Abstract
Recent advancements in the sensitivity of chemical instrumentation have led to increased interest in the use of microsamples for translational and biomedical research. Paper substrates are by far the most widely used media for biofluid collection, and mass spectrometry is the preferred method of analysis of the resultant dried blood spot (DBS) samples. Although there have been a variety of review papers published on DBS, there has been no attempt to unify the century old DBS methodology with modern applications utilizing modified paper and paper-based microfluidics for sampling, storage, processing, and analysis. This critical review will discuss how mass spectrometry (MS) has expanded the utility of paper substrates from sample collection and storage, to direct complex mixture analysis to on-surface reaction monitoring.
Keywords: microsampling, dried blood spot (DBS), paper spray (PS), ambient ionization, direct analysis, mass spectrometry, solid-phase extraction, microfluidic devices, direct reaction monitoring by paper spray
INTRODUCTION
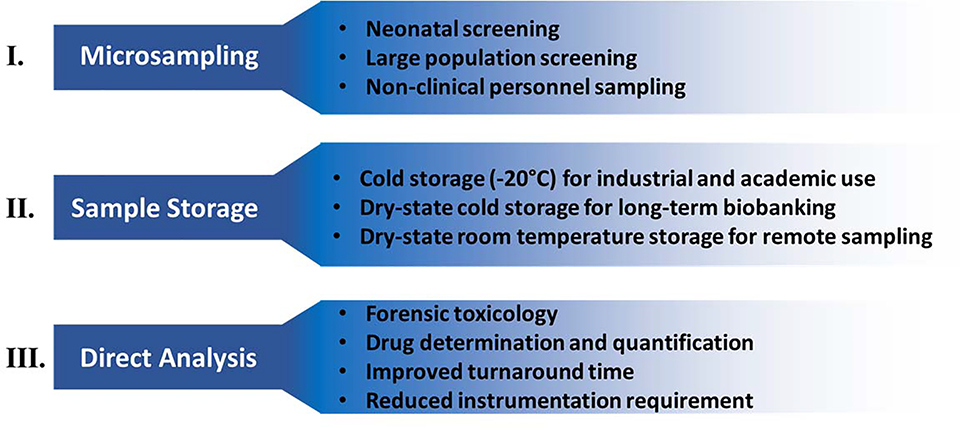
Paper substrates have been employed in biomedical research for several decades. Although dated, neonatal screening programs still heavily rely on paper for small blood collection, storage, and shipping. Paper is particularly suitable for remote sampling due to its unique characteristics, such as 1) the ease with which sample is collected, 2) its low cost and light weight, making it convenient for use in resource-limited settings, 3) ability to stabilize samples in the dry-state, which significantly reduces shipping and biohazard requirements, 4) ease of storage of the dried samples, and 5) the fact paper can be disposed of readily (via incineration or biodegradation) without environmental concerns. Recently, applications of paper have expanded beyond neonatal purposes to include precision medicine, forensic toxicology, population screenings for disease, and illicit drug detection. This expansion is largely due to simplified storage strategies in cold or ambient temperatures and the ability to analyze these samples with mass spectrometry (MS) through offline or online extraction, as outlined in Figure 1.
Figure 1.
Summary of current applications of paper including the impact with respect to microsampling, sample storage, and analysis.
The concept of microsampling (collection of <50 μL of biofluid) is an attractive alternative to venipuncture because it increases accessibility to populations that would otherwise have been overlooked in traditional large volume sampling. In particular, microsampling platforms that offer dry-state room temperature storage (e.g., dried blood spots and volumetric absorption microsampling) have been recognized as playing important roles in sustainable biobanking by 1) reducing operational cost via the elimination of cold storage and 2) creating social acceptance due to its potential to reduce bias sampling, which often culminate in convenience samples (selection of people who are easy to reach) and/or voluntary response samples (which favors people with strong interest in the subject). Recent focus on ease-of-use is demonstrated by commercialized self-contained microsampling devices, such as Shimadzu’s MSW2 (Shimadzu, 2017) and Trajan’s hemaPEN (Florian Lapierre et al.; Konstantinos Kouremenos et al.). Storage of liquid microsamples (blood, serum and plasma) is not commonly done; blood samples are typically diluted with water before freezing to lyse red blood cells and to facilitate downstream sample handling by reducing viscosity. This practice has been adopted for capillary microsampling (Spreadborough et al., 2013; White et al., 2014). Traditionally, analysis of stored samples requires significant offline sample processing, including protein precipitation, and centrifugation before subjecting the extracted sample to liquid chromatography (LC) mass spectrometry (MS) or gas chromatography (GC) MS characterizations. Dilution steps are also necessary to convert the microsample into a form that is compatible with large-volume analytical techniques. The use of MS offsets the intrinsic decrease in sensitivity associated sample dilution.
Recently, direct analysis of complex biological samples has been proposed to transcend tedious sample preparation procedures through an experiment known as ambient mass spectrometry. Specific examples of ambient ionization techniques include desorption electrospray ionization (DESI) (Takats, 2004; Takáts et al., 2005; Chernetsova and Morlock, 2011) and direct analysis in real time (DART) (Cody et al., 2005; Chernetsova and Morlock, 2011), which both can be paired with dried or fresh biofluid samples. The evolution of ambient ionization has been extensive discussed in several recent reviews (Alberici et al., 2010; Huang et al., 2011; Monge et al., 2013; Lebedev, 2015; Javanshad and Venter, 2017; Shelley et al., 2018; Feider et al., 2019). Overall, all ambient ionization techniques can be described by three distinguishing features: 1) sampling and ionization is performed outside of the mass spectrometer allowing full access and control of sample during analysis, 2) analysis is done in the native state of the sample and eliminates all preparation steps that are required in traditional analytical methods, and most importantly, 3) the analyte of interest (as opposed to the whole sample) is transferred into the mass spectrometer which significantly reduces matrix effects during ionization. Some notable techniques that integrate both sampling and ambient ionization include paper spray (Wang et al., 2010), touch spray (Kerian et al., 2014), thread spray (Jackson et al., 2018; Swiner et al., 2019), and blade spray (Gómez-Ríos and Pawliszyn, 2014). These techniques rely on sample collection directly on the substrate-based platform followed by elution of the target analyte with a spray solvent.
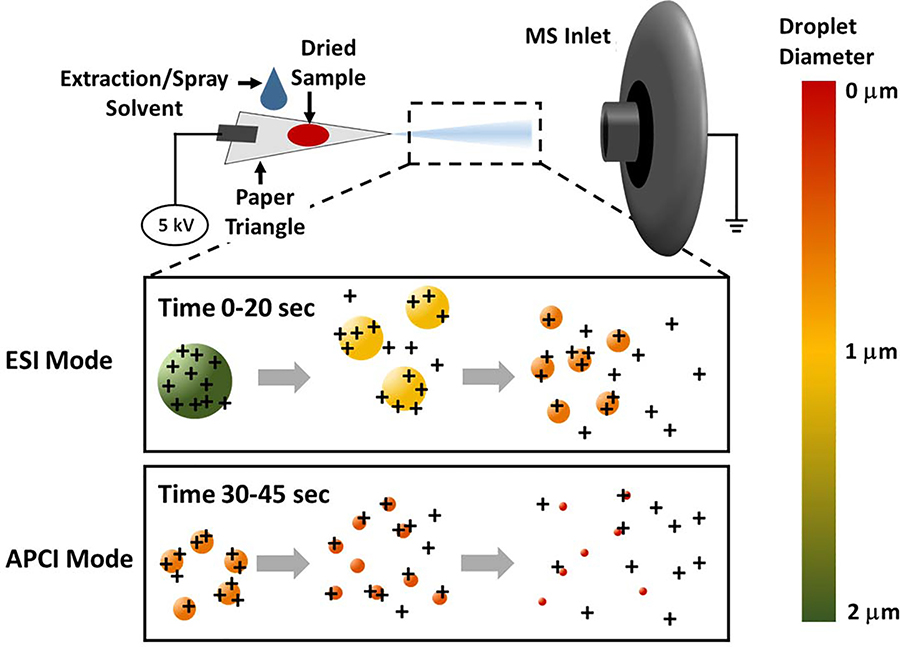
The current review focuses mostly on paper spray (PS) and its related substrate-based ambient ionization methods. Our objective is to provide adequate guidance both to the mass spectrometrists who wish to utilize the PS methodology in the field and to the application scientists considering adopting this method in bioanalysis, by describing practical challenges associated with PS and some recent approaches developed to improve its performance. PS is a more recent ambient ionization method which rapidly increased in popularity because of its simplicity, requiring no pneumatic assistance for ionization. PS MS was first reported by Cooks, Ouyang & co-workers in 2010 (Wang et al., 2010) and was first demonstrated by the analysis of therapeutic drugs in dried blood spots (DBS). In its simplest form, PS is performed by applying an extraction/spray solvent to a paper triangle that contains the dried sample (Figure 2). The solvent performs an online solid/liquid extraction. Simultaneously, a high DC voltage (typically 3–5 kV) is applied to the back-end of the paper through an alligator clip or electrode. Charges accumulate at the tiny tip of the paper triangle, and because the paper substrate is placed in front of the mass spectrometer inlet held at a ground (or other lower potential), charged droplets are generated from the applied solvent through the formation of a Taylor cone. Through this electrospray process, molecules extracted from the sample present on the paper are ionized and subsequently characterized by the mass spectrometer.
Figure 2.
Schematic for paper spray. Experiments show that when solvent is first applied to the paper and readily available, ESI mode results in large droplets and lower spray currents. As time progresses, APCI mode results in smaller diameter droplets and larger spray currents, possibly accessing alternative ionization routes.
Two spray modes of ionization can be distinguished in PS experiments: electrospray ionization (ESI) and atmospheric pressure chemical ionization (APCI) modes (inserts, Figure 2). (Espy et al., 2012b) ESI spray mode occurs at the beginning of the PS process (~0 – 10 s of spray time for a methanol/water system) where solvent flowrate is high and the resultant charged droplets are bigger (~1 – 2 μm). As the spray solvent evaporates, the initial droplets emitted from the tip of the paper triangle become smaller (<1 μm) resulting in concomitant decrease in droplet pH. At the same time, the reduced solvent flowrate from a paper triangle held at high DC voltage causes corona discharge, which is marked by a sharp increase in current. In most cases, the latter part of the PS is to be avoided, but in some cases, the onset of corona discharge can facilitate the detection of non-polar compounds. In this instance, higher voltages (>5 kV) are used to induced the APCI spray mode even at high solvent flowrates (Kim et al., 2017). As spray voltage is increased, the total current increases. The highest current has been observed for a macroscopic tip of 30°. Spray voltages exceeding 6 kV typically lead to severe corona discharge (Yang et al., 2012b). At the tip of the paper, there is a potential for field ionization/desorption to occur when the field strength is >107 Vcm-1. Field ionization can lead to field-enhanced electrospray, in which a single Taylor cone-jet coincides with corona discharge, giving rise to ionic species resembling those from APCI (Espy et al., 2012b). Corona discharge may also occur at lower spray voltages (~2 kV) due to field-assisted evaporation of non-polar solvents. This has been attributed to the lower surface tension of the non-polar solvents, which form dendrites that may aid in field desorption and ionization of the analyte (Li et al., 2011).
