Abstract
Objective
To report a patient with history of recurrent Bell’s Palsy who developed Bell’s Palsy 36 h after the administration of the second dose of the Pfizer-BioNTech COVID-19 vaccine.
Case
The patient is a 57-year-old female with past medical history of 3 episodes of Bell’s Palsy. She responded to prednisone treatment and returned to her baseline after each occurrence. Less than 36 h following the second dose of the vaccine, the patient developed a left Bell’s Palsy. The facial droop progressed in severity over the next 72 h.
Conclusion
Given the expedited production of the vaccine and the novelty associated with its production, there may be information pertaining to side effects and individual response that remain to be discovered. Since both the Moderna and Pfizer Vaccine trials reported Bell’s Palsy as medically attended adverse events, the association between vaccine administration and onset of symptomatic Bell’s Palsy may warrant further investigation.
1. Introduction
With medical and technological advancements, the ability to produce expedited, approved vaccines is now a reality as evidenced by the production of the COVID-19 Vaccines. Advancements in computational biology, protein engineering, and gene synthesis along with new manufacturing platforms have allowed for the production of vaccines with speed and precision (Graham, 2020). In addition to the known minor risks associated with vaccine administration (Spencer et al., 2017), some consideration may need to be given to potential novel side effect profile with expedited vaccine production.
In both the Pfizer-BioNTech COVID-19 Vaccine and Moderna COVID-19 Vaccine clinical trials, incidents of Bell’s Palsy were cited as medically attended adverse events (MAAE). In the Pfizer-BioNTech COVID-19 Vaccine trials, Bell’s Palsy was reported by 4 vaccine participants. From Dose 1 through 1 month after Dose 2, there were 3 reports of Bell’s Palsy in the vaccine group and none in the placebo group. This observed frequency of reported event is consistent with the expected background rate in the general population, which suggests that a causal relationship to the vaccine cannot be made (Pfizer-BioechD-19, 2020). In the Moderna trials, 3 vaccine recipients and 1 placebo recipient reported this MAAE. Considering the temporal association and biological plausibility, FDA recommends surveillance for cases of Bell’s Palsy with deployment of the Moderna COVID-19 Vaccine into larger populations (ModernaD-19 Vaccine, 2020). Such recommendation is not associated with the Pfizer vaccine.
Bell’s Palsy, an idiopathic Cranial Nerve 7 Palsy, occurs in 12–25 per 100,000 people in the general population. The association between vaccine administration and onset of Bell’s Palsy symptoms have been previously documented with the inactivated Influenza Vaccine (Zhou et al., 2004; Mutsch et al., 2004). So far, there has been no reporting of Bell’s Palsy incident in literature since the deployment of both of the COVID-19 Vaccines into the general population. We hereby report a patient who had three previous episodes of Bell’s Palsy and developed left sided Bell’s Palsy less than 36 h after the second dose of the Pfizer-BioNTech COVID-19 Vaccine.
2. Case
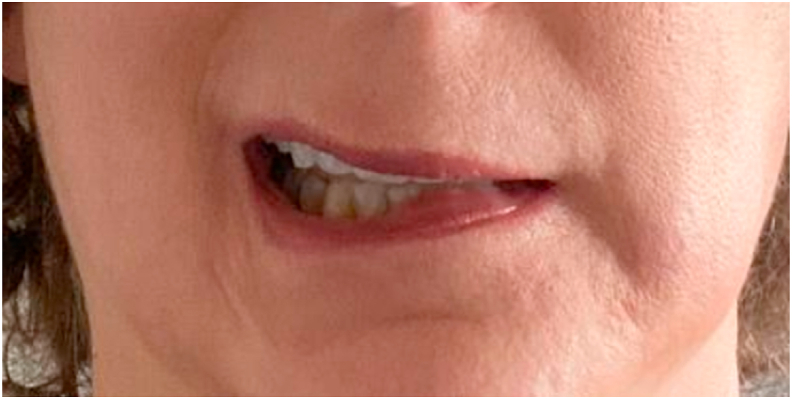
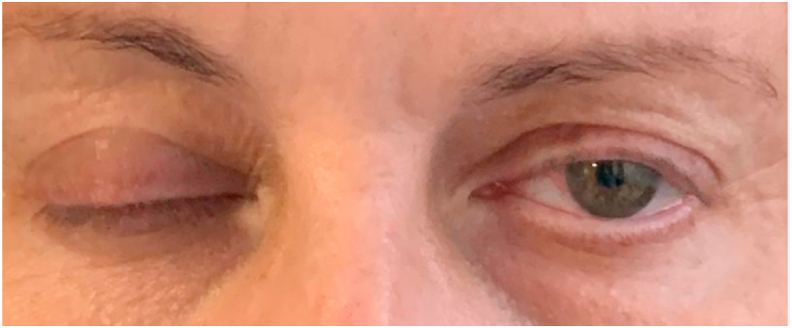
The patient is a 57-year-old Caucasian female with a past medical history of hypertension and Bell’s Palsy. Her hypertension was a consequence of prolonged corticosteroid administration for the treatment of multiple episodes of Bell’s Palsy. She had a total of three episodes of Bell’s Palsy prior to this report. Her first episode was in 2003 (right face), second episode in 2013 (left face) and the third one was in 2018 (right face). She responded to prednisone treatment and completely retuned to baseline after each episode. She had a negative head MRI in 2018. Of note, both sides of the face have been affected twice in previous and current episodes. She never had bilateral facial involvement at the same time. She does not smoke and her only medication is Losartan 50 mg daily. She has not been tested positive for SARS-COVID-2 PCR or antibodies, and she received her first dose of Pfizer COVID-19 vaccination at 11 a.m. December 17th, 2020. Patient reported injection site soreness as the sole complaint. On January 5th, 2021, patient received her 2nd Pfizer-BioNTech COVID-19 vaccination at 10 a.m. with minimal complaint for the rest of the day. On the 6th, patient developed general malaise and a maximum temperature of 99.1 F. Less than 36 h following the second dose of the vaccine, the patient noticed loss of taste sensation in addition to soreness behind her left ear above her jaw and discovered that she developed a facial droop as well as the inability to close her left eye completely. Examination on January 8th revealed an otherwise normal female without distress. She was afebrile and vital signs were stable. Her weight is 190 pounds. She was oriented and coherent. Cranial Nerve (CN) examination was significant for isolated left CN 7 palsy (Fig. 1, Fig. 2). There was left hyperacusis, which was also present back in 2018. On the 8th, ageusia has resolved. There was no oro-facial edema or tongue furrowing this time nor in 2018. Her motor, sensory, and cerebellar examination were normal. She walked with normal gait. Prednisone and an antiviral agent were started promptly. Besides the one time that her temperature reached 99.1F, she has always been afebrile. Her symptoms began to improve by Day 14.
