Abstract
Background
The current health concern to the entire world is the chronic respiratory disease caused by coronavirus 2 (COVID-19). A specific treatment or proper therapy is still lacking, and the investigations from across the world for proper drug/vaccine development towards disease control are in progress. The Coronavirus replication takes place by the conversion of the polypeptide into functional protein and this occurs due to the key enzyme Main protease (Mpro). Therefore, identification of natural and effective Mpro inhibitors could be a safe and promising approach for COVID-19 control.
Methods
The present in silico study evaluates the effect of bioactive compounds found in Eucalyptus and Corymbia species essential oil on Mpro by docking. Molecular docking of the major seven compounds of essential oil (citronellol, alpha-terpineol, eucalyptol, d-limonene, 3-carene, o-cymene, and alpha-pinene) with Mpro was studied by AutoDock 4.2, and the properties were analysed by PreADMET and Biovia Discovery Studio visualizer.
Results
The calculated parameters such as binding energy, hydrophobic interactions, and hydrogen bond interactions of 6LU7 (Mpro) with Eucalyptus and Corymbia volatile secondary metabolites represented its scope as an effective therapy option against covid-19. Among the docked compounds, eucalyptol shows the least binding energy without toxicity.
Conclusions
The outcome of this study reported that the essential oil of Eucalyptus and Corymbia species, mainly eucalyptol can be utilized as a potential inhibitor against COVID-19 and also it can be used in its treatment. Hence, further analysis was required to explore its potential application in medicine.
Keywords: Autodock, COVID-19, Eucalyptus, Corymbia eucalyptol, Molecular interaction analysis, Mpro
Introduction
Public health has always been a prime issue due to the continuous emerging of pandemics in last two decades. There were outbreaks of SARS-CoV-1, MERS-CoV, and now the current pandemic Coronavirus disease of 2019 (COVID-19) has become an extreme threat to human life, the livelihood, and also has brought about relentless social-economical along with political issues in the tainted countries [1]. The WHO has categorized COVID-19 as a pandemic [2]. The International Committee on Taxonomy of Viruses (ICTV) gave the name for this virus as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [3]. COVID-19 causes ARDS-Acute Respiratory Distress Syndrome, a condition in which fluids get collected in the air sacs of lung and causes organ oxygen deprivation. The virus has shown similarity with Bat SARS-like coronavirus of more than 87.99% uniqueness in sequencing, and also 80.0% uniqueness of nucleotide with the original SARS epidemic virus [4,5]. It was found that genome of CoV encodes two proteins ppla and pplb which are involved in the formation of nucleoprotein, spike, and membrane while it is also involved in the replicase, and polymerase activity of viruses [6].
The current treatments used for COVID-19 majorly include Convalescent plasma therapy which is based on the concept of passive immunity. Chenguang et al. (2020), carried out an experiment to find out if convalescent plasma transfusion can be beneficial in the cure of vitally infected patients of SARS-CoV-2 and it was found that the primary study showed positive outcome in the patient’s medical status [7]. Protease inhibitor is commonly used for the management of HIV and this was used for the cure of COVID-19 in a preliminary study [8]. Nuceloside analogues including umifenovir, neuraminidase inhibitors, tenofovir disoproxil, remdesivir and lamivudine has been reported by Siti et al. (2020) for the antiviral treatments for human pathogenic CoVs [9]. Even though large numbers of these drugs are showing action for coronavirus in both in vitro along with in vivo, their side effects in terms of pharmacokinetic and pharmacodynamic properties are yet to be studied on animal and human trials. Moreover it will take quite a long time to give dependable and reliable treatment to patients. All of the drug options for COVID-19 are being tested from the previous pandemics treatment experience. However, each treatment options have its own advantages and limitations.
In the course of recent years, the interest in nature origin medication has been on rise in different fields of medical treatment. In elective medicine and its medication, essential oils from medicinal plants are widely and generally utilized to treat several health ailments. The plant essential oils have been widely studied and are reported to have antiviral bioactivities [10]. Inhalation of steam with essential oil has previously showed positive impact for the treatment of respiratory difficulties and is suggested for managing bronchiolitis, communicable cold and disease, rhinosinusitis, allergies and flu asthma [11]. Among the conventional mainstream medication and medicines, the essential oil of Eucalyptus and Corymbia species has been utilized for the treatment of disorders associated with respiratory systems. Various researches on Eucalyptus species essential oil has been carried out to check the antibacterial and antiviral activity, which has shown positive results [[12], [13], [14]]. Few of the studies have been carried out on the antiviral activity of Eucalyptus essential oils and it has reported positive results for herpes simplex viruses and Adenovirus, mumps [3,15]. Various bioactivities shown by major compounds from both the essential oils has been detailed in Table 1 .
Table 1.
Bioactivities of compounds.
| Compounds | Activity |
|---|---|
| Citronellol | Antinociceptive, anti-inflammatory [41], antiviral activity [42] |
| Alpha-terpineol | Antimicrobial activity [43], antiviral activity [44] |
| o-Cymene | Anti-inflammatory activity [45], anti-influenza virus activity [46] |
| d-Limonene | Anti-viral activity [47] |
| Eucalyptol | Anti-viral activity [48], anti-inflammatory activity [49], antifungal activity [50] |
| Alpha-pinene | Anti-viral activity [47] |
| 3-Carene | - No major bioactivity has been shown |
As an initial step, it is highly desirable to screen and identify the potentiality of bioactive compounds present in oils of Eucalyptus and Corymbia against important therapeutic targets of SARS-CoV-2 with the help of molecular docking and its analysis. This will lead towards the progression of novel drug therapies for fighting COVID-19. However, selection of proper therapeutic target is highly crucial for an effective output. There are several therapeutic targets identified and reported for SARS-CoV-2, among which Mpro is ∼306 aa long main enzyme responsible for coronavirus duplication and dispensatings the functional proteins from viral polypeptide [16]. Mpro has also been mentioned as 3C-like protease (3CLpro) as it has the same restriction-site explicitness like picornavirus 3C protease (3Cpro). The main protease (Mpro) from COVID-19 was represented earlier as the latent and important target to stop the replication of CoV [17].
The current study is an effort carried out to understand the molecular interaction with bioactive volatiles from oils of Eucalyptus and Corymbia against SARS-CoV-2 specifically targeting Mpro and to predict the pharmacokinetics and toxological parameters of the volatile compounds taken for study. The findings and studies of this investigation will be useful to the general public and will give researchers and scientists to bring out the correct medication to fight against COVID-19 by focusing on Mpro.
Materials and methodology
Essential oil from Eucalyptus and Corymbia
The leaves of Eucalyptus globulus and Corymbia citrodora were collected from Palani hill areas. The images of both the species are represented in Fig. 1 . It was identified and authenticated at BSI Coimbatore. The oil from 4 kg of leaves was extracted by hydrodistillation method using copper distillation unit [18]. The procedure involves evaporation of essential oil by heating mixture of water and leaves followed by liquefaction of vapours in the condenser. The essential oils collected were stored at room temperature in air tight bottles.
Fig. 1.
(a) Eucalyptus globulus and (b) Corymbia citrodora.