The nature of the spray mode (e.g., ESI or APCI) and duration of the signal ion are impacted by various experimental parameters such as paper type, choice of solvent and volume (continuous or in aliquots), or applied voltage. Based on different surface interactions between the analyte in sample and paper substrate type, elution efficiency and sensitivity will vary (Ren et al., 2013). By varying solvent type, the transportation process and ionization step of the analyte can be affected. When utilizing solvent mixtures containing non- or less-polar solvent component of low boiling point, droplets of a more suitable size are formed, thus facilitating further processes of desolvation (Zhang et al., 2012c). An ample amount of solvent should be added such that the entire paper surface is covered/wetted. If the solvent evaporates, there is a potential for discharge to occur (presuming that the triangle is still moist enough to be conductive) (Manicke et al., 2011b). More volatile solvents such as hexanes will only persist for a few seconds on the paper, with the resulting droplets being extremely small. Less volatile solvents, such as dioxane, produce larger droplets, which was attributed to reduced affinity between solvent and paper substrate (Espy et al., 2012b). Differences in solvent properties necessitates the need to optimize applied spray voltage to maximize the ionization efficiency. Typically, the size of droplets in the resultant spray plume increases as the spray voltage is increased, so voltages below the spray onset and further beyond are not optimal for ionization (Kim et al., 2018b).
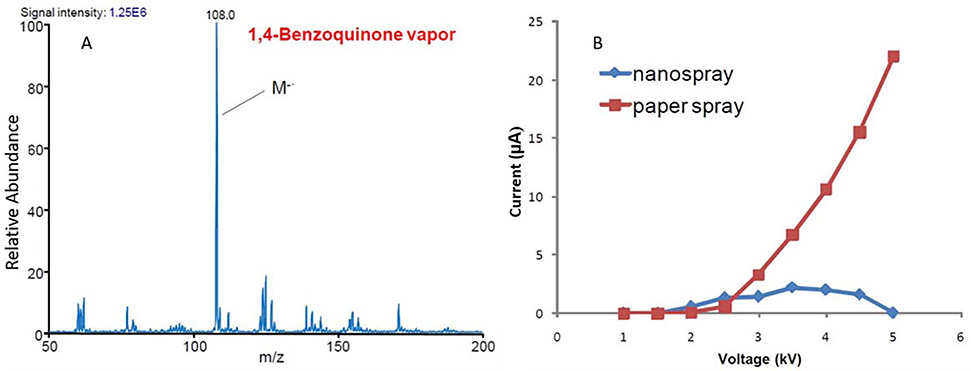
A third mechanism of ion formation in PS has been identified to involve gas-phase species that differs from APCI. At higher negative voltages, it was observed that both vapor phase electron capture and corona discharge occurred when methanol/water (1:1, vol./vol.) is used as the spray solvent (Wang et al., 2010). The Taylor cone formation during this vapor phase electron capture process was characterized by monitoring the current generated as a function of increasing voltage (Figure 3). Spray solvent with higher conductivity (due to electrolytes) gave rise to Taylor cones with a higher current and less viscous solvents (compared to water) gave rise to Taylor cones more easily (Aliaga-Aguilar, 2018).
Figure 3.
(A) Ion intensity of vapor-phase electron capture agent 1,4-Benzoquinone when a voltage of −4 kV is applied. (B) Spray current as a function of increasing voltage. Stable spray plumes were unable to form at higher voltages for PS. (Reprinted with permission from Wang et al., 2010, copyright 2010 John Wiley and Sons).
PS MS is convenient, as paper has natural wicking capabilities, allowing the solvent to spread without any pneumatic devices or pumps. With no nebulizer gases required for its operation, PS can more readily be used with portable MS in the field (Silva et al., 2019). Additionally, the paper’s cellulose fibers act as a stationary phase, suppressing matrix effects from complex samples such as blood or serum. Analysis by PS MS requires little or no sample preparation and the entire full MS or tandem mass spectrometry (MS/MS) experiment can be completed within seconds (< 1 minute). In comparison to other ambient ionization methods, PS integrates three analytical procedures: sample collection, separation, and ionization into a single experimental step, making it more attractive for rapid and direct analysis of analyte(s) in complex mixtures.
In terms of performance, PS MS has been characterized by different types of fibrous substrates (glass fiber, wool and chromatography filter paper), the pore size present in the paper substrate, and various classes of molecules such as drugs of abuse, proteins, and steroids to show versatility of the technique. Initial studies using cocaine as a model system for substrate type showed that glass wool exhibited poorest performance, while the best performance was obtained with chromatography filter paper. From these results, chromatography paper was selected for further investigations. With regards to varying spray solvent volume, it was determined that analysis time is maximized by fully wetting the triangle. The position of the macroscopic tip has been tested by using a 2D mounting stage. It was shown that the position of the tip may be in a 5 × 10 mm area (x by y) in front of the MS inlet (Liu et al., 2010). Chromatographic effects and on-paper separation of molecules have been studied using various dyes and hemoglobin. When the paper substrate is modified, the interaction between analyte and cellulose matrix may become altered, affecting the extraction efficiency. Elution and extraction profiles were also characterized, which showed that when spray solvent is added all at once, little to no separation occurs, whereas wicking of spray solvent (in smaller aliquots) allowed for elution of separate dye molecules. Depending on the nature of the applied spray solvent, ion suppression could be reduced or enhanced (Ren et al., 2013; Aliaga-Aguilar, 2018). Investigations concerning the geometry of the triangle tip (intervals of 30° to 150°) suggested that the diameter of the spray plume decreases as angle is increased. The total MS ion current was highest for the 30° tip (Yang et al., 2012b).
Further investigations into the paper substrate have been conducted to determine the effects of paper properties such as pore size on PS MS performance. The flow rate is directly impacted by the pore size. Slower chromatographic flow rates were obtained on smaller pore sizes. A decrease in recovery and increase in ionization efficiency was found to correlate with the filtration effectiveness of the paper, which is a function of the pore size. Other studies have suggested that ion suppression is highly compound- and matrix- dependent. Those analytes which ionize poorly exhibit high suppression, while suppression was much lower for those which ionize well (Vega et al., 2016). The paper thickness could also affect solvent transportation, allowing for greater interaction of the analyte and matrix (Bills et al., 2018). Different types of natural and synthetic paper fibers have been proposed to enhance the limit of detection (LOD) for molecules. Due to the thin and rigid structure of paper such as gampi paper, the sample evaporates quicker than it can absorb into the substrate, thus liberating a greater number of ions in a shorter amount of time during ionization (Lai et al., 2015).
PS has since been demonstrated for the analysis of a wide range of samples including biofluids (Manicke et al., 2011a; Espy et al., 2012a; Yang et al., 2012a), bio-tissues (Wang et al., 2011), protein complexes (Zhang et al., 2014), foodstuffs (Zhang et al., 2012b; Mazzotti et al., 2013; Taverna et al., 2013), beverages (Deng and Yang, 2013; Li et al., 2013), bacteria (Hamid et al., 2014), and biocides (Reeber et al., 2015). In addition to the wide range of applications, paper’s low cost and ease of use have led researchers to modify its properties to become more suited for selected specialized applications. PS has undergone various developments such as (but not limited to) covalently bond molecules for differential extraction, binding, and desorption; altered surface energy; metals or nanoparticles non-covalently bound to the surface or impregnated within the paper fibers; on-surface reactions for specificity in trace detection; high throughput implementation (Shen et al., 2013); and integration with solid phase extraction (Zhang and Manicke, 2015).
In the following sections of this review, we discuss the current usage of paper in sample collection, storage and direct analysis. We provide advances made possible via modified paper substrates, which are useful for online sample processing and reaction monitoring, all occurring during ionization enabling effective analysis and characterization by the mass spectrometer.
MICROSAMPLING
A. Biofluids on Paper
Paper-based substrates have become important tools for the analysis of both large and small molecules in complex mixtures. In the clinical setting, DBS have been extensively used for disease screening, toxicological studies, and precision medicine in the analysis of DNA (Lee and Li, 2014). DBS are created by depositing a small amount of blood (microliters) onto filter paper, which is then dried under ambient conditions (Guthrie and Susi, 1963). DBS are minimally invasive to the patient because it requires only micro-volumes of blood in contrast to venipuncture sample collections that require tens of milliliters. A simple prick of the finger or heel provides enough volume of sample, avoiding the need for use of an intravenous needle. Paper substrate allows for cheap and convenient sample collection, storage, and transportation of the DBS (Hamers; McDade et al., 2007; Elliott et al., 2014). These samples are analyzed by taking a partial punch followed by digestion and analysis via polymerase chain reaction (PCR), chromatography, electrophoresis, tandem mass spectrometry, and/or immunological assays (Johannessen, 2010; Melgaço et al., 2011; Wagner et al., 2016).
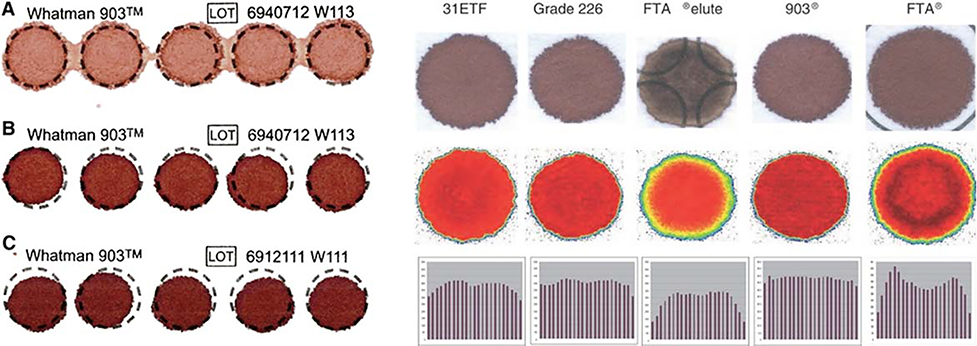
There are several challenges to performing quantitative bioanalysis of DBS. For example, the blood drying process differs greatly when hematocrit levels vary, resulting in fluctuating rates of absorption and diffusion, giving rise to a multitude of effects: (i) wetting area discrepancies, resulting in punch volume deviation; (ii) chromatographic effects (e.g., inhomogeneity throughout the spot); (iii) and analyte recovery (Zimmermann, 1939; Adam et al., 2000; Mei et al., 2001; Holub et al., 2006; Vu et al., 2011; Cobb et al., 2013; Hannon and Clinical and Laboratory Standards Institute, 2013). In many cases, concentrations of analytes are greater around the edge of the DBS due to the high rate of evaporation of water around the edges (Figure 4). As hematocrit levels increase, the resultant effect, known as the ‘volcano effect’ or ‘coffee-stain effect’, is less pronounce (Youhnovski et al., 2011; Fan and Lee, 2012; Lenk et al., 2015).
Figure 4.
