affect pain scores over time. None of the observed differences in the primary analysis exceeded an effect size of 0.1 and are therefore unlikely to have clinical implications. No clinically or statistically significant reductions in WOMAC Function or Stiffness were identified in either treatment group compared to placebo.
We saw a nominally statistically significant effect of vitamin D on WOMAC Pain in males over time. However, WOMAC Pain differed at only at the time of last follow-up, at which point males on active vitamin D had a WOMAC Pain score 4.5 points lower than those on placebo. Overall the interaction with sex was not statistically significant and furthermore, as these analyses were not adjusted for multiple comparisons this difference is unlikely to be clinically meaningful. Subgroup analyses by race, BMI, baseline fish consumption, randomization status to other treatment, baseline vitamin D level and baseline n-3 FA index did not modify the effect of either n-3 FA or vitamin D on WOMAC Pain over time.
Vitamin D and n-3 FA did not lead to reduction in receipt of TKR over placebo, an indicator of severely symptomatic knee OA. Additionally, we did not observe alteration in analgesics including opioids over the study period with supplementation of either vitamin D or n-3 FA.
Prior randomized controlled trials have been conflicting concerning the efficacy of vitamin D on knee pain in OA. In a 24-month trial of participants with symptomatic knee OA and low vitamin D (12.5 nmol/L to 60nmol/L) randomized to vitamin D versus placebo, there were no significant changes in WOMAC pain scores between the treatment groups.12 However, post hoc analysis revealed significant improvements in the vitamin D group for total WOMAC score and WOMAC function as well as a greater proportion of OMERACT-OARSI responders. In a 12-month pilot trial of participants with symptomatic knee OA and vitamin D insufficiency randomized to vitamin D versus placebo Sanghi et al reported significant improvement in WOMAC Pain and Function in the vitamin D group though these differences did not meet the threshold for minimal clinically improved difference.16 A randomized trial of vitamin D and placebo in 146 patients with symptomatic OA did not find significant differences in knee pain between treatment groups as measured by WOMAC over 24 months though the authors raised concerns about the possibility of a type 2 error.15 A subsequent and larger 3-year trial of 474 participants in the U.K. with symptomatic knee OA, 50% of whom were classified at vitamin D deficient, again did not find statistically significant differences between vitamin D and placebo in WOMAC Pain, Function, or Stiffness over the study period, despite reducing the number of participants with vitamin D deficiency by 80%.17 Our trial adds to this body of evidence through the use of a large racially-diverse population-based cohort with over four years of follow-up. Our findings further support that vitamin D supplementation doses not have a role in the reduction of knee pain in patients with OA even among the subgroup with low vitamin D.
Findings from the Multicenter Osteoarthritis Study (MOST) have suggested that higher systemic levels of total n-3 FAs were associated with less cartilage loss in the patellofemoral compartment.28 Conversely high levels of arachidonic acid, an n-6 FA considered to be pro-inflammatory, were associated with synovitis.28 Randomized trials have suggested benefit of n-3 FA in patients with rheumatoid arthritis.29,30 Trials using n-3 FA in OA have been limited. Hill et al compared high (4.5g EPA+DHA) versus low (0.45g EPA+DHA) dose fish oil supplementation in patients with symptomatic knee OA over 24 months and reported better WOMAC Pain and Function scores in the low-dose fish oil group.18 However, the low dose fish oil supplement also contained sunola oil, raising concern that perhaps this supplement conferred some benefit in knee OA symptoms.18 The current larger RCT allowed for comparison of n-3 FA acids to placebo and found that n-3 FA supplementation in knee OA did not confer symptomatic benefit.
Our study does have some limitations. Several knee pain cohort participants had late baseline questionnaire return and thus did not contribute to pre-randomization data. Their follow-up data contributed to our repeated measures model. Sensitivity analyses that did not include these subjects had similar findings to the primary analyses. Though the trial included fewer participants who met the definition of knee pain than anticipated in the initial power calculations, the sample size provided ample statistical power to detect differences in WOMAC Pain. We did not have knee radiographic data for participants to assess OA severity, therefore we are unable to assess whether vitamin D or n-3 FA supplementation may have a greater role in patients with more or less severe radiographic OA. Diagnosis of knee was based on patient-report which may be subject to bias; however, any bias should not differ by randomized treatment group. Moreover, in the subgroup of participants where medical records were requested and reviewed, the vast majority (92% of those reviewed) were confirmed to have knee OA. As we required at least a year of knee symptoms for eligibility, these results do not preclude that Vitamin D or n-3 FA may have a role in pain management in early disease. We queried the use of opioids and/or neuropathic pain medications with a single question and thus cannot report on reduction on use of opioids or neuropathic pain medications individually. The dose of vitamin D and n-3 FA were chosen as they represented the safest and most effective doses for the parent trial. We cannot rule out that different dosing regimens or supplement preparation may have changed our findings. This is a large community-based cohort with efforts to ensure inclusion of racially diverse participants increasing the generalizability of our findings. Participants were followed for five years, providing ample time to assess for changes in symptoms related to OA which is often a slowly progressive disease.
