Abstract
Objective
To examine whether the age distribution of COVID-19 deaths and the share of deaths in nursing homes changed in the second versus the first pandemic wave.
Eligible data
We considered all countries that had at least 4000 COVID-19 deaths occurring as of January 14, 2021, at least 200 COVID-19 deaths occurring in each of the two epidemic wave periods; and which had sufficiently detailed information available on the age distribution of these deaths. We also considered countries with data available on COVID-19 deaths of nursing home residents for the two waves.
Main outcome measures
Change in the second wave versus the first wave in the proportion of COVID-19 deaths occurring in people <50 years (“young deaths”) among all COVID-19 deaths and among COVID-19 deaths in people <70 years old; and change in the proportion of COVID-19 deaths in nursing home residents among all COVID-19 deaths.
Results
Data on age distribution were available for 14 eligible countries. Individuals <50 years old had small absolute difference in their share of the total COVID-19 deaths in the two waves across 13 high-income countries (absolute differences 0.0–0.4%). Their proportion was higher in Ukraine, but it decreased markedly in the second wave. The proportion of young deaths was lower in the second versus the first wave (summary prevalence ratio 0.81, 95% CI 0.71–0.92) with large between-country heterogeneity. The proportion of young deaths among deaths <70 years did not differ significantly across the two waves (summary prevalence ratio 0.96, 95% CI 0.86–1.06). Eligible data on nursing home COVID-19 deaths were available for 11 countries. The share of COVID-19 deaths that were accounted by nursing home residents decreased in the second wave significantly and substantially in 8 countries (prevalence ratio estimates: 0.36 to 0.78), remained the same in Denmark and Norway and markedly increased in Australia.
Conclusions
In the examined countries, age distribution of COVID-19 deaths has been fairly similar in the second versus the first wave, but the contribution of COVID-19 deaths in nursing home residents to total fatalities has decreased in most countries in the second wave.
Keywords: COVID-19, Age, Nursing home, Epidemic wave
Credit author statement
John P.A. Ioannidis: Conceptualization, Methodology, Formal analysis, Investigation, Writing – original draft, Project administration. Cathrine Axfors: Conceptualization, Software, Formal analysis, Investigation, Data curation, Writing – review & editing. Despina G. Contopoulos-Ioannidis: Conceptualization, Methodology, Investigation, Data curation, Writing – review & editing.
1. Introduction
Many countries around the world saw a pattern of the coronavirus disease 2019 (COVID-19) pandemic where a first wave occurred in the spring that substantially subsided during the summer, and a second wave emerged in the fall of 2020. A key question is whether the age distribution of COVID-19 fatalities in these locations remained steady between the two waves or not. COVID-19 has an extremely steep risk gradient for death across age groups (Williamson et al., 2020; Ioannidis et al., 2020; O'Driscoll et al., 2020). The relative share of infections among young, older, and debilitated people may shape the observed proportion of deaths in different demographic groups and the overall fatalities and infection fatality rate in the total population.
Data from a considerable number of seroprevalence studies done in different countries have suggested that not all age groups may have been equally infected during the spread of the virus in the first wave (Ioannidis, 2021). In several countries that did very well in the first wave, e.g. Singapore, Australia, or Iceland (Gudbjartsson et al., 2020) evidence suggests that elderly people were less likely to be infected and in particular nursing homes (long-term care facilities) were not contributing many fatalities (Ioannidis, 2021). The opposite pattern was seen in countries that had high rates of death, where nursing homes were massively infected during the first wave (Comas-Herrera et al., 2020a). One wonders whether preferential protection of older, higher-risk people and in particular of nursing home residents is possible and whether this might have happened more efficiently in the second wave (Smith and Spiegelhalter, 2020).
In order to answer these questions, we assessed the age distribution of COVID-19 fatalities in the two waves in countries with a substantial burden of fatalities as of January 2021, and we also assessed the relative contribution from deaths of nursing home residents in the two waves.
2. Methods
2.1. Data for comparison of age distribution of COVID-19 deaths in second versus first wave
We considered data from publicly available situational reports of countries that had a large number of deaths in both the first and second waves, so as to allow meaningful inferences in comparing the age distributions, and where the trough between the two waves had happened between May 15 and September 15, 2020. Specifically, we considered all countries that had at least 4000 COVID-19 deaths, at least 200 COVID-19 deaths occurring in the first wave, and at least 200 COVID-19 deaths occurring in the second wave; and which had information available on the age distribution of these deaths separately. Searches were last updated on January 14, 2021. In order to separate the two wave periods in a consistent manner, we used the date between the two peaks that had the trough (lowest number of deaths) for a 7-day average according to Worldometer data (Worldometers.info.D-, 2020). When two or more dates were tied for trough values, we picked the earliest one. In some countries, e.g. in the USA, for the second wave a separate peak may be discerned in late summer, followed by a higher peak in the late fall and winter. However, for consistency and convenience we separated all countries into two time periods called “first” and “second” wave.
