Abstract
Histologic chorioamnionitis is an inflammatory disorder of the placenta that commonly precedes preterm delivery. Preterm birth related to chorioamnionitis and fetal inflammation has been associated with a risk for serious inflammatory complications in infancy. In addition, preterm infants exposed to chorioamnionitis may be more susceptible to infection in the neonatal intensive care unit and possibly later in life. A significant body of work has established an association between chorioamnionitis and inflammatory processes. However, the potential consequences of this inflammation on postnatal immunity are less understood. In this review, we will discuss current knowledge regarding the effects of fetal exposure to inflammation on postnatal immune responses.
Introduction
The diagnosis of suspected clinical chorioamnionitis is based on non-specific symptoms such as maternal fever, leukocytosis, abdominal or uterine tenderness, or fetal tachycardia (1). However, the ‘gold standard’ for confirmation of this diagnosis rests on placental evidence of acute histological chorioamnionitis (HCA), represented by the infiltration of inflammatory neutrophils in maternal or fetal placental tissues (2). A more updated, but still controversial definition of chorioamnionitis, also referred to as intrauterine inflammation, infection or both (‘Triple I’), incorporates both clinical and histologic criteria (3). While clinical chorioamnionitis is commonly accompanied by HCA (4), the reverse situation may not be true. In fact, most cases of HCA occur without clinical symptoms in the mother or fetus and thus present ‘silently’ (5, 6). Despite the lack of clinical expression, however, asymptomatic placental inflammation is not innocuous even in the absence of infection (7). A diagnosis of HCA often precedes the delivery of extremely preterm infants (5), and like clinical chorioamnionitis, is associated with early-onset infection (8). Conversely, HCA was correlated with a decreased risk of late-onset neonatal infection with coagulase-negative staphylococci (9). HCA has also been closely linked to the pathogenesis of serious postnatal inflammatory disorders including bronchopulmonary dysplasia, brain injury, retinopathy of prematurity, and necrotizing enterocolitis (10–13). Preterm infants born to mothers with clinically suspected chorioamnionitis are typically identified as being at higher risk for infection and are typically screened (14). In contrast, in the absence of maternal symptoms, the possibility that a preterm infant has been exposed to HCA and a consideration of its inherent inflammatory and infectious risks may not be addressed in a timely fashion or even at all. This is particularly true given that a diagnosis of HCA rests on microscopic examination of the delivered placenta, and resulting information may not be available for days to weeks after birth.
A variety of approaches to identify gestations affected by HCA have been studied. The expression patterns of biological markers in amniotic fluid and cord blood, such as interleukin-6 and C-reactive protein, have been assessed for their predictive value in histological chorioamnionitis; however, sensitivity and specificity of these markers have not been consistent (15–17). Clinical prediction rules for HCA and funisitis have also been developed in order to identify newborns exposed to antenatal inflammation (18). The targeted clinical variables included the absence of pre-eclampsia, normal intrauterine growth, maternal or fetal evidence of clinical chorioamnionitis, prolonged premature rupture of membranes (PPROM), and vaginal delivery. Although these methods have shown clinical promise, to date none have been uniformly successful in identifying gestations with HCA.
The inflammatory complications associated with HCA have been well described (13, 19–24). Less appreciated is that affected preterm infants also may be at risk for immune consequences in addition to or in combination with the adverse effects of HCA-mediated inflammation (25, 26). Increasing evidence supports the concept that the ensuing neonatal immune dysfunction reflects the effects of inflammation on immune programming during critical developmental ‘windows’ (26). The goals of the present review article are to summarize the following: 1) The effects of inflammation during pregnancy on the reconfiguration of neonatal inflammatory and immune responses; and 2) The implications of intrauterine inflammatory exposure for immunity in the neonatal period and beyond. Understanding how in utero inflammation programs the postnatal immune response may reveal novel approaches to reduce inflammatory injury and the risk for infection in preterm infants.
Effects of antenatal inflammation on neonatal immunity
Inflammation and immunity.
Inflammatory exposure during intrauterine life is a pathologic force that can drive alterations of postnatal innate and adaptive immunity (Fig. 1). A growing body of data implicate myriad environmental exposures during pregnancy, many associated with inflammation, on subsequent immunity (reviewed in (25)). Recent evidence of a stereotypic developmental pattern of converging immune responses in preterm and term infants in the first three months of life, but divergent responses in infants with inflammatory exposure, additionally credits the role of intrauterine exposure at critical developmental windows in shaping immunity (26).
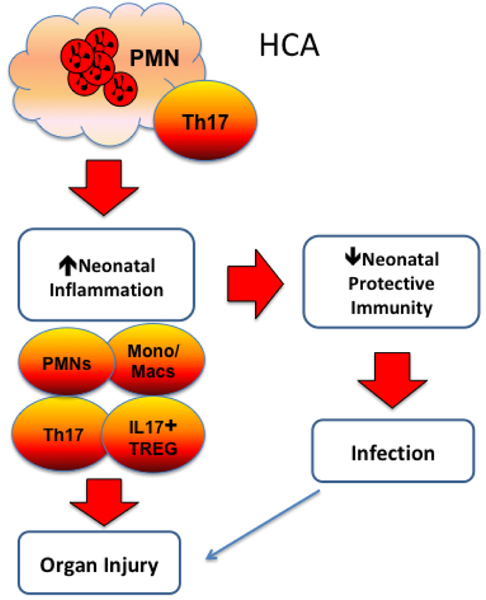
Figure 1. Potential effects of HCA on neonatal inflammatory and immune responses.
Studies in humans and in animal models have linked HCA, a neutrophil (PMN)-driven placental disorder associated with increased Th17 responses, with exaggerated inflammatory responses of both innate and adaptive immune cells. Neutrophil (PMN) production and activation may be increased, along with the release of inflammatory cytokines and chemokines that promote PMN infiltration and injury to major organs. Experimental fetal inflammation can induce functional maturation and activation of monocytes (Mono) and macrophages (Macs) that can also heighten inflammatory responses. Fetal inflammation enhances the generation of inflammatory Th17 cells and IL-17+ Treg cells; while IL-17 is important to host protection, high levels can induce organ injury, particularly in the brain. Exaggerated inflammatory responses may lead to suppression of protective immune responses, which increase risk for infection. Neonatal infection in the context of HCA exposure has also been shown to increase risk for organ injury and has been linked to bronchopulmonary dysplasia.
Studies in humans and in animal models have begun to define how inflammatory exposure can shape immune function in fetuses and in the newborn period; these are summarized in several recent reviews (27, 28). Acute HCA associated with fetal inflammation is a risk factor for numerous adverse neonatal outcomes (13, 19–24). Inflammatory injury related to HCA has been observed in both extremely preterm infants as well as in late preterm infants delivered after PPROM (29). However, HCA at the earliest gestations may more heavily influence neonatal immune responses, such as increased Th17 frequencies (30). This enhanced effect in very preterm newborns likely reflects the age-dependent ‘waves’ of immune cell populations with inflammatory or regulatory function that are generated in the developing fetus (31).
Immune priming and HCA.
In utero ‘priming’ or activation of the fetal immune system at critical developmental time points can lead to chronic inflammatory disorders as well as increased vulnerability to infection after birth (25). Maternal infections with chronic inflammation, such as HIV or malaria, during pregnancy were associated with fetal inflammation and alterations in infant B cell responses (32). Infants born to mothers with allergic disease had lower frequencies of Tregs, which in turn were impaired in their capacity to suppress effector T cells, particularly Th2 cells (33). This latter finding may be of particular relevance to exposed infants and future risk for asthma given its close association with Th2 polarization (34). Furthermore, even the low-grade systemic inflammation associated with maternal obesity was shown to induce placental and fetal inflammation (35).
