Abstract
We report a 16-year-old phenotypic female with 46,XY complete gonadal dysgenesis and metastatic dysgerminoma, unexpectedly discovered through direct-to-consumer (DTC) commercial genetic testing. This case underscores the importance of timely interdisciplinary care, including psychosocial intervention and consideration of gonadectomy, to optimize outcomes for individuals with differences of sex development. Her unique presentation highlights the implications of DTC genetic testing in a new diagnostic era, and informs general pediatricians, as well as specialists of non-genetic services, about the value, capabilities and limitations of DTC testing.
Table of Contents Summary:
Novel presentation of a girl with XY chromosomes discovered through direct-to-consumer genetic testing, highlighting importance of interdisciplinary care, and capabilities and limitations of such testing.
Introduction
XY Complete gonadal dysgenesis is a rare genetic difference of sex development (DSD), in which there is abnormal development of gonadal tissue resulting in the risk of malignant transformation of gonadal cells.1 Some aspects of health care for the DSD patient population remain without clear consensus to date, and historical (notably surgical) approaches to management are being challenged.2 In the area of surgical management, the risk of germ cell tumor has been estimated to be 15-35% for patients with gonadal dysgenesis, and early removal of gonads is typically recommended.3
The diagnostic evaluation of people with DSD has also entered a new era that involves improved genetic testing to make or confirm a specific diagnosis. In addition to emerging sequencing and analytic technologies, the new diagnostic era has seen the advent of direct-to-consumer (DTC) genetic testing, and the amount of health data available to the public has increased dramatically in the past few years.4 This diagnostic approach appeals to many who seek information about their ancestry or health, and success (and surprise) stories abound through public media, including social media.4 At the same time, DTC genetic testing raises other concerns, including lack of awareness of the extent and implications of the information that might be revealed, no recommendation for informed consent prior to test initiation, a shortage of genetic counseling support, and inadequate protection of patient privacy.4,5
We report a phenotypic female with XY complete gonadal dysgenesis who presented with stage IV metastatic dysgerminoma. Notably, the workup revealing this tumor was initiated due to the results of the patient’s DTC genetic testing. This case highlights the risk of gonadal cancer development in certain DSD conditions, and the importance of addressing DSD with an interdisciplinary approach in a timely manner.3 This case, to our knowledge, represents a novel report of sex chromosome / gender identity discordance revealed by DTC genetic testing. It also underscores the benefits, along with the complex and potentially life-changing implications, of such testing.
Case Presentation
A 16-year-old girl presented to her pediatrician because of primary amenorrhea and a surprising genetic test result. She had been gifted direct-to-consumer (DTC) commercial genetic testing (23andMe, Mountain View, CA) for her birthday to learn about her ancestry: the result reported an unexpected male sex. The pediatrician ordered a karyotype that was confirmed as 46,XY, and a pelvic ultrasound that revealed a large pelvic mass without visualization of uterus or gonads. She was referred to both the Pediatric Oncology and DSD hospital teams.
She was otherwise healthy. She had been assigned a female gender at birth, and identified herself as female. Her medical history included attention deficit and hyperactivity, generalized anxiety, and major depressive disorders. She was receiving weekly cognitive-behavioral counseling and being treated with an anti-depressive medication. She was performing well at high school and had a group of supportive friends. Her parents were divorced and both were involved in her care.
On examination, she was not dysmorphic, and her height, weight and body mass index were in the expected healthy female range. She had Tanner stage III breast development, but with minimal stimulation of the areolae and glandular tissue. Her external genitalia appeared female with a normal vulva, a normal vaginal opening with a separate urethral opening, and Tanner stage III pubic hair development.
