Abstract
The treatment of hypertrophic scars (HSs) is considered to be the most challenging task in wound rehabilitation. Conventional silicone sheet therapy has a positive effect on the healing process of HSs. However, the dimensions of the silicone sheet are typically larger than those of the HS itself which may negatively impact the healthy skin that surrounds the HS. Furthermore, the debonding and displacement of the silicone sheet from the skin are critical problems that affect treatment compliance. Herein, we propose a bespoke HS treatment design that integrates pressure sleeve with a silicone sheet and use of silicone gel using a workflow of three-dimensional (3D) printing, 3D scanning and computer-aided design, and manufacturing software. A finite element analysis (FEA) is used to optimize the control of the pressure distribution and investigate the effects of the silicone elastomer. The result shows that the silicone elastomer increases the amount of exerted pressure on the HS and minimizes unnecessary pressure to other parts of the wrist. Based on this treatment design, a silicone elastomer that perfectly conforms to an HS is printed and attached onto a customized pressure sleeve. Most importantly, unlimited scar treating gel can be applied as the means to optimize treatment of HSs while the silicone sheet is firmly affixed and secured by the pressure sleeve.
Keywords: Surgical scars, Hypertrophic scars, Finite element analysis, 3D-printing, 3D-scanning
1. Introduction
Wound healing is a complicated process that can be described as the restitution of natural anatomical relationships and physiological integrity of the injured tissues[1]. The wound healing process can be divided into overlapping phases with reference to the phase of recovery, including hemostasis, inflammation, proliferation, and remodeling. Hemostasis occurs immediately after the tissue or capillary blood vessels are damaged. The platelet degranulation creates a hemostatic plug to prevent the blood loss. With aid of the coagulation factors, the plug can be further stabilized by platelet aggregation and the formation of fibrin scaffold which are driven by the enzymatic cascade[1,2]. The second phase of wound healing is inflammation which occurs around the 5 days after the injury. In this phase, the immune system is triggered to activate the inflammatory reactions to prevent infection. Neutrophils and macrophages are recruited to remove any invading bacteria or foreign debris[3]. The third phase is the proliferation which occurs around 5 – 10 days after the injury and may last for 3 – 6 weeks. The main feature of this phase is the granulation tissue formation which includes fibroblasts, myofibroblasts, loose connective tissue, and capillaries. Extracellular matrix is excessively produced by fibroblasts and myofibroblasts for structural support for nascent capillaries which results in hypertrophic scars (HSs) formation[4-6]. At the end of this phase, the wound becomes an immature scar which is fragile and has a low tensile strength. The last phase of wound healing is the remodeling which turns the immature scar into a mature scar and this phase can last for 2 years. The excessive extracellular matrix is remodeled from the weak and disordered type III collagen into the stronger and more ordered type I collagen by matrix metalloproteinases. With the passage of time, the scar will be flattened because the newly synthesized collagen is weaved into the stable fibrils. Furthermore, the cellularity and vascularity of wound will be reduced due to the activity of matrix metalloproteinases[7].
Due to the protrude and erythematous appearance of the hypertrophic scarred skin, the quality of life of HS patients can even be significantly affected[8] as the scarring may reduce self-esteem or even affect the mental health of the patient due to their esthetically unpleasing appearance[9]. This problem is more obvious in female patients than male patients[8]. Apart from cosmetic issues, inappropriately managed HSs can cause functional difficulties; for instance, a limited range of motion is commonly found due to the reduced elasticity of the skin and HS hardened tissues[10]. The low elasticity of hypertrophic scarred skin is due to less elastin production in comparison to normal tissue[11,12]. Therefore, HSs can be said to be the most challenging problem in wound healing and rehabilitation process. In 2015, 67 million burn injuries were caused by fire and other sources of high heat worldwide and around 70% of burn patients develop an HS[13]. Another study also found that more than 70% of the patients in Hong Kong form an HS after surgery[14]. The high prevalence of HS formation with a lengthy treatment process can be a heavy burden on health care systems. In the United States, the cost for treating and managing scars is estimated at around US$ 20 billion every year[15].
Pressure, silicone sheet, and silicone gel therapies are the most common non-invasive treatments for HSs. For decades, pressure therapy has been used to accelerate the maturation of HSs and improve their appearance[16,17]. A number of studies conclude that exerting a continuous pressure of about 25 mmHg can inhibit the growth of HSs and encourage their maturation[16,18-21]. This is because exerted pressure may reduce the blood flow to the hypertrophic scarred tissues so as to limit nutrients and oxygen supply to the tissues, thereby effectively reducing the production of collagen[22,23]. Therefore, pressure garments should always be worn for optimal treatment results except when the patient is bathing or the garment needs to be washed[24]. Silicone sheet therapy is the application of a thin silicone sheet onto the HS which increases the skin temperature, hydrates the scar, and facilitates the polarization of scar tissues, thus contributing to a reduction of the HS[25-31]. Furthermore, silicone sheets can be used as a protective barrier against the external environment to prevent secondary damage or infection of the scar[27,32]. The differences between silicone sheets and silicone gel are the degree of crosslinking and polymerization. Silicone sheets have a longer and stronger polymer chain; therefore, they cannot be spread onto skin like silicone gel[26]. Although proven to be effective treatments for HSs, pressure therapy, silicone sheets, and silicone gel have their inherent limitations. For example, the complexities of the human body shape is a barrier for the pressure garments to exert pressure onto the concave parts of the body[33]. Moreover, silicone sheets do not work well on joints, such as elbows and knees, because they might debond from the body part during movement that requires joint use and a large range of motion. Although it is not a burden for the patients to apply the silicone gel twice a day, it is important to note that the gel might be easily wiped off during daily activities. Therefore, clinicians need to consider the location of the HS and determine which treatment should be used to obtain maximum treatment efficacy. In some cases, the treatments can be combined; for instance, silicone sheets and pressure therapy are often used together and proven to provide a better effect in reducing scar thickness and increasing scar pliability[26,34,35]. However, the size of the silicone sheet is usually larger than that of the HS to ensure that it is securely adhered to the skin, which may have negative impacts when covering healthy skin[26]. The negative impacts include excessive sweating, pruritus or even contact dermatitis[26,36]. To date, there have been few quantitative studies done on integrating pressure, silicone sheets, and silicone gel as a form of treatment to control the abnormal growth of hypertrophic scarred tissues.