For each eligible country, we found the most recent situational report that mentioned age distribution of deaths; and the situational report that mentioned age distribution of deaths as of the trough date (or a separation date for the two waves that was as close as possible to the trough, when data were not available specifically up to the trough date). It is acknowledged that a few deaths reported after the trough/separation dates may have happened earlier, but this is likely to pertain to very small numbers and it would not change the overall comparison.
Age cut-offs of interest were pre-specified to be 50 years and 70 years. In European countries that have seen two waves and in the USA, almost two-thirds of the population are younger than 50 years old and the age stratum of 50–69 accounts for another quarter of the population. In a few other countries that have also seen two waves, the proportion of the population younger than 50 years old even exceeds 80%, e.g. in Turkey or Pakistan. However, the lion's share of COVID-19 deaths happen in people above 70; moreover, in countries where elder care facilities are common, a large share of COVID-19 deaths occur in nursing home residents. Documentation of COVID-19 infection may be least systematic in this upper age stratum. The number of COVID-19 deaths may be most error prone and variable in its documentation across countries and even within the same country over time in elderly people, and nursing home residents in particular. Therefore, we aimed to assess whether (A) the proportion of COVID-19 deaths occurring in people <50 years old among all COVID-19 deaths changed in the second versus the first wave; and (B) whether the proportion of COVID-19 deaths occurring in people <50 years old among the COVID-19 deaths occurring in people <70 years old changed between the two waves. The analysis limited to deaths at age <70 years may be more unbiased in understanding whether the share of deaths in young people became relatively more (or less) common in the second wave versus the first wave. When data were not provided for the cut-offs of 50 and 70 years for COVID-19 deaths, we used the closest cut-offs available provided it was not more than 5 years off (45 and 65 years).
2.2. Data for COVID-19 deaths in nursing home residents in second versus first wave
We also extracted data on COVID-19 deaths occurring in nursing home residents. We preferred data on all COVID-19 deaths of such residents (occurring in the nursing homes or in hospitals) unless information was available only for COVID-19 deaths happening in nursing homes. We also preferred data on both confirmed and probable COVID-19 deaths, unless only data on confirmed deaths were available.
We compared proportions of these nursing home residents’ deaths among all COVID-19 deaths in the second versus first wave periods. The International Long-Term Care Policy Network has issued reports based on available country-level official data for the number of deaths among nursing home residents linked to COVID-19. An early report (June 26, 2020) (Comas-Herrera et al., 2020b) was considered. Given the substantial spread of COVID-19 occurring in many countries after their latest report, we restricted our analysis to the countries for which we could find updates that covered at least until the end of 2020 using the official sources cited in the International Long-Term Care Policy Network reports. Latest extraction of these data was performed between January 14 and January 20, 2021. We used as cutoff for the first wave the dates given in the June 26 report (between June 1 and June 23, 2020).
Of note, we use here the term “nursing home” broadly to include different types of long-term care facilities. The exact types of facilities included in the COVID-19 death data for each country may differ and we recorded the definition used in each country. We ensured also that the definition remained the same in the two waves, and recorded any noted any changes in the documentation of COVID-19 deaths in nursing homes.
2.3. Statistical analyses
All comparisons of proportions of COVID-19 deaths per country between the two periods used prevalence ratios (prevalence risk ratios) and 95% confidence intervals thereof. Meta-analysis of prevalence ratios used a random effects model. Heterogeneity was expressed with the I2 statistic and tested with the chi-squared-based Q test. P-values are two-tailed. Statistical analyses were run in STATA (StataCorp. Stata Statisti, 2017).
3. RESULTS
3.1. Eligible data for age distribution of COVID-19 deaths in the two waves
49 countries had at least 4000 deaths until January 14, 2021. Of those, 22 countries (Austria, Belgium, Canada, Czechia, France, Germany, Hungary, Italy, Japan, Netherlands, Pakistan, Poland, Portugal, Romania, Russia, Spain, Sweden, Switzerland, Turkey, Ukraine, United Kingdom, USA) also had at least 200 deaths in each wave and had a trough between May 15 and September 15. We could retrieve some data on the age distribution separately for the two waves for 17 of the 22 countries. However, we could not retrieve reports for Canada for deaths at age <50 in the first wave; Romania provided only graphs with percentages per age group and resolution was not sufficiently accurate in the <50 years old age group; and for Turkey there were no age-stratified data available after late October. Eventually, data from 14 countries were finally included (Table 1 ). As shown in Table 1, the trough separating the two waves occurred between June 1 and August 28 in all locations. The number of deaths in the second wave as of the date of the analysis was higher than the number of deaths in the first wave in all countries with eligible data, except for Spain and Sweden. Sources of information for the 14 countries appear in Supplementary Table 1.
Table 1.