Investigations showed the following serum hormone concentrations: estradiol 3.1 pg/mL (12-277 pg/mL), total testosterone 6 ng/dL (9-58 ng/dL), follicle stimulating hormone 105.8 mIU/mL (<2.5-23.7 mIU/mL), luteinizing hormone 51.2 mIU/mL (<3.0-18.0 mIU/mL), and undetectable anti-Mullerian hormone and inhibin B concentrations; these results were consistent with complete gonadal dysgenesis. A tumor marker panel was significant for elevated alpha-fetoprotein and CA-125 concentrations of 19.1 ng/mL (≤8.0 ng/mL) and 123 U/mL (<35 U/mL), respectively; her remaining tumor markers (beta-hCG, CA-199, carcinoembryonic antigen and inhibin B) were negative. A repeat karyotype was confirmed as 46,XY, with positive SRY fluorescence in situ hybridization. Magnetic resonance imaging, computed tomography and positron emission tomography scans showed a 14 cm pelvic mass with metastases to the pelvic and para-aortic lymph nodes and both lungs (Figure 1). Ultrasound-guided biopsy of the pelvic mass revealed dysgerminoma tissue that appeared to superimpose areas of gonadoblastoma. An examination under anesthesia at the time of the biopsy revealed a hypoestrogenic but otherwise normal vagina, and a small, patent cervix that was anteriorly displaced by the mass. A diagnosis of presumed stage IV dysgerminoma was made based on the presence of lung metastases on imaging (Figure 1). She was treated with two cycles of chemotherapy (bleomycin, etoposide and cisplatin), interval resection of the tumor, followed by two more cycles of chemotherapy. At surgery, a uterus that was small but otherwise normal