Abstract
Tissue spheroids consist of a three-dimensional model of cells which is capable of imitating the complicated composition of healthy and unhealthy human tissue. Due to their unique properties, they can bring innovative solutions to tissue engineering and regenerative medicine, where they can be used as building blocks for the formation of organ and tissue models used in drug experimentation. Considering the rapid transformation of the health industry, it is crucial to assess the research dynamics of this field to support the development of innovative applications. In this research, a scientometric analysis was performed as part of a Competitive Technology Intelligence methodology, to determine the main applications of tissue spheroids. Papers from Scopus and Web of Science published between 2000 and 2019 were organized and analyzed. In total, 868 scientific publications were identified, and four main categories of application were determined. Main subject areas, countries, cities, authors, journals, and institutions were established. In addition, a cluster analysis was performed to determine networks of collaborations between institutions and authors. This article provides insights into the applications of cell aggregates and the research dynamics of this field, which can help in the decision-making process to incorporate emerging and innovative technologies in the health industry.
Keywords: Scientometric analysis, Competitive technology intelligence, Bioprinting, Cell aggregates, Bioink
1. Introduction
Additive manufacturing (AM), commonly known as three-dimensional (3D) printing, is a rapidly growing area that fabricates a wide range of structures and complex geometries by depositing successive layers of materials on top of each other[1,2]. In the medical field, 3D bioprinting refers to different AM techniques able to print living cells and materials, in a specified location[1]. 3D bioprinting has brought new solutions to mimic the heterogeneous and complex native tissues. Its main goal is to develop 3D living human constructs with biological and physical properties that emulate the human tissues, being a solution to repair tissue defects and restore organ structure and function[3]. Through this innovative technology, constructs, or implants tailored to the geometrically complex and irregular shapes of the native tissues can be produced using computer designs or medical images. In addition, it is also possible to create biological connectivity by embedding cells with pore networks to deliver components such as drug or nutrients[4].
Ng et al.[5] identify seven main technologies for 3D bioprinting: extrusion, stereolithography, laser-assisted, inkjet, microvalve-based bioprinting, two-photon polymerization microfluidic printing, and acoustic bioprinting. The main working foundation for the first five techniques is:
(i) Extrusion: pneumatic-or mechanical extrusion, loading of bio-inks into cartridges
(ii) Stereolithography: photo-polymerization of photo-initiators, loading of bio-inks into vat
(iii) Laser-assisted: localized vaporization of energy-absorbing layer, coating of homogeneous ribbon layer
(iv) Inkjet: use of actuators to overcome surface tension, loading of bio-inks into cartridges
(v) Microvalve-based bioprinting: use of actuators to overcome surface tension, loading of bio-inks into cartridges.
Extrusion, stereolithography and microvalve-based bioprinting present the less difficulties to operate while laser-assisted involves more complex process and inkjet process is even more complex.
The material that is printed is referred to as “bioink” and it consists of multiple types of cells and biomaterials. Bioinks are analyzed in terms of their printability, biocompatibility, and bioactivity[6]. The printing resolution and dimensionality contribute to the overall shape fidelity of the bioprinted construct. Its dimensionality can be represented by z-resolution in printing and it mainly depends on specific printing parameters such as printing path height, path space, and the nozzle diameter, while material properties as material contraction/swelling, thixotropy, and the crosslinking mechanism affect the z-resolution. The principle for deposition varies according to the bioprinting technology to be applied which affects the print resolution and dimensionality differently[7].
To meet all mechanical and functional requirements to produce biomimetic tissue-like constructs, multicomponent bioinks have been developed recently. Also known as multimaterials or multicelular bioinks, they include more than one biomaterial, cell, and additive material or biomolecule[3]. Multicomponent bioinks can be characterized as:
(i) Bioinks having combination of natural materials, for example, alginate with gelatin/fibrin, silk fibroin with gelatin, agarose with collagen, chitosan with gelatin, cellulose with alginate, and hyaluronan with cellulose;
(ii) Bioinks comprising natural and synthetic components;
(iii) Bioinks involving synthetic biomaterials;
(iv) Bioinks fabricated with hydrogels and particles;
(v) Bioinks for 4D printing; and
(vi) Bioinks with different type of cells and soluble factors.
Moreover, materials innano scale can also be added to improve structure and functionality[3].
One crucial element to succeed in 3D bioprinting is the right selection of cells to print. Cells can be used as individually encapsulated, as cells in scaffolds or as cell aggregates (spheroids)[3,8]. As mentioned by Hospodiuk et al.[9] and Rezende et al.[10], tissue spheroids are a type of scaffold-free bioink that has a small-sized and ideal geometric shape for bioprinting. This novel bioink enhances cell-cell interaction, growth, differentiation, and resistance to the environment because of the high cell density in the assembly[11].
Tissue spheroids consist of 3D cell clusters that represent the intricacy of healthy and unhealthy human tissues[12,13]. One important characteristic of these cell aggregates is their self-assembly, which mimics developing tissue by fusion and reorganization[14]. Conversely, a major disadvantage is that the majority of the cells do not aggregate spontaneously in culture; therefore, they need to be induced by some means[1]. These cellular aggregates can be fabricated using a scaffold or scaffold-free[12].
The first time these multicellular spheroids were created was in 2003 by Garboc Forgacs at the University of Missouri[15]. Since then, several techniques have been used for the generation of tissue spheroids. The most commonly used techniques rely solely on the self-arranging properties of cells using micromolded recessed templates prepared in a non-adhesive hydrogel[16].
