Abstract
SARS-CoV-2 has triggered a major epidemic among people around the world, and it is the newest in the sequence to become prevalent among other infectious diseases. The drug repurposing concept has been utilized effectively for numerous viral infections. Considering the situation and the urgency, the idea of drug repurposing for coronavirus infection (COVID-19) is also being studied. The molecular docking method was used for the screening of 29 antiviral drugs against primary protease proteins (MPP) of SARS-CoV-2, spike ecto-domain, spike receptor binding domain, Nsp9 RNA binding protein, and HR2 domain. Among these drugs, in terms of least binding energy, Indinavir, Sorivudine, Cidofovir, and Darunavir showed minimum docking scores with all the key proteins. For ADMET (Absorption, Distribution, Metabolism, Excretion and Toxicity) analysis, the ADMET properties of the top 4 drug candidates were retrieved through literature study. This analysis revealed that these drug candidates are well metabolized, distributed, and bioavailable, but have some undesirable effects. Furthermore, some approved structural analogues, such as Telbivudine, Tenofovir, Amprenavir, Fosamprenavir, etc., were predicted as similar drugs which may also be used for treating viral infections. We highly recommend these drug candidates as potential fighters against the deadly SARS-CoV-2 virus, and suggest in vivo trials for experimental validation of our findings.
Keywords: SARS-CoV-2, Covid-19, Molecular docking, Drug repurposing, Antivirals
Graphical abstract
1. Introduction
The Health Authority of China notified the World Health Organization (WHO) about severe pneumonia cases in Wuhan City of Hubei Province in central China on December 31, 2019 [1,2]. Later, this emerging infectious disease was named novel coronavirus disease 2019 (COVID-19), and the causative agent was determined to be severe acute respiratory syndrome coronavirus 2 (SARSCoV-2) [3]. A well-known scientist in the field of SARS, Dr. Zhengli Shi, suggested the bats as the origin of SARS-CoV-2 [4], and other researchers in China also narrated that Middle East Respiratory Syndrome (MERS) and Severe Acute Respiratory Syndrome (SARS) like coronaviruses are likely to originate from bats in China [5,6]. This SARS-CoV-2 is an envelope and positive-sense single-stranded RNA (+ssRNA) virus [7]. It belongs to the genus Betacoronavirus, and shares about 79% and 50% genetic similarity with SARS-CoV and MERS-CoV, respectively [8]. The virus has become more perilous because of human-to-human transmission via respiratory droplets, especially when people are closely contacted (within 1–2 m) [[9], [10], [11]]. The disease may be symptomatic, paucisymptomatic, and asymptomatic [12]. Commonly appeared respiratory symptoms of this disease include fever, dry cough, dyspnoea, chest pain, fatigue, and myalgia. Besides, headache, dizziness, abdominal pain, diarrhea, nausea and vomiting are the less common symptoms of the disease [13,14]. After the emergence, the disease has spread so fast and extensively around the world that WHO announced it as a pandemic on March 11, 2020.
The pandemic stymied the strong health sectors of the leading countries, namely China, the UK, the United States, Russia, Germany, Canada, Italy, Spain, France, and others. As of 2 July 2020, a total of 10,694,288 people were infected with COVID-19, and 516,210 deaths were calculated worldwide [15]. Researchers from different countries are making every attempt to develop new vaccines and anti-illness medications. Many research and pharmaceutical companies are trying to develop new medicines and vaccines using their sophisticated and advanced laboratories [16,17]. However, it takes around a year before the drugs and/or vaccines to be available for patients because of the time-consuming process. In that case, repurposing of existing drugs can play a momentous role in reducing symptoms or treating the disease. In many studies, some drugs, such as antimalarial drugs (e.g. chloroquine, hydroxychloroquine) or anti-HIV drugs (e.g. lopinavir, ritonavir, saquinavir), showed positive results against COVID-19 [[18], [19], [20]].
Drug repurposing, alternatively known as repositioning, is considered as an important approach for speedy identification of the therapeutic drugs with proven safety profiles to fight novel infectious diseases [[21], [22], [23]]. This repurposing strategy was effective in identifying potential drugs that combat diseases such as hepatitis C virus infection, Zika virus infection, and Ebola disease [24,25,26,and27]]. Moreover, in-silico based screening has become a felicitous method for mitigating the drawbacks of antiviral drug discovery. This computational methods of drug screening, including molecular docking, save both money and time [28,29,30,31,and32]]. On the other hand, current licensed medicines of certain diseases, which are safe for human use, need to be proved as effective drugs against the target diseases [22,33]. Therefore, in silico repurposing can be a great way to identify suitable drugs which target essential proteins of SARS-CoV-2, such as proteins required for viral replication or proteins that bind to the human receptors (ACE2: angiotensin-converting enzyme 2). Our present research focused on virtual screening of a variety of antiviral drugs approved by the Food and Drug Administration (FDA). These drugs were screened against the promising targets, namely SARS-CoV-2 main protease (Mpro, PDB ID-6W63), which is very necessary for viral replication, and spike receptor binding domain (PDB ID-6MOJ), which is needed to bind to human receptor ACE2. Other drug targets include Nsp9 (Nonstructural protein-9) RNA binding protein, Spike Ectodomain and HR2 domain, which are involved in viral replication, receptor binding and fusion, and viral fusion with cell membrane, respectively. The studied FDA approved antiviral drugs such as Sorivudine, Tipranavir, Zalcitabine, Zidovudine, Indinavir, Nelfinavir, Nevirapine, etc. show efficacy against human immunodeficiency virus (HIV). Other screened drugs like Trifluridine, Valganciclovir, Vidarabine, Pritelivir, etc. are used for the treatment of human herpes virus disease. Along with these drugs, we also tested drugs that are workable against Influenza A virus, Influenza B virus, Hepatitis B virus, Hepatitis C virus, Respiratory Syncytial Virus (RSV), and other RNA/DNA viruses in order to detect their effectiveness against SARS-CoV-2.
2. Materials and methods
2.1. Retrieval of SARS-CoV-2 proteins/protein-domains and antiviral drugs
The 3D structures of SARS-CoV-2 main protease (PDB ID: 6W63), Nsp9 RNA binding protein (PDB ID: 6W4B), Spike receptor binding domain (PDB ID: 6M0J), spike ecto-domain (PDB ID: 6VYB), and HR2 Domain (PDB ID: 6LVN) were retrieved from the RCSB Protein Data Bank [34]. A total of 29 antiviral drugs previously used against various viruses (e.g., HIV, HSV, etc.) were collected in SDS (3D) format from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/) [35] (Supplementary file 1). These drugs were also crosschecked in DrugBank database (https://www.drugbank.ca/) [36] (Supplementary file 2). Then, OpenBabel v2.3 program was used to transform the retrieved SDS structures in PDB format for further analysis [37].
2.2. Molecular docking of antiviral drugs against SARS-CoV-2 proteins/protein-domains and dynamics simulation
Molecular docking is an efficient method that ranks docked compounds by the binding affinity of ligand-receptor complexes [[38], [39], [40]]. PatchDock server was used to measure the binding affinity of 29 antiviral drugs with different SARS-CoV-2 proteins/protein domains (drug targets/macromolecules) [41,42]. The docking was performed with the help of shape based complementary principal of docking alghorithm (provided by the patchdock server). The Crystal PDB structure of protein molecules were prepared for docking by removing all water molecules and hetatms [[41], [42], [43], [44]]. The spike glycans were excluded from this model because spike protein N-glycosylation occurs regularly at each site which made them more diverse and heterogeneous [45]. To refine the docked complexes, FireDock refinement tool [46] was employed. An experimental study claimed alpha-ketoamide (CID 6482451) as a primary protease inhibitor for SARS-CoV-2 [47]. Thus, it was docked against all five macromolecules being used as a positive control in this study. Finally, Discovery Studio v3.1 and PyMOL v2.0 were utilized to visualize the ligand receptor complexes [48,49]. Next, stabilization of the structure was determined through deformability analysis and calculation of Eigen value of the complexes. The deformability and Eigen value were predicted through iMOD server [50]. Molecular dynamics of the complexes were studied in water explicit model for 4ns using LARMD server, and from which RMSD and RMSF values were determined [51].
2.3. Analyzing drug profiles of active antiviral drugs
A typical drug candidate should have proper properties of absorption, distribution, metabolism, excretion, and toxicity (ADMET) along with sufficient efficacy against the therapeutic targets [52]. As the studied drugs are approved, the ADMET properties of these drugs were retrieved from the literature study, and then were analyzed.