and pubertal in appearance, normal bilateral fallopian tubes, a left streak gonad, and a large mass in the location of the right gonad were identified. Resection of the right-sided mass, the left streak gonad, and the bilateral fallopian tubes was performed (Figures 2A and B). Her final pathology revealed bilateral “burnt-out” gonadoblastoma and right focally necrotic dysgerminoma featuring primitive germ cells with abundant pale cytoplasm and fairly uniform nuclei (Figure 2C). The left adnexa contained “ovarian-like” stroma, smooth rounded microcalcifications, nests of sex cord derivatives, and Leydig cells (Figure 2D). She had an excellent response to treatment, with radiographic resolution of the metastases and undetectable tumor markers.
Figure 1.
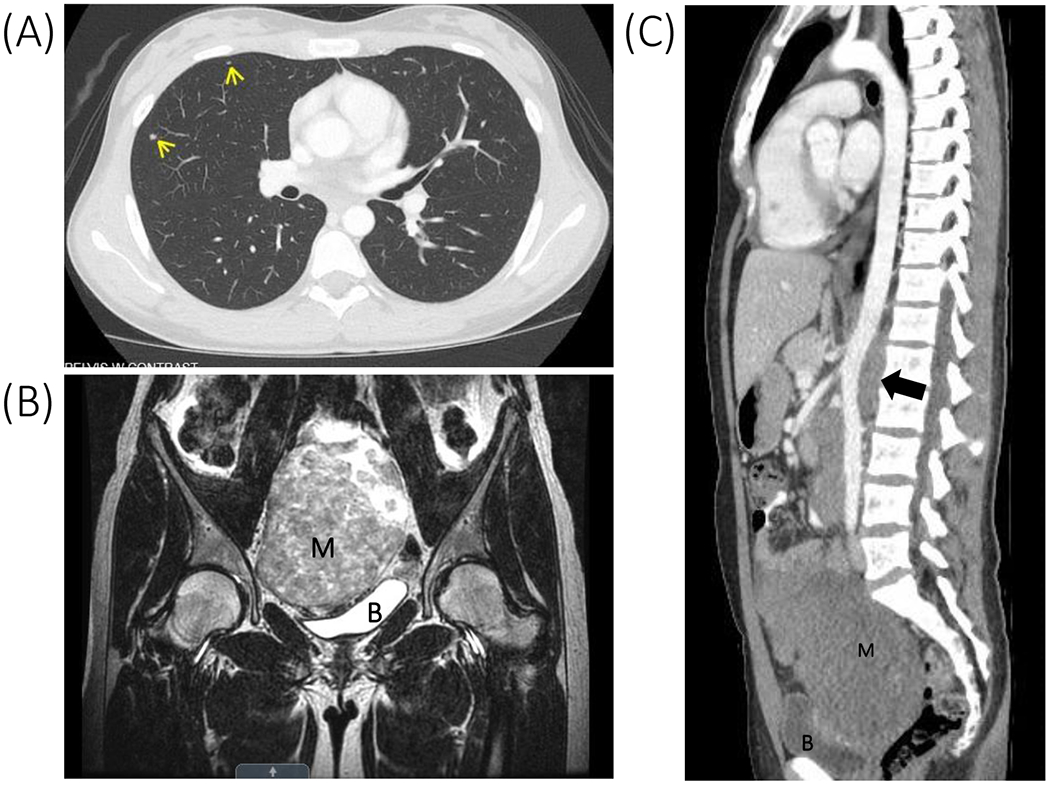
(A) Chest CT demonstrating pulmonary nodules (arrows) consistent with pulmonary metastases. (B) T2-weighted pelvic MRI demonstrating a 14-cm mass (M). Uterus not clearly visible. B represents bladder. (C) Chest/abdomen/pelvis CT demonstrating para-aortic lymph node metastases (arrow) raising abdominal aorta away from lumbar spine. M represents pelvic mass, B represents bladder.
Figure 2.
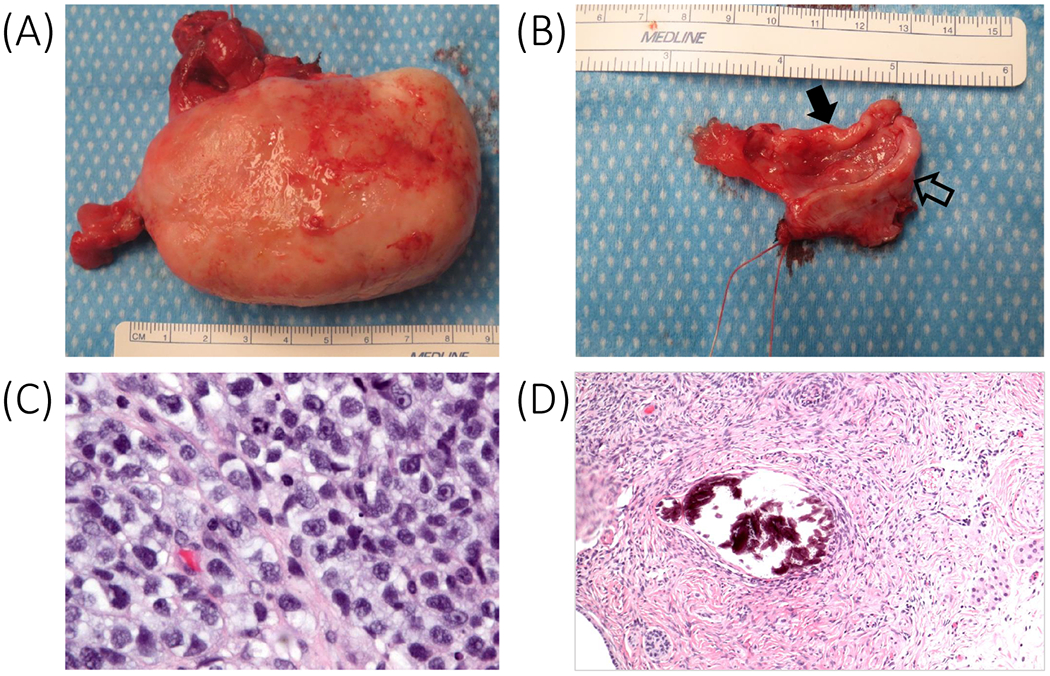
(A) Resected right pelvic mass (initially 14 cm; 9 cm by surgery) and fallopian tube. (B) Resected left fallopian tube (solid arrow) and streak gonad (outlined arrow). (C) Pre-therapy right gonad with dysgerminoma (magnification x40). (D) Post-therapy left gonad with gonadoblastoma, ovarian-type stroma, immature Sertoli and Leydig cells (magnification x20).