In general, the use of tissue spheroids serves two main purposes, as building blocks in tissue engineering or as tissue models used in the pharmaceutical industry[9]. Tissue engineering constitutes an important field of regenerative medicine for tissue repair as it offers the potential for developing patient-specific 3D tissue constructs for the treatment of human diseases. It represents a huge potential solution to overcome the current shortage of organs or tissues for transplantation. On the other hand, 3D in vitro systems have significantly advanced the drug screening processes as 3D tissue models can closely mimic native tissues and, in some cases, the physiological response to the drugs, thus improving the ability to predict the efficacy and toxicity of drug candidates[17].
This study was performed to analyze and describe the development of tissue spheroids, as these cell aggregates can contribute significantly to the advancement and innovation of tissue engineering and regenerative medicine.
2. Methodology
A scientometric analysis was performed as part of a Competitive Technology Intelligence (CTI) process to identify current applications and newly emerging areas related to tissue spheroids for regenerative medicine and tissue engineering. CTI is a cyclical process used to collect, analyze, and interpret data from different sources legally and ethically to produce valuable information for decision-making purposes pertaining to research and development (R&D) and innovation within an organization[18]. In this research, this process was conducted using the CTI hybrid model developed by Rodríguez-Salvador et al.[19], which comprises ten main steps: (i) process planning, (ii) primary and secondary source identification, (iii) establishment of the information collection strategy, (iv) information collection, (v) expert validation and adjustments, (vi) scientometric analysis, (vii) expert validation and adjustments, (viii) verification of the final results, (ix) results delivery, and (x) decision-making. Execution of CTI implies the collection of the most relevant information instead of collecting the largest number of documents. From this perspective, the identification of keywords and the design of a search query as accurately as possible is required; thereby, it involves expert consultation from the beginning through the validation of the final results[19]. Hence, the search query of this study was developed through an iterative process, to identify additional keywords, aside from the ones provided initially by experts on the topic, to improve its accuracy. The general structure of the search query employed is as follows:
((( spheroid* PRE/1 ( cell OR cellular )) OR (“3 d spheroid”) OR (( 3d OR “three dimensional” OR “3 dimensional” OR “three d”) PRE/1 spheroids ) OR ( cancer PRE/1 spheroids ) OR ( tumor* PRE/1 spheroids ) OR ( tumorspheres OR tumourspheres OR tumorospheres ) OR ( cell* PRE/4 spheroid*) OR ( multicell* PRE/3 spheroid*) OR ( tissue* PRE/2 spheroid*) OR (( hepat* OR liver OR pancrea* OR thyroid OR organotypic OR cardiomyocyte ) PRE/0 spheroid ) OR (“self assembl* spheroid”) OR cardiosphere OR ( cell AND ( spheroid PRE/0 ( formation OR invasion OR culture )))) AND (“tissue engineering” OR “regenerative medicine”) AND NOT ( plant OR graphite OR bacter* OR alga* OR “solar cell*” OR “eutectic cell*” OR yeast OR spheroidin OR alloy OR rhodopseudomonas OR phytoplankton OR mycobacteria OR larva OR protista OR volvox OR coli OR “non-spheroid*” OR anisotropic OR pollen OR coral OR biofilm OR sponge OR plankton OR microalga* OR dictyostelium OR microbial OR microbe OR phytoplankton OR saccharomyces OR eps OR candida OR sea OR food OR amoeba OR “date palm” OR kelvin OR peanut OR lanata OR yew OR roseus OR ajuga OR “protein aggregates” OR antenna OR batter* OR foam OR barnacle OR oblate OR review OR overview ))
Boolean operators AND, OR and NOT were used to include and exclude the terms and the PRE/# function that indicates the number of words that may be close to a specific term. Scopus and Web of Science (WoS) databases were selected to collect the scientific documents. Scopus contains more than 5000 publishers and 75 million items indexed dating back to 1970 across different disciplines in science[20]. In addition, WoS includes scientific documents from over 21,000 high-impact journals covering more than 100 years of scientific production[21].
Gathering scientific documents were conducted for journal articles and conference papers from both databases that were published between January 1, 2000, and June 5, 2019 (when the collection activity ended). The documents obtained from each database were exported and combined into a single list, where a manual cleaning process was performed to remove documents not complying with the purpose of the study, as well as those containing duplicated information. The resulting documents were classified according to the technological application of the tissue spheroids mentioned previously.
A scientometric analysis was applied to the collected data to identify the current and emerging areas of tissue spheroids applications. First, subject areas were identified according to the classification given by Scopus. For publications indexed in WoS but not in Scopus, their subject areas were adapted to Scopus classification to maintain homogeneity. Subsequently, the publishing growth dynamics within the time range selected (January 1, 2000, to June 5, 2019), along with the most prolific countries, cities, authors, journals, and institutions on the topic were identified. Finally, a cluster analysis was performed to determine networks of collaborations between institutions and authors.
3. Results and discussion
A total of 1296 scientific documents published between January 1, 2000, and June 5, 2019, were retrieved; 783 from Scopus and 513 from WoS. A deduplication and manual validation process was performed, resulting in 868 publications. These publications were classified according to the following categories.
3.1. Global trends
Scientific articles and conference papers retrieved in this study revealed four global trends depending on the application given to tissue spheroids. These are building blocks, drug testing and disease model, spheroid formation, and complementary studies. Each category is described in Table 1. The following tables (Tables 2-5) correspond to the most recent and representative studies from the documents analyzed for each global trend.
Table 1.