Emerging evidence also points to a critical role of activated fetal cells in driving intrauterine responses during chorioamnionitis. Gomez-Lopez et al. utilized DNA fingerprinting to show that predominance of inflammatory fetal neutrophils in the amniotic fluid of gestations with chorioamnionitis was highly associated with the delivery of extremely preterm neonates (36). Increased neonatal T cell activation has also been associated with preterm delivery (37). Frascoli et al. observed that activation of the fetal adaptive immune system suppressed maternal-fetal tolerance in the context of preterm labor (38). In that study, fetal blood showed early maturation of dendritic cells and enhanced maternal microchimerism in preterm relative to term gestations. Additionally, preterm (but not term) fetal T cells were alloreactive to maternal antigens, and maternal antigen-specific stimulation induced the proliferation of fetal Th1-type cells. Furthermore, the cytokines (IFNγ+ or TNF-α) released by proliferating T cells directly increased myometrial contractility in an in vitro assay, suggesting a directive role of activated fetal T cells in preterm labor. Although naïve T cells typically predominate in fetuses, high frequencies of memory (CD4+CD45+RO+RA-) T cells have been observed in association with preterm labor (37). This finding may be important given differing gene expression patterns and function in naïve vs. memory T cells (39).
The inflammatory processes induced by HCA also contribute to fetal immune activation. In a recent transcriptomic study, preterm infants exposed to HCA exhibited gene expression signatures indicative of immune priming (40). The most frequently upregulated genes in these neonates were associated with activation of innate and adaptive immune pathways. Notably, the microRNA, miR-155, was shown to be a top upstream regulator. MiR-155 is a master modulator of inflammatory and immune responses, and its elevated expression in immune cells has been associated with chronic inflammatory states including atopic dermatitis, multiple sclerosis and rheumatoid arthritis (reviewed in (41)). Pertinently, MiR-155, which is also expressed in activated CD4+ T cells, can promote pathogenic Th17-biased responses (42).
Evidence of immune priming was also observed in a murine model of LPS-induced antenatal inflammation followed by a postnatal ‘second hit’ immune challenge (43). In pups exposed to antenatal inflammation, infection with Sendai virus (the murine counterpart to the respiratory syncytial virus (RSV) that causes bronchiolitis in human infants) triggered strong inflammatory responses in the lungs, the primary site of infection, but also in distal organs such as the liver. This exaggerated correlation was not witnessed in infected control pups without antenatal LPS exposure. In addition, an inductive effect of maternal inflammation on lung CD4 T helper cell populations with a pro-inflammatory Th1- and Th17 phenotypes was most pronounced in exposed weanling pups relative to neonates. These findings suggested that the processes initiated in utero not only persisted but were possibly amplified beyond the neonatal period. Interestingly, a similar enhancement of lung Th17 cells was observed following secondary RSV challenge in adult mice that had survived severe sepsis (44).
Inflammatory innate immune responses in HCA.
A number of studies have shown that experimentally induced antenatal inflammation leads to exaggerated inflammatory immune responses in exposed offspring. In ex vivo studies of preterm fetal sheep, experimental chorioamnionitis promoted the functional maturation of lung monocytes and hastened their capacity to produce inflammatory cytokines in response to stimulation (45). Preterm piglets born after several doses of intra-amniotic LPS had increased systemic and organ specific (gut and lung) inflammatory responses at birth (46). In a murine model of antenatal inflammation, neonatal and weanling offspring of LPS-treated dams showed increased basal innate immune responses in the lungs and livers that were amplified following a ‘second hit’ viral infection (43).
The fetal inflammatory responses induced by HCA have also been shown to persist in newborn infants as systemic inflammation, much of it driven by neutrophils. As part of the ELGAN study, Chen et al. showed that the elevated levels of key neutrophil-associated inflammatory proteins (including myeloperoxidase, IL1β, IL-8, ICAM-3 and MMP9) in the cord blood of preterm infants born with funisitis (inflammation of cord blood vessels consistent with the fetal inflammatory response syndrome, FIRS) remained high on postnatal day 7 (47). Autopsies of human fetuses and newborn infants who died after severe chorioamnionitis also showed amplified neutrophil production (myelopoiesis) in hematopoietic organs (48, 49). These observations are consistent with the excessive neutrophil responses associated with this perinatal condition (36, 50–52) as well as the neutrophil-driven inflammatory responses in neonatal lungs and other organs (53, 54). Similar observations of neutrophil-driven inflammation have been observed in animal models. Antenatal inflammation was shown to promote neutrophil recruitment and the infiltration of organs such as the lungs and brain (43, 55, 56). High expression levels of inflammatory cytokines, including IL-1β, IL-6, IL-8, and IL-17 in the blood, thymi, lungs, and/or intestinal tracts of fetal sheep, macaques and piglets animals following experimental chorioamnionitis have been reported (46, 57–59). In addition, altered DNA methylation profiles have been observed in placentas with HCA, reflecting activation of innate immunity and neutrophil increases (60).
Antenatal fetal exposure has also been shown to induce inflammatory responses in the liver. In sheep studies of liver homeostasis and metabolism after LPS-induced chorioamnionitis, Vlassaks et al. found increased hepatic T-lymphocytes and apoptotic hepatocytes in term newborns and increased liver triglycerides and cholesterol levels at 7 weeks of life, indicating long-lasting postnatal effects on lipid metabolism (61). Endotoxin-induced chorioamnionitis also caused hepatic damage associated with disturbed lipid and glucose metabolism, reduced antioxidant capacity, and elevated liver enzymes (62). The adverse hepatic effects of fetal inflammation may have specific relevance to neonatal immunity, given the increasingly appreciated role of the liver in directing immune function (63)
Inflammatory adaptive immune responses after HCA.
T helper cell subsets belong to the adaptive arm of the immune system and can promote or suppress inflammatory responses. In addition to the effects of fetal inflammation on innate immunity, recent studies have identified the robust involvement of pro-inflammatory T helper cell lymphocyte subsets, such as Th17 cells, in fetuses or preterm infants with antenatal inflammation. Th17 cells characteristically function to protect the host against extracellular pathogens (64–66). However, under certain inflammatory conditions, Th17 cells may become pathogenic and promote tissue injury (67). Th17 cells release the canonical cytokine, IL-17, which is also produced by other immune cells such as γδ T cells and pro-inflammatory Treg cells (68). IL-17 plays a critical role in processes involved in FIRS associated with HCA (59). The developing brain is particularly sensitive to inflammatory injury, and exposure to IL-17 at critical ‘windows’ of immune development can induce microglial activation and white matter injury (reviewed in (69)). Furthermore, in addition to directly inducing tissue injury, Th17 cells can amplify inflammatory responses through cross-talk with neutrophils (70).
While Th17 cells play an important biological role in normal pregnancy (71), increased frequencies of pathogenic Th17 cells have been observed in placentas of women with recurrent miscarriages (72) and in gestations affected by chorioamnionitis (73). Higher circulating Th17 frequencies in mothers or in the cord blood of babies of preterm gestations with HCA have also been reported (74, 75). The exact mechanism(s) that promote Th17 responses in the context of HCA remain enigmatic. However, expression levels of several cytokines that are critical to the propagation of Th17 cells from naïve CD4 cells, including IL-1β and IL-6 (76), are also increased in the amniotic fluid in HCA (77, 78). The finding that inflammatory neutrophils promote in vitro propagation of Th17 cells (79) suggests their contribution to an intrauterine cytokine milieu that also modulates Th17 responses in HCA, as observed in the context of chronic inflammatory conditions such as rheumatoid arthritis (80).