(Left) Hematocrit effect: various blood samples containing different hematocrit levels spotted on the same type of paper substrate exhibit different drying properties. (Reprinted with permission from Wilhelm et. al, 2014, copyright 2014 Wilhelm et. al, 2014) (Right) Chromatographic effect: Top: Whole blood spiked with 14C radiolabeled Compound A from Isotope Chemistry and Metabolite Synthesis, sanofi-aventis, spotted on various types of paper substrate storage cards. Bottom: Autoradiogram of 14C distribution in each DBS. (Reprinted with permission from Ren et al., 2010, copyright 2010 Future Science Ltd.)
Blood is more viscous when the hematocrit level is high, which causes the blood to wick only slightly and take longer to dry. Blood wicks further and dries quicker at lower viscosity/hematocrit levels. The analytes contained within each of the aforementioned cases therefore have different rates of diffusion (Cobb et al., 2013). Several studies have shown the influence of filter paper type on the collection of blood due to various interactions with the blood matrix, which can further intensify the differences in concentrations of samples (Partington and Sinnott, 1964; Elliott et al., 2014). These factors are especially important when the entire blood spot is not sampled, and only a center punch is analyzed. As a consequence, a quality assurance program has been developed by the Centers for Disease Control, listing standard protocol for blood collection and use of certain U.S. Food and Drug Administration (FDA)-approved grades of chromatography papers (Mei et al., 2010). Humidity, the presence of anticoagulants, and origin of blood are also important factors to consider for DBS analysis method development (Greenland et al.; Cernik and Sayers, 1971; Holodniy et al., 1991; Kayiran et al., 2003; Adam et al., 2011; Lee and Li, 2014).
Hematocrit effects are unavoidable and differ with each patient. The hematocrit level, however, can be predicted and determined to correct for these effects. Pre-cut DBS have been utilized to perform an extraction of the whole sample rather than punch-outs, reducing chromatographic effects by analyzing a fixed blood volume (Youhnovski et al., 2011). A recent non-destructive technique, noncontact diffuse reflectance spectroscopy, was used to predict the hematocrit level based upon the hemoglobin content in a blood matrix. This technique relies on the spectral changes that occurs in hemoglobin upon aging, which arises due to oxidation of oxyhemoglobin into methemoglobin, followed by subsequent denaturation of methemoglobin into hemichrome. The sum of the signal response for these three forms of hemoglobin will remain constant over time and yields the quantitative amount of hematocrit in the blood sample. Volume corrections can then be applied to DBS volume calculations if the hematocrit level is known (Capiau et al., 2016). Other studies have utilized the concentration of potassium ions in whole blood. The potassium ion concentration in whole blood has been shown to directly correlate with the hematocrit level and does not widely vary amongst individuals. Additionally, the stability of potassium under ambient conditions allows for analysis of both old and newer samples without large deviation. The location of the punch did not significantly impact the concentration of potassium, allowing for an accurate prediction of hematocrit to be made. Prediction of hematocrit using potassium ion concentrations requires extensive sample preparation followed by analysis via potentiometry and hematology, so the former techniques are preferred (Capiau et al., 2013; De Kesel et al., 2014). Further attempts at correcting wetting area include the decrease of absorption of blood into the paper by chemical treatment. By treating the paper to decrease its surface energy and to make it hydrophobic, blood instead dries on the paper at a reproducible area independent of the hematocrit value (Damon et al., 2018).
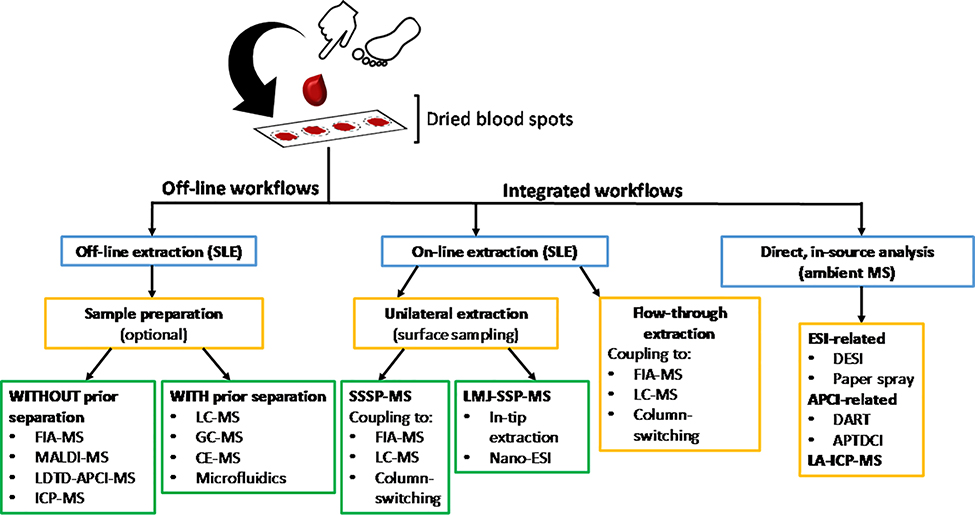
The clinical value of DBS drastically rose with the implementation of MS. MS enables multiplexed detection of diseases and high throughput screening of biological samples while greatly reducing uncertainty in results due to its inherent sensitivity. Two workflows are performed (Figure 5): offline workflows, which require separate extraction steps, and integrated workflows, which incorporate online extractions. Offline workflows are more commonly performed as they often require considerably less instrumentation. DBS are punched from their storage cards and are subjected to solid-liquid extraction using aqueous/organic solvent mixtures. These extracts are then either further subjected to separation by LC-MS (Rashed et al., 2005; Forni et al., 2010; Suyagh et al., 2010; Keevil, 2011; Déglon et al., 2012; Ellefsen et al., 2015; Zhang et al., 2019), GC-MS (Déglon et al., 2010; Ingels et al., 2010, 2011; Ellefsen et al., 2015), capillary electrophoresis mass spectrometry (CE-MS) (Shi et al., 1995; Chalcraft and Britz-McKibbin, 2009; Lyon et al., 2010; Jeong et al., 2013), or directly analyzed by techniques such as flow injection analysis with tandem mass spectrometry (FIA-MS/MS) (Carducci et al., 2006, 2006; Johnson et al., 2007; Turgeon et al., 2008) and APCI-MS (Sosnoff et al., 1996; Suyagh et al., 2010).
Figure 5.
Comparison of three basic workflows typically used in dried blood spot analysis: offline, online, and direct in-source MS strategies. The various acronyms are explained in Table 1 below. (Adapted and modified from Wagner M. et. al., 2016, copyright 2014 Wiley Periodicals, Inc).
MS/MS allows for the analysis of multiple analytes in a single sample and is a preferred method of analysis for newborn screening (NBS). There are several considerations for performing direct analysis without a prior separation. For example, isobaric peptides which have the same molecular weight (e.g., those containing leucine, valine, isoleucine, and alloisoleucine), and molecules which exist in several isomeric forms can be a significant challenge. Derivatization of the sample can allow for the analogs to be distinguished from each other. Use of different scan modes may also be performed to overcome these limitations. Single and multiple reaction monitoring (SRM, MRM) allows for unique mass-to-charge transitions to be monitored, which can differ for isomers and isobaric compounds. Neutral loss monitoring can also give rise to different types of structural losses due to the types of moieties present on a molecule which can help identify the species present.
As illustrated in Figure 5, two competing workflows are typically used for dried blood spot analysis: offline and integrated methods. The offline workflow can be performed with or without prior sample preparation. Offline methods requiring some sort of chromatography need prior sample workup as part of the workflow whilst those requiring no chromatography can be operated without extensive sample workup. The integrated workflow can be divided in two groups: online and direct, in-source analyses. In online methods, the extraction process is coupled to detection by analytical techniques such as MS. Samples may be extracted at a single surface interface (unilateral extraction) or from multiple surface interfaces (flow-through). Sealing surface-sampling probe MS (SSSP-MS) and liquid microjunction surface sampling probe MS (LMJ-SSP-MS) are employed as unilateral extraction techniques. LMJ-SSP-MS and SSSP-MS allow for the extraction of soluble materials (e.g., small molecules such as dyes, drugs, natural products (Gaissmaier et al., 2016), and larger molecules such as peptides and proteins) on surfaces (e.g., thin layer chromatography (TLC) plates, thin tissue sections, spotted sample arrays, affinity arrays, stainless steel, glass, glassy carbon, hydrophobic paper. etc.) coupled to direct analysis by MS (Van Berkel and Kertesz, 2009, 2013). In LMJ-SSP, a liquid microjunction is formed between the probe and the sample surface. Solvent moves through the probe, contacts the sample surface, and is subsequently introduced into an ionization source. This surface sampling technique is analogous to in situ microextraction and is applicable to all chemical species that can be dissolved and aspirated into the probe and subsequently analyzed by the respective ionization source (Van Berkel et al., 2008). LMJ-SSP-MS is useful for therapeutic drug monitoring in dried blood spots, which (i) minimizes additional sample preparation, (ii) allows for very small sample volumes (2 μL), and (iii) has enhance sensitivity compared to other direct analysis techniques such as DESI-MS. The enhanced sensitivity is attributed to the direct ESI processes (Gaissmaier et al., 2016).
The second group of methods used to achieve integrated workflow include the direct, in-source analytical platforms. These systems enable sample analysis in its native state. They incorporate a separation step during the analysis, which eliminates the need for separate sample preparation. Common methods of analysis include desorption electrospray ionization mass spectrometry and paper spray mass spectrometry. These techniques rely on the analysis of the whole DBS, and therefore are impacted by DBS characteristics in a different manner. Additional steps are commonly taken to avoid deviation in results due to hematocrit levels and DBS sample volume. For example, internal standards (IS) are used to correct for blood volume variations, but this can be challenging due to the solid nature of the DBS (Wagner et al., 2016).
The two most common direct, in-source analytical platforms for processing DBS are DESI and DART, which are spray-based and plasma-based ambient ionization methods, respectively. In DESI, an electrospray emitter is angled at the dried blood sample (collected onto paper or glass substrate) where primary droplets create a thin film to dissolve the target analyte. Impact of subsequent primary droplets in the thin film releases secondary droplets containing the dissolved analyte. These secondary droplets are transferred into the mass spectrometer. The DESI process is classified here as integrated workflow for DBS analysis because it does not require any sample preparation (Wiseman et al., 2010; Wiseman and Kennedy, 2014 Ifa et al., 2007).
Whereas DESI relies on liquid-phase processes for analyte extraction and ionization, DART uses gaseous molecules for interrogation of sample surface and subsequent ionization of analyte. In DART, gas-phase metastable atoms or molecules generated in the ion source are used for analyte desorption. After desorption, ionization in DART is thought to occur by one of three mechanisms: 1) Penning ionization – here, the metastable species (in electronically excited state) interacts directly with the target analyte and facilitates electron transfer between the two species, which leads to the formation of a radical cation; 2) Alternatively, the metastable atoms can interact with atmospheric water vapor, which through a series of cascade reactions culminate in the formation of protonated water clusters. These water clusters, in turn, transfer the extra proton to gaseous analytes via chemical reactions and the protonated analytes are then detected by the mass spectrometer; 3) In the negative mode, electrons produced by the DART ion source can interact with oxygen molecules, resulting in electron capture or deprotonation of the target analyte. DART has been applied to directly analyze DBS samples, either through online (Crawford et al., 2011; Meesters and Hooff, 2013) or offline methodologies (Wang et al., 2013a).