Eligible locations for analyses of COVID-19 deaths per age group.
| Countries | Date of trough | Deaths on trough | Deaths in first wave | Deaths in second wave | Separation date* | Latest date** |
|---|---|---|---|---|---|---|
| Austria | June 5 | 0 | 645 | 6183 | June 5 | Jan 14 |
| Belgium | July 14 | 1 | 9671 | 10623 | July 14 | Jan 14 |
| France | Aug 10 | 6 | 30354 | 38448 | Aug 11 | Jan 12 |
| Germany | Aug 1 | 3 | 9148 | 32429 | Aug 1 | Jan 12 |
| Italy | Aug 15 | 5 | 35614 | 39434 | Aug 30 | Jan 5 |
| Japan | July 6 | 0 | 977 | 2742 | July 6 | Jan 6 |
| Netherlands | July 14 | 0 | 6135 | 6428 | May 22 | Jan 12 |
| Portugal | Aug 7 | 1 | 1750 | 6634 | Aug 7 | Jan 13 |
| Spain | July 27 | 1 | 29856 | 23025 | July 27 | Jan 13 |
| Sweden | Aug 28 | 1 | 5739 | 4364 | Aug 28 | Jan 14 |
| Switzerland | June 1 | 0 | 1831 | 6755 | May 23 | Jan 14 |
| Ukraine | June 3 | 11 | 735 | 16437 | June 3 | Dec 23 |
| United Kingdom | Aug 21 | 7 | 56653 | 32590 | Aug 21 | Jan 1 |
| USA | July 5 | 518 | 130951 | 198660 | July 4 | Jan 9 |
*data were not always available to separate deaths in the first versus the second wave using the trough date, and in these cases the most proximal date with data available was used. **data were not always available until January 14, and in these cases the most proximal date with available data was used.
3.2. Age distribution of COVID-19 deaths in the two waves
Table 2 shows the age distribution of COVID-19 deaths in the first and second wave in each eligible country with data.
Table 2.
Proportion of COVID-19 deaths in specific age groups.
| Country |
FW < 50 |
FW 50-69 |
FW ≥ 70 |
SW < 50 |
SW 50-69 |
SW ≥ 70 |
Total FW ¤ |
Total SW ¤ |
|---|---|---|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |||
| Austria # | 3 (0.5) | 35 (5.4) | 607 (94.1) | 19 (0.3) | 365 (5.9) | 5799 (93.8) | 645 | 6183 |
| Belgium # | 40 (0.4) | 499 (5.2) | 9116 (94.4) | 56 (0.5) | 638 (5.9) | 9919 (93.5) | 9655 | 10613 |
| France #* | 212 (0.7) | 2055 (6.8) | 27976 (92.5) | 148 (0.4) | 1861 (4.9) | 36284 (94.8) | 30243 | 38293 |
| Germany | 114 (1.2) | 1212 (13.3) | 7817 (85.5) | 265 (0.8) | 3038 (9.4) | 29040 (89.8) | 9143 | 32343 |
| Italy | 400 (1.1) | 4815 (13.5) | 30399 (85.4) | 437 (1.1) | 4844 (12.3) | 34150 (86.6) | 35614 | 39431 |
| Japan | 11 (1.5) | 102 (14.3) | 602 (84.2) | 33 (1.2) | 288 (10.7) | 2378 (88.1) | 715 | 2699 |
| Netherlands | 46 (0.7) | 649 (10.6) | 5440 (88.7) | 48 (0.7) | 472 (7.3) | 5908 (91.9) | 6135 | 6428 |
| Portugal | 26 (1.5) | 213 (12.2) | 1511 (86.3) | 78 (1.2) | 716 (10.8) | 5840 (88.0) | 1750 | 6634 |
| Spain | 424 (1.4) | 3764 (12.7) | 25486 (85.9) | 375 (1.6) | 2753 (12.0) | 19747 (86.3) | 29674 | 22875 |
| Sweden | 72 (1.2) | 563 (9.7) | 5186 (89.1) | 42 (1.0) | 251 (5.8) | 4070 (93.3) | 5821 | 4363 |
| Switzerland | 9 (0.5) | 156 (9.5) | 1475 (89.9) | 25 (0.4) | 448 (7.2) | 5791 (92.5) | 1640 | 6264 |
| Ukraine | 99 (12.7) | 367 (47.0) | 315 (40.3) | 1080 (6.9) | 7085 (45.2) | 7500 (47.9) | 781 | 15665 |
| UK # | 592 (1.0) | 5338 (9.4) | 50723 (89.5) | 264 (0.8) | 2780 (8.5) | 29545 (90.7) | 56653 | 32589 |
| USA # | 3722 (2.8) | 22830 (17.4) | 104381 (79.7) | 4836 (2.4) | 31557 (15.9) | 162260 (81.7) | 130933 | 198653 |
FW: first wave; SW: second wave # Age groups: <45, 45–64, ≥65. ¤ Total number of deaths with age information. *deaths in chronic care establishments in France are counted in the ≥65 age group.
