Abstract
Ischemic stroke is a highly complex and devastating neurological disease. The sudden loss of blood flow to a brain region due to an ischemic insult leads to severe damage to that area resulting in the formation of an infarcted tissue, also known as the ischemic core. This is surrounded by the peri-infarct region or penumbra that denotes the functionally impaired but potentially salvageable tissue. Thus, the penumbral tissue is the main target for the development of neuroprotective strategies to minimize the extent of ischemic brain damage by timely therapeutic intervention. Given the limitations of reperfusion therapies with recombinant tissue plasminogen activator or mechanical thrombectomy, there is high enthusiasm to combine reperfusion therapy with neuroprotective strategies to further reduce the progression of ischemic brain injury. Till date, a large number of candidate neuroprotective drugs have been identified as potential therapies based on highly promising results from studies in rodent ischemic stroke models. However, none of these interventions have shown therapeutic benefits in stroke patients in clinical trials. In this review article, we discussed the urgent need to utilize preclinical models of ischemic stroke that more accurately mimic the clinical conditions in stroke patients by incorporating aged animals and animal stroke models with comorbidities. We also outlined the recent findings that highlight the significant differences in stroke outcome between young and aged animals, and how major comorbid conditions such as hypertension, diabetes, obesity and hyperlipidemia dramatically increase the vulnerability of the brain to ischemic damage that eventually results in worse functional outcomes. It is evident from these earlier studies that including animal models of aging and comorbidities during the early stages of drug development could facilitate the identification of neuroprotective strategies with high likelihood of success in stroke clinical trials.
Keywords: Aging, Comorbidity, Diabetes, Hyperlipidemia, Hypertension, Inflammation, Ischemic stroke, Neuroprotection, Obesity, Stroke models
1. Introduction
Stroke is defined as a sudden disruption in blood flow to the brain due to either occlusion (ischemic stroke) or rupture (hemorrhagic stroke) of a cerebral blood vessel. Transient ischemic attacks (TIA) or mini-strokes occur when blood supply to the brain is interrupted only briefly, and they are usually a warning of a full-blown stroke. Ischemic stroke or focal cerebral ischemia is mainly caused by either a thrombus, blood clot formed in a major brain artery, or by an embolus, blood clot formed outside of the brain, most commonly in the carotid circulation. These emboli formed in the periphery travel to the brain and can lodge in a major cerebral artery or in a penetrating arteriole. Blood clot formation is precipitated by atherosclerosis and atrial fibrillation, which are major risk factors for ischemic stroke (Elkind, 2006; Virani et al., 2020).
Interruption of cerebral blood flow dramatically impairs energy production resulting in the collapse of ionic homeostasis and excessive release of the neurotransmitter glutamate, which in turn leads to neuronal cell death and the development of a cerebral infarct. Focal ischemic stroke is characterized by an infarcted core, where cell death occurs within minutes after arterial occlusion and brain tissue in this region is generally considered unsalvageable. The peri-infarct region surrounding the ischemic core is termed penumbra (tissue at risk), where there is partial reduction in blood supply due to the presence of collateral vessels. Salvaging of the penumbra by prompt recanalization correlates with better neurological outcomes in stroke patients (Kakuda et al., 2008; Kidwell, 2013; Legrand et al., 2016; Ma et al., 2015). Therefore, the penumbral tissue is the main target for the development of neuroprotective drugs (Adibhatla and Hatcher, 2008; Hermann et al., 2019b).
Based on recent data from the World Health Organization, stroke is the leading cause of adult neurological disability and the second cause of death worldwide after ischemic heart disease (Johnson et al., 2019). With the increase in the aging population the burden of stroke is likely to increase dramatically in the coming years. According to recent statistics from the American Heart Association (Virani et al., 2020), ischemic stroke accounts for about 80–85% of all stroke cases in the Caucasian population. Between 15–20% of all the stroke cases are hemorrhagic, which comprise intracerebral hemorrhage (ICH) and subarachnoid hemorrhage (SAH). Globally, 68% of all strokes are ischemic and 32% are hemorrhagic (Lozano et al., 2012). This is mainly due to a higher incidence of ICH in many Asian countries (Hata and Kiyohara, 2013; Khan et al., 2017; Panel, 2000; Sudlow and Warlow, 1997; Venketasubramanian et al., 2017).
With the exception of reperfusion therapies utilizing recombinant tissue plasminogen activator (rtPA) or mechanical thrombectomy, there are no other therapeutic interventions to reduce ischemic brain injury and neurological deficits in stroke patients. Over the last two decades, the stroke research community has made significant progress in understanding the pathophysiological mechanisms of ischemic brain injury. A large number of candidate neuroprotective drugs have been identified as potential therapies based on highly promising results from studies utilizing ischemic stroke models in rodents. However, most of these drugs failed to show efficacy in randomized clinical trials conducted in ischemic stroke patients. The failure to translate preclinical studies in rodents to the clinic is due to many factors, including insufficient statistical power, poor experimental design, publication bias, lack of randomization and blinding in many preclinical studies, and unrealistic therapeutic time window (Berge et al., 2017; Dirnagl and Macleod, 2009; Sena et al., 2010; van der Worp et al., 2010). Numerous recent articles and commentaries have been published discussing the main causes of the translational roadblock in stroke research, as well as possible solutions to this problem (Bosetti et al., 2017; Fisher et al., 2009; Lalu et al., 2019; O’Collins et al., 2006; Philip et al., 2009; Savitz et al., 2019; Schmidt-Pogoda et al., 2020).
In this review, we aim to discuss the need to have better preclinical models of stroke that more accurately mimic the stroke population by incorporating aged and reproductively senescent rodents, as well as animals with comorbid conditions. We discuss recent findings highlighting the stark differences in the response to ischemic stroke between young and aged rodents, and how major comorbid conditions such as hypertension, diabetes, obesity, and hyperlipidemia dramatically alter stroke outcomes.
2. Neuroprotection in Stroke – Urgent Need of Novel Therapeutics in the Era of Thrombolysis
Clinical approaches that aimed at increasing perfusion of the ischemic territory with rtPA thrombolysis or with endovascular clot removal have been proven very efficacious when given in a timely manner upon hospital arrival. For many acute stroke patients, rtPA thrombolysis is recommended within the first 4.5 hours of symptoms onset (Demaerschalk et al., 2016; Emberson et al., 2014) or within the first 6 h for a subset of patients with large vessel occlusions (Goyal et al., 2016). However, this time line of thrombolytic treatment for acute stroke care has changed due to recent progress in perfusion imaging modalities using magnetic resonance imaging (MRI) and computed tomography (CT), which allows identification of brain infarcted tissue and surrounding penumbral tissue in stroke patients in a relatively timely manner. Data from recent studies suggest that extending pharmacological thrombolysis with rtPA or desmoteplase up to 9 h from stroke onset improves functional outcomes in patients with salvageable tissue as assessed by perfusion-diffusion MRI imaging (Campbell et al., 2019; Hacke et al., 2005; Ma et al., 2019; Thomalla et al., 2018; Zhao et al., 2019). Similarly, successful recanalization with mechanical thrombectomy has been shown to be beneficial when the endovascular procedure is performed within 24 h of stroke onset in patients with a significant amount of salvageable ischemic brain tissue (Albers et al., 2018a; Albers et al., 2018b; Casetta et al., 2020; Lansberg et al., 2005; Nogueira et al., 2018; Sarraj et al., 2019).
Despite the success of reperfusion therapies, a large percentage of stroke patients are not eligible to receive them due to several factors including late hospital arrival, potential for complications especially in older patients, and increased risk of hemorrhagic transformation associated with delayed rtPA treatment. Moreover, endovascular clot removal (thrombectomy) is only available at specialized hospitals and successful recanalization with this approach occurs in approximately two-thirds of stroke patients subjected to thrombolytic treatment (Flottmann et al., 2018). Treatment rates with rtPA or thrombectomy vary from 3.4 to 9.1% for acute ischemic stroke patients, according to several databases from clinical centers around the world. For intra-arterial rtPA delivery, the treatment rates are significantly lower (Fassbender et al., 2017). These statistics show that a very large number of patients never receive reperfusion therapies highlighting the urgent need for neuroprotective strategies to reduce the progressive cell death associated with worse neurological outcomes in ischemic stroke patients.
It is evident from recent clinical studies that post-ischemic cell death in the brain progresses over the course of several hours or days, even in patients that have been successfully recanalized (Elijovich et al., 2016; Federau et al., 2016; Labeyrie et al., 2012; Legrand et al., 2016; Seners et al., 2015; Soomro et al., 2020; Tisserand et al., 2016). As such, there is high enthusiasm to combine reperfusion therapies with neuroprotectants to reduce delayed ischemia-reperfusion injury. This is exemplified by recent trials testing the combination of rtPA with 3K3A-APC, a recombinant variant of human activated protein C (Lyden et al., 2019), rtPA with intravenous glibenclamide (glyburide), a blocker of sulfonylurea receptor 1 (Huang et al., 2020; Sheth et al., 2016), and nerinetide (NA-1, a peptide that interferes with post-synaptic density protein 95) combined with rtPA (Hill et al., 2020). Combining reperfusion approaches with protective drugs would also facilitate the access of the drug to the ischemic territory potentially increasing its therapeutic efficacy.
Neuroprotection is defined as a therapeutic intervention or combination of therapeutic strategies aimed at blocking, preventing, or interrupting the deleterious biochemical and molecular pathways that, if left unchecked, would eventually result in irreversible ischemic brain injury (Ginsberg, 2008). Most studies have focused on protecting the neurons, but several cell types die following a stroke. Protecting all cell types vulnerable to ischemic cell death should be the focus of neuroprotective strategies (Candelario-Jalil, 2009; Dirnagl et al., 1999). Ideally, a neuroprotectant would prevent cell death in the penumbral tissue, have a low risk of adverse effects, be easily administered in the pre-hospital setting (ambulance, emergency room, triage, imaging) and be given concomitantly with reperfusion therapy by pharmacological thrombolysis or endovascular approaches (Patel and McMullen, 2017; Shi et al., 2018). Agents targeting the vascular occlusion/clot such as thrombolytics, anti-thrombotic, anti-platelets, and fibrinogen-depleting drugs are excluded from the cerebroprotective/neuroprotective category since these classes of drugs act mainly via hemodynamic mechanisms (clot removal) rather than targeting injury mechanisms of the ischemic cascade (Ginsberg, 2008).
Pathophysiological mechanisms of ischemic stroke have been extensively investigated at the vascular, cellular, and molecular levels, which led to the identification of several potential targets to diminish brain injury, improve neurological outcomes, and promote neural repair (Carmichael, 2016; Iadecola, 2017; Iadecola and Anrather, 2011a, b; Iadecola et al., 2020; Moskowitz et al., 2010). Cellular and molecular events of the ischemic cascade are highly complex and interconnected, with several cell death mechanisms occurring simultaneously (Dirnagl et al., 1999; Kang and Yao, 2020; Malone et al., 2019; Moskowitz et al., 2010; Shi et al., 2019). A multi-pronged approach that targets multiple pathways with multifactorial drugs or a combination of drugs is expected to increase the chances of successful neuroprotection in stroke. Multiple earlier reviews have summarized some of the promising pharmacological and non-pharmacological strategies for treatment of ischemic stroke, and discussed their mechanisms of neuroprotection (Ginsberg, 2016; Grupke et al., 2015; Moretti et al., 2015a, b; Neuhaus et al., 2017). The pharmacological agents target excitotoxicity, oxidative stress, neuroinflammation, blood-brain barrier (BBB) breakdown and vasogenic edema, whereas the non-pharmacological approaches include induction of hypothermia, remote ischemic preconditioning, and cell-based therapies. In addition, growth factors and other agents aim to enhance neurorepair.
Many reasons for the failure of protective agents from preclinical to clinical studies have been extensively discussed in several review articles (Babadjouni et al., 2017; Berge et al., 2017; Bosetti et al., 2017; Cho and Yang, 2018; Fisher et al., 2009; Grupke et al., 2015; Lalu et al., 2019; O’Collins et al., 2006; Philip et al., 2009; Rajah and Ding, 2017; Savitz et al., 2019; Schmidt-Pogoda et al., 2020; Shi et al., 2018; Sutherland et al., 2012; van der Worp et al., 2010), and are summarized here in Figure 1.
Figure 1.
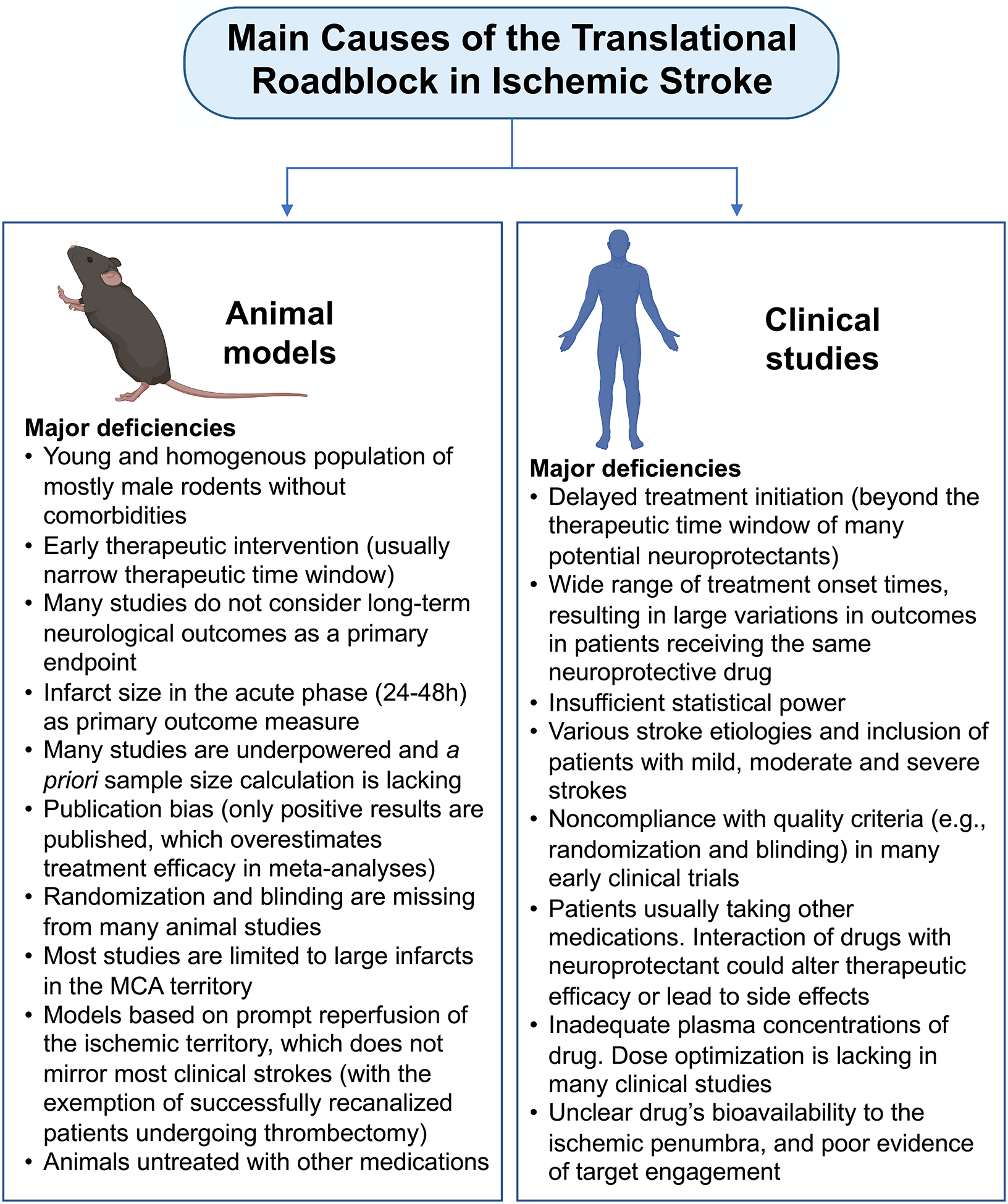
Main causes for the translational failure in ischemic stroke. Deficiencies in both preclinical animal models and clinical trials account for the failure to translate potential neuroprotective strategies into the clinic.
In subsequent sections of this review article, we will discuss recent studies highlighting the need to perform preclinical studies in aged rodents and rodent models with comorbidities in order to increase the likelihood of translating neuroprotective strategies to the clinic. Since the incidence of stroke is more prevalent in the aged population with comorbid conditions, preclinical studies evaluating the efficacy of potential neuroprotective drugs using healthy and young rodents have low external validity and may fail as they do not mimic the clinical situation (Schmidt-Pogoda et al., 2020; van der Worp et al., 2010).
3. Response of the aged brain to ischemic stroke
Age is the most significant non-modifiable risk factor for many human diseases, and the single most important risk factor for ischemic stroke (Popa-Wagner et al., 2020; Sacco, 1997; Sacco et al., 1997; Virani et al., 2020). With every decade of life, the incidence of stroke more than doubles (Mozaffarian et al., 2016). Aging promotes the development of many vascular risk factors such as hypertension, diabetes, hyperlipidemia, and obesity. Moreover, advanced age is associated with profound pathophysiological changes in both the CNS and the periphery, which underlie the increased susceptibility of the brain to ischemic injury, resulting in worse functional outcomes after stroke (Figure 2). Therefore, performing preclinical studies with neuroprotective agents in aged rodents might provide meaningful insights into the potential protective effects of the drugs in the elderly stroke population, increasing the translational impact and relevance of the findings.
Figure 2.
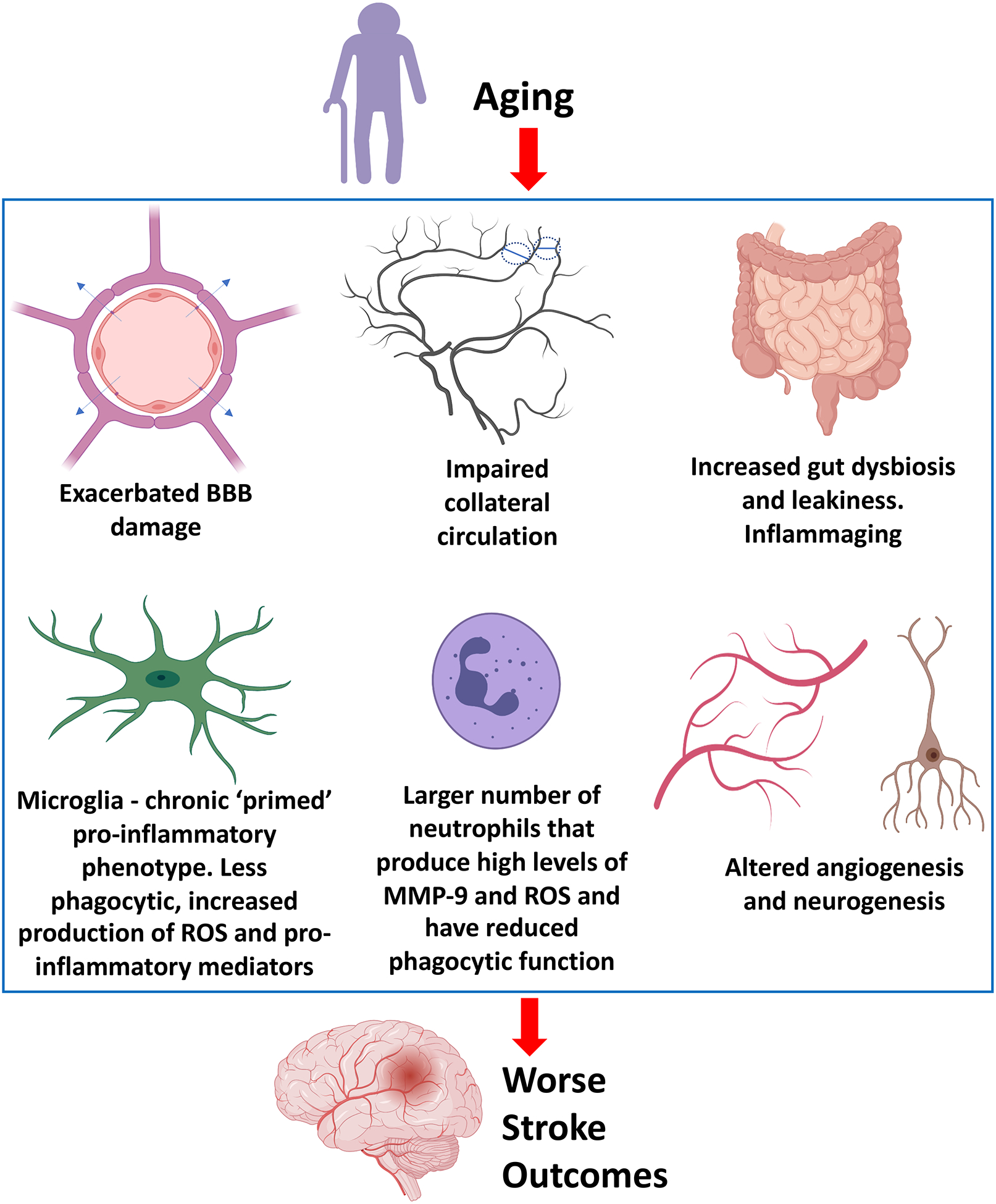
Aging and Ischemic Stroke. Advanced age is associated with many pathophysiological changes in both the CNS and the periphery. These changes contribute to an altered response to ischemic brain injury, which results in worse functional outcomes in aged compared to young individuals following an ischemic stroke. BBB, blood-brain barrier. MMP-9, matrix metalloproteinase-9. ROS, reactive oxygen species.