Tissue spheroid global trends.
| Global trend | Description |
|---|---|
| Building blocks | Tissue spheroids are used as basic units to biofabricate tissue constructs such as implants organ precursors. Tissue constructs are built placing the tissue spheroids with bioprinting or bioassembly techniques. In some cases, cells are bioprinted as bioinks to build the final tissue construct, but before its completion, cells first aggregate in spherical forms before they fusion |
| Drug testing and disease model | Cell aggregates are used as a 3D culture model for drug testing purposes or for mimicking a particular disease. The resulting model can be formed by a single tissue spheroid or by a tissue construct product of the fusion of several tissue spheroids made of one or different cell lines |
| Spheroid formation | This category is related to the improvement of the tissue spheroid formation, particularly to uniform the tissue spheroids characteristics (i.e. size and cells number) and to scale up the process for mass tissue spheroid formation. But no specific applications were discussed in documents analyzed |
| Complementary studies | Complementary studies for tissue spheroids management, such as the development of computer programs and mathematical models to simulate tissue spheroids behavior, and the production of novel accessories for imaging systems for tissue spheroids monitoring |
Table 2.
Global trend: Tissue spheroids as building blocks.
| Article | Year/Journal | Impact analysis |
|---|---|---|
| Machino, R. et al “Replacement of Rat Tracheas by Layered, Trachea-Like, Scaffold-Free Structures of Human Cells Using a Bio-3D Printing System”[22] | 2019/Advanced Healthcare Materials | “Human cartilage cells, human fibroblasts, human umbilical vein endothelial cells, and human mesenchymal stem cells from bone marrow are aggregated into 20,000 cell spheroids and placed into a Bio-3D printing system (Regenova) with dedicated needles positioned according to 3D configuration data (Kenzan Method), to develop scaffold-free trachea-like tubes.” |
| Daly, A. C., & Kelly, D. J. “Biofabrication of spatially organised tissues by directing the growth of cellular spheroids within 3D printed polymeric microchambers”[23] | 2019/ Biomaterials | “Novel biofabrication strategy that enables the engineering of structurally organized tissues by guiding the growth of cellular spheroids within arrays of 3D printed polymeric microchambers.” This research used bone marrow mesenchymal stem cells (BMSC) and chondrocytes in a concentration of 20,000 and 40,000 per microchamber using inkjet printing |
| Anada, T. et al ”Vascularized bone-mimetic hydrogel constructs by 3D bioprinting to promote osteogenesis and angiogenesis”[24] | 2019/ International Journal of Molecular Sciences | “Two-step digital light processing technique to fabricate a bone-mimetic 3D hydrogel construct based on octacalcium phosphate (OCP), spheroids of human umbilical vein endothelial cells (HUVEC), and gelatin methacrylate (GelMA) hydrogels”. In this research a spheroid culture chip was used, conformed by a solution of 25×104 cells/mL |
Table 5.
Global trend: Complementary studies.
| Article | Year/Journal | Impact Analysis |
|---|---|---|
| Nakagawa, K., & Kishimoto, T. “Unlabeled image analysis-based cell viability assay with intracellular movement monitoring”[31] | 2019/Biotechniques | “Unlabeled optical metabolic imaging of cultured living cells. This imaging technique is based on motion vector analysis with a block-matching algorithm to compare sequential time-lapse images. Motion vector analysis evaluates the movement of intracellular granules observed with a phase-contrast microscope. This assay can measure cellular viability at a single-cell level without requiring any reagents”. In this research, human osteosarcoma U2OS cells, human colon carcinoma Caco-2 cells and human hepatoma HepG2 cells were used. |
| Wu, H. et al. “Electrical impedance tomography for real-time and label-free cellular viability assays of 3D tumour spheroids”[32] | 2018/Analyst | “In silico and in vitro cell viability inside large cell spheroids can be monitored in real time and label-free with electrical impedance tomography (EIT). The results show the potential of EIT for non-destructive real-time and label-free cellular assays in the miniature sensor, providing physiological information in the applications of the 3D drug screening and tissue engineering.” MCF-7 breast cancer cells were used, and the liquid overlay technique was adopted to form cells spheroids on the hydrogel surface. Cell suspension with 1×104 cells were seeded onto each microplate well. |
| Parrish, J et al. “A 96-well microplate bioreactor platform supporting individual dual perfusion and high-throughput assessment of simple or biofabricated 3D tissue models”[33] | 2018/Lab on a Chip | “Platform to address the experimental and in vivo disparity in throughput and both system complexity (by supporting multiple in situ assessment methods) and tissue complexity (by adopting a construct-agnostic format). It describes the potential of a scalable dual perfusion bioreactor platform for parenchymal and barrier tissue constructs to support a broad range of multi-organ-in-a-chip applications”. In this research human umbilical cord-derived vascular endothelial cells (HUVEC), bone marrow-derived mesenchymal stromal cells (MSC), human ovarian cancer cells and human foreskin-derived fibroblast were used. |
Table 3.