2.4. Prediction of drug targets and available structural analogs
Screening of the top drugs was performed to find similar potential drugs that could be used for SARSCoV-2 therapy. To predict the probable macromolecular targets of the top drugs, Swiss Target Prediction tool was utilized [53]. Furthermore, the Swiss-Similarity web tool was used to evaluate the potential drug molecules that fight against SARS-CoV-2 by screening homology of the predicted top drugs [54]. The server uses several strategies, such as FP2 fingerprints, spectrophores, electroshape, and align-IT to predict approved drugs from DrugBank, which are commercially available, via virtual screening of numerous repositories of small molecules [54].
3. Results
3.1. Molecular docking of antiviral drugs against SARS-CoV-2 proteins/protein-domains and dynamics simulation
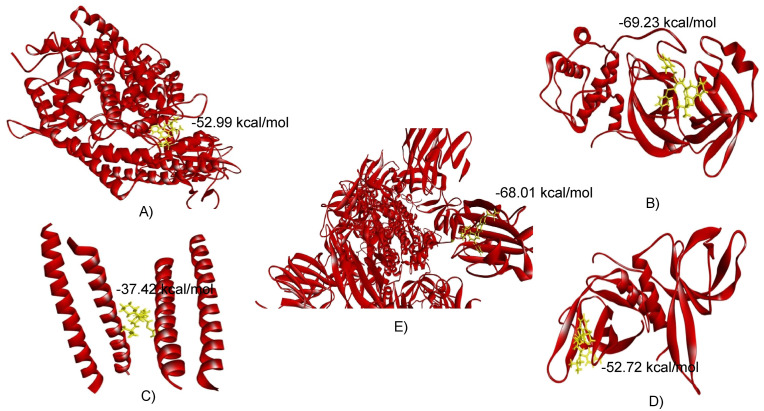
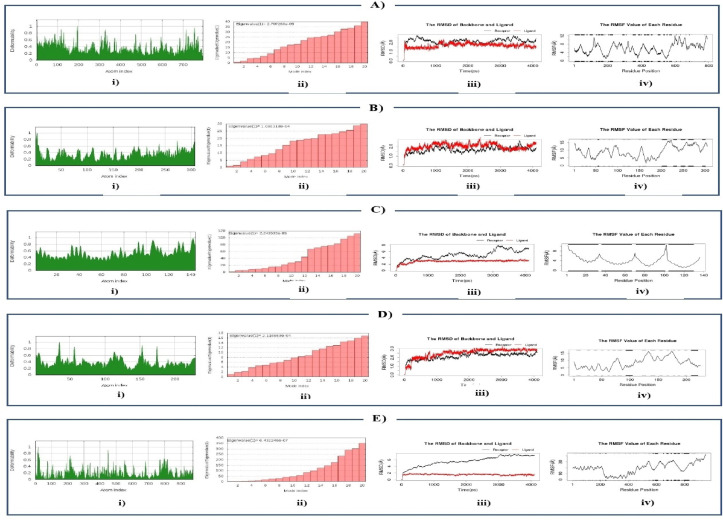
The retrieved structures of five SARS-CoV-2 proteins/protein-domains (macromolecules) and antiviral drugs (ligands) were optimized and executed for molecular docking to compute the binding affinity between the selected macromolecules and ligands. Based on binding energy, the antiviral drugs were ranked, and the drugs showing minimum binding energy were selected as top scorers (Supplementary File 3). In this way, four top scorers, Indinavir, Sorivudine, Cidofovir, and Darunavir, were chosen for further analysis (Table 1 , Fig. 1 ). Sorivudine, Darunavir, and Cidofovir showed the highest binding affinity with spike receptor binding domain (−52.99 kcal/mol), spike ecto-domain (−68.01 kcal/mol), and Nsp9 RNA binding protein (−52.74 kcal/mol), respectively, while Indinavir showed the best binding affinity with both HR2 Domain (−37.42 kcal/mol) and main protease (−69.23 kcal/mol) (Table 1 and Fig. 2, Fig. 3 ). Besides, Sorivudine also experienced excellent binding interactions with spike ecto-domain (−52.28 kcal/mol) and main protease (−59.62 kcal/mol), while Darunavir showed considerable interactions with spike receptor binding domain (−46.88 kcal/mol), Nsp9 RNA binding protein (−47.62 kcal/mol), and main protease (−55.06 kcal/mol). The observations of our analyzed complexes showed that each of them has a low propensity for flexibility, and exhibited resistance to deformation (Fig. 4 : A-i, B-i, C-i,D-i). Darunavir-spike ectodomain complex was only exceptional which showed a bit flexibility, and also had lower mobility (Fig. 4: E-i). The eigen value was found 2.707260 x 10- 05, 1.080318 x 10-04, 2.243535 x 10-05, 2.116669 x 10-04, and 6.432246 x 10-07 for Sorivudine-spike receptor-binding domain, Indinavir-main protease, Indinavir-HR2 Domain, Cidofovir-Nsp9 RNA binding protein, and Darunavir with spike ectodomain, respectively (Fig. 4: A-ii, B-ii, C-ii, Dii and E-ii). The lower eigen value displayed by the Darunavir-spike ectodomain complex made it's deformability easier than others (Fig. 4: E-ii). The RMSD plot of Sorivudine-spike receptor binding domain, Indinavir-main protease, and Cidofovir-Nsp9 RNA binding protein complex showed an equilibrium after 1ns (Fig. 4: A-iii, B-iii, D-iii) that justified the true binding pose, whereas the Indinavir-HR2 Domain and Darunavir-spike ectodomain complexes showed a bit fluctuation probably due to the presence of a loop region (Fig. 4: C-iii, E-iii). The RMSF plot revealed regular atomic fluctuation for complexes (Fig. 4: A-iv, B-iv, C-iv, D-iv), except Darunavir-spike ectodomain complex (Fig. 4: E-iv).
Table 1.
Analysis of binding energy of top five screened drugs (ligands).
| Macromolecules | Ligands | Global Energy | ACE | Score | Area | Binding sites |
|---|---|---|---|---|---|---|
| HR2 Domain (6LVN) | Indinavir | −37.42 | −2.33 | 5148 | 630.20 | Gln13, Lys14, Ile16, Asp17, Arg18, Asn20, Glu21, Lys24 |
| Sorivudine | −29.12 | −1.81 | 4782 | 592.80 | Gln13, Lys14, Asp17, Arg18, Asn20, Glu21, Lys24 | |
| Cidofovir | −28.02 | −2.75 | 5082 | 568.60 | Lys14, Asp17, Arg18, Asn20, Glu21, Lys24 | |
| Darunavir | −26.27 | −0.43 | 5102 | 592.10 | Lys14, Asp17, Arg18, Asn20, Glu21, Ala23, Lys24, Asn27 | |
| Spike receptor binding domain (6M0J) | Indinavir | −49.51 | −13.44 | 6372 | 765.30 | Leu95, Leu97, Gln98, Ala99, Gln101, Tyr196, Tyr202, Trp203, Gly205, Asp206, Glu208, Val209, Asn210, Ala396, Lys562, Glu564, Pro565, Trp566 |
| Sorivudine | −52.99 | −10.46 | 6254 | 787.40 | Leu95, Gln98, Ala99, Gln102, Tyr196, Gly205, Asp206, Glu208, Val209, Asn210, Ala396, Glu398, Lys562, Glu564, Pro565, Trp566 | |
| Cidofovir | −49.19 | −13.26 | 6206 | 795.80 | Lys94, Leu95, Gln98, Ala99, Gln102, Tyr196, Tyr202, Gly205, Asp206, Tyr207, Glu208, Val209, Asn210, Ala396, Lys562, Glu564, Pro565, Trp566 | |
| Darunavir | −46.88 | −14.07 | 5456 | 667.50 | Leu95, Gln102, Asn103, Asn194, Tyr196, Tyr202, Trp203, Gly205, Asp206, Tyr207, Glu208, Val209, Ala396, Lys562, Glu564, Pro565, Trp566 | |
| Spike ecto-domain (6VYB) | Indinavir | −37.29 | −4.09 | 7150 | 854.60 | Arg765, Ala766, Thr768, Gly769, Ile770, Val772, Glu773, Lys776, Glu780, Lys947, Asp950, Gln954, Gln957, Gln1010, Leu1012, Ile1013, Arg1014, Glu1017, Arg1019 |