Clinical studies have shown that age is an independent predictor of neurological outcome in acute ischemic stroke patients following reperfusion therapies. Despite similar rates of arterial recanalization, aged patients perform worse than younger individuals, and the viable penumbral tissue is more rapidly recruited into the infarct in the aged population, as assessed by diffusion-weighted imaging (DWI)/Perfusion-weighted imaging (PWI) mismatch on MRI (Ay et al., 2005; Kruetzelmann et al., 2011; Mishra et al., 2010; Sharma et al., 2020; Weimar et al., 2004). In line with the clinical findings, animal studies consistently show that aged rodents develop worse neurological deficits, impaired long-term functional recovery, exacerbated BBB damage and vasogenic edema, as well as increased mortality compared with young animals (Andersen et al., 1999; Brown et al., 2003; Buchhold et al., 2007; Chen and Sun, 2007; Crapser et al., 2016; Davis et al., 1995; DiNapoli et al., 2008; Lindner et al., 2003; Ritzel et al., 2018; Sutherland et al., 1996; Wang et al., 2003; Won et al., 2006; Zhang et al., 2005). Interestingly, there are some discrepancies in the ischemic infarct size observed in aged animal models of stroke. Compared to the young control group, some studies show that aged animals have larger stroke volumes (DiNapoli et al., 2008; Doyle et al., 2010; Kelly et al., 2009; Ma et al., 2020; Suenaga et al., 2015), while others report the opposite (Liu et al., 2010; Liu et al., 2012; Liu and McCullough, 2012). Sex seems to be an important variable in the observed differences in infarct volume between young and aged rodents. Middle-aged females display larger infarcts compared to young females or aged mice of either sex (Manwani et al., 2013). A recent study showed that aged males have greater mortality and sensorimotor impairment than aged female mice after stroke (Ahnstedt et al., 2020). These studies emphasize the complex interactions between sex, hormonal changes, and the aging process in response to focal ischemic brain injury.
3.1. Aging increases stroke-induced neurovascular damage
Aging has a profound impact on the cerebrovasculature with damaging consequences in the context of stroke. During normal human brain aging, there are structural and functional changes in cells composing the neurovascular unit (endothelial cells, neurons, astrocytes, pericytes, microglia) that result in significant alterations in brain perfusion and permeability of the BBB. Human studies have shown 25–40% reduction in cerebral blood flow (CBF) and oxygen consumption between 30 and 89 years of age (Ainslie et al., 2008; De Vis et al., 2015; Matteis et al., 1998; Pantano et al., 1984). Conflicting data exist regarding changes in the BBB permeability associated with normal aging. Compared to the young brain, some studies found the BBB to be leakier to various tracers in aged subjects, which is associated with modifications in tight junction proteins (Farrall and Wardlaw, 2009; Goodall et al., 2019; Goodall et al., 2018; Hafezi-Moghadam et al., 2007; Janota et al., 2015; Montagne et al., 2015; Popescu et al., 2009; Senatorov et al., 2019; Shin et al., 2015; Stamatovic et al., 2019; Yang et al., 2020), while others show that the BBB remains intact in the aged brain (Banks et al., 2000; Mooradian and McCuskey, 1992; Vorbrodt and Dobrogowska, 1994; Wadhwani et al., 1991).
In ischemic stroke models, there is exacerbated BBB damage leading to vasogenic edema and higher mortality in aged animals when compared to young animals (DiNapoli et al., 2008; Kelly et al., 2009; Tan et al., 2015; Yu et al., 2019). The underlying mechanisms for the increased vulnerability of the aged brain to neurovascular injury are complex and involve higher susceptibility to oxidative damage, increased pro-inflammatory cytokines (e.g., interleukin-1β and tumor necrosis factor-α) and production of matrix metalloproteinase-9 (MMP-9). The neurovascular unit is particularly susceptible to hypoxia in the aged brain with neurons and endothelial cells being most vulnerable and undergo rapid cell death (Macri et al., 2010; Ostergaard et al., 2016; Popa-Wagner et al., 2007a). This could explain the earlier appearance of infarcted brain tissue assessed by MRI in aged animals when compared to young animals following focal ischemic injury (Canese et al., 1998; Titova et al., 2014).
3.2. Altered neurovascular coupling in aged animals
Spreading depolarization (SD) is a major cause of neuronal damage and expansion of the infarct in ischemic stroke. This phenomenon has been well documented in both animals and stroke patients (Dohmen et al., 2008; Dreier, 2011; Dreier et al., 2018; Lapilover et al., 2012; Ostergaard et al., 2015; Strong et al., 2007). Neuronal death related to SD events after stroke is thought to be caused by an insufficient hyperemic response, where tissue acidosis seems to play an important role (Menyhart et al., 2017). Compared to young animals, the magnitude of the SD-evoked CBF response is significantly reduced with aging, which could prolong tissue acidosis and increase neuronal vulnerability after ischemic injury (Balint et al., 2019; Hertelendy et al., 2019; Menyhart et al., 2015; Menyhart et al., 2017).
3.3. Impaired collateral circulation in aging
Aging significantly impairs the cerebral collateral circulation by promoting the rarefaction of collateral vessels (Faber et al., 2011). This process leads to a significant reduction in the blood flow to the penumbral tissue, accelerating tissue infarction and edema, which ultimately results in worse stroke outcomes. Leptomeningeal (pial) anastomotic connections between adjacent vascular territories serve as a ‘backup’ mechanism, or vascular redundancy, for when blood flow to a particular region is reduced due to vessel occlusion as it occurs during ischemic stroke (Ginsberg, 2016; Winship, 2015). By restoring flow to the affected region from other vascular territories, the leptomeningeal collateral circulation plays a key role in limiting the recruitment of the ischemic penumbra into the infarct core.
In a recent study using two-photon laser scanning microscopy combined with laser speckle contrast imaging, pial collaterals between the middle cerebral artery (MCA) and the anterior cerebral artery (ACA) were monitored during distal MCA occlusion in young and aged rats. Following MCA occlusion, there is a significant decline in collateral perfusion in both aged and young rats, with aged rats showing a more dramatic decline in penumbral perfusion via leptomeningeal collaterals, which translated into larger areas of ischemic brain injury (Ma et al., 2020).
3.4. Aging significantly alters the neurogenic and angiogenic responses following ischemic brain injury
Neurogenesis is significantly impaired with aging, both under normal conditions and in response to stroke (Apple et al., 2017; Cutler and Kokovay, 2019; Daynac et al., 2016). The number of proliferating neural progenitor cells (NPCs) in the subventricular zone and the dentate gyrus subgranular zone are lower in old compared to young rats after stroke. Also, the ability of newly formed progenitor cells to differentiate into neurons is significantly impaired in the aged animals (Darsalia et al., 2005; Jin et al., 2004). Surprisingly, there is increased neurogenesis in the unaffected (contralateral) hemisphere in the aged, but not young, mice after focal ischemia. In an elegant study using mice expressing luciferase in doublecortin positive cells, Adamczak et al showed that in middle-aged and old mice there is a significant upregulation of neurogenesis in the contralesional hemisphere in response to stroke (Adamczak et al., 2017). Neurorehabilitation with forced limb-use is highly effective in increasing neurogenesis and neurological recovery after stroke in aged rats (Qu et al., 2015). In a very recent study, electrical stimulation was utilized to stimulate neurogenesis, leading to improved functional outcomes in aged rats following permanent cortical stroke (Balseanu et al., 2020).
Similar to what occurs to the neurogenic response, aging studies in humans and animals show that there is progressive failure of brain angiogenesis in old compared to young during both physiological conditions and in response to injury, which is associated with a significant alteration in the expression of several angiogenesis-associated genes (Black et al., 1989; Murugesan et al., 2012; Popa-Wagner et al., 2010a; Riddle et al., 2003). Impaired neurogenesis and angiogenesis could be an important contributor to poorer outcomes after ischemic injury in the aged brain. Inhibition of angiogenesis dramatically impairs the survival of migrating neuroblasts after stroke, suggesting that the neurogenic response is highly dependent on angiogenesis (Nih et al., 2012). Recent studies have shown that it is possible to therapeutically enhance neurogenesis and angiogenesis in the aged brain to improve functional recovery after stroke. Growth differentiation factor-11 (GDF11) significantly increases angiogenesis, improves white matter integrity, and reduces sensorimotor deficits in aged mice subjected to transient focal cerebral ischemia (Hudobenko et al., 2020). In other studies, post-ischemia treatment with omega-3 polyunsaturated fatty acids resulted in a significant improvement in neurological function associated with enhanced angiogenesis and reduction in white matter damage in a permanent distal middle cerebral artery occlusion (MCAO) model in aged mice (Cai et al., 2017; Jiang et al., 2019). Increased neurogenesis and differentiation of neuroblasts by granulocyte-colony stimulating factor (G-CSF) treatment significantly increases motor recovery in aged rats after stroke (Popa-Wagner et al., 2010b).
3.5. Inflammaging and gut dysbiosis exacerbate ischemic stroke outcomes
Chronic and heightened inflammation associated with aging, a phenomenon coined ‘inflammaging’ (Franceschi et al., 2018; Furman et al., 2019) seems to play a key role in ischemic stroke outcomes. A large body of evidence from animal and human studies shows that inflammaging is triggered by various stimuli including viral and bacterial infections, cell debris, as well as misfolded and oxidatively-modified proteins. Gut microbiota and intestinal immune responses take center stage in inflammaging. Age-related gut dysbiosis (altered ratio of Firmicutes to Bacteroidetes and reduced bacterial diversity) and increased gut leakiness have been documented in animals and humans (Biagi et al., 2011; Franceschi et al., 2018; Thevaranjan et al., 2017). Age-related changes in microbiota composition drive gut permeability, increased systemic inflammation, and altered immune cell function (Thevaranjan et al., 2017), which could exacerbate pathological processes in many diseases including stroke. Inflammaging increases the vulnerability of the aged brain to ischemia and enhances the post-stroke inflammatory response.
Several studies have found increased intestinal permeability, enhanced translocation and dissemination of commensal bacteria, and gut dysbiosis in response to ischemic stroke (Ahnstedt et al., 2020; Blasco et al., 2020; Chen et al., 2019; Crapser et al., 2016; Ferrara et al., 2020; Houlden et al., 2016; Kurita et al., 2020; Liu et al., 2019; Singh et al., 2016; Stanley et al., 2016; Stanley et al., 2018; Wen et al., 2019; Xia et al., 2019). Importantly, aged animals show worse gut dysbiosis and intestinal permeability after stroke (Crapser et al., 2016; Wen et al., 2019). The aged biome seems to worsen ischemic stroke outcomes, at least in part, by increasing levels of systemic pro-inflammatory mediators. Fecal transplant gavage of microbiota from young to aged mice significantly reduces mortality and improves neurobehavioral outcomes following MCAO (Spychala et al., 2018).
Production of short-chain fatty acids (SCFAs), primarily butyrate, acetate and propionate, is significantly reduced in aged animals (Lee et al., 2020; Spychala et al., 2018). SCFAs are important signaling molecules mainly produced by bacterial metabolism in the gut. Recent evidence demonstrates that increasing levels of SCFAs improves ischemic stroke outcomes in animal models (Lee et al., 2020; Sadler et al., 2020). The study by Lee et al. showed for the first time that the worse stroke recovery in aged mice can be reversed by post-ischemia “bacteriotherapy” with SCFA-producing bacterial strains given by oral gavage (Lee et al., 2020).
3.6. Microglial and astroglial responses to ischemic damage in the aged brain
Age-associated changes in microglia/macrophage function could have a big impact on neuroinflammation and neurovascular function after ischemic brain injury. Microglia in the aged brain show a chronic ‘primed’ pro-inflammatory phenotype, are less phagocytic, produce high levels of reactive oxygen species (ROS), and secrete more pro-inflammatory mediators associated with damaging pathogenic events (Frank et al., 2006; Godbout et al., 2005; Kim and Cho, 2016; Marschallinger et al., 2020; Mosher and Wyss-Coray, 2014; Salas et al., 2020; Streit et al., 2004; Streit and Xue, 2010). Overall, these microglial changes associated with aging make the microenvironment of the CNS more pro-inflammatory. Exaggerated neuroinflammation in the aged brain could worsen ischemic stroke pathology and interfere with neurorepair and functional recovery. Interestingly, the gut microbiota is a critical regulator of microglial function mainly via production of SCFAs and aryl hydrocarbon (AhR) ligands (Erny and Prinz, 2020). A very recent study showed that SCFA supplementation in the drinking water improved outcomes in models of ischemic stroke mainly through reduction in microglial activation (Sadler et al., 2020).
Using RNA sequencing, recent studies compared microglial cells isolated from 2.5-month-old and 18-month-old mice subjected to permanent distal MCAO. Significant increase in transcription of many pro-inflammatory genes was observed in microglia obtained from aged naïve mice, suggesting heightened on-going inflammation in the aged brain. In response to stroke, aged mice showed impaired transcriptional activation of genes involved in immune cell chemotaxis, tissue remodeling, cell-cell interactions, and inflammatory responses, which may contribute to enhanced vulnerability and worse recovery in aged animals after ischemic stroke (Jiang et al., 2020; Shi et al., 2020). Aged mice exhibited a reduced number of microglia/macrophages expressing phenotypic markers of alternative activation (M2 polarization) compared with young animals following focal cerebral ischemia (Suenaga et al., 2015).
Aging also accelerates astrocytic responses to ischemic injury. Earlier formation of an astroglial scar has been documented in aged rats (20-month old) compared to young animals in response to ischemic stroke (Popa-Wagner et al., 2007b; Popa-Wagner et al., 2006). This age-associated premature astroglial scarring could be an impediment for neuronal plasticity and neurorepair in the aftermath of an ischemic event.
3.7. More neutrophils infiltrate the aged brain after stroke
Infiltration of peripheral immune cells into the ischemic brain is a critical event in the ischemic cascade, having a huge impact on tissue fate, functional outcomes, and neurorepair processes. Among the infiltrated immune cells, neutrophils are the first responders to injury and they have been shown to worsen stroke pathology by releasing proteolytic enzymes (e.g., MMP-9, neutrophil elastase, cathepsin G), disrupting the BBB, producing blockage of blood vessels due to their local accumulation in the ischemic territory (no-reflow phenomenon), and releasing ROS (Lambertsen et al., 2019; Otxoa-de-Amezaga et al., 2019a; Otxoa-de-Amezaga et al., 2019b; Perez-de-Puig et al., 2015). Compared to the young, the aged ischemic brain shows a larger number of neutrophils that have a reduced phagocytic function and produce high levels of MMP-9 and ROS (Ritzel et al., 2018). Since microglial phagocytosis controls the accumulation and fate of invading neutrophils after stroke (Otxoa-de-Amezaga et al., 2019b), and aging impairs the phagocytic capacity of microglia, it is tempting to speculate that increased neutrophil infiltration in the aged ischemic brain is due to alterations in microglial-mediated clearance of extravasated neutrophils. However, this hypothesis needs to be demonstrated experimentally.
3.8. Oxidative stress in the ischemic aged brain
Shortly after ischemic brain damage, there is excessive ROS production and, at the same time, cellular antioxidant mechanisms become deficient, leading to oxidative stress (Heo et al., 2005). Compared to other organs, the brain is highly susceptible to oxidative stress, and the aged brain is more so. There is a powerful association between the antioxidant status and longevity, suggesting that increased ROS production precipitates the aging process. Aging is associated with mitochondrial dysfunction, which makes the body more susceptible to increased oxidative stress after injury. Oxidative damage to endothelial cells that line the brain vasculature contributes to vasogenic edema after stroke (Chan, 2001). Cellular antioxidant mechanisms are decreased in older individuals contributing to exaggerated tissue damage after stroke (Popa-Wagner et al., 2018).
4. Hypertension and stroke outcomes
Hypertension is defined by a systolic/diastolic arterial blood pressure above 130/80 mmHg, based on a recently-revised definition (Whelton et al., 2018). Hypertension induces endothelial dysfunction and angiopathy in large and small vessels. Arterial hypertension is more frequent in older individuals (≥ 60 years) (Benjamin et al., 2017), and it dramatically increases ischemic brain injury in both humans and animal models. Blood pressure lowering therapies significantly reduce stroke risk (Hong, 2017). Hypertension ranks at the top of modifiable risks factors for stroke and up to 75% of stroke patients have hypertension (AlSibai and Qureshi, 2016; Hong, 2017; van der Worp and van Gijn, 2007; Virani et al., 2020). Thus, it is surprising that only about 10% of the preclinical studies use animals with hypertension when testing potential neuroprotective drugs (van der Worp et al., 2010).
In acute ischemic stroke patients, hypertension is associated with increased mortality, worse functional outcomes, and a higher risk of intracranial hemorrhage after thrombolytic therapy (Ahmed et al., 2009; Leonardi-Bee et al., 2002; Maïer et al., 2017; Maïer et al., 2018). Hypertension is also associated with cognitive decline through mechanisms involving impaired neurovascular coupling and altered cerebral blood vessel reactivity (Iadecola, 2017).
There are several preclinical rodent models to study hypertension (Maier and Kubis, 2019). These include the spontaneously hypertensive rat (SHR), Dahl salt-sensitive rats that develop different degrees of hypertension depending on salt intake, surgically-induced models involving constriction of one or two renal arteries with or without kidney ablation, and pharmacologically-induced hypertension with angiotensin II, deoxycorticosterone acetate (DOCA) or the nitric oxide synthase inhibitor L-N-nitroarginine-methyl ester (L-NAME). The spontaneously hypertensive rat (SHR) is among the most widely utilized animal model of hypertension. This inbred strain start exhibiting high blood pressure around 6 weeks of age and reach levels of 180–200 mmHg at ~18 weeks. The SHR stroke prone (SHRSP) rat is a sub-strain of the SHR and they develop spontaneous strokes. The infarct size in SHRSP rats subjected to 1h of MCAO is twice as large as that measured in normotensive Wistar-Kyoto (WKY) rats, the strain from which SHRSP was derived. In addition, SHRSP rats subjected to stroke exhibit less neurological recovery than WKY control animals (McCabe et al., 2009; McGill et al., 2005). This is associated with stronger microglial responses and enhanced neuroinflammation in SHRSP rats compared to normotensive controls (Marks et al., 2001).
Arterial hypertension is accompanied by microglial activation in the hypothalamic paraventricular nucleus (PVN), which results in increased neuroinflammation (Shen et al., 2015; Shi et al., 2010). The involvement of inflammation in the onset and maintenance of the hypertensive condition is supported by studies showing that anti-inflammatory treatment with minocycline or overexpression of interleukin-10 (IL-10), an anti-inflammatory cytokine, attenuates hypertension in both SHR rats and in the chronic angiotensin II-infused hypertensive rat model (Santisteban et al., 2015; Shi et al., 2010). Enhanced microglial activation in autonomic brain regions plays a key role in neurogenic hypertension. Increased sympathetic nerve activity seen in hypertension leads to a significant increase in bone marrow-derived peripheral pro-inflammatory cells and enhances activation of leukocytes in the spleen (Ahmari et al., 2019; Ganta et al., 2005; Santisteban et al., 2015). Collectively, these studies suggest that hypertension is characterized by a highly pro-inflammatory environment that is likely to contribute to the worse tissue injury and limited functional recovery seen in hypertensive ischemic stroke patients.
Using a photothrombotic stroke model, Möller et al compared the post-ischemic inflammatory response between SHR and WKY normotensive control rats. Infarct volume was significantly larger in the SHR, which showed a strong correlation with the number of invading CD45high leukocytes present in the ischemic hemisphere. Brain infiltrating myeloid cells had a higher surface level of intercellular adhesion molecule-1 (ICAM-1) in SHR compared to normotensive animals. Similarly, hypertensive rats had a significant increase in the number of infiltrating neutrophils, monocytes, and macrophages compared to WKY rats, which correlated with higher expression of chemokines (CCL2, CXCL2, and CCL7) known to participate in the transmigration of immune cells to the brain after stroke (Möller et al., 2015).
Stroke patients with good collateral status have a larger penumbra region and are more likely to respond favorably to thrombolytic therapy and/or potential neuroprotectants (Ginsberg, 2016; Rusanen et al., 2015; Vagal et al., 2018). There is convincing evidence that hypertension accelerates the rate at which the penumbral tissue is incorporated into the infarct. This is mainly due to worse perfusion of the penumbra via the leptomeningeal collateral circulation (Campbell et al., 2013). In acute ischemic stroke, chronic hypertension has a detrimental effect on collateral flow in patients with large-vessel occlusions (Fujita et al., 2019). Luminal narrowing of blood vessels due to atherosclerosis is further exacerbated by hypertension (Sabbatini et al., 2001).
Remodeling capacity in hypertension seems to be age-dependent. Comparing young (3-month-old) and middle-aged SHR rats (12-month-old) that were subjected to focal cerebral ischemia, Liang et al. found that, while they exhibited similar infarct size, neurobehavioral recovery was significantly impaired in the 12-month-old SHR rats compared to the 3-month-old controls. This impaired recovery after stroke was associated with decreased neurogenesis and oligodendrogenesis in aged hypertensive rats (Liang et al., 2016).