Global trend: Tissue spheroids for drug testing and disease models.
| Article | Year/Journal | Impact Analysis |
|---|---|---|
| Lee, C. et al. “Bioprinting a novel glioblastoma tumor model using a fibrin-based bioink for drug screening”[25]. | 2019/Materials Today Chemistry | “Printed cells spontaneously formed spheroids with upregulated levels of the proteins CD133 and DCX markers associated with cancer stem cells and metastatic invasiveness, respectively. Printed scaffolds were treated with a novel chemical treatment method previously tested in 2D culture and showed significant resistance, indicating the 3D printed glioblastoma model’s potential as a more accurate representation of the in vivo response to drug treatment.” Glioblastoma multiforme and human-induced pluripotent stem cells where printed using an Aspect Biosystems RX1 printer, which uses a microfluidic technology. |
| Kingsley, D. M. et al. ”Laser-based 3D bioprinting for spatial and size control of tumor spheroids and embryoid bodies”[26] | 2019/Acta Biomaterialia | “Impact analysis of the aggregate size on the uptake of a commonly employed ligand for receptor-mediated drug delivery, Transferrin, indicating that larger tumor spheroids exhibit greater spatial heterogeneity in ligand uptake” For this research, human breast cancer cells and CCE mouse embryonic stem cells (mESCs) were printed using laser direct write (LDW) bioprinting. |
| Trisno, S. L.. et al. ” Esophageal Organoids from Human Pluripotent Stem Cells Delineate Sox2 Functions during Esophageal Specification”[27] | 2018/Cell Stem Cell | “Dorsal anterior foregut (AFG) spheroids grown in a 3D matrix formed human esophageal organoids (HEOs), and HEO cells could be transitioned into two-dimensional cultures and grown as esophageal organotypic rafts. HEOs present a powerful platform for modeling human pathologies and tissue engineering.” In this research pluripotent stem cells (PCSs) signaling pathways´ were manipulated to differentiate into esophageal organoids. Suspension method was used for spheroid formation. |
Table 4.
Global trend: Spheroid formation.
| Article | Year/Journal | Impact Analysis |
|---|---|---|
| Miller, A. J. et al. “Generation of lung organoids from human pluripotent stem cells in vitro”[28]. | 2019/Nature Protocols | “Protocol that recapitulates several stages like induction, patterning, lung specification, budding, morphogenesis; to differentiate human pluripotent stem cells (hPSCs) into ventral–anterior foregut spheroids and further into two distinct types of organoids: human lung organoids and bud tip progenitor organoids.” |
| Lee, W. et al. ” Dispersible hydrogel force sensors reveal patterns of solid mechanical stress in multicellular spheroid cultures”[29] | 2019/Nature Communications | “Development of ultrasoft mechanosensors that visibly deform under <10 Pascals of cell-generated stress. By incorporating mechanosensors into multicellular spheroids, the patterns of internal stress that arise during spheroid formation where captured. This technique can provide a quantitative basis to design tissues that leverage the mechanical activity of constituent cells to evolve towards a desired form and function.” In this research, HS-5 fibroblasts were used as well as an aqueous two-phase droplet printing technique by an automated liquid handler in a concentration of 6×107 cells/mL. |
| Heo, D. N. et al. ” Synergistic interplay between human MSCs and HUVECs in 3D spheroids laden in collagen/fibrin hydrogels for bone tissue engineering”[30] | 2019/Acta Biomaterialia | “To enhance stem cell function and generate pre-vascularized network, a collagen/fibrin hydrogel was employed as an encapsulation matrix for the incorporation of human mesenchymal stem cell/human umbilical vein endothelial cell (MSC/HUVEC) spheroids, and their cellular behavior (including cell viability, morphology, proliferation, and gene expression profile) was investigated and compared to that of cell suspension- or MSC spheroids-laden hydrogels.” In this study, microwells in AggreWell plates were used. Cell suspension at a density of 1.2×106 cells/well was seeded. MSC-only and MSC/HUVEC (75%/25%) spheroids were used. |
Results of these tables show the specific focus of the different group trends identified. There is a diversity of bioinks and cell types, ranging from healthy cells, such as human fibroblasts, human umbilical vein endothelial cells (HUVECs), human mesenchymal stem cells from bone marrow (hMSCs), human-induced pluripotent cells (hPCSs), and carcinogenic cells (e.g., human breast cancer, osteosarcoma, colon carcinoma, hepatoma, and ovarian cancer cells). Among them, the HUVECs, MSCs, and PCSs are the most used cell types. For example, stem cells offer interesting advantages as they can be obtained from various sources and differentiated into various lineages[3].
Our findings also exhibit that there is no single predominant 3D bioprinting process, this technology is evolving rapidly and different approaches exist depending on the main goal to achieve. Moreover, our results also show that spheroid resolution can be manipulated depending on the purpose of the study provided that a certain spheroid size is not yet defined.
According to Ng et al.,[5] a key dilemma lies in the need of obtaining a balance between achieving the nano-scale resolution that emulate the extracellular matrix (ECM) of human tissues/organs and improving the speed for fabrication of human-scale tissues/organs.
Ashammakhi et al.[3] published on the challenges involving multicomponent bioinks that are related to the development of appropriate materials having shear-thinning properties with cell-friendly capability and other desired biological characteristics for different tissue engineering applications. As Ng et al.[5] indicate, it is also important to know more about the composition and spatial arrangement of living cells and ECM within tissue constructs along with the development of advanced bioprinting strategies.
3.2. Scientometric analysis results
As shown in Figure 1, of the 868 publications obtained, 597 publications (69%) exhibited the analysis of spheroid formation, 135 publications (16%) described the use of tissue spheroids as building blocks, 100 (11%) relates to tissue spheroids for drug testing and disease model and finally, and 36 (4%) comprise complementary studies of tissue spheroids.
Figure 1.
