Abstract
Objectives
To investigate the effects of the COVID-19 pandemic on the treatment course of neovascular age-related macular degeneration (nAMD) patients who received anti-VEGF injection therapy with real-life data.
Methods
This retrospective study consisted of 116 eyes of 106 patients. Ophthalmic examination, assessment of best-corrected visual acuity (BCVA), optical coherence tomography (OCT) findings and data of last two visits before restrictions (V-2 and V-1) and the first visit (V0) after the release of national lockdown and subsequent visits (V1 and Vlast) were recorded. The lockdown period was determined by the time interval between March 11 and June 1, 2020.
Main results
The injection interval before V-1 was significantly longer than the interval after V0 (2.56 ± 0.9 vs. 2.14 ± 0.8 months, P = 0.02). While the median central macular thickness (CMT) was significantly increased at V0 compared to V-1 [274(132–711) vs. 238(136–628), P < 0.001], the median CMT was significantly lower at V1 compared to V0 [256 (136–591) vs. 274(132–711), P = 0.003]. The median BCVA was 0.67(0.1–1.1) logMAR at V-1 and significantly worsened to 0.78 (0.1–1.2) logMAR at V0 (P = 0.003). Although the median BCVA improved to 0.69 logMAR (0.1–1.2) at Vlast, the difference did not reach statistical significance compared to V0 (P = 0.08).
Conclusion
Treatment delay due to the COVID-19 pandemic cause progression of nAMD and visual impairment. To plan more frequent anti-VEGF treatments and visits may be an appropriate approach until the disease stabilizes. However, it should be kept in mind that despite the improvement in OCT findings, the desired success in VA could not be achieved in the short term.
Keywords: COVID-19, Age-related macular degeneration, Anti-VEGF, Pandemic
Résumé
Objectif
Étudier les effets de la pandémie de COVID-19 sur l’évolution du traitement des patients atteints de la forme néovasculaire de la dégénérescence maculaire liée à l’âge (DMLA) ayant reçu un traitement par injection d'anti-VEGF.
Méthodes
Cette étude rétrospective a été effectuée sur 116 yeux de 106 patients. L'examen ophtalmique, l'évaluation de la meilleure acuité visuelle corrigée (MAVC), les résultats de la tomographie en cohérence optique (OCT) et les données des deux dernières visites avant les restrictions (V-2 et V-1) et de la première visite (V0) après l’annonce de la fin du confinement et des visites ultérieures (V1 et Vlast) ont été enregistrés. La période du confinement a été déterminée entre le 11 mars et le 1er juin 2020.
Résultats
L’intervalle d’injection avant V-1 était significativement plus long que l’intervalle après V0 (2,56 ± 0,9 vs 2,14 ± 0,8 mois, p = 0,02). Alors que l’épaisseur maculaire centrale médiane (EMC) avait significativement augmenté à V0 par rapport à V-1 (274 [132–711] contre 238 [136–628], p < 0,001), l'EMC médiane était significativement plus faible à V1 par rapport à V0 (256 [136–591] contre 274 [132–711], p = 0,003). La MAVC médiane était de 0,67 (0,1–1,1) logMAR à V-1 et s’est significativement aggravée à 0,78 (0,1–1,2) logMAR à V0 (p = 0,003). Bien que la MAVC médiane se soit améliorée à 0,69 logMAR (0,1–1,2) à Vlast, la différence n’a pas atteint une signification statistique par rapport à V0 (p = 0,08).
Conclusion
Le retard de traitement dû à la pandémie de COVID-19 entraîne une progression de la DMLA et une déficience visuelle. Planifier des traitements anti-VEGF et des visites plus fréquents peut être une approche appropriée jusqu’à ce que la maladie se stabilise. Cependant, il convient de garder à l’esprit que malgré l’amélioration des résultats des OCT, le succès souhaité en matière d’AV n’a pas pu être atteint à court terme.