The above studies highlighted the significant differences in the response to ischemic brain injury between hypertensive and normotensive animals and humans. Therefore, incorporating hypertension as a crucial comorbid condition to our preclinical models will likely increase the likelihood of translation of potential neuroprotective strategies from the bench to the bedside. Evidence from recent studies further help to emphasize the need to have better preclinical models to test neuroprotective agents. While some of these studies show that administration of adipose tissue-derived mesenchymal stem cells (MSCs) are highly protective in young animals after focal cerebral ischemia (Gutiérrez-Fernández et al., 2015; Gutiérrez-Fernández et al., 2013; Ikegame et al., 2011), similar treatment with MSCs fails to modify stroke outcomes in hypertensive animals (Diekhorst et al., 2020; Mangin et al., 2019).
5. Stroke Outcomes in Animal Models of Metabolic Disease
5.1. Diabetes
Diabetes mellitus (DM) is a metabolic disease characterized by hyperglycemia caused by defects in insulin secretion, insulin action, or both. The chronic hyperglycemic state results in organ damage, especially to the eyes, nerves, kidney, heart, and blood vessels (Gavin et al., 2003). Based on recent statistics, 9.8% of the population in the USA has been diagnosed with DM, and 37.6% have pre-diabetes. Type 1 diabetes constitutes 5–10% of DM patients, while type 2 diabetes represents 90–95% of all DM cases (Virani et al., 2020). Type 1 DM is an autoimmune disease that triggers a dramatic loss of insulin-producing β cells in the pancreas. Type 2 DM is characterized by peripheral insulin resistance and hyperinsulinemia caused by obesity, excessive food intake, and lack of physical activity (Gavin et al., 2003).
A large percentage of ischemic stroke patients have DM, which is associated with increased brain injury and mortality, as well as worse neurological impairment (Ergul et al., 2009). Hyperglycemia at hospital admission is an independent predictor of neurological worsening and intracerebral hemorrhage after endovascular clot removal (Laredo et al., 2020; Soomro et al., 2020), rtPA thrombolysis (Alvarez-Sabín et al., 2004; Bruno et al., 2002), as well as in patients not receiving any recanalization therapy (Baird et al., 2003; Capes et al., 2001; Yong and Kaste, 2008). Similarly, in rodent models of ischemic stroke, diabetic hyperglycemia increases brain injury and worsens neurological deficits (Chen et al., 2011; Gómez-de Frutos et al., 2019; Kusaka et al., 2004; Martini and Kent, 2007; Mayanagi et al., 2008; Shukla et al., 2017; Tureyen et al., 2011). A more dramatic reduction in CBF in the peri-infarct region is seen in animals exposed to acute hyperglycemic conditions induced by infusion of glucose immediately before stroke (Kawai et al., 1997) or chronic diabetes induced by the administration of streptozotocin (STZ), a toxin that destroys β cells of the pancreatic Langerhans islets (Martini and Kent, 2007). Thus, impaired brain reperfusion after vessel recanalization is an important mechanism underlying the detrimental effects of hyperglycemia on ischemic stroke outcomes.
Diabetic hyperglycemia induces major biochemical changes within endothelial cells. These changes include overproduction of superoxide radicals by the mitochondria leading to oxidative stress. In addition, increased formation of diacylglycerol (DAG) results in increased PKC activation, which in turn has several pathogenic consequences including reduction in endothelial nitric oxide synthase (eNOS) levels, increased expression of vascular endothelial growth factor (VEGF), activation of NF-κB-dependent inflammatory gene expression, and increased NADPH oxidase levels (Brownlee, 2001). These changes contribute to the hyperglycemic endothelium being highly vulnerable to ischemia.
A dysregulated inflammatory response seems to be a hallmark of ischemic stroke under diabetic conditions. Mice fed with high-fat diet for 8 weeks to model diabetes have increased basal MCP-1 levels in the plasma and in peritoneal macrophages. In response to focal ischemia, diabetic mice have larger strokes and a marked increase in brain swelling compared to control animals (Kim et al., 2014). Interestingly, mRNA levels of MCP-1, IL-6, and CCR2 are significantly reduced in the ischemic brain in diabetic mice compared to normoglycemic controls (Kim et al., 2014). In another study using diabetic mice subjected to hypoxia-ischemia, a delayed and diminished inflammatory response, as assessed by lower levels of brain TNF-α, IL-1α and IL-1β, have been observed (Kumari et al., 2007). As speculated by Kim et al., the inability of the diabetic animals to launch a proper immune response to ischemic brain injury may prolong the acute inflammatory phase, leading to more infiltration of peripheral immune cells resulting in worse outcomes (Kim et al., 2014).
Not all studies using diabetic animals have found reduced pro-inflammatory gene expression in response to ischemic injury. Due to a G-to-T point mutation in the diabetic gene (db) encoding for the leptin receptor, db/db mice have defective leptin signaling leading to obesity and hyperinsulinemia, resembling some aspects of human type 2 diabetes. Starting around 12 weeks of age, these mice have dramatic hyperglycemia (Hummel et al., 1966). Using the db/db mouse model of type II diabetes, a previous study found much higher levels of IL-1β, IL-6, MIP-1α, MCP-1, P-selectin, and E-selectin in the ischemic brain of diabetic animals compared to normoglycemic controls at 12h after transient MCAO. Increased immunoreactivity for ICAM-1 in brain vasculature and marked increase in microglia/macrophage activation were seen in db/db mice compared to controls. These inflammatory changes correlated with larger strokes, worse brain swelling, and more infiltration of neutrophils into the ischemic brain of diabetic mice compared to normoglycemic controls (Tureyen et al., 2011). In another study using a rat model of permanent MCAO, acute hyperglycemia induced by intraperitoneal administration of D-glucose before MCAO induction resulted in significantly higher levels of IL-1β and cyclooxygenase-2 (COX-2) in hyperglycemic rats compared to normoglycemic rats or hyperglycemic sham controls (Bémeur et al., 2005). In Zucker Diabetic Fatty (ZDF) rats subjected to 2h of focal cerebral ischemia and 24h of reperfusion, there is a dramatic increase in the levels of ICAM-1 leading to more neutrophil adhesion to the cerebral endothelium and infiltration into the ischemic brain, which correlates with larger strokes, worse edema formation, and poorer neurological function in ZDF compared to lean normoglycemic control rats (Ritter et al., 2011). Thus, increased inflammation might be a contributing factor to the exacerbated brain injury observed under diabetic conditions (Venkat et al., 2017). However, differences between the studies in the selection of diabetes models, severity of brain injury determined by the duration of MCAO, and degree of reperfusion (transient vs permanent occlusion models) could explain some of the differences in the published literature regarding the role of inflammation in stroke outcomes in diabetes.
Impaired polarization of monocytes/macrophages to an anti-inflammatory phenotype seems to be one of the mechanisms underlying the detrimental effects of diabetic hyperglycemia in ischemic stroke. In a mouse model of permanent distal MCAO, hyperglycemia induced by intraperitoneal injection of glucose at the time of vessel cauterization significantly increased infarct volume and reduced the number of non-inflammatory monocytes (Ly-6Clow Ly-6G−; Arginase-1+) infiltrating the injured brain. Interestingly, monocyte ablation induced by diphtheria toxin (DT) administration to CD11b-DTR mice, abrogated the hyperglycemia-induced exacerbation of ischemic brain injury, suggesting that hyperglycemia produces its damaging effects on stroke, at least in part, through monocytes (Khan et al., 2016).
In animal models, diabetes increases neovascularization in the brain, as demonstrated by greater vascular density, volume and surface area, increased number and diameter of collateral vessels, as well as increased anastomoses between MCA branches. Diabetes-augmented angiogenesis is dysfunctional since new vessels are immature, as indicated by reduced pericyte coverage, increased vascular permeability, and higher percentage of nonperfused vessels in the diabetic animals compared to normoglycemic controls (Ergul et al., 2014; Li et al., 2010; Prakash et al., 2013; Prakash et al., 2012). It is important to emphasize that irrespective of infarct size, there is increased intracerebral bleeding after ischemic stroke in diabetic animals compared to controls (Ergul et al., 2007; Mishiro et al., 2014). A greater hemorrhagic transformation and brain swelling in diabetic conditions correlates with increased mortality and worse outcomes after ischemia. A higher level of MMP-9 in diabetes is critically involved in abnormal cerebrovascular remodeling that contributes to greater hemorrhagic transformation and edema following stroke (Elgebaly et al., 2010).
Hyperglycemia induced by STZ given three days before stroke increases ROS generation and activation of MMP-9 leading to exacerbation of BBB damage and dramatically increasing vasogenic edema after focal cerebral ischemia in rats. Transgenic rats overexpressing human SOD1, an antioxidant enzyme, have reduced hyperglycemia-induced BBB opening, vasogenic edema, and MMP-9 activation after ischemic stroke compared with control, non-transgenic animals (Kamada et al., 2007). More recent studies have shown that MMP-3 and MMP-9 play an important role in the damage to the neurovascular unit following focal cerebral ischemia in diabetic animals (Elgebaly et al., 2011; Elgebaly et al., 2010; Hafez et al., 2016; Hafez et al., 2017; Kumari et al., 2011).
Angiopoietins are a family of vascular growth factors that modulate endothelial cell function and angiogenesis. Angiopoietin (Ang) 1 and Ang2 are the most widely studied angiopoietins and they seem to have opposing effects on vasculogenesis. Ang1 plays a critical role in vessel maturation by promoting the migration, adhesion and survival of endothelial cells, while Ang2 disrupts the connections between the endothelium and perivascular cells and promotes endothelial cell death and vessel regression (Fagiani and Christofori, 2013). Alterations in the levels of Ang1 and Ang2, as well as their receptor Tie2, seem to play an important role in the increased vascular damage in diabetic animals after stroke. The Ang1/Tie2 signaling pathway contributes to endothelial cell survival and is important in vascular stability by promoting the recruitment of pericytes to the blood vessels (Brindle et al., 2006; Teichert et al., 2017). Ang2-mediated signaling has opposing effects leading to endothelial cell apoptosis and BBB breakdown (Nag et al., 2005). Utilizing the db/db type-2 diabetes mouse model, Cui et al. found decreased Ang1/Tie2 and increased Ang2 levels in diabetic animals compared to controls after focal ischemic brain injury. This was associated with worse BBB disruption and loss of tight junction proteins in db/db mice compared to normoglycemic controls after stroke (Cui et al., 2011).
5.2. Obesity and stroke outcomes
Obesity is a major health concern worldwide and a well-known risk factor for diabetes, hypertension, cardiovascular disease and stroke. Obesity is considered an independent risk factor for ischemic stroke (Lu et al., 2014; Strazzullo et al., 2010), but a few epidemiological studies suggest that obesity is associated with reduced long-term mortality and better functional recovery after stroke (Doehner et al., 2013; Vemmos et al., 2011). Other studies report the opposite: worse outcomes in obese stroke patients (Bazzano et al., 2010; Yi et al., 2009). The obesity-stroke paradox and contradictory clinical data may be explained by poor design of the human epidemiological studies or the influence of other factors such as age, ethnicity, and sex-specific differences on the interpretation and analysis of the data, as discussed in recent reviews (Haley and Lawrence, 2016; Scherbakov et al., 2011). What is clear from preclinical studies is that obesity results in exacerbated brain injury and more BBB disruption and brain edema, which leads to worse neurobehavioral outcomes in rodent models of focal cerebral ischemia (Deng et al., 2014; Deutsch et al., 2009; Haley et al., 2019; Haley and Lawrence, 2017; Haley et al., 2017; Langdon et al., 2011; Li et al., 2013; Maysami et al., 2015; Osmond et al., 2010; Ritter et al., 2011).
Mice fed a high-fat diet for 10 weeks have increased cerebrovascular tortuosity and decreased lumen diameter of the middle cerebral artery. In response to transient ischemic stroke, these animals develop larger strokes compared to lean controls on a normal diet, and have a dramatic increase in MMP-9-mediated BBB damage, edema and hemorrhagic transformation. Of interest is the finding that MMP-9 deficient mice on a high fat diet have attenuated vascular remodeling and less infarct volume and neurovascular injury compared to obese wild-type controls, suggesting that MMP-9 activation plays a key role in obesity-induced worsening of stroke outcomes (Deng et al., 2014).
Excessive accumulation of fat, impaired metabolic processes, and chronic low-grade inflammation are among the most salient features of obesity (Virani et al., 2020). Increased adipose tissue inflammation results from cellular stress, since the capacity of adipocytes to store lipids is exceeded in obesity. Inflamed adipose tissue releases several pro-inflammatory mediators and adipokines into the blood stream, which produce many effects on different organs and alter various physiological functions (Chan et al., 2019; Haley et al., 2017). Using metabolomics, Haley et al. showed that obesity produces marked changes in the acute metabolic and inflammatory response to ischemic stroke. In naïve ob/ob mice, there was a significant increase in plasma free fatty acids compared to ob/− control mice. Stroke induced a further increase in these metabolites only in the obese mice. Similarly, inflammatory mediators (IL-6, G-CSF, CXCL1) were increased in plasma, adipose tissue, and liver after stroke, and this increase was greater in obese mice (Haley et al., 2017).
Adiponectin, leptin, and resistin are the most important adipokines produced by the adipose tissue. Before stroke, obese ob/ob mice have lower levels of resistin and adiponectin in adipose tissue, which is further decreased by ischemic brain injury (Haley et al., 2017), suggesting that stroke dramatically alters the release of adipokines from adipose tissue under obese conditions. Reduced levels of adiponectin in obese mice after stroke could significantly impact outcomes since there is a large body of evidence indicating that adiponectin is protective in the context of ischemic stroke (Li et al., 2017; Miao et al., 2013; Nishimura et al., 2008).
5.3. Hyperlipidemia
Hyperlipidemia is associated with atherosclerosis of blood vessels in humans and is one of the main risk factors for coronary artery disease and cerebrovascular disease (Virani et al., 2020). A few animal models of hypercholesterolemia have been widely utilized to study the impact of this comorbid condition on stroke outcomes. Mice with genetic deletion of apolipoprotein E (ApoE), a fat-binding protein critically involved in cholesterol metabolism, have several fold increases in plasma cholesterol levels, which are further elevated in ApoE−/− mice on a high-cholesterol diet. Another popular mouse model of hyperlipidemia is the low-density lipoprotein receptor (Ldlr−/−) knockout mice. By binding to ApoE and ApoB, the LDL receptor controls cellular uptake of LDL and VLDL lipoproteins from the blood. On a regular diet, the Ldlr−/− mice have ~2 times elevated cholesterol concentrations in blood, and this increase is greater when maintained on a high-cholesterol diet.
Several lines of evidence indicate that hyperlipidemia exacerbates ischemic brain damage through different mechanisms including increased oxidative stress, inflammation, BBB damage, impaired CBF regulation, and deficient collateral perfusion (Ayata et al., 2013; Cao et al., 2015; ElAli et al., 2011). Hypercholesterolemia also dramatically affects the cerebrovasculature under resting conditions and in response to focal cerebral ischemia (Ayata et al., 2013; ElAli et al., 2011). Using intravital microscopy, a previous study showed that high-fat diet significantly increases the interactions of platelets and leukocytes with the cerebral endothelium, a process that depends on ROS production by NADPH oxidase and increased levels of P-selectin. In response to ischemic brain injury, high-fat diet further exaggerated the platelet- and leukocyte-endothelial cell interactions, which could contribute to inflammation and focal thrombosis (Ishikawa et al., 2004). Massive neutrophil infiltration has been documented in ApoE−/− mice fed a high-cholesterol diet compared to wild-type controls on a normal diet in response to transient focal cerebral ischemia. Blockade of CXCR2, a neutrophil receptor that binds to the chemokines CXCL1 and CXCL2/3, significantly reduces hyperlipidemia-exacerbated neurological deficits and brain tissue infarction after experimental ischemic stroke (Herz et al., 2015). Hyperlipidemia induced by high-fat diet increases pro-inflammatory mediators in the brain including IL-6, TNF-α, ICAM-1, and VCAM-1, and these changes are greater in rats subjected to 2h of MCAO. Worse neurological deficits, larger infarct volumes and increased apoptosis were observed in stroked rats fed with high-fat diet compared to controls (Cao et al., 2015). Hyperlipidemia is associated with increased inflammation not only in the brain, but also in the periphery. These changes are even more dramatic in response to stroke (ElAli et al., 2011; Herz et al., 2014), which might explain the higher vulnerability to ischemic brain injury in hyperlipidemic conditions.
A key role for CD36/fatty acid translocase, a scavenger receptor with a high affinity for lipids, in the exacerbation of ischemic stroke pathology in hyperlipidemia has been demonstrated in previous studies. Infarct size and brain swelling were significantly increased in ApoE−/− mice on a high-fat diet compared to wild-type controls on a normal diet, which was associated with a significant increase in CD36 and MCP-1 in the brain and the periphery. Genetic deletion of CD36 ameliorated stroke-induced inflammation, edema, and neuronal injury (Kim et al., 2008). Infiltrating monocyte-derived macrophages are the major source of CD36 in the ischemic brain of hyperlipidemic mice (Kim et al., 2012). Effective pharmacological targeting of CD36 in hyperlipidemic stroke might be challenging based on data from a recent study which showed that only chronic administration of CD36 inhibitors prior to stroke was beneficial in reducing brain swelling after stroke. Treatment with CD36 inhibitors after the stroke onset, a more clinically relevant therapeutic schedule, failed to impact stroke outcomes in hyperlipidemic mice (Kim et al., 2020).
Deficits in cerebral perfusion and the status of the leptomeningeal collateral circulation are associated with hyperlipidemia (Hermann et al., 2019a; Zechariah et al., 2013), contributing to the increased susceptibility of the brain to ischemic stroke in hyperlipidemic conditions. Compared to controls, ApoE−/− mice fed a high-fat diet have impaired cerebrovascular responses, as demonstrated by reduced resting CBF, altered vasodilatory reflexes, and worse perfusion deficits after distal occlusion of the middle cerebral artery (Ayata et al., 2013). Vascular dysfunction in hyperlipidemic stroked mice is ameliorated by Rho-associated kinase (ROCK) inhibition (Shin et al., 2014).
As with other comorbidities discussed before, hyperlipidemia is associated with worse stroke outcomes. However, it is important to discuss a potential caveat of utilizing genetic models combined with high-fat diet to study the therapeutic effects of potential neuroprotectants in stroke under hyperlipidemic conditions. When maintained on high-fat diet, ApoE and Ldlr knockout mice have dramatically elevated levels of cholesterol in their blood, which models familial hypercholesterolemia, a rare human hereditary condition. Thus, it is not clear how well these animal models reflect the hyperlipidemic conditions seen clinically in stroke patients. As discussed in a recent article (Hermann et al., 2019a), potentially neuroprotective strategies could be prematurely abandoned (missed opportunity) by using preclinical models that do not accurately represent the clinical situation.
6. Challenges of modeling ischemic stroke in aged and/or comorbid animals
Most preclinical stroke studies fail to include aged and/or comorbid animals. In a recent analysis of data from preclinical systematic reviews of therapeutic interventions for ischemic stroke, only 11.4% of studies included an aged or comorbid model (McCann and Lawrence, 2020). The main reasons for the lack of attention to aging and comorbidities in preclinical stroke research include the high costs and increased mortality in aged and/or comorbid animals subjected to ischemic stroke. Moreover, the time that it takes to perform the experiments is dramatically increased since animals need to be maintained for long periods of time to reach a certain age (aging experiments) or to induce a specific phenotype by maintaining the animals on a particular diet for several weeks/months (e.g., rodent models of hyperlipidemia, obesity, and diabetes).
Inducing intraluminal MCAO or embolic stroke in aged mice or rats is methodologically challenging. Aged rodents, especially outbred rat strains fed ad libitum, show a wide range of body weights, making the intraluminal approach of vessel occlusion more complicated due to varying diameters of cerebral vessels, which require altering the caliber of the occluding filament for each range of animal weights (Turner et al., 2013). Moreover, in heavier or obese animals, it is harder to dissect the arteries due to increased adiposity. Inbred Fischer-344 and Fischer-344/Brown-Norway hybrid rats are widely utilized in aging research. These rat strains are unsuitable for intraluminal MCAO due to kinking of the internal carotid artery, as assessed by magnetic resonance angiography. Surgical complications in these strains are mainly due to inability to advance the intraluminal filament, usually resulting in subarachnoid hemorrhage and high mortality (Dittmar et al., 2006).
Based on our experience using aged rodents to model ischemic stroke (Bennion et al., 2017; DeMars et al., 2019; Yang et al., 2017), a special attention should be paid to the level of anesthesia and breathing patterns during surgery. Furthermore, the variability in infarct volumes is higher in aged animals compared to young ones, despite similar degree of CBF reduction during MCAO. Thus, more animals per group are needed to detect differences between treatment conditions.
Mortality rate in aged ischemic rodents is significantly higher than in young animals when ischemic stroke is induced by the intraluminal approach. In some studies, the mortality rate is as high as 50% (Crapser et al., 2016). Shortening the occlusion time helps reduce mortality in the intraluminal filament stroke model in aged and comorbid animals. Based on our own experience, aged stroked rodents require careful and more frequent post-operative monitoring to avoid dehydration and excessive weight loss, which are a major cause of mortality in the intraluminal MCAO model. Importantly, mortality rate is extremely low in aged and comorbid rodents when focal ischemia is induced by distal MCAO (DeMars et al., 2019; Hermann et al., 2019b), which makes this stroke model very useful for studies using aged and/or comorbid animals. Similar to the distal MCAO, the photothrombotic stroke model induces smaller infarcts with lower mortality rates and this model has been employed to overcome the challenge of high mortality typically seen in aged animals or in the db/db mouse model of diabetes.
