Abstract
Three-dimensional (3D) bioprinting is an important technology for fabricating artificial tissue. To effectively reconstruct the multiscale structure and multi-material gradient of natural tissues and organs, 3D bioprinting has been increasingly developed into multi-process composite mode. The current 3D composite bioprinting is a combination of two or more printing processes, and oftentimes, physical field regulation that can regulate filaments or cells during or after printing may be involved. Correspondingly, both path planning strategy and process control all become more complex. Hence, the computer-aided design and computer-aided manufacturing (CAD/CAM) system that is traditionally used in 3D printing system is now facing challenges. Thus, the scale information that cannot be modeled in the CAD process should be considered in the design of CAM by adding a process management module in the traditional CAD/CAM system and add more information reflecting component gradient in the path planning strategy.
Keywords: 3D composite bioprinting, Biofabrication, Computer-aided design and computer-aided manufacturing, Multiscale structure, Physical field control
1. Introduction
Driven by clinical needs, tissue engineering, bio-fabrication, and additive manufacturing have been deeply intersected, and this multi-disciplinary intersection effectively promotes the rapid development of three-dimensional (3D) bioprinting technology. 3D bioprinting not only inherits the principle of additive manufacturing but also has obvious particularity in printing ink and printing object. Specifically, the “ink” for 3D bioprinting refers mostly to biological materials, cells, drugs, growth factors, etc.[1,2] The ideal printing process needs to effectively fabricate items that imitate the structure and composition of natural biological tissues and organs as well as take into account the regulation of the behavior of printed biological tissues/organs in the later cultivation and growth process[3,4].
It is well known that 3D bioprinting is divided into the following types: Material extrusion (mechanical/pneumatic extrusion)[5-7], material jetting (inkjet, microvalve-based, laser-assisted, electrohydrodynamic printing)[8-11], and vat polymerization (stereolithography, digital light processing, and two-photon polymerization)[12-14]. Each printing process is unique for its characteristics. Since natural tissues, such as the heart, nerves, and blood vessels, tend to have unique anisotropic fiber structures of exceptionally rich internal components[15], it is quite difficult to fabricate a bioconstruct that features multi-scale and heterogeneous microstructures using a single-step printing process[16]. Therefore, an increasing number of researches have begun to integrate two or more printing processes with different forming principles to prepare complex biological structures or functional scaffolds, which is also the origin of 3D composite bioprinting. In fact, 3D composite bioprinting has become a research hotspot in the field of artificial biological tissue and organ construction. The current 3D composite bioprinting not only features a combination of two or more printing processes but also often involves physical field regulation that can regulate filaments or cells during or after printing.
It should be pointed out that the steps of multi-physical field regulation are often adopted in the process of 3D composite bioprinting to form micro-scale structure inside the macro-scale 3D printing structure. Correspondingly, the computer-aided design and computer-aided manufacturing (CAD/CAM) software for 3D composite bioprinting are different from the traditional 3D printing system. In other words, the CAM software for 3D composite bioprinting should be able to combine the scale information that cannot be modeled in CAD software with the modellable information from the CAD software, presenting new challenges to the design of 3D composite bioprinting system.
2. The process of 3D composite bioprinting
The intention of 3D composite bioprinting is to effectively overcome the limitations of a single-step printing process and ensure the inclusion of multiscale heterogeneous characteristics in the final construction by the integration of multiple process technologies. However, according to the status quo of research, two principles are very important for good integration: (i) The material structure formed by different printing processes can form a good composite interface and (ii) the integration of different printing processes has engineering realizability in the system implementation.
2.1. Combination of extrusion printing and dynamic crosslinking
Extrusion printing is the most typical and common process method. It uses air pressure or mechanical force as the driving energy to controllably extrude bioink, and by the spatial motion of the platform and the print nozzle, different two-dimensional patterns can be depicted and stacked to form a 3D structure. In extrusion printing, materials with different viscosity can be used as bioink. The highly viscous materials can be extruded to form continuous fibers, while the lower ones can be applied to obtain discrete droplets. Therefore, various materials are available for extrusion printing which are beneficial for manufacturing structures with good mechanical properties. Recently, many studies on 3D composite bioprinting based on extrusion printing have been carried out and the most representative is the combination of dynamic crosslinking technologies, which specifically refer to a class of technologies that can achieve various degrees of crosslinking in extrusion printing process through online control of process parameters or dynamic adjustment of external physical field.
At present, by adding temperature gradient in the process of extrusion printing, printing technologies and equipment with the capacity of imposing different temperature conditions have been developed[17-20]. Direct extrusion of biomaterials such as gel, slurry, particle, and filament that represent temperature-dependent phase transition is the main operating mode and the printing path can be obtained from conventional 3D modeling, slicing, and path planning methods and software. One research hotspot in this field is to improve the mechanical properties and biocompatibility of the printed construct at the same time using a composite printing system which combines the printing processes under different temperature field; some progress has already been made. Chen et al. built a hierarchical construct by alternately depositing the Wharton’s jelly mesenchymal stem cells-coated polydopamine (PDA)-coated calcium silicate/polycaprolactone (PCL) fibers and HUVECs-laden hydrogel in a composite printing system combining melt extrusion, normal temperature extrusion, and electrospray processes to obtain a bone scaffold with good mechanical properties and the ability to promote angiogenesis and osteogenesis[21]. Mekhileri et al. designed a singularization device that was capable of capturing and extruding a single microtissue of hydrogel spheroid with more than 80% accuracy. The integrated system of this device with melt extrusion equipment was capable of precisely delivering a single microstructure to a specific position in the scaffold during the printing process, thereby realizing the preparation of complex hierarchical bioconstruct (Figure 1A)[22].
Figure 1.
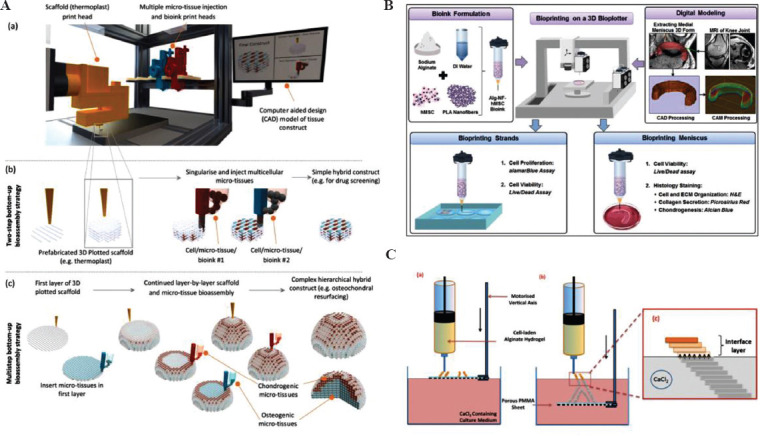
Fabrication of bioconstruct through the composite forming technology by combining extrusion printing with dynamic crosslinking. (A) Complex hierarchical construct made by the integrated system combining melt extrusion and singularization printing (from ref.[22] licensed under Creative Commons Attribution 3.0 license). (B) Meniscus printing based on the extrusion of bioink containing polylactic acid (PLA) fibers, human adipose-derived stem cells, and alginate, and crosslinking by CaCl2 (Reprinted with permission from Narayanan LK, Huebner P, Fisher MB, et al., 2016, 3D-Bioprinting of Polylactic Acid (PLA) Nanofiber–Alginate Hydrogel Bioink Containing hASCs. ACS Biomater. Sci. Eng., 2(10):1732–1742[27]. Copyright © 2016 American Chemical Society). (C) Vascular structure formed by stacking pre-crosslinking alginate patterns and subsequently enhanced by adding Ba2+ (from ref.[28] licensed under Creative Commons Attribution 3.0 license).