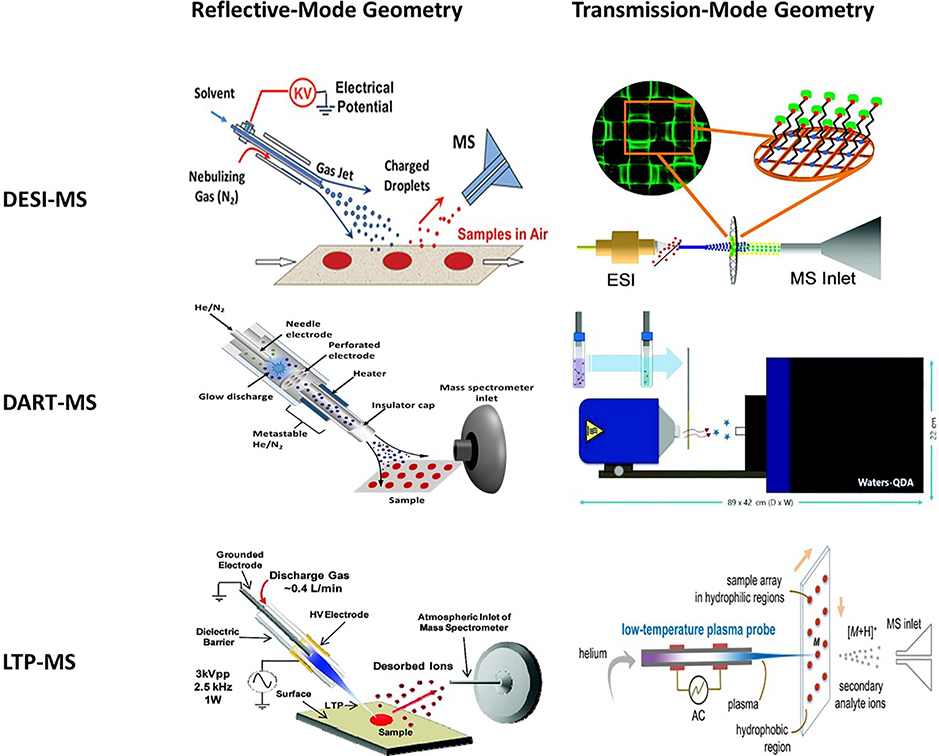
For online DBS analysis, both DESI and DART can offer additional spatial analytical information. Because only a portion of the DBS sample is observed at a single time point, the analytical concentration of a target molecule will vary across the DBS due to chromatographic effects. As a result, the majority of the analyte may not be accounted for (Ren et al., 2010; Wilhelm et al., 2014). The experimental setups for both DESI and DART utilize reflective-mode geometries (Figure 6), which can sometimes pose challenges in experiment optimization and limit signal quantitative accuracies. In this regard, transmission-mode (TM) experimental setups have been proposed as geometry-independent methods to ease MS analysis (Chipuk et al., 2010b, 2010a; Gómez-Ríos et al., 2017b). DART analysis of DBS prepared in paper is limited due to the high temperature of the He gas (100 – 400°C), which can burn the paper substrate and cause interference in MS analysis. A transmission-mode low temperature plasma (LTP) probe has potential of enabling dried sample analysis on paper (Zhao et al., 2016).
Figure 6.
(Left column) Reflective-mode geometry configuration for desorption electrospray ionization (Reprinted with permission from Ifa et al., 2010, copyright 2010 The Royal Society of Chemistry), direct analysis in real time (Adapted and modified with permission from Hajslova et al., 2011, copyright 2010 Elsevier Ltd.), and low-temperature plasma ionization sources (Reprinted with permission from Harper et al., 2008, copyright 2008 American Chemical Society). (Right column) Transmission-mode geometry configurations for desorption electrospray ionization (Reprinted with permission from Chipuk et al., 2010b, copyright 2009 American Chemical Society), direct analysis in real time (Reprinted with permission from Gómez-Ríos et al., 2017b, copyright 2017 The Royal Society of Chemistry), and low-temperature plasma ionization sources (Reprinted with permission from Zhao et al., 2016, copyright 2016 American Chemical Society).
B. Other Microsampling Platforms
Conventional liquid sampling (e.g., venipuncture) is not always feasible due to the biohazardous nature of biofluids, availability of sample, trained personnel, and instrumentation. A vast number of animals are additionally required for toxicokinetic (TK) and pharmacokinetic (PK) studies, which can become costly, in addition to raising ethical concerns for animal health and welfare. Microsampling techniques such as plasma extraction cards, volumetric absorptive microsampling, and capillary microsampling (Figures 6a, 6b and 6c) are employed to collect very small volumes (~10–100 μL) (Jonsson et al., 2012b; Bowen et al., 2013; Jonsson et al., 2013; Chapman et al., 2014; White et al., 2014; Kar et al., 2015; Spooner et al., 2015; Mercolini et al., 2016; Niu et al., 2016; Barco et al., 2017; van den Broek et al., 2017; Kita and Mano, 2017; Qu et al., 2017; Fang et al., 2018; Koponen et al., 2018) which greatly reduces exposure to biohazards, eliminates the need for a large number of satellite animals, and allows for the collection of samples from individuals who are not able to provide large sample volumes such as newborn infants, the elderly, and the critically ill (White et al., 2014).
1. Plasma Collection Paper Cards
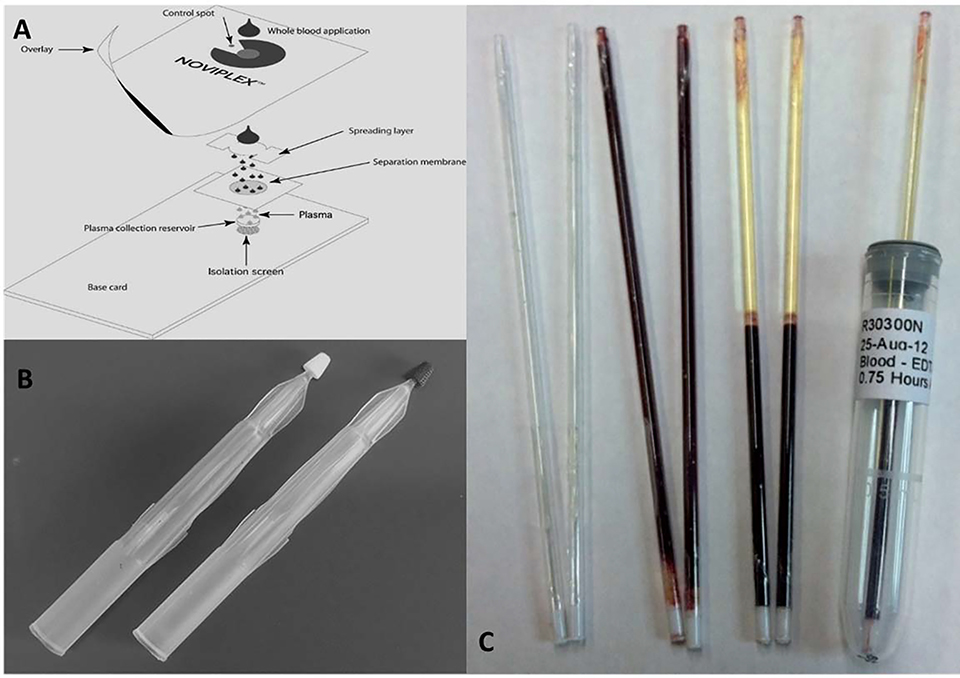
Plasma is an ideal sample medium for clinical diagnostics due to reduced chemical complexity from the absence of erythrocytes (red blood cells, RBCs), leukocytes, platelets, and other cellular interferences found in whole blood. Plasma, however, requires fractionation of whole blood, which is achieved through centrifugation and other separation techniques such as sedimentation. These methods are often time extensive, requires large sample volumes (mL), trained personnel, and automated instrumentation, all of which may not be readily available (Songjaroen et al., 2012). To overcome these challenges, portable microfluidic devices containing plasma filtration membranes are used to provide an on-site, single-step separation and sample collection (Figure 7a) (Kim et al., 2013). These devices take advantage of active and passive separation methods; passive methods do not rely on external forces and are influenced by biophysical effects, cell behavior, hydrodynamic forces and channel geometry. Deterministic lateral deviation (DLD) or a principle mechanism analogous to size exclusion chromatography (SEC) are two common methods of passive separation used in portable microfluidic devices. In DLD, cellular components of varying sizes experience different flow paths as they pass through a channel obstructed by several obstacles such as micropost arrays. These flow paths differ due to altered fluid dynamics. The second method of passive separation, the SEC-like separation, utilizes layered membrane filters (Figure 7a) such as bound glass fiber which have a defined pore size; cellular components of blood have a much larger size and viscosity relative to plasma and are retained while plasma may pass through (Tripathi et al., 2015).
Figure 7.
(a) Plasma extraction card device for rapid extraction of plasma from blood for subsequent analysis by MS (Adapted and modified with permission from Kim et al., 2013, copyright 2013 American Chemical Society); (b) volumetric absorptive device for acquiring fixed micro-volumes of biofluid sample (Adapted and modified with permission from Denniff and Spooner, 2014 copyright 2014 American Chemical Society); and (c) capillary microsampling of blood in EDTA-coated tubes containing thixotropic gel for plasma separation (Reprinted with permission from Bowen et al., 2013, copyright 2013 Future Science Ltd.).
Recent advancement in field-ready diagnostic testing has moved towards the fabrication of portable 3D devices for on-site sample collection and storage. Lei Ge. et. al. devised a multichannel wax-printed 3D origami device that integrates plasma separation with multiplex detection of four cancer biomarkers via chemiluminescence sandwiched-based immunoassay. 6 μL of sample allows for the detection of four cancer biomarkers, which have a corresponding signal proportional to the concentration found within the sample itself. Above a certain threshold, an individual can be diagnosed with the corresponding cancer. (Ge et al., 2012). Ryona et. al. also devised a separate book-type dried plasma spot (DPS) card for collection of plasma for small volume analysis coupled to automated instrumentation. These spots are not subject to hematocrit effects like DBS, making them more suitable for replicate sample collection and analysis(Ryona and Henion, 2016).
Plasma separating devices address one of the main challenges of sampling with DBS: the hematocrit effect. DPS and DBS extraction methods, however, can be laborious and impractical if samples are punched followed by offline solid-phase or liquid-liquid extractions. Development of automation of DPS and DBS sampling can be challenging due to solvent composition – for example, a higher organic solvent composition will favor the extraction of non-polar molecules. A high organic solvent composition, however, will reduce retention on a solid-phase extraction cartridge, which can negatively impact subsequent analysis by LC-MS/MS. A second challenge faced is the extraction of molecules that have a high affinity for RBCs; these molecules will be in much lower quantity in DPS as they will be filtered out with the RBCs. The cost of specialized filter paper for plasma separation can be quite costly compared to the types of filter paper that are used for conventional DBS sampling (Nilghaz and Shen, 2015; Ryona and Henion, 2016).