An important consideration in conducting stroke preclinical work using aged rodents is the very high cost of acquiring and using these animals. When establishing an aging colony, per diem animal housing charges add up quickly. Similarly, procuring aged animals from commercial suppliers is expensive. Overall, the cost of using aged rodents could be several times higher than performing studies in young-adult animals.
7. Concluding remarks
Experimentally-induced focal cerebral ischemia in young and healthy rodents does not mimic the highly heterogenous and complex nature of human stroke and could lead to false conclusions regarding therapeutic efficacy of potential neuroprotective approaches. Improving the success in translating preclinical stroke research into the clinic will require incorporating better animal models to mimic human stroke. Use of aged animals and/or animals suffering from comorbidities in preclinical stroke modeling is clinically more relevant. Underlying molecular mechanisms of protection by drugs or non-pharmacological approaches could be significantly altered in aged animals compared to young ones. This is a critical factor to be considered in the road to translation from animal studies to the clinic. Moreover, our preclinical models should incorporate a more realistic therapeutic time window, as well as clinically-relevant endpoints to assess long-term recovery of neurological function.
Acknowledgements
The authors thank Jonathan Larochelle, B.S., Dr. Changjun Yang, and Dr. Lei Liu for valuable comments and suggestions.
Funding sources
Authors are supported by grants from the National Institute of Neurological Disorders and Stroke, NINDS/NIH (R01 NS103094 and R01 NS109816 to E.C.J and R01 NS059962 to S.P.).
Footnotes
Declaration of Competing Interests
The authors declare no competing interests.
References
- Adamczak J, Aswendt M, Kreutzer C, Rotheneichner P, Riou A, Selt M, Beyrau A, Uhlenkuken U, Diedenhofen M, Nelles M, Aigner L, Couillard-Despres S, Hoehn M, 2017. Neurogenesis upregulation on the healthy hemisphere after stroke enhances compensation for age-dependent decrease of basal neurogenesis. Neurobiol Dis 99, 47–57. 10.1016/j.nbd.2016.12.015 [DOI] [PubMed] [Google Scholar]
- Adibhatla RM, Hatcher JF, 2008. Tissue plasminogen activator (tPA) and matrix metalloproteinases in the pathogenesis of stroke: therapeutic strategies. CNS & neurological disorders drug targets 7, 243–253. 10.2174/187152708784936608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmari N, Santisteban MM, Miller DR, Geis NM, Larkin R, Redler T, Denson H, Khoshbouei H, Baekey DM, Raizada MK, Zubcevic J, 2019. Elevated bone marrow sympathetic drive precedes systemic inflammation in angiotensin II hypertension. Am J Physiol Heart Circ Physiol 317, H279–h289. 10.1152/ajpheart.00510.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed N, Wahlgren N, Brainin M, Castillo J, Ford GA, Kaste M, Lees KR, Toni D, 2009. Relationship of blood pressure, antihypertensive therapy, and outcome in ischemic stroke treated with intravenous thrombolysis: retrospective analysis from Safe Implementation of Thrombolysis in Stroke-International Stroke Thrombolysis Register (SITS-ISTR). Stroke 40, 2442–2449. 10.1161/strokeaha.109.548602 [DOI] [PubMed] [Google Scholar]
- Ahnstedt H, Patrizz A, Chauhan A, Roy-O’Reilly M, Furr JW, Spychala MS, D’Aigle J, Blixt FW, Zhu L, Bravo Alegria J, McCullough LD, 2020. Sex differences in T cell immune responses, gut permeability and outcome after ischemic stroke in aged mice. Brain, behavior, and immunity 87, 556–567. 10.1016/j.bbi.2020.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainslie PN, Cotter JD, George KP, Lucas S, Murrell C, Shave R, Thomas KN, Williams MJ, Atkinson G, 2008. Elevation in cerebral blood flow velocity with aerobic fitness throughout healthy human ageing. J Physiol 586, 4005–4010. 10.1113/jphysiol.2008.158279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, McTaggart RA, Torbey MT, Kim-Tenser M, Leslie-Mazwi T, Sarraj A, Kasner SE, Ansari SA, Yeatts SD, Hamilton S, Mlynash M, Heit JJ, Zaharchuk G, Kim S, Carrozzella J, Palesch YY, Demchuk AM, Bammer R, Lavori PW, Broderick JP, Lansberg MG, Investigators D, 2018a. Thrombectomy for Stroke at 6 to 16 Hours with Selection by Perfusion Imaging. N Engl J Med 378, 708–718. 10.1056/NEJMoa1713973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albers GW, Marks MP, Lansberg MG, 2018b. Thrombectomy for Stroke with Selection by Perfusion Imaging. N Engl J Med 378, 1849–1850. 10.1056/NEJMc1803856 [DOI] [PubMed] [Google Scholar]
- AlSibai A, Qureshi AI, 2016. Management of Acute Hypertensive Response in Patients With Ischemic Stroke. Neurohospitalist 6, 122–129. 10.1177/1941874416630029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Sabín J, Molina CA, Ribó M, Arenillas JF, Montaner J, Huertas R, Santamarina E, Rubiera M, 2004. Impact of admission hyperglycemia on stroke outcome after thrombolysis: risk stratification in relation to time to reperfusion. Stroke 35, 2493–2498. 10.1161/01.STR.0000143728.45516.c6 [DOI] [PubMed] [Google Scholar]
- Andersen MB, Zimmer J, Sams-Dodd F, 1999. Specific behavioral effects related to age and cerebral ischemia in rats. Pharmacology, biochemistry, and behavior 62, 673–682. 10.1016/s0091-3057(98)00204-4 [DOI] [PubMed] [Google Scholar]
- Apple DM, Solano-Fonseca R, Kokovay E, 2017. Neurogenesis in the aging brain. Biochemical pharmacology 141, 77–85. 10.1016/j.bcp.2017.06.116 [DOI] [PubMed] [Google Scholar]
- Ay H, Koroshetz WJ, Vangel M, Benner T, Melinosky C, Zhu M, Menezes N, Lopez CJ, Sorensen AG, 2005. Conversion of ischemic brain tissue into infarction increases with age. Stroke 36, 2632–2636. 10.1161/01.STR.0000189991.23918.01 [DOI] [PubMed] [Google Scholar]
- Ayata C, Shin HK, Dileköz E, Atochin DN, Kashiwagi S, Eikermann-Haerter K, Huang PL, 2013. Hyperlipidemia disrupts cerebrovascular reflexes and worsens ischemic perfusion defect. J Cereb Blood Flow Metab 33, 954–962. 10.1038/jcbfm.2013.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babadjouni RM, Walcott BP, Liu Q, Tenser MS, Amar AP, Mack WJ, 2017. Neuroprotective delivery platforms as an adjunct to mechanical thrombectomy. Neurosurg Focus 42, E4 10.3171/2017.1.FOCUS16514 [DOI] [PubMed] [Google Scholar]
- Baird TA, Parsons MW, Phan T, Butcher KS, Desmond PM, Tress BM, Colman PG, Chambers BR, Davis SM, 2003. Persistent poststroke hyperglycemia is independently associated with infarct expansion and worse clinical outcome. Stroke 34, 2208–2214. 10.1161/01.Str.0000085087.41330.Ff [DOI] [PubMed] [Google Scholar]
- Balint AR, Puskas T, Menyhart A, Kozak G, Szenti I, Konya Z, Marek T, Bari F, Farkas E, 2019. Aging Impairs Cerebrovascular Reactivity at Preserved Resting Cerebral Arteriolar Tone and Vascular Density in the Laboratory Rat. Front Aging Neurosci 11, 301 10.3389/fnagi.2019.00301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balseanu AT, Grigore M, Pinosanu L-R, Slevin M, Hermann DM, Glavan D, Popa-Wagner A, 2020. Electric Stimulation of Neurogenesis Improves Behavioral Recovery After Focal Ischemia in Aged Rats. Frontiers in neuroscience 14 10.3389/fnins.2020.00732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks WA, Farr SA, Morley JE, 2000. Permeability of the blood-brain barrier to albumin and insulin in the young and aged SAMP8 mouse. J Gerontol A Biol Sci Med Sci 55, B601–606. 10.1093/gerona/55.12.b601 [DOI] [PubMed] [Google Scholar]
- Bazzano LA, Gu D, Whelton MR, Wu X, Chen CS, Duan X, Chen J, Chen JC, He J, 2010. Body mass index and risk of stroke among Chinese men and women. Ann Neurol 67, 11–20. 10.1002/ana.21950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bémeur C, Ste-Marie L, Desjardins P, Vachon L, Butterworth RF, Hazell AS, Montgomery J, 2005. Dehydroascorbic acid normalizes several markers of oxidative stress and inflammation in acute hyperglycemic focal cerebral ischemia in the rat. Neurochem Int 46, 399–407. 10.1016/j.neuint.2004.11.007 [DOI] [PubMed] [Google Scholar]
- Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P, 2017. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation 135, e146–e603. 10.1161/CIR.0000000000000485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennion DM, Isenberg JD, Harmel AT, DeMars K, Dang AN, Jones CH, Pignataro ME, Graham JT, Steckelings UM, Alexander JC, Febo M, Krause EG, de Kloet AD, Candelario-Jalil E, Sumners C, 2017. Post-stroke angiotensin II type 2 receptor activation provides long-term neuroprotection in aged rats. PloS one 12, e0180738 10.1371/journal.pone.0180738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berge E, Al-Shahi Salman R, van der Worp HB, Stapf C, Sandercock P, Sprigg N, Macleod MR, Kelly PJ, Nederkoorn PJ, Ford GA, European Stroke Organisation Trials Network, C., 2017. Increasing value and reducing waste in stroke research. Lancet Neurol 16, 399–408. 10.1016/S1474-4422(17)30078-9 [DOI] [PubMed] [Google Scholar]
- Biagi E, Candela M, Franceschi C, Brigidi P, 2011. The aging gut microbiota: new perspectives. Ageing Res Rev 10, 428–429. 10.1016/j.arr.2011.03.004 [DOI] [PubMed] [Google Scholar]
- Black JE, Polinsky M, Greenough WT, 1989. Progressive failure of cerebral angiogenesis supporting neural plasticity in aging rats. Neurobiol Aging 10, 353–358. 10.1016/0197-4580(89)90048-1 [DOI] [PubMed] [Google Scholar]
- Blasco MP, Chauhan A, Honarpisheh P, Ahnstedt H, d’Aigle J, Ganesan A, Ayyaswamy S, Blixt F, Venable S, Major A, Durgan D, Haag A, Kofler J, Bryan R, McCullough LD, Ganesh BP, 2020. Age-dependent involvement of gut mast cells and histamine in post-stroke inflammation. J Neuroinflammation 17, 160 10.1186/s12974-020-01833-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosetti F, Koenig JI, Ayata C, Back SA, Becker K, Broderick JP, Carmichael ST, Cho S, Cipolla MJ, Corbett D, Corriveau RA, Cramer SC, Ferguson AR, Finklestein SP, Ford BD, Furie KL, Hemmen TM, Iadecola C, Jakeman LB, Janis S, Jauch EC, Johnston KC, Kochanek PM, Kohn H, Lo EH, Lyden PD, Mallard C, McCullough LD, McGavern LM, Meschia JF, Moy CS, Perez-Pinzon MA, Ramadan I, Savitz SI, Schwamm LH, Steinberg GK, Stenzel-Poore MP, Tymianski M, Warach S, Wechsler LR, Zhang JH, Koroshetz W, 2017. Translational Stroke Research: Vision and Opportunities. Stroke 48, 2632–2637. 10.1161/STROKEAHA.117.017112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brindle NP, Saharinen P, Alitalo K, 2006. Signaling and functions of angiopoietin-1 in vascular protection. Circulation research 98, 1014–1023. 10.1161/01.Res.0000218275.54089.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AW, Marlowe KJ, Bjelke B, 2003. Age effect on motor recovery in a post-acute animal stroke model. Neurobiol Aging 24, 607–614. 10.1016/s0197-4580(02)00129-x [DOI] [PubMed] [Google Scholar]
- Brownlee M, 2001. Biochemistry and molecular cell biology of diabetic complications. Nature 414, 813–820. 10.1038/414813a [DOI] [PubMed] [Google Scholar]
- Bruno A, Levine SR, Frankel MR, Brott TG, Lin Y, Tilley BC, Lyden PD, Broderick JP, Kwiatkowski TG, Fineberg SE, 2002. Admission glucose level and clinical outcomes in the NINDS rt-PA Stroke Trial. Neurology 59, 669–674. 10.1212/wnl.59.5.669 [DOI] [PubMed] [Google Scholar]
- Buchhold B, Mogoanta L, Suofu Y, Hamm A, Walker L, Kessler C, Popa-Wagner A, 2007. Environmental enrichment improves functional and neuropathological indices following stroke in young and aged rats. Restorative neurology and neuroscience 25, 467–484. [PubMed] [Google Scholar]
- Cai M, Zhang W, Weng Z, Stetler RA, Jiang X, Shi Y, Gao Y, Chen J, 2017. Promoting Neurovascular Recovery in Aged Mice after Ischemic Stroke - Prophylactic Effect of Omega-3 Polyunsaturated Fatty Acids. Aging and disease 8, 531–545. 10.14336/AD.2017.0520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell BC, Christensen S, Tress BM, Churilov L, Desmond PM, Parsons MW, Barber PA, Levi CR, Bladin C, Donnan GA, Davis SM, 2013. Failure of collateral blood flow is associated with infarct growth in ischemic stroke. J Cereb Blood Flow Metab 33, 1168–1172. 10.1038/jcbfm.2013.77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell BCV, Ma H, Ringleb PA, Parsons MW, Churilov L, Bendszus M, Levi CR, Hsu C, Kleinig TJ, Fatar M, Leys D, Molina C, Wijeratne T, Curtze S, Dewey HM, Barber PA, Butcher KS, De Silva DA, Bladin CF, Yassi N, Pfaff JAR, Sharma G, Bivard A, Desmond PM, Schwab S, Schellinger PD, Yan B, Mitchell PJ, Serena J, Toni D, Thijs V, Hacke W, Davis SM, Donnan GA, Extend E, Investigators E, 2019. Extending thrombolysis to 4.5–9 h and wake-up stroke using perfusion imaging: a systematic review and meta-analysis of individual patient data. Lancet 394, 139–147. 10.1016/S0140-6736(19)31053-0 [DOI] [PubMed] [Google Scholar]
- Candelario-Jalil E, 2009. Injury and repair mechanisms in ischemic stroke: considerations for the development of novel neurotherapeutics. Curr Opin Investig Drugs 10, 644–654. [PubMed] [Google Scholar]
- Canese R, Fortuna S, Lorenzini P, Podo F, Michalek H, 1998. Transient global brain ischemia in young and aged rats: differences in severity and progression, but not localisation, of lesions evaluated by magnetic resonance imaging. MAGMA 7, 28–34. 10.1007/BF02592254 [DOI] [PubMed] [Google Scholar]
- Cao XL, Du J, Zhang Y, Yan JT, Hu XM, 2015. Hyperlipidemia exacerbates cerebral injury through oxidative stress, inflammation and neuronal apoptosis in MCAO/reperfusion rats. Exp Brain Res 233, 2753–2765. 10.1007/s00221-015-4269-x [DOI] [PubMed] [Google Scholar]
- Capes SE, Hunt D, Malmberg K, Pathak P, Gerstein HC, 2001. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke 32, 2426–2432. 10.1161/hs1001.096194 [DOI] [PubMed] [Google Scholar]
- Carmichael ST, 2016. The 3 Rs of Stroke Biology: Radial, Relayed, and Regenerative. Neurotherapeutics 13, 348–359. 10.1007/s13311-015-0408-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casetta I, Fainardi E, Saia V, Pracucci G, Padroni M, Renieri L, Nencini P, Inzitari D, Morosetti D, Sallustio F, Vallone S, Bigliardi G, Zini A, Longo M, Francalanza I, Bracco S, Vallone IM, Tassi R, Bergui M, Naldi A, Saletti A, De Vito A, Gasparotti R, Magoni M, Castellan L, Serrati C, Menozzi R, Scoditti U, Causin F, Pieroni A, Puglielli E, Casalena A, Sanna A, Ruggiero M, Cordici F, Di Maggio L, Duc E, Cosottini M, Giannini N, Sanfilippo G, Zappoli F, Cavallini A, Cavasin N, Critelli A, Ciceri E, Plebani M, Cappellari M, Chiumarulo L, Petruzzellis M, Terrana A, Cariddi LP, Burdi N, Tinelli A, Auteri W, Silvagni U, Biraschi F, Nicolini E, Padolecchia R, Tassinari T, Filauri P, Sacco S, Pavia M, Invernizzi P, Nuzzi NP, Marcheselli S, Amista P, Russo M, Gallesio I, Craparo G, Mannino M, Mangiafico S, Toni D, Italian Registry of Endovascular Treatment in Acute, S., 2020. Endovascular Thrombectomy for Acute Ischemic Stroke Beyond 6 Hours From Onset: A Real-World Experience. Stroke 51, 2051–2057. 10.1161/STROKEAHA.119.027974 [DOI] [PubMed] [Google Scholar]
- Chan CC, Damen M, Alarcon PC, Sanchez-Gurmaches J, Divanovic S, 2019. Inflammation and Immunity: From an Adipocyte’s Perspective. Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research 39, 459–471. 10.1089/jir.2019.0014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan PH, 2001. Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab 21, 2–14. 10.1097/00004647-200101000-00002 [DOI] [PubMed] [Google Scholar]
- Chen J, Cui X, Zacharek A, Cui Y, Roberts C, Chopp M, 2011. White matter damage and the effect of matrix metalloproteinases in type 2 diabetic mice after stroke. Stroke 42, 445–452. 10.1161/STROKEAHA.110.596486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Liang J, Ouyang F, Chen X, Lu T, Jiang Z, Li J, Li Y, Zeng J, 2019. Persistence of Gut Microbiota Dysbiosis and Chronic Systemic Inflammation After Cerebral Infarction in Cynomolgus Monkeys. Frontiers in neurology 10, 661 10.3389/fneur.2019.00661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Sun FY, 2007. Age-related decrease of striatal neurogenesis is associated with apoptosis of neural precursors and newborn neurons in rat brain after ischemia. Brain Res 1166, 9–19. 10.1016/j.brainres.2007.06.043 [DOI] [PubMed] [Google Scholar]
- Cho S, Yang J, 2018. What Do Experimental Models Teach Us About Comorbidities in Stroke? Stroke 49, 501–507. 10.1161/strokeaha.117.017793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crapser J, Ritzel R, Verma R, Venna VR, Liu F, Chauhan A, Koellhoffer E, Patel A, Ricker A, Maas K, Graf J, McCullough LD, 2016. Ischemic stroke induces gut permeability and enhances bacterial translocation leading to sepsis in aged mice. Aging (Albany NY) 8, 1049–1063. 10.18632/aging.100952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X, Chopp M, Zacharek A, Ye X, Roberts C, Chen J, 2011. Angiopoietin/Tie2 pathway mediates type 2 diabetes induced vascular damage after cerebral stroke. Neurobiol Dis 43, 285–292. 10.1016/j.nbd.2011.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler RR, Kokovay E, 2019. Rejuvenating subventricular zone neurogenesis in the aging brain. Curr Opin Pharmacol 50, 1–8. 10.1016/j.coph.2019.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darsalia V, Heldmann U, Lindvall O, Kokaia Z, 2005. Stroke-induced neurogenesis in aged brain. Stroke 36, 1790–1795. 10.1161/01.STR.0000173151.36031.be [DOI] [PubMed] [Google Scholar]
- Davis M, Mendelow AD, Perry RH, Chambers IR, James OF, 1995. Experimental stroke and neuroprotection in the aging rat brain. Stroke 26, 1072–1078. 10.1161/01.str.26.6.1072 [DOI] [PubMed] [Google Scholar]
- Daynac M, Morizur L, Chicheportiche A, Mouthon MA, Boussin FD, 2016. Age-related neurogenesis decline in the subventricular zone is associated with specific cell cycle regulation changes in activated neural stem cells. Sci Rep 6, 21505 10.1038/srep21505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vis JB, Hendrikse J, Bhogal A, Adams A, Kappelle LJ, Petersen ET, 2015. Age-related changes in brain hemodynamics; A calibrated MRI study. Hum Brain Mapp 36, 3973–3987. 10.1002/hbm.22891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaerschalk BM, Kleindorfer DO, Adeoye OM, Demchuk AM, Fugate JE, Grotta JC, Khalessi AA, Levy EI, Palesch YY, Prabhakaran S, Saposnik G, Saver JL, Smith EE, American Heart Association Stroke, C., Council on, E., Prevention, 2016. Scientific Rationale for the Inclusion and Exclusion Criteria for Intravenous Alteplase in Acute Ischemic Stroke: A Statement for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 47, 581–641. 10.1161/STR.0000000000000086 [DOI] [PubMed] [Google Scholar]