The advancement of three-dimensional (3D) printing, 3D scanning and computer-aided design, and manufacturing software has led to a variety of applications in the medical field, such as artificial implants, orthoses, and prostheses, which can be customized and 3D printed to fit the needs of individual patients[37-39]. For example, Kang et al.[40] demonstrated the ability of creating vascularized cell-laden bone constructs with tunable mechanical properties using integrated tissue-organ printer. It is worth noting that the 3D printed face shields play a significant role during the outbreak of the COVID-19 in 2019[41,42]. Hale et al.[43] also developed bespoke orthoses for neck stability using 3D printing and scanning technologies. To improve the control over the pressure distribution or heat transfer of custom-engineered products, biomechanical finite element models (FEMs) are commonly used to enhance the design of orthoses or prostheses through numerical simulation. For instance, Zolfagharian et al.[44] demonstrated the use of FE analysis (FEA) in the design of a patient-specific 3D-printed splint for mallet finger injury to ensure that the mechanical properties of the design meet the requirements but production consumes the least amount of material. These customized products first incorporated the geometric shape of the body part obtained through 3D scanning to cater to each patient based on his/her needs. After that, FEA can be adopted to modify the design to enhance the performance of the product based on the material properties of the 3D printed materials without any further trial and error fittings, thus minimizing the volume of material waste during the design process.
In this study, we propose a novel framework to manufacture a bespoke garment for HSs using a workflow of 3D scanning, 3D printing, FEA, and computer-aided design and manufacturing software. The 3D printed silicone insert which conforms to the profile of the HS is attached to a pressure garment to eliminate the problem of its displacement. Hence, scar treating gel can be applied with a silicone insert, thus helping patients toward a quicker recovery. The integration of pressure, silicone sheet, and silicone gel therapies will surely open new paths of development in HS treatment. A female participant was invited for a clinical study to observe the feasibility of the suggested therapy. Our proposed framework is also valuable for different types of customized products in the clinical field.
2. Materials and methods
2.1. Details of clinical case
A 51-year-old female patient with a body mass index of 21.5 kg/m2 who has undergone surgical operation on her right wrist was recruited for the study. The patient has given her written informed consent to publish the pictures and her personal data. In December 2018, the patient suffered a radial bone fracture near her right wrist. During surgery, metal screws were applied to stabilize the radial during the healing process. About 1 month after surgery, an HS with a raised and dark red appearance developed on her hand. Afterward, she underwent silicone sheet therapy to increase scar maturation and improve the appearance of the HS. However, the silicone sheet frequently debonded. For example, the silicone sheet would debond when she sweated (wet skin), came into contact with other surfaces due to the friction (excessive friction between the silicone sheet with the desk when typing on a keyboard, or with the bed sheets while she slept at night). To address these problems, a bespoke HS treatment that combines pressure, silicone gel, and silicone sheet therapies was designed for the patient. During the study, she was ordered to wear a customized pressure sleeve with a silicone insert at all times except when she had to bathe or apply the silicone gel, which was done twice a day. The study was approved by the Human Subjects Ethics Sub-committee of The Hong Kong Polytechnic University (Reference Number: HSEARS20171214001) and Joint Chinese University of Hong Kong-New Territories East Cluster Clinical Research Ethics Committee (CREC Ref No: 2018.467).
2.2. Evaluation of current treatment
Current treatment with silicone sheets is problematic in that the silicone sheet debonds from the skin. To evaluate the adhesion between the silicone sheet and skin under different conditions, ASTM D5169 Standard Test Method for Shear Strength (Dynamic Method) of Hook and Loop Touch Fasteners was referenced and the Instron 5566 universal mechanical test frame was used. Pigskin was used to represent human skin, which was cleaned using a nonionic detergent and then padded dry with paper towel. The size of the specimens is 2.54 × 7.62 cm. The temperature of the wrist is approximately 34 – 36°C depending on the activity and the environment[45,46]. Testing was therefore carried out in a temperature-controlled chamber and the specimens were kept at a temperature of 35°C to simulate normal skin temperature. In the experiment, two different commercial silicone sheets, CICA-CARE and Mepiform®, were evaluated under four different skin conditions, which are normal dry skin and skin separately coated with three different types of scar care gels, which are SILBIONE BLEND 4001 (Elkem), Hiruscar®, and Contractubex®. The suggested dosage and mode of application are discussed as follows.
2.3. Design framework
Figure 1 illustrates the overall design framework that involves the combination of three different types of therapies. The details will be discussed in the following sections.
Figure 1.
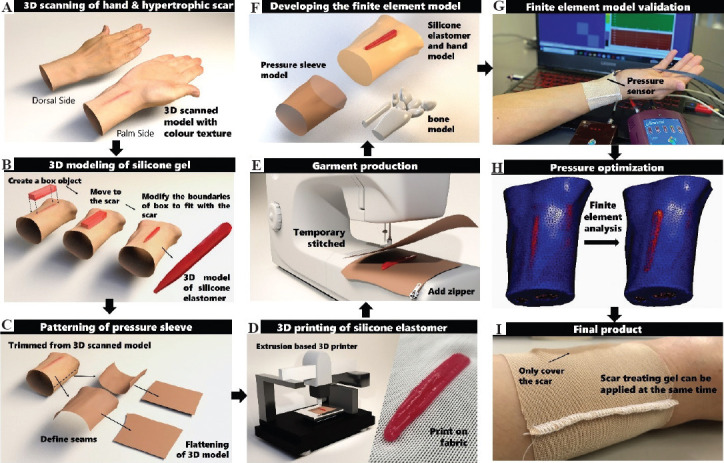
Schematic of workflow of patient specific pressure sleeve with silicone elastomer insert. (A) 3D scanning to obtain geometry and texture of hand and HS. (B) 3D modeling of a silicone elastomer which fits the HS. (C) Flattening of 3D model as pattern for pressure sleeve. (D) 3D printing of silicone elastomer on fabric by using extrusion-based 3D printer and photo of the printed silicone on fabric. (E) Producing pressure sleeve by applying stitches on the patterns. (F) Developing the FEM including the pressure sleeve, bone, silicone, and hand models. (G) Validation of the FEM for pressure through wear trial. (H) Pressure optimization through FEA. (I) Photo of the final pressure sleeve with customized silicone elastomer insert.