Identification of metabolites of essential oil using GC–MS
The essential oils of E. globulus and C. citrodora were analysed using GC—MS analysis according to the method described by Dey and Harborne (1997) with slight modifications. Agilent 7890B gas chromatography system with Agilent 5977B MS detector was employed for compound separation and identification [19]. The settings of GC: the initial temperature of oven was kept at 60 °C for 1 min which was raised at 10 °C min−1 to 180 °C then it kept for 1 min, and increased at 20 °C min−1 to 280 °C and maintained there for 15 min. Carrier gas namely helium was utilized with 1.0 ml min−1 flow rate. Spectrum was recorded with the scan ranges from 20 to 550 m/z at 2 scans s−1. The compounds of essential oils were analysed based on alkane series compounds (C4–C28) that determined by their retention time which compared with the chemical constituents grouped by the Adams table [20], and the mass spectrum which is similar with those of grouped compounds in the NIST-MS library was shown in the article [21].
Protein preparation
The crystallographic structure of SARS corona virus main protease (COVID-19 3CLpro/Mpro) was retrieved from the structure database (PDB) and its PDB id is 6LU7-A Chain from Homo sapiens [22,23].
Ligand preparation
ACD/ChemSketch is a chemical structure depicting tool that allows us to draw compound structures. The SMILES format of the compounds, namely, citronellol, alpha-terpineol, o-cymene, d-limonene, eucalyptol, alpha-pinene, and 3-carene were retrieved from PubChem database. The 2D structure was generated through SMILES structure, converted to 3D structure, and 3D optimization was also carried out and saved in MDL-MOL File format and converted in to the PDB format using converter program (open babel) [24].
PreADMET assessment of bioactive compounds
The pharmacokinetic and toxicological properties of citronellol, alpha-terpineol, o-cymene, d-limonene, eucalyptol, alpha-pinene, and 3-carene were predicted using PreADMET server. The pharmacokinetic properties such as Caco-2 values which is an in vitro cell permeability parameter (Caco-2 cell model), human intestinal absorption (HIA value), plasma protein binding (PPB value), the blood–brain barrier (BBB) penetration, P-glycoprotein (Pgp) binding parameter, and metabolic parameters (phase 1 and phase 2 enzymes) were carried out using ADME property prediction. In addition, the compounds were virtually screened to calculate and detect the toxicological properties such as mutagenicity, and carcinogenicity using toxicity prediction.
Docking analysis and interpretations
Molecular interaction analysis was carried out to understand the molecular affinity between the compounds, namely, citronellol, alpha-terpineol, o-cymene, d-limonene, eucalyptol, alpha-pinene, and 3-carene, against Mpro of novel corona virus using AutoDock 4.2. To the 3D Mpro structure of macromolecular structure hydrogen atoms (polar) and kollman charges were added. In the ligand preparation centre node and rotatable bonds were selected and saved in PDBQT format. In the grid preparation, active sites were selected and grids were adjusted with the spacing of 0.375 Å and dimensions of x, y, and z-axes was set to 64 × 66 × 66 points and saved in grid parameter file (GPF) format and run. In docking process, the Lamarckian Genetic Algorithm (LGA) was selected which utilizes local minimisation that enables gene population modification to the complex structures [25]. The parameters of genetic algorithm were set to 10 runs with 150 population size; the number of evaluations was set to 25,000,000; generation numbers as 27,000 with default mutation rate (0.02) and crossover rate (0.8). Docking simulation was performed by utilizing AutoDock 4.2 version [26].
Docking visualization
The protein–compound interactions such as bonded and other non-bonded energies among the citronellol, alpha-terpineol, o-cymene, d-limonene, eucalyptol, alpha-pinene, and 3-careneagainst Mpro of novel corona virus were depicted by utilizing Biovia Discovery Studio visualizer. This software visualizes the molecular interaction such as hydrogen bond, hydrophobic interaction and van der Waals interactions.
Results
Identification of metabolites
The compounds identified GC–MS chromatograms of E. globulus and C. citrodora are represented in Table 2 . Eucalyptol is the major compound (52.47%) present in E. globulus. The compound citronellol (59.95%) is the major component of C. citrodora. The following compounds citronellol, alpha-terpineol, o-cymene, d-limonene, eucalyptol, alpha-pinene and 3-carene were taken for further study based upon the high percentage availability in the essential oils and also all these compounds are commonly found in both the Eucalyptus species taken for study.
Table 2.
Chemical composition of the essential oils (% total peak area).
| Volatile compounds | Eucalyptus globulus | Corymbia citrodora |
|---|---|---|
| Citronellol | – | 59.31% |
| Alpha-terpineol | 0.53% | 1.34% |
| o-Cymene | 5.06% | 1.37% |
| d-Limonene | 4.49% | 3.15% |
| Eucalyptol | 52.47% | 13.54% |
| Alpha-pinene | 17.00% | 2.21% |
| 3-Carene | 1.70% | 5.39% |
Prediction of active sites in Mpro-novel corona virus
The crystal structure of Mpro was uploaded in the Computed Atlas for Surface Topography of Proteins (CASTp) server to predict the active sites of protein. The following active sites were predicted from the pocket 1: THR24, THR25, THR26, LEU27, HIS41, CYS44, THR45, THR45, SER46, MET49, PRO52, TYR54, PHE140, LEU141, ASN142, GLY143, SER144, CYS145, HIS163, HIS164, MET165, GLU166, LEU167, PRO168, HIS172, ASP187, ARG188, GLN189, THR190, and GLN192.
Drug likeliness assessment of the compounds from the essential oils
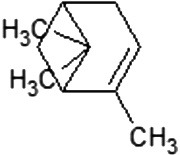
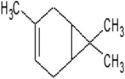
The volatile compounds, namely, citronellol, alpha-terpineol, o-cymene, d-limonene, eucalyptol, alpha-pinene, and 3-carene from E. globulus and C. citrodora were analysed for molecular properties. All the compounds had molecular weight below 500, hydrogen bond donor ≤5, hydrogen bond acceptor ≤10, and logP value ≤5. Hence, all compounds obey Lipinski’s rule of 5. The PubChem id, 2D, 3D structures, Lipinski’s rule are shown in Table 3 .
Table 3.
Properties of COVID-19 Mpro potential inhibitor compounds.