In a recent human study, cord blood from preterm and term infants with HCA had increased frequencies of Th17 cells relative to unaffected controls (74). Th17 cells were highest in the cord blood of extremely preterm infants, who also exhibited increased T cells with an effector memory phenotype associated with Th17-type responses (81). In addition, the elevated circulating Th17 frequencies observed at birth in preterm neonates exposed to chorioamnionitis persisted in the first month of life (75). Increased Th17-type responses have been observed in the cord blood of human infants following both acute and chronic HCA (73), and in animal models in the context of antenatal inflammation. Fetal macaques exposed to LPS-induced chorioamnionitis had increased splenic IL-17+ and IL-22+ Th17 cells (59), while weanling murine pups exposed to LPS exhibited increased lung Th17 responses (43).
T regulatory (Treg) cells constitute a T helper cell subset that typically functions to suppress activated cells and inflammatory responses, including those mediated by Th17 cells (82, 83). Chorioamnionitis has been variably associated with decreased T regulatory (Treg) cell frequencies or reduced Treg suppressor function (84). Fetal rhesus monkeys and sheep exposed to experimental chorioamnionitis had an increased ratio of IL-17 producing cells to Tregs in lymphoid organs (85). Exposure was also associated with decreased frequencies of circulating Tregs in extremely preterm human neonates and in fetal macaques (59, 74). However, the majority of Tregs in these two studies also co-expressed the canonical Th17 transcription factor, RORγt, and/or IL-17, consistent with a pro-inflammatory rather than a regulatory phenotype (86, 87). Pertinently, IL-17+ Tregs can serve as a major source of IL-17 during inflammation (88).
The enhanced Th17-type responses observed in conjunction with antenatal inflammation have been linked to inflammatory injury in the lungs or brain. Elevated frequencies of IL-17 producing cells in fetal rhesus monkeys with chorioamnionitis were associated with lung inflammation in neonates (85). When LPS-induced antenatal inflammation was combined with neonatal hypoxic-ischemic brain injury in a rat pup model, Th17-like lymphocytes migrated to the brain to direct neuroinflammatory responses (89). Th17 cells appear to be the major cell group mediating this inflammatory IL-17 effect; while γδ t cells also produce IL-17 (90), experimental HCA did not measurably alter this lymphocyte population in exposed lambs (91).
Other lymphocyte subsets may have the capacity to contribute to neonatal inflammatory responses in HCA that are not mediated by IL-17. A higher proportion of Th1 cells were determined in the umbilical cord blood of human neonates with clinical evidence of perinatal infection (92). A recently described subset of lymphocytes unique to cord blood produces IL-8/CXCL8 and can activate neutrophils and γδ t cells (93), although whether and how HCA influences these lymphocytes is not clear.
Immune suppression and HCA.
In contrast to the hyper-inflammatory responses associated with HCA exposure, protective immune responses may be suppressed. Immune suppressive mechanisms in chorioamnionitis may be selectively quantitative. Human fetuses and neonates exposed to chorioamnionitis have been shown to exhibit both thymic involution and depletion of splenic T cells (94, 95). Studies in fetal sheep affected by chorioamnionitis found reductions in CD8+ but not CD4+ T cells in thymic cell populations (96).
Intrauterine inflammatory exposure may also lead to qualitative alterations in neonatal innate or adaptive immune function. A relationship between an inflammatory antenatal environment and immune suppression is suggested by the enhanced HIV-positivity observed in human infants born to HIV-affected mothers in the context of chorioamnionitis (97), possibly due to activated fetal lymphocytes (98). A recent study also showed suppressed transcriptional responses to Staphyloccoccus epidermidis in ex vivo monocytes from preterm human neonates with chorioamnionitis (99). Studies in animal models are supportive of this premise: Repetitive intrauterine LPS exposure in sheep induced ‘immune paralysis’ of ex vivo fetal and neonatal monocytes following stimulation with LPS or other Toll-like receptor ligands (45, 100). Similarly, chronic, but not acute, intra-amniotic infection with Ureaplasma parvum resulted in suppressed ‘second hit’ LPS-induced cytokine responses in the fetal lung (101, 102). This evidence further supports the idea that prenatal exposure to HCA-mediated inflammation, particularly if longstanding, can alter postnatal immune response patterns. Pertinently, septic human neonates have been observed to exhibit early hyper-inflammatory responses followed by suppressed immune responses (103), a pattern reminiscent of that observed in infants exposed to HCA. Similarly, Azizia et al. found a correlation between prematurity, neonatal sepsis, and reduced monocyte MHC class II expression associated with immune paralysis in HCA-exposed gestations, with an increased risk for sepsis and organ dysfunction (104).
Potential mechanisms of immune suppression in HCA.
The immune system in preterm infants is developmentally restricted in its capacity to protect the host against infection (105). The added burden of intrauterine inflammatory exposure during sensitive developmental ‘windows’ to already impaired immune function also remain incompletely understood, but may involve developmentally regulated epigenetic processes (106). In studies of short-term antenatal LPS exposure in preterm sheep, the role of timing rather than the specific inflammatory trigger was found to have a greater impact on abnormal neurological findings in the fetal brain (107). However, the mechanisms involved in the inflammation-induced immune suppression of infants exposed to HCA, like the immune dysfunction associated with neonatal sepsis (103), remain incompletely understood (108).
A variety of quantitative and qualitative alterations of immune function that are biologically prevalent in preterm infants can contribute to processes that suppress immunity (31, 109) (Fig. 2). The characteristic limitations of neutrophil production and storage that are typical in preterm infants can lead to rapid depletion and severe neutropenia during periods of increased utilization, such as sepsis (110). In addition, neonatal neutrophils and monocytes exhibit intrinsic dysfunction, including hyporesponsiveness to stimulation and impaired antimicrobial capacity (111, 112) that may be additionally affected by inflammation-induced immune paralysis. HCA can induce excessive fetal neutrophil production (granulopoiesis), suggesting a fetal capacity to overcome or circumvent developmental restrictions under inflammatory conditions (113). However, the functionality of these newly-minted neutrophils may also be impaired. Inflammation can lead to hypofunctional T cells through a process that downregulates the T cell receptor (TCR) zeta-chain (114), although whether this functions as a suppressive mechanism in the context of HCA is unknown. Conversely, while neonatal immune cells are also at a developmental disadvantage in terms of generating protective cytokines, such as IFNγ, neonatal Th1 cell frequencies may be increased following HCA exposure (115).
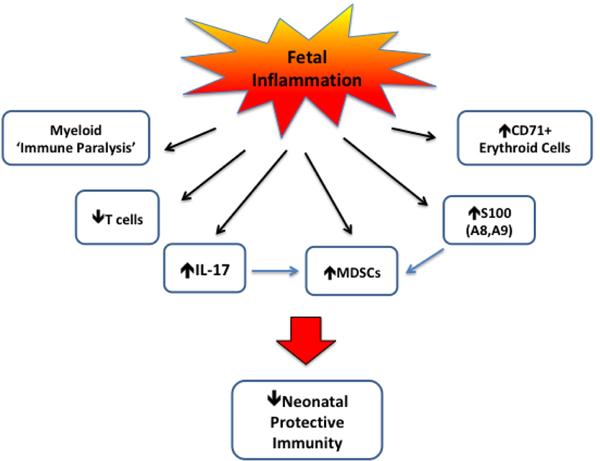
Figure 2. Potential mechanisms of suppressed protective immunity in neonates exposed to fetal inflammation associated with HCA.