In addition, the introduction of ionic crosslinking and coaxial nozzle enables the extrusion process to effectively construct vessels like microchannels[23-26]. Dynamic reactive extrusion printing technology, which has shown good potential in the realization of cell printing under room temperature, is a growing research interest. Narayanan et al. produced meniscus by printing human adipose-derived stem cells (hASCs) with polylactic acid (PLA) fibers and alginate hydrogel. Previous studies indicated that the composite fiber structure enhanced cell proliferation and promoted extracellular matrix (ECM) secretion and chondrogenic differentiation (Figure 1B)[27]. Tabriz et al. prepared a 3D cellular biological structure by extruding pre-crosslinked sodium alginate into calcium ion bathing. After that, the structural stability was further enhanced by barium ion crosslinking (Figure 1C)[28]. Lozano et al. printed 3D brain-like structures composed of discrete layers of primary cortical neural cells with a coaxial nozzle. The result showed that the cortical cells inside the structure could develop into 3D neuronal networks in <5 days[29]. Wang et al. prepared in vitro glioma model by coaxially extruding materials into the calcium chloride (CaCl2) solution. The shell consisted of sodium alginate and glioma stem cells (GSC23), while the cell suspension containing glioma cell U118 was taken as the core material. The experimental results showed that the glioma model prepared by this method could mimic the glioma microenvironment and had enhanced drug resistance[30].
In summary, the integration of dynamic crosslinking means is an important driving force for the development of extrusion printing technology; especially, the combination of coaxial extrusion printing and materials with ionic crosslinking properties gives great advantages in the construction of vessel-like structures. Compared with the existing two-dimensional lamellar microchannel manufacturing technology, this method has obvious advantages and potentials in the integration forming process with living cells. Besides, the vessel-like constructs made by this method can better mimic the tubular structure and is more convenient for 3D bioprinting. In addition, the aforementioned dynamic crosslinking technology usually achieves various degrees of crosslinking in the whole extrusion printing process through online control of process parameters or dynamic adjustment of the external physical field. Hence, this kind of method accords with the characteristics of the above-mentioned 3D composite bioprinting.
2.2. Combination of electrohydrodynamics and extrusion printing
Electrohydrodynamics refers to the dynamics of electrically charged fluid, which constitute the basis of electrospinning, material jetting, and electrostatic direct writing. The process of electrospinning, electrospray, or electrostatic direct writing is achieved similarly by applying a voltage between the nozzle and the receiving plate. However, due to different material properties, the resultant forces formed on the charged fluid surface are different, resulting in different shapes of material after they leave the nozzle.
Compared with other methods, electrospinning is more widely used to fabricate tissue engineering scaffolds because the structure made by electrospinning resembles ECM. However, the electrospinning structure does not have enough mechanical properties and cannot form a 3D structure with a certain thickness; therefore, many researchers start to combine electrospinning with extrusion printing. Besides, some of the research results pointed out that electrostatic direct writing can generate micron-scale fibers and properly control the fiber deposition position. However, electrostatic direct writing encounters challenges when constructing thick structures[31]. According to recent studies, the composite forming process based on electrospinning/electrostatic direct writing and extrusion printing has emerged as a powerful technique in the field of developing new scaffolds, including vessel, bone, and skin.
Among various attempts, the most representative one lies in the preparation of the artificial blood vessel, which contains the integration of micro-nano fibers generated by electrospinning and the macrostructure formed by extrusion printing to mimic the multilayer structure of the blood vessel wall and regulate the mechanical properties[32-36]. Wu et al. utilized melt extrusion printing and electrospinning to prepare a bi-layered vascular graft with 3D interconnected circumferential microchannels. The bi-layered structure was fabricated by casting and electrospinning poly(l-lactic acid-co-ε-caprolactone) while the microchannels in the inner layer were formed by sacrificing the extruded sugar fiber[37]. By combining electrospinning with melt extrusion printing process, Lee et al. proposed a fabrication method of building a composite artificial vessel which using electrospinning PCL membranes with highly-aligned fiber surface as the inner layer and extruding PCL grid structure as the outer layer. After PDA coating and vascular endothelial growth factor immobilization, this vessel scaffold achieved good mechanical properties and biocompatibility[38]. Due to the tubular structure of the artificial blood vessel, most of the composite printing process in this field can adjust the properties by controlling the rotation of the receiving axis instead of the planning printing path by CAD/CAM.
In the field of bone and skin-repairing, the composite fabrication method containing electrospinning, electrostatic direct writing, and extrusion printing has drawn lots of attention as it is capable of controllably shaping both macro and micro characteristics[39-42]. The core idea of this kind of technology is to take advantage of the respective characteristics of different processes. Extrusion printing, capable of forming mesoscale filaments, is chosen to provide mechanical support for the scaffold. Meanwhile, the microstructure formed by sub-10-micrometer fibers can be readily manufactured by electrohydrodynamic processes. Along with the development of this technology and increasing fulfilment of various application requirements, this method has begun using materials with different attributes. At the same time, with the improvement of technology, the number of cross-scale features is progressively increasing, putting this technology to great advantages for manufacturing bioconstructs with stable mechanical properties and controllable cell distribution. Recently, for the 1st time, de Ruijter et al. verified the hydrogel extrusion printing and electrostatic direct writing composite forming system. The system can achieve the preparation of bioconstructs with stable mechanical properties as well as controllable cell distribution[43]. Rajzer et al. used fused deposition modeling (FDM) and electrospinning to prepare a kind of layered scaffold for the reconstruction of nasal cartilage and subchondral bone. The upper layer of the scaffold was made of osteogenon-gelatin by electrospinning to promote cell adhesion and proliferation. The lower layer of the scaffold was prepared by printing poly-L-lactide with FDM. The porous grid structure could not only provide the mechanical strength for the scaffold as well as convenient in vivo fixation of the implant but also promote the tissue growth and the penetration of gelatin[44]. Diloksumpan et al. proposed a method to prepare bone cartilage scaffold by composite technology. In this method, PCL framework was constructed by melt electrowriting and then the printable calcium phosphate-based materials (pCaP) subchondral bone was directly built on the PCL layer by extrusion printing. After that, the cartilage was prepared by injecting methacryloyl-modified gelatin (GelMA) into the former framework. The experimental results showed that the PCL framework improved the interfacial shear strength between GelMA and pCaP by 6.5 times. Furthermore, the PCL grid embedded in GelMA increases the compression stiffness of the cartilage layer, increasing its resemblance to the natural one (Figure 2)[45].
Figure 2.
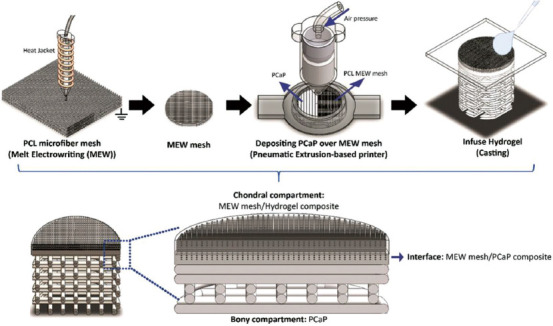
Manufacture of bioconstruct using composite forming technology that combines electrohydrodynamics and extrusion printing. Bone cartilage scaffolds are successively made by three processes – melt electrowriting, fused deposition modeling, and hydrogel casting (from ref.[45] licensed under Creative Commons Attribution 4.0 license).