| Properties | Citronellol | Eucalyptol | Alpha-terpineol | d-Limonene | o-Cymene | Alpha-pinene | 3-Carene a |
|---|---|---|---|---|---|---|---|
| Pubchem id | 8842 | 2758 | 17,100 | 440,917 | 10,703 | 440,968 | 26,049 |
| 2D structure | 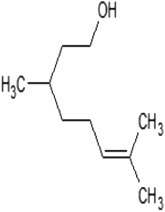 |
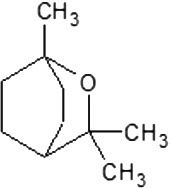 |
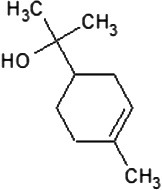 |
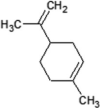 |
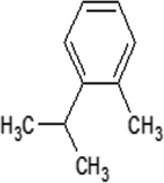 |
 |
 |
| 3D structure |  |
 |
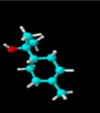 |
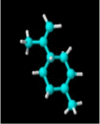 |
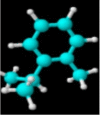 |
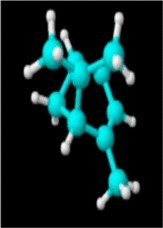 |
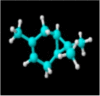 |
| Molecular formula | C10H20O | C10H18O | C10H18O | C10H16 | C10H14 | C10H16 | C10H16 |
| Molecular weight | 156.26 | 154.25 | 154.25 | 136.23 | 134.22 | 136.23 | 136.23 |
| Log P | 3.2 | 2.72 | 1.8 | 3.4 | 3.4 | 3.54 | 2.8 |
| Hydrogen bond donor | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| Hydrogen bond acceptor | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
PreADMET assessment of compounds from Eucalyptus and Corymbia essential oil
The absorption, metabolism and distribution parameters of citronellol, alpha-terpineol, o-cymene, d-limonene, eucalyptol, alpha-pinene, and 3-carene were assessed using the PreADMET server. The calculated pharmacokinetic parameters are summarized in Table 4 . The predicted absorption values for Caco-2 cell (PCaco-2) were found to be between 4 and 70. Hence all compounds taken for analysis were moderately permeable. The compounds taken for study can be well absorbed through the intestinal cells as the predicted HIA values for all the compounds was found to be 100%. The predicted value of PPB for citronellol, alpha-terpineol, d-limonene, eucalyptol, alpha-pinene, and 3-carene was 100.00%, indicating that the compounds are strongly bound chemicals, whereas cymene has 88.39% indicating it as weakly bound chemical. The ratio of Cbrain/Cblood value for eucalyptol was found to be 1.46. All other compounds with a Cbrain/Cblood value of greater than 2.0 indicate increase absorption of compounds to CNS, suggesting compounds ability to cross BBB. The compounds such as d-limonene, alpha-pinene and 3-carene were found as inhibitors of Pgp, whereas citronellol, cymene, and eucalyptol were found to be non-inhibitors of Pgp. The computed metabolism of compounds showed that citronellol, alpha-terpineol, o-cymene, d-limonene, eucalyptol, alpha-pinene, and 3-carene are inhibitors of CYP2C9. Other enzyme inhibition details (CYP_2C19, CYP_2D6, CYP_3A4) are displayed in Table 3. The term “negative” suggests compounds having carcinogenic activity and term “positive” suggests absence of carcinogenic activity. It was found that although all compounds found to be mutagenic in the in silico AMES test model. Citronellol, cymene, and eucalyptol showed non-carcinogenicity in the mouse model and d-limonene, eucalyptol, alpha-pinene, and 3-carene show noncarcinogenicity in the rat model.
Table 4.
ADME and toxicity results of the seven compounds.
| Properties | Citronellol | Alpha-terpineol | o-Cymene | d-Limonene | Eucalyptol | Alpha-pinene | 3-Carene | |
|---|---|---|---|---|---|---|---|---|
| ADME | ||||||||
| BBB | 7.09219 | 5.5333 | 4.96983 | 8.27823 | 1.46723 | 5.5333 | 5.5333 | |
| Buffer_solubility_mg_L | 303.591 | 1368.72 | 328.927 | 14.068 | 1339.31 | 1368.72 | 1368.72 | |
| Caco2 | 8.91366 | 23.6313 | 23.4337 | 23.6317 | 21.895 | 23.6322 | 23.6313 | |
| CYP_2C19_inhibition | Inhibitor | Non inhibitor | Inhibitor | Inhibitor | Non inhibitor | Non Inhibitor | Non Inhibitor | |
| CYP_2C9_inhibition | Inhibitor | Inhibitor | Inhibitor | Inhibitor | Inhibitor | Inhibitor | Inhibitor | |
| CYP_2D6_inhibition | Non Inhibitor | Non Inhibitor | Non Inhibitor | Non Inhibitor | Non Inhibitor | Non Inhibitor | Non Inhibitor | |
| CYP_2D6_substrate | Non Inhibitor | Non Inhibitor | Non Inhibitor | Non Inhibitor | Non Inhibitor | Non Inhibitor | Non Inhibitor | |
| CYP_3A4_inhibition | Non Inhibitor | Non Inhibitor | Inhibitor | Non Inhibitor | Inhibitor | Non | Non | |
| CYP_3A4_substrate | Weakly | Substrate | Weakly | Substrate | Weakly | Substrate | Substrate | |
| HIA | 100 | 100 | 100 | 100 | 100 | 100 | 100 | |
| Pgp_inhibition | Non Inhibitor | Inhibitor | Non Inhibitor | Inhibitor | Non Inhibitor | Inhibitor | Inhibitor | |
| Plasma_Protein_Binding (%) | 100 | 100 | 88.39 | 100 | 100 | 100 | 100 | |
| TOXICITY | ||||||||
| Ames_test | Mutagen | Mutagen | Mutagen | Mutagen | Mutagen | Mutagen | Mutagen | |
| Carcino_Mouse | Positive | Negative | Positive | Negative | Positive | Negative | Negative | |
| Carcino_Rat | Negative | Negative | Negative | Positive | Positive | Positive | Positive | |
Molecular docking analysis of compounds from Eucalyptus and Corymbia oil with Mpro
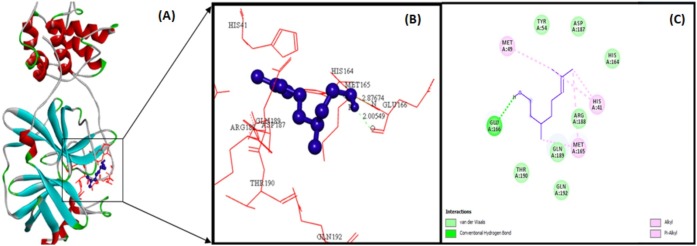
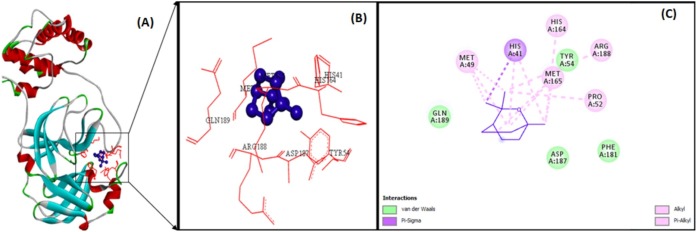
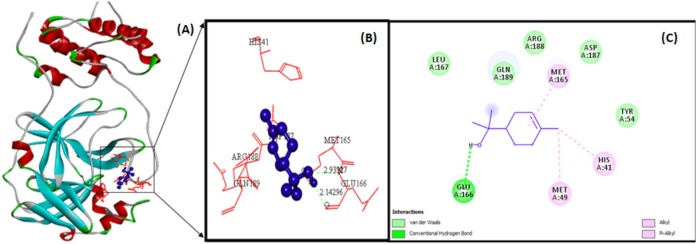
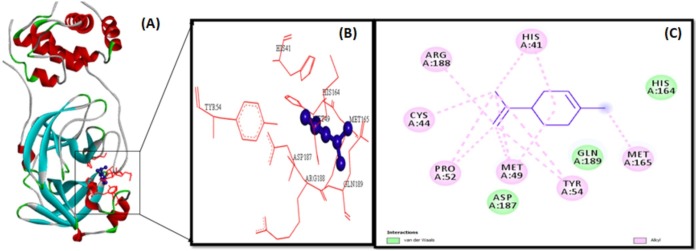
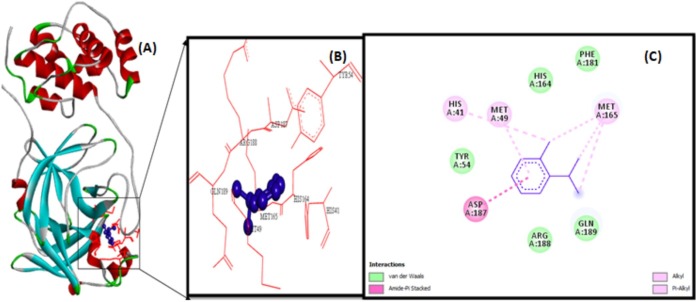
The docking analysis of citronellol, alpha-terpineol, o-cymene, d-limonene, eucalyptol, alpha-pinene, and 3-carene against Mpro by utilizing AutoDock software 4.2 version. The docking simulation generated 10 conformations of the protein–compound complex which are displayed based on least binding free energy (ΔG). The best conformations were chosen based on the least energy and minimal solvent accessibility. The interactions such as hydrogen, hydrophobic, and other non-bonded terms between the bioactive compounds and Mpro are visualized using Biovia Discovery Studio Visualizer software. The Mpro structure was represented as solid ribbon model, key residues were represented in red colour line model, and the compounds were represented in violet colour ball and stick model is shown in Fig. 2 . The citronellol, eucalyptol, alpha-terpineol, d-limonene, o-cymene, alpha-pinene, and 3-carene showing hydrogen, hydrophobic, van der Waals interactions within active binding sites of MPro are shown in Fig. 2.