(1) Three-dimensional scanning and modeling
The first and second steps of the design framework are to conduct 3D scanning and modeling (Figures 1A and B). The geometry of the wrist and shape of the HS of the patient was obtained using a structured light handheld 3D scanner (Artec Eva, Luxembourg). The scanner captures the texture and color of the scanned object which is convenient during the silicone insert modeling process to differentiate between the healthy skin and HS. The scanned data were registered using Artec Studio 13 and then imported into a 3D model processing software (3ds Max, Autodesk). To create a model that corresponds with the scar, a box object with a similar length and width of the HS was first created and the thickness had to be larger than the height of the HS. Then, the box model was moved to the area where the HS was submerged into the box model. The boundaries of the box model were adjusted to the shape of the HS boundaries based on the scanned color of the HS and hand by moving the vertex or edges of the box model. Note that the number of length and width segments of the box model has to be adequate to carry out the boundary adjustment process. Once the boundaries of the silicone elastomer model fit with those of the HS, a Boolean operation can be done to subdivide the overlap volume of the silicone elastomer and hand model. The modeling process is shown in Figure 1B. The created model can be saved as a stereolithography (stl) file for the 3D printing process. Once the insert model was created, the coordinations of the model were rotated and adjusted to the proper position for the 3D printing of silicone onto fabric.
(2) 3D printing and patterning
The third and fourth steps of the design framework are 3D printing of silicone elastomer on the pressure sleeve and the patterning of the pressure sleeve (Figures 1C and D). Before the silicone insert model was imported into the 3D-printer (3D-Bioplotter® Manufacturer Series), a slicing process was done to determine the number of printed layers and the path of printing for each layer. A biocompatible silicone elastomer (SILBIONE® RTV 4410 1:1 A&B) was mixed with 0.2 and 2 wt% of a silicone thickener (THI-VEXTM) and degassed respectively for the 3D printing process. The settings of the 3D printing process and the specifications of the warp knitted fabric are shown in Supplementary Tables 1 (125.5KB, pdf) and 2 (125.5KB, pdf) , respectively. A multi-viscosity printing technique developed by our team was adopted. The silicone mixture with lower viscosity was extruded through the nozzle of the 3D printer and deposited onto the warp knitted fabric to improve the adhesion between the silicone and fabric. Apart from the first layer of printing, higher viscosity print mixture was adopted to maintain the form accuracy of the silicone part. The shear force of the sample constructed using the multi-viscosity technique was increased from about 10 to 60 N in comparison to normal printing approaches which should be sufficient for the application[47]. Once the silicone insert was printed and cured on the fabric, it was ready for adherence to the pressure sleeve. The sleeve pattern was created using ExactFlat for Rhino 3D software based on the scanned geometry of the patient hand. The hand model was separated at the radial and ulnar sides of the wrist with two patterns. Since the created patterns are based on the scanned image of the hand geometry, no pressure can be exerted onto an HS and inhibit its growth. Therefore, a reduction of the sleeve circumference is required to create an effective level of pressure to treat the HS. In this study, a reduction factor of 10% was applied which is a common value for pressure garments [48,49]. After the patterns were reduced to the appropriate size, the patterns were stitched together in the fifth step of the workflow (Figure 1E).
(3) Finite element analysis for pressure optimization
After the pressure sleeve was produced, sub-models of the carpal bones, pressure sleeve, silicone elastomer, and hand were constructed using FEA software (MSC Marc/Mentat) (Figure 1F). The material properties of the hand, sleeve, and silicone were obtained with reference to the literature and the experimental results in Yu et al. and Wu et al.[50,51]. The material properties and parameters are listed in Table S3 (125.5KB, pdf) . To validate the accuracy of the FE contact model, the subject was invited to participate in a wear trial to measure the interface pressure produced by the pressure garment and silicone elastomer using the NOVEL Pliance X system (Figure 1G). The system has been objectively evaluated and validated for accuracy by different scholars[52,53]. In total, four positions were marked on the subject, including the center of the HS, and the ulnar, radial, and back of the hand which are horizontally aligned with the HS landmark. Through the FEA, the pressure distribution on the hand from the silicone elastomer samples with five different thicknesses of 1, 2, 3, 4, and 5 mm (the smallest thickness was considered) and two pressure sleeves with circumference reduction factors of 5% and 10% were systematically evaluated (Figure 1H). A pressure threshold of 25 mmHg was exerted onto the hypertrophic scarred area for effective treatment while preserving the wear comfort with the least amount of pressure on the other parts of the hand; this is as the optimal design criterion of the pressure sleeve (Figure 1I).
3. Results and discussion
3.1. Evaluation of current treatments
Conventionally, the silicone sheet applied onto an HSs is larger than the HS itself, especially with smaller scars. Otherwise, the silicone sheet would easily debond, causing inconvenience to the patient. Moreover, there are negative impacts, such as excessive sweating, when the silicone covers the healthy skin[26]. To minimize these unfavorable effects, we adopted 3D printing to fabricate a customized silicone elastomer so that it is the same size as the HS. The elastomer is directly attached to the pressure sleeve so that the debonding of the silicone sheet is remedied. Figure 2A shows the differences between a conventional silicone sheet and our 3D printed silicone elastomer.
Figure 2.
