More than 5% of women treated for breast cancer with anthracyclines develop cardiotoxicity1. The mechanism of injury is not fully understood, but mitochondrial damage may play a causal role. Early detection of myocardial damage due to anthracyclines is an important but unrealized objective. Hyperpolarized (HP) 13C MR spectroscopy detects fluxes through reactions essential for normal energy metabolism including pyruvate dehydrogenase (PDH) and lactate dehydrogenase (LDH)2. This study tested the hypothesis that exams of cardiac metabolism using HP [1-13C1]pyruvate are feasible before and after conventional neoadjuvant doxorubicin chemotherapy in women with breast cancer, and that production of HP [13C]bicarbonate or HP [1-13C1]lactate may be sensitive to chemotherapy.
The study (clinicaltrials.gov/ct2/show/NCT03685175) was approved by the relevant institutional committees (STU 072016-058), under an Investigational New Drug approval (133229). All patients provided written informed consent, were > 18 years old, without diabetes, with biopsy-proven breast cancer requiring neoadjuvant doxorubicin (cumulative 240mg/m2). Patients with metastatic lesions, significant kidney, liver, cardiovascular or pulmonary disease and MR safety restrictions were excluded. Participants continued their ordinary nutrition, activity and medications and received standard care for breast cancer including screening with echocardiography.
Experimental procedures were similar to recently published exams in human subjects 3-5. Following an overnight fast, participants arrived for study at 9:00 AM, and thirty minutes prior to the exam ingested 48 grams of glucose. Sterile [1-13C1]pyruvate was prepared in a laminar flow hood by a licensed pharmacist. Polarization and quality assurance testing were performed using a SPINlab™ (GE Healthcare). Prior to injection, the solution passed through a 0.22μm filter. MR studies were performed on a wide-bore 3T clinical scanner (GE Discovery 750w). The positioning of the 13C coils, a transmit Helmholtz and 8-channel receive array, was determined by participant comfort and body habitus with one or both coils anterior to the heart (Figure). 13C data were acquired from a 10-cm long-axis slice ECG-triggered in mid diastole. The excitation RF pulse was 10° every 2.8-3.8 seconds for 80 timepoints, total 3.7-5 minutes. 13C data were reconstructed and analyzed using MATLAB. Data from each coil were weighted according to the distance from the center of the LV cavity based on fiducials in a 1H image acquired with the body coil. HP 13C spectra were averaged over 90 seconds from injection for peak quantification and normalized to the total 13C signal (TC). Second study was completed 11 ± 0.5 days after doxorubicin. Data analysis was completed in a blinded fashion, using objective measurement criteria. Statistical significance was evaluated using a paired t-test (α = 0.05, two-tailed analysis). The sample size was 9 except echocardiography (n = 8) due to missing post-doxorubicin test from Patient #4. Data are presented as mean ± standard error.
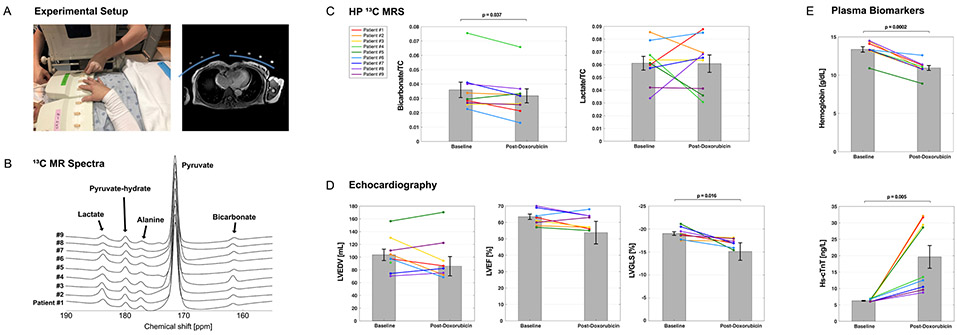
Figure. Experimental setup, hyperpolarized 13C NMR spectra, and clinical results.
(A) Positioning of 8-channel paddle radiofrequency (RF) array receive coils is shown, along with an axial 1H MRI with position of the 8-channel paddle array coils (blue arcs), with fiducial markers indicating the approximate location of each loop. (B) 13C MR spectra from each participant, acquired over approximately 90 seconds from a bolus injection of hyperpolarized [1-13C1]pyruvate is shown. (C) There was a significant decrease in [13C]bicarbonate but not [1-13C1]lactate after doxorubicin treatment, normalized to the total 13C signal (TC). (D) There was no significant change in left ventricular end-diastolic volume (LVEDV) or left ventricular ejection fraction (LVEF) measured by echocardiography, but left ventricular global longitudinal strain (LVGLS) deteriorated after therapy. (E) Compared to pre-treatment baseline, plasma hemoglobin decreased and high sensitivity troponin (hs-cTnT) was slightly above the upper limit of normal. The error bars denote standard error of the mean for a sample size of 9 for panels C and E, and 8 for panel D.