Mots clés: COVID-19, Dégénérescence maculaire liée à l’âge, Anti-VEGF, Pandémie
Introduction
The novel coronavirus disease (COVID-19) caused by “severe acute respiratory syndrome coronavirus type 2” (SARS-CoV-2) primarily manifests as an acute upper and lower respiratory tract infection and could affect multiple organs and systems, including the heart, intestines, kidneys, blood, and nervous system. The disease's main way of transmission is especially by droplets and human-to-human direct contact [1]. Furthermore, SARS-CoV-2 was detected in tears, and conjunctival secretions of infected patients and the disease also showed ocular manifestations consisting of chemosis, epiphora, and conjunctival hyperemia [2]. The SERPICO-19 study has reported COVID-19 associated retinal findings such as hemorrhage, cotton wool spots, dilated veins, and tortuous vessels [3]. The total estimated frequency of severe cases and mortality were reported as 25% (17.4–34.9) and 3.6% (1.1–7.2), respectively, and COVID-19 was recognized as a pandemic by the World Health Organization (WHO) on March 11, 2020 [4]. The rapid and uncontrolled spread of the disease all around the world forced governments to lockdown cities, apply social distancing rules, cancel scientific meetings, and limit non-essential works [5].
The American Academy of Ophthalmology (AAO) alerted ophthalmologists, health workers, and hospitals to manage their workflow, including surgeries, treatment modalities, and patient visit intervals, during the outbreak of COVID-19 to minimize the risk of infection transmission and prevent spread among patients and health workers [6]. They recommended eye protection, frequent and careful disinfection of clinics, and personal protective equipment such as face coverings [7]. As a part of daily ophthalmology practice, patients with retinal diseases are generally elderly and had multiple comorbid conditions, which could facilitate the morbidity and mortality of COVİD-19 disease [4]. However, retinal diseases, particularly proliferative diabetic retinopathy, diabetic macular edema, and neovascular age-related macular degeneration (nAMD), mostly need anti-vascular endothelial growth factor (VEGF) injections for treatment and can cause permanent vision loss if left untreated [5].
Age-related macular degeneration (AMD) is the leading cause of severe vision loss in people aged over 50 years [8]. Neovascular age-related macular degeneration, a late stage of the disease, is characterized by the development of macular neovascularization (MNV), which may be responsible for irreversible damage of photoreceptors and retinal pigment epithelium [9]. Macular neovascularization is a new terminology that is accepted instead of choroidal neovascularization in nAMD [10]. Anti- VEGF agents have been used as a gold standard treatment method in the nAMD to prevent disease progression and provide anatomical and functional improvement. However, it is crucial to apply the injections at appropriate intervals for the success of the treatment [11].
During the ongoing outbreak of COVID-19, it has been an important challenge to manage patients with retinal diseases to prevent disease progression, avoid vision loss, and protect healthcare professionals and high-risk patients. For this purpose, different treatment algorithms and guidelines have been recommended by different clinics to continue applying intravitreal anti-VEGF injections for patients, particularly those with nAMD [5], [6], [12]. However, restrictions (social distancing rules, lockdowns, curfews), increased anxiety levels of patients, and difficulty in accessing hospitals caused delays in treatment protocols.
In this study, we aimed to investigate the effects of the COVID-19 pandemic on the treatment course of nAMD patients who received anti-VEGF injection therapy with real-life data.
Materials and methods
Study population
This retrospective study was conducted in the retina clinic of the Department of Ophthalmology at Sivas Cumhuriyet University School of Medicine. From the beginning of the restrictions (March 11, 2020) to the immediately end of the restrictions in Turkey (June 1, 2020), patients who were followed up in our department but could not attend follow up visits and injections due to lockdown were enrolled in this study. The Health Ministry of Turkey Republic and the local Ethics Committee of Sivas Cumhuriyet University approved the study's design and procedures in agreement with the principles of the Declaration of Helsinki and ethical standards for human experimentation. Written informed consent was obtained from all participants. The inclusion criteria for study enrollment were to be aged over 50 years, diagnosed with nAMD who had a history of anti-VEGF treatment. Patients who had a disciform scar or geographic atrophy with no need for anti-VEGF treatment were diagnosed with choroidal neovascularization (CNV) associated with other ocular conditions, had concomitant retinal vascular diseases such as diabetic retinopathy, retinal vein occlusion, glaucoma, and high refractive errors (> 6D myopia), or had a history of photodynamic therapy or intraocular surgery except cataract surgery were excluded from the study.