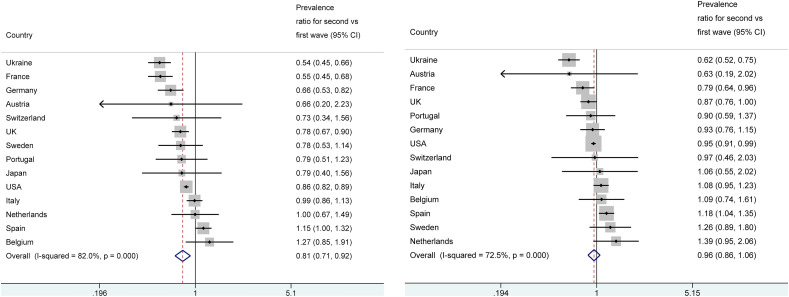
The proportion of COVID-19 deaths <50 years among all COVID-19 deaths in the first wave did not exceed 1.5% in any high-income country except for the USA (2.8% for a cut-off of <45 years) and it was much higher in Ukraine (12.7%). In the second wave, the absolute difference versus the first wave was only 0.0–0.4% in high-income countries, with the largest decreases (0.4%) in the share of deaths <50 years occurring in USA (2.4% from 2.8% in the first wave) and in Germany (0.8% from 1.2%). Decreases of 0.3% were seen in Sweden, Portugal, Japan, and France; decreases of 0.2% occurred in the UK and Austria and 0.1% in Switzerland, while there was no change at all in Italy and in the Netherlands, and minor increases in Belgium (0.1%) and Spain (0.2%). A far more major decrease was seen in Ukraine (from 12.7% to 6.9%). As shown in Fig. 1 a, there was very large heterogeneity when results were expressed in a prevalence ratio comparing the two waves (I2 = 82%, p < 0.001 for heterogeneity) and the summary prevalence ratio was 0.81, 95% CI 0.71–0.92, suggesting fewer young deaths in the second wave. In 5 countries (Ukraine, France, Germany, UK, USA) the proportions were significantly less for young deaths in the second wave, while in Spain there was a borderline significance in the opposite direction.
Fig. 1.
Panel a: COVID-19 deaths in people <50 years among all COVID-19 deaths: prevalence ratio for the second versus first wave. Panel b: COVID-19 deaths in people <50 years among COVID-19 deaths in people <70 years: prevalence ratio for the second versus first wave.
When estimated only among the COVID-19 deaths in people <70 years old, the proportion of COVID-19 deaths in individuals <50 years old showed no major differences on average in the second wave versus the first wave (Fig. 1b) with a summary estimate of the prevalence ratio of 0.96 (95% CI 0.86–1.06). There was again significant between-country heterogeneity (I2 = 73%, p < 0.001 for heterogeneity). In 4 countries (Ukraine, France, UK, USA) the proportion were significantly lower for young deaths in the second wave, while the opposite pattern was seen in Spain.
3.3. COVID-19 deaths of nursing homes residents
Out of 21 countries with any documented COVID-19 nursing home deaths as per the International Long-Term Care Policy Network report on June 26 (Comas-Herrera et al., 2020b), eligible data on nursing home residents’ COVID-19 deaths in the first versus second wave could be obtained for 11 countries (Table 3 ). Sources of data appear in Supplementary Table 2. The definitions of COVID-19 deaths and nursing home institutions differed across the different countries. In four countries, nursing home deaths data included only deaths that occurred in the nursing home environment (i.e. in-hospital deaths of nursing home residents were excluded). In five countries, only confirmed COVID-19 nursing home deaths were considered. Definitions of nursing home facilities and other included facilities appear also in Table 3.
Table 3.
Proportion of COVID-19 deaths occurring in nursing home residents.
| Countries |
Nursing home resident deaths in FW |
End date of FW (2020) |
Nursing home resident deaths in SW |
Latest date (2021) |
Total deaths in FW # |
Total deaths in SW # |
Definition of nursing home deaths $ |
Definition of nursing home and other included facilities |
|---|---|---|---|---|---|---|---|---|
| N (%) | N (%) | |||||||
| Australia | 29 (28.4) | June 21 | 656 (81.3) | Jan 14 | 102 | 807 | R, C | Government-subsidized residential age care facilities |
| Belgium | 4892 (50.5) | June 20 | 3880 (36.8) | Jan 10 | 9696 | 10554 | O, C/P | Nursing homes |
| Canada | 6236 (85.1) | June 1 | 5737 (59.0) | Jan 14 | 7326 | 9726 | R, C/P | Nursing and retirement homes |
| Denmark | 211 (35.3) | June 15 | 400 (39.0) | Jan 12 | 598 | 1025 | R, C | Nursing homes |
| Finland | 147 (45.0) | June 23 | 62 (21.5) | Jan 14 | 327 | 289 | O, C | Social care 24-h units |
| France | 14341 (48.5) | June 16 | 6662 (17.4) | Jan 10 | 29547 | 38396 | R, C/P | Social and medico-social establishments (ESMS) |
| Germany | 3491 (39.2) | June 23 | 8493 (24.3) | Jan 14 | 8895 | 34986 | R, C | Communal settings (e.g., nursing homes, homeless shelters, accommodation for refugees, prisons) |
| Norway | 144 (59.0) | June 19 | 174 (61.5) | Jan 20 | 244 | 283 | O, C | Other health institutions than hospitals: nursing homes and other institutions |
| Sweden | 2280 (47.4) | June 15 | 2283 (37.1) | Jan 18 | 4810 | 6149 | R, C/P | Nursing homes £ |
| UK | 16598 (31.1) | June 14 ¤ | 7438 (20.0) | Jan 1 # | 53403 | 37710 | O, C/P | Nursing homes |
| USA | 50185 (45.0) | June 18 | 80993 (34.7) | Jan 5 | 111522 | 233683 | R, C/P | Nursing facilities, assisted living facilities, adult care centers, intermediate care centers, and/or other long-term care facilities |
FW: first wave; SW: second wave.