The composite forming method combining extrusion printing and electrospinning has been proven to be able to effectively prepare the scaffold with a multiscale pore structure, which has obvious advantages in realizing the composite forming of multiscale micro-nano structures. However, most of the existing research results are still limited in the use of biomaterials to prepare artificial regeneration scaffolds while the research on the direct printing of cells, growth factors, and scaffolds materials to achieve the composite forming of active biological structures is still at the stage of exploration.
2.3. Combining cell printing and hybrid additive/subtractive manufacturing
Cell printing technology has advantages in achieving direct cell assembly, but most of the technologies with high cell printing resolution are often unable to directly construct large-scale complex biostructures[46-49]. For this reason, tackling this bottleneck requires combining them with scaffold printing technologies. Due to the complexity of the vascularization process, the use of artificial biological tissue is limited to clinical application at present[50-53]. It is necessary take into account the requirements of cell metabolism in the process of preparing biological structures and the role of scaffold materials, cells, and growth factors from macro, meso, and micro scales[54-56]. In this context, as hybrid additive/subtractive manufacturing has already archived many positive results in the preparation of prevascularized tissues[57-61], the composite forming technology combining cell printing and hybrid additive/subtractive manufacturing is applied to the manufacture of biological structure.
At present, in the process of the preparation of biostructures, the composite forming technology combining cell printing with hybrid additive/subtractive manufacturing has been able to initially realize the structural shaping in different scales and the position control of different materials and cells, which proves it to be a potential technical means to construct heterogeneous biostructures with a multiscale vascular network. Kim et al. used extrusion printing to form human preadipocytes, human dermal fibroblasts, and gelatin to construct subcutaneous tissue, dermis, and vascular channels between them, respectively. After that, primary human epidermal keratinocytes were ink-jetted on the surface of the dermis to form the epidermis layer to complete the manufacture of the vascularized full-layer skin model in vitro. Compared with the in vitro skin model only with the epidermis or dermis layer, the cell morphology and functional expression markers in the dynamically cultured, vascularized full-thickness skin model were similar to those in the natural one and can better simulate the complexity of real skin[62]. Kang et al. proposed an integrated tissue–organ printer for human-scale organ manufacturing, which uses PCL and F-127 as the structural support frame while composite of gelatin, fibrin, hyaluronic acid, and glycerin as the cell carrier. Through the integrated printing of these materials, the method could fabricate vascularized tissue structures that resemble the size and stable mechanical structure of human organs[63]. Noor et al. verified the possibility of making a personalized vascularized heart patch with no immune response by capitalizing on multi-head extrusion printing with the patient’s cells and acellular matrix. On this basis, through the combination of embedded printing, the fabrication of a human heart with natural structure characteristics was achieved (Figure 3)[64].
Figure 3.
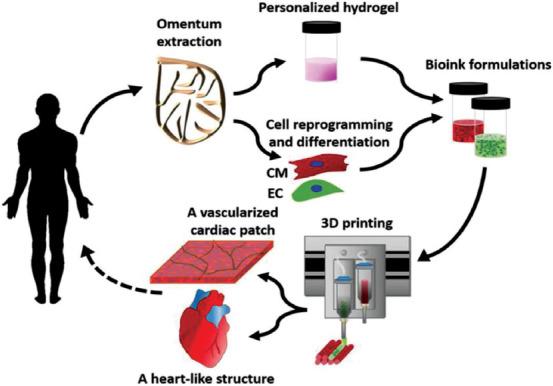
Manufacture of bioconstructs using the composite forming process of cell printing and hybrid additive/subtractive manufacturing. This example shows the preparation of personalized perfusable cardiac patches and cellularized human heart using multi-head extrusion printing of autologous stem cells and acellular matrix (from ref.[64] licensed under Creative Commons Attribution 4.0 license).
In summary, hybrid additive/subtractive manufacturing has been proven to have great potential in the construction of vessel-like microchannel structure, highlighting its significance in the formation of vascularized bioconstructs using cell printing technology. The printing of sacrificial materials into the whole biological structure not only helps provide mechanical support in the manufacturing process but also is important for the construction of microchannel structures.
2.4. 3D composite bioprinting integrated with light, magnetic and acoustic field control
As 3D bioprinting not only constructs structures that mimic the structure and component distribution of natural biological tissues and organs but also take into account the regulation of cell behavior in the printing process, the research of combining non-contact field regulation technology, such as light, magnetism, and sound, with traditional 3D bioprinting technology has attracted more attention, especially in combination with environmentally responsive intelligent materials.
Some innovative research results have been achieved recently. Yang et al. combined the external DC electric field and the photocuring mask projection process so that the modified multiwalled carbon nanotubes (mwcnt-s) embedded in the photosensitive resin could be controllably arranged by the electric field imposed. The tensile test shows that the arrangement of mwcnt-s will produce anisotropic elastic modulus, which is higher in the direction parallel to mwcnt-s, but lower in the vertical one. Using this approach, a reinforced artificial meniscus with carbon nanotubes aligned in the circumferential and radial directions was fabricated[65]. Chansoria et al. developed an ultrasound-assisted bio 3D printing technology that used the standing bulk acoustic wave produced by an ultrasonic alignment chamber around the printing platform to arrange the cells in the printed structure to construct single or multi-layer anisotropic cell structure (Figure 4A)[66]. Kirillova et al. performed two-dimensional extrusion printing with methacrylated alginate or methacrylated hyaluronic acid on the glass substrate. By taking advantage of the photo-crosslinking gradient between the upper and lower surface of the structure, the printed film would have a self-folding behavior to form a tubular structure of which the internal diameter could achieve as low as 20 μm[67].
Figure 4.
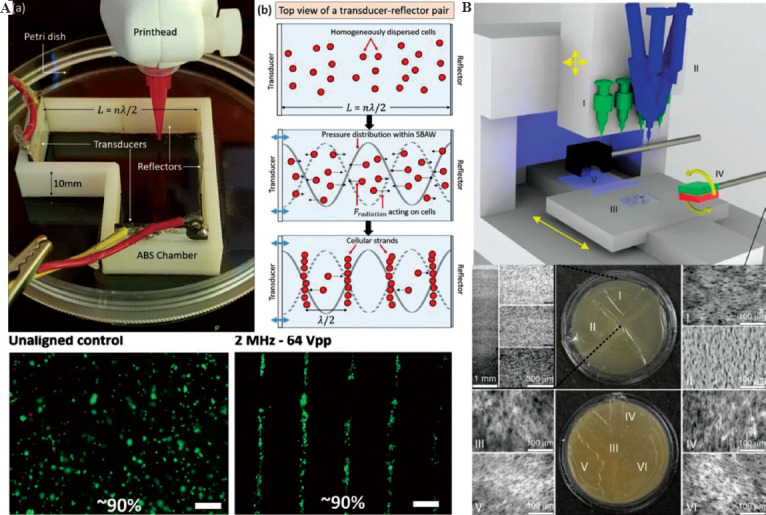
Bio-composite printing assisted by non-contact field regulation technology. (A) The ultrasonic-assisted extrusion printing with the capacity of no-contact cell arrangement based on the acoustophoretic principle (from ref.[66] licensed under Creative Commons Attribution 4.0 license). (B) The extrusion printing system combined with the external magnetic field and material component mixing printhead for adjusting the material concentration and particle orientation during printing procedure (from ref.[68] licensed under Creative Commons Attribution 4.0 license).