In parallel with her tumor management, the patient and her family requested a meeting with the interdisciplinary DSD team, including specialists from Genetics, Endocrinology, Urology, Gynecology, and Psychology, for further DSD education, diagnosis, treatment planning and psychosocial support. A comprehensive genetic panel for 46,XY gonadal dysgenesis revealed a pathogenic hemizygous mutation in the SRY (c.294G>A) gene, confirming the genetic etiology for her DSD. This change results in premature protein termination, as reported previously in a female with XY complete gonadal dysgenesis.6 Information about her condition, including implications for reproductive health and hormone replacement, was reviewed with the patient and her family. They had undertaken extensive online research related to her DSD, and were knowledgeable, but also eager to learn new information. At the time of this initial DSD care conference, both the patient and her parents appeared to be coping well. The patient reported being able to discuss her DSD diagnosis openly with her friends who had been supportive, and she maintained that her diagnosis was important information pertaining to herself, but did not define who she was or change her identity, including her female gender identity. Psychoeducation included differentiating between sex chromosomes, gender identity and sexual orientation. Ongoing DSD psychological services were not deemed necessary due to outpatient counseling services and oncology psychosocial services already in place. It was decided that the patient and her family would focus on her oncology treatment and return to the DSD clinic when chemotherapy was completed.
At her DSD follow-up visit 7 months post-cancer diagnosis, the patient had completed chemotherapy, and estrogen replacement was initiated. From a psychosocial standpoint, she was maintaining her relationships with her friends and had begun to attend school more regularly. She reported a worsening of depression symptoms as she continued to come to terms with her DSD and oncology diagnoses; her apathy was confounded by cancer-related fatigue. She had resumed active counseling with her therapist (which had been intermittent during chemotherapy due to hospitalizations and treatment-related symptoms). She remained free of recurrent tumor with post-treatment surveillance.
Discussion
We report a phenotypic female with XY complete gonadal dysgenesis due to a pathogenic mutation in SRY, who had an unusual presentation with stage IV metastatic dysgerminoma, discovered because of DTC commercial genetic testing. This patient’s case highlights the risk of gonadal tumor development in certain DSD conditions, the importance of appropriately timed discussions addressing DSD (especially psychosocial) aspects of care in the midst of oncology management, and issues raised by an emerging paradigm of genetic diagnosis preceding other clinical information. This case has important lessons for primary care and specialist providers and teams, and implications for public consumers of DTC testing.
XY Complete gonadal dysgenesis is a rare condition caused by a defect in the normal differentiation of testicular cells, and can lead to subsequent transformation into germ cell cancer,1 as strikingly illustrated by our case. It is critical that the diagnosis of gonadal dysgenesis be made early to ensure optimal care, including prophylactic gonadectomy, hormone replacement, fertility and reproductive counseling, and psychosocial intervention.3,7 Achieving a definitive diagnosis also enables and enhances comprehensive care and psychosocial adaptation. In an era when indications for irreversible surgery, particularly decisions regarding gonadectomy, in patients with DSD are being challenged,2,3 our patient highlights the real risk for gonadal malignancy and its sequelae. The options and risks should be openly discussed, with consideration of appropriately-timed prophylactic gonadectomy in individuals with this diagnosis,3 and development of an informed plan that respects the patient’s / parents’ preferences. This case also serves to remind the medical community, including primary pediatricians and pediatric specialists, about the importance of timely evaluation for girls who do not enter or progress through puberty normally. In this case, the family had already planned a near-future workup with the pediatrician regarding the patient’s primary amenorrhea, which is within the standard of care.8 However, given the manner in which the events unfolded, the patient attributed the revelation of her diagnosis to DTC genetic testing, which she felt had saved her life.