- DeMars KM, Yang C, Candelario-Jalil E, 2019. Neuroprotective effects of targeting BET proteins for degradation with dBET1 in aged mice subjected to ischemic stroke. Neurochem Int 10.1016/j.neuint.2019.03.004. 10.1016/j.neuint.2019.03.004 [DOI] [PubMed] [Google Scholar]
- Deng J, Zhang J, Feng C, Xiong L, Zuo Z, 2014. Critical role of matrix metalloprotease-9 in chronic high fat diet-induced cerebral vascular remodelling and increase of ischaemic brain injury in mice†. Cardiovasc Res 103, 473–484. 10.1093/cvr/cvu154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch C, Portik-Dobos V, Smith AD, Ergul A, Dorrance AM, 2009. Diet-induced obesity causes cerebral vessel remodeling and increases the damage caused by ischemic stroke. Microvasc Res 78, 100–106. 10.1016/j.mvr.2009.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekhorst L, Gómez-de Frutos MC, Laso-García F, Otero-Ortega L, Fuentes B, Jolkkonen J, Detante O, Moisan A, Leyva L, Martínez-Arroyo A, Díez-Tejedor E, Gutiérrez-Fernández M, 2020. Mesenchymal Stem Cells From Adipose Tissue Do not Improve Functional Recovery After Ischemic Stroke in Hypertensive Rats. Stroke 51, 342–346. 10.1161/strokeaha.119.027133 [DOI] [PubMed] [Google Scholar]
- DiNapoli VA, Huber JD, Houser K, Li X, Rosen CL, 2008. Early disruptions of the blood-brain barrier may contribute to exacerbated neuronal damage and prolonged functional recovery following stroke in aged rats. Neurobiol Aging 29, 753–764. 10.1016/j.neurobiolaging.2006.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirnagl U, Iadecola C, Moskowitz MA, 1999. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci 22, 391–397. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Macleod MR, 2009. Stroke research at a road block: the streets from adversity should be paved with meta-analysis and good laboratory practice. Br J Pharmacol 157, 1154–1156. 10.1111/j.1476-5381.2009.00211.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittmar MS, Vatankhah B, Fehm NP, Schuierer G, Bogdahn U, Horn M, Schlachetzki F, 2006. Fischer-344 rats are unsuitable for the MCAO filament model due to their cerebrovascular anatomy. J.Neurosci.Methods 156, 50–54. [DOI] [PubMed] [Google Scholar]
- Doehner W, Schenkel J, Anker SD, Springer J, Audebert HJ, 2013. Overweight and obesity are associated with improved survival, functional outcome, and stroke recurrence after acute stroke or transient ischaemic attack: observations from the TEMPiS trial. European heart journal 34, 268–277. 10.1093/eurheartj/ehs340 [DOI] [PubMed] [Google Scholar]
- Dohmen C, Sakowitz OW, Fabricius M, Bosche B, Reithmeier T, Ernestus RI, Brinker G, Dreier JP, Woitzik J, Strong AJ, Graf R, Co-Operative Study of Brain Injury, D., 2008. Spreading depolarizations occur in human ischemic stroke with high incidence. Ann Neurol 63, 720–728. 10.1002/ana.21390 [DOI] [PubMed] [Google Scholar]
- Doyle KP, Cekanaviciute E, Mamer LE, Buckwalter MS, 2010. TGFbeta signaling in the brain increases with aging and signals to astrocytes and innate immune cells in the weeks after stroke. J Neuroinflammation 7, 62 10.1186/1742-2094-7-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreier JP, 2011. The role of spreading depression, spreading depolarization and spreading ischemia in neurological disease. Nat Med 17, 439–447. 10.1038/nm.2333 [DOI] [PubMed] [Google Scholar]
- Dreier JP, Lemale CL, Kola V, Friedman A, Schoknecht K, 2018. Spreading depolarization is not an epiphenomenon but the principal mechanism of the cytotoxic edema in various gray matter structures of the brain during stroke. Neuropharmacology 134, 189–207. 10.1016/j.neuropharm.2017.09.027 [DOI] [PubMed] [Google Scholar]
- ElAli A, Doeppner TR, Zechariah A, Hermann DM, 2011. Increased blood-brain barrier permeability and brain edema after focal cerebral ischemia induced by hyperlipidemia: role of lipid peroxidation and calpain-1/2, matrix metalloproteinase-2/9, and RhoA overactivation. Stroke 42, 3238–3244. 10.1161/strokeaha.111.615559 [DOI] [PubMed] [Google Scholar]
- Elgebaly MM, Ogbi S, Li W, Mezzetti EM, Prakash R, Johnson MH, Bruno A, Fagan SC, Ergul A, 2011. Neurovascular injury in acute hyperglycemia and diabetes: A comparative analysis in experimental stroke. Translational stroke research 2, 391–398. 10.1007/s12975-011-0083-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgebaly MM, Prakash R, Li W, Ogbi S, Johnson MH, Mezzetti EM, Fagan SC, Ergul A, 2010. Vascular protection in diabetic stroke: role of matrix metalloprotease-dependent vascular remodeling. J Cereb Blood Flow Metab 30, 1928–1938. 10.1038/jcbfm.2010.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elijovich L, Goyal N, Mainali S, Hoit D, Arthur AS, Whitehead M, Choudhri AF, 2016. CTA collateral score predicts infarct volume and clinical outcome after endovascular therapy for acute ischemic stroke: a retrospective chart review. J Neurointerv Surg 8, 559–562. 10.1136/neurintsurg-2015-011731 [DOI] [PubMed] [Google Scholar]
- Elkind MS, 2006. Inflammation, atherosclerosis, and stroke. Neurologist 12, 140–148. 10.1097/01.nrl.0000215789.70804.b0 [DOI] [PubMed] [Google Scholar]
- Emberson J, Lees KR, Lyden P, Blackwell L, Albers G, Bluhmki E, Brott T, Cohen G, Davis S, Donnan G, Grotta J, Howard G, Kaste M, Koga M, von Kummer R, Lansberg M, Lindley RI, Murray G, Olivot JM, Parsons M, Tilley B, Toni D, Toyoda K, Wahlgren N, Wardlaw J, Whiteley W, del Zoppo GJ, Baigent C, Sandercock P, Hacke W, Stroke Thrombolysis Trialists’ Collaborative, G., 2014. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet 384, 1929–1935. 10.1016/S0140-6736(14)60584-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ergul A, Abdelsaid M, Fouda AY, Fagan SC, 2014. Cerebral neovascularization in diabetes: implications for stroke recovery and beyond. J Cereb Blood Flow Metab 34, 553–563. 10.1038/jcbfm.2014.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ergul A, Elgebaly MM, Middlemore ML, Li W, Elewa H, Switzer JA, Hall C, Kozak A, Fagan SC, 2007. Increased hemorrhagic transformation and altered infarct size and localization after experimental stroke in a rat model type 2 diabetes. BMC neurology 7, 33 10.1186/1471-2377-7-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ergul A, Li W, Elgebaly MM, Bruno A, Fagan SC, 2009. Hyperglycemia, diabetes and stroke: focus on the cerebrovasculature. Vascul Pharmacol 51, 44–49. 10.1016/j.vph.2009.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erny D, Prinz M, 2020. How microbiota shape microglial phenotypes and epigenetics. Glia 68, 1655–1672. 10.1002/glia.23822 [DOI] [PubMed] [Google Scholar]
- Faber JE, Zhang H, Lassance-Soares RM, Prabhakar P, Najafi AH, Burnett MS, Epstein SE, 2011. Aging causes collateral rarefaction and increased severity of ischemic injury in multiple tissues. Arteriosclerosis, thrombosis, and vascular biology 31, 1748–1756. 10.1161/ATVBAHA.111.227314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagiani E, Christofori G, 2013. Angiopoietins in angiogenesis. Cancer letters 328, 18–26. 10.1016/j.canlet.2012.08.018 [DOI] [PubMed] [Google Scholar]
- Farrall AJ, Wardlaw JM, 2009. Blood-brain barrier: ageing and microvascular disease--systematic review and meta-analysis. Neurobiol Aging 30, 337–352. 10.1016/j.neurobiolaging.2007.07.015 [DOI] [PubMed] [Google Scholar]
- Fassbender K, Grotta JC, Walter S, Grunwald IQ, Ragoschke-Schumm A, Saver JL, 2017. Mobile stroke units for prehospital thrombolysis, triage, and beyond: benefits and challenges. Lancet Neurol 16, 227–237. 10.1016/S1474-4422(17)30008-X [DOI] [PubMed] [Google Scholar]
- Federau C, Mlynash M, Christensen S, Zaharchuk G, Cha B, Lansberg MG, Wintermark M, Albers GW, 2016. Evolution of Volume and Signal Intensity on Fluid-attenuated Inversion Recovery MR Images after Endovascular Stroke Therapy. Radiology 280, 184–192. 10.1148/radiol.2015151586 [DOI] [PubMed] [Google Scholar]
- Ferrara F, Zeisig V, Pietsch S, Rutten R, Dreyer AY, Pieper L, Schatzl AK, McLeod DD, Barthel H, Boltze J, Schrodl W, Nitzsche B, 2020. Hypothesis and Theory: A Pathophysiological Concept of Stroke-Induced Acute Phase Response and Increased Intestinal Permeability Leading to Secondary Brain Damage. Frontiers in neuroscience 14, 272 10.3389/fnins.2020.00272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M, Feuerstein G, Howells DW, Hurn PD, Kent TA, Savitz SI, Lo EH, Group S, 2009. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke 40, 2244–2250. 10.1161/STROKEAHA.108.541128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flottmann F, Leischner H, Broocks G, Nawabi J, Bernhardt M, Faizy TD, Deb-Chatterji M, Thomalla G, Fiehler J, Brekenfeld C, 2018. Recanalization Rate per Retrieval Attempt in Mechanical Thrombectomy for Acute Ischemic Stroke. Stroke 49, 2523–2525. 10.1161/STROKEAHA.118.022737 [DOI] [PubMed] [Google Scholar]
- Franceschi C, Garagnani P, Parini P, Giuliani C, Santoro A, 2018. Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat Rev Endocrinol 14, 576–590. 10.1038/s41574-018-0059-4 [DOI] [PubMed] [Google Scholar]
- Frank MG, Barrientos RM, Biedenkapp JC, Rudy JW, Watkins LR, Maier SF, 2006. mRNA up-regulation of MHC II and pivotal pro-inflammatory genes in normal brain aging. Neurobiol Aging 27, 717–722. 10.1016/j.neurobiolaging.2005.03.013 [DOI] [PubMed] [Google Scholar]
- Fujita K, Tanaka K, Yamagami H, Ide T, Ishiyama H, Sonoda K, Satow T, Takahashi JC, Ihara M, Koga M, Yokota T, Toyoda K, 2019. Detrimental Effect of Chronic Hypertension on Leptomeningeal Collateral Flow in Acute Ischemic Stroke. Stroke 50, 1751–1757. 10.1161/strokeaha.119.025142 [DOI] [PubMed] [Google Scholar]
- Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, Ferrucci L, Gilroy DW, Fasano A, Miller GW, Miller AH, Mantovani A, Weyand CM, Barzilai N, Goronzy JJ, Rando TA, Effros RB, Lucia A, Kleinstreuer N, Slavich GM, 2019. Chronic inflammation in the etiology of disease across the life span. Nat Med 25, 1822–1832. 10.1038/s41591-019-0675-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganta CK, Lu N, Helwig BG, Blecha F, Ganta RR, Zheng L, Ross CR, Musch TI, Fels RJ, Kenney MJ, 2005. Central angiotensin II-enhanced splenic cytokine gene expression is mediated by the sympathetic nervous system. Am J Physiol Heart Circ Physiol 289, H1683–1691. 10.1152/ajpheart.00125.2005 [DOI] [PubMed] [Google Scholar]
- Gavin JR, Alberti K, Davidson MB, DeFronzo RA, Drash A, Gabbe SG, Genuth S, Harris MI, Khan R, Keen H, Knowler WC, Lebovitz H, Maclaren NK, Palmer JP, Raskin P, Rizza RA, Stern MP, 2003. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes care 26 Suppl 1, S5–20. 10.2337/diacare.26.2007.s5 [DOI] [PubMed] [Google Scholar]
- Ginsberg MD, 2008. Neuroprotection for ischemic stroke: past, present and future. Neuropharmacology 55, 363–389. 10.1016/j.neuropharm.2007.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg MD, 2016. Expanding the concept of neuroprotection for acute ischemic stroke: The pivotal roles of reperfusion and the collateral circulation. Prog Neurobiol 145–146, 46–77. 10.1016/j.pneurobio.2016.09.002 [DOI] [PubMed] [Google Scholar]
- Godbout JP, Chen J, Abraham J, Richwine AF, Berg BM, Kelley KW, Johnson RW, 2005. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. FASEB J 19, 1329–1331. 10.1096/fj.05-3776fje [DOI] [PubMed] [Google Scholar]
- Gómez-de Frutos MC, Laso-García F, Diekhorst L, Otero-Ortega L, Fuentes B, Jolkkonen J, Detante O, Moisan A, Martínez-Arroyo A, Díez-Tejedor E, Gutiérrez-Fernández M, 2019. Intravenous delivery of adipose tissue-derived mesenchymal stem cells improves brain repair in hyperglycemic stroke rats. Stem Cell Res Ther 10, 212 10.1186/s13287-019-1322-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodall EF, Leach V, Wang C, Cooper-Knock J, Heath PR, Baker D, Drew DR, Saffrey MJ, Simpson JE, Romero IA, Wharton SB, 2019. Age-Associated mRNA and miRNA Expression Changes in the Blood-Brain Barrier. International journal of molecular sciences 20 10.3390/ijms20123097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodall EF, Wang C, Simpson JE, Baker DJ, Drew DR, Heath PR, Saffrey MJ, Romero IA, Wharton SB, 2018. Age-associated changes in the blood-brain barrier: comparative studies in human and mouse. Neuropathol Appl Neurobiol 44, 328–340. 10.1111/nan.12408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, Davalos A, Majoie CB, van der Lugt A, de Miquel MA, Donnan GA, Roos YB, Bonafe A, Jahan R, Diener HC, van den Berg LA, Levy EI, Berkhemer OA, Pereira VM, Rempel J, Millan M, Davis SM, Roy D, Thornton J, Roman LS, Ribo M, Beumer D, Stouch B, Brown S, Campbell BC, van Oostenbrugge RJ, Saver JL, Hill MD, Jovin TG, collaborators H, 2016. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 387, 1723–1731. 10.1016/S0140-6736(16)00163-X [DOI] [PubMed] [Google Scholar]
- Grupke S, Hall J, Dobbs M, Bix GJ, Fraser JF, 2015. Understanding history, and not repeating it. Neuroprotection for acute ischemic stroke: from review to preview. Clinical neurology and neurosurgery 129, 1–9. 10.1016/j.clineuro.2014.11.013 [DOI] [PubMed] [Google Scholar]
- Gutiérrez-Fernández M, Rodríguez-Frutos B, Ramos-Cejudo J, Otero-Ortega L, Fuentes B, Vallejo-Cremades MT, Sanz-Cuesta BE, Díez-Tejedor E, 2015. Comparison between xenogeneic and allogeneic adipose mesenchymal stem cells in the treatment of acute cerebral infarct: proof of concept in rats. J Transl Med 13, 46 10.1186/s12967-015-0406-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez-Fernández M, Rodríguez-Frutos B, Ramos-Cejudo J, Teresa Vallejo-Cremades M, Fuentes B, Cerdán S, Díez-Tejedor E, 2013. Effects of intravenous administration of allogenic bone marrow- and adipose tissue-derived mesenchymal stem cells on functional recovery and brain repair markers in experimental ischemic stroke. Stem Cell Res Ther 4, 11 10.1186/scrt159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacke W, Albers G, Al-Rawi Y, Bogousslavsky J, Davalos A, Eliasziw M, Fischer M, Furlan A, Kaste M, Lees KR, Soehngen M, Warach S, Group DS, 2005. The Desmoteplase in Acute Ischemic Stroke Trial (DIAS): a phase II MRI-based 9-hour window acute stroke thrombolysis trial with intravenous desmoteplase. Stroke 36, 66–73. 10.1161/01.STR.0000149938.08731.2c [DOI] [PubMed] [Google Scholar]
- Hafez S, Abdelsaid M, El-Shafey S, Johnson MH, Fagan SC, Ergul A, 2016. Matrix Metalloprotease 3 Exacerbates Hemorrhagic Transformation and Worsens Functional Outcomes in Hyperglycemic Stroke. Stroke 47, 843–851. 10.1161/STROKEAHA.115.011258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafez S, Abdelsaid M, Fagan SC, Ergul A, 2017. Peroxynitrite-Induced Tyrosine Nitration Contributes to Matrix Metalloprotease-3 Activation: Relevance to Hyperglycemic Ischemic Brain Injury and Tissue Plasminogen Activator. Neurochemical research 10.1007/s11064-017-2411-9. 10.1007/s11064-017-2411-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafezi-Moghadam A, Thomas KL, Wagner DD, 2007. ApoE deficiency leads to a progressive age-dependent blood-brain barrier leakage. Am J Physiol Cell Physiol 292, C1256–1262. 10.1152/ajpcell.00563.2005 [DOI] [PubMed] [Google Scholar]
- Haley MJ, Krishnan S, Burrows D, de Hoog L, Thakrar J, Schiessl I, Allan SM, Lawrence CB, 2019. Acute high-fat feeding leads to disruptions in glucose homeostasis and worsens stroke outcome. J Cereb Blood Flow Metab 39, 1026–1037. 10.1177/0271678X17744718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley MJ, Lawrence CB, 2016. Obesity and stroke: Can we translate from rodents to patients? J Cereb Blood Flow Metab 36, 2007–2021. 10.1177/0271678X16670411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley MJ, Lawrence CB, 2017. The blood-brain barrier after stroke: Structural studies and the role of transcytotic vesicles. J Cereb Blood Flow Metab 37, 456–470. 10.1177/0271678X16629976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley MJ, Mullard G, Hollywood KA, Cooper GJ, Dunn WB, Lawrence CB, 2017. Adipose tissue and metabolic and inflammatory responses to stroke are altered in obese mice. Disease models & mechanisms 10, 1229–1243. 10.1242/dmm.030411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata J, Kiyohara Y, 2013. Epidemiology of stroke and coronary artery disease in Asia. Circulation journal : official journal of the Japanese Circulation Society 77, 1923–1932. [DOI] [PubMed] [Google Scholar]
- Heo JH, Han SW, Lee SK, 2005. Free radicals as triggers of brain edema formation after stroke. Free Radic Biol Med 39, 51–70. [DOI] [PubMed] [Google Scholar]
- Hermann DM, Doeppner TR, Popa-Wagner A, 2019a. Opportunities and Limitations of Vascular Risk Factor Models in Studying Plasticity-Promoting and Restorative Ischemic Stroke Therapies. Neural plasticity 2019, 9785476 10.1155/2019/9785476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann DM, Popa-Wagner A, Kleinschnitz C, Doeppner TR, 2019b. Animal models of ischemic stroke and their impact on drug discovery. Expert Opin Drug Discov 14, 315–326. 10.1080/17460441.2019.1573984 [DOI] [PubMed] [Google Scholar]
- Hertelendy P, Varga DP, Menyhart A, Bari F, Farkas E, 2019. Susceptibility of the cerebral cortex to spreading depolarization in neurological disease states: The impact of aging. Neurochem Int 127, 125–136. 10.1016/j.neuint.2018.10.010 [DOI] [PubMed] [Google Scholar]
- Herz J, Hagen SI, Bergmüller E, Sabellek P, Göthert JR, Buer J, Hansen W, Hermann DM, Doeppner TR, 2014. Exacerbation of ischemic brain injury in hypercholesterolemic mice is associated with pronounced changes in peripheral and cerebral immune responses. Neurobiol Dis 62, 456–468. 10.1016/j.nbd.2013.10.022 [DOI] [PubMed] [Google Scholar]
- Herz J, Sabellek P, Lane TE, Gunzer M, Hermann DM, Doeppner TR, 2015. Role of Neutrophils in Exacerbation of Brain Injury After Focal Cerebral Ischemia in Hyperlipidemic Mice. Stroke 46, 2916–2925. 10.1161/strokeaha.115.010620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MD, Goyal M, Menon BK, Nogueira RG, McTaggart RA, Demchuk AM, Poppe AY, Buck BH, Field TS, Dowlatshahi D, van Adel BA, Swartz RH, Shah RA, Sauvageau E, Zerna C, Ospel JM, Joshi M, Almekhlafi MA, Ryckborst KJ, Lowerison MW, Heard K, Garman D, Haussen D, Cutting SM, Coutts SB, Roy D, Rempel JL, Rohr AC, Iancu D, Sahlas DJ, Yu AYX, Devlin TG, Hanel RA, Puetz V, Silver FL, Campbell BCV, Chapot R, Teitelbaum J, Mandzia JL, Kleinig TJ, Turkel-Parrella D, Heck D, Kelly ME, Bharatha A, Bang OY, Jadhav A, Gupta R, Frei DF, Tarpley JW, McDougall CG, Holmin S, Rha JH, Puri AS, Camden MC, Thomalla G, Choe H, Phillips SJ, Schindler JL, Thornton J, Nagel S, Heo JH, Sohn SI, Psychogios MN, Budzik RF, Starkman S, Martin CO, Burns PA, Murphy S, Lopez GA, English J, Tymianski M, Investigators E-N, 2020. Efficacy and safety of nerinetide for the treatment of acute ischaemic stroke (ESCAPE-NA1): a multicentre, double-blind, randomised controlled trial. Lancet 395, 878–887. 10.1016/S0140-6736(20)30258-0 [DOI] [PubMed] [Google Scholar]