Fig. 2.
Citronellol (violet colour ball and stick model) interactions with MPro represented in (A) solid ribbon model with; (B) active sites of amino acid residues represented in red colour line model; (C) hydrogen bond (green dotted lines represents) and hydrophobic interactions (alkyl and pi-alkyl are shown in light pink colour dotted lines), van der Waals interactions (light green colour circles). Solvent accessible surfaces of the interacting residues are represented by a blue halo around the residues.
Binding interaction of the compounds from Eucalyptus and Corymbia oil with Mpro
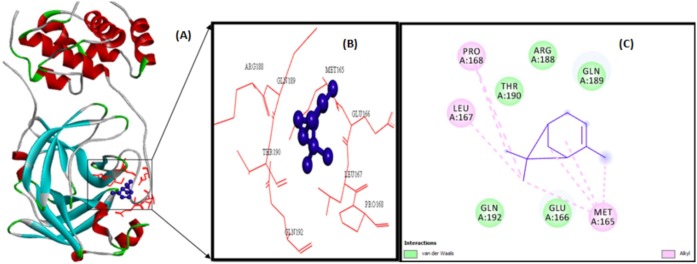
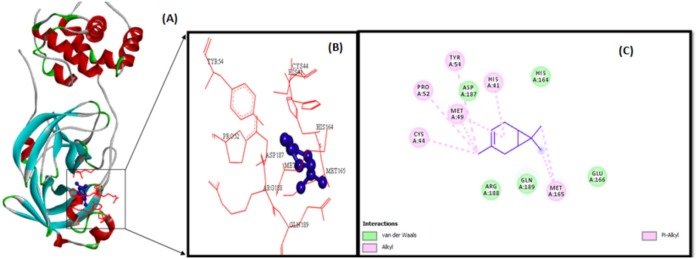
The illustration of molecular interaction of citranellol, eucalyptol, alpha-terpineol, d-limonene, o-cymene, alpha-pinene, and 3-carene in the key residues of Mpro are shown in Table 5 . The docking simulation of Mpro to citranellol has showed that citranellol forms two hydrogen bonds (GLU166) with Mpro; five hydrophobic bonds formed with HIS41, MET165, and MET49; and seven van der Waals forces with TYR54, ASP187, HIS164, ARG188, GLN189, GLN192, and THR190, which are displayed in Fig. 3 with a interaction energy of −4.94 kcal/mol and 239.53 μM inhibitory constant (Table 6 ). The molecular interaction analysis of Mpro with eucalyptol utilizes the least energy of –5.86 kcal/mol and 50.78 μM inhibitory constant. The Mpro–eucalyptol conformation depicts 11 hydrophobic bonds with MET49, MET165, HIS164, ARG188, and PRO52 amino acids; one pi-sigma interaction with HIS41; and four van der Waals forces with ASP187, GLN189, TYR54, and PHE181 residues [Fig. 4 ] Mpro docking analysis with alpha-terpineol showed two hydrogen bonds with GLU166; three hydrophobic alkyl and pi-alkyl bonds with MET49, MET165, and HIS41 residues; and five van der Waals forces with TYR54, LEU167, GLN189, ARG188, and ASP187 [Fig. 5 (A)–(C)] with a binding energy value of –5.43 kcal/mol and 105.43 μM inhibitory constant. The docking study of Mpro with d-limonene has utilized a energy value of –5.18 kcal/mol and 159.51 μM inhibitory constant and 12 hydrophobic bonds with MET49, PRO52, TYR54, MET165, HIS41, CYS44, and ARG188 and three van der Waals interaction with HIS164, GLN189, and ASP187 residues were formed [Fig. 6 (A)–(C)]. The Mpro-o-cymene complex formation utilized a energy value of –4.99 kcal/mol and 219.4 μM inhibitory constant and results in seven hydrophobic bonds with MET165, MET49, HIS41 (alkyl and pi-alkyl interactions),and ASP187 (amide-pi stacked interaction) and five van der Waals interactions with PHE181, HIS164, TYR54, GLN189, and ARG188 residues shown in Fig. 7 (A)–(C). Mpro with alpha-pinene utilizes a binding energy of –5.61 kcal/mol and 77.63 μM inhibitory constant and showed seven hydrophobic bonds with PRO168, LEU167, and MET165 and five van der Waals interactions with GLN189, ARG188, THR190, GLU166, and GLN194 residues [Fig. 8 (A)–(C)]. Finally, docking simulation of Mpro with 3-carene showed seven hydrophobic bonds with the Mpro amino acids MET165, MET49, HIS41, TYR54, PRO52, and CYS44 (alkyl and pi-alkyl interactions) and five van der Waals interactions with GLN189, ARG188, ASP187, GLU166, and HIS164 with a binding energy –5.43 kcal/mol and 103.88 μM inhibitory constant. Table 5 shows the least energy values, vdW + Hbond + desolv energy, torsional energy, intermolecular energy, electrostatic energy, unbound energy, inhibition constants, and refRMS value of compounds with Mpro. All the energy values are given in Kcal/mol.
Table 5.