| Sorivudine | −52.28 | −14.36 | 6848 | 824.30 | Arg319, Phe541, Thr547, Gly548, Thr549, Asp571, Thr572, Thr573, Pro589, Cys590, Phe593, Met740, Cys743, Gly744, Asp745, Ser746, Asn856, Leu966, Ser975, Val976, Leu977, Asn978, Arg1000 | |
| Cidofovir | −42.21 | −9.27 | 6714 | 774.20 | Leu368, Tyr369, Asn370, Ser371, Ala372, Ser373, Phe374, Ser375, Thr376, Phe377, Arg403, Asp405, Glu406, Arg408, Gln409, Thr415, Gly416, Lys417, Asn437, Tyr453 | |
| Darunavir | −68.01 | −25.44 | 6174 | 845.70 | Ser349, Val350, Tyr351, Ala352, Trp353, Asn354, Arg355, Asp398, Ile410, Ala411, Asn422, Tyr423, Lys424, Leu425, Pro426, Phe429, Thr430, Gly431, Cys432, Val433, Pro463, Phe464, Arg466, Val512, Ser514, Phe515 | |
| Nsp9 RNA binding protein (6W4B) | Indinavir | −43.27 | −13.58 | 5908 | 755.00 | Met13, Ser14, Thr36, Lys37, Gly38, Gly39, Arg40, Phe41, Val42, Phe57, Pro58, Lys59, Ser60, Asp61, Ile66, Thr68 |
| Sorivudine | −25.09 | −10.17 | 5506 | 643.50 | Met13, Gly38, Gly39, Arg40, Phe41, Val42, Phe57, Pro58, Lys59, Ser60, Asp61, Thr63, Ile66 | |
| Cidofovir | −52.74 | −18.32 | 5456 | 683.20 | Met13, Tyr33, Gly39, Arg40, Phe41, Val42, Phe57, Pro58, Lys59, Ser60, Asp61, Ile66, Thr68 | |
| Darunavir | −47.62 | −17.90 | 5350 | 640.90 | Met13, Ser14, Gly39, Arg40, Phe41, Val42, Phe57, Pro58, Lys59, Ser60, Asp61, Ile66, Thr68 | |
| Main protease (6W63) | Indinavir | −69.23 | −21.97 | 5584 | 695.70 | Thr25, Leu27, His41, Val42, Cys44, Thr45, Ser46, Met49, Phe140, Leu141, Asn142, Gly143, Ser144, Cys145, His163, His164, Met165, Glu166, Leu167, Pro168, His172, Val186, Asp187, Arg188, Gln189, Gln192 |
| Sorivudine | −59.62 | −19.53 | 5816 | 704.90 | Thr25, Leu27, His41, Cys44, Thr45, Ser46, Met49, Tyr54, Phe140, Leu141, Asn142, Gly143, Ser144, Cys145, His163, His164, Met165, Glu166, Leu167, Pro168, His172, Asp187, Arg188, Gln189, | |
| Cidofovir | −56.49 | −18.88 | 6074 | 720.90 | Thr24, Thr25, Thr26, Leu27, His41, Val42, Cys44, Ser46, Met49, Phe140, Leu141, Asn142, Gly143, Ser144, Cys145, His163, His164, Met165, Glu166, His172, Val186, Asp187, Arg188, Gln189, Gln192 | |
| Darunavir | −55.06 | −19.64 | 5240 | 670.40 | Thr25, Leu27, His41, Val42, Cys44, Thr45, Ser46, Met49, Phe140, Leu141, Asn142, Ser144, Cys145, His163, His164, Met165, Glu166, His172, Val186, Asp187, Arg188, Gln189, Thr190 |
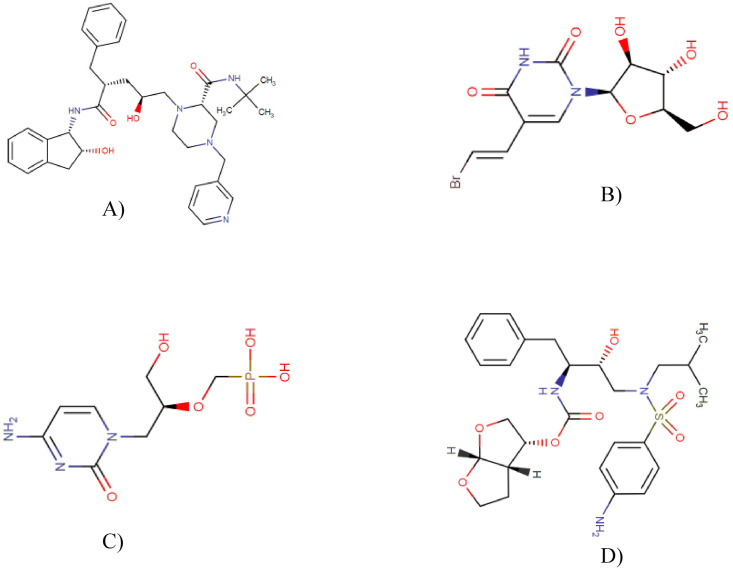
Fig. 1.
Chemical structures of Indinavir (A), Sorivudine (B), Cidofovir (C) and Darunavir (D).
Fig. 2.
Molecular interaction of Sorivudine with spike receptor-binding domain (A), Indinavir with main protease(B), Indinavir with HR2 Domain (C), Cidofovir with Nsp9 RNA binding protein (D) and (E) Darunavir with spike ectodomain.
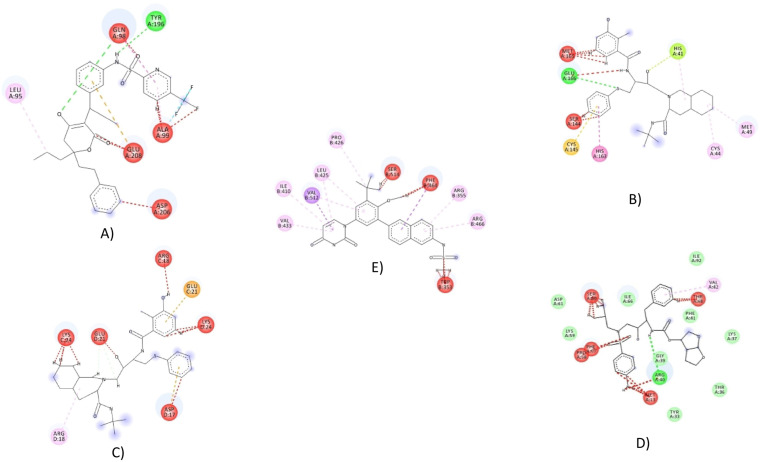
Fig. 3.
Ligand binding site of Sorivudine with spike receptor-binding domain (A), Indinavir with main protease(B), Indinavir with HR2 Domain (C), Cidofovir with Nsp9 RNA binding protein (D) and (E) Darunavir with spike ectodomain.
Fig. 4.
Molecular Dynamics: Deformability analysis: A-i) Sorivudine with spike receptor-binding domain, B-i) Indinavir with main protease, C-i) Indinavir with HR2 Domain, D-i) Cidofovir with Nsp9 RNA binding protein and (E) Darunavir with spike ectodomain;Eigen value: A-ii) Sorivudine with spike receptor-binding domain, B-ii) Indinavir with main protease, C-ii) Indinavir with HR2 Domain, D-ii) Cidofovir with Nsp9 RNA binding protein and E-ii) Darunavir with spike ectodomain;RMSD plot: A-iii) Sorivudine with spike receptor-binding domain, B-iii) Indinavir with main protease, C-iii) Indinavir with HR2 Domain, D-iii) Cidofovir with Nsp9 RNA binding protein and E-iii) Darunavir with spike ectodomain;RMSF plot: A-iv) Sorivudine with spike receptor-binding domain, B-iv) Indinavir with main protease, C-iv) Indinavir with HR2 Domain, D-iv) Cidofovir with Nsp9 RNA binding protein and E-iv) Darunavir with spike ectodomain.
3.2. ADMET analysis of selected top drugs
All the top drug candidates except cidofovir show good bioavailability. Darunavir and Indinavir are extensively metabolized by CYP3A4 enzymes, while Cidofovir is converted via cellular enzymes, and Sorivudine metabolism is found higher in animals. These drugs show binding capacity with plasma proteins. In healthy people, approximately 79.5% and 13.9% of the administered dose of radiolabeled darunavir was obtained in the feces and the urine, respectively, whereas the proportion of eliminated unchanged indinavir in the urine was found approximately 11%, and >47% in the feces. Cidofovir and Sorivudine are excreted greatly by the kidneys, and are eliminated as almost unchanged in the urine. These drugs have some toxic effects. Limited cytotoxicity and drug-induced hepatitis (e.g. acute hepatitis, cytolytic hepatitis) have been reported with darunavir. No cytotoxicity was detected for indinavir, but it can cause transient and usually asymptomatic elevations in serum aminotransferase levels. Moreover, it can cause mild elevations in indirect bilirubin concentration that creates a risk of acute renal failure. Cidofovir creates a risk of nephrotoxicity, and it has carcinogenic potential based on animal studies, while there is absence of hepatotoxicity. Lastly, Sorivudine shows a lethal effect when co-administrated with 5-fluorouracil anti-cancer drugs (Table 2 ).