In summary, this trial did not reveal that either vitamin D or n-3 FA compared to placebo was associated with improvement in knee symptoms in older adults with chronic daily knee pain and self-reported knee OA. These results are in agreement with past smaller randomized control trials and suggest that supplementation of vitamin D or n-3 FA does not have a role in the management of symptomatic knee pain due to osteoarthritis.
Supplementary Material
Acknowledgments
Dr. MacFarlane has consulted for Flexion Therapeutics and receives research support from Flexion Therapeutics, Samumed and Amgen. Dr. Iversen received funding as a speaker for Janssen Pharmaceuticals and grant support from the Swedish Rheumatism Foundation. Dr. Buring has a family member on the scientific board of Pharmavite. Dr. Katz received research support from Flexion Therapeutics and Samumed.
Source of funding: NIH R01 AR059086, K24 AR066109, U01 CA13892, R01 CA13892, P30 AR072577, RRF SDA
Footnotes
References
- 1.Mantyselka P, Kumpusalo E, Ahonen R, et al. Pain as a reason to visit the doctor: a study in Finnish primary health care. Pain 2001;89:175–80. [DOI] [PubMed] [Google Scholar]
- 2.Deshpande BR, Katz JN, Solomon DH, et al. Number of Persons With Symptomatic Knee Osteoarthritis in the US: Impact of Race and Ethnicity, Age, Sex, and Obesity. Arthritis Care Res 2016;68:1743–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peat G, McCarney R, Croft P. Knee pain and osteoarthritis in older adults: a review of community burden and current use of primary health care. Ann Rheum Dis 2001;60:91–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Ambulatory Medical Care Survey: 2015 State and National Summary Tables. 2015. (Accessed 5/20/2019, at http://www.cdc.gov/nchs/ahcd/ahcd_products.htm.)
- 5.Nguyen US, Zhang Y, Zhu Y, Niu J, Zhang B, Felson DT. Increasing prevalence of knee pain and symptomatic knee osteoarthritis: survey and cohort data. Ann Intern Med 2011;155:725–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jordan KM, Arden NK, Doherty M, et al. EULAR Recommendations 2003: an evidence based approach to the management of knee osteoarthritis: Report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis 2003;62:1145–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kwan Tat S, Pelletier JP, Lajeunesse D, Fahmi H, Lavigne M, Martel-Pelletier J. The differential expression of osteoprotegerin (OPG) and receptor activator of nuclear factor kappaB ligand (RANKL) in human osteoarthritic subchondral bone osteoblasts is an indicator of the metabolic state of these disease cells. Clin Exp Rheumatol 2008;26:295–304. [PMC free article] [PubMed] [Google Scholar]
- 8.Bischoff-Ferrari HA, Dietrich T, Orav EJ, et al. Higher 25-hydroxyvitamin D concentrations are associated with better lower-extremity function in both active and inactive persons aged > or =60 y. Am J Clin Nutr 2004;80:752–8. [DOI] [PubMed] [Google Scholar]
- 9.Jeffery LE, Raza K, Hewison M. Vitamin D in rheumatoid arthritis-towards clinical application. Nat Rev Rheumatol 2016;12:201–10. [DOI] [PubMed] [Google Scholar]
- 10.Bagga D, Wang L, Farias-Eisner R, Glaspy JA, Reddy ST. Differential effects of prostaglandin derived from omega-6 and omega-3 polyunsaturated fatty acids on COX-2 expression and IL-6 secretion. Proc Natl Acad Sci U S A 2003;100:1751–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Curtis CL, Hughes CE, Flannery CR, Little CB, Harwood JL, Caterson B. n-3 fatty acids specifically modulate catabolic factors involved in articular cartilage degradation. J Biol Chem 2000;275:721–4. [DOI] [PubMed] [Google Scholar]
- 12.Jin X, Jones G, Cicuttini F, et al. Effect of Vitamin D Supplementation on Tibial Cartilage Volume and Knee Pain Among Patients With Symptomatic Knee Osteoarthritis: A Randomized Clinical Trial. Jama 2016;315:1005–13. [DOI] [PubMed] [Google Scholar]
- 13.Laslett LL, Quinn S, Burgess JR, et al. Moderate vitamin D deficiency is associated with changes in knee and hip pain in older adults: a 5-year longitudinal study. Ann Rheum Dis 2014;73:697–703. [DOI] [PubMed] [Google Scholar]