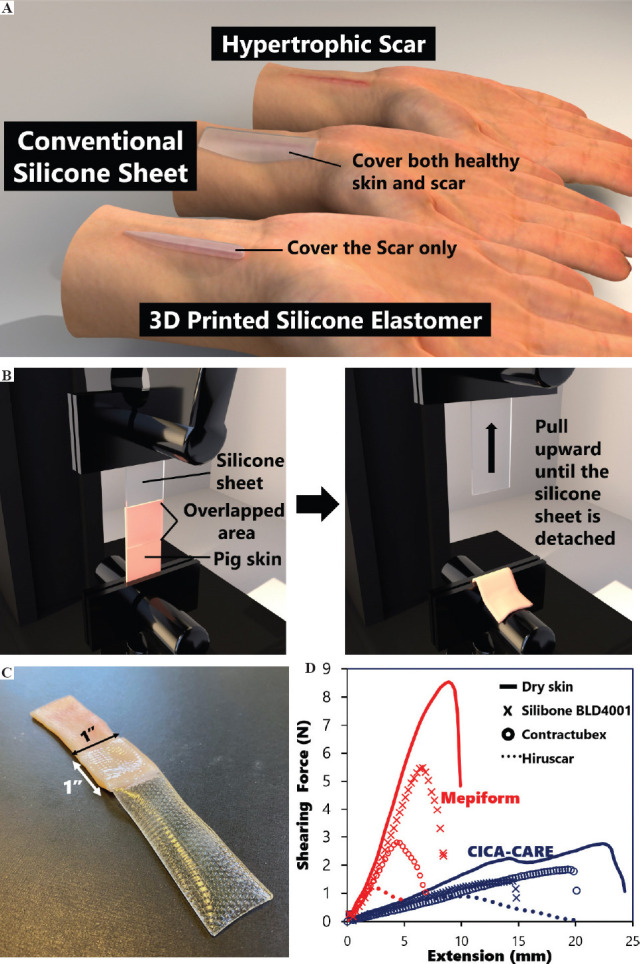
Evaluation of the silicone sheet therapy. (A) Illustration of the difference between conventional silicone sheet and 3D printed silicone elastomer on covered area. (B) Schematic of ASTM D5169 setup. (C) Photo of sample based on ASTM D5169. (D) Adhesion force of different silicone sheets (Mepiform® and CICA-CARE) under different skin conditions.
Another issue that we observed about the silicone sheet is that it may not adhere to skin with just any type of scar treating gel. To further confirm this issue, we used ASTM D5169 to assess the adhesion between the silicone and skin under various conditions. The schematic of the test and photo of the sample are shown in Figures 2B and C, respectively. Figure 2D shows that when any random type of scar treating gel is used, lower shear forces results when compared to skin without the use of any treating gel. When comparing the effect of the three different types of gels, SILBIONE BLEND 4001 has a small effect on the shear force, while Hiruscar® has the worst performance. The pigskin samples that used Hiruscar® showed an approximately 3-fold and 8-fold reduction of the maximum shear force with the CICA-CARE and Mepiform® silicone sheets, respectively. This indicates that the application of scar treating gel can further aggravate the problem of silicone sheet debonding. The poor adhesion of the silicone sheet to gel-coated skin might be a possible challenge, so HS therapy that combines the use of a silicone sheet and silicone gel, such as an onion extract gel, has been seldom discussed in the literature. In our proposed therapy, the silicone elastomer is attached to the pressure sleeve which is secured by the corresponding pressure so that the adhesion of the silicone to the skin with silicone gel is not a problematic issue.
3.2. Finite element model and validation of simulated result
Figure 3 illustrates the components of the developed FEM and the simulation process. The FEM simulates the wear process of the hand sleeve. The silicone elastomer was secured onto the model of the hand to prevent any unanticipated movement during the simulation process. Since the circumference of the pressure sleeve is smaller than that of the hand, a face load was applied on the shell elements of the pressure sleeve so that it stretched to fully fit and came into contact with the hand. During the stretching of the pressure sleeve, the sleeve was shifted toward the hand, and then the face load was applied. The pressure sleeve then recovered to its original size and came into contact with the silicone elastomer and the hand to simulate the pressure applied by the pressure sleeve. In this study, the friction between the hand and the fabric of the sleeve was neglected. The interface pressure produced by the pressure sleeve and silicone elastomer was observed at the end of the simulation. The sleeve size and the thickness of the silicone elastomer were adjusted before the next simulation was carried out until all the parameters were tested. To validate the accuracy of the FEM, the simulation result of a 2 mm thick silicone elastomer and a reduction factor of 10% of the sleeve were compared with the experimental result. The landmark positions are shown in Figure 4A. The experimentally obtained and simulated interface is compared in Figure 4B. The differences among the four different positions are within 5%, which is an acceptable margin of error to predict the amount of pressure.
Figure 3.
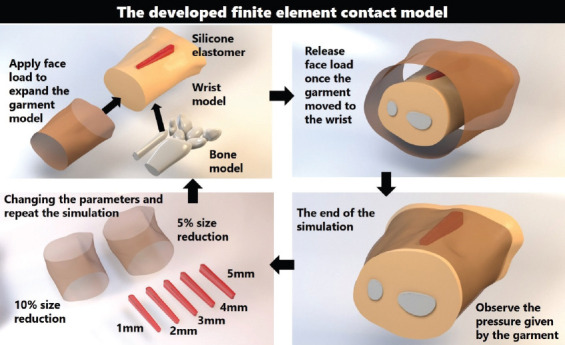
Illustration of the developed FEM for predicting the interface pressure.
Figure 4.
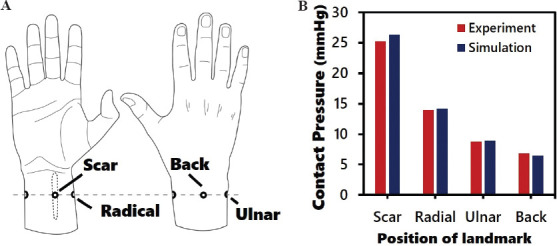
Validation of the developed FEM. (A) Illustration of the positions for pressure testing using pressure sensor. (B) Comparison of experimental and simulated results of pressure level.