Table 2.
ADMET properties of these approved drugs.
| Properties | Darunavir | Indinavir | Cidofovir | Sorivudine |
|---|---|---|---|---|
| Bioavailability | The absolute oral bioavailability of one single 600 mg dose of darunavir alone and with 100 mg of ritonavir twice a day was 37% and 82%, respectively. The bioavailability of oral darunavir is increased by about 30% when taken with food [55]. | After oral administration, indinavir is rapidly absorbed in the fasting state (70%) [56]. | Cidofovir has poor oral bioavailability (<5%) and is therefore administered intravenously [57]. | BV-araU shows good bioavailability [58]. |
| Distribution | Darunavir appears to bind to serum proteins, particularly α1-acid glycoprotein [59], 95% binding to plasma proteins [60]. | Plasma protein binding of indinavir is approximately 60% [56]. | Binding of cidofovir to plasma proteins is negligible (<7%) [57]. | Found in plasma after oral administration [61]. |
| Metabolism | Darunavir is extensively metabolized by CYP3A4 enzymes [60]. | Indinavir undergoes extensive metabolism by cytochrome P-450-CYP3A4 isoenzymes [62,63]. | Cidofovir is converted via cellular enzymes to the pharmacologically active cidofovir diphosphate [64]. | Their metabolism in animals were higher [58]. |
| Excretion | In healthy people, approximately 79.5% and 13.9% of the administered dose of radiolabeled darunavir was obtained in the feces and urine, respectively, when ritonavir was also added with it [55]. | In healthy people, the proportion of eliminated unchanged indinavir in the urine was approximately 11% [65] and indinavir metabolites in the feces accounted for >47% [56]. | Cidofovir is excreted extensively by the kidneys and is eliminated almost entirely as unchanged drug in the urine (>90% within 24 h) [66]. | Higher Urinary excretion [58]. |
| Toxicity | Shows lethal effect when co-administrated with 5-fluorouracil anti-cancer drugs [72]. |
3.3. Prediction of effective drug targets and structural drug analogs from DrugBank
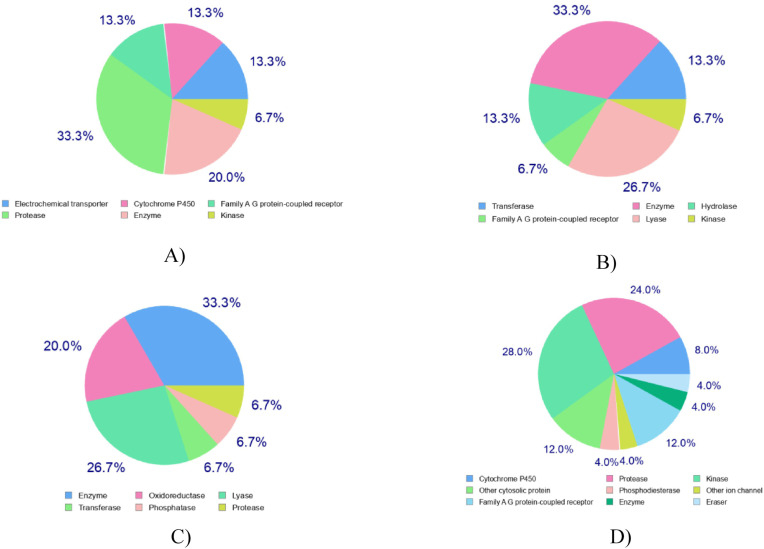
Prediction of effective drug targets against the top drugs revealed some other similar drugs that may be potential against SARS CoV2. Maximum targets belong to protease, transferase and enzyme class. Other target class includes electrochemical transporter, cytochrome p450, family A G protein-coupled receptor, phosphodiesterase, oxidoreductase, transferase DNA polymerase alpha subunit transferase, etc. (Table 3 , Fig. 5 ). One of the most significant concepts in chemoinformatics, particularly for drug design, is chemical similarity [73]. This concept has been successful and widely applied for the identification of novel inhibitors of various targets of biological importance [[74], [75], [76], [77], [78], [79]]. To predict structural similar bioactive small compounds from DrugBank that act against SARS-CoV-2, a ligand-based screening strategy was employed (Table 4 ). Quinapril (DB00881) and Sirolimus (DB00877), two approved drugs along with an experimental drug, L-756,423 (DB02009), were found as analogous to Indinavir with a score of 0.048, 0.014, and 0.906, respectively. Sorivudine predicted two similar approved drugs, Telbivudine (DB01265) and Idoxuridine (DB00249), while Cidofovir predicted Tenofovir (DB00300) and Riboflavin (DB00140) as similar approved drugs. Besides, Darunavir also predicted two similar approved drugs, Amprenavir (DB00701) and Fosamprenavir (DB01319). These similarity findings indicate the efficacy of these related drugs against SARS-CoV-2, and suggest further experimental studies.
Table 3.
Predicted drug targets for Indinavir, Sorivudine Cidofovir and Darunavir.
| Drugs | Drug Targets | Common Name | Uniprot ID | ChEMBL ID | Target Class | Probability* |
|---|---|---|---|---|---|---|
| Indinavir | Multidrug and toxin extrusion protein 1 | SLC47A1 | Q96FL8 | CHEMBL1743126 | Electrochemical transporter |  |
| Multidrug and toxin extrusion protein 2 | SLC47A2 | Q86VL8 | CHEMBL1743127 | Electrochemical transporter |  |
|
| Neurokinin 2 receptor | TACR2 | P21452 | CHEMBL2327 | Family A G protein-coupled receptor |  |
|
| Renin | REN | P00797 | CHEMBL286 | Protease |  |
|
| Sorivudine | Thymidine kinase, cytosolic | TK1 | P04183 | CHEMBL2883 | Transferase |  |
| Cytidine deaminase | CDA | P32320 | CHEMBL4502 | Enzyme |  |
|
| Thymidine kinase, mitochondrial | TK2 | O00142 | CHEMBL4580 | Enzyme |  |
|
| Cidofovir | Thymidine phosphorylase | TYMP | P19971 | CHEMBL3106 | Enzyme | |
| Hypoxanthine-guanine phosphoribosyltransferase | HPRT1 | P00492 | CHEMBL2360 | Enzyme | ||
| Purine nucleoside phosphorylase | PNP | P00491 | CHEMBL4338 | Enzyme | ||
| Darunavir | Cathepsin D | CTSD | P07339 | CHEMBL2581 | Protease |  |
| Cytochrome P450 3A4 | CYP3A4 | P08684 | CHEMBL340 | Cytochrome P450 |  |
|
| Complement factor D | CFD | P00746 | CHEMBL2176771 | Protease |  |
Fig. 5.
Prediction of drug targets for Indinavir (A), Sorivudine (B), Cidofovir (C) and Darunavir (D).
Table 4.