Thus, it can be observed that multi-physics field control has unique advantages in improving printing flexibility. Although some studies have not yet been applied in the field of biological manufacturing, it can be predicted from the latest technical characteristics analysis that these methods will provide new solutions for biological manufacturing. Kokkinis et al. proposed a “5D” printing method. By integrating the printhead with component mixing function as well as rotating magnetic field in the extrusion printing system, the composite system allowed additional control over component concentration and orientation of magnetic particles, which realized the printing of the structures with anisotropic texture arrangement (Figure 4B)[68]; Kim et al. added a magnetic coil around the extrusion printing nozzle to achieve the desired orientation of ferromagnetic particles contained in the materials. This method could pattern the magnetic polarity of the printed filament so that fast transformations between complex 3D structures through magnetic actuation could be realized[69].
3. 3D composite bioprinting system
With the continuous development of 3D printing technology for biological purposes, the research of bio-CAD/CAM/3D printing forming system is also progressing. However, it should be pointed out that most of the early research on biological manufacturing is based on commercial 3D printers, and some commercial CAD/CAM software has been widely used. The representative software includes Makerbot proposed by Makerbot, which is a slicing software customized for Makerbot printer; Xbuilder of Xery 3D printing technology, a Chinese software supporting STL, G-Code, obj, and other formats; Cura developed by Ultimaker, which has a high degree of integration and the best support for Ultimaker’s products; Skeinforge, an open-source program composed of Python scripts that can convert 3D models into G-Code files. Focusing on the needs of medical applications, the Magics developed by Materialise is the most representative program, as Magics can process not only the CAD model generated through 3D model reconstruction by Mimics but also the general CAD data output by the CAD/Re software system. The generated model files by Magics are saved in STL format and can be fabricated by a rapid prototyping machine.
With the continuous improvement in biomanufacturing and 3D bioprinting technology, the application field has also expanded from the preparation of biological scaffolds to cell printing, cell assembly, construction of complex biological structures, etc., which make the technical route based on industrial 3D printers no longer meet the demand. Many research institutions develop corresponding bio-CAD/CAM/3D printing systems for specific bioprinting processes. Shim et al. combined air pressure extrusion with piston extrusion to prepare a multi-nozzle extrusion printing platform that is suitable for 3D printing of various materials[70]. The NovoGen MMX bioprinter of Organovo has two printheads for printing cell-laden materials and cell-free materials. Two vials housed on the instrument can adjust the temperature of materials. Besides, the device is also equipped with a laser calibration device to calibrate the position of the micropipette[71]. Whatley et al. developed a 3D additive manufacturing equipment to prepare the biomimetic elastic intervertebral disk scaffold[72]. The 3D bioprinting system of RegenHU uses air pressure for the extrusion of materials to satisfy the requirements of biological fabrication. The printer has strong adaptability and is easy to operate. The inlet air pressure can be adjusted according to different viscosity of the materials so that the fabrication of bioconstruct consisting of multi-materials can be carried out. The 3D-Bioplotter printing platform of EnvisionTec has high mobile precision and multiple printheads. The printheads have embedded heating modules and can be swiftly changed. A high-resolution camera is used to position the nozzle while the z-axis sensor can accurately control the height between the nozzle and the receiving platform. Lu et al. used CAD software to design the 3D model of the internal micropore structure of the bionic bone scaffold and fabricated it based on the path planned by an independently developed path planning software[73]. Yan et al. put forward the low-temperature 3D bioprinting technology and equipment and made a number of achievements in the preparation of bone scaffolds[74]. Liu et al. introduced the inertial force injection (AVIFJ) and extrusion printing composite forming system, which can achieve precise deposition of droplets containing a single cell and the printing of cell-laden hydrogel[75]. Xie et al. introduced a printing system, which integrates extrusion printing and electrospinning for forming structures with micro-nano scale features. The system had a micro-level printing resolution to meet the requirements of forming exquisite complex cross-scale structures[76]. Liu et al. proposed and preliminarily provided the test platform of the bio-CAD/CAM/3D composite printing technology, abbreviated as a composite forming test platform[77,78].
It can be found that combining the process with micro-nano forming precision and 3D bioprinting effectively solves the problem of limited precision and the inability of forming micro-scale structures inside the structure, which are evident in the formation process of traditional 3D printing. This feature makes the corresponding bio-CAD/CAM process different from the traditional CAD/CAM, that is, the biological CAM process needs to merge the scale information that cannot be modeled in the CAD process with the information from the CAD process. In addition, the composite forming process, which combines printing technology and micro-nano forming technology, often integrates multiple physical fields, cross-linked reaction, and other effects so as to effectively regulate the interface effect in the composite structure which make the prepared scaffold satisfy the requirements of cell attachment and growth while taking into account the structural controllability and mechanical properties. These also call out challenges upon the corresponding printing system and the designation of the CAD/CAM function module. To solve these problems, Liu Yuanyuan et al. from Shanghai University proposed a type of a composite forming system consisting of multiple functional subsystems with which the bio-CAD/CAM software system was studied. The system designed the automatic processing flow and detail implementer method that encompass the following: Obtaining STL models of injury tissue from manual designs or medical imaging modalities reconstruction, generating printing path information and drive file containing processing information by post-processing. Importantly, an approach of adding process management module which innovates the bio-CAD/CAM system and realizes the use of bio-CAM processing for fabricating structures which could not be modeled in the CAD processing was put forward (Figure 5) [79].
Figure 5.
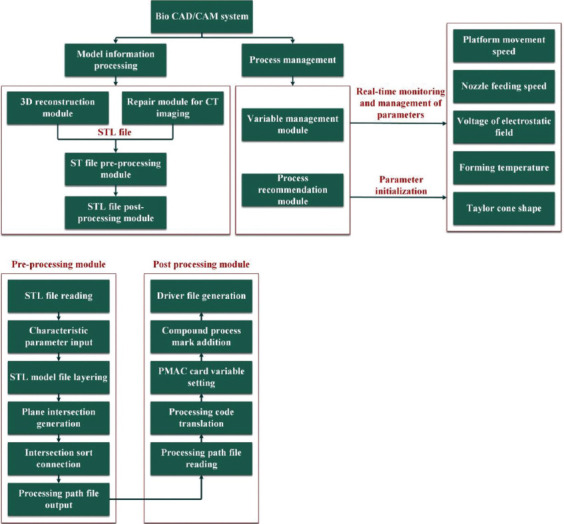
Software composition and model information processing flow of the bio-computer-aided design/computer-aided manufacturing/3D composite printing system.
In summary, for tissue engineering scaffold printing, the CAD/CAM system for 3D bioprinting is the same as the traditional 3D printing system if the scale of the printing structure is single or common and the material used is suitable for the current mainstream 3D printing technology and system. Otherwise, if the goal of printing is to assemble components such as cells, drugs, biomaterials, etc., both path planning strategy and process control can become more complex. Hence, it is necessary to add a process management module in a traditional CAD/CAM system and supplement with more information reflecting component gradient in the path planning strategy. Besides, it should be pointed out that at present, the 3D composite bioprinting system proposed by different institutions involves different processes, so there will be differences in the scope of applicable materials and specific application objects. As for the index advantage analysis from the engineering and technology perspectives, the detailed parameter comparison is not available at present because most of the systems are not commercial.
4. Discussion and prospects
According to the researches and demands for tissue engineering and regenerative medicine, the core issue of the regeneration of tissue and organ by biofabrication technologies lies in the effective assembly and behavior regulation of cells. A typical way of cell assembly is using the biodegradable scaffolds with autologous stem cells seeded and then co-cultured on it in vitro. After that, the scaffold can be planted in vivo to repair the injured tissue and organ. In this context, to ensure the effective adhesion of the inoculated cells and further realize the accurate positioning and reasonable distribution of cells or cell clusters, the solution of precisely controlling the components and microstructures of scaffolds as required has become an important goal of the development of 3D printing technologies and equipment. Another route is the technology of direct assembly of cells which is capable of precisely depositing living cells in the spatiotemporal dimension. Although great progress has been made in this field in obtaining a 3D biological structure, its shortcomings still lie in the manufacturing efficiency, achievable scale, and complexity of the structure; these problems pose new challenges to the biological printing process and equipment technology.