3.3. Effect of silicone elastomer and garment size
The results of the simulated interface pressure that is exerted onto the HS, radial, ulnar, and back of the hand with the five different thicknesses of the silicone elastomer and two different pressure sleeve conditions are shown in Figures 5A-D, respectively. The interface pressure is proportional to the thickness of the silicone elastomer for both sleeve conditions (Figure 5A). A reduction factor of 10% in the circumference of the sleeve without the use of a silicone elastomer and a reduction factor of 5% with a 1 mm thick silicone elastomer show an exerted pressure of 20 mmHg onto the HS area. However, the pressure exerted onto the other areas with a reduction factor of 5% in the circumference of the sleeve along with a 1 mm thick silicone elastomer is significantly lower than the sleeve with a reduction factor of 10% in circumstance, with a decline of about 43% (radial and ulnar) and 50% (back). This indicates that customized 3D printed silicone elastomers can exert localized pressure onto the front side of the hand. The sleeve with a reduction factor of 5% also enables ease of wear and preserves the wear comfort in respect to the practical use of the pressure garment. When the garment size is much smaller than the body part, the patient will be not able to independently don the garment by him/herself and his/her skin might even get caught by the fastener. In considering the efficacy of treatment, we used a silicone elastomer with a thickness of 2 mm and a reduction factor of 5% of the circumference of the sleeve to exert approximately 25 mmHg of pressure onto the HS, as recommended in the literature. Compared to the treatment regimen of conventional pressure therapy that prescribes a reduction factor of 10% without the use of a silicone insert, the optimal pressure dosage applied to the HS here is on average about 20 – 25 mmHg. Furthermore, the pressure dosage on the other parts of the body is reduced by around 60%, 56%, and 80% for the ulnar, radial, and back of the hand, respectively, which result in reduced pressure discomfort. Figure 6 shows the complete pressure distribution of the hand based on the treatment regimen of conventional therapy and the optimized parameters in this study. The literature on pressure garment therapy indicates that when the pressure dosage exceeds 30 – 40 mmHg, discomfort, and potential harm to the body part, such as maceration and paresthesia may occur[26,54-56]. To prevent these issues, the insertion of a silicone elastomer into a garment with a reduction factor of 10% and the insertion of a silicone elastomer with a thickness of 4 mm or more into a garment with a reduction factor of 5% should be avoided when the mechanical properties of the fabric used are similar to those in this study. The plotted force extension of the warp knitted fabric is shown in Supplementary Figure 1 (125.5KB, pdf) .
Figure 5.
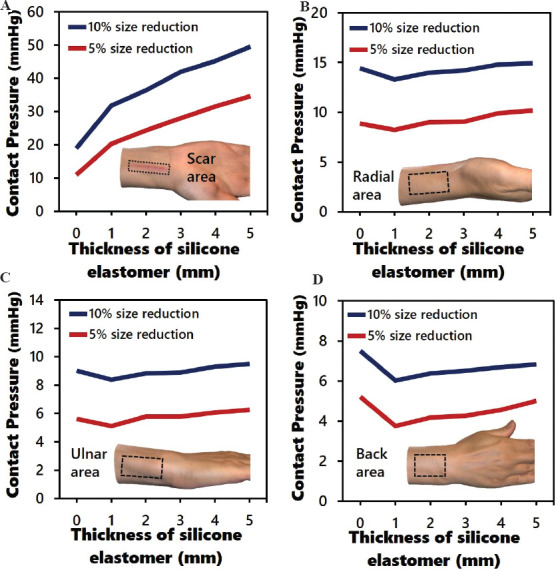
Simulated average interface pressure with different thicknesses of silicone elastomer (0 – 5 mm) and circumferences of sleeve (reduction factors of 5% and 10%) on various regions of hand: (A) scar area; (B) radial; (C) ulnar; and (D) back of hand.
Figure 6.
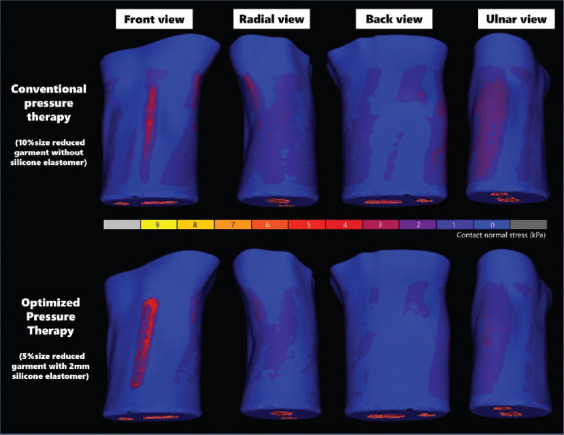
Comparison of pressure distribution provided by optimal size of pressure sleeve and silicone elastomer versus conventional pressure sleeve.
3.4. Demonstration of the combined therapies
Figure 7 demonstrates the appearance of the HS before the patient underwent the prescribed combined therapy in this study, and after 1 year of following the therapy. The sleeve was worn for at least 23 h each day and silicone gel was applied twice a day. It can be clearly observed that the HS was reduced in thickness and pigmentation, thus showing an improved appearance. The problem of the debonding of the silicone sheet was resolved so that the negative effect of the treatment was minimized. The patient reported that the placement of the silicone elastomer remained securely attached even during a badminton game and she did not experience any negative impacts in comparison to conventional silicone sheet treatment. Since HS treatment usually requires more than a year, the tension of the fabric of the pressure garment can gradually decline, resulting in a lower level of pressure exerted. Therefore, it is suggested that the level of pressure should be monitored every 3 months to ensure treatment efficacy. If the amount of induced pressure significantly declines, the pressure garment will need to be adjusted or a new garment will need to be prescribed. Although the application of this combined therapy can reduce the scar thickness and pigmentation, further investigation of the effect of the use of the silicone sheet, silicone gel, and pressure as a form of combined therapy should be conducted through randomized clinical trials in the future.
Figure 7.
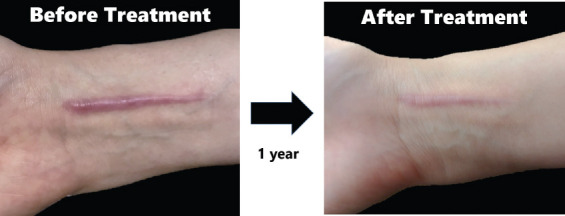
Effect of combined therapies for HS including the use of silicone sheet, silicone gel, and pressure.
4. Conclusion
Conventional silicone sheet treatment has a positive effect in the healing process of HSs. However, the debonding of the silicone sheet may affect the treatment compliance and therefore the treatment efficacy. In this study, we found that the scar treating gel reduces the adhesion of the silicone sheet. Therefore, using both silicone sheet and scar treating gel is not recommended for treatment of HSs. Instead, we propose a novel bespoke HS treatment involving a combination of 3D scanning, 3D printing, FEA, computer-aided design, and manufacturing software. The 3D printed silicone elastomer that only covers the HS itself is secured to a bespoke pressure sleeve. Since the silicone sheet is secured by the pressure from the sleeve, any type of scar treating gel can be applied at the same time. Using an FEA, the pressure dosage for the HS can be accurately modified, which in this case, is approximately 25 mmHg for the effective management of HS. Most importantly, the unnecessary pressure exerted on normal skin can be reduced by approximately 60%, 56%, and 80% at the ulnar, radial, and back of the hand, respectively. In this case study, the suggested treatment provides a significant effect in reducing the scar thickness and pigmentation. Also, there is no adverse effects of the hybrid pressure and silicone therapy found. The developed design framework and production of the pressure garment can contribute to new insights for future HS therapies and new customized clinical products for scars.