Study design
All patients were receiving “pro re nata” (PRN-“as needed”) anti-VEGF treatment regimen in our clinical routine. “Pro re nata” therapy was performed after three consecutive monthly injections. All patients underwent a complete baseline ophthalmic examination, including intraocular pressure (IOP) measurement, assessment of best-corrected visual acuity (BCVA) measured by using a Snellen chart and converted to the logarithm of the minimum angle of resolution (logMAR), slit-lamp biomicroscopy, dilated fundus examination, and spectral domain-optical coherence tomography (SD-OCT) imaging (Nidek RS-3000 Advance, Japan). We defined central macular thickness (CMT) as the mean retinal thickness in the central 1000 μm area. Additionally, exudative features of MNV as described before, including subretinal fluid (SRF), intraretinal fluid (IRF), and subretinal hyperreflective material (SRHM), were evaluated by three independent and experienced specialist [10]. We retrospectively collected the records of at least two previous visits (V-2 and V-1), which contains complete ophthalmology examinations before the restrictions. First visit after restrictions recorded as “V0” and the subsequent visits recorded as “V1” and “Vlast.” V1 was described as the first visit after the first injection, and Vlast was determined as the last visit. All patients included in the study with the V-2 visit consisted of patients who had completed the loading phase (at least three injections) of anti-VEGF treatment prior to the V-2 visit. In addition, the PRN treatment regimen was continued without a new three consecutive monthly injections protocol after V0 visit.
Study outcomes
Demographic characteristics (age, gender) were recorded. Also, BCVA, CMT, and OCT findings for all visits (V-1, V-2, V0, V1, and Vlast), the total number of injections, type of anti-VEGF injections, and time interval between injections before V-1 and after V0 were evaluated. We also determined the associated factors with increased CMT and BCVA. Additionally, the attendance rates in relation to visits and anti-VEGF treatments during the pandemic period were compared with the same dates of last year from the hospital records.
Statistical analysis
All statistical analyses were performed using Statistical Package for the Social Sciences version 20 (IBM Corp., SPSS for Windows. Armonk, NY, USA). Distributions were assessed for normality using the Kolmogorov–Smirnov test. The homogeneity of variables was determined using one-way ANOVA homogeneity of variance tests. In the text and tables, numerical variables are presented as the mean ± standard deviation or median (minimum-maximum) according to the data distribution. Categorical variables are presented as numbers (n) and percentages (%). We used the Chi2 test for a comparison of the categorical variables. We used the Wilcoxon test for the comparison of consecutive measurements of CMT and BCVA. Linear regression analysis was used to determine associated factors with CMT and BCVA. The level of statistical significance was set to P ≤ 0.05.
Results
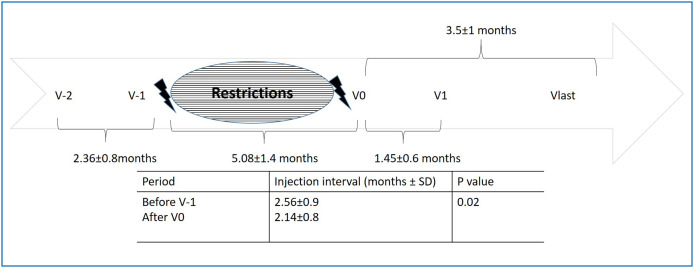
During the national lockdown period, the mean number of patient visits decreased from 2824 ± 34.6 to 854.3 ± 758.4 (P = 0.04), and the mean number of intravitreal injections decreased from 238.3 ± 50.4 to 47.6 ± 52.4 (P = 0.04) compared to the same months of last year (March, April, May 2019). With the normalization period (June 1, 2020), patient visits increased, and patients were highly anxious about their sight as well. The mean injection interval before V-1 was 2.56 ± 0.9 months. Patients received the first injection after restrictions at V0, and the time interval between the first injection and first visit after injection was 1.45 ± 0.6 months. On the other hand, the injection interval after V0 was 2.14 ± 0.8 months and significantly different compared to the injection interval before V-1 (P = 0.02) (Fig. 1 ).
Figure 1.
Follow-up of patients with neovascular age related macular degeneration before and after restrictions during COVID-19 pandemic.
A total of 116 eyes from 106 patients were enrolled in this study. The mean age of the total study population was 73.2 ± 8.1 years, and 52% (55/106) of patients were female. Demographics and patient characteristics were summarized in Table 1 . In our study population, all of the patients had MNV, and change in exudative features of MNV was demonstrated in Table 2 . It was observed that the OCT findings of the patients significantly increased with the disruption of their treatment (P < 0.001, P = 0.02, and P = 0.01, for SRF, IRF, and SRHM, respectively), and these findings regressed to the frequency of the pre-pandemic visits with the initiation of treatment (P < 0.001, P = 0.002 and P = 0.003 for SRF, IRF, and SRHM, respectively) (Fig. 2 ).