The Eucalyptus essential oil compounds interacting residues of the Mpro proteins of novel corona virus are summarized with the number of hydrophobic interactions and the number of hydrogen bonds.
| Compounds from Eucalyptus essential oil | Hydrogen bonds |
Hydrophobic interactions (pi alkyl; alkyl-alkyl; pi-sigma; amide-pi stacked) | van der Waals interactions | |||
|---|---|---|---|---|---|---|
| Mpro |
Atom in bio-active compound | Distance (Å) | ||||
| Aminoacid | Atom | |||||
| Citronellol | GLU166 | O | H | 2.14 | HIS41(2) | TYR54 |
| GLU166 | N | N | 2.93 | MET165(2) | ASP187 | |
| MET49(1) | HIS164 | |||||
| ARG188 | ||||||
| GLN189 | ||||||
| GLN192 | ||||||
| THR190 | ||||||
| Eucalyptol | – | – | – | – | MET49(3) | TYR54 |
| MET165(3) | ASP187 | |||||
| HIS164(1) | GLN189 | |||||
| ARG188(1) | PHE181 | |||||
| PRO52(1) | ||||||
| HIS41(2) | ||||||
| Alpha-terpineol | GLU166 | O | H | 2.14 | MET49(1) | TYR54 |
| GLU166 | N | N | 2.93 | MET165(1) | LEU167 | |
| HIS41(1) | GLN189 | |||||
| ARG188 | ||||||
| ASP187 | ||||||
| d-Limonene | – | – | – | – | MET49(3) | HIS164 |
| PRO52(2) | GLN189 | |||||
| TYR54(2) | ASP187 | |||||
| MET165(1) | ||||||
| HIS41(2) | ||||||
| CYS44(1) | ||||||
| ARG188(1) | ||||||
| o-Cymene | – | – | – | – | MET165(3) | PHE181 |
| MET49(2) | HIS164 | |||||
| HIS41(1) | TYR54 | |||||
| ASP187(1) | GLN189 | |||||
| ARG188 | ||||||
| alpha-Pinene | – | – | – | – | PRO168(2) | GLN189 |
| LEU167(1) | ARG188 | |||||
| MET165(4) | THR190 | |||||
| GLU166 | ||||||
| GLN194 | ||||||
| 3-Carene | – | – | – | – | MET165(2) | GLN189 |
| MET49(1) | ARG188 | |||||
| HIS41(1) | ASP187 | |||||
| 0TYR54(1) | GLU166 | |||||
| PRO52(1) | HIS164 | |||||
| CYS44(1) | ||||||
Fig. 3.
Eucalyptol (violet colour ball and stick model) interactions with MPro represented in (A) solid ribbon model with; (B) active site amino acid residues amino acid residues represented in red colour line model; (C) hydrophobic interactions (alkyl and pi-alkyl are shown in light pink colour dotted lines; pi-sigma interactions are shown in dark purple), van der Waals interactions (light green colour circles).
Table 6.
Inhibition constant, energy values of docking simulation of Mpro with compounds.
| Mpro docking with compounds | Inhibition constant (μM) | Binding energy | Ligand efficiency | Intermolecular energy | vdW + Hbond + desolv energy | Electrostatic energy | Torsional energy | Total internal unbound | refRMS |
|---|---|---|---|---|---|---|---|---|---|
| Citronellol | 239.53 | –4.94 | –0.45 | –6.73 | –6.63 | –0.1 | 1.79 | –0.24 | 73.29 |
| Eucalyptol | 50.78 | –5.86 | –0.53 | –5.86 | –5.86 | 0.0 | 0.0 | 0.0 | 73.76 |
| Alpha-terpineol | 105.14 | –5.43 | –0.49 | –6.02 | –5.95 | –0.08 | 0.6 | –0.15 | 71.16 |
| d-Limonene | 159.51 | –5.18 | –0.52 | –5.48 | –5.49 | 0.01 | 0.3 | –0.13 | 75.11 |
| o-Cymene | 219.4 | –4.99 | –0.5 | –5.29 | –5.29 | 0.0 | 0.3 | –0.2 | 74.42 |
| Alpha-pinene | 77.63 | –5.61 | –0.56 | –5.61 | –5.61 | 0.01 | 0.0 | 0.0 | 71.1 |
| 3-Carene | 103.88 | –5.43 | –0.54 | –5.43 | –5.44 | 0.0 | 0.0 | 0.0 | 74.49 |
All energy values are given in Kcal/mol.
Fig. 4.
Alpha-terpineol (violet colour ball and stick model) interactions with MPro represented in (A) solid ribbon model with; (B) active sites ofamino acid residues represented in red colour line model; (c) hydrogen bond (green dotted lines represents), hydrophobic interactions (alkyl and pi-alkyl are shown in light pink colour dotted lines; pi-sigma interactions are shown in dark purple), and van der Waals interactions (light green colour circles). Solvent accessible surfaces of the interacting residues are represented by a blue halo around the residues.
Fig. 5.
d-Limonene (violet colour ball and stick model) interactions with MPro represented in (A) solid ribbon model with; (B) active sitesof amino acid residues represented in red colour line model; (C) hydrophobic interactions (alkyl are shown in light pink colour dotted lines), van der Waals interactions (light green colour circles).
Fig. 6.
o-Cymene (violet colour ball and stick model) interactions with MPro represented in (A) solid ribbon model with; (B) active sites ofamino acid residues represented in red colour line model; (C) hydrophobic interactions (alkyl and pi-alkyl are shown in light pink colour dotted lines; amide-pi stacked interaction are shown in dark pink colour), van der Waals interactions (light green colour circles). Solvent accessible surfaces of the interacting residues are represented by a blue halo around the residues.
Fig. 7.
Alpha-pinene (violet colour ball and stick model) interactions with MPro represented in (A) solid ribbon model with; (B) active sites of amino acid residues represented in red colour line model; (c) hydrophobic interactions (alkyl are shown in light pink colour dotted lines), van der Waals interactions (light green colour circles). Solvent accessible surfaces of the interacting residues are represented by a blue halo around the residues.
Fig. 8.
3-Carene (violet colour ball and stick model) interactions with MPro represented in (A) solid ribbon model with; (B) active sites of amino acid residues represented in red colour line model; (c) hydrophobic interactions (alkyl and pi-alkyl are shown in light pink colour dotted lines), van der Waals interactions (light green colour circles). Solvent accessible surfaces of the interacting residues are represented by a blue halo around the residues.
Discussion
Corona viruses have been included under the set of RNA viruses which infect humans as well as some animals, and the recent outbreak of Coronavirus has been induced by beta-coronavirus. The liver, respiratory, digestive, and mostly the focal sensory systems of humans and animals are the infection sites affected due to COVID-19 [15]. The prime objective for the progress of effective antiviral agents is SARS coronavirus main peptidase (SARS-CoV Mpro) which has a major role in virus as it supports its life cycle in case of COVID-19 infection. The Mpro is been considered as the important and significant protein and also an imperative part which brings about the proteolytic development of the infective virus and also it checks the expanded multiplication of contamination by repressing the cleavage of the viral polyprotein [27]. The finding of the structure of Mpro has provided a major opening to recognize and identify the potent drug against COVID-19. Hence the following study has aimed the main proteases in Coronavirus (3CLpro/Mpro) specifically PDB ID 6LU7, as important target proteins for COVID-19 management and treatment. Herbal medicines are been used worldwide for the curing of wide range of diseases either by consuming it or by taking it in the form of drugs. Several essential oil compounds obtained from plant parts have reported to show anti-viral activities [14]. The inhibitory mechanism of Eucalyptus essential oil has been found to be dependent on the blockage activity of the influenza virus by suppressing the main external proteins. [28]. Among seven compounds taken for the study, six compounds eucalyptol, alpha-pinene, alpha-terpineol, 3-carene, d-limonene, o-cymene present in both E. globulus and C. citrodora in different compositions. The compound eucalyptol found as a major compound in E. globulus (52.47%), whereas in C. citrodora composition was found as 13.54%. The compound citronellol was major component (59.31%) present in C. citrodora. Eucalyptol along with other compounds like alpha-terpinene, gamma-terpinene, alpha-pinene, p-cymene, terpinen-4-ol, alpha-terpineol, thymol, citral obtained from essential oils have shown good potential against (HSV-1) herpes simplex virus type 1 in vitro research, but among all the compounds α-pinene and α-terpineol were slightly superior in their anti-viral activity [29].