Fig. 1.
Patient with a left facial droop.
Fig. 2.
Patient unable to close her left eye.
3. Discussion
With the dissemination of the COVID-19 Vaccine to the general population, it will be important to monitor individual response to determine side effect profile and any contraindications that may not have been previously elucidated. The Center for Disease Control and Prevention (CDC) recently updated guidelines for contraindication to the vaccine to include anyone with a history of allergic reaction to polysorbate or polyethylene glycol (The Center for Disease Co, 2021). In this same report, the CDC stated that post-authorization safety surveillance will be important to monitor Bell’s Palsy symptoms, in patients with prior episodes, following vaccine administration to further assess any potential causal association. As more of the population becomes vaccinated, more information will likely be validated. Given these considerations, the CDC recommends it is important to monitor response and report findings to the Vaccine Adverse Event Reporting System (VAERS).
The current patient’s response to the Pfizer-BioNTech COVID-19 Vaccine warrants some attention. With previous association found between the administration of the inactivated Influenza Vaccine and onset of Bell’s Palsy symptoms, there remains the possibility of a causal association between these symptoms and the COVID-19 vaccine. The Pfizer data did not indicate if any of the 4 patients in their trial had previous history of Bell’s Palsy or the time frame of these occurrences. Our patient does not have the triad of Melkersson-Rosenthal Syndrome. Generally, no particular etiologies have been identified for people with recurrent Bell’s Palsy (Dong et al., 2019). In our patient, although Bell’s Palsy recurrence took place within 36 h after the second dose of Pfizer COVID-19 vaccination, proof of association as well as exact pathophysiology await further investigation. Whether history of Bell’s Palsy predisposes a person to a higher chance of recurrence after COVID-19 vaccination is also to be determined.
The patient has read this draft and given us consent to submit for publication. The incident has also been reported by the vaccine administration site to the Vaccine Adverse Event Reporting System (VAERS) per FDA requirement.
Declaration
The authors:
Michael Repajic, Xue Lei Lai, Prissilla Xu, and Antonio Liu have no financial or any other interest to declare.
The patient has read this draft (including the picture/photo) and has given us consent to submit for publication. The incident has also been reported by the vaccine administration site to the Vaccine Adverse Event Reporting System (VAERS) per FDA requirement.
References
- Dong S., Jung A., Jung J. Recurrent Bell’s palsy. Clin. Otolaryngol. 2019 May;44(3):305–312. doi: 10.1111/coa.13293. [DOI] [PubMed] [Google Scholar]
- Graham B.S. Rapid COVID-19 vaccine development. Science. 2020 May 29;368(6494):945–946. doi: 10.1126/science.abb8923. Epub 2020 May 8. PMID: 32385100. [DOI] [PubMed] [Google Scholar]
- Moderna COVID-19 vaccine vaccines and related biological products advisory committee meeting (VRBPAC) Briefing Document December 17, 2020.
- Mutsch M., Zhou W., Rhodes P., Bopp M., Chen R.T., Linder T., Spyr C., Steffen R. Use of the inactivated intranasal influenza vaccine and the risk of Bell’s palsy in Switzerland. N. Engl. J. Med. 2004 Feb 26;350(9):896–903. doi: 10.1056/NEJMoa030595. PMID: 14985487. [DOI] [PubMed] [Google Scholar]
- Pfizer-BioNTech COVID-19 vaccine vaccines and related biological products advisory committee meeting (VRBPAC) Briefing Document December 10, 2020.
- Spencer J.P., Trondsen Pawlowski R.H., Thomas S. Vaccine adverse events: separating myth from reality. Am. Fam. Physician. 2017 Jun 15;95(12):786–794. PMID: 28671426. [PubMed] [Google Scholar]
- The Center for Disease Control and Prevention: Interim Clinical Considerations for Use of mRNA COVID-19 Vaccines Currently Authorized in the United States January vol. 6, 2021.
- Zhou W., Pool V., DeStefano F., Iskander J.K., Haber P., Chen R.T., VAERS Working Group A potential signal of Bell’s palsy after parenteral inactivated influenza vaccines: reports to the Vaccine Adverse Event Reporting System (VAERS)--United States, 1991-2001. Pharmacoepidemiol. Drug Saf. 2004 Aug;13(8):505–510. doi: 10.1002/pds.998. PMID: 15317028. [DOI] [PubMed] [Google Scholar]