Table 1.
Demographic characteristics.
| Age (mean ± SD) | 73.2 ± 8.1 years |
| Gender (F/M) | 55/51 |
| Total number of anti-VEGF injections (mean ± SD) | |
| Before restrictions | 10.7 ± 5.03 |
| After end of the restrictions | 2.1 ± 0.7 |
| Anti-VEGF injection type (n/%) | |
| Bevacizumab | 23/20 |
| Ranibizumab | 61/53 |
| Aflibercept | 32/27 |
Anti-VEGF: Anti -vascular endothelial growth factor.
Table 2.
Spectral domain optical coherence tomography findings of patients before and after restrictions.
| SRF | IRF | SRHM | |
|---|---|---|---|
| V-2 n(%) | 79(68%) | 44(38%) | 15(13%) |
| V-1 n(%) | 57(49%) | 38(33%) | 16(14%) |
| V0 n(%) | 93(80%) | 59(51%) | 36(31%) |
| V1 n(%) | 58(50%) | 41(35%) | 15(13%) |
| Vlast n(%) | 41(35%) | 34(29%) | 13(11%) |
| V-1 vs. V0 (P-value) | < 0.001 | 0.02 | 0.01 |
| V0 vs. V1 (P-value) | < 0.001 | 0.03 | 0.009 |
| V0 vs. Vlast (P-value) | < 0.001 | 0.002 | 0.003 |
V-2 and V-1: Two previous visits before the restrictions;V0: First visit after restrictions; V1: First visit after the first injection after the restrictions; Vlast: Last visit after restrictions;SRF: Subretinal Fluid; IRF: Intraretinal Fluid; SRHM: Subretinal hyperreflective material.
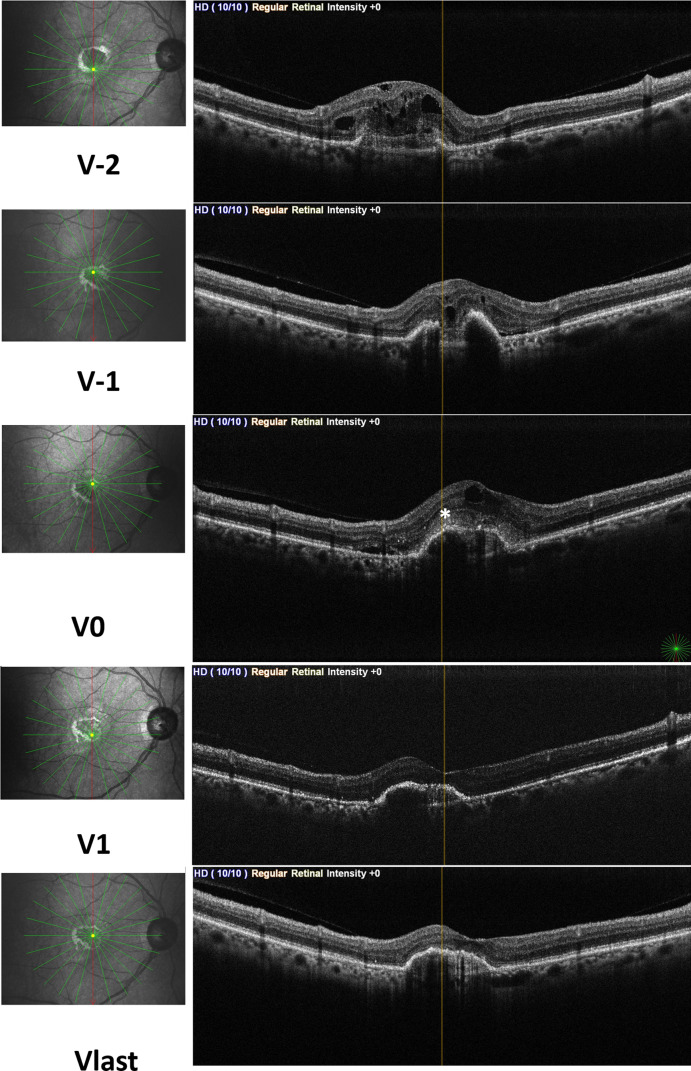
Figure 2.
Structural optical coherence tomography images of a patient with neovascular age related macular degeneration shows change of exudative features of macular neovascularization according to the visits. Subretinal fluid (SRF) and intraretinal fluid (IRF) were observed at V-1 and V-2. Subretinal hyperreflective material (asterix) was added to SRF and IRF at V0 visit. At V1 and Vlast visit all of the exudative features were regressed.