In such a context, the research interest in 3D composite bioprinting based on the integration of two or more printing processes with different forming principles is gradually intensifying (Table 1). 3D composite bioprinting is a combination of two or more printing processes, and it may often involve physical field regulation that can regulate filaments or cells during or after printing.
Table 1.
Current efforts toward 3D composite bioprinting
| Combined processes | Main characteristics | References | |
| Phase transition through temperature gradient | Extrusion printing | Improve the mechanical properties and biocompatibility of the printed construction at the same time | [21,22] |
| Ionic crosslinking | Extrusion printing | Provide more fabrication flexibility by controlling ion diffusion and material extrusion speed; perform cell printing under room temperature; effectively construct vessels like microchannels | [27-30] |
| Electrohydrodynamics | Extrusion printing | Fabricate scaffolds with good mechanical properties and large scales, and the structure can mimic natural extracellular matrix on the micro-nano scale; effectively prepare the scaffolds with a multiscale pore structure | [37,38,43-45] |
| Cell printing | Hybrid additive/ subtractive manufacturing | Construct vascularized bio constructs with certain mechanical strength and complex microstructure | [62-64] |
| Physical field control | Extrusion printing | Improve printing flexibility, especially with environmentally responsive intelligent materials; regulate materials and cells in the printing process | [65-69] |
Some research results in the field of tissue damage repairing demonstrated a technology with broad application prospects. For example, 3D composite bioprinting which combines extrusion forming with electrospinning technology has been proven to be able to effectively prepare tissue scaffold with a multiscale pore structure, showing obvious advantages in the formation of multi-scale micro-nano composite structure. Besides, hybrid additive/subtractive manufacturing has been proven to have great potential in the construction of vessel-like microchannel structure, which is of great significance in the formation of vascularized bioconstructs. Moreover, the printing of sacrificial materials into the whole biological structure not only helps provide mechanical support in the manufacturing process but also is very important for the construction of microchannel structures. On the other hand, the 3D composite bioprinting integrated with light, magnetic, and acoustic field control would have a greater development potential if it is combined with environmentally responsive smart materials. Recently, some innovative research achievements have been made, such as the manufacture of artificial blood vessels. However, it is still limited by the difficulties in the construction of large-scale artificial regeneration tissue as well as repairing large area tissue damage. Furthermore, how to attain rapid vascularization of large-scale artificial regeneration tissue remains an urgent question to address.
In terms of 3D composite bioprinting process and system research, the printing process, whether extrusion printing, electrospinning or the existing 3D composite bioprinting technology, is being further integrated with the technologies such as direct cell assembly, dynamic cross-linking formation, cell behavior guiding, controlled drug-releasing, etc. as required. The constant expansion and enrichment of the research scope of 3D composite bioprinting promote further application and innovation of this technology in the field of tissue and organ repairing and regeneration. The existing interface problems in the integration of multiscale structure and multi-gradient components and in the technical feasibility of multi-process integration remain to be addressed in parallel with the continuous innovation of the 3D composite bioprinting process and system.
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No. 61973206, 61703265, 61803250, 61933008) and Shanghai Science and Technology Committee Rising-Star Program (No. 19QA1403700).
References
- 1.Groll J, Burdick JA, Cho DW, et al. A Definition of Bioinks and their Distinction from Biomaterial Inks [J] Biofabrication. 2018;11(1):013001. doi: 10.1088/1758-5090/aaec52. https://doi.org/10.1088/1758-5090/aaec52. [DOI] [PubMed] [Google Scholar]
- 2.Gungor-Ozkerim PS, Inci I, Zhang YS, et al. Bioinks for 3D Bioprinting:An Overview [J] Biomater Sci. 2018;6(5):915–46. doi: 10.1039/c7bm00765e. https://doi.org/10.1039/c7bm00765e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ng WL, Chua CK, Shen YF. Print Me An Organ!Why We Are Not There Yet [J] Prog Polym Sci. 2019;97:101145. https://doi.org/10.1016/j.progpolymsci.2019.101145. [Google Scholar]
- 4.Ng WL, Chan A, Ong YS, et al. Deep Learning for Fabrication and Maturation of 3D Bioprinted Tissues and Organs [J] Virtual Phys Prototyp. 2020;15(3):340–58. [Google Scholar]
- 5.Ozbolat IT, Hospodiuk M. Current Advances and Future Perspectives in Extrusion-based Bioprinting [J] Biomaterials. 2016;76:321–43. doi: 10.1016/j.biomaterials.2015.10.076. https://doi.org/10.1016/j.biomaterials.2015.10.076. [DOI] [PubMed] [Google Scholar]
- 6.Emmermacher J, Spura D, Cziommer J, et al. Engineering Considerations on Extrusion-based Bioprinting:Interactions of Material Behavior, Mechanical Forces and Cells in the Printing Needle [J] Biofabrication. 2020;12(2):025022. doi: 10.1088/1758-5090/ab7553. https://doi.org/10.1088/1758-5090/ab7553. [DOI] [PubMed] [Google Scholar]
- 7.Gao G, Kim BS, Jang J, et al. Recent Strategies in Extrusion-Based Three-Dimensional Cell Printing toward Organ Biofabrication [J] ACS Biomater Sci Eng. 2019;5(3):1150–69. doi: 10.1021/acsbiomaterials.8b00691. https://doi.org/10.1021/acsbiomaterials.8b00691. [DOI] [PubMed] [Google Scholar]
- 8.Gudapati H, Dey M, Ozbolat I. A Comprehensive Review on Droplet-based Bioprinting:Past, Present and Future [J] Biomaterials. 2016;102:20–42. doi: 10.1016/j.biomaterials.2016.06.012. https://doi.org/10.1016/j.biomaterials.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 9.Ng WL, Lee JM, Yeong WY, et al. Microvalve-based Bioprinting Process, Bio-inks and Applications [J] Biomater Sci. 2017;5(4):632–47. doi: 10.1039/c6bm00861e. https://doi.org/10.1039/c6bm00861e. [DOI] [PubMed] [Google Scholar]