Experimental HCA has been associated with ‘immune paralysis’ as suggested by decreased LPS responsiveness in fetal sheep monocytes. HCA has been variably associated with quantitative and qualitative defects in T cells. Conversely, increases in Th17 and inflammatory Treg cells promote IL-17 release. While IL-17 provides immune protective function, it can also promote the generation of myeloid-derived suppressor cells (MDSCs), which adversely affect protective immunity. The increased expression of S100 proteins, particularly S100A8 and S100A9, may promote host protection; however, high levels can increase MDSC generation. Recent evidence also indicates an immunosuppressive role of CD71+ erythroid cells, which could potentially be increased with HCA.
Cells with regulatory function may serve to further suppress immune function in newborns exposed to HCA (Fig. 2). Granulocytic myeloid-derived suppressor cells (Gr-MDSC), an immature neutrophil subset with high frequencies in neonates, suppress T cell function (116, 117). This action may occur through the reduction of L-arginine levels (118), which are biologically low in preterm infants (119). Pertinently, increased expression of arginase 1 and subsequent depletion of L-arginine were observed in exposed offspring in a rat model of LPS-induced chorioamnionitis (120). MDSCs are also important negative regulators of inflammatory responses (121, 122). Elevated circulating frequencies of granulocytic MDSCs have been reported in extremely preterm infants in association with clinical inflammation, though not specifically HCA (123). Importantly, these MDSCs persisted for several months beyond the immediate neonatal period, suggesting an immunosuppressive role in later infancy. Neonatal inflammatory neutrophils and monocytes also release the alarmins, S100A8 and S100A9, which may suppress hyper-inflammatory responses through the expansion of MDSCs (124, 125). Pertinently, increased S100 protein expression levels in amniotic fluid have been observed in gestations with HCA (126).
Lymphocytes with intrinsic suppressive function can also inhibit immune responses in preterm infants. Tregs are critical to the suppression of T cell responses to self and maternal antigens that is necessary for maternal-fetal tolerance (127, 128). Although Tregs in preterm infants with HCA may exhibit a pro-inflammatory (Th17-like) phenotype (74), conversely their release of IL-17 could attract MDSCs to mediate immune suppression (129). Regulatory B cells, another type of immune cell, can modulate neonatal inflammatory responses (130) and promote Th2 skewing in neonatal mice through suppressive actions on dendritic cells (131).
Recent evidence also points to a role of a unique subset of CD71+ erythroid cells in modulating myeloid and T cell responses (132). Pertinently, these regulatory erythroid cells are found in high numbers in preterm but not term neonates. CD71+ cells were shown to suppress protective immune responses to pertussis infection in neonatal mice, in part through actions mediated by arginase and expression of programmed death ligand-1 (133).
Steroid associated effects on immune responses in HCA.
While current treatment guidelines for chorioamnionitis are institutionally varied, antenatal steroids (in the form of betamethasone) are commonly administered for preterm labor. A recent meta-analysis showed that steroid administration in the setting of HCA was associated with reduced mortality and incidence of respiratory distress, patent ductus arteriosus, intraventricular hemorrhage (IVH) and severe IVH; in the setting of clinical chorioamnionitis, steroid administration reduced severe IVH and periventricular leukomalacia (134). Although several studies suggest that antenatal steroids can dampen the inflammatory cascade, their effects on fetal inflammation are not well defined. Evidence of anti-inflammatory effects of steroid administration includes the inhibition of intrauterine TFG-β signaling associated with fetal lung inflammation and the partial prevention of the structural lung changes induced by LPS exposure (135, 136).
The antenatal timing of steroid administration may also influence inflammatory responses. Kuypers et al. showed that while steroid administration prior to intrauterine LPS exposure reduced the adverse effects of inflammation on the brain in fetal sheep, conversely steroids aggravated inflammatory changes in the brain and thymus in the context of pre-existing inflammation (137, 138). These observations suggest that, in the presence of chorioamnionitis, steroids could potentially amplify fetal injury in an organ-specific manner. In studies of fetal sheep exposed to intra-amniotic endotoxin and subsequently treated with steroids, inflammatory responses in ex vivo monocytes were initially suppressed but were followed by a later activation, possibly the result of steroid-induced functional maturation (139).
Summary
Histologic chorioamnionitis is a common disorder that is tightly linked to preterm delivery and dysregulated immune function. Inroads are being made towards better defining the immune effects of antenatal inflammatory exposure on the fetus and newborn, which includes a pattern of hyper-inflammation combined with immune suppression. However, much remains to be learned regarding the underlying mechanisms so that potential therapeutic targets can be identified.
Perinatal inflammation has clear implications for human health. Mounting evidence points to a negative impact of early inflammatory exposure of any origin on the developing immune program (140, 141). Chorioamnionitis has been identified as contributing factor in childhood asthma (142, 143), possibly through a mechanism involving Th2 skewing (144). However, much remains to be learned in this regard. Numerous factors aside from microbial exposure have been shown to induce systemic maternal inflammation and/or chorioamnionitis, including nutritional and psychosocial factors (reviewed in (140)). Of great concern are the observations linking perinatal inflammation from various causes with immune dysfunction and abnormal stress responses, not only in the immediate postnatal period but possibly throughout life or even into the next generation (145). Thus, the importance of advancing knowledge of perinatal inflammation and its causes cannot be overstated.
Acknowledgments
Statement of Financial Support
This work was supported in part by funding from the Saint Louis University Department of Pediatrics, the Cardinal Glennon Foundation, the National Institutes of Health (AI094478, AI138096), and The Gerber Foundation (all, J.M.K.)
Footnotes
Disclosure
The authors declare no conflicts of interest.
Category of Study: Review article
This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Tita AT, Andrews WW. Diagnosis and management of clinical chorioamnionitis. Clin Perinatol 2010;37:339–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Redline RW. Inflammatory response in acute chorioamnionitis. Semin Fetal Neonatal Med 2012;17:20–25 [DOI] [PubMed] [Google Scholar]
- 3.Peng CC, Chang JH, Lin HY, Cheng PJ, Su BH. Intrauterine inflammation, infection, or both (Triple I): A new concept for chorioamnionitis. Pediatr Neonatol 2018;59:231–237 [DOI] [PubMed] [Google Scholar]
- 4.Suzuki S Association between clinical chorioamnionitis and histological funisitis at term. J Neonatal Perinatal Med 2019;12:37–40 [DOI] [PubMed] [Google Scholar]