# Total number of deaths with information on nursing home residence status or place of death. $ By place of residence (R) or by place of occurrence (O; i.e., not counting in-hospital deaths of nursing home residents); confirmed COVID-19 (C); confirmed or probable COVID-19 (C/P). ¤ England, Wales, and Northern Ireland: June 12, 2020; Scotland: June 14, 2020. # England and Wales and Northern Ireland: January 1, 2021; Scotland: January 4, 2021. £ Different definitions of place of residence are used for Swedish FW and SW data (change reported by the Swedish National Board of Health and Welfare on August 24, 2020).
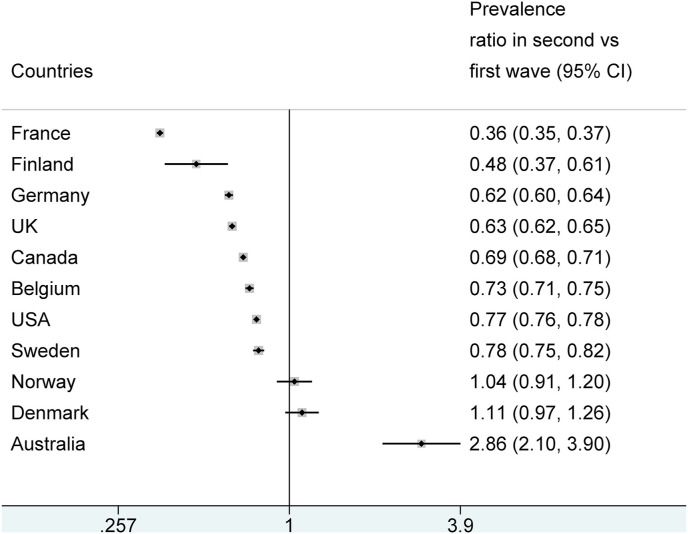
The proportion of nursing home COVID-19 deaths among all COVID-19 deaths was lower in the second wave than in the first wave in 8 of the 11 countries (Fig. 2 ). There were large and statistically significant reductions in these 8 countries (prevalence ratios 0.36 to 0.78). There were no significant differences in Denmark and Norway where few deaths overall were recorded in both waves, and a major increase in the proportion of nursing home COVID-19 deaths in Australia (prevalence ratio 2.9, 95% CI 2.1–3.9) in the second wave. This resulted in extreme between-country heterogeneity (I2 = 99.7%, p < 0.001 for heterogeneity) making a single summary odds ratio not meaningful to obtain.
Fig. 2.
COVID-19 deaths in nursing home residents: prevalence ratio for the second versus first wave.
4. Discussion
4.1. Main findings
The age distribution of COVID-19 deaths did not change much within the examined high-income countries between the first and second waves. Deaths in individuals <50 years accounted for approximately 1% of all deaths in European high-income countries, and modestly higher proportion in the USA. Some countries even saw modest decreases of this percentage in the second wave compared with the first. Concurrently, there was a strong pattern for a decreasing share of COVID-19 deaths of nursing home residents in the second wave versus the first wave, with Australia being an exception to this pattern. Data from one middle-income country (Ukraine) showed overall much higher proportion of COVID-19 deaths among people <50 years old, with a substantial decrease in the second wave.
4.2. Nursing home COVID-19 deaths
The decreasing share of nursing home resident COVID-19 deaths in the second wave may reflect multiple factors. Higher awareness of the extreme fatality risk of nursing home residents and raised efforts to protect nursing homes (learning from experience) (Burton et al., 2020; Salcher-Konrad et al., 2020) may be primarily responsible. Perhaps hygiene measures, infection control, testing of personnel and residents, and avoidance of staff working across multiple nursing homes have become more routine during the second wave. Another potential contributing factor may be better treatment and management. For example, dexamethasone (Horby et al., 2020) has been available in the second wave; conversely, use of mortality-increasing hydroxychloroquine (Axfors et al., 2020) and suboptimal mechanical ventilation practices may have been reduced in some countries. Cohort effects are also possible: e.g. the first wave may have killed some of the most frail residents and/or may have already preferentially devastated nursing homes with poorer standards and thus higher infection risk. In countries with the heaviest toll of nursing home deaths, a large share of nursing home residents and personnel (perhaps even the majority) may have been infected by January 2021. E.g. according to (The Atlantic Monthly Grou, 2021) as of January 21, 2021 there are 1,183,661 documented COVID-19 cases and 146,294 COVID-19 deaths in nursing homes in the USA. With such extremely high infection rates, the residual pool of infected nursing home residents has shrunk markedly, and this may explain in part the decreasing share of nursing home deaths to total COVID-19 deaths.