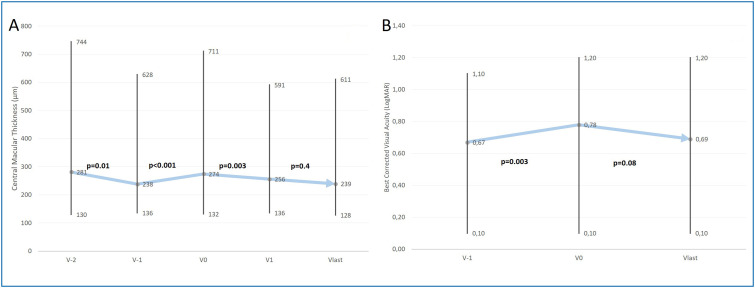
While the median CMT tended to decrease at the last two clinical visits before the pandemic, measurements had an upward trend, and they were significantly increased during the lockdown compared to the last visit before the lockdown [274(132–711) vs. 238(136–628), P < 0.001] (Fig. 3A). Also, we observed new developed submacular hemorrhages in 7 of the patients. During the normalization period after restrictions, patients attended the visits, and the injections were initiated immediately. The median CMT was reduced, and it was significantly lower at V1 compared to V0 [256 (136–591) vs. 274(132–711), P = 0.003]. After the first visit, the median CMT was stabilized and returned the pre-restriction values. Linear logistic regression analysis revealed that there were no associated factors with the CMT change between V0 and Vlast (Table 3 ). The median BCVA was 0.67 (0.1–1.1) logMAR at the last visit before the pandemic and significantly worsened to 0.78 (0.1–1.2) logMAR at the end of the restriction period (P = 0.003). Although the median BCVA improved to 0.69 (0.1–1.2) logMAR at the last visit, the difference did not reach statistical significance compared to V0 (P = 0.08) (Fig. 3B). Linear logistic regression analysis showed that the time interval between V0 and V1 was associated with BCVA change (from Vlast to V0)
Figure 3.
A) Change in central macular thickness before and after restrictions during COVID-19 pandemic (minimum-median-maximum). B) Change in best corrected visual acuity before and after restrictions during COVID-19 pandemic (minimum-median-maximum).
Table 3.
Evaluation of factors related to central macular thickness and best corrected visual acuity change after the restrictions due to the COVID-19 pandemic.
| Central macular thickness |
Best corrected visual acuity |
|||||
|---|---|---|---|---|---|---|
| B | t | P-value | B | t | P-value | |
| Age | −0.911 | −0.497 | 0.6 | −1.116 | −0.332 | 0.5 |
| Gender | −10.334 | −0.344 | 0.7 | −0.029 | −0.476 | 0.6 |
| Best corrected visual acuity | ||||||
| Change after restrictions | −51.417 | −0.632 | 0.5 | |||
| Injection interval after restrictions | 16.809 | 0.096 | 0.5 | −0.035 | −0.211 | 0.8 |
| Time interval between V0 and Vlast | 23.545 | 0.965 | 0.3 | 0.096 | 2.534 | 0.02 |
| Time interval between V-1 and V0 | −17.348 | −1.601 | 0.1 | 0.034 | 2.119 | 0.06 |
| Injection counts after pandemic | 23.720 | 1.163 | 0.2 | 0.013 | 0.057 | 0.8 |
| Central macular thickness | ||||||
| Change after restrictions | −28.234 | −0.548 | 0.6 | |||
Discussion
We anticipated deterioration in the course of the nAMD disease during the COVID-19 pandemic due to the delay in treatment. However, it was important to evaluate the real-life data to determine how much degradation might occur and how much recovery might be observed. In this study, we found an increase in CMT after releasing the restrictions, while it was in a downward trend before the pandemic. However, CMT showed significant improvement and reached the before restriction period value within a short time with the start of the anti-VEGF injections. In spite of the favor in CMT after injections, we did not observe similar improvements in the visual acuity of the patients. On the other hand, exudative features of MNV increased due to the treatment delay but regressed with the initiation of injections.
The COVID-19 outbreak is a global crisis that affects and changes the world order, even causing more than one million death to date. In this period, the social lives of people have been restricted, the economic burden has increased, and perhaps the most important effect of the pandemic was delaying the diagnosis and treatment of chronic diseases that require regular follow-up. Thus, this situation creates difficulty for maintaining the balance between providing treatments for ocular diseases against the risk of cross-infection of COVID-19 between patients and healthcare staff [13].