Conflicts of interest
The authors report no potential conflicts of interest.
Author contribution
L.C. designed the workflow, methodology, data curation and prepared the original draft of the manuscript and figures. Co-authors Y.S., M.S.H.L and M.Y.K involved in the investigation and analysis of results. K.L.Y. supervised and administered the project. K.L.Y., S.P.N, A.Y., J.Y. and Y.F.C. acquired the project funding, and reviewed and edited the manuscript.
Ethics approval and consent to participate
This study was approved by the Human Subjects Ethics Sub-committee of The Hong Kong Polytechnic University and Joint Chinese University of Hong Kong-New Territories East Cluster Clinical Research Ethics Committee. Written informed consent was obtained from the participant before data collection.
Consent for publication
The patient provided written informed consent to publish the pictures and her personal data.
Acknowledgments
We acknowledge the financial support of the Research Grant Council for funding this research project (project account PolyU 152097/18E).
References
- 1.Ogawa R. Total Scar Management :From Lasers to Surgery for Scars, Keloids, and Scar Contractures. Springer, Singapore. 2020 https://doi.org/10.1007/978-981-32-9791-3. [Google Scholar]
- 2.Tredget EE, Nedelec B, Scott PG, et al. Hypertrophic Scars, Keloids, and Contractures:The Cellular and Molecular Basis for Therapy. Surg Clin North Am. 1997;77(3):701–30. doi: 10.1016/s0039-6109(05)70576-4. https://doi.org/10.1016/s0039-6109(05)70576-4. [DOI] [PubMed] [Google Scholar]
- 3.Grose R, Werner S. Wound-healing Studies in Transgenic and Knockout Mice. Appl Biochem Biotechnol Part B Mol Biotechnol. 2004;28(2):147–66. doi: 10.1385/MB:28:2:147. https://doi.org/10.1385/mb:28:2:147. [DOI] [PubMed] [Google Scholar]
- 4.Son D, Harijan A. Overview of Surgical Scar Prevention and Management. J Korean Med Sci. 2014;29(6):751–7. doi: 10.3346/jkms.2014.29.6.751. https://doi.org/10.3346/jkms.2014.29.6.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Enoch S, Leaper DJ. Basic Science of Wound Healing. Surgery (Oxford) 2008;26(2):31–7. https://doi.org/10.1016/j.mpsur.2007.11.005. [Google Scholar]
- 6.Werner S, Krieg T, Smola H. Keratinocyte-fibroblast Interactions in Wound Healing. J Investig Dermatol. 2007;127(5):998–1008. doi: 10.1038/sj.jid.5700786. https://doi.org/10.1038/sj.jid.5700786. [DOI] [PubMed] [Google Scholar]
- 7.Nagase H, Woessner JF. Matrix Metalloproteinases. J Biol Chem. 1999;274(31):21491–4. doi: 10.1074/jbc.274.31.21491. https://doi.org/10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- 8.Bock O, Schmid-Ott G, Malewski P, et al. Quality of Life of Patients with Keloid and Hypertrophic Scarring. Arch Dermatol Res. 2006;297(10):433–8. doi: 10.1007/s00403-006-0651-7. https://doi.org/10.1007/s00403-006-0651-7. [DOI] [PubMed] [Google Scholar]
- 9.Mazharinia N, Aghaei S, Shayan Z. Dermatology life quality index (DLQI) scores in burn victims after revival. J Burn Care Res. 2007;28(2):312–7. doi: 10.1097/BCR.0B013E318031A151. https://doi.org/10.1097/bcr.0b013e318031a151. [DOI] [PubMed] [Google Scholar]
- 10.Kwan P, Hori K, Ding J, et al. Scar and Contracture:Biological Principles. Hand Clin. 2009;25(4):511–28. doi: 10.1016/j.hcl.2009.06.007. https://doi.org/10.1016/j.hcl.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 11.van Vlimmeren MA, Driessen-Mol A, van Den Broek M, et al. Controlling matrix formation and cross-linking by hypoxia in cardiovascular tissue engineering. J Appl Physiol (Bethesda, Md. 1985) 2010;109(5):1483. doi: 10.1152/japplphysiol.00571.2010. https://doi.org/10.1152/japplphysiol.00571.2010. [DOI] [PubMed] [Google Scholar]
- 12.Teekakirikul P, Eminaga S, Toka O, et al. Cardiac Fibrosis in Mice with Hypertrophic Cardiomyopathy is Mediated by Non-myocyte Proliferation and Requires Tgf-[Beta] J Clin Investig. 2010;120(10):3520–9. doi: 10.1172/JCI42028. https://doi.org/10.1172/jci42028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kassebaum NJ, Arora M, Barber RM, et al. Global, Regional, and National Disability-adjusted Life-years (DALYs) for 315 Diseases and Injuries and Healthy Life Expectancy (HALE), 1990-2015:A Systematic Analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1603–58. doi: 10.1016/S0140-6736(16)31460-X. https://doi.org/10.3410/f.726827339.793524296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li-Tsang CW, Lau JC, Chan CH. Prevalence of Hypertrophic Scar Formation and its Characteristics among the Chinese Population. Burns. 2005;31(5):610–6. doi: 10.1016/j.burns.2005.01.022. https://doi.org/10.1016/j.burns.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 15.Block L, Gosain A, King TW. Emerging Therapies for Scar Prevention. Adv Wound Care (New Rochelle) 2015;4(10):607–614. doi: 10.1089/wound.2015.0646. https://doi.org/10.1089/wound.2015.0646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Puzey G. The Use, of Pressure Garments on Hypertrophic Scars. J Tissue Viability. 2002;12(1):11–5. doi: 10.1016/s0965-206x(02)80004-3. https://doi.org/10.1016/s0965-206x(02)80004-3. [DOI] [PubMed] [Google Scholar]