- 14.McAlindon TE, Felson DT, Zhang Y, et al. Relation of dietary intake and serum levels of vitamin D to progression of osteoarthritis of the knee among participants in the Framingham Study. Ann Intern Med 1996;125:353–9. [DOI] [PubMed] [Google Scholar]
- 15.McAlindon T, LaValley M, Schneider E, et al. Effect of vitamin D supplementation on progression of knee pain and cartilage volume loss in patients with symptomatic osteoarthritis: a randomized controlled trial. Jama 2013;309:155–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanghi D, Mishra A, Sharma AC, et al. Does vitamin D improve osteoarthritis of the knee: a randomized controlled pilot trial. Clinical orthopaedics and related research 2013;471:3556–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arden NK, Cro S, Sheard S, et al. The effect of vitamin D supplementation on knee osteoarthritis, the VIDEO study: a randomised controlled trial. Osteoarthritis and cartilage 2016;24:1858–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hill CL, March LM, Aitken D, et al. Fish oil in knee osteoarthritis: a randomised clinical trial of low dose versus high dose. Ann Rheum Dis 2016;75:23–9. [DOI] [PubMed] [Google Scholar]
- 19.Manson JE, Cook NR, Lee IM, et al. Vitamin D Supplements and Prevention of Cancer and Cardiovascular Disease. The New England journal of medicine 2019;380:33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manson JE, Cook NR, Lee IM, et al. Marine n-3 Fatty Acids and Prevention of Cardiovascular Disease and Cancer. The New England journal of medicine 2019;380:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bassuk SS, Manson JE, Lee IM, et al. Baseline characteristics of participants in the VITamin D and OmegA-3 TriaL (VITAL). Contemporary clinical trials 2016;47:235–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manson JE, Bassuk SS, Lee IM, et al. The VITamin D and OmegA-3 TriaL (VITAL): rationale and design of a large randomized controlled trial of vitamin D and marine omega-3 fatty acid supplements for the primary prevention of cancer and cardiovascular disease. Contemporary clinical trials 2012;33:159–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.LaValley M, McAlindon TE, Evans S, Chaisson CE, Felson DT. Problems in the development and validation of questionnaire-based screening instruments for ascertaining cases with symptomatic knee osteoarthritis: the Framingham Study. Arthritis and rheumatism 2001;44:1105–13. [DOI] [PubMed] [Google Scholar]
- 24.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 1988;15:1833–40. [PubMed] [Google Scholar]
- 25.Whitehouse SL, Lingard EA, Katz JN, Learmonth ID. Development and testing of a reduced WOMAC function scale. J Bone Joint Surg Br 2003;85:706–11. [PubMed] [Google Scholar]
- 26.MacFarlane LA, Kim E, Cook NR, et al. Racial Variation in Total Knee Replacement in a Diverse Nationwide Clinical Trial. J Clin Rheumatol 2018;24:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Collins JE, Katz JN, Dervan EE, Losina E. Trajectories and risk profiles of pain in persons with radiographic, symptomatic knee osteoarthritis: data from the osteoarthritis initiative. Osteoarthritis and cartilage 2014;22:622–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baker KR, Matthan NR, Lichtenstein AH, et al. Association of plasma n-6 and n-3 polyunsaturated fatty acids with synovitis in the knee: the MOST study. Osteoarthritis and cartilage 2012;20:382–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kremer JM, Lawrence DA, Petrillo GF, et al. Effects of high-dose fish oil on rheumatoid arthritis after stopping nonsteroidal antiinflammatory drugs. Clinical and immune correlates. Arthritis and rheumatism 1995;38:1107–14. [DOI] [PubMed] [Google Scholar]
- 30.Nielsen GL, Faarvang KL, Thomsen BS, et al. The effects of dietary supplementation with n-3 polyunsaturated fatty acids in patients with rheumatoid arthritis: a randomized, double blind trial. European journal of clinical investigation 1992;22:687–91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.