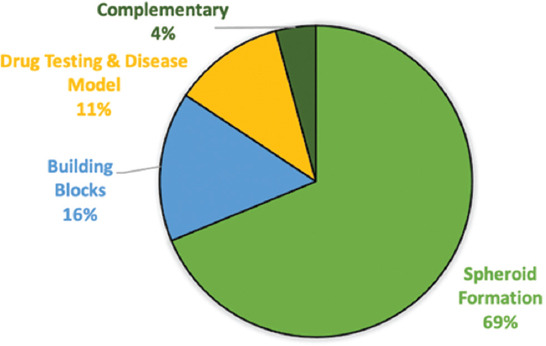
Distribution of scientific publications by categories
Subject areas were identified based on the classification of science disciplines in the Scopus database. For publications indexed in WoS but not in Scopus, their subject areas were adapted to Scopus categorization to maintain homogeneity. In this study, the analysis of all 868 publications revealed nine subjects following the distribution displayed in Figure 2: biochemistry, genetics and molecular biology (25%), engineering (19%), materials science (16%), chemical engineering (11%), medicine (10%), chemistry (4%), immunology and microbiology (3%), applied physics (3%), and other (9%). However, biochemistry, genetics and molecular biology, engineering, and materials science account for more than half of all the publications with 60% of all the documents.
Figure 2.
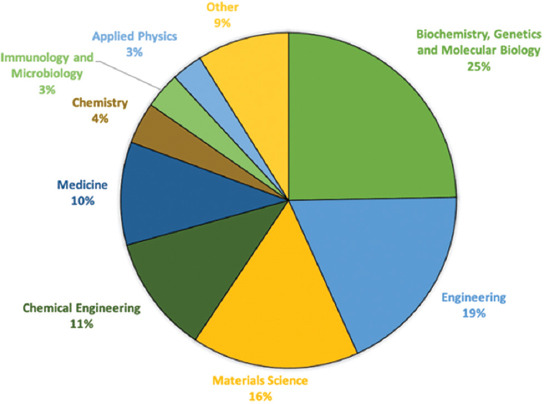
Distribution of the subject areas of publication on the use of tissue spheroids.
The growth dynamics of publications on tissue spheroids were defined as shown in Figure 3. In terms of publications, number of papers by year of publication did not exhibit a strictly patterned behavior (e.g., linear or exponential); nevertheless, publications regarding tissue spheroids showed an increased growth from 2 publications in 2000 to 122 publications in 2018. The year 2019 was not depicted in the graph since the retrieval period ended on June 5; thus far there had been 42 overall. The biggest growth was seen from 2015 to 2016, with a 35.5% increase, going from 76 documents to 103. Of the 868 scientific documents, 51% were published in the past 5 years (2015–2019).
Figure 3.
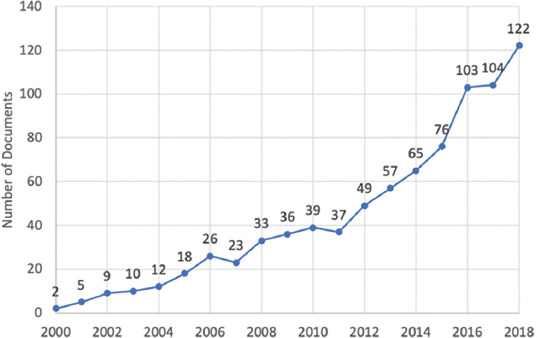
Number of documents on tissue spheroids by year of publication.
The affiliations of authors indexed in high-impact scientific databases are an indicator, of which countries and organizations have patterns of research concentration. The top countries and cities with the largest numbers of publications on tissue spheroids were also determined; results are presented in Figure 4A and B. The United States is the most prolific country with 288 publications, followed by Japan with a total of 155, China with 93, and Germany with 84 published articles. These four countries account for more than half (55%) of the total documents. The remaining countries on the top ten published between 26 and 73 scientific articles and are located either in western Europe or eastern Asia – except for Canada, which holds the ninth position.
Figure 4.
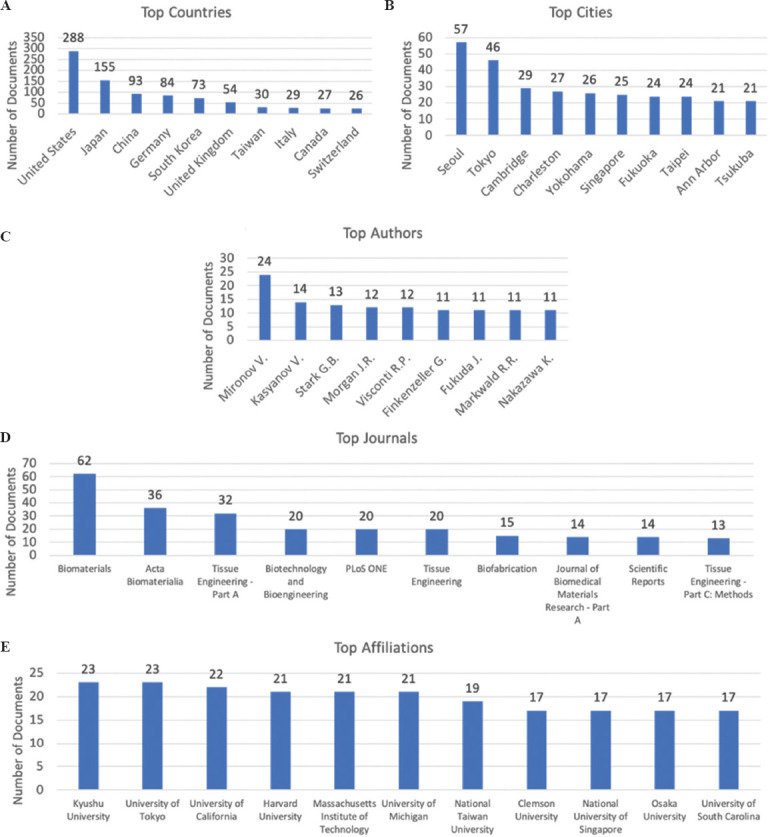
Global scientific trends in tissue spheroids. A summary of the publications that are indexed in Scopus and Web of Science according to (A) the ten most frequent affiliation countries and (B) cities of the authors; (C) the ten most cited authors (D) the ten journals with the most occurrences of the search terms; and (E) the ten most frequent organizational affiliations of the authors.