- 5.Lahra MM, Jeffery HE. A fetal response to chorioamnionitis is associated with early survival after preterm birth. Am J Obstet Gynecol 2004;190:147–151 [DOI] [PubMed] [Google Scholar]
- 6.Horvath B, Lakatos F, Toth C, Bodecs T, Bodis J. Silent chorioamnionitis and associated pregnancy outcomes: a review of clinical data gathered over a 16-year period. J Perinat Med 2014;42:441–447 [DOI] [PubMed] [Google Scholar]
- 7.Park JW, Park KH, Jung EY. Clinical significance of histologic chorioamnionitis with a negative amniotic fluid culture in patients with preterm labor and premature membrane rupture. PLoS One 2017;12:e0173312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hillier SL, Martius J, Krohn M, Kiviat N, Holmes KK, Eschenbach DA. A case-control study of chorioamnionic infection and histologic chorioamnionitis in prematurity. N Engl J Med 1988;319:972–978 [DOI] [PubMed] [Google Scholar]
- 9.Strunk T, Doherty D, Jacques A, et al. Histologic chorioamnionitis is associated with reduced risk of late-onset sepsis in preterm infants. Pediatrics 2012;129:e134–e141 [DOI] [PubMed] [Google Scholar]
- 10.Rocha G Chorioamnionitis and lung injury in preterm newborns. Crit Care Res Pract 2013;2013:890987. doi: 10.1155/2013/890987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anblagan D, Pataky R, Evans MJ, et al. Association between preterm brain injury and exposure to chorioamnionitis during fetal life. Sci Rep 2016;6:37932. doi: 10.1038/srep37932.:37932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Villamor-Martinez E, Cavallaro G, Raffaeli G, et al. Chorioamnionitis as a risk factor for retinopathy of prematurity: An updated systematic review and meta-analysis. PLoS One 2018;13:e0205838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Been JV, Lievense S, Zimmermann LJ, Kramer BW, Wolfs TG. Chorioamnionitis as a risk factor for necrotizing enterocolitis: a systematic review and meta-analysis. J Pediatr 2013;162:236–242 [DOI] [PubMed] [Google Scholar]
- 14.Sung JH, Choi SJ, Oh SY, Roh CR, Kim JH. Revisiting the diagnostic criteria of clinical chorioamnionitis in preterm birth. BJOG 2017;124:775–783 [DOI] [PubMed] [Google Scholar]
- 15.Tasci Y, Dilbaz B, Uzmez OB, et al. The value of cord blood interleukin-6 levels for predicting chorioamnionitis, funisitis and neonatal infection in term premature rupture of membranes. Eur J Obstet Gynecol Reprod Biol 2006;128:34–39 [DOI] [PubMed] [Google Scholar]
- 16.Yoon BH, Romero R, Shim JY, Shim SS, Kim CJ, Jun JK. C-reactive protein in umbilical cord blood: a simple and widely available clinical method to assess the risk of amniotic fluid infection and funisitis. J Matern Fetal Neonatal Med 2003;14:85–90 [DOI] [PubMed] [Google Scholar]
- 17.Yoon BH, Romero R, Park JS, et al. The relationship among inflammatory lesions of the umbilical cord (funisitis), umbilical cord plasma interleukin 6 concentration, amniotic fluid infection, and neonatal sepsis. Am J Obstet Gynecol 2000;183:1124–1129 [DOI] [PubMed] [Google Scholar]
- 18.Been JV, Vanterpool SF, de Rooij JD, et al. A clinical prediction rule for histological chorioamnionitis in preterm newborns. PLoS One 2012;7:e46217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McElrath TF, Hecht JL, Dammann O, et al. Pregnancy disorders that lead to delivery before the 28th week of gestation: an epidemiologic approach to classification. Am J Epidemiol 2008;168:980–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gomez R, Romero R, Ghezzi F, Yoon BH, Mazor M, Berry SM. The fetal inflammatory response syndrome. Am J Obstet Gynecol 1998;179:194–202 [DOI] [PubMed] [Google Scholar]
- 21.Dessardo NS, Mustac E, Dessardo S, et al. Chorioamnionitis and chronic lung disease of prematurity: a path analysis of causality. Am J Perinatol 2012;29:133–140 [DOI] [PubMed] [Google Scholar]
- 22.Malaeb S, Dammann O. Fetal inflammatory response and brain injury in the preterm newborn. J Child Neurol 2009;24:1119–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoon BH, Jun JK, Romero R, et al. Amniotic fluid inflammatory cytokines (interleukin-6, interleukin-1beta, and tumor necrosis factor-alpha), neonatal brain white matter lesions, and cerebral palsy. Am J Obstet Gynecol 1997;177:19–26 [DOI] [PubMed] [Google Scholar]
- 24.Chen ML, Allred EN, Hecht JL, et al. Placenta microbiology and histology and the risk for severe retinopathy of prematurity. Invest Ophthalmol Vis Sci 2011;52:7052–7058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rychlik KA, Sille FCM. Environmental exposures during pregnancy: Mechanistic effects on immunity. Birth Defects Res 2019;111:178–196 [DOI] [PubMed] [Google Scholar]
- 26.Olin A, Henckel E, Chen Y, et al. Stereotypic Immune System Development in Newborn Children. Cell 2018;174:1277–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kallapur SG, Presicce P, Rueda CM, Jobe AH, Chougnet CA. Fetal immune response to chorioamnionitis. Semin Reprod Med 2014;32:56–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Well GTJ, Daalderop LA, Wolfs T, Kramer BW. Human perinatal immunity in physiological conditions and during infection. Mol Cell Pediatr 2017;4:4–0070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim SA, Park KH, Lee SM. Non-Invasive Prediction of Histologic Chorioamnionitis in Women with Preterm Premature Rupture of Membranes. Yonsei Med J 2016;57:461–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rito DC, Viehl LT, Buchanan PM, Haridas S, Koenig JM. Augmented Th17-type immune responses in preterm neonates exposed to histologic chorioamnionitis. Pediatr Res 2017;81:639–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang X, Zhivaki D, Lo-Man R. Unique aspects of the perinatal immune system. Nat Rev Immunol 2017;17:495–507 [DOI] [PubMed] [Google Scholar]
- 32.Yeo KT, Embury P, Anderson T, et al. HIV, Cytomegalovirus, and Malaria Infections during Pregnancy Lead to Inflammation and Shifts in Memory B Cell Subsets in Kenyan Neonates. J Immunol 2019;202:1465–1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meng SS, Gao R, Yan BD, et al. Maternal allergic disease history affects childhood allergy development through impairment of neonatal regulatory T-cells. Respir Res 2016; 17:114–0430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Noutsios GT, Floros J. Childhood asthma: causes, risks, and protective factors; a role of innate immunity. Swiss Med Wkly 2014;144:w14036. doi: 10.4414/smw.2014.14036.eCollection;2014.:w14036 [DOI] [PubMed] [Google Scholar]
- 35.Aye IL, Lager S, Ramirez VI, et al. Increasing maternal body mass index is associated with systemic inflammation in the mother and the activation of distinct placental inflammatory pathways. Biol Reprod 2014;90:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gomez-Lopez N, Romero R, Xu Y, et al. Are amniotic fluid neutrophils in women with intraamniotic infection and/or inflammation of fetal or maternal origin? Am J Obstet Gynecol 2017;217:693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luciano AA, Yu H, Jackson LW, Wolfe LA, Bernstein HB. Preterm labor and chorioamnionitis are associated with neonatal T cell activation. PLoS One 2011;6:e16698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frascoli M, Coniglio L, Witt R, et al. Alloreactive fetal T cells promote uterine contractility in preterm labor via IFN-gamma and TNF-alpha. Sci Transl Med 2018;10:10–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Booth NJ, McQuaid AJ, Sobande T, et al. Different proliferative potential and migratory characteristics of human CD4+ regulatory T cells that express either CD45RA or CD45RO. J Immunol 2010;184:4317–4326 [DOI] [PubMed] [Google Scholar]