Structural similar bioactive molecules from drug bank.
| Drugs | Similar structure Drug bank id | Name | Score | Status |
|---|---|---|---|---|
| Indinavir | Quinapril | DB00881 | 0.048 | Approved |
| Sirolimus | DB00877 | 0.014 | Approved | |
| L-756,423 | DB02009 | 0.906 | Experimental | |
| Sorivudin | Telbivudine | DB01265 | 0.933 | Approved |
| Idoxuridine | DB00249 | 0.863 | Approved | |
| Cidofovir | Tenofovir | DB00300 | 0.811 | Approved |
| Riboflavin | DB00140 | 0.127 | Approved | |
| Darunavir | Amprenavir | DB00701 | 0.983 | Approved |
| Fosamprenavir | DB01319 | 0.503 | Approved |
4. Discussion
SARS-CoV-2 belongs to a group of viruses that can contaminate humans as well as vertebrate animals. There has no recorded or approved potent drug or vaccine for treating the patient infected with SARS-CoV-2. However, there are a few candidates within the investigational stages, but many of them raised controversial issues [4,80]. In this study, attempts were taken to screen and suggest some FDA approved antiviral drugs as inhibitory agents of SARS-CoV-2 using molecular docking strategy. The study suggested that Indinavir, Sorivudine, Cidofovir, and Darunavir along with their top derivatives may be effective against SARS-CoV-2. The drug repurposing is one of the exciting applications of computational pharmacology for finding new uses of existing drugs. Computer-based analysis can speed up the identification of drug targets, and facilitate the screening and refinement of drug candidates. It also simplifies the detection of side effects, and forsees the patterns of drug resistance. Antiviral drugs such as Ledipasvir, Elbasvir, Nelfinavir, Danoprevir, Darunavir, lopinavir, and ritonavir were previously used as the inhibiting agents for HCV and HIV [81]. Lopinavir, Ritonavir, and Nelfinavir have been reported as potential drug candidates in earlier studies, which used repurposing strategies targeting Main protease protein (Mpro) of SARS-CoV-2 [82]. Besides, a recent study focused on alpha-ketoamide as a Mpro inhibitor to determine it's efficacy against SARS-Cov-2 [47]. Main protease proteins (Mpro) or RNAdependent RNA polymerase of SARS-CoV-2 were used as possible drug targets in almost all previous experiments. In this study, we assessed the potency of 29 FDA approved commercially available antiviral drugs against SARS-Cov-2 main protease (6W63), spike ecto-domain (6VYB), spike receptor binding domain (6M0J), Nsp9(Non-structural protein-9) RNA binding protein (6W4B), and HR2 domain (6LVN) using molecular docking approach [83]. SARS-CoV-2 Mpro is a desirable pharmacological target for designing covid-19 drugs because the cleavage of Mpro polyprotein facilitates the formation of helicase and RNA-dependent RNA polymerase (RdRp), which are the prerequisites for the initiation of viral replication [84,85]. Besides, human proteases have no cleavage specificity that resembles coronavirus proteases, which is why the inhibitors of these proteins are considered safe [47]. ORF1a encodes Nsp9 RNA binding protein that is involved in the synthesis of viral RNA. Nsp9 has evolved, possibly, from a protease, and it is a dimeric protein. This Nsp9 communicates with nsp8 that may be crucial for its function. Viral replication complexes are also connected with membranes, and in this case, Nsp9 aids. In the replication Complex, Nsp9 may have the RNA binding activity as a putative component. In this way, it makes a difference in viral replication by binding with single stranded RNA. SARS-Cov-2, moreover, has a surface structural spike glycoprotein (S) which plays a crucial role in association with the cell receptor, and subsequent viral passage into the cell. The S protein is composed of two subunits, the S1 (receptor-binding) and the S2 (membrane fusion) domain [40]. Interaction between the HR1 and HR2 domains in the membrane fusion subunit is enabled via the attachment of the receptor-binding subunit to the receptor, and forms a six-helix bundle. This conformational shift results in a close apposition of the fusion peptide that leads to virus-cell membrane fusion [86]. Hence, spike protein binds to human ACE2 and CLEC4M/DC- SIGNR receptors, and the internalization of the virus into the host cell endosomes results in the conformational changes in the S glycoprotein [87]. Therefore, all these macromolecules are the potential targets for repurposing study.
Based on global energy, four drugs among our studied 29 drugs showed comparatively well binding affinity against our targeted macromolecules. Indinavir had highest binding affinity with SARS-CoV-2 main protease (−69.23 kcal/mol) and HR2 domain (−37.42 kcal/mol). The remaining three drug candidates, i.e. Sorivudine, Cidofovir and Darunavir, had the highest binding affinity with Spike receptor binding domain (−52.99 kcal/mol), Nsp9 RNA binding protein (−52.74 kcal/mol) and Spike ecto-domain(-68.01 kcal/mol), respectively. The ligands showed the highest binding interaction in 38–68 regions of Nsp9 RNA binding protein (6W4B) where Gly38, Gly39, Phe41, Val42, Phe57, Pro58, Ile66, and Thr68 were most dominant. Again, the residues from 94 to 99 and from 563 to 566 regions were identified as top surface hotspots for spike receptor binding domain (6M0J) where the position Lys94, Leu95, Tyr196, Asp 206, Lys562, Pro565, and Trp566 were most dominant (Table 1). The top candidates were well fitted into the active pocket of MPP, in which several hydrophobic amino acid residues, including Met49, Gly143, Cys145, and Met165, compose a relatively hydrophobic environment that may help to stabilize it's conformation [7]. In the present study, we revealed the molecular interactions of top drug candidates with SARS-CoV-2 key proteins (Fig. 2, Fig. 3; Table 1). The binding sites for each ligand occupied at the catalytic domain of SARS-CoV-2 main protease protein [88]. Among the common binding residues, His41 and Cys145 form the catalytic dyad which act as substrate recognition sites [7,88]. The crucial binding sites of Nsp9 protein (39–73 region) are characterized by positively charged, glycine rich β-loops, which were proposed to be involved in RNA binding [89]. Moreover, we targeted three distinct domains of SARS-CoV-2 spike protein, all of which play essential roles in the mechanism of viral entry into the host cell [90]. To unravel the drug surface hotspots of the studied SARS-CoV-2 proteins, the structural conformation of the docked complexes was analyzed. The pattern of ligand binding residues interacting with their respective positions had been studied (Table 1). Results showed that the amino acids from 41 to 54 and from 142 to 190 positions were significant for the binding interactions of SARS CoV-2 main protease protein (6W63). Besides, the docked complexes were formed in His41, Cys44, Met49, Gly143, Asn142, Cys145, and Met165 in maximum cases. Indinavir, an alpha-amino acid amide protease inhibitor, is used in the treatment of Human immunodeficiency virus (HIV) infection. Indinavir inhibits enzyme activity by binding to the active site of the protease. This inhibition facilitates the formation of immature non-infectious viral particles by preventing cleavage of the viral polyproteins [91]. Sorivudine is an antimetabolite and synthetic analogue of thymidine Kinase activity of thymidine induces sorivudine phosphorylation in the body, and is absorbed into the viral DNA instead of the correct nucleoside [92]. Thus, the viral DNA cannot be replicated, and the virus cannot grow because sorivudine is a competitive inhibitor of DNA polymerase. Cidofovir, a nucleotide analogue, is active against chronic hepatitis B and herpes cytomegalovirus (CMV) retinitis infection. It works selectively by inhibiting viral DNA polymerase. As a result, Cidofovir reduces the synthesis of viral DNA. Darunavir is a non-peptide protease inhibitor, with a distinct chemical structure that enhances the binding affinity of the drug [93]. This antiviral drug prevents HIV replication by binding to the enzyme that leads to the cessation of the catalytic activity, and dimerization of HIV-1 protease. Specifically, it inhibits the cleavage of HIV encoded Gag-Pol proteins in virus infected cells, by blocking the formation of mature virus particles that are required to spread the infection [94]. The molecular dynamics study showed that the complexes were resistant to deform with higher eigen value, and were fluctuated almost regularly in RMSD and RMSF plots (Fig. 4). ADMET data is crucial in drug development projects whether it is determined by in vitro, in vivo, or computational approaches because many drug development projects previously failed during clinical trials due to poor ADMET data [95]. The ADMET analysis of these drugs showed that these are well metabolized, distributed, and bioavailable, but have some undesirable effects. Most of the target class for the top drug candidates fall into the enzyme categories such as electrochemical transporter, protease, transferase, Family A G protein-coupled receptor, etc., (Table 3).
Ligand based drug similarity analysis identified three structural analogs of Indinavir where two (Quinapril, Sirolimus) are approved, and another one (L-756,423) is in the experimental stage. Quinapril, an ACE (angiotensin converting enzyme) inhibitor, is used for treating heart failure and hypertension [96]. As we know that, SARS-CoV-2 enters the host cell by interacting with ACE-2 receptor, thus this analog could be a drug of choice to treat Covid-19. Besides, drug similarity analysis revealed two (Telbivudine & Idoxuridine) approved analogs for Sorivudine, and both of them act by incorporating into viral DNA in place of thymidine resulting in the termination of replication process. Telbivudine and Idoxuridine are used to treat hepatitis B virus (HBV) and Herpes simplex virus, respectively [97,98]. Amprenavir and Fosamprenavir are two approved analogs of Darunavir, and both of them are protease inhibitors. These analogs prevent the processing of viral Gag and Gag- Pol polyprotein, and produce noninfectious and immature viral particles that are harmless to host cell [99,100]. The findings suggest that all these compounds may be used against SARS-CoV-2 as potential drug candidates.