- 17.Perkins K, Davey R, Wallis K. Silicone Gel:A New Treatment for Burn Scars and Contractures. Burns. 1983;9(3):201–4. doi: 10.1016/0305-4179(83)90039-6. https://doi.org/10.1016/0305-4179(83)90039-6. [DOI] [PubMed] [Google Scholar]
- 18.Leung P, Ng M. Pressure Treatment for Hypertrophic Scars Resulting from Burns. Burns. 1980;6(4):244–50. https://doi.org/10.1016/s0305-4179(80)80007-6. [Google Scholar]
- 19.Rivers E, Strate R, Solem L. The Transparent Face Mask. Am J Occup Ther. 1979;33(2):108–13. [PubMed] [Google Scholar]
- 20.Staley MJ, Richard RL. Use of Pressure to Treat Hypertrophic Burn Scars. Adv Wound Care. 1997;10(3):44–6. [PubMed] [Google Scholar]
- 21.Ai JW, Liu JT, Pei SD, et al. The Effectiveness of Pressure Therapy (15-25 mmHg) for Hypertrophic Burn Scars:A Systematic Review and Meta-analysis. Sci Rep. 2017;7(1):40185. doi: 10.1038/srep40185. https://doi.org/10.1038/srep40185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolfram D, Tzankov A, Pülzl P, et al. Hypertrophic Scars and Keloids a Review of Their Pathophysiology, Risk Factors, and Therapeutic Management. Dermatol Surg. 2009;35(2):171–81. doi: 10.1111/j.1524-4725.2008.34406.x. https://doi.org/10.1111/j.1524-4725.2008.34406.x. [DOI] [PubMed] [Google Scholar]
- 23.Klöti J, Pochon J. Conservative Treatment Using Compression Suits for Second and Third Degree Burns in Children. Burns. 1982;8(3):180–7. doi: 10.1016/0305-4179(82)90085-7. https://doi.org/10.1016/0305-4179(82)90085-7. [DOI] [PubMed] [Google Scholar]
- 24.Pratt J. In: Pressure Garments:A Manual on Their Design and Fabrication. 1st ed. West G, Withinshaw B, editors. Oxford, Boston: Butterworth-Heinemann; 1995. [Google Scholar]
- 25.Hoeksema H, De Vos M, Verbelen J, et al. Scar Management by Means of Occlusion and Hydration:A Comparative Study of Silicones Versus a Hydrating Gel-cream. Burns. 2013;39(7):1437–48. doi: 10.1016/j.burns.2013.03.025. https://doi.org/10.1016/j.burns.2013.03.025. [DOI] [PubMed] [Google Scholar]
- 26.Van den Kerckhove E, Stappaerts K, Boeckx W, et al. Silicones in the Rehabilitation of Burns:A Review and Overview. Burns. 2001;27(3):205–14. doi: 10.1016/s0305-4179(00)00102-9. https://doi.org/10.1016/s0305-4179(00)00102-9. [DOI] [PubMed] [Google Scholar]
- 27.Gilman TH. Silicone Sheet for Treatment and Prevention of Hypertrophic Scar:A New Proposal for the Mechanism of Efficacy. Wound Repair Regen. 2003;11(3):235–6. doi: 10.1046/j.1524-475x.2003.11313.x. https://doi.org/10.1046/j.1524-475x.2003.11313.x. [DOI] [PubMed] [Google Scholar]
- 28.Ko WJ, Na YC, Suh BS, et al. The Effects of Topical Agent (kelo-cote or contractubex) Massage on the Thickness of Post-burn Scar Tissue Formed in Rats. Arch Plast Surg. 2013;40(6):697–704. doi: 10.5999/aps.2013.40.6.697. https://doi.org/10.5999/aps.2013.40.6.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berman B, Perez OA, Konda S, et al. A Review of the Biologic Effects, Clinical Efficacy, and Safety of Silicone Elastomer Sheeting for Hypertrophic and Keloid Scar Treatment and Management. Malden, USA. 2007:1291–303. doi: 10.1111/j.1524-4725.2007.33280.x. https://doi.org/10.1111/j.1524-4725.2007.33280.x. [DOI] [PubMed] [Google Scholar]
- 30.Saulis AS, Chao JD, Telser A, et al. Silicone Occlusive Treatment of Hypertrophic Scar in the Rabbit Model. Aesthetic Surg J. 2002;22(2):147–53. doi: 10.1067/maj.2002.123023. https://doi.org/10.1067/maj.2002.123023. [DOI] [PubMed] [Google Scholar]
- 31.Li-Tsang CW, Lau JC, Choi J, et al. A Prospective Randomized Clinical Trial to Investigate the Effect of Silicone Gel Sheeting (Cica-Care) on Post-traumatic Hypertrophic Scar among the Chinese Population. Burns. 2006;32(6):678–83. doi: 10.1016/j.burns.2006.01.016. https://doi.org/10.1016/j.burns.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 32.Puri N, Talwar A. The Efficacy of Silicone Gel for the Treatment of Hypertrophic Scars and Keloids. J Cutan Aesthet Surg. 2009;2(2):104–6. doi: 10.4103/0974-2077.58527. https://doi.org/10.4103/0974-2077.58527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu A, Yick KL, Ng SP, et al. Orthopaedic Textile Inserts for Pressure Treatment of Hypertrophic Scars. Textile Res J. 2016;86(14):1549–62. https://doi.org/10.1177/0040517515573409. [Google Scholar]
- 34.Li-Tsang CW, Zheng YP, Lau JC. A Randomized Clinical Trial to Study the Effect of Silicone Gel Dressing and Pressure Therapy on Posttraumatic Hypertrophic Scars. J Burn Care Res. 2010;31(3):448–57. doi: 10.1097/BCR.0b013e3181db52a7. https://doi.org/10.1097/bcr.0b013e3181db52a7. [DOI] [PubMed] [Google Scholar]
- 35.Muangman P, Kongkor A, Namviriyachote N, et al. Effectiveness of Silicone Gel Combined with Pressure Garment for Prevention of Post-Burn Hypertrophic Scar:A Randomized Controlled Trial. J Med Assoc Thailand. 2020;103(5):39–43. [Google Scholar]
- 36.Uslu A, Sürücü A, Korkmaz MA, et al. Acquired Localized Hypertrichosis Following Pressure Garment and/or Silicone Therapy in Burn Patients. Ann Plast Surg. 2019;82(2):158–61. doi: 10.1097/SAP.0000000000001686. https://doi.org/10.1097/sap.0000000000001686. [DOI] [PubMed] [Google Scholar]