In developed countries, AMD is the major cause of legal blindness in the adult population. Neovascular AMD, one of the advanced forms of the disease, is characterized by MNV, which causes hemorrhage, fluid accumulation, and macular fibrosis [14]. The expression of VEGF is responsible for increased angiogenesis and vascular permeability. Thus, effective treatment for nAMD is based on targeting VEGF inhibition [15]. Anti-VEGF agents, including bevacizumab, ranibizumab, and aflibercept, effectively limit progression and improve vision in nAMD patients [16]. However, the application of these agents at appropriate intervals is crucial to control the activation of the disease, prevent progression and improve vision. Studies on the optimal treatment regimen intervals are still ongoing. The monthly application of bevacizumab and ranibizumab and bimonthly application of aflibercept was specifically supported in initial studies [17], [18], [19].
The higher number of injections may provide a better visual acuity but also causes an increase in the number of sight-threatening complications, such as endophthalmitis. In addition, more injections may lead to an economic burden for patients and increase healthcare costs. To reduce these disadvantages, different treatment regimens, such as quarterly, PRN (as needed), treat-and-extend, and observe-and-plan regimens, have been developed [20]. Despite these treatment algorithms, delay or discontinuation of treatment can lead to permanent visual loss due to progressive retinal pigment epithelium death and photoreceptor loss. While prompt treatment may provide less macular disruption, increasing delay duration may lead to irreversible changes in the macular anatomy. Lim et al. indicated that 21 weeks or longer treatment delays resulted in a 5-fold increased worsening of visual outcomes after treatment compared to a delay of 7 weeks or shorter between the first symptoms and their first anti-VEGF treatment [14]. Kim et al. reported an increasing deterioration in visual acuity at 6, 12, and 24 months after treatment discontinuation in nAMD patients who needed treatment but left the treatment [21]. Additionally, real-world studies confirmed that delay of active disease re-treatment could lead to worse visual outcomes and less reduction in central subfield macular thickness [22]. Furthermore, a higher CRT increase was found to be associated with visual acuity loss [23]. Our patients with active disease had approximately five months of treatment delay. Similar to real-life studies, there was a significant increase in CMT in our study at V0, and this increase may be responsible for worsening visual acuity.
On the other hand, we showed significant improvement in CMT and reached the before restriction period value within a short time with the start of the anti-VEGF injections, but we could not achieve a similar improvement in visual acuity. This may be explained by the fact that long-lasting damage to the photoreceptors and retinal pigment epithelium affects the visual prognosis more negatively, even if structural success is achieved. In a previous study, it was reported that a 23.5 ± 10.4 day delay between anti-VEGF indication to treatment caused an increase in central retinal thickness (CRT) by 31.5 ± 69.6 μm with VA loss (−2.2 ± 5.0 letters) [24]. After the treatment initiation, they showed that CRT was regressed 47.7 ± 117.5 μm with worse regain in VA (0.4 ± 7.4 letters) [24]. They suggested that functional deterioration despite morphologic response to therapy is likely to be caused by the loss of photoreceptors.
Borelli et al. evaluated the short-term effects of the COVID-19 pandemic on the outcomes of 112 eyes with nAMD[11]. They showed that delayed visits were significantly associated with worse structural and functional outcomes. Furthermore, increased exudative disease activity has also been shown, as we have demonstrated in our study. Various studies have demonstrated that the PRN regimen can provide comparable results to a fixed dosing regimen concerning visual acuity improvement, but it is important to provide prompt injections if needed [25]. Unfortunately, during this COVID-19 pandemic, patients could not receive the needed injections. Delayed or omitted injections have resulted in increased CMT, which leads to visual deterioration. In addition to the study mentioned above, we also evaluated the treatment effects after the lockdown period and found a regression in exudative features and disease activation during follow-up. Before the pandemic period, injection intervals were longer than intervals after V0. Starting anti-VEGF injections immediately after the restrictions were ended, and applying more frequent injections in a short time may lead to the regression of the disease activation and even more positive results. In previous studies, higher treatment frequency was reported associated with favorable outcomes [26].