Despite the patient’s oncologic diagnosis and management, it was vital to not overlook the interdisciplinary care for her DSD, in particular the assessment and intervention for her psychosocial needs. She had baseline psychosocial concerns that were escalated by her cancer and DSD diagnoses. Although she was receiving ongoing outpatient and inpatient psychosocial services through the oncology division, the focused assessment and input of a psychologist with expertise in DSD was essential, in particular to address interpersonal information-sharing and gender-related concerns. Given the developmental timing of these diagnoses, numerous potential psychosocial concerns warranted ongoing assessment and intervention, including support around the impact of both the DSD and oncology treatments on fertility, navigating intimate relationships, and the cascading effect of multiple diagnoses on the development of trauma symptoms.7 In addition, providing coping interventions to the patient’s parents was important to enhance the quality of family support.7
DTC testing is quite popular and clearly has raised awareness of genetics as a powerful influence on people’s lives. This supports some degree of value to the potential consumer. This should be acknowledged and addressed in discussion around genetic testing for general interest and as applied to health maintenance. Nevertheless, the unexpected result from our patient’s commercial DTC testing underscores potential issues surrounding the consumer’s understanding of the capabilities and limitations of the test. Individuals are required to agree to the company’s terms of service prior to testing by clicking on a box within the website, a “click-wrap” agreement to an online contract. The information provided is vague in describing possible medical diagnoses that could be revealed. Furthermore, the terms of service are lengthy, written at reading levels above that recommended for consumer health literacy, and therefore difficult to read and understand. Although the terms of service must be agreed to by someone over the age of 18, this could be on behalf of a (younger) person for whom the adult has legal authorization and who may provide a sample. In our patient’s case, her father consented for the testing, and received email notification that his child’s results were ready for review. The patient’s father noticed that the report referenced “your son”, without other explanation. When he contacted the company to question this, the company verified the result and suggested further discussion with a medical professional.
People may choose to undergo DTC genetic testing to find out more information about their family or ancestry,9 to determine their risk for certain health conditions, or determine their carrier status for certain diseases. Many consumers are not aware of the potential for unexpected findings from the testing, or implications regarding privacy of their genetic information. For example, while DTC tests can provide clues for someone’s ancestry, this report can change over time as the company updates the information. This can lead to confusion in identity for the consumer.9 Further, DTC ancestry testing can uncover non-paternity, or children born from affairs that were unknown to the family, which consumers may not be prepared to discover and could disrupt family dynamics. Similarly, regarding health information that DTC testing provides, consumers and providers may not understand the limitations of testing.4 For example, while DTC testing offers screening for genes that confer increased risk of breast cancer, only three mutations in the BRCA genes that are common in the Ashkenazi Jewish population are currently tested. A negative result may falsely reassure a consumer or provider about a patient’s risk of development of cancer.10 One study evaluating screening for hereditary breast and ovarian cancers by DTC testing recommended that, regardless of a positive or negative result, confirmatory clinical testing should be performed.10 Finally, the privacy of an individual’s (including child’s) genetic information may not be fully protected (for example, if they seek disability or life insurance, or enter the military) and can be shared with law enforcement agencies.
It is clear from the popularity of DTC testing that the service has value to consumers. It is less clear whether the service is being used primarily out of curiosity or for health-related questions. DTC companies offer genetic counseling for their customers to discuss their testing results. However, these services have not promoted pretest counseling and the availability of counseling may not be known of by the consumers. As in our case, consumers are often encouraged to reach out to their primary physicians for help in interpreting the genetic findings. This can be intimidating for the provider who may have little experience with interpreting genetic tests11. One study evaluating primary care physicians’ comfort levels with DTC genetic testing revealed that most providers did not feel confident in their ability to help patients understand these results. This confidence level increased significantly after completing DTC testing themselves, reflecting an increase in familiarity with the testing, its limitations and results. However, pre- and post-test evaluation of genetics knowledge did not change, highlighting the need for continuing education in genomics for primary care providers to ensure proper patient counseling with regards to DTC testing.