Ten patients were enrolled. One patient discontinued because of technical issues related to polarization. Of the nine participants (age 47 ± 5 years, 3 Black, 6 non-Hispanic white) who completed exams before and after therapy, none developed congestive heart failure. After therapy, patients had small but significant changes in hemoglobin, high-sensitivity troponin (hs-cTnT) and peak left ventricular global longitudinal strain (LVGLS) (Figure). All participants tolerated the HP exam well, without any adverse effects. Baseline 13C NMR spectra were similar for all participants. The fraction of [13C]bicarbonate, [1-13C1]lactate, and [1-13C1]alanine, relative to TC was 0.036 ± 0.005, 0.448 ± 0.023, and 0.045 ± 0.004, respectively. After 4 cycles of doxorubicin and cyclophosphamide, there was a small decrease in HP [13C]bicarbonate relative to TC (bicarbonate/TC = 0.032 ± 0.005, p = 0.037) but no significant change in HP [1-13C1]lactate (lactate/TC = 0.44 ± 0.04, p = 0.9) or [1-13C1]alanine (alanine/TC = 0.045 ± 0.006, p = 0.9). To examine the reproducibility of HP data acquisition during the same session, five patients before chemotherapy and two patients after chemotherapy had two injections of HP [1-13C1]pyruvate separated by 30 min. Total signal at the first and second exam in a single session for [13C]bicarbonate/TC (0.037 ± 0.007; 0.038 ± 0.007), [1-13C1]lactate/TC (0.061 ± 0.005 vs. 0.062 ± 0.006), and [1-13C1]alanine/TC (0.051 ± 0.005 vs. 0.050 ± 0.005) were not different.
In conclusion, myocardial HP 13C spectra acquired from patients with breast cancer were sensitive to cardiotoxic chemotherapy. Data were reproducible within the same visit, and serial exams are feasible and well-tolerated by patients. Doxorubicin was associated with a decrease in HP [13C]bicarbonate/TC, consistent with subtle mitochondrial injury. Other biomarkers such as hemoglobin, hs-cTnT and LVGLS (Figure) also changed in association with chemotherapy. Direct comparison to [18F]fluorodeoxyglucose imaging with positron emission tomography and image-based localization of the [13C]bicarbonate signal is desirable. The clinical relevance of HP methods awaits further evaluation.
Acknowledgments
SOURCES OF FUNDING
The National Institute of Health (S10OD018468, S10RR029119, P41EB015908, R01NS107409), the Welch Foundation (I-2009-20190330), The Texas Institute for Brain Injury and Repair, The Cancer Prevention and Research Institute of Texas (RP180404 and RP170003) and a donation from the Ben E. Keith Foundation.
Footnotes
Clinical Trial Registration: NCT03685175
DATA AND MATERIALS AVAILABILITY
Datasets are available from the corresponding author on reasonable request, reviewed by the Institutional Review Board and the Data Safety Monitoring Committee of the Harold C. Simmons Comprehensive Cancer Center, University of Texas Southwestern Medical Center.
DISCLOSURE
G.D.R. is an employee of GE Healthcare.
REFERENCES
- 1.Mehta LS, Watson KE, Barac A, et al. Cardiovascular Disease and Breast Cancer: Where These Entities Intersect: A Scientific Statement From the American Heart Association. Circulation. 2018;137:e30–e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Merritt ME, Harrison C, Storey C, Jeffrey FM, Sherry AD and Malloy CR. Hyperpolarized 13C allows a direct measure of flux through a single enzyme-catalyzed step by NMR. Proceedings of the National Academy of Sciences. 2007;104:19773–19777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cunningham CH, Lau JY, Chen AP, Geraghty BJ, Perks WJ, Roifman I, Wright GA, Connelly KA. Hyperpolarized 13C Metabolic MRI of the Human Heart: Initial Experience. Circ Res. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gallagher FA, Woitek R, McLean MA, et al. Imaging breast cancer using hyperpolarized carbon-13 MRI. Proc Natl Acad Sci U S A. 2020;117:2092–2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rider OJ, Apps A, Miller J, et al. Noninvasive In Vivo Assessment of Cardiac Metabolism in the Healthy and Diabetic Human Heart Using Hyperpolarized (13)C MRI. Circ Res. 2020;126:725–736. [DOI] [PMC free article] [PubMed] [Google Scholar]