Furthermore, Romano et al. reported an increased number (41 of 6568 patients) of submacular hemorrhages and a decrease in visual acuity (0.57 to 1.66 logMAR) in patients with AMD as a result of non-attendance to scheduled appointments due to COVID-19 lockdown [27]. In our study, newly developed submacular hemorrhages occurred in 7 patients, and the rate was higher from the study above. This may be explained by a further delay in our treatments and appointments.
Our study has some limitations. Firstly, our study population was heterogeneous, and this may be associated with a wide range in CMT, visual acuity, and injection numbers. Secondly, follow up time after restrictions were short. This may be insufficient to predict the increase in visual acuity and regression in OCT findings. To observe stabilization, we need studies with longer follow up.
Conclusion
Treatment delay due to the COVID-19 pandemic caused the progression of the disease and the visual impairment in patients with nAMD. After treatment delay or discontinuation, to plan anti-VEGF treatment immediately and more frequent visit intervals may be appropriate until the disease stabilizes. However, it should be kept in mind that despite the improvement in CMT, the desired success in visual acuity could not be achieved in the short term.
Financial Support
None.
Disclosure of interest
The authors declare that they have no competing interest.
References
- 1.Emparan JPO-d, Sardi-Correa C., López-Ulloa J.A., Viteri-Soria J., Penniecook J.A., Jimenez-Román J., et al. COVID-19 and the eye: how much do we really know?. A best evidence review. Arq Bras Oftalmol. 2020;83:250–261. doi: 10.5935/0004-2749.20200067. [DOI] [PubMed] [Google Scholar]
- 2.Chen L., Deng C., Chen X., Zhang X., Chen B., Yu H., et al. Ocular manifestations and clinical characteristics of 534 cases of COVID-19 in China: a cross-sectional study. Acta Ophthalmol. 2020 doi: 10.1111/aos.14472. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Invernizzi A., Torre A., Parrulli S., Zicarelli F., Schiuma M., Colombo V., et al. Retinal findings in patients with COVID-19: Results from the SERPICO-19 study. EClinicalMedicine. 2020 doi: 10.1016/j.eclinm.2020.100550. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fu L., Wang B., Yuan T., Chen X., Ao Y., Fitzpatrick T., et al. Clinical characteristics of coronavirus disease 2019 (COVID-19) in China: a systematic review and meta-analysis. J Infect. 2020;80:656–665. doi: 10.1016/j.jinf.2020.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corradetti G., Corvi F., Nguyen T.V., Sadda S.R. Management of neovascular age-related macular degeneration during the COVID-19 pandemia. Ophthalmol Retina. 2020;4:757–759. doi: 10.1016/j.oret.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corazza P., D’Alterio F.M., Younis S. Proposed algorithm during COVID-19 pandemic for patient management in medical retina clinic. Int J Retina Vitreous. 2020;6:1–5. doi: 10.1186/s40942-020-00226-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Danthuluri V., Grant M.B. Update and recommendations for ocular manifestations of COVID-19 in adults and children: a narrative review. Ophthalmol Ther. 2020 doi: 10.1007/s40123-020-00310-5. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bressler N.M. Age-related macular degeneration is the leading cause of blindness. Jama. 2004;291:1900–1901. doi: 10.1001/jama.291.15.1900. [DOI] [PubMed] [Google Scholar]
- 9.Querques L., Parravano M., Borrelli E., Chiaravalloti A., Tedeschi M., Sacconi R., et al. Anatomical and functional changes in neovascular AMD in remission: comparison of fibrocellular and fibrovascular phenotypes. Br J Ophthal. 2020;104:47–52. doi: 10.1136/bjophthalmol-2018-313685. [DOI] [PubMed] [Google Scholar]