The molecular properties including bioavailability and membrane permeability have been linked with many fundamental molecular descriptors like logP (partition coefficient), molecular weight (MW), or number of hydrogen bond acceptors and donors in a molecule. The “rule of five” has been formulated by using these molecular properties [30]. It shows that the majority molecules having fine membrane permeability have molecular weight less than or equal to 500, calculated octanol–water partition coefficient, log P ≤ 5, hydrogen bond donors less than or equal to 5, and acceptors less than or equal to 10 [31]. Hence, Lipinski’s Rule of Five has been used to predict and test the biological availability of properties like absorption, distribution, metabolism, and elimination (ADME) of the main compounds. In the following research work, citronellol, alpha-terpineol, o-cymene, d-limonene, eucalyptol, alpha-pinene, and 3-carene obeyed the Lipinski’s rule of five, as well as the compound possess properties of drug likeliness.
Caco-2 cells are made up from human colon adenocarcinoma and contain various numbers of drug transport pathways along with the intestinal epithelium. The predicted Caco-2 values of the compounds shown compounds have moderate intestinal epithelium absorption [32]. HIA information is the expansion of bioavailability and amalgamation assessed from the proportion of discharge or aggregate discharge in urine, bile, and defecation products [33]. In the following study, the calculated HIA of all compounds was shown to be 100%, which suggests that the compounds can get properly absorbed through the intestinal cell [34]. PPB predicts the unbound amount of drugs that help in the inhibition of drug metabolizing transporters and enzymes, which potentially and specifically precipitate an unfavourable reaction [35]. Mostly the strongly bound chemicals are classified as the compounds with more than 90% of PPB while the weakly bound chemicals are classified as the compound with less than 90% of PPB (https://preadmet.bmdrc.kr/adme-prediction/). The systematic circulation will have less free form of compounds because the predicted value of PPB indicating that all compounds are strongly bound to chemicals except cymene which is moderately bound to chemical. The BBB penetration term finds out whether compounds can move across the blood–brain barrier. Based on the Cbrain/Cbloodratio, all chemicals are categorized within three sections – elevated absorption to CNS where Cbrain/Cbloodvalue will be more than 2.0, medium absorption to CNS where Cbrain/Cblood value is within 2.0–0.1, and low absorption to CNS where Cbrain/Cblood ratio value will be below 0.1 [36]. The following values are vital in the pharmaceutical sphere because CNS-active compounds should bypass through it and inactive CNS compounds should not surpass across it in order to evade off the side effects of CNS. Except eucalyptol, all the compounds value >2.0 is showing high absorption to CNSP-glycoprotein (Pgp) which is due to result of the multi drug resistance (MDR) gene alongwith an ATP-dependent efflux transporter which affects the assimilation, allocation and emission of medically significant drugs [37]. The most important factor of drug discovery is to identify molecules that interact with Pgp transporters, but it is usually confirmed by arduous in vitro and in vivo studies. However, to predict the likeliness to be substrate for Pgp and the examining of molecules, it can be done through computational classification [38]. From the following study, alpha-terpineol, limonene, and alpha-pinene were found to inhibit Pgp. Cytochrome P450 (CYP) which was brought out by the administered drugs that can direct to unwanted effects involved with increase in plasma concentrations or considerable in efficiency of drug [39]. All compounds found to be an inhibitor of CYP_2C9 and non-inhibitor of CYP_2D6. O-Cymene and eucalyptol inhibits CYP_3A4, whereas other compounds fond to be a non-inhibitor. The carcinogenicity of the compounds were evaluated and determined by PreADMET server (https://preadmet.bmdrc.kr/). From the AMES test, all the seven compounds were found to be mutagen and eucalyptol was found to be non-carcinogenic in rat and mouse models. The toxicity hazard and the bioavailability of citronellol, alpha-terpineol, eucalyptol, d-limonene, 3-carene, o-cymene, and alpha-pinene were predicted based on their ADMET profile. The compound eucalyptol possesses good bioavailability, BBB penetration, and inhibitor of CYP_2C9 and CYP_3A4 and non-carcinogenic in rat and mouse models which the major constituent in the leaves of E. globulus essential oil.
The inhibition of targeted and main enzyme is caused by knowing the binding affinity and bond formation (hydrogen and hydrophobic) of the most potent molecule. From docking analysis, the compound eucalyptol utilizes slightest binding energy of –5.86 k calorie/mole with Mpro than other compounds. The compounds citronellol and alpha-terpineol form two hydrogen bond interactions with GLU166. Hydrophobic amino acids HIS41, MET165, MET49, and PRO52 residues were found to be key residues that aid in the development of hydrophobic interactions with majority of the compounds. The molecular docking study in this paper showed the inhibition capability of compounds from Eucalyptus species and ranked by binding energy (ΔG), eucalyptol > alpha-pinene > alpha-terpineol > 3-carene > d-limonene > o-cymene > citronellol. The results of this study were consistent with that of Belhassan et al. (2020), who performed molecular docking analysis of the SARS-Cov-2 main protease with oseltamivir derivatives. Hence, the volatile compounds against COVID-9 may work as potent inhibitors of the virus replication and transcription process by inhibiting Mpro, which is a novel CoV enzyme making these compounds as an important drug target and objective for COVID-19 virus [40]. However, more number of experimental works and studies are required to validate the efficiency of these lead molecules present in E. globulus and C. citrodora.
Conclusion
Currently, the search for proper medication and vaccine for COVID-19 is in progress. The use of Eucalyptus and Corymbia species essential oil and the main compounds such as eucalyptol and citronellol has shown positive antiviral action in various research studies. We utilized the recently published 3D structure of Mpro structure from SARS-CoV-2 to predict molecular interaction analysis of volatile compounds from E. globulus and C. citrodora using Autodock 4.2 software. The docking results attributed various kinds of binding interaction of the drug with Mpro among which some interactions are said to be favourable. Hence, we have suggested that the two species essential oils, and their bioactive compounds mainly eucalyptol may act as possible inhibitor of Mpro, and also further research is essential to explore the possible uses of the Eucalyptus and Corymbia essential oils and their compounds.