5. Conclusion
The results indicate that it may be possible for Indinavir, Sorivudine, Cidofovir, and Darunavir to fight SARS-CoV-2 infection. Furthermore, several biologically active structural analogs from DrugBank, i.e. Telbivudine, Tenofovir, Fosamprenavir, Tenofovir, etc., may also be successful against the viral pathogen. We strongly recommend these drug candidates as potential warriors because of promising results, and refer to in vivo trials for experimental confirmation of our findings.
Funding information
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Department of Molecular Biology and Genetic Engineering, Sylhet Agricultural University for their technical support.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.imu.2021.100531.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paules C.I., Marston H.D., Fauci A.S. Coronavirus infections—more than just the common cold. JAMA, J Am Med Assoc. 2020;323(8):707–708. doi: 10.1001/jama.2020.0757. [DOI] [PubMed] [Google Scholar]
- 3.Lai C.C., Shih T.P., Ko W.C., Tang H.J., Hsueh P.R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and corona virus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. 2020;55:105924. doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xia S., Lan Q., Pu J., Wang C., Liu Z., Xu W., Wang Q., Liu H., Jiang S., Lu L. Potent MERS- CoV fusion inhibitory peptides identified from HR2 domain in spike protein of bat coronavirus HKU4. Viruses. 2019;11:56. doi: 10.3390/v11010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yuan Y., Qi J., Peng R., Li C., Lu G., Yan J., Wang Q., Gao G.F. Molecular basis of binding between Middle East respiratory syndrome coronavirus and CD26 from seven bat species. J Virol. 2020;94 doi: 10.1128/JVI.01387-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu C., Liu Y., Yang Y., Zhang P., Zhong W., Wang Y., et al. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm Sin B. 2020;10:766–788. doi: 10.1016/j.apsb.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu R., Zhao X., Li J., et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.CDC . Centers for Disease Control and Prevention (CDC); United States: 2020. Coronavirus disease 2019 (COVID-19)—Transmission. [Google Scholar]
- 10.CDC . Centers for Disease Control and Prevention (CDC); U.S: 2020. How COVID-19 spreads. [Google Scholar]
- 11.WHO . World Health Organization (WHO); 2020. Q&a on coronaviruses. [Google Scholar]
- 12.WHO . World Health Organization (WHO); 2020. Coronavirus disease (COVID-2019) situation report-73. [Google Scholar]
- 13.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hos- pitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA, J Am Med Assoc. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.COVID-19 dashboard by the center for systems science and engineering (CSSE) at Johns Hopkins University (JHU)". ArcGIS. Johns Hopkins University. Retrieved 2 July 2020.
- 16.Herst C.V., Burkholz S., Sidney J., Sette A., Harris P., Massey S., Brasel T., Cunha- Neto E., Rosa D., Chao W., et al. An effective CTL peptide vaccine for Ebola Zaire based on survivors' CD8+ targeting of a particular nucleocapsid protein epitope with potential implications for COVID-19 vaccine design. Vaccine. 2020;38:4464–4475. doi: 10.1016/j.vaccine.2020.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamey G., Schäferhoff M., Hatchett R., Pate M., Zhao F., McDade K.K. Ensuring global access to COVID-19 vaccines. Lancet. 2020;395:1405–1406. doi: 10.1016/S0140-6736(20)30763-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao J., Tian Z., Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. BioSci Trends. 2020;14:72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 19.Gautret P., Lagier J.C., Parola P., Hoang V.T., Meddeb L., Mailhe M., Doudier B., Courjon J., Giordanengo V., Vieira V.E., et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;56:105949. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Stebbing J., Phelan A., Griffin I., Tucker C., Oechsle O., Smith D., Richardson P. COVID- 19: combining antiviral and anti-inflammatory treatments. Lancet Infect Dis. 2020;20:400–402. doi: 10.1016/S1473-3099(20)30132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.AshburnandK T.T., Thor B. Drug repositioning: identifying and developing new uses for existing drugs. Nat Rev Drug Discov. 2004;3:673–683. doi: 10.1038/nrd1468. [DOI] [PubMed] [Google Scholar]
- 22.Li J., Zheng S., Chen B., Butte A.J., Swamidass S.J., Lu Z. A survey of current trends in computational drug repositioning. Briefings Bioinf. 2016;17:2–12. doi: 10.1093/bib/bbv020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chopra G., Samudrala R. Exploring polypharmacology in drug discovery and Repurposing using the CANDO platform. Curr Pharmaceut Des. 2016;22:3109–3123. doi: 10.2174/1381612822666160325121943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kouznetsova J., Sun W., Martinez-Romero C., Tawa G., Shinn P., Chen C.Z., Schimmer A., Sanderson P., McKew J.C., Zheng W., Garcia-Sastre A. Identification of 53 compounds that block Ebola virus-like particle entry via a repurposing screen of approved drugs. Emerg Microb Infect. 2014;3:1–7. doi: 10.1038/emi.2014.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johansen L.M., DeWald L.E., Shoemaker C.J., Hoffstrom B.G., Lear-Rooney C.M., Stossel A., Nelson E., Delos S.E., Simmons J.A., Grenier J.M., Pierce L.T. A screen of approved drugs and molecular probes identifies therapeutics with anti–Ebola virus activity. Sci Transl Med. 2015;7:290ra89. doi: 10.1126/scitranslmed.aaa5597. [DOI] [PubMed] [Google Scholar]
- 26.He S., Lin B., Chu V., Hu Z., Hu X., Xiao J., Wang A.Q., Schweitzer C.J., Li Q., Imamura M., Hiraga N. Repurposing of the antihistamine chlorcyclizine and related compounds for treatment of hepatitis C virus infection. Sci Transl Med. 2015;7:282ra49. doi: 10.1126/scitranslmed.3010286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barrows N.J., Campos R.K., Powell S.T., Prasanth K.R., Schott-Lerner G., Soto- Acosta R., Galarza-Munoz G., McGrath E.L., Urrabaz-Garza R., Gaoand J., Wu P. A screen of FDA-approved drugs for inhibitors of Zika virus infection. Cell Host Microbe. 2016;20:259–270. doi: 10.1016/j.chom.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones G., Willett P., Glen R.C., Leach A.R., Taylor R. Development and validation of a genetic algorithm for flexible docking. J Mol Biol. 1997;267:727–748. doi: 10.1006/jmbi.1996.0897. [DOI] [PubMed] [Google Scholar]
- 29.Friesner R.A., et al. Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J Med Chem. 2004;47:1739–1749. doi: 10.1021/jm0306430. [DOI] [PubMed] [Google Scholar]
- 30.Irwin J.J., Shoichet B.K. Docking screens for novel ligands conferring new Biology. J Med Chem. 2016;59:4103–4120. doi: 10.1021/acs.jmedchem.5b02008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mohanasundaram N., Sekhar T. Computational studies of molecular targets regarding the adverse effects of isoniazid drug for tuberculosis. Curr Pharmacogenomics Personalized Med (CPPM) 2018;16:210–218. doi: 10.2174/1875692116666181108145230. [DOI] [Google Scholar]