Some additional factors need to be considered. Within the same country, the second wave may have spread to different communities with different shares of nursing home populations compared with the first wave. For example, in the USA there have been distinct waves of the pandemic that have impacted various regions, e.g., northeast and northwest in the spring of 2020, the sunbelt region in the summer, the Midwest and California in the late summer, and widespread outbreaks by late fall/winter 2020, especially in areas that had been relatively spared earlier. Also some data artefact cannot be fully excluded, in particular if some nursing home COVID-19 deaths in the second wave suffer reporting delays. Finally, we tried to ensure that definitions of deaths and nursing home facilities were similar in the two waves, but subtle changes cannot be excluded, e.g. Sweden made some changes in the defining documentation of nursing home deaths during the summer months. Regardless, the difference between the two waves in overall COVID-19 deaths in most countries is quite large, thus unlikely to be only a data or methods artefact.
Despite the clear improvement, the proportion of COVID-19 deaths that occurred among nursing home residents remained very large in western European countries and the USA. Moreover, not all countries have seen improvements in the second wave. Australia witnessed a major increase, and this may be explained by non-remedied dysfunctions in its elder care (Cousins, 2020). The Australian aged care system has long been criticized for understaffing, and using low-pay staff with poor skills who work across multiple facilities. Most facilities are privately run for-profit enterprises. Similar problems with nursing home care inefficiencies have been described also in other countries with a large share of deaths in nursing homes, e.g. in Canada (Liu et al., 2020); however, in other countries, COVID-19 already hit nursing homes rapidly during the first wave.
4.3. Age distribution
The relative stability of the age distribution of COVID-19 deaths in the same country between the two waves suggests that country-specific population demographics are the key driver of the age distribution of infections, which then get reflected also in the age distribution of deaths (Spiegelhalter, 2020). Other features that may also affect COVID-19 mortality and its age distribution (e.g. population density, deprivation, ethnicity, frequencies of pre-existing conditions, occupational profile, and environmental factors) also remain steady within the same country between the two waves.
While the absolute share of deaths at age <50 among all COVID-19 deaths had small fluctuations between the two waves, in relative terms several countries (Germany, USA, UK, France, Ukraine) had a clearly smaller share of young deaths in the second wave. One may expect shifts in the age distribution of COVID-19 deaths in the second wave if the epidemic within a country is spreading to new epicenters and populations with different demographics. Alternatively, documentation of COVID-19 may have become more aggressive in the second wave with more testing being performed, especially among deceased elderly.
Overall, in a meta-analysis of all countries with eligible data, the relative share of COVID-19 deaths at age <50 years among all COVID-19 deaths decreased statistically significantly in the second wave versus the first wave, but this difference was not evident when deaths at age >70 years were excluded from the calculations in a sensitivity analysis. The sensitivity analysis may be more unbiased given that the attribution of deaths to COVID-19 may be more difficult in the elderly and may also have differed between the two waves. However, given that the share of deaths accounted by elderly nursing home residents declined in the second wave, an alternative possibility is that the share of deaths in the community-dwelling elderly genuinely increased in the second wave. Ideally one would have liked to see more emphasis on protecting the vulnerable community-dwelling elderly rather than the younger community-dwelling people in the second wave, learning from the lessons of the first wave. However, this did not seem to happen.
We had data only from one middle-income country (Ukraine) and no data from low-income countries. Differential demographics in the two waves (e.g. higher infection rates in urban and congested young populations in the first wave, followed by older provincial populations later) may be more prominent in lower-income countries. For example, Turkey is another country with very high proportion of deaths occurring in young people in the first wave (6.8% among those <50). During the first wave, the highest numbers of cases per million population were seen in Istanbul and southern Turkey (Ministry of Health.D, 2020). Both epicenters are characterized by a very young population, younger than the inhabitants of other locations that were hit more in the second wave. Data on age distribution of deaths were available for Turkey only until late October, showing a decrease of the proportion of deaths among those <50 years (4.8%). Alternatively, non-documentation of COVID-19 deaths, especially among the elderly and even more so in the first wave when testing was more limited might have been even more prominent in developing than in high-income countries.
4.4. Implications
The observed patterns of diminished COVID-19 deaths in nursing homes in many countries may act in the direction of decreasing the infection fatality rate of the pandemic in several high-income countries in the second wave and any subsequent waves that might occur. For an equal number of total infections, when nursing homes are spared, the number of total COVID-19 deaths will be substantially less (Ioannidis, 2020). With prioritization of vaccination for nursing home residents and elderly individuals in early 2021, the share of nursing home deaths and of deaths among the elderly in general may decrease further. This may induce an increase in the share of young deaths among the total. Paradoxically, having a larger share of young deaths would not be a bad sign, but may indirectly be an indicator of better protection of vulnerable elderly individuals. This pattern will not be observed, however, if uptake of vaccines is not preferentially higher among the most vulnerable, if vaccines are less effective among the most vulnerable, and if there is larger risk compensation among the vulnerable who are vaccinated (e.g. marked decrease in other protections taken with non-pharmaceutical measures). For countries where the pandemic shifts from urban centers with young population to areas with older populations, infection fatality rate may increase. The same applies to any countries that successfully protected their nursing homes in the first wave, but have been less successful in the second wave.