Funding
This work was supported by TNSCSCT/DST, reference number TNSCSCT/DST-PRG/TD-AWE/VR/06/2017/3033. This project was supported by Researchers Supporting Project number (RSP-2020/257) King Saud University, Riyadh, Saudi Arabia. This work was supported by the Soonchunhyang University Research Fund.
Competing interests
None declared.
Ethical approval
Not required.
Acknowledgements
This work was supported by TNSCSCT/DST, reference number TNSCSCT/DST-PRG/TD-AWE/VR/06/2017/3033. This project was supported by Researchers Supporting Project number (RSP-2020/257) King Saud University, Riyadh, Saudi Arabia. This work was supported by the Soonchunhyang University Research Fund.
References
- 1.Indranil C., Prasenjit M. COVID-19 outbreak: migration, effects on society, global environment and prevention. Sci Total Environ. 2020;728:138882. doi: 10.1016/j.scitotenv.2020.138882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization . 2020. Clinical Management of Severe Acute Respiratory Infection When Novel Coronavirus (2019-nCoV) Infection Is Suspected: Interim Guidance. [Google Scholar]
- 3.Gorbalenya A.E., Baker S.C., Baric R.S., Groot R.J., Drosten C., Gulyaeva A.A. The species severe acute respiratory syndrome-related coronavirus: classifying 2019-n CoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jansi R.S., Khusro A., Agastian P., Alfarhan A., Al-Dhabi N.A., Arasu M.V. Emerging paradigms of viral diseases and paramount role of natural resources as antiviral agents. Sci Total Environ. 2021;759:143539. doi: 10.1016/j.scitotenv.2020.143539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Indu Purushothaman, Rameshkumar MarimuthuRagavan, Arunagirinathan Narasingam, Al-Dhabi NaifAbdullah, Arasu MariadhasValan, Ignacimuthu Savarimuthu. Raltegravir, Indinavir, Tipranavir, Dolutegravir, and Etravirine against main protease and RNA-dependent RNA polymerase of SARS-CoV-2: a molecular docking and drug repurposing approach. J Infect Public Health. 2020;13(12):1856–1861. doi: 10.1016/j.jiph.2020.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu X., Wang X.J. Potential inhibitors against 2019-nCoV coronavirus M protease from clinically approved medicine. J Genet Genom. 2020;47(2):119–121. doi: 10.1016/j.jgg.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chenguang S., Zhaoqin W., Fang Z., Yang Y., Jinxiu L., Jing Y. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. J Amer Med Assoc. 2020;323(16):1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu H. Drug treatment options for the 2019-new coronavirus (2019-nCoV) Biosci Trends. 2020;14(1):69–71. doi: 10.5582/bst.2020.01020. [DOI] [PubMed] [Google Scholar]
- 9.Siti K., Hendra K., Rizki A., Suhartati S., Soetjipto S. Potential inhibitor of COVID-19 main protease (Mpro) from several medicinal plant compounds by molecular docking study. Med Pharmacology, Pharmacology & Toxicology. 2020 doi: 10.20944/preprints202003.0226.v1. [DOI] [Google Scholar]
- 10.Zakaryan H., Arabyan E., Oo A., Zandi K. Flavonoids: promising natural compounds against viral infections. Arch Virol. 2017;162(9):2539–2551. doi: 10.1007/s00705-017-3417-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meghana K., Smitha L., Pooja R., Preethi T., Suresh R. A comparative study to assess the effect of steam inhalation v/s Tulsi leaves inhalation on the sign and symptoms of cold and cough among adult group in selected areas of Pune city. Int J Med Res. 2017;2(2):24–26. [Google Scholar]
- 12.Harkenthal M., Reichling J., Geiss H.K., Saller R. Comparative study on the in vitro antibacterial activity of Australian tea tree oil, cajuput oil, niaouli oil, manuka oil, kanuka oil, and Eucalyptus oil. Pharmazie. 1999;54:460–463. PMID: 10399193. [PubMed] [Google Scholar]
- 13.Salari M.H., Amine G., Shirazi M.H., Hafezi R., Mohammadypour M. Antibacterial effects of Eucalyptus globulus leaf extract on pathogenic bacteria isolated from specimens of patients with respiratory tract disorders. Clin Microbiol Infect. 2006;12:194–196. doi: 10.1111/j.1469-0691.2005.01284.x. [DOI] [PubMed] [Google Scholar]
- 14.Claudio C., Anna F., Giuliana F., Paola Q. Effect of Eucalyptus essential oil on respiratory bacteria and viruses. Curr Microbiol. 2008;56:89–92. doi: 10.1007/s00284-007-9045-0. [DOI] [PubMed] [Google Scholar]
- 15.Fabio A., Cermelli C., Fabio G., Nicoletti P., Quaglio P. Screening of the antibacterial effects of a variety of essential oils on microorganisms responsible for respiratory infections. Phytother Res. 2007;21:374–377. doi: 10.1002/ptr.1968. [DOI] [PubMed] [Google Scholar]
- 16.Heshman H.A.R., Abdurahman H.N., Rosli M.Y. Techniques for extraction of essential oils from plants: a review. Aust J Basic Appl Sci. 2016;10(16):117–127. [Google Scholar]
- 17.Dey P.M., Harborne J.B. 1st edition. Academic Press; London: 1997. Plant biochemistry. [Google Scholar]
- 18.Derwich E., Benziane Z., Taouil R. GC/MS analysis of volatile compounds of the essential oil of the leaves of Mentha pulegium growing in Morocco. Chem Bull "POLITEHNICA" Univ (Timisoara) 2010;55(69) [Google Scholar]
- 19.Pala Paul J., Perez Alonso M.J., Velasco Negueruela V., Ramos Vazquez P., Gomez Contreras F., Sanz J. Essential oil of Santolina rosmarinifolia L. ssp. rosmarinifolia: first isolation of capillene, a diacetylene derivative. Flavour Frag J. 1999;14(2):131–134. [Google Scholar]
- 20.Berman H.M., Westbrook J., Feng Z., Gilliland G., Bhat T.N., Weissig H. The protein data bank. Nucleic Acids Res. 2000;28:235–242. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin Z., Du X., Xu Y., Deng Y., Liu M., Zhao Y. Structure of Mpro from COVID-19 virus and discovery of its inhibitors. Nature. 2020;582:289–293. doi: 10.1038/s41586-020-2223-y. [DOI] [PubMed] [Google Scholar]
- 22.Boyle O.N.M., Banck M., James C.A., Morley C., Vandermeersch T. Open babel: an open chemical toolbox. J Cheminform. 2011;33 doi: 10.1186/1758-2946-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Atilgan E., Hu J. Improving protein docking using sustainable genetic algorithms. Int J Comput Inform Syst Ind Manag Appl. 2011;3:248–255. [Google Scholar]