2. Volumetric Absorptive Microsampling
Volumetric absorptive microsampling (VAMS) is a recent dried microsampling approach well-suited for collection of biological matrices such as blood (Denniff and Spooner, 2014). This device contains a molded plastic handle with a porous hydrophilic tip that absorbs a fixed volume of sample by passive capillary action controlled by the properties and amount of the tip substrate present. VAMS offers several advantages over DBS, such as sample volume accuracy, greater reduction of hematocrit effects, little to no pre-treatment, and automation. Most notably, samples collected via VAMS may be shipped at room temperature and can be classified as non-hazardous (Kok and Fillet, 2018; Protti et al., 2019).
Denniff et. al. first devised the VAMS device, and since commercially named Neoteryx’s Mitra (Kovač et al., 2018; Taylor et al., 2018; Li et al., 2019), in 2014 to address the major issues of DBS, most notably the hematocrit effect, sample volume bias, and inhomogeneity. In its simplest form (Figure 7b), the VAMS device is directly applied to a bleed site caused by a small needle, eliminating the need for subsequent sample transfer for further processing, such as with plasma extraction techniques. The sample collected is the sample analyzed, so subsampling/punching is eliminated. In initial findings, the tip of the VAMS device was optimized at a 45° angle relative to the sampling surface and collects a blood volume of ~ 10 μL. The tip is held at the bleed site for an additional 2 seconds after total saturation, making the total sampling time ~ 4–6 s. The tip is subsequently dried for a minimum of 2 h under ambient laboratory conditions. (Denniff and Spooner, 2014).
DBS and VAMS both allow for collection of very small samples with non-invasive procedures and no required phlebotomist or trained personnel. Sample volumes and inhomogeneity are problematic for DBS when collected without using a calibrated instrument such as a micropipette. This results in sub-sampling/punching of DBS, which is subject to hematocrit and chromatographic effects. VAMS devices are calibrated to contain specified volumes of blood and exhibits minimal sample volume variation for samples containing different levels of hematocrit and does not suffer from inhomogeneity (Spooner et al., 2015; Qu et al., 2017). These devices are extremely useful for TK and PK studies using animals, as it reduces sample volume and number of animals, which follows the three ‘R’s’ guidelines of reduce, refine, and replace for in situ sampling to preserve animal health and welfare (Houbart et al., 2015). By reducing the number of animals, the inter-individual differences in PK profiles is reduced (Kita and Mano, 2017). To help reduce bias in VAMS assays, samples should be collected at room temperature or 37 °C, but not at colder temperatures such as 4 °C (Fang et al., 2018), with a recommended drying time of 2 hours (Parker et al., 2016). For optimal reduction in hematocrit effects, the type of extraction solvent has been found to be extremely important as it affects analyte recoveries (Ye and Gao, 2017). Additional workup steps, such as sonication, pre-wetting, and liquid-liquid extraction may also be performed to further improve the recovery (Xie et al., 2018).
3. Capillary Microsampling
Using paper as a microsampling platform may be inexpensive and effective, but the volumetric precision is often inadequate and has inspired several alternate or modified sampling strategies. A popular alternate is using microcapillaries to measure volume through capillary action and then storing the sample in a freezer while still in the capillary or wicked onto a paper substrate.
Trajan’s HemaPen uses four adjacent capillaries to accurately measure 2.74 μL of whole blood, which is then stored onto paper discs. By sampling with four capillaries simultaneously, true replicates from the same blood drop is collected by a user-friendly interface (Florian Lapierre et al.; Konstantinos Kouremenos et al.).
Shimadzu’s MSW2 is an enclosed polycarbonate capillary coated with EDTA in a U-shape. When one end of the capillary is touched to a source of whole blood, such as a bleed site, 23 μL of whole blood is drawn into the device through capillary action without the use of a pipette. Because of the U-shape, the device (individually called an MSWing or microsampling wing) can be loaded into an array of 14 slots (MSWindmill or microsampling windmill) and centrifuged to separate plasma from red blood cells. The design of the U-shape capillary allows the analyst to snap off the upper-part of the U-shape containing the separated plasma with light pressure. The volume contained in this reservoir is 2 × 2.8 μL with an additional volume compartment nearer the red blood cells containing 2.5 μL. Sample can then be removed from the capillary by vortexing in a tube containing solvent or blank plasma (Shimadzu, 2017).
Instead of paper, capillary microsampling (CMS) employs glass capillaries to collect microliter volumes of liquid blood sample (~4–32 μL) through passive capillary forces (Nilsson et al., 2013; White et al., 2014; Nys et al., 2017). The European Bioanalysis Forum describes CMS for biosampling as follows: blood is sampled in a capillary containing anticoagulant, with or without subsequent washout using a diluent at the time of sample concentration; plasma is obtained by centrifuging blood sampled in a capillary coated with anticoagulant. Low volumes of plasma (typically 4 or 8 μL) are transferred to a second capillary and washout/dilution is performed immediately or at the start of analysis (MacDonald et al., 2014). CMS is commonly used in the clinical setting for point-of-care health monitoring as it eliminates the need for use of venous blood collection methods, which can be invasive and potentially painful to the patient. Less sample is required when sampling via capillary, which is crucial if multiple or repeated analysis are to be performed.
Bowen et. al. devised a novel CMS device capable of collection and isolating plasma from a rodent. The capillaries were coated with EDTA to prevent coagulation of sample and contained thixotropic gel (Figure 7c). At the end of the capillary was a self-sealing plug that allowed for sample centrifugation. After collecting 75 μL blood samples from rodents, the capillaries were centrifuged; the thixotropic gel migrates to the middle of the capillary based on density, forming a stable barrier between the erythrocytes and plasma. The plug at the end can be used to dispense the plasma. A PK study was performed on rodents by administering either acetaminophen or moxifloxacin. The concentrations of acetaminophen and moxifloxacin were compared for samples collected by CMS and by caudal vasculature (200 μL sample volumes). It was found that for both methods of collection, the AUC (μg*h/mL), Cmax (μg/mL), and Tmax (h) were similar for acetaminophen and moxifloxacin. Freeze-thaw cycles had minimal effect on the samples stored in the tubes, and assay performance was consistent with precision and accuracy requirements as set by regulatory standards (Bowen et al., 2013).
CMS is also commonly used for TK studies to link circulating drug concentrations with functional or pathological changes in animals during a specific dosing time point. Small sampling of animals is imperative to (i) reduce the number of animals used in a given study, and (ii) to allow for multiple sampling from a single individual (Niu et al., 2016). The Institutional Animal Care and Use Committee guidelines vary, but strongly recommends 7.5% total blood volume to be collected over a period of 24 h; for a 6-week-old rat containing ~ 11 mL for a female weighing less than 200 g, only about 800 μL of blood would be able to be collected, which amounts to about 3 or 4 samples if taken using conventional methods. Using CMS, however, a much greater number of samples may be collected (Bowen et al., 2013). Compared to VAMS and DBS, CMS is most commonly used for TK/PK studies due to the ability to collect a sample in native liquid state; the sample itself can be stored ‘wet’ by freezer (Verhaeghe et al., 2017). Since the sample is in liquid state, it can be handled similarly to conventional methods of processing samples (Jonsson et al., 2012a). CMS may also be used to circumvent hematocrit effects observed in DBS sampling. However, for some classes of molecules such as drugs, sample processing can be complex and exhibit nonspecific binding or a lack of stability (Youhnovski et al., 2017).
SAMPLE STABILITY
The acceptance and rejection of sample qualities require the establishment of defined parameters for reliable application. For DBS, physical parameter factors such size of the printed blood collection circle on the collection card, drying time, elution times, and filter paper type are stringently controlled to maintain consistency in sample quality. Regardless, storage is the main determining factor governing the analyte stability in blood. Because of recent use of DBS in population-based screening, the evaluation of analyte stability in the filter paper has become an important parameter. In many parts of the world, (e.g., Africa and Middle East), the paper cards can be exposed to high temperatures (37–50°C) and high humidity (>70%) during collection and transportation, (Elliott et al., 2014) especially in the remote areas where cold storage might be not immediately available. Even for the well-established newborn screening program, cold storage of the paper cards is considered a requirement (Olshan, 2007; Lacher et al., 2013).
A. Storage and Stability of Small Molecules
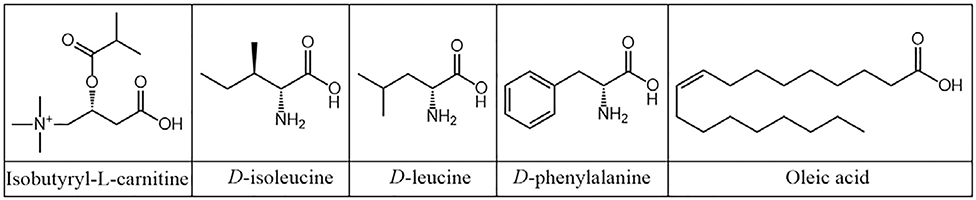
The stability of metabolites such as amino acids, cathinones, and carnitines have been investigated under various storage environments (Figure 8). The general workflow for offline DBS analysis utilizing LC-MS/MS is as follows: DBS (~3 mm, diameter) is placed in 96-well plate and extracted using appropriate solvent system. The corresponding IS is typically added to the extraction solvent. The extract containing the punched-out DBS and IS is vortexed and/or sonicated for a short amount of time (~5–10 minutes), followed by centrifugation. Modified versions of this extraction protocol, such as freezing, can be performed to enhance precipitation of some molecules. The supernatant is then analyzed by LC-MS/MS to determine quantity of analyte present under specific storage condition (Chace, 2003; Allard et al., 2004; Turgeon et al., 2008, 2010; Li et al., 2012; Johnson et al., 2013; Scott et al., 2013; Koster et al., 2015).
Figure 8.
Chemical structures of common metabolite biomarkers for disease monitoring.
Metabolites stored under ambient conditions registered a greater change in concentration relative to storage at −20 °C and −80°C, although they maintain some degree of stability. Detection was possible for up to 90 days for some of the molecules. Metabolites that degraded at a quicker rate were attributed to enhanced structural affinity for enzymes. Other potential explanations include the formation of alcohol-derived molecules under alkaline conditions and anti-oxidants present in the whole blood (Zytkovicz et al., 2001; Prentice et al., 2013; da Cunha et al., 2018).
Particle retention, pore size, thickness of paper, and loading capacity of the paper substrate all impact the interactions between the blood matrix and cellulosic fibers. DBS should be dried completely prior to storage in a breathable paper bag containing a desiccant package and humidity indicator card (Edelbroek et al., 2009). Blank blood should additionally be collected to perform calibrations and quality control samples. Concentrations of metabolites may be different between the plasma and cellular component of a blood spot, and as a result, are subject to changes in hematocrit. This effect was observed in a study of 31 metabolites in a series of five increasing hematocrit levels for neonatal whole blood samples (Holub et al., 2006). Additionally, the stability of molecules has been shown to be impacted by temperature. Some molecules, such as ester- and amide-containing compounds, are prone to abrupt enzymatic degradation upon deposition onto paper substrate. Enzyme inhibitors cannot easily be added to samples prior to spotting DBS, making analysis more difficult to perform. Artificial whole blood and serum may be used to overcome this limitation, although it may not address inter-laboratory variability of results (Li and Tse, 2010; Keevil, 2011).