- Hong KS, 2017. Blood Pressure Management for Stroke Prevention and in Acute Stroke. J Stroke 19, 152–165. 10.5853/jos.2017.00164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houlden A, Goldrick M, Brough D, Vizi ES, Lenart N, Martinecz B, Roberts IS, Denes A, 2016. Brain injury induces specific changes in the caecal microbiota of mice via altered autonomic activity and mucoprotein production. Brain, behavior, and immunity 57, 10–20. 10.1016/j.bbi.2016.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K, Ji Z, Wu Y, Huang Y, Li G, Zhou S, Yang Z, Huang W, Yang G, Weng G, Chen P, Pan S, 2020. Safety and efficacy of glibenclamide combined with rtPA in acute cerebral ischemia with occlusion/stenosis of anterior circulation (SE-GRACE): study protocol for a randomized controlled trial. BMC neurology 20, 239 10.1186/s12883-020-01823-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudobenko J, Ganesh BP, Jiang J, Mohan EC, Lee S, Sheth S, Morales D, Zhu L, Kofler JK, Pautler RG, McCullough LD, Chauhan A, 2020. Growth differentiation factor-11 supplementation improves survival and promotes recovery after ischemic stroke in aged mice. Aging (Albany NY) 12, 8049–8066. 10.18632/aging.103122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel KP, Dickie MM, Coleman DL, 1966. Diabetes, a new mutation in the mouse. Science 153, 1127–1128. 10.1126/science.153.3740.1127 [DOI] [PubMed] [Google Scholar]
- Iadecola C, 2017. The Neurovascular Unit Coming of Age: A Journey through Neurovascular Coupling in Health and Disease. Neuron 96, 17–42. 10.1016/j.neuron.2017.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C, Anrather J, 2011a. The immunology of stroke: from mechanisms to translation. Nat Med 17, 796–808. 10.1038/nm.2399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C, Anrather J, 2011b. Stroke research at a crossroad: asking the brain for directions. Nature neuroscience 14, 1363–1368. 10.1038/nn.2953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C, Buckwalter MS, Anrather J, 2020. Immune responses to stroke: mechanisms, modulation, and therapeutic potential. J Clin Invest 130, 2777–2788. 10.1172/JCI135530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegame Y, Yamashita K, Hayashi S, Mizuno H, Tawada M, You F, Yamada K, Tanaka Y, Egashira Y, Nakashima S, Yoshimura S, Iwama T, 2011. Comparison of mesenchymal stem cells from adipose tissue and bone marrow for ischemic stroke therapy. Cytotherapy 13, 675–685. 10.3109/14653249.2010.549122 [DOI] [PubMed] [Google Scholar]
- Ishikawa M, Stokes KY, Zhang JH, Nanda A, Granger DN, 2004. Cerebral microvascular responses to hypercholesterolemia: roles of NADPH oxidase and P-selectin. Circulation research 94, 239–244. 10.1161/01.Res.0000111524.05779.60 [DOI] [PubMed] [Google Scholar]
- Janota CS, Brites D, Lemere CA, Brito MA, 2015. Glio-vascular changes during ageing in wild-type and Alzheimer’s disease-like APP/PS1 mice. Brain Res 1620, 153–168. 10.1016/j.brainres.2015.04.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Mu H, Xu F, Xie D, Su W, Xu J, Sun Z, Liu S, Luo J, Shi Y, Leak RK, Wechsler LR, Chen J, Hu X, 2020. Transcriptomic and functional studies reveal undermined chemotactic and angiostimulatory properties of aged microglia during stroke recovery. J Cereb Blood Flow Metab 10.1177/0271678×20902542, 271678×20902542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Suenaga J, Pu H, Wei Z, Smith AD, Hu X, Shi Y, Chen J, 2019. Post-stroke administration of omega-3 polyunsaturated fatty acids promotes neurovascular restoration after ischemic stroke in mice: Efficacy declines with aging. Neurobiol Dis 126, 62–75. 10.1016/j.nbd.2018.09.012 [DOI] [PubMed] [Google Scholar]
- Jin K, Minami M, Xie L, Sun Y, Mao XO, Wang Y, Simon RP, Greenberg DA, 2004. Ischemia-induced neurogenesis is preserved but reduced in the aged rodent brain. Aging Cell 3, 373–377. 10.1111/j.1474-9728.2004.00131.x [DOI] [PubMed] [Google Scholar]
- Johnson CO, Nguyen M, Roth GA, Nichols E, Alam T, Collaborators, G.B.D.S., 2019. Global, regional, and national burden of stroke, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 18, 439–458. 10.1016/S1474-4422(19)30034-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakuda W, Lansberg MG, Thijs VN, Kemp SM, Bammer R, Wechsler LR, Moseley ME, Marks MP, Albers GW, 2008. Optimal definition for PWI/DWI mismatch in acute ischemic stroke patients. J Cereb Blood Flow Metab 28, 887–891. 10.1038/sj.jcbfm.9600604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamada H, Yu F, Nito C, Chan PH, 2007. Influence of hyperglycemia on oxidative stress and matrix metalloproteinase-9 activation after focal cerebral ischemia/reperfusion in rats: relation to blood-brain barrier dysfunction. Stroke 38, 1044–1049. 10.1161/01.STR.0000258041.75739.cb [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang M, Yao Y, 2020. Basement Membrane Changes in Ischemic Stroke. Stroke 51, 1344–1352. 10.1161/STROKEAHA.120.028928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai N, Keep RF, Betz AL, 1997. Hyperglycemia and the vascular effects of cerebral ischemia. Stroke 28, 149–154. 10.1161/01.str.28.1.149 [DOI] [PubMed] [Google Scholar]
- Kelly KA, Li X, Tan Z, VanGilder RL, Rosen CL, Huber JD, 2009. NOX2 inhibition with apocynin worsens stroke outcome in aged rats. Brain Res 1292, 165–172. 10.1016/j.brainres.2009.07.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MA, Schultz S, Othman A, Fleming T, Lebron-Galan R, Rades D, Clemente D, Nawroth PP, Schwaninger M, 2016. Hyperglycemia in Stroke Impairs Polarization of Monocytes/Macrophages to a Protective Noninflammatory Cell Type. J Neurosci 36, 9313–9325. 10.1523/JNEUROSCI.0473-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan NA, McAlister FA, Pilote L, Palepu A, Quan H, Hill MD, Fang J, Kapral MK, 2017. Temporal trends in stroke incidence in South Asian, Chinese and white patients: A population based analysis. PloS one 12, e0175556 10.1371/journal.pone.0175556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidwell CS, 2013. MRI biomarkers in acute ischemic stroke: a conceptual framework and historical analysis. Stroke 44, 570–578. 10.1161/STROKEAHA.111.626093 [DOI] [PubMed] [Google Scholar]
- Kim E, Cho S, 2016. Microglia and Monocyte-Derived Macrophages in Stroke. Neurotherapeutics 13, 702–718. 10.1007/s13311-016-0463-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Febbraio M, Bao Y, Tolhurst AT, Epstein JM, Cho S, 2012. CD36 in the periphery and brain synergizes in stroke injury in hyperlipidemia. Ann Neurol 71, 753–764. 10.1002/ana.23569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Tolhurst AT, Cho S, 2014. Deregulation of inflammatory response in the diabetic condition is associated with increased ischemic brain injury. Journal of Neuroinflammation 11, 83 10.1186/1742-2094-11-83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Tolhurst AT, Qin LY, Chen XY, Febbraio M, Cho S, 2008. CD36/fatty acid translocase, an inflammatory mediator, is involved in hyperlipidemia-induced exacerbation in ischemic brain injury. J Neurosci 28, 4661–4670. 10.1523/jneurosci.0982-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Yang J, Woo Park K, Cho S, 2020. Preventative, but not post-stroke, inhibition of CD36 attenuates brain swelling in hyperlipidemic stroke. J Cereb Blood Flow Metab 40, 885–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruetzelmann A, Kohrmann M, Sobesky J, Cheng B, Rosenkranz M, Rother J, Schellinger PD, Ringleb P, Gerloff C, Fiehler J, Thomalla G, 2011. Pretreatment diffusion-weighted imaging lesion volume predicts favorable outcome after intravenous thrombolysis with tissue-type plasminogen activator in acute ischemic stroke. Stroke 42, 1251–1254. 10.1161/STROKEAHA.110.600148 [DOI] [PubMed] [Google Scholar]
- Kumari R, Willing LB, Krady JK, Vannucci SJ, Simpson IA, 2007. Impaired wound healing after cerebral hypoxia-ischemia in the diabetic mouse. J Cereb Blood Flow Metab 27, 710–718. 10.1038/sj.jcbfm.9600382 [DOI] [PubMed] [Google Scholar]
- Kumari R, Willing LB, Patel SD, Baskerville KA, Simpson IA, 2011. Increased cerebral matrix metalloprotease-9 activity is associated with compromised recovery in the diabetic db/db mouse following a stroke. J Neurochem 119, 1029–1040. 10.1111/j.1471-4159.2011.07487.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurita N, Yamashiro K, Kuroki T, Tanaka R, Urabe T, Ueno Y, Miyamoto N, Takanashi M, Shimura H, Inaba T, Yamashiro Y, Nomoto K, Matsumoto S, Takahashi T, Tsuji H, Asahara T, Hattori N, 2020. Metabolic endotoxemia promotes neuroinflammation after focal cerebral ischemia. J Cereb Blood Flow Metab 10.1177/0271678X19899577, 271678X19899577. 10.1177/0271678X19899577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusaka I, Kusaka G, Zhou C, Ishikawa M, Nanda A, Granger DN, Zhang JH, Tang J, 2004. Role of AT1 receptors and NAD(P)H oxidase in diabetes-aggravated ischemic brain injury. Am J Physiol Heart Circ Physiol 286, H2442–2451. 10.1152/ajpheart.01169.2003 [DOI] [PubMed] [Google Scholar]
- Labeyrie MA, Turc G, Hess A, Hervo P, Mas JL, Meder JF, Baron JC, Touze E, Oppenheim C, 2012. Diffusion lesion reversal after thrombolysis: a MR correlate of early neurological improvement. Stroke 43, 2986–2991. 10.1161/STROKEAHA.112.661009 [DOI] [PubMed] [Google Scholar]
- Lalu MM, Fergusson DA, Cheng W, Avey MT, Corbett D, Dowlatshahi D, Macleod MR, Sena ES, Moher D, Shorr R, McCann SK, Gray LJ, Hill MD, O’Connor A, Thayer K, Haggar F, Dobriyal A, Chung HS, Welton NJ, Hutton B, 2019. Identifying stroke therapeutics from preclinical models: A protocol for a novel application of network meta-analysis. F1000Res 8, 11 10.12688/f1000research.15869.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambertsen KL, Finsen B, Clausen BH, 2019. Post-stroke inflammation-target or tool for therapy? Acta Neuropathol 137, 693–714. 10.1007/s00401-018-1930-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langdon KD, Clarke J, Corbett D, 2011. Long-term exposure to high fat diet is bad for your brain: exacerbation of focal ischemic brain injury. Neuroscience 182, 82–87. 10.1016/j.neuroscience.2011.03.028 [DOI] [PubMed] [Google Scholar]
- Lansberg MG, Fields JD, Albers GW, Jayaraman MV, Do HM, Marks MP, 2005. Mechanical thrombectomy following intravenous thrombolysis in the treatment of acute stroke. Arch Neurol 62, 1763–1765. 10.1001/archneur.62.11.1763 [DOI] [PubMed] [Google Scholar]
- Lapilover EG, Lippmann K, Salar S, Maslarova A, Dreier JP, Heinemann U, Friedman A, 2012. Peri-infarct blood-brain barrier dysfunction facilitates induction of spreading depolarization associated with epileptiform discharges. Neurobiol Dis 48, 495–506. 10.1016/j.nbd.2012.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laredo C, Renú A, Llull L, Tudela R, López-Rueda A, Urra X, Macías NG, Rudilosso S, Obach V, Amaro S, Chamorro Á, 2020. Elevated glucose is associated with hemorrhagic transformation after mechanical thrombectomy in acute ischemic stroke patients with severe pretreatment hypoperfusion. Sci Rep 10, 10588 10.1038/s41598-020-67448-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, d’Aigle J, Atadja L, Quaicoe V, Honarpisheh P, Ganesh BP, Hassan A, Graf J, Petrosino JF, Putluri N, Zhu L, Durgan DJ, Bryan RM Jr., McCullough LD, Venna VR, 2020. Gut Microbiota-Derived Short-Chain Fatty Acids Promote Post-Stroke Recovery in Aged Mice. Circulation research 10.1161/CIRCRESAHA.119.316448. 10.1161/CIRCRESAHA.119.316448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrand L, Tisserand M, Turc G, Edjlali M, Calvet D, Trystram D, Roca P, Naggara O, Mas JL, Meder JF, Baron JC, Oppenheim C, 2016. Fluid-Attenuated Inversion Recovery Vascular Hyperintensities-Diffusion-Weighted Imaging Mismatch Identifies Acute Stroke Patients Most Likely to Benefit From Recanalization. Stroke 47, 424–427. 10.1161/STROKEAHA.115.010999 [DOI] [PubMed] [Google Scholar]
- Leonardi-Bee J, Bath PM, Phillips SJ, Sandercock PA, 2002. Blood pressure and clinical outcomes in the International Stroke Trial. Stroke 33, 1315–1320. 10.1161/01.str.0000014509.11540.66 [DOI] [PubMed] [Google Scholar]
- Li W, Prakash R, Chawla D, Du W, Didion SP, Filosa JA, Zhang Q, Brann DW, Lima VV, Tostes RC, Ergul A, 2013. Early effects of high-fat diet on neurovascular function and focal ischemic brain injury. Am J Physiol Regul Integr Comp Physiol 304, R1001–1008. 10.1152/ajpregu.00523.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Prakash R, Kelly-Cobbs AI, Ogbi S, Kozak A, El-Remessy AB, Schreihofer DA, Fagan SC, Ergul A, 2010. Adaptive cerebral neovascularization in a model of type 2 diabetes: relevance to focal cerebral ischemia. Diabetes 59, 228–235. 10.2337/db09-0902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Guo H, Zhao L, Wang B, Liu H, Yue L, Bai H, Jiang H, Gao L, Feng D, Qu Y, 2017. Adiponectin attenuates NADPH oxidase-mediated oxidative stress and neuronal damage induced by cerebral ischemia-reperfusion injury. Biochim Biophys Acta Mol Basis Dis 1863, 3265–3276. 10.1016/j.bbadis.2017.08.010 [DOI] [PubMed] [Google Scholar]
- Liang AC, Mandeville ET, Maki T, Shindo A, Som AT, Egawa N, Itoh K, Chuang TT, McNeish JD, Holder JC, Lok J, Lo EH, Arai K, 2016. Effects of Aging on Neural Stem/Progenitor Cells and Oligodendrocyte Precursor Cells After Focal Cerebral Ischemia in Spontaneously Hypertensive Rats. Cell transplantation 25, 705–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner MD, Gribkoff VK, Donlan NA, Jones TA, 2003. Long-lasting functional disabilities in middle-aged rats with small cerebral infarcts. J Neurosci 23, 10913–10922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Akella P, Benashski SE, Xu Y, McCullough LD, 2010. Expression of Na-K-Cl cotransporter and edema formation are age dependent after ischemic stroke. Exp Neurol 224, 356–361. 10.1016/j.expneurol.2010.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Benashski SE, Persky R, Xu Y, Li J, McCullough LD, 2012. Age-related changes in AMP-activated protein kinase after stroke. Age (Dordr) 34, 157–168. 10.1007/s11357-011-9214-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, McCullough LD, 2012. Interactions between age, sex, and hormones in experimental ischemic stroke. Neurochem Int 61, 1255–1265. 10.1016/j.neuint.2012.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Johnson EM, Lam RK, Wang Q, Bo Ye H, Wilson EN, Minhas PS, Liu L, Swarovski MS, Tran S, Wang J, Mehta SS, Yang X, Rabinowitz JD, Yang SS, Shamloo M, Mueller C, James ML, Andreasson KI, 2019. Peripheral TREM1 responses to brain and intestinal immunogens amplify stroke severity. Nature immunology 20, 1023–1034. 10.1038/s41590-019-0421-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, De Leo D, Degenhardt L, Delossantos A, Denenberg J, Des Jarlais DC, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez-Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, Hotez PJ, Hoy D, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Karthikeyan G, Kassebaum N, Keren A, Khoo JP, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lipnick M, Lipshultz SE, Ohno SL, Mabweijano J, MacIntyre MF, Mallinger L, March L, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGrath J, Mensah GA, Merriman TR, Michaud C, Miller M, Miller TR, Mock C, Mocumbi AO, Mokdad AA, Moran A, Mulholland K, Nair MN, Naldi L, Narayan KM, Nasseri K, Norman P, O’Donnell M, Omer SB, Ortblad K, Osborne R, Ozgediz D, Pahari B, Pandian JD, Rivero AP, Padilla RP, Perez-Ruiz F, Perico N, Phillips D, Pierce K, Pope CA 3rd, Porrini E, Pourmalek F, Raju M, Ranganathan D, Rehm JT, Rein DB, Remuzzi G, Rivara FP, Roberts T, De Leon FR, Rosenfeld LC, Rushton L, Sacco RL, Salomon JA, Sampson U, Sanman E, Schwebel DC, Segui-Gomez M, Shepard DS, Singh D, Singleton J, Sliwa K, Smith E, Steer A, Taylor JA, Thomas B, Tleyjeh IM, Towbin JA, Truelsen T, Undurraga EA, Venketasubramanian N, Vijayakumar L, Vos T, Wagner GR, Wang M, Wang W, Watt K, Weinstock MA, Weintraub R, Wilkinson JD, Woolf AD, Wulf S, Yeh PH, Yip P, Zabetian A, Zheng ZJ, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA, 2012. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380, 2095–2128. 10.1016/S0140-6736(12)61728-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Hajifathalian K, Ezzati M, Woodward M, Rimm EB, Danaei G, 2014. Metabolic mediators of the effects of body-mass index, overweight, and obesity on coronary heart disease and stroke: a pooled analysis of 97 prospective cohorts with 1.8 million participants. Lancet 383, 970–983. 10.1016/S0140-6736(13)61836-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyden P, Pryor KE, Coffey CS, Cudkowicz M, Conwit R, Jadhav A, Sawyer RN Jr., Claassen J, Adeoye O, Song S, Hannon P, Rost NS, Hinduja A, Torbey M, Lee JM, Benesch C, Rippee M, Rymer M, Froehler MT, Clarke Haley E, Johnson M, Yankey J, Magee K, Qidwai J, Levy H, Mark Haacke E, Fawaz M, Davis TP, Toga AW, Griffin JH, Zlokovic BV, Neuro NCTNNNI, 2019. Final Results of the RHAPSODY Trial: A Multi-Center, Phase 2 Trial Using a Continual Reassessment Method to Determine the Safety and Tolerability of 3K3A-APC, A Recombinant Variant of Human Activated Protein C, in Combination with Tissue Plasminogen Activator, Mechanical Thrombectomy or both in Moderate to Severe Acute Ischemic Stroke. Ann Neurol 85, 125–136. 10.1002/ana.25383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Campbell BCV, Parsons MW, Churilov L, Levi CR, Hsu C, Kleinig TJ, Wijeratne T, Curtze S, Dewey HM, Miteff F, Tsai CH, Lee JT, Phan TG, Mahant N, Sun MC, Krause M, Sturm J, Grimley R, Chen CH, Hu CJ, Wong AA, Field D, Sun Y, Barber PA, Sabet A, Jannes J, Jeng JS, Clissold B, Markus R, Lin CH, Lien LM, Bladin CF, Christensen S, Yassi N, Sharma G, Bivard A, Desmond PM, Yan B, Mitchell PJ, Thijs V, Carey L, Meretoja A, Davis SM, Donnan GA, Investigators E, 2019. Thrombolysis Guided by Perfusion Imaging up to 9 Hours after Onset of Stroke. N Engl J Med 380, 1795–1803. 10.1056/NEJMoa1813046 [DOI] [PubMed] [Google Scholar]
- Ma H, Wright P, Allport L, Phan TG, Churilov L, Ly J, Zavala JA, Arakawa S, Campbell B, Davis SM, Donnan GA, 2015. Salvage of the PWI/DWI mismatch up to 48 h from stroke onset leads to favorable clinical outcome. International journal of stroke : official journal of the International Stroke Society 10, 565–570. 10.1111/ijs.12203 [DOI] [PubMed] [Google Scholar]