Some measures taken to contain the pandemic may affect differentially people at different ages and may have different real-world effectiveness for people at different ages. More data need to be accumulated as the pandemic progresses, as different types of lockdown measures are used and relieved, and as vaccines become more widely deployed. Some countries like Italy have presented data where the median age of fatalities during the first wave started at lower values, increased during draconian lockdown, and then declined again as restrictions were relieved (Instituto Superiore di Sa, 2020). Such differences over time may have been due to chance. However, an alternative explanation might be that draconian lockdown increases the level of protection more prominently for the young than for the elderly compared with pre- or post-lockdown behavior. E.g. the elderly may be taking severe precautions anyhow, regardless of government-imposed lockdown status.
4.5. Limitations
Some limitations need to be discussed. First, age information was missing on some deaths, but this pertained to very few fatalities and it is unlikely to have created systematic bias in the comparison of first versus second waves. Second, many nursing home deaths have substantial ambiguity in their attribution to COVID-19, especially when there is no test confirmation. We tried to use data with consistent definitions and approaches in the two waves, but the two periods may still differ, e.g. typically more testing was done in the second wave. If anything, this would usually tend to increase the number of confirmed COVID-19 deaths in nursing homes in the second wave. Third, it would be useful to understand whether there are differences between the two waves in the share of deaths in people without underlying conditions and/or specific risk profiles. This could give additional insights about the relative exposure and protection of these groups in the two time periods. However, such data are very sparse in currently available situational reports (e.g., Santé publique France., 2020; Rijksinstituut voor Volks, 2020). Fourth, we did not find sufficiently detailed data on age distribution of COVID-19 deaths even for some high-income countries. This is a deficiency that could be quickly corrected in country-level situational reports worldwide.
Finally, we found very sparse data from middle-income countries and no data from low-income countries. However, most low-income countries have not had a trough separating two waves. Instead, they have typically seen continuous epidemic activity comprising a single wave. The share of COVID-19 death that are accounted by young people is probably greater in middle- and low-income countries and in countries with many impoverished people. E.g., in Colombia (an upper middle-income country, 8% of COVID-19 deaths are accounted by ages <50 years and the proportion is 6% even in Chile which qualifies for a high-income country but has a large rich-poor gap and many impoverished citizens (Instituto Nacional de Sal, 2021; Gobierno Digital Minister, 2021); in Indonesia (an country transitioning from lower to upper middle-income country), 20.4% of COVID-19 deaths are accounted by ages <45 years (Komite PenangananD-1, 2021); and in India (a lower middle-income country), 14.4% of COVID-19 deaths are accounted by ages <40 (Ioannidis et al., 2020). Moreover, in all countries disadvantaged people with lower socioeconomic status are often more hit by the pandemic (as well as the measures taken, e.g. in terms of unemployment, loss of health insurance, food insecurity, mental health burden, housing instability, and loss of preventive healthcare). Overall, during 2020 only ~1% of COVID-19 deaths in high-income European countries were in ages <50 (accounting for only ~4000 fatalities), while the overall proportion across the rest of the world may have been ~5% or higher (accounting for ~100,000 or more fatalities). The impact of measures taken on mid-term and long-term mortality worldwide needs careful study and it may affect more prominently young people than COVID-19 itself.
4.6. Future questions
Acknowledging these caveats, demographic profile changes in the future evolution of the COVID-19 pandemic activity should be monitored as the pandemic progresses with the advent of new mutations and variants. Moreover, the effects of different non-pharmaceutical measures, as well as prospective vaccinations during 2021, on the distribution of deaths warrants close attention. It would be useful to understand the extent to which the demographic footprint of fatalities can be efficiently modulated by appropriate interventions in different settings.
Patient and public involvement
No involvement.
Data sharing
All the data are in the manuscript and tables.
Ethics approval
Not required for this type of study.
Funding
METRICS is supported by grants by the Laura and John Arnold Foundation. CA is supported by postdoctoral grants from Uppsala University, the Swedish Society of Medicine, the Blanceflor Foundation, and the Sweden-America Foundation. The funders had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; nor in the decision to submit the article for publication.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envres.2021.110856.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Axfors C., Schmitt A.M., Janiaud P., van ’t Hooft J., Abd-Elsalam S., Abdo E.F., et al. Mortality outcomes with hydroxychloroquine and chloroquine in COVID-19: an international collaborative meta-analysis of randomized trials. medRxiv. 2020 doi: 10.1038/s41467-021-22446-z. 2020.09.16.20194571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton J.K., Bayne G., Evans C., Garbe F., Gorman D., Honhold N., et al. Evolution and effects of COVID-19 outbreaks in care homes: a population analysis in 189 care homes in one geographical region of the UK. The Lancet Healthy Longevity. 2020;1(1):e21–e31. doi: 10.1016/S2666-7568(20)30012-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comas-Herrera A, Zalakaín J, Lemmon E, Henderson D, Litwin C, Hsu A, et al. Mortality Associated with COVID-19 in Care Homes: International Evidence. Article in LTCcovid.org, International Long-Term Care Policy Network, CPEC-LSE, 14 October2020.