- 40.Weitkamp JH, Guthrie SO, Wong HR, Moldawer LL, Baker HV, Wynn JL. Histological chorioamnionitis shapes the neonatal transcriptomic immune response. Early Hum Dev 2016;98:1–6. doi: 10.1016/j.earlhumdev.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mahesh G, Biswas R. MicroRNA-155: A Master Regulator of Inflammation. J Interferon Cytokine Res 2019; 20. doi: 10.1089/jir.2018.0155.:10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu R, Huffaker TB, Kagele DA, et al. MicroRNA-155 confers encephalogenic potential to Th17 cells by promoting effector gene expression. J Immunol 2013;190:5972–5980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gleditsch DD, Shornick LP, Van Steenwinckel J, Gressens P, Weisert RP, Koenig JM. Maternal inflammation modulates infant immune response patterns to viral lung challenge in a murine model. Pediatr Res 2014;76:33–40 [DOI] [PubMed] [Google Scholar]
- 44.Mukherjee S, Allen RM, Lukacs NW, Kunkel SL, Carson WF. STAT3-mediated IL-17 production by postseptic T cells exacerbates viral immunopathology of the lung. Shock 2012;38:515–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kramer BW, Ikegami M, Moss TJ, Nitsos I, Newnham JP, Jobe AH. Endotoxin-induced chorioamnionitis modulates innate immunity of monocytes in preterm sheep. Am J Respir Crit Care Med 2005;171:73–77 [DOI] [PubMed] [Google Scholar]
- 46.Nguyen DN, Thymann T, Goericke-Pesch SK, et al. Prenatal intra-amniotic endotoxin induces fetal gut and lung immune responses and postnatal systemic inflammation in preterm pigs. Am J Pathol 2018;188:2629–2643 [DOI] [PubMed] [Google Scholar]
- 47.Chen ML, Allred EN, Hecht JL, et al. Placenta microbiology and histology and the risk for severe retinopathy of prematurity. Invest Ophthalmol Vis Sci 2011;52:7052–7058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pfisterer C, Faber R, Horn LC. Chorioamnionitis-induced changes of fetal extramedullar hematopoiesis in the second trimester of gestation. Is diagnosis from fetal autopsy possible? Virchows Arch 2005;446:150–156 [DOI] [PubMed] [Google Scholar]
- 49.Miranda RN, Omurtag K, Castellani WJ, De las Casas LE, Quintanilla NM, Kaabipour E. Myelopoiesis in the liver of stillborns with evidence of intrauterine infection. Arch Pathol Lab Med 2006;130:1786–1791 [DOI] [PubMed] [Google Scholar]
- 50.Sampson JE, Theve RP, Blatman RN, et al. Fetal origin of amniotic fluid polymorphonuclear leukocytes. Am J Obstet Gynecol 1997;176:77–81 [DOI] [PubMed] [Google Scholar]
- 51.Andrews WW, Goldenberg RL, Faye-Petersen O, Cliver S, Goepfert AR, Hauth JC. The Alabama Preterm Birth study: polymorphonuclear and mononuclear cell placental infiltrations, other markers of inflammation, and outcomes in 23- to 32-week preterm newborn infants. Am J Obstet Gynecol 2006;195:803–808 [DOI] [PubMed] [Google Scholar]
- 52.Howman RA, Charles AK, Jacques A, et al. Inflammatory and haematological markers in the maternal, umbilical cord and infant circulation in histological chorioamnionitis. PLoS One 2012;7:e51836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cheah FC, Jobe AH, Moss TJ, Newnham JP, Kallapur SG. Oxidative stress in fetal lambs exposed to intra-amniotic endotoxin in a chorioamnionitis model. Pediatr Res 2008;63:274–279 [DOI] [PubMed] [Google Scholar]
- 54.Hikino S, Ohga S, Kinjo T, et al. Tracheal aspirate gene expression in preterm newborns and development of bronchopulmonary dysplasia. Pediatr Int 2012;54:208–214 [DOI] [PubMed] [Google Scholar]
- 55.Hudalla H, Karenberg K, Kuon RJ, Poschl J, Tschada R, Frommhold D. LPS-induced maternal inflammation promotes fetal leukocyte recruitment and prenatal organ infiltration in mice. Pediatr Res 2018;84:757–764 [DOI] [PubMed] [Google Scholar]
- 56.Presicce P, Park CW, Senthamaraikannan P, et al. IL-1 signaling mediates intrauterine inflammation and chorio-decidua neutrophil recruitment and activation. JCI Insight 2018;3:98306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kuypers E, Wolfs TG, Collins JJ, et al. Intraamniotic lipopolysaccharide exposure changes cell populations and structure of the ovine fetal thymus. Reprod Sci 2013;20:946–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schmidt AF, Kannan PS, Kemp MW, et al. Intra-amniotic LPS modulates expression of antimicrobial peptides in the fetal sheep lung. Pediatr Res 2014;76:441–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rueda CM, Presicce P, Jackson CM, et al. Lipopolysaccharide-Induced Chorioamnionitis Promotes IL-1-Dependent Inflammatory FOXP3+ CD4+ T Cells in the Fetal Rhesus Macaque. J Immunol 2016;196:3706–3715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Konwar C, Price EM, Wang LQ, Wilson SL, Terry J, Robinson WP. DNA methylation profiling of acute chorioamnionitis-associated placentas and fetal membranes: insights into epigenetic variation in spontaneous preterm births. Epigenetics Chromatin 2018;11:63–0234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vlassaks E, Gavilanes AW, Bieghs V, et al. Antenatal exposure to chorioamnionitis affects lipid metabolism in 7-week-old sheep. J Dev Orig Health Dis 2012;3:103–110 [DOI] [PubMed] [Google Scholar]
- 62.Bieghs V, Vlassaks E, Custers A, et al. Chorioamnionitis induced hepatic inflammation and disturbed lipid metabolism in fetal sheep. Pediatr Res 2010;68:466–472 [DOI] [PubMed] [Google Scholar]
- 63.Kubes P, Jenne C. Immune Responses in the Liver. Annu Rev Immunol 2018;36:247–277. doi: 10.1146/annurev-immunol-051116-052415. [DOI] [PubMed] [Google Scholar]
- 64.Happel KI, Zheng M, Young E, et al. Cutting edge: roles of Toll-like receptor 4 and IL-23 in IL-17 expression in response to Klebsiella pneumoniae infection. J Immunol 2003;170:4432–4436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huang W, Na L, Fidel PL, Schwarzenberger P. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J Infect Dis 2004;190:624–631 [DOI] [PubMed] [Google Scholar]
- 66.Ishigame H, Kakuta S, Nagai T, et al. Differential roles of interleukin-17A and −17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity 2009;30:108119 [DOI] [PubMed] [Google Scholar]
- 67.Lee Y, Awasthi A, Yosef N, et al. Induction and molecular signature of pathogenic TH17 cells. Nat Immunol 2012;13:991–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jin W, Dong C. IL-17 cytokines in immunity and inflammation. Emerg Microbes Infect 2013;2:e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lawrence SM, Wynn JL. Chorioamnionitis, IL-17A, and fetal origins of neurologic disease. Am J Reprod Immunol 2018;79:e12803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pelletier M, Maggi L, Micheletti A, et al. Evidence for a cross-talk between human neutrophils and Th17 cells. Blood 2010;115:335–343 [DOI] [PubMed] [Google Scholar]
- 71.Figueiredo AS, Schumacher A. The T helper type 17/regulatory T cell paradigm in pregnancy. Immunology 2016;148:13–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee SK, Kim JY, Lee M, Gilman-Sachs A, Kwak-Kim J. Th17 and regulatory T cells in women with recurrent pregnancy loss. Am J Reprod Immunol 2012;67:311–318 [DOI] [PubMed] [Google Scholar]