- Ma J, Ma Y, Shuaib A, Winship IR, 2020. Impaired Collateral Flow in Pial Arterioles of Aged Rats During Ischemic Stroke. Translational stroke research 11, 243–253. 10.1007/s12975-019-00710-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macri MA, D’Alessandro N, Di Giulio C, Di Iorio P, Di Luzio S, Giuliani P, Esposito E, Pokorski M, 2010. Region-specific effects on brain metabolites of hypoxia and hyperoxia overlaid on cerebral ischemia in young and old rats: a quantitative proton magnetic resonance spectroscopy study. Journal of biomedical science 17, 14 10.1186/1423-0127-17-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maïer B, Gory B, Taylor G, Labreuche J, Blanc R, Obadia M, Abrivard M, Smajda S, Desilles JP, Redjem H, Ciccio G, Lukaszewicz AC, Turjman F, Riva R, Labeyrie PE, Duhamel A, Blacher J, Piotin M, Lapergue B, Mazighi M, 2017. Mortality and Disability According to Baseline Blood Pressure in Acute Ischemic Stroke Patients Treated by Thrombectomy: A Collaborative Pooled Analysis. Journal of the American Heart Association 6 10.1161/jaha.117.006484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier B, Kubis N, 2019. Hypertension and Its Impact on Stroke Recovery: From a Vascular to a Parenchymal Overview. Neural plasticity 2019, 6843895 10.1155/2019/6843895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maïer B, Turc G, Taylor G, Blanc R, Obadia M, Smajda S, Desilles JP, Redjem H, Ciccio G, Boisseau W, Sabben C, Ben Machaa M, Hamdani M, Leguen M, Gayat E, Blacher J, Lapergue B, Piotin M, Mazighi M, 2018. Prognostic Significance of Pulse Pressure Variability During Mechanical Thrombectomy in Acute Ischemic Stroke Patients. Journal of the American Heart Association 7, e009378 10.1161/jaha.118.009378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone K, Amu S, Moore AC, Waeber C, 2019. Immunomodulatory Therapeutic Strategies in Stroke. Frontiers in pharmacology 10, 630 10.3389/fphar.2019.00630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangin G, Cogo A, Moisan A, Bonnin P, Maïer B, Kubis N, 2019. Intravenous Administration of Human Adipose Derived-Mesenchymal Stem Cells Is Not Efficient in Diabetic or Hypertensive Mice Subjected to Focal Cerebral Ischemia. Frontiers in neuroscience 13, 718 10.3389/fnins.2019.00718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manwani B, Liu F, Scranton V, Hammond MD, Sansing LH, McCullough LD, 2013. Differential effects of aging and sex on stroke induced inflammation across the lifespan. Exp Neurol 249, 120–131. 10.1016/j.expneurol.2013.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks L, Carswell HV, Peters EE, Graham DI, Patterson J, Dominiczak AF, Macrae IM, 2001. Characterization of the microglial response to cerebral ischemia in the stroke-prone spontaneously hypertensive rat. Hypertension 38, 116–122. 10.1161/01.hyp.38.1.116 [DOI] [PubMed] [Google Scholar]
- Marschallinger J, Iram T, Zardeneta M, Lee SE, Lehallier B, Haney MS, Pluvinage JV, Mathur V, Hahn O, Morgens DW, Kim J, Tevini J, Felder TK, Wolinski H, Bertozzi CR, Bassik MC, Aigner L, Wyss-Coray T, 2020. Lipid-droplet-accumulating microglia represent a dysfunctional and proinflammatory state in the aging brain. Nature neuroscience 23, 194–208. 10.1038/s41593-019-0566-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini SR, Kent TA, 2007. Hyperglycemia in acute ischemic stroke: a vascular perspective. J Cereb Blood Flow Metab 27, 435–451. 10.1038/sj.jcbfm.9600355 [DOI] [PubMed] [Google Scholar]
- Matteis M, Troisi E, Monaldo BC, Caltagirone C, Silvestrini M, 1998. Age and sex differences in cerebral hemodynamics: a transcranial Doppler study. Stroke 29, 963–967. 10.1161/01.str.29.5.963 [DOI] [PubMed] [Google Scholar]
- Mayanagi K, Katakam PV, Gáspár T, Domoki F, Busija DW, 2008. Acute treatment with rosuvastatin protects insulin resistant (C57BL/6J ob/ob) mice against transient cerebral ischemia. J Cereb Blood Flow Metab 28, 1927–1935. 10.1038/jcbfm.2008.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maysami S, Haley MJ, Gorenkova N, Krishnan S, McColl BW, Lawrence CB, 2015. Prolonged diet-induced obesity in mice modifies the inflammatory response and leads to worse outcome after stroke. J Neuroinflammation 12, 140 10.1186/s12974-015-0359-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe C, Gallagher L, Gsell W, Graham D, Dominiczak AF, Macrae IM, 2009. Differences in the evolution of the ischemic penumbra in stroke-prone spontaneously hypertensive and Wistar-Kyoto rats. Stroke 40, 3864–3868. 10.1161/STROKEAHA.109.559021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann SK, Lawrence CB, 2020. Comorbidity and age in the modelling of stroke: are we still failing to consider the characteristics of stroke patients? BMJ Open Science 4, e100013 10.1136/bmjos-2019-100013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill JK, Gallagher L, Carswell HV, Irving EA, Dominiczak AF, Macrae IM, 2005. Impaired functional recovery after stroke in the stroke-prone spontaneously hypertensive rat. Stroke 36, 135–141. 10.1161/01.STR.0000149629.32525.b7 [DOI] [PubMed] [Google Scholar]
- Menyhart A, Makra P, Szepes BE, Toth OM, Hertelendy P, Bari F, Farkas E, 2015. High incidence of adverse cerebral blood flow responses to spreading depolarization in the aged ischemic rat brain. Neurobiol Aging 36, 3269–3277. 10.1016/j.neurobiolaging.2015.08.014 [DOI] [PubMed] [Google Scholar]
- Menyhart A, Zolei-Szenasi D, Puskas T, Makra P, Bari F, Farkas E, 2017. Age or ischemia uncouples the blood flow response, tissue acidosis, and direct current potential signature of spreading depolarization in the rat brain. Am J Physiol Heart Circ Physiol 313, H328–H337. 10.1152/ajpheart.00222.2017 [DOI] [PubMed] [Google Scholar]
- Miao J, Shen LH, Tang YH, Wang YT, Tao MX, Jin KL, Zhao YJ, Yang GY, 2013. Overexpression of adiponectin improves neurobehavioral outcomes after focal cerebral ischemia in aged mice. CNS neuroscience & therapeutics 19, 969–977. 10.1111/cns.12198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishiro K, Imai T, Sugitani S, Kitashoji A, Suzuki Y, Takagi T, Chen H, Oumi Y, Tsuruma K, Shimazawa M, Hara H, 2014. Diabetes mellitus aggravates hemorrhagic transformation after ischemic stroke via mitochondrial defects leading to endothelial apoptosis. PloS one 9, e103818 10.1371/journal.pone.0103818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra NK, Diener HC, Lyden PD, Bluhmki E, Lees KR, Collaborators V, 2010. Influence of age on outcome from thrombolysis in acute stroke: a controlled comparison in patients from the Virtual International Stroke Trials Archive (VISTA). Stroke 41, 2840–2848. 10.1161/STROKEAHA.110.586206 [DOI] [PubMed] [Google Scholar]
- Möller K, Pösel C, Kranz A, Schulz I, Scheibe J, Didwischus N, Boltze J, Weise G, Wagner DC, 2015. Arterial Hypertension Aggravates Innate Immune Responses after Experimental Stroke. Frontiers in cellular neuroscience 9, 461 10.3389/fncel.2015.00461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagne A, Barnes SR, Sweeney MD, Halliday MR, Sagare AP, Zhao Z, Toga AW, Jacobs RE, Liu CY, Amezcua L, Harrington MG, Chui HC, Law M, Zlokovic BV, 2015. Blood-brain barrier breakdown in the aging human hippocampus. Neuron 85, 296–302. 10.1016/j.neuron.2014.12.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooradian AD, McCuskey RS, 1992. In vivo microscopic studies of age-related changes in the structure and the reactivity of cerebral microvessels. Mech Ageing Dev 64, 247–254. 10.1016/0047-6374(92)90082-o [DOI] [PubMed] [Google Scholar]
- Moretti A, Ferrari F, Villa RF, 2015a. Neuroprotection for ischaemic stroke: current status and challenges. Pharmacology & therapeutics 146, 23–34. 10.1016/j.pharmthera.2014.09.003 [DOI] [PubMed] [Google Scholar]
- Moretti A, Ferrari F, Villa RF, 2015b. Pharmacological therapy of acute ischaemic stroke: Achievements and problems. Pharmacology & therapeutics 153, 79–89. 10.1016/j.pharmthera.2015.06.004 [DOI] [PubMed] [Google Scholar]
- Mosher KI, Wyss-Coray T, 2014. Microglial dysfunction in brain aging and Alzheimer’s disease. Biochemical pharmacology 88, 594–604. 10.1016/j.bcp.2014.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskowitz MA, Lo EH, Iadecola C, 2010. The science of stroke: mechanisms in search of treatments. Neuron 67, 181–198. 10.1016/j.neuron.2010.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB, 2016. Executive Summary: Heart Disease and Stroke Statistics--2016 Update: A Report From the American Heart Association. Circulation 133, 447–454. 10.1161/CIR.0000000000000366 [DOI] [PubMed] [Google Scholar]
- Murugesan N, Demarest TG, Madri JA, Pachter JS, 2012. Brain regional angiogenic potential at the neurovascular unit during normal aging. Neurobiol Aging 33, 1004 e1001–1016. 10.1016/j.neurobiolaging.2011.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nag S, Papneja T, Venugopalan R, Stewart DJ, 2005. Increased angiopoietin2 expression is associated with endothelial apoptosis and blood-brain barrier breakdown. Lab Invest 85, 1189–1198. 10.1038/labinvest.3700325 [DOI] [PubMed] [Google Scholar]
- Neuhaus AA, Couch Y, Hadley G, Buchan AM, 2017. Neuroprotection in stroke: the importance of collaboration and reproducibility. Brain 140, 2079–2092. 10.1093/brain/awx126 [DOI] [PubMed] [Google Scholar]
- Nih LR, Deroide N, Lere-Dean C, Lerouet D, Soustrat M, Levy BI, Silvestre JS, Merkulova-Rainon T, Pocard M, Margaill I, Kubis N, 2012. Neuroblast survival depends on mature vascular network formation after mouse stroke: role of endothelial and smooth muscle progenitor cell co-administration. Eur J Neurosci 35, 1208–1217. 10.1111/j.1460-9568.2012.08041.x [DOI] [PubMed] [Google Scholar]
- Nishimura M, Izumiya Y, Higuchi A, Shibata R, Qiu J, Kudo C, Shin HK, Moskowitz MA, Ouchi N, 2008. Adiponectin prevents cerebral ischemic injury through endothelial nitric oxide synthase dependent mechanisms. Circulation 117, 216–223. 10.1161/CIRCULATIONAHA.107.725044 [DOI] [PubMed] [Google Scholar]
- Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, Yavagal DR, Ribo M, Cognard C, Hanel RA, Sila CA, Hassan AE, Millan M, Levy EI, Mitchell P, Chen M, English JD, Shah QA, Silver FL, Pereira VM, Mehta BP, Baxter BW, Abraham MG, Cardona P, Veznedaroglu E, Hellinger FR, Feng L, Kirmani JF, Lopes DK, Jankowitz BT, Frankel MR, Costalat V, Vora NA, Yoo AJ, Malik AM, Furlan AJ, Rubiera M, Aghaebrahim A, Olivot JM, Tekle WG, Shields R, Graves T, Lewis RJ, Smith WS, Liebeskind DS, Saver JL, Jovin TG, Investigators DT, 2018. Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct. N Engl J Med 378, 11–21. 10.1056/NEJMoa1706442 [DOI] [PubMed] [Google Scholar]
- O’Collins VE, Macleod MR, Donnan GA, Horky LL, van der Worp BH, Howells DW, 2006. 1,026 experimental treatments in acute stroke. Ann Neurol 59, 467–477. 10.1002/ana.20741 [DOI] [PubMed] [Google Scholar]
- Osmond JM, Mintz JD, Stepp DW, 2010. Preventing increased blood pressure in the obese Zucker rat improves severity of stroke. Am J Physiol Heart Circ Physiol 299, H55–61. 10.1152/ajpheart.01111.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostergaard L, Dreier JP, Hadjikhani N, Jespersen SN, Dirnagl U, Dalkara T, 2015. Neurovascular coupling during cortical spreading depolarization and -depression. Stroke 46, 1392–1401. 10.1161/STROKEAHA.114.008077 [DOI] [PubMed] [Google Scholar]
- Ostergaard L, Engedal TS, Moreton F, Hansen MB, Wardlaw JM, Dalkara T, Markus HS, Muir KW, 2016. Cerebral small vessel disease: Capillary pathways to stroke and cognitive decline. J Cereb Blood Flow Metab 36, 302–325. 10.1177/0271678X15606723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otxoa-de-Amezaga A, Gallizioli M, Pedragosa J, Justicia C, Miró-Mur F, Salas-Perdomo A, Díaz-Marugan L, Gunzer M, Planas AM, 2019a. Location of Neutrophils in Different Compartments of the Damaged Mouse Brain After Severe Ischemia/Reperfusion. Stroke 50, 1548–1557. 10.1161/strokeaha.118.023837 [DOI] [PubMed] [Google Scholar]
- Otxoa-de-Amezaga A, Miró-Mur F, Pedragosa J, Gallizioli M, Justicia C, Gaja-Capdevila N, Ruíz-Jaen F, Salas-Perdomo A, Bosch A, Calvo M, Márquez-Kisinousky L, Denes A, Gunzer M, Planas AM, 2019b. Microglial cell loss after ischemic stroke favors brain neutrophil accumulation. Acta Neuropathol 137, 321–341. 10.1007/s00401-018-1954-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panel AASA, 2000. Stroke epidemiological data of nine Asian countries. Asian Acute Stroke Advisory Panel (AASAP). J Med Assoc Thai 83, 1–7. [PubMed] [Google Scholar]
- Pantano P, Baron JC, Lebrun-Grandie P, Duquesnoy N, Bousser MG, Comar D, 1984. Regional cerebral blood flow and oxygen consumption in human aging. Stroke 15, 635–641. 10.1161/01.str.15.4.635 [DOI] [PubMed] [Google Scholar]
- Patel RAG, McMullen PW, 2017. Neuroprotection in the Treatment of Acute Ischemic Stroke. Prog Cardiovasc Dis 59, 542–548. 10.1016/j.pcad.2017.04.005 [DOI] [PubMed] [Google Scholar]
- Perez-de-Puig I, Miró-Mur F, Ferrer-Ferrer M, Gelpi E, Pedragosa J, Justicia C, Urra X, Chamorro A, Planas AM, 2015. Neutrophil recruitment to the brain in mouse and human ischemic stroke. Acta Neuropathol 129, 239–257. 10.1007/s00401-014-1381-0 [DOI] [PubMed] [Google Scholar]
- Philip M, Benatar M, Fisher M, Savitz SI, 2009. Methodological quality of animal studies of neuroprotective agents currently in phase II/III acute ischemic stroke trials. Stroke 40, 577–581. 10.1161/STROKEAHA.108.524330 [DOI] [PubMed] [Google Scholar]
- Popa-Wagner A, Badan I, Walker L, Groppa S, Patrana N, Kessler C, 2007a. Accelerated infarct development, cytogenesis and apoptosis following transient cerebral ischemia in aged rats. Acta Neuropathol 113, 277–293. 10.1007/s00401-006-0164-7 [DOI] [PubMed] [Google Scholar]
- Popa-Wagner A, Carmichael ST, Kokaia Z, Kessler C, Walker LC, 2007b. The response of the aged brain to stroke: too much, too soon? Curr Neurovasc Res 4, 216–227. 10.2174/156720207781387213 [DOI] [PubMed] [Google Scholar]
- Popa-Wagner A, Dinca I, Yalikun S, Walker L, Kroemer H, Kessler C, 2006. Accelerated delimitation of the infarct zone by capillary-derived nestin-positive cells in aged rats. Curr Neurovasc Res 3, 3–13. 10.2174/156720206775541732 [DOI] [PubMed] [Google Scholar]
- Popa-Wagner A, Glavan DG, Olaru A, Olaru DG, Margaritescu O, Tica O, Surugiu R, Sandu RE, 2018. Present Status and Future Challenges of New Therapeutic Targets in Preclinical Models of Stroke in Aged Animals with/without Comorbidities. International journal of molecular sciences 19 10.3390/ijms19020356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popa-Wagner A, Petcu E, Capitanescu B, Hermann D, Radu E, Gresita A, 2020. Ageing as a Risk Factor for Cerebral Ischemia. Underlying Mechanisms and Therapy in Animal Models and in the Clinic. Mech Ageing Dev 10.1016/j.mad.2020.111312, 111312 10.1016/j.mad.2020.111312 [DOI] [PubMed] [Google Scholar]
- Popa-Wagner A, Pirici D, Petcu EB, Mogoanta L, Buga AM, Rosen CL, Leon R, Huber J, 2010a. Pathophysiology of the vascular wall and its relevance for cerebrovascular disorders in aged rodents. Curr Neurovasc Res 7, 251–267. 10.2174/156720210792231813 [DOI] [PubMed] [Google Scholar]
- Popa-Wagner A, Stocker K, Balseanu AT, Rogalewski A, Diederich K, Minnerup J, Margaritescu C, Schabitz WR, 2010b. Effects of granulocyte-colony stimulating factor after stroke in aged rats. Stroke 41, 1027–1031. 10.1161/STROKEAHA.109.575621 [DOI] [PubMed] [Google Scholar]
- Popescu BO, Toescu EC, Popescu LM, Bajenaru O, Muresanu DF, Schultzberg M, Bogdanovic N, 2009. Blood-brain barrier alterations in ageing and dementia. J Neurol Sci 283, 99–106. 10.1016/j.jns.2009.02.321 [DOI] [PubMed] [Google Scholar]
- Prakash R, Johnson M, Fagan SC, Ergul A, 2013. Cerebral neovascularization and remodeling patterns in two different models of type 2 diabetes. PloS one 8, e56264 10.1371/journal.pone.0056264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash R, Somanath PR, El-Remessy AB, Kelly-Cobbs A, Stern JE, Dore-Duffy P, Johnson M, Fagan SC, Ergul A, 2012. Enhanced cerebral but not peripheral angiogenesis in the Goto-Kakizaki model of type 2 diabetes involves VEGF and peroxynitrite signaling. Diabetes 61, 1533–1542. 10.2337/db11-1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu HL, Zhao M, Zhao SS, Xiao T, Song CG, Cao YP, Jolkkonen J, Zhao CS, 2015. Forced limb-use enhanced neurogenesis and behavioral recovery after stroke in the aged rats. Neuroscience 286, 316–324. 10.1016/j.neuroscience.2014.11.040 [DOI] [PubMed] [Google Scholar]
- Rajah GB, Ding Y, 2017. Experimental neuroprotection in ischemic stroke: a concise review. Neurosurg Focus 42, E2 10.3171/2017.1.FOCUS16497 [DOI] [PubMed] [Google Scholar]
- Riddle DR, Sonntag WE, Lichtenwalner RJ, 2003. Microvascular plasticity in aging. Ageing Res Rev 2, 149–168. 10.1016/s1568-1637(02)00064-8 [DOI] [PubMed] [Google Scholar]
- Ritter L, Davidson L, Henry M, Davis-Gorman G, Morrison H, Frye JB, Cohen Z, Chandler S, McDonagh P, Funk JL, 2011. Exaggerated neutrophil-mediated reperfusion injury after ischemic stroke in a rodent model of type 2 diabetes. Microcirculation 18, 552–561. 10.1111/j.1549-8719.2011.00115.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritzel RM, Lai YJ, Crapser JD, Patel AR, Schrecengost A, Grenier JM, Mancini NS, Patrizz A, Jellison ER, Morales-Scheihing D, Venna VR, Kofler JK, Liu F, Verma R, McCullough LD, 2018. Aging alters the immunological response to ischemic stroke. Acta Neuropathol 136, 89–110. 10.1007/s00401-018-1859-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusanen H, Saarinen JT, Sillanpää N, 2015. Collateral Circulation Predicts the Size of the Infarct Core and the Proportion of Salvageable Penumbra in Hyperacute Ischemic Stroke Patients Treated with Intravenous Thrombolysis. Cerebrovasc Dis 40, 182–190. 10.1159/000439064 [DOI] [PubMed] [Google Scholar]
- Sabbatini M, Strocchi P, Vitaioli L, Amenta F, 2001. Microanatomical changes of intracerebral arteries in spontaneously hypertensive rats: a model of cerebrovascular disease of the elderly. Mech Ageing Dev 122, 1257–1268. 10.1016/s0047-6374(01)00234-2 [DOI] [PubMed] [Google Scholar]
- Sacco RL, 1997. Risk factors, outcomes, and stroke subtypes for ischemic stroke. Neurology 49, S39–44. [DOI] [PubMed] [Google Scholar]
- Sacco RL, Benjamin EJ, Broderick JP, Dyken M, Easton JD, Feinberg WM, Goldstein LB, Gorelick PB, Howard G, Kittner SJ, Manolio TA, Whisnant JP, Wolf PA, 1997. American Heart Association Prevention Conference. IV. Prevention and Rehabilitation of Stroke. Risk factors. Stroke 28, 1507–1517. [DOI] [PubMed] [Google Scholar]
- Sadler R, Cramer JV, Heindl S, Kostidis S, Betz D, Zuurbier KR, Northoff BH, Heijink M, Goldberg MP, Plautz EJ, Roth S, Malik R, Dichgans M, Holdt LM, Benakis C, Giera M, Stowe AM, Liesz A, 2020. Short-Chain Fatty Acids Improve Poststroke Recovery via Immunological Mechanisms. J Neurosci 40, 1162–1173. 10.1523/JNEUROSCI.1359-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas IH, Burgado J, Allen NJ, 2020. Glia: victims or villains of the aging brain? Neurobiol Dis 143, 105008 10.1016/j.nbd.2020.105008 [DOI] [PubMed] [Google Scholar]