- 32.Murgueitio M.S., Bermudez M., Mortier J., Wolber G. Insilico virtual screening approaches for anti-viral drug discovery. Drug Discov Today Technol. 2012;9:e219–e225. doi: 10.1016/j.ddtec.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hurle M.R., Yang L., Xie Q., Rajpal D.K., Sanseau P., Agarwal P. Computational drug repositioning: from data to therapeutics. Clin Pharmacol Therapeut. 2013;93:335–341. doi: 10.1038/clpt.2013.1. [DOI] [PubMed] [Google Scholar]
- 34.Rose P.W., Prlić A., Altunkaya A., Bi C., Bradley A.R., Christie C.H., et al. The RCSB protein data bank: integrative view of protein, gene and 3D structural information. Nucleic Acids Res. 2017;45 doi: 10.1093/nar/gkw1000. D271–D81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim S., Thiessen P.A., Bolton E.E., Chen J., Fu G., Gindulyte A., Han L., He J., He S., Shoemaker B.A., Wang J. PubChem substance and compound databases. Nucleic Acids Res. 2016;44:D1202–D1213. doi: 10.1093/nar/gkv951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wishart D.S., Feunang Y.D., Guo A.C., Lo E.J., Marcu A., Grant J.R., Assempour N. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2018;46:D1074–D1082. doi: 10.1093/nar/gkx1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O'Boyle N.M., Banck M., James C.A., Morley C., Vandermeersch T., Hutchison G.R. Open Babel: an open chemical toolbox. J Cheminf. 2011;3:33. doi: 10.1186/1758-2946-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meng X.Y., Zhang H.X., Mezei M., Cui M. Molecular docking: a powerful approach for structure-based drug discovery. Curr Comput Aided Drug Des. 2011;7:146–157. doi: 10.2174/157340911795677602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang S.Y., Zou X. Advances and challenges in protein-ligand docking. Int J Mol Sci. 2010;11:3016–3034. doi: 10.3390/ijms11083016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.López-Vallejo F., Caulfield T., Martínez-Mayorga K., Giulianotti M.A., Houghten R.A., Nefzi A., Medina-Franco J.L. Integrating virtual screening and combinatorial chemistry for accelerated drug discovery. Comb Chem High Throughput Screen. 2011;14:475–487. doi: 10.2174/138620711795767866. [DOI] [PubMed] [Google Scholar]
- 41.Schneidman-Duhovny D., Inbar Y., Nussinov R., Wolfson H.J. PatchDock and SymmDock: servers for rigid and symmetric docking. Nucleic Acids Res. 2005;33:W363–W367. doi: 10.1093/nar/gki481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Azim K.F., Ahmed S.R., Banik A., Khan M.M.R., Deb A., Somana S.R. Screening and druggability analysis of some plant metabolites against SARS- CoV-2: an integrative computational approach. Inf Med Unlocked. 2020;20:100367. doi: 10.1016/j.imu.2020.100367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zainab B., Ayaz Z., Alwahibi M.S., Khan S., Rizwana H., Soliman D.W., Alawaad A., Abbasi A.M. In-silico elucidation of Moringaoleifera phytochemicals against diabetes mellitus. Saudi J Biol Sci. 2020;27(9):2299–2307. doi: 10.1016/j.sjbs.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Banik A., Ahmed S.R., Sajib E.H., Deb A., Sinha S., Azim K.F. 2020. Identification of potential inhibitory analogs of metastasis tumor antigens (MTAs) using bioactive compounds: revealing therapeutic option to prevent malignancy. bioRxiv. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Y., Zhao W., Mao Y., Chen Y., Wang S., Zhong Y., Su T., Gong M., Du D., Lu X., Cheng J. Site-specific N-glycosylation characterization of recombinant SARS-CoV-2 spike proteins. Mol Cell Proteomics. 2020 doi: 10.1074/mcp.RA120.002295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mashiach E., Schneidman-Duhovny D., Andrusier N., Nussinov R., Wolfson H.J. FireDock: a web server for fast interaction refinement in molecular docking. Nucleic Acids Res. 2008;36:W229–W232. doi: 10.1093/nar/gkn186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang L., Lin D., Sun X., Curth U., Drosten C., Sauerhering L., Becker S., Rox K., Hilgenfeld R. Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors. Science. 2020;368:409–412. doi: 10.1126/science.abb3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Q., He J., Wu D., Wang J., Yan J., Li H. Interaction of α-cyperone with human serum albumin: determination of the binding site by using Discovery Studio and via spectroscopic methods. J Lumin. 2015;164:81–85. doi: 10.1016/j.jlumin.2015.03.025. [DOI] [Google Scholar]
- 49.DeLano W.L. CCP4 Newsletter on protein crystallography. Vol. 40. 2002. Pymol: an open-source molecular graphics tool; pp. 82–92. 1. [Google Scholar]
- 50.López-Blanco J.R., Aliaga J.I., Quintana-Ortí E.S., Chacón P. iMODS: internal coordinates normal mode analysis server. Nucleic Acids Res. 2014;42:W271–W276. doi: 10.1093/nar/gku339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang J.F., Wang F., Chen Y.Z., Hao G.F., Yang G.F. LARMD: integration of bioinformatic resources to profile ligand-driven protein dynamics with a case on the activation of estrogen receptor. Briefings Bioinf. 2019;21:2206–2218. doi: 10.1093/bib/bbz141. [DOI] [PubMed] [Google Scholar]
- 52.Guan L., Yang H., Cai Y., et al. ADMET-score - a comprehensive scoring function for evaluation of chemical drug-likeness. Med Chem Comm. 2018;10(1):148–157. doi: 10.1039/c8md00472b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Daina A., Michielinand O., Zoete V. SwissTargetPrediction: updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019;47:W357–W364. doi: 10.1093/nar/gkz382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zoete V., Daina A., Bovigny C., Michielin O. SwissSimilarity: a web tool for low to ultra high throughput ligand-based virtual screening. J Chem Inf Model. 2016;56:1399–1404. doi: 10.1021/acs.jcim.6b00174. [DOI] [PubMed] [Google Scholar]
- 55.McKeage K., Perry C.M., Keam S.J. Darunavir. Drugs. 2009;69:477–503. doi: 10.2165/00003495-200969040-00007. [DOI] [PubMed] [Google Scholar]
- 56.Plosker G.L., Noble S. Indinavir. Drugs. 1999;58:1165–1203. doi: 10.2165/00003495-199958060-00011. [DOI] [PubMed] [Google Scholar]
- 57.Cundy K.C. Clinical pharmacokinetics of the antiviral nucleotide analogues cidofovir and adefovir. Clin Pharmacokinet. 1999;36:127–143. doi: 10.2165/00003088-199936020-00004. [DOI] [PubMed] [Google Scholar]
- 58.Ashida N., Ijichi K., Watanabe Y., Machida H. Metabolism of 5′-ether prodrugs of 1-β-d-Arabinofuranosyl-E-5-(2-bromovinyl) uracil in rats. Biochem Pharmacol. 1993;46:2201–2207. doi: 10.1016/0006-2952(93)90610-9. [DOI] [PubMed] [Google Scholar]
- 59.De Meyer S., Azijn H., Surleraux D., et al. TMC114, a novel Angeles (CA) human immunodeficiency virus type 1 protease inhibitor active against protease inhibitor-resistant viruses, including a broad range of clinical isolates. Antimicrob Agents Chemother. 2005;49:2314. doi: 10.1128/AAC.49.6.2314-2321.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tibotec Therapeutics PREZISTATM US prescribing informa- tion. http://www.prezista.com/docs/us_package_insert.pdf [online] 2007 Dec 1.