Pharmaceutical compounds and therapeutic drugs are used as markers for the validation of bioanalytical quantitative studies of DBS. All method validation studies conform to the guideline on bioanalytical method validation set forth by the European Medicines Agency (2018). Sampling methods and comparative studies between whole blood and plasma matrices have been investigated (e.g., intravenous whole blood collection versus finger prick). The results showed no significant variation of concentration of analytes amongst samples (Koal et al., 2005, 2005; Kromdijk et al., 2012; Vu et al., 2012; van der Elst et al., 2013). The half-life and rate of exposure to drugs has been monitored by DBS analysis and yields similar results to that of whole blood and plasma analysis (Castillo-Mancilla et al., 2013). The differences in peak area ratios (Intensity of analyte/Intensity of IS) between DBS dried under ambient conditions compared to cold storage conditions was negligible and was determined that molecules are stable for prolonged periods of time (> 30 days) (Barfield et al., 2008; Spooner et al., 2009; Vu et al., 2011, 2014). Punch size has been determined to affect recovery due to different sample volumes being analyzed. Analyte loss during the extraction has also been investigated and found not to be significant, although the effect was observed to be analyte dependent. Drugs localized on different regions within the DBS showed no significant variation amongst spots (Koal et al., 2005; ter Heine et al., 2008; Liang et al., 2009). The type of paper card used also deserves consideration since variable results have been reported on different paper substrates (Möller et al., 2012). There is also no significant variations in spot size that can be attributed to lot variation or location within the lot suggesting acceptable manufacturing reproducibility (Wilhelm et al., 2014). Matrix effects are not observed to a large degree for the DBS extracts, although some ion suppression can occur prior to elution around the column dead volume during LC separation (Hoogtanders et al., 2007; Barfield et al., 2008; ter Heine et al., 2008; van der Heijden et al., 2009; ter Heine et al., 2009b; Liang et al., 2009; Vu et al., 2011; Möller et al., 2012, 2018). Chelator additives have been shown to reduce matrix interactions which further enhances recovery (Vu et al., 2014). The specificity and selectivity of analytes present in plasma and whole blood have been monitored. No co-eluting peaks were observed, and deviations less than 20% for the nominal concentration are reported (Heine et al., 2007; Hoogtanders et al., 2007; van der Heijden et al., 2009; ter Heine et al., 2009a, 2011; Vu et al., 2011; 2018).
Drugs of abuse have additionally been studied using DBS. Labile drugs stored in solution undergo degradation during transportation which can be problematic for quantitative analysis. The stability of drugs on Guthrie Card 903 stored at room temperature, refrigeration, and freezing has been investigated. The stability of benzodiazepines and cocaine was shown to be greater when stored on filter paper than in solution (Alfazil and Anderson, 2008). The detection and identification of sub-nanogram-per-milliliter concentration of drugs in sample is necessitated by advancements in high resolution mass spectrometry. A simple modification of a mass spectrometer design allows for a single extraction protocol to be performed, allowing for drugs of various classes to be analyzed using the same method. Results of this study abided by the standards laid out by the bioanalytical guidelines and therefore makes this a quick method of analysis suitable for doping control analysis (Thomas et al., 2012).
Attempts to increase the stability by storing in 3-dimensional dried blood spheroids on hydrophobic treated paper as opposed to 2D DBS cards was performed at room temperature. The stability of diazepam was studied in the dry-state blood spheroids, and increased stability was found, with at least 70% of signal remaining after 7 days when blood spheroids on hydrophobic paper was used, compared to 25–30% of signal in dried blood spots over the same time frame. When stability of cocaine was studied, about 90% of signal was lost after one day of storage in a dried blood spot, while the analyte intensity remained constant for 28 days in a spheroid. The inability of the blood to wet through the fiber core of the hydrophobic paper suggests that there are reduced interactions between cocaine and paper surface. The amount of free cocaine in the spheroids are greater as a result, which leads to increased analyte signal. The stability of cocaine was determined to be affected by the volume of sample deposited on grade 903 paper. For a 4 μL sample of blood containing 5 ppm cocaine, the signal degraded quickly on untreated paper. For a 50 μL sample, however, the signal was relatively stable for a greater duration of time (Alfazil and Anderson, 2008; Damon et al., 2018).
B. Storage and Stability of Large Molecules
Infectious diseases may also be diagnosed and monitored in remote areas by semi-quantitative analysis of DBS via LC-MS/MS. Diseases detected in the early stages of development allows for treatment prior to onset of symptoms. Antibodies and viral particles are detected via MS with sensitivity and specificity matching that of the established targeted assays such as nucleic acid amplification tests and enzyme immunological assays (Bellisario et al., 2001; Judd et al., 2003; Blanchard et al., 2008; Elliott et al., 2014). Antibodies have been shown to be stable for 2–4 months and in some cases up to 4 years under freezing conditions (Castro et al., 2008; Marques et al., 2012). Compared with immunoassays and PCR tests, higher throughput can be attained using LC-ESI-MS/MS due to reduced sample preparation steps (Lai et al., 2001).
For certain diseases (e.g., lysosomal storage diseases), there is a need to establish between latent and active stages. Therefore, protein activity and stability monitoring has become popular using DBS analysis. The target enzyme is naturally found within the blood sample, while the enzyme substrate is added during extraction procedures. Currently, there are two available methodologies used for measurement of enzymatic activities: digital microfluidics fluorimetry and MS/MS. Prior to analysis via fluorescence or MS/MS, the substrate is added, and the activity of the bound enzyme-substrate complex is subtracted from that of the inhibited enzyme-catalyzed reaction (Hamilton et al., 2012). Different sample collection and handling methods (e.g., test tubes containing anti-clotting factors and storage conditions such as ethylenediaminetetraacetic acid, heparin, and collection of samples on filter paper) have been compared and no statistical differences were observed (de Castilhos et al., 2011). Both MS/MS and fluorescent detection platforms offer multiplexing capability. Advantage of MS/MS over fluorescence may include sensitivity, specificity, and the ability to monitor enzymatic activities without the use of tag molecules. However, the fluorescence detection can be simpler and easy to maintain compared with a mass spectrometer.
Aside from monitoring protein activity by detecting the presence of enzymatic reaction product, MS/MS also allows for a high throughput proteomics workflow by detection of peptides derived from an enzymatic (e.g., proteases) digestion of a particular protein. In this way, performing MRM enables the confirmation of the presence of clinically significant proteins in sample. The feasibility of this workflow has been demonstrated for the detection of hemoglobin variants associated with a β-thalassemia phenotype, as well as other newborn diseases such as medium chain acyl-CoA dehydrogenase deficiency (Li, 2004; Daniel et al., 2007; Dajnoki et al., 2008, 2010; Oerton et al., 2011; Martin et al., 2013). Proteins were shown to be stable for over 100 days when stored under freezing conditions, although activity drastically decreases as much as 94% after 4 days stored under ambient conditions (De Jesus et al., 2009; de Castilhos et al., 2011; Hamilton et al., 2012). Later studies providing data on incubation of DBS has shown that proteins can withstand storage at elevated temperatures and can remain stable for 2–6 weeks (Fujita et al., 2007). In a biobank study, proteins stored at 4°C or −24°C for up to 30 years were analyzed. It was found that the drying of protein did not influence its detectability and lower temperatures reduced the rate of decay (Björkesten et al., 2017). Inflammatory markers were measured in serum and plasma after the exposure of blood to different environmental conditions before centrifugation. The stability of these markers was compared to those that were separated immediately after collection and stored at −20°C. As more time passes, there is a greater difference between the concentration in plasma and in serum. A greater increase was found to be in serum, which was likely due to a release of these markers by the coagulation process (Skogstrand et al., 2008).
DIRECT ANALYSIS OF MOLECULES IN NATIVE STATE
A. Applications of PS MS
PS MS has been employed for various applications including but not limited to: biomedical and clinical studies (Manicke et al., 2011a, 2011b; Espy et al., 2012a; Yang et al., 2012a; Shi et al., 2015; Jeong et al., 2016; Shi et al., 2016; Yannell et al., 2017; Zhang et al., 2017b), illicit drugs (Wiseman and Manicke, 2014; Manicke et al., 2016; Vega et al., 2016; Jett et al., 2017; Michely et al., 2017; de Paula et al., 2018; Liu et al., 2018; McKenna et al., 2018), chemical warfare agents (Dhummakupt et al., 2017a; McKenna et al., 2017), agrochemical analysis (Pereira et al., 2016b), environmental and industrial monitoring (Jjunju et al., 2013, 2016; Maher et al., 2016), and for studying on-surface chemical reactions (Yan et al., 2013; Bag et al., 2015; Zhou et al., 2015; Maher et al., 2016). PS MS is advantageous in the clinical setting as it provides a means of rapid quantification of drugs and metabolites while minimizing sample preparation steps. Tissue samples have also been analyzed by collecting a biopsy punch and transferring it to paper substrate (Wang et al., 2011). The substrate itself can readily be modified to enhance sensitivity and can be designed for automation to provide auxiliary components such as continuous solvent flow. 3D printed cartridges have been created to allow for online sample enrichment of proteins (Zhang et al., 2017b). For biological samples, internal standards and blood samples are pre-loaded onto paper which is then cut into triangles. A study on pre-spotting internal standards onto various paper devices showed similar results to when the internal standard is mixed in with the sample itself (Yannell et al., 2017). Methanol/water (1:1) has shown to be very versatile as a spray solvent; however, the signal for some molecules, such as acylcarnitines, quickly decayed. Spray solvents containing formic acid often yielded reproducible signal that increases several manifolds (Yang et al., 2012a). Acetonitrile/water has also been shown to be useful for extracting drugs in urine (Michely et al., 2017). For pharmaceutical applications, other solvents such as chloroform were used for greater extraction efficiencies (Shi et al., 2015). MS/MS is used to overcome issues pertaining to isobaric compounds; a selected target molecule can be fragmented to give rise to a unique fragment, which is subsequently monitored either via SRM or MRM (Manicke et al., 2011b).