The top cities are highly correlated with the top ten countries; however, the rankings are much closer in the total output, except Seoul, with 57 and Tokyo with 46 scientific documents which have almost twice as much as the output from other cities in the top ten. We can conclude that a significant amount (78%) of the papers produced in South Korea are centralized in Seoul, whilst in Japan, most of the papers were contributed from four main cities, that is, Tokyo, Yokohama, Fukuoka, and Tsukuba, which are ranked in the top ten of most prolific cities.
The top authors in this study are presented in Figure 4C. A total of 4,069 authors were identified among all the publications. The first and second most prolific authors were Vladimir Mironov and Vladimir Kasyanov with 24 and 14 publications each. The third most prolific author is Gerhard Björn Stark with 13 scientific articles. These findings can correlate directly to the recently published paper “Bioprinting in the Russian Federation: Can Russians Compete?” by Peter Timashev and Vladimir Mironov in which they state five main achievements made by Russian bioprintists that have contributed to global technology in the field, such as the development of original 3D bioprinters, natural bioinks and the world’s first functional and vascularized organ construct[34].
Figure 4D shows the journals with the highest number of publications on tissue spheroids. Biomaterials is the most prolific journal with 62 documents, followed by Acta Biomaterialia which has almost half the number of articles with 36. Tissue Engineering – Part A claims the third place with 32 publications. These three journals comprise 52% of all the documents in the top ten list. These journals focus on either biomaterial structure, function, and clinical application or in therapeutic strategies to regenerate tissue – the topics closely related to tissue spheroids.
Furthermore, the institutions with the highest numbers of publications were also identified, as shown in Figure 4E. Overall, 840 institutions were identified worldwide but the most prolific institutions are directly correlated with the most prolific countries mentioned before. Japan has the most prolific institutions with 23 publications each from Kyushu University and the University of Tokyo. The University of California in the United States published a total of 22 articles, and in the same country, we found a triple tie with 21 documents: Harvard University, Massachusetts Institute of Technology, and the University of Michigan.
Finally, Figure 5A and B shows network maps of the authors and institutions’ collaborations, respectively. In these illustrations, the nodes’ size is proportional to the number of publications. Vladimir Mironov was identified as the most prolific author who engages in close collaboration with Vladimir Kasyanov, Rodrigo Alvarenga Rezende, Jorge Vicente Lopes da Silva, Roger R. Markwald, and Richard P Visconti; these authors represent the biggest collaborating network. Other main authors, such as Yasuyuki Sakai, Gerhard Björn Stark, and Jeffrey R. Morgan, were also visualized working with their own research groups.
Figure 5.
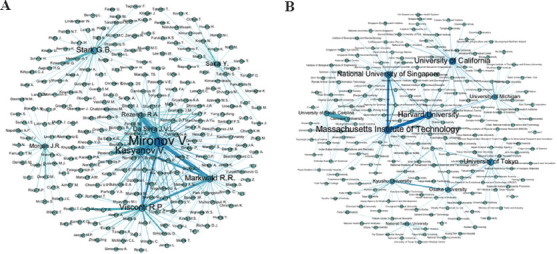
Co-occurrence network maps. (A) Top authors cooccurrence. (B) Top affiliations cooccurrence.
As to the institutions, we identified that the University of South Carolina has the closest collaboration with the Clemson University. Another substantial collaborator is the Massachusetts Institute of Technology with both Harvard University and the National University of Singapore. Finally, the Kyushu University collaborates closely with Osaka University.
4. Conclusions
This study assessed the scientific research dynamics of tissue spheroids through a CTI process using a scientometric analysis. To accomplish this, scientific publications published between January 1, 2000, and June 5, 2019, were retrieved from Scopus and WoS, before organization and analysis. Four fundamental trends were detected: tissue spheroids as building blocks, tissue spheroids for drug testing and disease models, spheroid formation analysis, and complementary studies. Different types of bioinks and cells, ranging from healthy cells to carcinogenic cells, were also identified. In addition, subject area distributions as well as the most prolific countries, cities, authors, journals, and institutions regarding this topic were identified to determine the overall research publications landscape, as well as a network of collaborations between institutions and authors.
Our results exhibit that tissue spheroids research covers nine subject areas: biochemistry, genetics and molecular biology, engineering, materials science, chemical engineering, medicine, chemistry, immunology and microbiology, applied physics, and others, with an emphasis on biochemistry, genetics, and molecular biology, engineering, and materials science that constitute 60% of the publications.