- 24.Morris G.M., Ruth H., Lindstrom W., Belew R.K., Goodsell D.S., Olson A.J. Software news and updates AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem. 2009;30:2785–2791. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu J., Zhao S., Teng T., Abdalla A.E., Zhu W. Systematic comparison of two animal-to-human transmitted human coronaviruses: SARS-CoV-2 and SARS Co. Viruses. 2020;12(2):E244. doi: 10.3390/v12020244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu R.X., Zhang B., Jin Z., Yang H.Z. The crystal structure of 2019-nCoV main protease in complex with an inhibitor N3. Nature. 2020;582 doi: 10.2210/pdb6LU7/pdb. http://wwwrcsborg/structure/6LU7 Available from: [DOI] [Google Scholar]
- 27.Akram A., Jurgen R., Paul S. Screening for antiviral activities of isolated compounds from essential oils. Evid Based Compl Altern Med. 2011 doi: 10.1093/ecam/nep187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Panyod S., Ho C.T., Sheen L.Y. Dietary theraphy and herbal medicine for COVID-19 prevention: a review and prespective. J Tradit Complement Med. 2020;10(4):420–427. doi: 10.1016/j.jtcme.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oleg V.P., Evgeny V.U., Olga P., Igor E.A. Inactivation of airborne influenza virus by tea tree and Eucalyptus oils. Aerosol Sci Technol. 2012;462(12):1295–1302. doi: 10.1080/02786826.2012.708948. [DOI] [Google Scholar]
- 30.Lipinski C.A., Lombardo F., Dominy B.W., Feeney P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2001;46(1–3):3–26. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 31.Muegge I. Selection criteria for drug-like compounds. Med Res Rev. 2003;23(3):302–321. doi: 10.1002/med.10041. [DOI] [PubMed] [Google Scholar]
- 32.Yazdanian M., Glynn S.L., Wright J.L., Hawi A. Correlating partitioning and Caco-2 cell permeability of structurally diverse small molecular weight compounds. Pharm Res. 1998;15(9):1490–1494. doi: 10.1023/a:1011930411574. [DOI] [PubMed] [Google Scholar]
- 33.Zhao Y.H., Le J., Abraham M.H., Eddershaw P.J., Luscombe C.N., Butina D. Evaluation of human intestinal absorption data and subsequent derivation of a quantitative structure-activity relationship (QSAR) with the Abraham descriptors. J Pharm Sci. 2001;90(6):749–784. doi: 10.1002/jps.1031. [DOI] [PubMed] [Google Scholar]
- 34.Yee S. In vitro permeability across Caco-2 cells (colonic) can predict in vivo (small intestinal) absorption in man—fact or myth. Pharm Res. 1999;14(6):763–766. doi: 10.1023/a:1012102522787. [DOI] [PubMed] [Google Scholar]
- 35.Bohnert T., Gan L.S. Plasma protein binding: from discovery to development. J Pharm Sci. 2013;102(9):2953–2994. doi: 10.1002/jps.23614. [DOI] [PubMed] [Google Scholar]
- 36.Chen C., Yang J. Predictive model of blood-brain barrier penetration of organic compounds. Acta Pharmacol Sin. 2005;26(4):500–512. doi: 10.1111/j.1745-7254.2005.00068.x. [DOI] [PubMed] [Google Scholar]
- 37.Schinkel A.H. P-Glycoprotein, a gatekeeper in the blood–brain barrier. Adv Drug Deliv Rev. 1999;36(2):179–194. doi: 10.1016/s0169-409x(98)00085-4. [DOI] [PubMed] [Google Scholar]
- 38.Joung J.Y., Kim H.J., Kim H.J.M., Ahn S.K., Nam K.Y., No K.T. Prediction models of p-glycoprotein substrates using simple 2d and 3d descriptors by a recursive partitioning approach. Bull Korean Chem Soc. 2012;33(4):1123–1127. [Google Scholar]
- 39.Backman J.T., Wang J.S., Wen X., Kivisto K.T., Neuvonen P.J. Mibefradil but not isradipine substantially elevates the plasma concentrations of the CYP3A4 substrate triazolam. Clin Pharmacol Ther. 1999;66(4):401–407. doi: 10.1053/cp.1999.v66.a101461. [DOI] [PubMed] [Google Scholar]
- 40.Belhassan A., Chtita S., Zaki H., Lakhlifi T., Bouachrine M. Molecular docking analysis of N-substituted oseltamivir derivatives with the SARS-Cov-2 main protease. Bioinformation. 2020;16(5):404–410. doi: 10.6026/97320630016404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brito R.G., Guimarães A.G., Quintans J.S., Santos M.R., De Sousa D.P., Badaue-Passos D., Jr. Citronellol, a monoterpene alcohol, reduces nociceptive and inflammatory activities in rodents. J Nat Med. 2012;66(October (4)):637–644. doi: 10.1007/s11418-012-0632-4. Epub 2012 February 21. PMID: 22350215. [DOI] [PubMed] [Google Scholar]
- 42.Selvarani V., James H. American anti-influenza virus activity of essential oils and vapors. J Essent Oils Nat Prod. 2014;2(1):47–53. [Google Scholar]
- 43.Park S.N., Lim Y.K., Freire M.O., Cho E., Jin D., Kook J.K. Antimicrobial effect of linalool and α-terpineol against periodontopathic and cariogenic bacteria. Anaerobe. 2012;18(June (3)):369–372. doi: 10.1016/j.anaerobe.2012.04.001. Epub 2012 April 17. PMID: 22537719. [DOI] [PubMed] [Google Scholar]
- 44.Astani A., Reichling J., Schnitzler P. Comparative study on the antiviral activity of selected monoterpenes derived from essential oils. Phytother Res. 2010;24(May (5)):673–679. doi: 10.1002/ptr.2955. PMID: 19653195; PMCID: PMC7167768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marchese A., Arciola C.R., Barbieri R., Silva A.S., Nabavi S.F., Sokeng A.J.T. Update on monoterpenes as antimicrobial agents: a particular focus on p-cymene. Materials (Basel) 2017;10(8):947. doi: 10.3390/ma10080947. Published 2017 August 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gavanji S., Sayedipour S.S., Larki B., Bakhtari A. Antiviral activity of some plant oils against herpes simplex virus type 1 in vero cell culture. J Acute Med. 2015;5:62–68. [Google Scholar]
- 47.Astani A., Schnitzler P. Antiviral activity of monoterpenes beta-pinene and limonene against herpes simplex virus in vitro. Iran J Microbiol. 2014;6(3):149–155. [PMC free article] [PubMed] [Google Scholar]
- 48.Asif M., Saleem M., Saadullah M., Yaseen H.S., Zarzour R.A. COVID-19 and therapy with essential oils having antiviral, anti-inflammatory, and immunomodulatory properties. Inflammopharmacol. 2020;28:1153–1161. doi: 10.1007/s10787-020-00744-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Juergens U.R., Dethlefsen U., Steinkamp G., Gillissen A., Repges R., Vetter H. Anti-inflammatory activity of 1.8-cineol (eucalyptol) in bronchial asthma: a double-blind placebo-controlled trial. Respir Med. 2003;97(March (3)):250–256. doi: 10.1053/rmed.2003.1432. PMID: 12645832. [DOI] [PubMed] [Google Scholar]
- 50.Morcia C., Malnati M., Terzi V. In vitro antifungal activity of terpinen-4-ol, eugenol, carvone, 1,8-cineole (eucalyptol) and thymol against mycotoxigenic plant pathogens. Food Addit Contam A Chem Anal Control Expo Risk Assess. 2012;29(3):415–422. doi: 10.1080/19440049.2011.643458. Epub 2012 January 19. PMID: 22257275. [DOI] [PubMed] [Google Scholar]