Our patient exemplifies an ongoing call for broader discussions as to whether online “click-wrap” terms of use agreements and privacy policies are appropriate in cases that may involve actionable or clinically important genetic information.12 We recommend that DTC genetic testing companies clearly delineate potentially important considerations about the results to consumers prior to proceeding with testing, and that this be included in a formal request for pretest informed consent that explicitly lists the kinds of information that may be reported. It is also important that health care providers, including primary care pediatricians and subspecialists of non-genetic services, be aware of the limitations of DTC genetic testing, specifically that the list of health problems screened or carrier testing performed by DTC testing is not exhaustive, that the testing has very incomplete coverage even for the problems listed for screening, and that health screening results on DTC testing should be clinically confirmed. Finally, we recommend that questions on DTC genetic testing results be referred to local genetic providers for complete discussion.
Acknowledgments
We would like to thank our patient and her family for reviewing and agreeing to the publication of this manuscript, and for what they have taught (and continue to teach) us. This work was supported in part by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number R01HD093450-01A1.
Funding Source: This work was supported in part by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number R01HD093450-01A1.
Financial Disclosure Statement: The authors have no financial relationships relevant to this article to disclose.
Abbreviations:
- DSD
Difference(s) of sex development
- DTC
Direct-to-consumer
Footnotes
Conflict of Interest Statement: The authors have no conflicts of interest relevant to this article to disclose.
References
- 1.Wong YS, Tam YH, Pang KKY, et al. Clinical heterogeneity in children with gonadal dysgenesis associated with non-mosaic 46,XY karyotype. J Pediatr Urol. 2017;13(5):508 e501–508 e506. [DOI] [PubMed] [Google Scholar]
- 2.HUMAN RIGHTS WATCH. “I Want to Be Like Nature Made Me” Medically Unnecessary Surgeries on Intersex Children in the US. https://interactadvocatesorg/. 2017.
- 3.Looijenga LH, Hersmus R, Oosterhuis JW, Cools M, Drop SL, Wolffenbuttel KP. Tumor risk in disorders of sex development (DSD). Best Pract Res Clin Endocrinol Metab. 2007;21(3):480–495. [DOI] [PubMed] [Google Scholar]
- 4.Crow D A New Wave of Genomics for All. Cell. 2019;177(1):5–7. [DOI] [PubMed] [Google Scholar]
- 5.Hendricks-Sturrup RM, Prince AER, Lu CY. Direct-to-Consumer Genetic Testing and Potential Loopholes in Protecting Consumer Privacy and Nondiscrimination. JAMA. 2019;321(19):1869–1870. [DOI] [PubMed] [Google Scholar]
- 6.Paliwal P, Sharma A, Birla S, Kriplani A, Khadgawat R, Sharma A. Identification of novel SRY mutations and SF1 (NR5A1) changes in patients with pure gonadal dysgenesis and 46,XY karyotype. Mol Hum Reprod. 2011;17(6):372–378. [DOI] [PubMed] [Google Scholar]
- 7.Sandberg DE, Gardner M, Cohen-Kettenis PT. Psychological aspects of the treatment of patients with disorders of sex development. Semin Reprod Med. 2012;30(5):443–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Care CoAH. ACOG Committee Opinion No. 651: Menstruation in Girls and Adolescents: Using the Menstrual Cycle as a Vital Sign. Obstet Gynecol. 2015;126(6):e143–146. [DOI] [PubMed] [Google Scholar]
- 9.Padawer R Decoding the story of yourself. The New York Times Magazine; November 18, 2018. [Google Scholar]
- 10.Espli ED, Haverfield E, Yang S, et al. Limitations of HBOC Direct-To-Consumer Genetic Screening: False Positives, False Negatives and Everything in Between. Cancer Research. 2019;79(4):Supplement. [Google Scholar]
- 11.Haga SB, Kim E, Myers RA, Ginsburg GS. Primary Care Physicians’ Knowledge, Attitudes, and Experience with Personal Genetic Testing. J Pers Med. 2019;9(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hendricks-Sturrup RM, Lu CY. Direct-to-Consumer Genetic Testing Data Privacy: Key Concerns and Recommendations Based on Consumer Perspectives. J Pers Med. 2019;9(2). [DOI] [PMC free article] [PubMed] [Google Scholar]