Our findings also revealed a growing interest on tissue spheroids research, evidenced by the biggest leap of scientific production particularly in the past 5 years. The United States and Japan were found to be the most prolific countries, for being ranked in the top ten positions and authoring more than half of the documents analyzed. Nevertheless, the most prolific city was Seoul, South Korea; this might be due to the centralization of the research centers in this capital. The most prolific author was found to be Vladimir Mironov, followed by Vladimir Kasyanov, probably due to the fact that they collaborate closely, representing an interesting finding in the network of authors collaboration. The top three journals identified were Biomaterials, Acta Biomaterialia, and Tissue Engineering – Part A. The main institutions identified are directly related to the most prolific countries. For instance, Kyushu University and the University of Tokyo in Japan were both tied at number one position, followed by the institutions in the United States: University of California in the second place whereas Harvard University, Massachusetts Institute of Technology, and the University of Michigan tied at the third place.
Insights obtained in this study show the main trends of published research in tissue spheroids. These findings may help guide research efforts in the tissue engineering and regenerative medicine, supporting the development of new technological applications that would revolutionize the health industry in the coming years.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
The authors acknowledge institutional funding received from Tecnológico de Monterrey and Consejo Nacional de Ciencia y Tecnología (CONACyT).
References
- 1.Donderwinkel I, Hest JC, Cameron NR. Bio-inks for 3D Bio-Printing:Recent Advances and Future Prospects. Polym Chem. 2017;8:4451–71. https://doi.org/10.1039/c7py00826k. [Google Scholar]
- 2.Ngo TD, Kashami A, Imbalzano G, et al. Additive Manufacturing (3D Printing):A Review of Materials, Methods, Applications and Challenges. Compos B Eng. 2018;143:172–96. https://doi.org/10.1016/j.compositesb.2018.02.012. [Google Scholar]
- 3.Ashammakhi N, Ahadian S, Xu C, et al. Bioinks and Bio-Printing Technologies to Make Heterogeneous and Biomimetic Tissue Constructs. Mater Today Bio. 2019;1:100008. doi: 10.1016/j.mtbio.2019.100008. https://doi.org/10.1016/j.mtbio.2019.100008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwab A, Levato R, D'Este M, et al. Printability and Shape Fidelity of Bioinks in 3D Bioprinting. Chem Rev. 2020;120:11028–55. doi: 10.1021/acs.chemrev.0c00084. https://doi.org/10.1021/acs.chemrev.0c00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ng WL, Chua CK, Shen YF. Print Me An Organ!Why We Are Not There Yet. Prog Polym Sci. 2019;97:101145. https://doi.org/10.1016/j.progpolymsci.2019.101145. [Google Scholar]
- 6.Ji S, Guvendiren M. Recent Advances in Bioink Design for 3D Bio-Printing of Tissues and Organs. Front Bioeng Biotechnol. 2017;5:23. doi: 10.3389/fbioe.2017.00023. https://doi.org/10.3389/fbioe.2017.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee JM, Ng WL, Yeong WY. Resolution and Shape in Bio-Printing:Strategizing Towards Complex Tissue and Organ Printing. Appl Phys Rev. 2019;6:11307. https://doi.org/10.1063/1.5053909. [Google Scholar]
- 8.Colosi C, Shin SR, Manoharan V, et al. Microfluidic Bio-Printing of Heterogeneous 3D Tissue Constructs Using Low-Viscosity Bioink. Adv Mater. 2016;28:677–84. doi: 10.1002/adma.201503310. https://doi.org/10.1002/adma.201503310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hospodiuk M, Dey M, Sosnoski D, et al. The Bioink:A Comprehensive Review on Bio-Printable Materials. Biotechnol Adv. 2017;35:217–39. doi: 10.1016/j.biotechadv.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 10.Rezende RA, Pereira FD, Kasyanov V, et al. In:Procedia CIRP. Vol. 5. Amsterdam, Elsevier; 2013. Scalable biofabrication of tissue spheroids for organ printing; pp. 276–81. https://doi.org/10.1016/j.procir.2013.01.054. [Google Scholar]
- 11.Sriphutkiat Y, Kasetsirikul S, Zhou Y. Formation of Cell Spheroids Using Standing Surface Acoustic Wave (SSAW) Int J Bioprint. 2018;4:130. doi: 10.18063/IJB.v4i1.130. https://doi.org/10.18063/ijb.v4i1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Costa EC, Melo-Diogo DD, Moreira AF, et al. Spheroids Formation on Non-Adhesive Surfaces by Liquid Overlay Technique:Considerations and Practical Approaches. Biotechnol J. 2017;13:1002. doi: 10.1002/biot.201700417. https://doi.org/10.1002/biot.201700417. [DOI] [PubMed] [Google Scholar]
- 13.Gopinathan J, Noh I. Recent Trends in Bio-Inks for 3D Printing. Biomater Res. 2018;22:11. doi: 10.1186/s40824-018-0122-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murphy SV, Atala A. 3D Bio-Printing of Tissues and Organs. Nat Biotechnol. 2014;32:773–85. doi: 10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- 15.Jose RR, Rodriguez MJ, Dixon TA, et al. Evolution of Bio-Inks and Additive Manufacturing Technologies for 3D Bio-Printing. ACS Biomater Sci Eng. 2016;2:1662–78. doi: 10.1021/acsbiomaterials.6b00088. [DOI] [PubMed] [Google Scholar]
- 16.Mehesz AN, Brown J, Hajdu Z, et al. Scalable Robotic Bio-Fabrication of Tissue Spheroids. Biofabrication. 2011;3:025002. doi: 10.1088/1758-5082/3/2/025002. https://doi.org/10.1088/1758-5082/3/2/025002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peng W, Unutmaz D, Ozbolat IT. Bio-Printing towards Physiologically Relevant Tissue Models for Pharmaceutics. Trends Biotechnol. 2016;34:722–32. doi: 10.1016/j.tibtech.2016.05.013. https://doi.org/10.1016/j.tibtech.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 18.Toit AS. Competitive Intelligence Research:An Investigation of Trends in the Literature. J Intell Stud Bus. 2015;5:14–21. [Google Scholar]
- 19.Rodríguez-Salvador M, Villarreal-Garza D, Álvarez MM, et al. Analysis of the Knowledge Landscape of Three-Dimensional Bio-Printing in Latin America. Int J Bioprint. 2019;5:240. doi: 10.18063/ijb.v5i2.2.240. https://doi.org/10.18063/ijb.v5i2.3.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elsevier, 2019. [Last accessed on 2020 Oct 20];Scopus. Available from: https://www.elsevier.com/solutions/scopus .