- 37.Ng WL, Chan A, Ong YS, et al. Deep Learning for Fabrication and Maturation of 3D Bioprinted Tissues and Organs. Virtual Phys Prototyp. 2020;15(3):340–58. [Google Scholar]
- 38.Sun W, Starly B, Daly AC, et al. The Bioprinting Roadmap. Biofabrication. 2020;12(2):5158. doi: 10.1088/1758-5090/ab5158. [DOI] [PubMed] [Google Scholar]
- 39.Ng WL, Chua CK, Shen YF. Print Me An Organ!Why We Are Not There Yet. Prog Polym Sci. 2019;97:101145. https://doi.org/10.1016/j.progpolymsci.2019.101145. [Google Scholar]
- 40.Kang HW, Lee SJ, Ko IK, et al. A 3D Bioprinting System to Produce Human-scale Tissue Constructs with Structural Integrity. Nat Biotechnol. 2016;34(3):312–9. doi: 10.1038/nbt.3413. https://doi.org/10.1038/nbt.3413. [DOI] [PubMed] [Google Scholar]
- 41.Oladapo BI, Ismail SO, Afolalu TD, et al. Review on 3D Printing:Fight against COVID-19. Mater Chem Phys. 2021;258:123943. doi: 10.1016/j.matchemphys.2020.123943. https://doi.org/10.1016/j.matchemphys.2020.123943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rendeki S, Nagy B, Bene M, et al. An Overview on Personal Protective Equipment (PPE) Fabricated with Additive Manufacturing Technologies in the Era of COVID-19 Pandemic. Polymers. 2020;12(11):1–18. doi: 10.3390/polym12112703. https://doi.org/10.3390/polym12112703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hale L, Linley E, Kalaskar DM. A Digital Workflow for Design and Fabrication of Bespoke Orthoses Using 3D Scanning and 3D Printing, a Patient-based Case Study. Sci Rep. 2020;10(1):7028–7. doi: 10.1038/s41598-020-63937-1. https://doi.org/10.1038/s41598-020-63937-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zolfagharian A, Gregory TM, Bodaghi M, et al. Patient-Specific 3D-printed Splint for Mallet Finger Injury. Int J Bioprint. 2020;6(2):1–13. doi: 10.18063/ijb.v6i2.259. https://doi.org/10.18063/ijb.v6i2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Holt SG, Yo JH, Karschimkus C, et al. Monitoring Skin Temperature at the Wrist in Hospitalised Patients May Assist in the Detection of Infection. Intern Med J. 2020;50(6):685–90. doi: 10.1111/imj.14748. https://doi.org/10.1111/imj.14748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen G, Xie J, Dai G, et al. Validity of the Use of Wrist and Forehead Temperatures in Screening the General Population for COVID-19:A Prospective Real-World Study. Iran J Public Health. 2020;49(supple 1):3670. doi: 10.18502/ijph.v49iS1.3670. https://doi.org/10.18502/ijph.v49is1.3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chow L, Yick KL, Kwan MY, et al. Customized Fabrication Approach for Hypertrophic Scar Treatment:3D Printed Fabric Silicone Composite. Int J Bioprint. 2020;6(2):262. doi: 10.18063/ijb.v6i2.262.. https://doi.org/10.18063/ijb.v6i2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boone LA. Development of a Customized Pattern Drafting System for Interim Burnscar Pressure Garments Utilizing Fabric Properties and Circumference Measurements. University of Alberta, Edmonton, Alta 1995 [Google Scholar]
- 49.Yu A. Development of Pressure Therapy Gloves for Hypertrophic Scar Treatment. The Hong Kong Polytechnic University, Hong Kong 2015 [Google Scholar]
- 50.Yu A, Yick KL, Ng SP, et al. Numerical Simulation of Pressure Therapy Glove by Using Finite Element Method. Burns. 2016;42(1):141–51. doi: 10.1016/j.burns.2015.09.013. https://doi.org/10.1016/j.burns.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 51.Wu JZ, Dong RG, Rakheja S, et al. Simulation of Mechanical Responses of Fingertip to Dynamic Loading. Med Eng Phys. 2002;24(4):253–64. doi: 10.1016/s1350-4533(02)00018-8. [DOI] [PubMed] [Google Scholar]
- 52.Lai CH, Li-Tsang CW. Validation of the Pliance X System in measuring interface pressure generated by pressure garment. Burns. 2009;35(6):845–51. doi: 10.1016/j.burns.2008.09.013. https://doi.org/10.1016/j.burns.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 53.Wiseman J, Simons M, Kimble R, et al. Reliability and Clinical Utility of the Pliance X for Measuring Pressure at the Interface of Pressure Garments and Burn Scars in Children. Burns. 2018;44(7):1820–8. doi: 10.1016/j.burns.2018.05.002. https://doi.org/10.1016/j.burns.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 54.Reid W, Evans J, Naismith R, et al. Hypertrophic Scarring and Pressure Therapy. Burns. 1987;13:S29–32. doi: 10.1016/0305-4179(87)90090-8. https://doi.org/10.1016/0305-4179(87)90090-8. [DOI] [PubMed] [Google Scholar]
- 55.Leung K, Cheng J, Ma G, et al. Complications of Pressure Therapy for Post-burn Hypertrophic Scars:Biomechanical Analysis Based on 5 Patients. Burns. 1984;10(6):434–8. doi: 10.1016/0305-4179(84)90085-8. https://doi.org/10.1016/0305-4179(84)90085-8. [DOI] [PubMed] [Google Scholar]
- 56.Miyatsuji A, Matsumoto T, Mitarai S, et al. Effects of Clothing Pressure Caused by Different Types of Brassieres on Autonomic Nervous System Activity Evaluated by Heart Rate Variability Power Spectral Analysis. J Physiol Anthropol Appl Hum Sci. 2002;21(1):67–74. doi: 10.2114/jpa.21.67. https://doi.org/10.2114/jpa.21.67. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