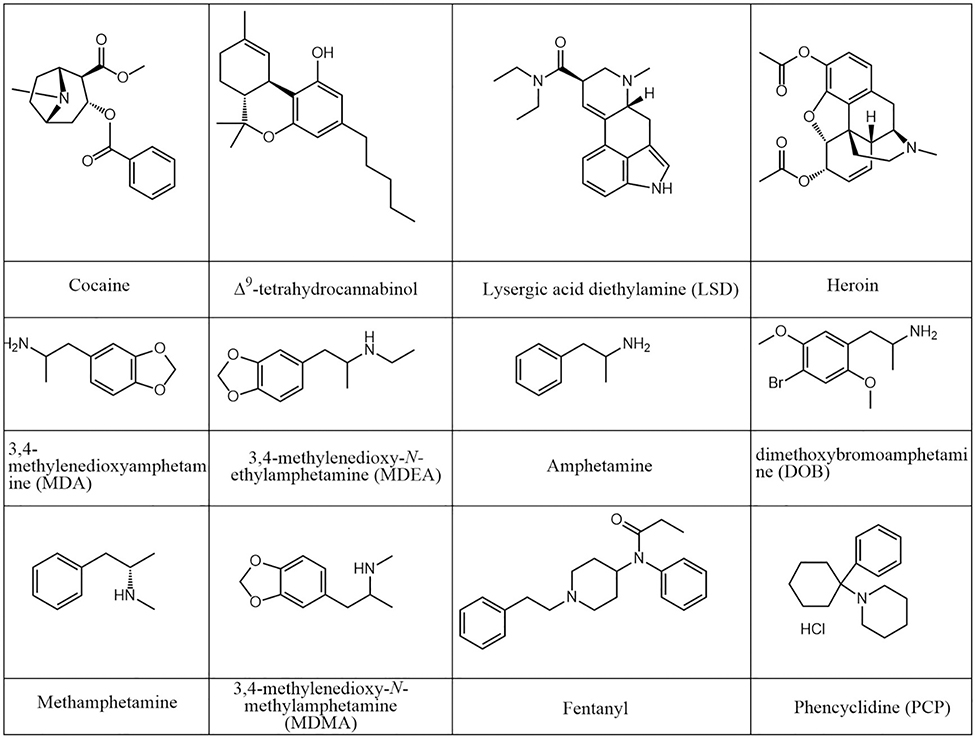
There is an increasing need for field analysis of drugs of abuse, most notably in the forensic community. PS MS provides a more sensitive solution to perform road-side testing for fresh blood collected onto paper, compared to the conventional colorimetric techniques (Su et al., 2013; Wang et al., 2013b; Espy et al., 2014; Ma et al., 2015; Costa et al., 2017; Domingos et al., 2017; Teunissen et al., 2017; Kennedy et al., 2018; Yang et al., 2018). PS MS is effective for detecting structurally different small organic compounds (Figure 9).
Figure 9.
Unmodified chemical structures of common drugs of abuse.
Without any treatment, raw blood and urine samples have been analyzed by slug-flow microextraction PS MS, which is well suited for very small amounts of analyte while reducing matrix effects (Yang et al., 2018). Continuous solvent flow PS MS of DBS has shown to be comparable to LC-MS/MS without needing to perform offline extraction steps (Vandergrift et al., 2018). Dichloromethane and hydroxylamine have been used in spray solvents for enhanced extraction of amphetamines (Teunissen et al., 2017). Various designer drugs have been screened via PS MS by direct blotter analysis, and structures were subsequently elucidated (Carvalho et al., 2016). Chemical warfare agents were detected in nanogram amounts without requiring any sample preparation beforehand (McKenna et al., 2017). For explosives analysis, isopropanol was the best spray solvent with added ammonium nitrate to enhance analyte signal (Tsai et al., 2017). PS MS is additionally well suited for dopant control analysis, such as within the sports industry. A blind quantitative study was performed on several ephedrine analogues spiked in human urine utilizing high resolution PS MS with a lab-built paper spray instrument. The accuracy ranged from 96.4 to 106.1%, while precision ranged from 0.98 to 6.28%. Sample run times were approximately 0.8 min., and the device enhanced selectivity of analyte from matrix interference, making this platform an excellent candidate for rapid, accurate, and precise quantitation studies (Jeong et al., 2016).
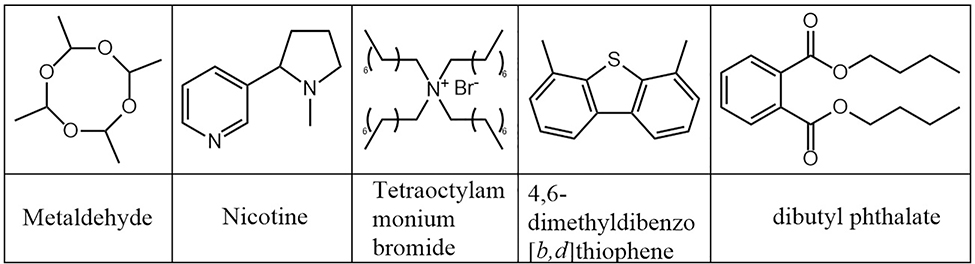
Environmental monitoring is important for quality assurance of food and beverages, and examples of analytes of interest in this area are shown in Figure 10. Quantification of pesticides and packaging products in fruits and vegetables were shown to be reproducible due to the soft ionization nature of paper spray (Chen et al., 2017; Di Donna et al., 2017; Teodoro et al., 2017; Cody et al., 2018). The surfaces of foodstuffs can be “swiped” with paper substrate (an equivalent to microsampling) and subsequently analyzed. The sample may additionally be pre-extracted prior to PS MS analysis (Ma et al., 2015; Pereira et al., 2016b). Soil analysis has also been performed for trace toxic organic components such as tetrabromobisphenol A by utilizing isotopic distributions in the mass spectrum (Liu et al., 2019). Water quality tests have been performed for metaldehydes in run-off samples (Damon et al., 2016b; Maher et al., 2016).
Figure 10.
Chemical structures of molecules monitored in food, beverages, and the environment.
Additionally, oil spills and degradation products are easily monitored with a reduction in salt effects via PS MS. In the negative electrospray mode, no discernable ion signals were detected in oils; using paper spray chemical ionization in the negative ion mode, abundant ion signal from several aromatic compounds were observed and characterized in oils (Kim et al., 2018a). Anti-corrosive agents in water has also been characterized, allowing for the detection of long-chain aliphatic primary polydiamines, which are not readily detected by conventional techniques such as GC-MS (Jjunju et al., 2016). Field analysis of corrosion inhibitors in oils is possible due to the ambient nature of PS MS (Jjunju et al., 2013). Quality control of plant-based products can readily be performed in a high throughput manner, which is not possible using analytical techniques such as GC- and LC-MS (Keating et al., 2018). In a more recent publication, adulterants and contamination in alcoholic beverages has been investigated. Samples were quantified using PS MS, and the extent of mixing was determined by using various chemometric methods such as principal component analysis and partial least squares (Li et al., 2013; Mazzotti et al., 2013; Pereira et al., 2016a; Tosato et al., 2018; Yu et al., 2018). Perfumes have been analyzed in a similar manner to adulterants in alcohol (Guo et al., 2017). Foodstuffs such as olive oil have been characterized using PS MS with enhanced ionization efficiency by doping the sample with silver nitrate (Lara-Ortega et al., 2018).
B. On-surface Reaction Monitoring and Reactive PS MS
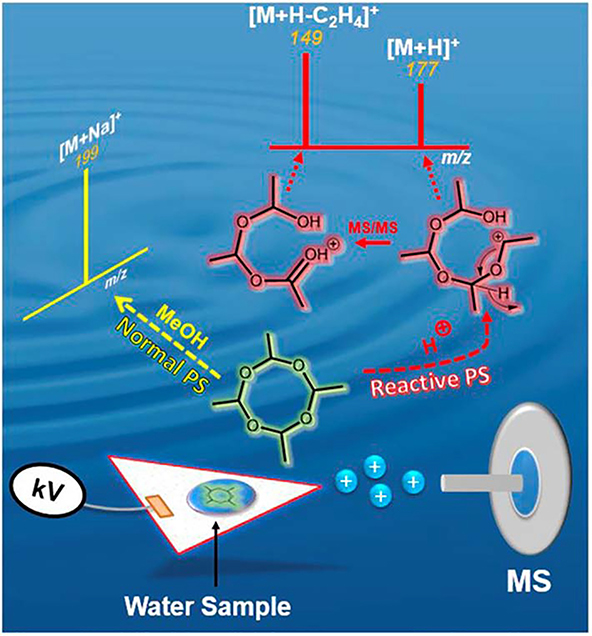
After drying, the weight of aqueous-based samples can decrease by >90%, which can facilitate transportation to a central laboratory. Recent advances in chemical instrumentation have enabled the development of miniaturized instruments that allow the lab to be brought to the field and analysis made at the sampling point. With reduced resolution of these miniature mass spectrometers, matrix effects from isobaric compound can severely impede analyte identification in the field, especially when no sample pre-treatment is employed. Selective chemical reactions can thus become a useful tool to differentiate between analytes of interest and background unrelated compounds. An example is shown for the analysis of metaldehyde (CH3CHO)4 analyte (MW 176 Da), a cyclic tetramer of acetaldehyde that is extensively used around the world as a molluscicide in agriculture. In traditional PS MS utilizing methanol spray solvent, metaldehyde is registered as a sodium adduct (M+Na)+ at m/z 199 via the inclusion of the sodium ion into the intact native cyclic structure. In reactive PS MS performed in the presence of formic acid, however, there is instantaneous ring opening after proton attachment to give ion at m/z 177, which goes on to dissociate spontaneously to yield an ion at m/z 149 via the elimination of ethylene (CH2=CH2, MW 28 Da) (Maher et al., 2016). The presence of the two ions m/z 177 and 149 in full MS during reactive PS MS serves as a second layer of confidence in metaldehyde detection in complex mixtures (Figure 11). Interestingly, MS/MS analysis of the ring (M+Na)+ and linear (M+H)+ structures of metaldehyde show distinctively different product ions, which also can be used to differentiate isobaric background ions from metaldehyde.
Figure 11.
Online derivatization of metaldehyde simultaneously analyzed via reactive PS MS which gives rise to an additional unique m/z transition. Reprinted with permission from Maher et al., 2016, with no modifications to the figure, copyright 2016 Maher et. al., 2016). Licensing: https://creativecommons.org/licenses/by/4.0/
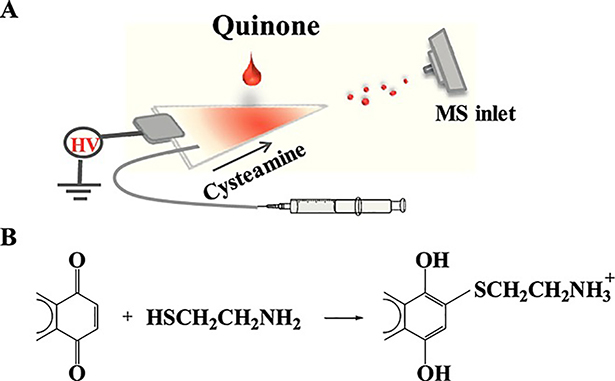
Aside from intramolecular dissociative reactions, online bimolecular derivatization reactions (Figure 12) have been reported using reactive PS MS that enhanced the detection of polycyclic aromatic hydrocarbons and aliphatic aromatic aldehydes without pre-treatment. Ionization efficiency was found to be much greater than the matrix, making this a quick approach (Bag et al., 2015; Zhou et al., 2015). Highly volatile chemical warfare agents (CWA) such as the G-series can be modified both in solution and vapor phase using 2-[(dimethylamino)methyl] phenol. This paper substrate dopant causes lower volatility complexes to form, extending the time CWAs are absorbed for PS analysis (Mach et al., 2018). Ozonolysis of double bonds has been achieved for polar lipids, which can subsequently be characterized by MS/MS (Oradu and Cooks, 2012).
Figure 12.