- Comas-Herrera A, Zalakaín J, Litwin C, Hsu A, Lemmon E, Henderson D, et al. Mortality Associated with COVID-19 in Care Homes: Early International Evidence. Article in LTCcovid.org, International Long-Term Care Policy Network, CPEC-LSE, 26 June2020.
- Cousins S. Experts criticise Australia's aged care failings over COVID-19. Lancet. 2020;396:1322–1323. doi: 10.1016/S0140-6736(20)32206-6. 10259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobierno Digital Ministerio Secretaría General de la Presidencia, Ministerio del Interior y Ministerio de Ciencia Tecnología Conocimiento e Innovación. Cifras Oficiales COVID-19 en Chile: La Realidad Nacional en Datos.https://www.gob.cl/coronavirus/cifrasoficiales/Accessed 2021-01-24.
- Gudbjartsson D.F., Helgason A., Jonsson H., Magnusson O.T., Melsted P., Norddahl G.L., et al. Spread of SARS-CoV-2 in the Icelandic population. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2006100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., Linsell L., et al. Dexamethasone in hospitalized patients with covid-19 - preliminary report. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Instituto Nacional de Salud COVID-19 en Colombia. http://www.ins.gov.co/Noticias/Paginas/coronavirus-casos.aspx
- Instituto Superiore di Sanità (ISS) Caratteristiche dei pazienti deceduti positivi all’infezione da SARS-CoV-2 in Italia. 2020. https://www.epicentro.iss.it/en/coronavirus/sars-cov-2-integrated-surveillance-data Dati al 11 november 2020.
- Ioannidis J.P.A. Global perspective of COVID-19 epidemiology for a full-cycle pandemic. Eur. J. Clin. Invest. 2020;50(12) doi: 10.1111/eci.13423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannidis JP. Precision shielding for COVID-19: metrics of assessment and feasibility of deployment. BMJ Glob. Health. 2021;6(1):e004614. doi: 10.1136/bmjgh-2020-004614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannidis J.P.A., Axfors C., Contopoulos-Ioannidis D.G. Population-level COVID-19 mortality risk for non-elderly individuals overall and for non-elderly individuals without underlying diseases in pandemic epicenters. Environ. Res. 2020;188 doi: 10.1016/j.envres.2020.109890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komite Penanganan COVID-19 Dan Pemulihan Ekonomi Nasional Situasi virus COVID-19 di Indonesia. Peta Sebaran. https://covid19.go.id/peta-sebaran
- Liu M., Maxwell C.J., Armstrong P., Schwandt M., Moser A., McGregor M.J., et al. COVID-19 in long-term care homes in Ontario and British Columbia. Can. Med. Assoc. J. 2020;192(47):E1540. doi: 10.1503/cmaj.201860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministry of Health COVID-19 weekly situation report 29/06/2020 - 05/02/2020 Turkey. 2020. https://covid19.saglik.gov.tr/
- O'Driscoll M., Dos Santos G.R., Wang L., Cummings D.A.T., Azman A.S., Paireau J., et al. Age-specific mortality and immunity patterns of SARS-CoV-2. Nature. 2020 doi: 10.1038/s41586-020-2918-0. [DOI] [PubMed] [Google Scholar]
- Rijksinstituut voor Volksgezondheid en Milieu – RIVM Epidemiologische situatie COVID-19 in nederland. 2020. https://www.rivm.nl 10 november 2020, 10:00.
- Salcher-Konrad M., Jhass A., Naci H., Tan M., El-Tawil Y., Comas-Herrera A. COVID-19 related mortality and spread of disease in long-term care: a living systematic review of emerging evidence. medRxiv. 2020 2020.06.09.20125237. [Google Scholar]
- Santé publique France COVID-19. Point épidémiologique hebdomadaire du 12 novembre 2020. 2020. https://www.santepubliquefrance.fr
- Smith G.D., Spiegelhalter D. Shielding from covid-19 should be stratified by risk. BMJ. 2020;369:m2063. doi: 10.1136/bmj.m2063. [DOI] [PubMed] [Google Scholar]
- Spiegelhalter D. Use of “normal” risk to improve understanding of dangers of covid-19. BMJ. 2020;370:m3259. doi: 10.1136/bmj.m3259. [DOI] [PubMed] [Google Scholar]
- StataCorp. Stata Statistical Software: Release 15. StataCorp LLC; College Station, TX: 2017. [Google Scholar]
- The Atlantic Monthly Group The COVID tracking Project at the atlantic: the long-term care COVID tracker. https://covidtrackingcom/data/long-term-care Accessed 2021-01-21.
- Williamson E., Walker A.J., Bhaskaran K.J., Bacon S., Bates C., Morton C.E., et al. OpenSAFELY: factors associated with COVID-19-related hospital death in the linked electronic health records of 17 million adult NHS patients. medRxiv. 2020 2020.05.06.20092999. [Google Scholar]
- Worldometers.info COVID-19 CORONAVIRUS PANDEMIC dover. https://www.worldometers.info/coronavirus/#countries Delaware, USA2020 [updated November 26, 2020. Available from:
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.