- 61.Nishimoto Takashi. Studies on the metabolic fate of Brovavir (YN-72) II; metabolism in rats. IyakuhinKenkyu. 1990;21:378–389. [Google Scholar]
- 62.Chiba M., Hensleigh M., Nishime J.A., et al. Role of cytochrome P450 3A4 in human metabolism of MK-639, a potent human immunodeficiency virus protease inhibitor. Drug Metabol Dispos. 1996;24:307–314. [PubMed] [Google Scholar]
- 63.Koudriakova T., Iatsimirskaia E., Utkin I., et al. Metabolism of the human immunodeficiency virus protease inhibitors indinavir and ritonavir by human intestinal microsomes and expressed cytochrome P4503A4/3A5: mechanism-based in activation of cytochrome P4503A by ritonavir. Drug Metabol Dispos. 1998;26:552–561. [PubMed] [Google Scholar]
- 64.McEvoy G.K. American Society of Health-System Pharmacists, Inc.; Bethesda, MD: 2003. American Hospital formulary service - drug information 2003. (Plus Supplements) 540. [Google Scholar]
- 65.Balani S.K., Woolf E.J., Hoagland V.L., et al. Disposition of indinavir, a potent HIV-1 protease inhibitor, after an oral dose in humans. Drug Metabol Dispos. 1996;24:1389–1394. [PubMed] [Google Scholar]
- 66.Plosker G.L., Noble S. Cidofovir. Drugs. 1999;58:325–345. doi: 10.2165/00003495-199958020-00015. [DOI] [PubMed] [Google Scholar]
- 67.Farèse-Di Giorgio A., Rouquayrol M., Greiner J., et al. Synthesis and anti-HIV activity of prodrugs derived from saquinavir and indinavir. Antivir Chem Chemother. 2000;11:97–110. doi: 10.1177/095632020001100202. [DOI] [PubMed] [Google Scholar]
- 68.LiverTox: clinical and research information on drug-induced liver injury. National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda (MD): 2012. [Internet] Indinavir. [Updated 2017 Sep 1] [PubMed] [Google Scholar]
- 69.Reilly R.F., Tray K., Perazella M.A. Indinavir nephropathy revisited: a pattern of insidious renal failure with identifiable risk factors. Am J Kidney Dis. 2001;38:E23. doi: 10.1053/ajkd.2001.27732. PMID: 11576910. [DOI] [PubMed] [Google Scholar]
- 70.Soma M.A., Albert D.M. Cidofovir: to use or not to use? Curr Opin Otolaryngol Head Neck Surg. 2008;16:86–90. doi: 10.1097/MOO.0b013e3282f43408. PMID: 18197029. [DOI] [PubMed] [Google Scholar]
- 71.LiverTox: clinical and research information on drug-induced liver injury. National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda (MD): 2012. https://www.ncbi.nlm.nih.gov/books/NBK548417/ [Internet] Cidofovir. [Updated 2017 Oct 10] [PubMed] [Google Scholar]
- 72.Ashida N., Sakata S., Machida H. Metabolic fate of [3H] 1-β-D-arabinofuranosyl-5-[(E)-2-bromovinyl] uracil in herpes simplex virus type 1-infected cells. Microbiol Immunol. 1997;41:957–964. doi: 10.1111/j.1348-0421.1997.tb01955.x. [DOI] [PubMed] [Google Scholar]
- 73.Johnson M.A., Maggiora G.M. Wiley; 1990. Concepts and applications of molecular similarity. [Google Scholar]
- 74.Martin Y.C., KofronL J.L., Traphagen M. Do structurally similar molecules have similar biological activity? J Med Chem. 2002;45:4350–4358. doi: 10.1021/jm020155c. [DOI] [PubMed] [Google Scholar]
- 75.Franke L., Schwarz O., Kuhrt L.M., Hoernig C., Fischer L., George S., Tanrikulu Y., Schneider P., Werz O., Steinhilber D., Schneider G. Identification of natural-product-derived inhibitors of 5- lipoxygenase activity by ligand-based virtual screening. J Med Chem. 2007:2640–2646. doi: 10.1021/jm060655w. [DOI] [PubMed] [Google Scholar]
- 76.Betzi S., Restouin A., Opi S., Arold S.T., Parrot I., Guerlesquin F., Morelli X., Collette Y. Protein−protein interaction inhibition (2P2I) combining high throughput and virtual screening: application to the HIV-1 Nefprotein. Proc Natl Acad Sci USA. 2007;104:19256–19261. doi: 10.1073/pnas.0707130104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Willett P. Similarity-based virtual screening using 2D fingerprints. Drug Discov Today. 2006;11:1046–1053. doi: 10.1016/j.drudis.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 78.Amaro R.E., Schnaufer A., Interthal H., Hol W., Stuart K.D., McCammon J.A. Discovery of drug-like inhibitors of an essential RNAediting ligase in Trypanosomabrucei. Proc Natl Acad Sci USA. 2008;105:17278–17283. doi: 10.1073/pnas.0805820105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Langdon S.R., Westwood I.M., van Montfort R.L.M., Brown N., Blagg J. Scaffold-focused virtual screening: prospective application to the discovery of TTK inhibitors. J Chem Inf Model. 2013;53:1100–1112. doi: 10.1021/ci400100c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dong L., Hu S., Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19) Drug Discov Therapeut. 2020;14:58–60. doi: 10.5582/ddt.2020.01012. [DOI] [PubMed] [Google Scholar]
- 81.Rosa S.G.V., Santos W.C. Clinical trials on drug repositioning for COVID- 19 treatment. RevistaPanamericana de SaludPública. 2020;44:1–13. doi: 10.26633/RPSP.2020.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sekhar T. Virtual Screening based prediction of potential drugs for COVID-19. Comb Chem High Throughput Screen. 2020;23 doi: 10.2174/1386207323666200814132149. [DOI] [PubMed] [Google Scholar]
- 83.Chang M.W., Ayeni C., BreuerandB S., Torbett E. Virtual screening for HIV protease inhibitors: a comparison of AutoDock 4 and Vina. PloS One. 2010;5 doi: 10.1371/journal.pone.0011955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Thiel V., Ivanov K.A., Putics A., Hertzig T., Schelle B., Bayer S., Gorbalenya A.E. Mechanisms and enzymes involved in SARS coronavirus genome expression. J Gen Virol. 2003;84:2305–2315. doi: 10.1099/vir.0.19424-0. [DOI] [PubMed] [Google Scholar]
- 85.Chan J.F., Lau S.K., To K.K., Cheng V.C., Woo P.C., Yuen K.Y. Middle East respiratory syndrome coronavirus: another zoonotic betacoronavirus causing SARS-like disease. Clin Microbiol Rev. 2015;28:465–522. doi: 10.1128/CMR.00102-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.MooreandR J.P., Doms W. The entry of entry inhibitors: a fusion of science and medicine. Proc Natl Acad Sci USA. 2003;100:10598–10602. doi: 10.1073/pnas.1932511100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tortorici M.A., Veesler D. Structural insights into coronavirus entry. Adv Virus Res. 2019;105:93–116. doi: 10.1016/bs.aivir.2019.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mesecar A. 2020. A taxonomically-driven approach to development of potent, broad-spectrum inhibitors of coronavirus main protease including SARS-CoV-2 (COVID-19) Be Published. [Google Scholar]
- 89.Littler D.R., Gully B.S., Colson R.N., Rossjohn J. Crystal structure of the SARS-CoV-2 non-structural protein 9, Nsp9. Iscience. 2020;23:101258. doi: 10.1016/j.isci.2020.101258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shang J., Wan Y., Luo C., Ye G., Geng Q., Auerbach A., Li F. Cell entry mechanisms of SARS-CoV-2. Proc Natl Acad Sci Unit States Am. 2020;117:11727–11734. doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hoetelmans R.M.W., Koks C.H.W., Beijnen J.H., Meenhorst P.L., Mulder J.W., Burger D.M. Clinical pharmacology of HIV protease inhibitors: focus on saquinavir, indinavir, and ritonavir. Pharm World Sci. 1997;19:159–175. doi: 10.1023/A:1008629608556. [DOI] [PubMed] [Google Scholar]
- 92.Diasio R.B. Sorivudine and 5‐fluorouracil; a clinically significant drug‐drug interaction due to inhibition of dihydropyrimidine dehydrogenase. Br J Clin Pharmacol. 1998;46:1–4. doi: 10.1046/j.1365-2125.1998.00050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pasquau L.J., Hidalgo T.C. Chemical characteristics, mechanism of action and antiviral activity of darunavir. Enferm Infecc Microbiol Clín. 2008;26:3–9. doi: 10.1016/s0213-005x(08)76547-9. [DOI] [PubMed] [Google Scholar]
- 94.Davis D.A., Soule E.E., Davidoff K.S., Daniels S.I., Naiman N.E., Yarchoan R. Activity of human immunodeficiency virus type 1 protease inhibitors against the initial autocleavage in Gag-Pol polyprotein processing. Antimicrob Agents Chemother. 2012;56:3620–3628. doi: 10.1128/AAC.00055-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shin H.K., Kang Y.M., No K.T. Handbook of computational chemistry. 2017. Predicting ADME properties of chemicals; pp. 1–37. [DOI] [Google Scholar]
- 96.Kieback A.G., Felix S.B., Reffelmann T. Quinaprilat: a review of its pharmacokinetics, pharmacodynamics, toxicological data and clinical application. Expet Opin Drug Metabol Toxicol. 2009;5:1337–1347. doi: 10.1517/17425250903282773. [DOI] [PubMed] [Google Scholar]
- 97.Seth A.K., Misraand A., Umrigar D. Topical liposomal gel of idoxuridine for the treatment of herpes simplex: pharmaceutical and clinical implications. Pharmaceut Dev Technol. 2005;9:277–289. doi: 10.1081/PDT-200031432. [DOI] [PubMed] [Google Scholar]
- 98.LuiandH Y.Y., Chan L. Treatment of chronic hepatitis B: focus on telbivudine. Expert Rev Anti-infect Ther. 2009;7:259–268. doi: 10.1586/eri.09.6. [DOI] [PubMed] [Google Scholar]
- 99.Smith K.Y., Weinberg W.G., DeJesus E., Fischl M.A., Liao Q., Rossand L.L., Lancaster C. Fosamprenavir or atazanavir once daily boosted with ritonavir 100 mg, plus tenofovir/emtricitabine, for the initial treatment of HIV infection: 48-week results of ALERT. AIDS Res Ther. 2008;5:5. doi: 10.1186/1742-6405-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sadler B.M., Hanson C.D., Chittick G.E., Symonds W.T., Roskell N.S. Safety and pharmacokinetics of amprenavir (141W94), a human immunodeficiency virus (HIV) type 1 protease inhibitor, following oral administration of single doses to HIV-infected adults. Antimicrob Agents Chemother. 1999;43:1686–1692. doi: 10.1128/AAC.43.7.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.