- 21.Clarivate Analytics. [Last accessed on 2020 Oct 20];Databases. 2019 Available from: https://www.clarivate.com/products/web-of-science/databases .
- 22.Machino R, Matsumoto K, Taniguchi D, et al. Replacement of Rat Tracheas by Layered, Trachea-Like, Scaffold-Free Structures of Human Cells Using a Bio-3D Printing System. Adv Healthc Mater. 2019;8:1800983. doi: 10.1002/adhm.201800983. https://doi.org/10.1002/adhm.201800983. [DOI] [PubMed] [Google Scholar]
- 23.Daly AC, Kelly DJ. Bio-Fabrication of Spatially Organised Tissues by Directing the Growth of Cellular Spheroids within 3D Printed Polymeric Microchambers. Biomaterials. 2019;197:194–206. doi: 10.1016/j.biomaterials.2018.12.028. https://doi.org/10.1016/j.biomaterials.2018.12.028. [DOI] [PubMed] [Google Scholar]
- 24.Anada T, Pan CC, Stahl AM, et al. Vascularized Bone-Mimetic Hydrogel Constructs by 3D Bioprinting to Promote Osteogenesis and Angiogenesis. Int J Mol Sci. 2019;20:1096. doi: 10.3390/ijms20051096. https://doi.org/10.3390/ijms20051096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee C, Abelseth E, de la Vega L, et al. Bioprinting a Novel Glioblastoma Tumor Model Using a Fibrin-Based Bio-Ink for Drug Screening. Mater Today Chem. 2019;12:78–84. https://doi.org/10.1016/j.mtchem.2018.12.005. [Google Scholar]
- 26.Kingsley DM, Roberge CL, Rudkouskaya A, et al. Laser-Based 3D Bio-Printing for Spatial and Size Control of Tumor Spheroids and Embryoid Bodies. Acta Biomater. 2019;95:357–70. doi: 10.1016/j.actbio.2019.02.014. https://doi.org/10.1016/j.actbio.2019.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trisno SL, Philo K, McCracken KW, et al. Esophageal Organoids from Human Pluripotent Stem Cells Delineate Sox2 Functions during Esophageal Specification. Cell Stem Cell. 2018;23:501–15.e7. doi: 10.1016/j.stem.2018.08.008. https://doi.org/10.1016/j.stem.2018.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller AJ, Dye BR, Ferrer-Torres D, et al. Generation of Lung Organoids from Human Pluripotent Stem Cells in Vitro. Nat Protoc. 2019;14:518–40. doi: 10.1038/s41596-018-0104-8. https://doi.org/10.1038/s41596-018-0104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee W, Kalashnikov N, Mok S, et al. Dispersible Hydrogel Force Sensors Reveal Patterns of Solid Mechanical Stress in Multicellular Spheroid Cultures. Nat Commun. 2019;10:144. doi: 10.1038/s41467-018-07967-4. https://doi.org/10.1038/s41467-018-07967-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heo DN, Hospodiuk M, Ozbolat IT. Synergistic Interplay Between Human MSCs and HUVECs in 3D Spheroids Laden in Collagen/Fibrin Hydrogels for Bone Tissue Engineering. Acta Biomater. 2019;95:348–56. doi: 10.1016/j.actbio.2019.02.046. https://doi.org/10.1016/j.actbio.2019.02.046. [DOI] [PubMed] [Google Scholar]
- 31.Nakagawa K, Kishimoto T. Unlabeled Image Analysis-Based Cell Viability Assay with Intracellular Movement Monitoring. Biotechniques. 2019;66(3):128–33. doi: 10.2144/btn-2018-0157. https://doi.org/10.2144/btn-2018-0157. [DOI] [PubMed] [Google Scholar]
- 32.Wu H, Yang Y, Bagnaninchi PO, et al. Electrical Impedance Tomography for Real-Time and Label-Free Cellular Viability Assays of 3D Tumour Spheroids. Analyst. 2018;143:4189–98. doi: 10.1039/c8an00729b. https://doi.org/10.1039/c8an00729b. [DOI] [PubMed] [Google Scholar]
- 33.Parrish J, Lim KS, Baer K, et al. A 96-Well Microplate Bioreactor Platform Supporting Individual Dual Perfusion and High-Throughput Assessment of Simple or Bio-Fabricated 3D Tissue Models. Lab Chip. 2018;18:2757–75. doi: 10.1039/c8lc00485d. https://doi.org/10.1039/c8lc00485d. [DOI] [PubMed] [Google Scholar]
- 34.Timashev P, Mironov V. Bio-Printing in the Russian Federation:Can Russians Compete? Int J Bioprint. 2020;6:303. doi: 10.18063/ijb.v6i3.303. https://doi.org/10.18063/ijb.v6i3.303. [DOI] [PMC free article] [PubMed] [Google Scholar]