- 10.Koch L, Brandt O, Deiwick A, et al. Laser-assisted Bioprinting at Different Wavelengths and Pulse Durations with a Metal Dynamic Release Layer:A Parametric Study [J] Int J Bioprint. 2017;3(1):42–53. doi: 10.18063/IJB.2017.01.001. https://doi.org/10.18063/ijb.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Onses MS, Sutanto E, Ferreira PM, et al. Mechanisms, Capabilities, and Applications of High-Resolution Electrohydrodynamic Jet Printing [J] Small. 2015;11(34):4237–66. doi: 10.1002/smll.201500593. https://doi.org/10.1002/smll.201500593. [DOI] [PubMed] [Google Scholar]
- 12.Ng WL, Lee JM, Zhou M, et al. Vat Polymerization-based Bioprinting Process, Materials, Applications and Regulatory Challenges [J] Biofabrication. 2020;12(2):022001. doi: 10.1088/1758-5090/ab6034. https://doi.org/10.1088/1758-5090/ab6034. [DOI] [PubMed] [Google Scholar]
- 13.Li W, Mille LS, Robledo JA, et al. Recent Advances in Formulating and Processing Biomaterial Inks for Vat Polymerization-Based 3D Printing [J] Adv Healthc Mater. 2020;9(15):2000156. doi: 10.1002/adhm.202000156. https://doi.org/10.1002/adhm.202000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quan H, Zhang T, Xu H, et al. Photo-curing 3D Printing Technique and its Challenges [J] Bioact Mater. 2020;5(1):110–5. doi: 10.1016/j.bioactmat.2019.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jun I, Han HS, Edwards JR, et al. Electrospun Fibrous Scaffolds for Tissue Engineering:Viewpoints on Architecture and Fabrication [J] Int J Mol Sci. 2018;19(3):745. doi: 10.3390/ijms19030745. https://doi.org/10.3390/ijms19030745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liashenko I, Rosell-Llompart J, Cabot A. Ultrafast 3D Printing with Submicrometer Features Using Electrostatic Jet Deflection [J] Nat Commun. 2020;11(1):753. doi: 10.1038/s41467-020-14557-w. https://doi.org/10.1038/s41467-020-14557-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang C, Meng G, Zhang L, et al. Physical Properties and Biocompatibility of a Core-sheath Structure Composite Scaffold for Bone Tissue Engineering In Vitro [J] J Biomed Biotechnol. 2012;2012:579141. doi: 10.1155/2012/579141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kolesky DB, Homan KA, Skylar-Scott MA, et al. Three-dimensional Bioprinting of thick Vascularized Tissues [J] Proc Natl Acad Sci U S A. 2016;113(12):3179–84. doi: 10.1073/pnas.1521342113. https://doi.org/10.1073/pnas.1521342113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Skylar-Scott MA, Uzel SG, Nam LL, et al. Biomanufacturing of Organ-specific Tissues with High Cellular Density and Embedded Vascular Channels [J] Sci Adv. 2019;5(9):eaaw2459. doi: 10.1126/sciadv.aaw2459. https://doi.org/10.1126/sciadv.aaw2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang W, Junior JR, Nalesso PR, et al. Engineered 3D Printed Poly(ɛ-caprolactone)/Graphene Scaffolds for Bone Tissue Engineering [J] Mater Sci Eng C. 2019;100:759–70. doi: 10.1016/j.msec.2019.03.047. https://doi.org/10.1016/j.msec.2019.03.047. [DOI] [PubMed] [Google Scholar]
- 21.Chen YW, Shen YF, Ho CC, et al. Osteogenic and Angiogenic Potentials of the Cell-laden Hydrogel/Mussel-inspired Calcium Silicate Complex Hierarchical Porous Scaffold Fabricated by 3D Bioprinting [J] Mater Sci Eng C. 2018;91:679–87. doi: 10.1016/j.msec.2018.06.005. https://doi.org/10.1016/j.msec.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 22.Mekhileri NV, Lim KS, Brown GC, et al. Automated 3D Bioassembly of Micro-tissues for Biofabrication of Hybrid Tissue Engineered Constructs [J] Biofabrication. 2018;10(2):024103. doi: 10.1088/1758-5090/aa9ef1. https://doi.org/10.1088/1758-5090/aa9ef1. [DOI] [PubMed] [Google Scholar]
- 23.Gao Q, He Y, Fu JZ, et al. Coaxial Nozzle-assisted 3D Bioprinting with Built-in Microchannels for Nutrients Delivery [J] Biomaterials. 2015;61:203–15. doi: 10.1016/j.biomaterials.2015.05.031. https://doi.org/10.1016/j.biomaterials.2015.05.031. [DOI] [PubMed] [Google Scholar]
- 24.Andrique L, Recher G, Alessandri K, et al. A Model of Guided Cell Self-organization for Rapid and Spontaneous Formation of Functional Vessels [J] Sci Adv. 2019;5(6):eaau6562. doi: 10.1126/sciadv.aau6562. https://doi.org/10.1126/sciadv.aau6562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jia WT, Gungor-Ozkerim PS, Zhang YS, et al. Direct 3D Bioprinting of Perfusable Vascular Constructs Using a Blend Bioink [J] Biomaterials. 2016;106:58–68. doi: 10.1016/j.biomaterials.2016.07.038. https://doi.org/10.1016/j.biomaterials.2016.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Millik SC, Dostie AM, Karis DG, et al. 3D Printed Coaxial Nozzles for the Extrusion of Hydrogel Tubes Toward Modeling Vascular Endothelium [J] Biofabrication. 2019;11(4):045009. doi: 10.1088/1758-5090/ab2b4d. https://doi.org/10.1088/1758-5090/ab2b4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Narayanan LK, Huebner P, Fisher MB, et al. 3D-Bioprinting of Polylactic Acid (PLA) Nanofiber-Alginate Hydrogel Bioink Containing Human Adipose-Derived Stem Cells [J] ACS Biomater Sci Eng. 2016;2(10):1732–42. doi: 10.1021/acsbiomaterials.6b00196. https://doi.org/10.1021/acsbiomaterials.6b00196. [DOI] [PubMed] [Google Scholar]
- 28.Tabriz AG, Hermida MA, Leslie NR, et al. Three-dimensional Bioprinting of Complex Cell Laden Alginate Hydrogel Structures [J] Biofabrication. 2015;7(4):045012. doi: 10.1088/1758-5090/7/4/045012. https://doi.org/10.1088/1758-5090/7/4/045012. [DOI] [PubMed] [Google Scholar]
- 29.Lozano R, Stevens L, Thompson BC, et al. 3D Printing of Layered Brain-like Structures Using Peptide Modified Gellan Gum Substrates [J] Biomaterials. 2015;67:264–73. doi: 10.1016/j.biomaterials.2015.07.022. https://doi.org/10.1016/j.biomaterials.2015.07.022. [DOI] [PubMed] [Google Scholar]
- 30.Wang X, Li X, Dai X, et al. Coaxial Extrusion Bioprinted Shell-core Hydrogel Microfibers Mimic Glioma Microenvironment and Enhance the Drug Resistance of Cancer Cells [J] Colloids Surf B Biointerfaces. 2018;171:291–9. doi: 10.1016/j.colsurfb.2018.07.042. https://doi.org/10.1016/j.colsurfb.2018.07.042. [DOI] [PubMed] [Google Scholar]
- 31.Abel SB, Ballarin FM, Abraham GA. Combination of Electrospinning with other Techniques for the Fabrication of 3D Polymeric and Composite Nanofibrous Scaffolds with Improved Cellular Interactions [J] Nanotechnology. 2020;31(17):172002. doi: 10.1088/1361-6528/ab6ab4. https://doi.org/10.1088/1361-6528/ab6ab4. [DOI] [PubMed] [Google Scholar]
- 32.Lee SJ, Heo DN, Park JS, et al. Characterization and Preparation of Bio-tubular Scaffolds for Fabricating Artificial Vascular Grafts by Combining Electrospinning and a 3D Printing System [J] Phys Chem Chem Phys. 2015;17(5):2996–9. doi: 10.1039/c4cp04801f. https://doi.org/10.1039/c4cp04801f. [DOI] [PubMed] [Google Scholar]