- Santisteban MM, Ahmari N, Carvajal JM, Zingler MB, Qi Y, Kim S, Joseph J, Garcia-Pereira F, Johnson RD, Shenoy V, Raizada MK, Zubcevic J, 2015. Involvement of bone marrow cells and neuroinflammation in hypertension. Circulation research 117, 178–191. 10.1161/circresaha.117.305853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarraj A, Mlynash M, Savitz SI, Heit JJ, Lansberg MG, Marks MP, Albers GW, 2019. Outcomes of Thrombectomy in Transferred Patients With Ischemic Stroke in the Late Window: A Subanalysis From the DEFUSE 3 Trial. JAMA neurology 76, 682–689. 10.1001/jamaneurol.2019.0118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz SI, Baron JC, Fisher M, Consortium SX, 2019. Stroke Treatment Academic Industry Roundtable X: Brain Cytoprotection Therapies in the Reperfusion Era. Stroke 50, 1026–1031. 10.1161/STROKEAHA.118.023927 [DOI] [PubMed] [Google Scholar]
- Scherbakov N, Dirnagl U, Doehner W, 2011. Body weight after stroke: lessons from the obesity paradox. Stroke 42, 3646–3650. 10.1161/strokeaha.111.619163 [DOI] [PubMed] [Google Scholar]
- Schmidt-Pogoda A, Bonberg N, Koecke MHM, Strecker JK, Wellmann J, Bruckmann NM, Beuker C, Schabitz WR, Meuth SG, Wiendl H, Minnerup H, Minnerup J, 2020. Why Most Acute Stroke Studies Are Positive in Animals but Not in Patients: A Systematic Comparison of Preclinical, Early Phase, and Phase 3 Clinical Trials of Neuroprotective Agents. Ann Neurol 87, 40–51. 10.1002/ana.25643 [DOI] [PubMed] [Google Scholar]
- Sena ES, van der Worp HB, Bath PM, Howells DW, Macleod MR, 2010. Publication bias in reports of animal stroke studies leads to major overstatement of efficacy. PLoS biology 8, e1000344 10.1371/journal.pbio.1000344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senatorov VV Jr., Friedman AR, Milikovsky DZ, Ofer J, Saar-Ashkenazy R, Charbash A, Jahan N, Chin G, Mihaly E, Lin JM, Ramsay HJ, Moghbel A, Preininger MK, Eddings CR, Harrison HV, Patel R, Shen Y, Ghanim H, Sheng H, Veksler R, Sudmant PH, Becker A, Hart B, Rogawski MA, Dillin A, Friedman A, Kaufer D, 2019. Blood-brain barrier dysfunction in aging induces hyperactivation of TGFbeta signaling and chronic yet reversible neural dysfunction. Sci Transl Med 11 10.1126/scitranslmed.aaw8283 [DOI] [PubMed] [Google Scholar]
- Seners P, Turc G, Oppenheim C, Baron JC, 2015. Incidence, causes and predictors of neurological deterioration occurring within 24 h following acute ischaemic stroke: a systematic review with pathophysiological implications. J Neurol Neurosurg Psychiatry 86, 87–94. 10.1136/jnnp-2014-308327 [DOI] [PubMed] [Google Scholar]
- Sharma A, Sharma VK, Ahmad A, Gupta D, Khan K, Shuaib A, Alexandrov AV, Saqqur M, 2020. Effect of Age on Arterial Recanalization and Clinical Outcome in Thrombolyzed Acute Ischemic Stroke in CLOTBUST Cohort. Ann Indian Acad Neurol 23, 189–194. 10.4103/aian.AIAN_434_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen XZ, Li Y, Li L, Shah KH, Bernstein KE, Lyden P, Shi P, 2015. Microglia participate in neurogenic regulation of hypertension. Hypertension 66, 309–316. 10.1161/hypertensionaha.115.05333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheth KN, Elm JJ, Molyneaux BJ, Hinson H, Beslow LA, Sze GK, Ostwaldt AC, Del Zoppo GJ, Simard JM, Jacobson S, Kimberly WT, 2016. Safety and efficacy of intravenous glyburide on brain swelling after large hemispheric infarction (GAMES-RP): a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol 15, 1160–1169. 10.1016/S1474-4422(16)30196-X [DOI] [PubMed] [Google Scholar]
- Shi K, Tian DC, Li ZG, Ducruet AF, Lawton MT, Shi FD, 2019. Global brain inflammation in stroke. Lancet Neurol 18, 1058–1066. 10.1016/S1474-4422(19)30078-X [DOI] [PubMed] [Google Scholar]
- Shi L, Rocha M, Leak RK, Zhao J, Bhatia TN, Mu H, Wei Z, Yu F, Weiner SL, Ma F, Jovin TG, Chen J, 2018. A new era for stroke therapy: Integrating neurovascular protection with optimal reperfusion. J Cereb Blood Flow Metab 38, 2073–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Rocha M, Zhang W, Jiang M, Li S, Ye Q, Hassan SH, Liu L, Adair MN, Xu J, Luo J, Hu X, Wechsler LR, Chen J, Shi Y, 2020. Genome-wide transcriptomic analysis of microglia reveals impaired responses in aged mice after cerebral ischemia. J Cereb Blood Flow Metab 10.1177/0271678×20925655, 271678×20925655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi P, Diez-Freire C, Jun JY, Qi Y, Katovich MJ, Li Q, Sriramula S, Francis J, Sumners C, Raizada MK, 2010. Brain microglial cytokines in neurogenic hypertension. Hypertension 56, 297–303. 10.1161/hypertensionaha.110.150409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin HK, Huang PL, Ayata C, 2014. Rho-kinase inhibition improves ischemic perfusion deficit in hyperlipidemic mice. J Cereb Blood Flow Metab 34, 284–287. 10.1038/jcbfm.2013.195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JA, Jeong SI, Kim M, Yoon JC, Kim HS, Park EM, 2015. Visceral adipose tissue inflammation is associated with age-related brain changes and ischemic brain damage in aged mice. Brain, behavior, and immunity 50, 221–231. 10.1016/j.bbi.2015.07.008 [DOI] [PubMed] [Google Scholar]
- Shukla V, Shakya AK, Perez-Pinzon MA, Dave KR, 2017. Cerebral ischemic damage in diabetes: an inflammatory perspective. J Neuroinflammation 14, 21 10.1186/s12974-016-0774-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh V, Roth S, Llovera G, Sadler R, Garzetti D, Stecher B, Dichgans M, Liesz A, 2016. Microbiota Dysbiosis Controls the Neuroinflammatory Response after Stroke. J Neurosci 36, 7428–7440. 10.1523/JNEUROSCI.1114-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soomro J, Zhu L, Savitz SI, Sarraj A, 2020. Predictors of Acute Neurological Worsening after Endovascular Thrombectomy. Interv Neurol 8, 172–179. 10.1159/000499973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spychala MS, Venna VR, Jandzinski M, Doran SJ, Durgan DJ, Ganesh BP, Ajami NJ, Putluri N, Graf J, Bryan RM, McCullough LD, 2018. Age-related changes in the gut microbiota influence systemic inflammation and stroke outcome. Ann Neurol 84, 23–36. 10.1002/ana.25250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatovic SM, Martinez-Revollar G, Hu A, Choi J, Keep RF, Andjelkovic AV, 2019. Decline in Sirtuin-1 expression and activity plays a critical role in blood-brain barrier permeability in aging. Neurobiol Dis 126, 105–116. 10.1016/j.nbd.2018.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley D, Mason LJ, Mackin KE, Srikhanta YN, Lyras D, Prakash MD, Nurgali K, Venegas A, Hill MD, Moore RJ, Wong CH, 2016. Translocation and dissemination of commensal bacteria in post-stroke infection. Nat Med 22, 1277–1284. 10.1038/nm.4194 [DOI] [PubMed] [Google Scholar]
- Stanley D, Moore RJ, Wong CHY, 2018. An insight into intestinal mucosal microbiota disruption after stroke. Sci Rep 8, 568 10.1038/s41598-017-18904-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strazzullo P, D’Elia L, Cairella G, Garbagnati F, Cappuccio FP, Scalfi L, 2010. Excess body weight and incidence of stroke: meta-analysis of prospective studies with 2 million participants. Stroke 41, e418–426. 10.1161/STROKEAHA.109.576967 [DOI] [PubMed] [Google Scholar]
- Streit WJ, Sammons NW, Kuhns AJ, Sparks DL, 2004. Dystrophic microglia in the aging human brain. Glia 45, 208–212. 10.1002/glia.10319 [DOI] [PubMed] [Google Scholar]
- Streit WJ, Xue QS, 2010. The Brain’s Aging Immune System. Aging and disease 1, 254–261. [PMC free article] [PubMed] [Google Scholar]
- Strong AJ, Anderson PJ, Watts HR, Virley DJ, Lloyd A, Irving EA, Nagafuji T, Ninomiya M, Nakamura H, Dunn AK, Graf R, 2007. Peri-infarct depolarizations lead to loss of perfusion in ischaemic gyrencephalic cerebral cortex. Brain 130, 995–1008. 10.1093/brain/awl392 [DOI] [PubMed] [Google Scholar]
- Sudlow CL, Warlow CP, 1997. Comparable studies of the incidence of stroke and its pathological types: results from an international collaboration. International Stroke Incidence Collaboration. Stroke 28, 491–499. 10.1161/01.str.28.3.491 [DOI] [PubMed] [Google Scholar]
- Suenaga J, Hu X, Pu H, Shi Y, Hassan SH, Xu M, Leak RK, Stetler RA, Gao Y, Chen J, 2015. White matter injury and microglia/macrophage polarization are strongly linked with age-related long-term deficits in neurological function after stroke. Exp Neurol 272, 109–119. 10.1016/j.expneurol.2015.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland BA, Minnerup J, Balami JS, Arba F, Buchan AM, Kleinschnitz C, 2012. Neuroprotection for ischaemic stroke: translation from the bench to the bedside. International journal of stroke : official journal of the International Stroke Society 7, 407–418. 10.1111/j.1747-4949.2012.00770.x [DOI] [PubMed] [Google Scholar]
- Sutherland GR, Dix GA, Auer RN, 1996. Effect of age in rodent models of focal and forebrain ischemia. Stroke 27, 1663–1667; discussion 1668. 10.1161/01.str.27.9.1663 [DOI] [PubMed] [Google Scholar]
- Tan Z, Lucke-Wold BP, Logsdon AF, Turner RC, Tan C, Li X, Hongpaison J, Alkon DL, Simpkins JW, Rosen CL, Huber JD, 2015. Bryostatin extends tPA time window to 6 h following middle cerebral artery occlusion in aged female rats. Eur J Pharmacol 764, 404–412. 10.1016/j.ejphar.2015.07.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teichert M, Milde L, Holm A, Stanicek L, Gengenbacher N, Savant S, Ruckdeschel T, Hasanov Z, Srivastava K, Hu J, Hertel S, Bartol A, Schlereth K, Augustin HG, 2017. Pericyte-expressed Tie2 controls angiogenesis and vessel maturation. Nature communications 8, 16106 10.1038/ncomms16106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevaranjan N, Puchta A, Schulz C, Naidoo A, Szamosi JC, Verschoor CP, Loukov D, Schenck LP, Jury J, Foley KP, Schertzer JD, Larche MJ, Davidson DJ, Verdu EF, Surette MG, Bowdish DME, 2017. Age-Associated Microbial Dysbiosis Promotes Intestinal Permeability, Systemic Inflammation, and Macrophage Dysfunction. Cell Host Microbe 21, 455–466 e454 10.1016/j.chom.2017.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomalla G, Simonsen CZ, Boutitie F, Andersen G, Berthezene Y, Cheng B, Cheripelli B, Cho TH, Fazekas F, Fiehler J, Ford I, Galinovic I, Gellissen S, Golsari A, Gregori J, Gunther M, Guibernau J, Hausler KG, Hennerici M, Kemmling A, Marstrand J, Modrau B, Neeb L, Perez de la Ossa N, Puig J, Ringleb P, Roy P, Scheel E, Schonewille W, Serena J, Sunaert S, Villringer K, Wouters A, Thijs V, Ebinger M, Endres M, Fiebach JB, Lemmens R, Muir KW, Nighoghossian N, Pedraza S, Gerloff C, Investigators, W.-U., 2018. MRI-Guided Thrombolysis for Stroke with Unknown Time of Onset. N Engl J Med 379, 611–622. 10.1056/NEJMoa1804355 [DOI] [PubMed] [Google Scholar]
- Tisserand M, Turc G, Charron S, Legrand L, Edjlali M, Seners P, Roca P, Lion S, Naggara O, Mas JL, Meder JF, Baron JC, Oppenheim C, 2016. Does Diffusion Lesion Volume Above 70 mL Preclude Favorable Outcome Despite Post-Thrombolysis Recanalization? Stroke 47, 1005–1011. 10.1161/STROKEAHA.115.012518 [DOI] [PubMed] [Google Scholar]
- Titova EM, Ghosh N, Valadez ZG, Zhang JH, Bellinger DL, Obenaus A, 2014. The late phase of post-stroke neurorepair in aged rats is reflected by MRI-based measures. Neuroscience 283, 231–244. 10.1016/j.neuroscience.2014.09.028 [DOI] [PubMed] [Google Scholar]
- Tureyen K, Bowen K, Liang J, Dempsey RJ, Vemuganti R, 2011. Exacerbated brain damage, edema and inflammation in type-2 diabetic mice subjected to focal ischemia. J Neurochem 116, 499–507. 10.1111/j.1471-4159.2010.07127.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner RC, Lucke-Wold B, Lucke-Wold N, Elliott AS, Logsdon AF, Rosen CL, Huber JD, 2013. Neuroprotection for ischemic stroke: moving past shortcomings and identifying promising directions. International journal of molecular sciences 14, 1890–1917. 10.3390/ijms14011890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagal A, Aviv R, Sucharew H, Reddy M, Hou Q, Michel P, Jovin T, Tomsick T, Wintermark M, Khatri P, 2018. Collateral Clock Is More Important Than Time Clock for Tissue Fate. Stroke 49, 2102–2107. 10.1161/strokeaha.118.021484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Worp HB, Howells DW, Sena ES, Porritt MJ, Rewell S, O’Collins V, Macleod MR, 2010. Can animal models of disease reliably inform human studies? PLoS medicine 7, e1000245 10.1371/journal.pmed.1000245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Worp HB, van Gijn J, 2007. Clinical practice. Acute ischemic stroke. N Engl J Med 357, 572–579. 10.1056/NEJMcp072057 [DOI] [PubMed] [Google Scholar]
- Vemmos K, Ntaios G, Spengos K, Savvari P, Vemmou A, Pappa T, Manios E, Georgiopoulos G, Alevizaki M, 2011. Association between obesity and mortality after acute first-ever stroke: the obesity-stroke paradox. Stroke 42, 30–36. 10.1161/STROKEAHA.110.593434 [DOI] [PubMed] [Google Scholar]
- Venkat P, Chopp M, Chen J, 2017. Blood-Brain Barrier Disruption, Vascular Impairment, and Ischemia/Reperfusion Damage in Diabetic Stroke. Journal of the American Heart Association 6 10.1161/jaha.117.005819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venketasubramanian N, Yoon BW, Pandian J, Navarro JC, 2017. Stroke Epidemiology in South, East, and South-East Asia: A Review. J Stroke 19, 286–294. 10.5853/jos.2017.00234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Shay CM, Spartano NL, Stokes A, Tirschwell DL, VanWagner LB, Tsao CW, American Heart Association Council on, E., Prevention Statistics, C., Stroke Statistics, S., 2020. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation 141, e139–e596. 10.1161/CIR.0000000000000757 [DOI] [PubMed] [Google Scholar]
- Vorbrodt AW, Dobrogowska DH, 1994. Immunocytochemical evaluation of blood-brain barrier to endogenous albumin in adult, newborn and aged mice. Folia Histochem Cytobiol 32, 63–70. [PubMed] [Google Scholar]
- Wadhwani KC, Koistinaho J, Balbo A, Rapoport SI, 1991. Blood-nerve and blood-brain barrier permeabilities and nerve vascular space in Fischer-344 rats of different ages. Mech Ageing Dev 58, 177–190. 10.1016/0047-6374(91)90091-d [DOI] [PubMed] [Google Scholar]
- Wang RY, Wang PS, Yang YR, 2003. Effect of age in rats following middle cerebral artery occlusion. Gerontology 49, 27–32. 10.1159/000066505 [DOI] [PubMed] [Google Scholar]
- Weimar C, Konig IR, Kraywinkel K, Ziegler A, Diener HC, German Stroke Study C, 2004. Age and National Institutes of Health Stroke Scale Score within 6 hours after onset are accurate predictors of outcome after cerebral ischemia: development and external validation of prognostic models. Stroke 35, 158–162. 10.1161/01.STR.0000106761.94985.8B [DOI] [PubMed] [Google Scholar]
- Wen SW, Shim R, Ho L, Wanrooy BJ, Srikhanta YN, Prame Kumar K, Nicholls AJ, Shen SJ, Sepehrizadeh T, de Veer M, Srikanth VK, Ma H, Phan TG, Lyras D, Wong CHY, 2019. Advanced age promotes colonic dysfunction and gut-derived lung infection after stroke. Aging Cell 18, e12980 10.1111/acel.12980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr., Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr., Williamson JD, Wright JT Jr., 2018. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 71, e13–e115. 10.1161/hyp.0000000000000065 [DOI] [PubMed] [Google Scholar]
- Winship IR, 2015. Cerebral collaterals and collateral therapeutics for acute ischemic stroke. Microcirculation 22, 228–236. 10.1111/micc.12177 [DOI] [PubMed] [Google Scholar]
- Won SJ, Xie L, Kim SH, Tang H, Wang Y, Mao X, Banwait S, Jin K, 2006. Influence of age on the response to fibroblast growth factor-2 treatment in a rat model of stroke. Brain Res 1123, 237–244. 10.1016/j.brainres.2006.09.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia GH, You C, Gao XX, Zeng XL, Zhu JJ, Xu KY, Tan CH, Xu RT, Wu QH, Zhou HW, He Y, Yin J, 2019. Stroke Dysbiosis Index (SDI) in Gut Microbiome Are Associated With Brain Injury and Prognosis of Stroke. Frontiers in neurology 10, 397 10.3389/fneur.2019.00397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang AC, Stevens MY, Chen MB, Lee DP, Stahli D, Gate D, Contrepois K, Chen W, Iram T, Zhang L, Vest RT, Chaney A, Lehallier B, Olsson N, du Bois H, Hsieh R, Cropper HC, Berdnik D, Li L, Wang EY, Traber GM, Bertozzi CR, Luo J, Snyder MP, Elias JE, Quake SR, James ML, Wyss-Coray T, 2020. Physiological blood-brain transport is impaired with age by a shift in transcytosis. Nature 583, 425–430. 10.1038/s41586-020-2453-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, DeMars KM, Alexander JC, Febo M, Candelario-Jalil E, 2017. Sustained Neurological Recovery After Stroke in Aged Rats Treated With a Novel Prostacyclin Analog. Stroke 48, 1948–1956. 10.1161/STROKEAHA.117.016474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi SW, Odongua N, Nam CM, Sull JW, Ohrr H, 2009. Body mass index and stroke mortality by smoking and age at menopause among Korean postmenopausal women. Stroke 40, 3428–3435. 10.1161/strokeaha.109.555144 [DOI] [PubMed] [Google Scholar]
- Yong M, Kaste M, 2008. Dynamic of hyperglycemia as a predictor of stroke outcome in the ECASS-II trial. Stroke 39, 2749–2755. 10.1161/strokeaha.108.514307 [DOI] [PubMed] [Google Scholar]
- Yu P, Venkat P, Chopp M, Zacharek A, Shen Y, Liang L, Landschoot-Ward J, Liu Z, Jiang R, Chen J, 2019. Deficiency of tPA Exacerbates White Matter Damage, Neuroinflammation, Glymphatic Dysfunction and Cognitive Dysfunction in Aging Mice. Aging and disease 10, 770–783. 10.14336/AD.2018.0816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zechariah A, ElAli A, Hagemann N, Jin F, Doeppner TR, Helfrich I, Mies G, Hermann DM, 2013. Hyperlipidemia attenuates vascular endothelial growth factor-induced angiogenesis, impairs cerebral blood flow, and disturbs stroke recovery via decreased pericyte coverage of brain endothelial cells. Arteriosclerosis, thrombosis, and vascular biology 33, 1561–1567. 10.1161/atvbaha.112.300749 [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhang RL, Wang Y, Zhang C, Zhang ZG, Meng H, Chopp M, 2005. Functional recovery in aged and young rats after embolic stroke: treatment with a phosphodiesterase type 5 inhibitor. Stroke 36, 847–852. 10.1161/01.STR.0000158923.19956.73 [DOI] [PubMed] [Google Scholar]
- Zhao J, Zhao H, Li R, Li J, Liu C, Lv J, Li Y, Liu W, Ma D, Hao H, Xiao X, Liu J, Yin Y, Liu R, Yu Q, Wei Y, Li P, Wang Y, Wang R, 2019. Outcome of multimodal MRI-guided intravenous thrombolysis in patients with stroke with unknown time of onset. Stroke Vasc Neurol 4, 3–7. 10.1136/svn-2018-000151 [DOI] [PMC free article] [PubMed] [Google Scholar]