- 73.Singh AM, Sherenian MG, Kim KY, et al. Fetal cord blood and tissue immune responses to chronic placental inflammation and chorioamnionitis. Allergy Asthma Clin Immunol 2018; 14:66. doi: 10.1186/s13223-018-0297-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rito DC, Viehl LT, Buchanan PM, Haridas S, Koenig JM. Augmented Th17-type immune responses in preterm neonates exposed to histologic chorioamnionitis. Pediatr Res 2017;81:639–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jackson CM, Wells CB, Tabangin ME, Meinzen-Derr J, Jobe AH, Chougnet CA. Pro-inflammatory immune responses in leukocytes of premature infants exposed to maternal chorioamnionitis or funisitis. Pediatr Res 2017;81:384–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huang G, Wang Y, Chi H. Regulation of Th17 cell differentiation by innate immune signals. Cell Mol Immunol 2012;9:287–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Romero R, Sepulveda W, Kenney JS, Archer LE, Allison AC, Sehgal PB. Interleukin 6 determination in the detection of microbial invasion of the amniotic cavity. Ciba Found Symp 1992;167:205–20; discussion 220–3.:205–220 [DOI] [PubMed] [Google Scholar]
- 78.Baud O, Emilie D, Pelletier E, et al. Amniotic fluid concentrations of interleukin-1beta, interleukin-6 and TNF-alpha in chorioamnionitis before 32 weeks of gestation: histological associations and neonatal outcome. Br J Obstet Gynaecol 1999;106:72–77 [DOI] [PubMed] [Google Scholar]
- 79.Lin J, Haridas S, Barenkamp SJ, et al. Neonatal neutrophils stimulated by group B Streptococcus induce a proinflammatory T-helper cell bias. Pediatr Res 2018;83:739–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang W, Shao S, Jiao Z, Guo M, Xu H, Wang S. The Th17/Treg imbalance and cytokine environment in peripheral blood of patients with rheumatoid arthritis. Rheumatol Int 2012;32:887–893 [DOI] [PubMed] [Google Scholar]
- 81.Liu H, Rohowsky-Kochan C. Regulation of IL-17 in human CCR6+ effector memory T cells. J Immunol 2008;180:7948–7957 [DOI] [PubMed] [Google Scholar]
- 82.Stewart CA, Metheny H, Iida N, et al. Interferon-dependent IL-10 production by Tregs limits tumor Th17 inflammation. J Clin Invest 2013;123:4859–4874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhao H, Liao X, Kang Y. Tregs: Where we are and what comes next? Front Immunol 2017. 8:1578. doi: 10.3389/fimmu.2017.01578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rueda CM, Wells CB, Gisslen T, Jobe AH, Kallapur SG, Chougnet CA. Effect of chorioamnionitis on regulatory T cells in moderate/late preterm neonates. Hum Immunol 2015;76:65–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kallapur SG, Presicce P, Senthamaraikannan P, et al. Intra-amniotic IL-1beta induces fetal inflammation in rhesus monkeys and alters the regulatory T cell/IL-17 balance. J Immunol 2013;191:1102–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pandiyan P, Zhu J. Origin and functions of pro-inflammatory cytokine producing Foxp3+ regulatory T cells. Cytokine 2015;76:13–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jung MK, Kwak JE, Shin EC. IL-17A-producing Foxp3(+) regulatory T cells and human diseases. Immune Netw 2017;17:276–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pandiyan P, Zhu J. Origin and functions of pro-inflammatory cytokine producing Foxp3+ regulatory T cells. Cytokine 2015;76:13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yang D, Sun YY, Bhaumik SK, et al. Blocking lymphocyte trafficking with FTY720 prevents inflammation-sensitized hypoxic-ischemic brain injury in newborns. J Neurosci 2014;34:16467–16481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Papotto PH, Ribot JC, Silva-Santos B. IL-17(+) gammadelta T cells as kick-starters of inflammation. Nat Immunol 2017;18:604–611 [DOI] [PubMed] [Google Scholar]
- 91.Lee AJ, Lambermont VA, Pillow JJ, et al. Fetal responses to lipopolysaccharide-induced chorioamnionitis alter immune and airway responses in 7-week-old sheep. Am J Obstet Gynecol 2011;204:364–24 [DOI] [PubMed] [Google Scholar]
- 92.Matsuoka T, Matsubara T, Katayama K, Takeda K, Koga M, Furukawa S. Increase of cord blood cytokine-producing T cells in intrauterine infection. Pediatr Int 2001;43:453–457 [DOI] [PubMed] [Google Scholar]
- 93.Gibbons D, Fleming P, Virasami A, et al. Interleukin-8 (CXCL8) production is a signatory T cell effector function of human newborn infants. Nat Med 2014;20:1206–1210 [DOI] [PubMed] [Google Scholar]
- 94.Toti P, De Felice C, Stumpo M, et al. Acute thymic involution in fetuses and neonates with chorioamnionitis. Hum Pathol 2000;31:1121–1128 [DOI] [PubMed] [Google Scholar]
- 95.Toti P, De Felice C, Occhini R, et al. Spleen depletion in neonatal sepsis and chorioamnionitis. Am J Clin Pathol 2004;122:765–771 [DOI] [PubMed] [Google Scholar]
- 96.Melville JM, Bischof RJ, Meeusen EN, Westover AJ, Moss TJ. Changes in fetal thymic immune cell populations in a sheep model of intrauterine inflammation. Reprod Sci 2012;19:740–747 [DOI] [PubMed] [Google Scholar]
- 97.Lawn SD, Butera ST, Folks TM. Contribution of immune activation to the pathogenesis and transmission of human immunodeficiency virus type 1 infection. Clin Microbiol Rev 2001;14:753–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bernstein HB, Jackson RW, Anderson J, Kinter AL. The effect of elective cesarean delivery and intrapartum infection on fetal lymphocyte activation and susceptibility to HIV infection. Am J Obstet Gynecol 2002;187:1283–1289 [DOI] [PubMed] [Google Scholar]
- 99.De Jong E, Hancock DG, Wells C, et al. Exposure to chorioamnionitis alters the monocyte transcriptional response to the neonatal pathogen Staphylococcus epidermidis. Immunol Cell Biol 2018;96:792–804 [DOI] [PubMed] [Google Scholar]
- 100.Kallapur SG, Jobe AH, Ball MK, et al. Pulmonary and systemic endotoxin tolerance in preterm fetal sheep exposed to chorioamnionitis. J Immunol 2007;179:8491–8499 [DOI] [PubMed] [Google Scholar]
- 101.Kallapur SG, Kramer BW, Knox CL, et al. Chronic fetal exposure to Ureaplasma parvum suppresses innate immune responses in sheep. J Immunol 2011;187:2688–2695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Snyder CC, Wolfe KB, Gisslen T, et al. Modulation of lipopolysaccharide-induced chorioamnionitis by Ureaplasma parvum in sheep. Am J Obstet Gynecol 2013;208:399–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hibbert JE, Currie A, Strunk T. Sepsis-Induced Immunosuppression in Neonates. Front Pediatr 2018;6:357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Azizia M, Lloyd J, Allen M, Klein N, Peebles D. Immune status in very preterm neonates. Pediatrics 2012;129:e967–e974 [DOI] [PubMed] [Google Scholar]
- 105.Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat Rev Immunol 2004;4:553–564 [DOI] [PubMed] [Google Scholar]
- 106.Adkins B Neonatal immunology: responses to pathogenic microorganisms and epigenetics reveal an “immunodiverse” developmental state. Immunol Res 2013;57:246–257 [DOI] [PubMed] [Google Scholar]
- 107.Gussenhoven R, Westerlaken RJJ, Ophelders DRMG, et al. Chorioamnionitis, neuroinflammation, and injury: timing is key in the preterm ovine fetus. J Neuroinflammation 2018; 15:113–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gervassi AL, Horton H. Is Infant Immunity Actively Suppressed or Immature? Virology (Auckl ) 2014;2014:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Koenig JM, Yoder MC. Neonatal neutrophils: the good, the bad, and the ugly. Clin Perinatol 2004;31:39–51 [DOI] [PubMed] [Google Scholar]
- 110.Melvan JN, Bagby GJ, Welsh DA, Nelson S, Zhang P. Neonatal sepsis and neutrophil insufficiencies. Int Rev Immunol 2010;29:315–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Goenka A, Kollmann TR. Development of immunity in early life. J Infect 2015;71 Suppl 1:S112–20. doi: 10.1016/j.jinf.2015.04.027. Epub;%2015 Apr 28.:S112-S120 [DOI] [PubMed] [Google Scholar]