- 10.Spaide R.F., Jaffe G.J., Sarraf D., Freund K.B., Sadda S.R., Staurenghi G., et al. Consensus nomenclature for reporting neovascular age-related macular degeneration data: consensus on neovascular age-related macular degeneration nomenclature study group. Ophthalmology. 2020;127:616–636. doi: 10.1016/j.ophtha.2019.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borrelli E., Grosso D., Vella G., Sacconi R., Battista M., Querques L., et al. Short-term outcomes of patients with neovascular exudative AMD: the effect of COVID-19 pandemic. Graefe's Arch Clin Exp Ophthalmol. 2020;3:1–8. doi: 10.1007/s00417-020-04955-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Antaki F., Dirani A. Treating neovascular age-related macular degeneration in the era of COVID-19. Graefe's Arch Clin Exp Ophthalmol. 2020 doi: 10.1007/s00417-020-04693-w. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheung S.S., Wong C.Y., Chan J.C., Chan C.K., Lam N., Yuen H.K., et al. Ophthalmology in the time of COVID-19: experience from Hong Kong Eye Hospital. Int J Ophthalmol. 2020;13:851. doi: 10.18240/ijo.2020.06.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lim J.H., Wickremasinghe S.S., Xie J., Chauhan D.S., Baird P.N., Robman L.D., et al. Delay to treatment and visual outcomes in patients treated with anti-vascular endothelial growth factor for age-related macular degeneration. Am J Ophthalmol. 2012;153:678–686. doi: 10.1016/j.ajo.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller J.W., Le Couter J., Strauss E.C., Ferrara N. Vascular endothelial growth factor a in intraocular vascular disease. Ophthalmology. 2013;120:106–114. doi: 10.1016/j.ophtha.2012.07.038. [DOI] [PubMed] [Google Scholar]
- 16.Solomon S.D., Lindsley K., Vedula S.S., Krzystolik M.G., Hawkins B.S. Anti-vascular endothelial growth factor for neovascular age-related macular degeneration. Cochrane Database Syst Rev. 2019;3:CD005139. doi: 10.1002/14651858.CD005139.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenfeld P.J., Brown D.M., Heier J.S., Boyer D.S., Kaiser P.K., Chung C.Y., et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 18.Heier J.S., Brown D.M., Chong V., Korobelnik J.-F., Kaiser P.K., Nguyen Q.D., et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119:2537–2548. doi: 10.1016/j.ophtha.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 19.Khanna S., Komati R., Eichenbaum D.A., Hariprasad I., Ciulla T.A., Hariprasad S.M. Current and upcoming anti-VEGF therapies and dosing strategies for the treatment of neovascular AMD: a comparative review. BMJ Open Ophthalmol. 2019;4:e000298. doi: 10.1136/bmjophth-2019-000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al-Zamil W.M., Yassin S.A. Recent developments in age-related macular degeneration: a review. Clin Interv Aging. 2017;12:1313. doi: 10.2147/CIA.S143508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim J.H., Chang Y.S., Kim J.W. Natural course of patients discontinuing treatment for age-related macular degeneration and factors associated with visual prognosis. Retina. 2017;37:2254–2261. doi: 10.1097/IAE.0000000000001494. [DOI] [PubMed] [Google Scholar]
- 22.Teo K.Y.C., Saxena N., Gan A., Wong T.Y., Gillies M.C., Chakravarthy U., et al. Detrimental effect of delayed retreatment of active disease on outcomes in neovascular age related macular degeneration-RAMPS study. Ophthalmol Retina. 2020 doi: 10.1016/j.oret.2020.03.017. in press. [DOI] [PubMed] [Google Scholar]
- 23.Muether P.S., Hermann M.M., Koch K., Fauser S. Delay between medical indication to anti-VEGF treatment in age-related macular degeneration can result in a loss of visual acuity. Graefe's Arch Clin Exp Ophthalmol. 2011;249:633–637. doi: 10.1007/s00417-010-1520-9. [DOI] [PubMed] [Google Scholar]
- 24.Muether P.S., Hoerster R., Hermann M.M., Kirchhof B., Fauser S. Long-term effects of ranibizumab treatment delay in neovascular age-related macular degeneration. Graefe's Arch Clin Experim Ophthalmol. 2013;251:453–458. doi: 10.1007/s00417-012-2038-0. [DOI] [PubMed] [Google Scholar]
- 25.Gerding H., Loukopoulos V., Riese J., Hefner L., Timmermann M. Results of flexible ranibizumab treatment in age-related macular degeneration and search for parameters with impact on outcome. Graefe's Arch Clin Exp Ophthalmol. 2013;251:453–458. doi: 10.1007/s00417-011-1636-6. [DOI] [PubMed] [Google Scholar]
- 26.Wecker T., Grundel B., Reichl S., Stech M., Lange C., Agostini H., et al. Anti-VEGF injection frequency correlates with visual acuity outcomes in pro re nata neovascular AMD treatment. Sci Rep. 2019;9:1–8. doi: 10.1038/s41598-019-38934-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Romano F., Monteduro D., Airaldi M., Zicarelli F., Parrulli S., Cozzi M., et al. Increased number of submacular hemorrhages as a consequence of COVID-19 lockdown. Ophthalmol Retina. 2020 doi: 10.1016/j.oret.2020.06.027. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]