- 33.Akentjew TL, Terraza C, Suazo C, et al. Rapid Fabrication of Reinforced and Cell-laden Vascular Grafts Structurally Inspired by Human Coronary Arteries [J] Nat Commun. 2019;10(1):3098. doi: 10.1038/s41467-019-11090-3. https://doi.org/10.1038/s41467-019-11446-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jungst T, Pennings I, Schmitz M, et al. Heterotypic Scaffold Design Orchestrates Primary Cell Organization and Phenotypes in Cocultured Small Diameter Vascular Grafts [J] Adv Funct Mater. 2019;29(43):1905987. https://doi.org/10.1002/adfm.201905987. [Google Scholar]
- 35.Wang K, Zheng W, Pan Y, et al. Three-Layered PCL Grafts Promoted Vascular Regeneration in a Rabbit Carotid Artery Model [J] Macromol Biosci. 2016;16(4):608–18. doi: 10.1002/mabi.201500355. https://doi.org/10.1002/mabi.201500355. [DOI] [PubMed] [Google Scholar]
- 36.Liu Y, Xiang K, Chen H, et al. Composite Vascular Repair Grafts via Micro-imprinting and Electrospinning [J] AIP Adv. 2015;5(4):041318. https://doi.org/10.1063/1.4906571. [Google Scholar]
- 37.Wu P, Wang L, Li W, et al. Construction of Vascular Graft with Circumferentially Oriented Microchannels for Improving Artery Regeneration [J] Biomaterials. 2020;242:119922. doi: 10.1016/j.biomaterials.2020.119922. https://doi.org/10.1016/j.biomaterials.2020.119922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee SJ, Kim ME, Nah H, et al. Vascular Endothelial Growth Factor Immobilized on Mussel-inspired Three-dimensional Bilayered Scaffold for Artificial Vascular Graft Application:In Vitro and In Vivo Evaluations [J] J Colloid Interface Sci. 2019;537:333–44. doi: 10.1016/j.jcis.2018.11.039. https://doi.org/10.1016/j.jcis.2018.11.039. [DOI] [PubMed] [Google Scholar]
- 39.Chen X, Ergun A, Gevgilili H, et al. Shell-core bi-layered Scaffolds for Engineering of Vascularized Osteon-like Structures [J] Biomaterials. 2013;34:8203–12. doi: 10.1016/j.biomaterials.2013.07.035. https://doi.org/10.1016/j.biomaterials.2013.07.035. [DOI] [PubMed] [Google Scholar]
- 40.Costa PF, Vaquette C, Zhang Q, et al. Advanced Tissue Engineering Scaffold Design for Regeneration of the Complex Hierarchical Periodontal Structure [J] J Clin Periodontol. 2014;41(3):283–94. doi: 10.1111/jcpe.12214. https://doi.org/10.1111/jcpe.12214. [DOI] [PubMed] [Google Scholar]
- 41.Vaquette C, Fan W, Xiao Y, et al. A Biphasic Scaffold Design Combined with Cell Sheet Technology for Simultaneous Regeneration of Alveolar Bone/Periodontal Ligament Complex [J] Biomaterials. 2012;33(22):5560–73. doi: 10.1016/j.biomaterials.2012.04.038. https://doi.org/10.1016/j.biomaterials.2012.04.038. [DOI] [PubMed] [Google Scholar]
- 42.Kumar PT, Hashimi S, Saifzadeh S, et al. Additively Manufactured Biphasic Construct Loaded with BMP-2 for Vertical Bone Regeneration:A Pilot Study in Rabbit [J] Mater Sci Eng C. 2018;92:554–64. doi: 10.1016/j.msec.2018.06.071. https://doi.org/10.1016/j.msec.2018.06.071. [DOI] [PubMed] [Google Scholar]
- 43.De Ruijter M, Ribeiro A, Dokter I, et al. Simultaneous Micropatterning of Fibrous Meshes and Bioinks for the Fabrication of Living Tissue Constructs [J] Adv Healthc Mater. 2019;8(7):1800418. doi: 10.1002/adhm.201800418. https://doi.org/10.1002/adhm.201800418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rajzer I, Kurowska A, Jabłoński A, et al. Layered Gelatin/PLLA Scaffolds Fabricated by Electrospinning and 3D Printing for Nasal Cartilages and Subchondral Bone Reconstruction [J] Mater Des. 2018;155:297–306. https://doi.org/10.1016/j.matdes.2018.06.012. [Google Scholar]
- 45.Diloksumpan P, De Ruijter M, Castilho M, et al. Combining Multi-scale 3D Printing Technologies to Engineer Reinforced Hydrogel-ceramic Interfaces [J] Biofabrication. 2020;12(2):025014. doi: 10.1088/1758-5090/ab69d9. https://doi.org/10.1088/1758-5090/ab69d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park JA, Lee HR, Park SY, et al. Self-Organization of Fibroblast-Laden 3D Collagen Microstructures from Inkjet-Printed Cell Patterns [J] Adv Biosyst. 2020;4(5):1900280. doi: 10.1002/adbi.201900280. https://doi.org/10.1002/adbi.201900280. [DOI] [PubMed] [Google Scholar]
- 47.Kérourédan O, Bourget JM, Rémy M, et al. Micropatterning of Endothelial Cells to Create a Capillary-like Network with Defined Architecture by Laser-assisted Bioprinting [J] J Mater Sci Mater Med. 2019;30(2):28. doi: 10.1007/s10856-019-6230-1. https://doi.org/10.1007/s10856-019-6230-1. [DOI] [PubMed] [Google Scholar]
- 48.Kérourédan O, Hakobyan D, Rémy M, et al. In Situ Prevascularization Designed by Laser-assisted Bioprinting:Effect on Bone Regeneration [J] Biofabrication. 2019;11(4):045002. doi: 10.1088/1758-5090/ab2620. https://doi.org/10.1088/1758-5090/ab2620. [DOI] [PubMed] [Google Scholar]
- 49.Chen H, Liu Y, Hu Q. A Novel Bioactive Membrane by Cell Electrospinning [J] Exp Cell Res. 2015;338(2):261–6. doi: 10.1016/j.yexcr.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 50.Redd MA, Zeinstra N, Qin W, et al. Patterned Human Microvascular Grafts Enable Rapid Vascularization and Increase Perfusion in Infarcted Rat Hearts [J] Nat Commun. 2019;10(1):584. doi: 10.1038/s41467-019-08388-7. https://doi.org/10.1038/s41467-019-08388-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clyne AM, Swaminathan S, Lantada AD. Biofabrication Strategies for Creating Microvascular Complexity [J] Biofabrication. 2019;11(3):032001. doi: 10.1088/1758-5090/ab0621. https://doi.org/10.1088/1758-5090/ab0621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chandra P, Atala A. Engineering Blood Vessels and Vascularized Tissues:Technology Trends and Potential Clinical Applications [J] Clin Sci. 2019;133(9):1115–35. doi: 10.1042/CS20180155. https://doi.org/10.1042/cs20180155. [DOI] [PubMed] [Google Scholar]
- 53.Rouwkema J, Khademhosseini A. Vascularization and Angiogenesis in Tissue Engineering:Beyond Creating Static Networks [J] Trends Biotechnol. 2016;34(9):733–45. doi: 10.1016/j.tibtech.2016.03.002. https://doi.org/10.1016/j.tibtech.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 54.Kinstlinger IS, Miller JS. 3D-printed Fluidic Networks as Vasculature for Engineered Tissue [J] Lab Chip. 2016;16(11):2025–43. doi: 10.1039/c6lc00193a. https://doi.org/10.1039/c6lc00193a. [DOI] [PubMed] [Google Scholar]