- 112.Kollmann TR, Crabtree J, Rein-Weston A, et al. Neonatal innate TLR-mediated responses are distinct from those of adults. J Immunol 2009;183:7150–7160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Stallmach T, Karolyi L. Augmentation of fetal granulopoiesis with chorioamnionitis during the second trimester of gestation. Hum Pathol 1994;25:244–247 [DOI] [PubMed] [Google Scholar]
- 114.Baniyash M TCR zeta-chain downregulation: curtailing an excessive inflammatory immune response. Nat Rev Immunol 2004;4:675–687 [DOI] [PubMed] [Google Scholar]
- 115.Kollmann TR, Kampmann B, Mazmanian SK, Marchant A, Levy O. Protecting the newborn and young infant from infectious diseases: Lessons from immune ontogeny. Immunity 2017;46:350–363 [DOI] [PubMed] [Google Scholar]
- 116.Gervassi A, Lejarcegui N, Dross S, et al. Myeloid derived suppressor cells are present at high frequency in neonates and suppress in vitro T cell responses. PLoS One 2014;9:e107816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rieber N, Gille C, Kostlin N, et al. Neutrophilic myeloid-derived suppressor cells in cord blood modulate innate and adaptive immune responses. Clin Exp Immunol 2013;174:45–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Raber P, Ochoa AC, Rodriguez PC. Metabolism of L-arginine by myeloid-derived suppressor cells in cancer: mechanisms of T cell suppression and therapeutic perspectives. Immunol Invest 2012;41:614–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Badurdeen S, Mulongo M, Berkley JA. Arginine depletion increases susceptibility to serious infections in preterm newborns. Pediatr Res 2015;77:290–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dedja A, Gucciardi A, Giordano G, et al. Lipopolysaccharide-induced chorioamnionitis and postnatal lung injury: The beneficial effects of L-citrulline in newborn rats. Exp Lung Res 2018;44:226–240 [DOI] [PubMed] [Google Scholar]
- 121.He YM, Li X, Perego M, et al. Transitory presence of myeloid-derived suppressor cells in neonates is critical for control of inflammation. Nat Med 2018;24:224–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kostlin N, Vogelmann M, Spring B, et al. Granulocytic myeloid-derived suppressor cells from human cord blood modulate T-helper cell response towards an anti-inflammatory phenotype. Immunology 2017;152:89–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schwarz J, Scheckenbach V, Kugel H, et al. Granulocytic myeloid-derived suppressor cells (GR-MDSC) accumulate in cord blood of preterm infants and remain elevated during the neonatal period. Clin Exp Immunol 2018;191:328–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ulas T, Pirr S, Fehlhaber B, et al. S100-alarmin-induced innate immune programming protects newborn infants from sepsis. Nat Immunol 2017;18:622–632 [DOI] [PubMed] [Google Scholar]
- 125.Heinemann AS, Pirr S, Fehlhaber B, et al. In neonates S100A8/S100A9 alarmins prevent the expansion of a specific inflammatory monocyte population promoting septic shock. FASEB J 2017;31:1153–1164 [DOI] [PubMed] [Google Scholar]
- 126.Buhimschi CS, Buhimschi IA, Abdel-Razeq S, et al. Proteomic biomarkers of intra-amniotic inflammation: relationship with funisitis and early-onset sepsis in the premature neonate. Pediatr Res 2007;61:318–324 [DOI] [PubMed] [Google Scholar]
- 127.Mold JE, McCune JM. Immunological tolerance during fetal development: from mouse to man. Adv Immunol 2012;115:73–111. doi: 10.1016/B978-0-12-394299-9.00003-5.:73-111 [DOI] [PubMed] [Google Scholar]
- 128.Mold JE, Michaelsson J, Burt TD, et al. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science 2008;322:1562–1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kong X, Sun R, Chen Y, Wei H, Tian Z. gammadeltaT cells drive myeloid-derived suppressor cell-mediated CD8+ T cell exhaustion in hepatitis B virus-induced immunotolerance. J Immunol 2014;193:1645–1653 [DOI] [PubMed] [Google Scholar]
- 130.Esteve-Sole A, Luo Y, Vlagea A, et al. B regulatory cells: Players in pregnancy and early life. Int J Mol Sci 2018; 19:ijms19072099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sun CM, Deriaud E, Leclerc C, Lo-Man R. Upon TLR9 signaling, CD5+ B cells control the IL-12-dependent Th1-priming capacity of neonatal DCs. Immunity 2005;22:467–477 [DOI] [PubMed] [Google Scholar]
- 132.Elahi S, Ertelt JM, Kinder JM, et al. Immunosuppressive CD71+ erythroid cells compromise neonatal host defence against infection. Nature 2013;504:158–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Delyea C, Bozorgmehr N, Koleva P, et al. CD71(+) Erythroid suppressor cells promote fetomaternal tolerance through arginase-2 and PDL-1. J Immunol 2018;200:4044–4058 [DOI] [PubMed] [Google Scholar]
- 134.Been JV, Lievense S, Zimmermann LJ, Kramer BW, Wolfs TG. Chorioamnionitis as a risk factor for necrotizing enterocolitis: a systematic review and meta-analysis. J Pediatr 2013;162:236–242 [DOI] [PubMed] [Google Scholar]
- 135.Collins JJ, Kunzmann S, Kuypers E, et al. Antenatal glucocorticoids counteract LPS changes in TGF-beta pathway and caveolin-1 in ovine fetal lung. Am J Physiol Lung Cell Mol Physiol 2013;304:L438–L444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Collins JJ, Kuypers E, Nitsos I, et al. LPS-induced chorioamnionitis and antenatal corticosteroids modulate Shh signaling in the ovine fetal lung. Am J Physiol Lung Cell Mol Physiol 2012;303:L778–L787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kuypers E, Jellema RK, Ophelders DR, et al. Effects of intra-amniotic lipopolysaccharide and maternal betamethasone on brain inflammation in fetal sheep. PLoS One 2013;8:e81644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kuypers E, Wolfs TG, Collins JJ, et al. Intraamniotic lipopolysaccharide exposure changes cell populations and structure of the ovine fetal thymus. Reprod Sci 2013;20:946–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kramer BW, Ikegami M, Moss TJ, Nitsos I, Newnham JP, Jobe AH. Antenatal betamethasone changes cord blood monocyte responses to endotoxin in preterm lambs. Pediatr Res 2004;55:764–768 [DOI] [PubMed] [Google Scholar]
- 140.McDade TW. Early environments and the ecology of inflammation. Proc Natl Acad Sci U S A 2012;109 Suppl 2:17281–8. doi: 10.1073/pnas.1202244109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.McDade TW, Hoke M, Borja JB, Adair LS, Kuzawa C. Do environments in infancy moderate the association between stress and inflammation in adulthood? Initial evidence from a birth cohort in the Philippines. Brain Behav Immun 2013;31:23–30. doi: 10.1016/j.bbi.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Getahun D, Strickland D, Zeiger RS, et al. Effect of chorioamnionitis on early childhood asthma. Arch Pediatr Adolesc Med 2010;164:187–192 [DOI] [PubMed] [Google Scholar]
- 143.Zhu T, Zhang L, Qu Y, Mu D. Meta-analysis of antenatal infection and risk of asthma and eczema. Medicine (Baltimore) 2016;95:e4671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Belderbos M, Levy O, Bont L. Neonatal innate immunity in allergy development. Curr Opin Pediatr 2009;21:762–769 [DOI] [PubMed] [Google Scholar]
- 145.Kuzawa CW, Tallman PS, Adair LS, Lee N, McDade TW. Inflammatory profiles in the nonpregnant state predict offspring birth weight at Cebu: evidence for inter-generational effects of low grade inflammation. Ann Hum Biol 2012;39:267–274 [DOI] [PMC free article] [PubMed] [Google Scholar]