- 55.Miri AK, Khalilpour A, Cecen B, et al. Multiscale Bioprinting of Vascularized Models [J] Biomaterials. 2019;198:204–16. doi: 10.1016/j.biomaterials.2018.08.006. https://doi.org/10.1016/j.biomaterials.2018.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sharma D, Ross D, Wang G, et al. Upgrading Prevascularization in Tissue Engineering:A Review of Strategies for Promoting Highly Organized Microvascular Network Formation [J] Acta Biomater. 2019;95:112–30. doi: 10.1016/j.actbio.2019.03.016. https://doi.org/10.1016/j.actbio.2019.03.016. [DOI] [PubMed] [Google Scholar]
- 57.Daly AC, Pitacco P, Nulty J, et al. 3D Printed Microchannel Networks to Direct Vascularisation during Endochondral Bone Repair [J] Biomaterials. 2018;162:34–46. doi: 10.1016/j.biomaterials.2018.01.057. https://doi.org/10.1016/j.biomaterials.2018.01.057. [DOI] [PubMed] [Google Scholar]
- 58.Pimentel CR, Ko SK, Caviglia C, et al. Three-dimensional Fabrication of Thick and Densely Populated Soft Constructs with Complex and Actively Perfused Channel Network [J] Acta Biomater. 2018;65:174–84. doi: 10.1016/j.actbio.2017.10.047. https://doi.org/10.1016/j.actbio.2017.10.047. [DOI] [PubMed] [Google Scholar]
- 59.Negrini NC, Bonnetier M, Giatsidis G, et al. Tissue-mimicking Gelatin Scaffolds by Alginate Sacrificial Templates for Adipose Tissue Engineering [J] Acta Biomater. 2019;87:61–75. doi: 10.1016/j.actbio.2019.01.018. https://doi.org/10.1016/j.actbio.2019.01.018. [DOI] [PubMed] [Google Scholar]
- 60.Ji S, Almeida E, Guvendiren M. 3D Bioprinting of Complex Channels within Cell-laden Hydrogels [J] Acta Biomater. 2019;95:214–24. doi: 10.1016/j.actbio.2019.02.038. https://doi.org/10.1016/j.actbio.2019.02.038. [DOI] [PubMed] [Google Scholar]
- 61.Ouyang L, Armstrong JP, Chen Q, et al. Void-Free 3D Bioprinting for In Situ Endothelialization and Microfluidic Perfusion [J] Adv Funct Mater. 2020;30(1):1908349. doi: 10.1002/adfm.201908349. https://doi.org/10.1002/adfm.201908349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim BS, Gao G, Kim JY, et al. 3D Cell Printing of Perfusable Vascularized Human Skin Equivalent Composed of Epidermis, Dermis, and Hypodermis for Better Structural Recapitulation of Native Skin [J] Adv. Healthcare Mater. 2019;8(7):1801019. doi: 10.1002/adhm.201801019. https://doi.org/10.1002/adhm.201801019. [DOI] [PubMed] [Google Scholar]
- 63.Kang HW, Lee SJ, Ko IK, et al. A 3D Bioprinting System to Produce Human-scale Tissue Constructs with Structural Integrity [J] Nat Biotechnol. 2016;34(3):312–9. doi: 10.1038/nbt.3413. https://doi.org/10.1038/nbt.3413. [DOI] [PubMed] [Google Scholar]
- 64.Noor N, Shapira A, Edri R, et al. 3D Printing of Personalized Thick and Perfusable Cardiac Patches and Hearts [J] Adv Sci. 2019;6(11):1900344. doi: 10.1002/advs.201900344. https://doi.org/10.1002/advs.201900344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang Y, Chen Z, Song X, et al. Biomimetic Anisotropic Reinforcement Architectures by Electrically Assisted Nanocomposite 3D Printing [J] Adv Mater. 2017;29(11):1605750. doi: 10.1002/adma.201605750. https://doi.org/10.1002/adma.201605750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chansoria P, Shirwaiker R. Characterizing the Process Physics of Ultrasound-Assisted Bioprinting [J] Sci Rep. 2019;9(1):13889. doi: 10.1038/s41598-019-50449-w. https://doi.org/10.1038/s41598-019-50449-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kirillova A, Maxson R, Stoychev G, et al. 4D Biofabrication Using Shape-Morphing Hydrogels [J] Adv Mater. 2017;29(46):1703443. doi: 10.1002/adma.201703443. https://doi.org/10.1002/adma.201703443. [DOI] [PubMed] [Google Scholar]
- 68.Kokkinis D, Schaffner M, Studart AR. Multimaterial Magnetically Assisted 3D Printing of Composite Materials [J] Nat Commun. 2015;6(1):8643. doi: 10.1038/ncomms9643. https://doi.org/10.1038/ncomms9643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim Y, Yuk H, Zhao R, et al. Printing Ferromagnetic Domains for Untethered Fast-transforming Soft Materials [J] Nature. 2018;558(7709):274–9. doi: 10.1038/s41586-018-0185-0. https://doi.org/10.1038/s41586-018-0185-0. [DOI] [PubMed] [Google Scholar]
- 70.Shim JH, Lee JS, Kim JY, et al. Bioprinting of a Mechanically Enhanced Three-dimensional Dual Cell-laden Construct for Osteochondral Tissue Engineering Using a Multi-head Tissue/organ Building System [J] J Micromech Microeng. 2012;22(8):085014. https://doi.org/10.1088/0960-1317/22/8/085014. [Google Scholar]
- 71.Marga F, Jakab K, Khatiwala C, et al. Toward Engineering Functional Organ Modules by Additive Manufacturing [J] Biofabrication. 2012;4(2):022001. doi: 10.1088/1758-5082/4/2/022001. https://doi.org/10.1088/1758-5082/4/2/022001. [DOI] [PubMed] [Google Scholar]
- 72.Whatley BR, Kuo J, Shuai C, et al. Fabrication of a Biomimetic Elastic Intervertebral Disk Scaffold Using Additive Manufacturing [J] Biofabrication. 2011;3(1):015004. doi: 10.1088/1758-5082/3/1/015004. https://doi.org/10.1088/1758-5082/3/1/015004. [DOI] [PubMed] [Google Scholar]
- 73.Chen Z, Li D, Lu B, et al. Fabrication of Artificial Bioactive Bone Using Rapid Prototyping [J] Rapid Prototyp J. 2004;10(5):327–33. https://doi.org/10.1108/13552540410562368. [Google Scholar]
- 74.Liu L, Xiong Z, Yan Y, et al. Porous Morphology, Porosity, Mechanical Properties of Poly(a-hydroxy acid)-Tricalcium Phosphate Composite Scaffolds Fabricated by Low-temperature Deposition [J] J Biomed Mater Res Part A. 2007;82(A(3)):618–29. doi: 10.1002/jbm.a.31177. https://doi.org/10.1002/jbm.a.31177. [DOI] [PubMed] [Google Scholar]
- 75.Liu TK, Pang Y, Zhou ZZ, et al. An Integrated Cell Printing System for the Construction of Heterogeneous Tissue Models [J] Acta Biomater. 2019;95:245–57. doi: 10.1016/j.actbio.2019.05.052. https://doi.org/10.1016/j.actbio.2019.05.052. [DOI] [PubMed] [Google Scholar]
- 76.Xie C, Gao Q, Wang P, et al. Structure-induced Cell Growth by 3D Printing of Heterogeneous Scaffolds with Ultrafine Fibers [J] Mater Des. 2019;181:108092. https://doi.org/10.1016/j.matdes.2019.108092. [Google Scholar]
- 77.Liu D. Multiphysics Coupling Analysis and Experiment of Low-temperature Deposition Manufacturing and Electrospinning for Multi-scale Tissue Engineering Scaffold [J] J Mech Eng. 2012;48(15):137–43. https://doi.org/10.3901/jme.2012.15.137. [Google Scholar]
- 78.Dali L, Jun G, Shuhui F, et al. On-line Monitor System for Nanoscale Fiber Manufacturing Based on Multi-featured Pattern Recognition [J] Opt Precis Eng. 2012;20(2):360–8. https://doi.org/10.37h88/OPE.20122002.0360. [Google Scholar]
- 79.Liu Y. CAD/CAM. System and Experimental Study of Biological 3D Printing Composite Process. J Mech Eng. 2014;50(15):147. https://doi.org/10.3901/jme.2014.15.147. [Google Scholar]