(A) Paper spray schematic for the detection of derivatized quinones. (B) In-situ reaction for derivatization of polycyclic aromatic hydrocarbons in the presence of cysteamine. (Reprinted with permission from Zhou et al., 2015, copyright 2014 John Wiley and Sons, Ltd.)
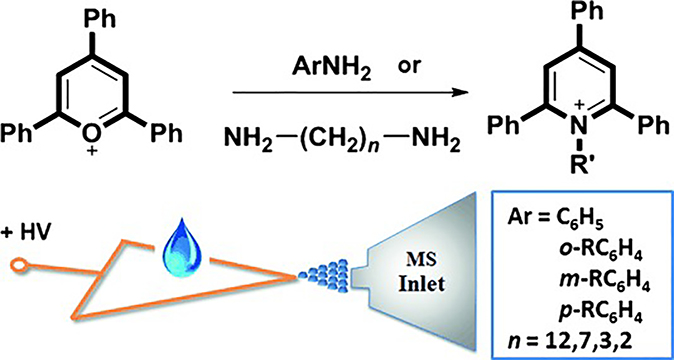
On-surface reactions monitoring have also been performed using reactive PS MS. In a typical experiment, reagents are transferred to the paper surface by drop-casting; charged droplets containing product are subsequently released by field evaporation and are analyzed by PS MS. In the case of Katritzky chemistry (Figure 13), several parameters were determined to affect the rate of product formation, which included rate of solvent evaporation and steric hindrance studied using the reaction between a pyrylium salt and mono-, di-amines (Yan et al., 2013).
Figure 13.
Katritzky reactions between 2,4,6-triphenyl pyrylium cation and mono-, di-amines monitored by PS MS. (Reprinted with permission from Yan et al., 2013, copyright 2013 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.)
On-surface reactions are performed under ambient conditions. Modified paper surfaces provide altered surface energies which can accelerate reactions by reducing absorption of reactants into the pores of the paper substrate (Davis and Badu-Tawiah, 2017). Heterogeneously catalyzed reactions can thus be performed by embedding paper substrate with nanoparticles, which is promising direction for reaction monitoring and mechanistic studies (Banerjee et al., 2016). In a similar fashion, photocatalysts can be coated onto paper substrate to perform photocatalytic reactions which can be used to monitor dye activity (Resende et al., 2017). Colorimetric reactions on chromatography filter paper was investigated in its application for direct detection and quantification of drugs (Santos et al., 2017). Different modes of PS may be utilized to perform either accelerated synthesis or chemical analysis (Bain et al., 2016). PS chemical ionization has been utilized for the analysis of low/non-polar aromatic analytes that do not readily ionize via ESI (Kim et al., 2017).
A photochemistry-assisted paper spray experiment was recently described, where a laser and high DC voltage are concomitantly applied during PS MS (Basuri et al., 2018). The presence of photons causes a reduction in ionization potential, allowing the detection of non-polar molecules such as polycyclic aromatic hydrocarbons in the positive and negative modes through field assisted photoionization. The conventional PS experiment mainly forms protonated species from polar analytes. The combination of laser and high voltage enabled the formation of all kinds of ion types, including radical cations (M+•), deprotonated molecules (M–H)–, and radical anions (M–•). Analysis of C60 showed a 103-fold enhancement in signal in negative mode full MS when the 10 mW 523 nm laser was used. In addition to enhancing ionization of non-polar analytes, the use of laser also enabled photochemical reactions to be monitored. This was demonstrated with the decarboxylation of para-mercaptobenzoic acid in negative mode on silver nanoparticle-coated paper and the dehydrogenation of 2,3-dihydro-1H-idoindole in positive mode.
C. Modified Paper Surfaces for Biological and Non-biological Samples
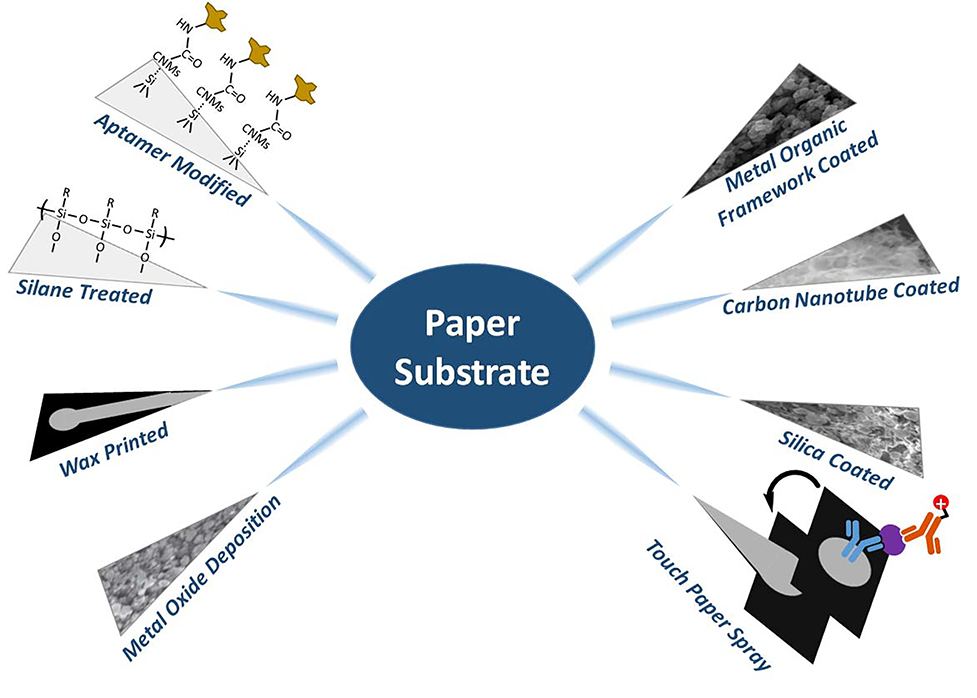
The utility of PS can be further expanded by storing samples, detecting lower abundance molecules or those that are more difficult to ionize, or performing online reactions. Modifying paper in general has focused on i) increasing sensitivity; ii) increasing the range of applicable analyte types; and iii) decreasing power required for ionization. Paper fiber is abundant with hydroxyl (OH) groups, which form a strong interaction with a large variety of chemical compounds. This increases the difficulty of elution, and thus decreases abundance of ions detected by the mass spectrometer. The key to increasing sensitivity is to prevent these sample-paper interactions, and secondly to increase ionization efficiency. To prevent interaction, a large amount of research is focused on either chemically or physically blocking these hydroxyl groups prior to sample deposition (Figure 14). Although this strategy has shown incredible improvements in sensitivity and detection limits, summarized in Table 2, possible complications involve the choice of spray solvent, which may be required to change due to the new surface properties and possibility of non-wetting or unfavorable partitioning. Additional complications may arise from solvents causing the deposited chemical to desorb or chemically react, contaminating a mass spectrometer. Therefore, special care is required when using these methods.
Figure 14.
Summary of several modification methods used during paper spray.
Table 2.
Alphabetical listing of quantification of analytes using modified paper spray
| Analyte | Surface modification | Instrument | Matrix | Range (ng/mL) | LOD (ng/mL) | LLOQ (ng/mL) | Ref. |
|---|---|---|---|---|---|---|---|
| Amisulpride | UiO-66(Zr) Coated | TSQ Quantum Access Max | Human Blood | 0.65 | (Wang et al., 2016) | ||
| Amisulpride | Polystyrene Microsphere Coated | TSQ Quantum Access Max | Bovine blood | 0.05–100 | 0.040 | (Wang et al., 2017a) | |
| Amisulpride | Polystyrene Microsphere Coated | TSQ Quantum Access Max | Bovine serum | 0.048 | (Wang et al., 2017a) | ||
| Amisulpride | Polystyrene Microsphere Coated | TSQ Quantum Access Max | Synthetic urine | 0.054 | (Wang et al., 2017a) | ||
| Amisulpride | Silica Coated | TSQ Quantum Access Max | Bovine blood | 0.50 | (Zheng et al., 2015) | ||
| Amisulpride | Silica Coated | TSQ Quantum Access Max | Human urine | 0.05 | (Zheng et al., 2015) | ||
| Amitriptyline | Polystyrene Microsphere Coated | TSQ Quantum Access Max | Bovine blood | 0.005–100 | 0.055 | (Wang et al., 2017a) | |
| Amitriptyline | Polystyrene Microsphere Coated | TSQ Quantum Access Max | Bovine serum | 0.084 | (Wang et al., 2017a) | ||
| Amitriptyline | Polystyrene Microsphere Coated | TSQ Quantum Access Max | Synthetic urine | 0.064 | (Wang et al., 2017a) | ||
| Amitriptyline | Silica Coated | Mini 12 | Bovine blood | 10 | (Zhang et al., 2012c) | ||
| Amitriptyline | Silica Coated | TSQ Quantum Access Max | Bovine blood | 0.10 | (Zheng et al., 2015) | ||
| Amitriptyline | Silica Coated | TSQ Quantum Access Max | Human urine | 0.50 | (Zheng et al., 2015) | ||
| Amitriptyline | Zirconia coated | TSQ Quantum Access Max | Bovine blood | 1.38 | (Zheng et al., 2016a) | ||
| Amphetamine | Hydrophobic | LTQ Velos Pro | Human Blood | 10–500 | 0.11 | 0.69 | (Damon et al., 2018) |
| Amphetamine | Hydrophobic | LTQ Velos Pro | Raw Human Blood | 0.06 | (Damon et al., 2016a) | ||
| Amphetamine | Hydrophobic | LTQ Velos Pro | Raw Human Serum | 0.02 | (Damon et al., 2016a) | ||
| Amphetamine | Hydrophobic | LTQ Velos Pro | Raw Human Urine | 0.08 | (Damon et al., 2016a) | ||
| Amphetamine | Wax Printed | LTQ Velos Pro | Human urine | 0.33 | 0.38 | (Damon et al., 2016b) | |
| Amphetamine | Wax Printed | LTQ Velos Pro | Fresh human urine | 0.76 | 1.40 | (Damon et al., 2016b) | |
| Angiotension II | Polystyrene Impregnated | TSQ Quantum Access Max | 0.1 M Tris Buffer | 1–1000 | 1 | (Li et al., 2018) | |
| Aripiprazole | UiO-66(Zr) Coated | TSQ Quantum Access Max | Human Blood | 0.36 | (Wang et al., 2016) | ||
| Aripiprazole | Silica Coated | TSQ Quantum Access Max | Bovine blood | 0.10 | (Zheng et al., 2015) | ||
| Aripiprazole | Silica Coated | TSQ Quantum Access Max | Human urine | 0.50 | (Zheng et al., 2015) | ||
| Aripiprazole | Zirconia Coated | TSQ Quantum Access Max | Bovine blood | 0.12 | (Zheng et al., 2016a) | ||
| Benzoylecgonine | Hydrophobic | LTQ Velos Pro | Human Blood | 10–500 | 0.48 | 0.79 | (Damon et al., 2018) |
| Benzoylecgonine | Hydrophobic | LTQ Velos Pro | Raw Human Blood | 0.13 | (Damon et al., 2016a) | ||
| Benzoylecgonine | Hydrophobic | LTQ Velos Pro | Raw Human Serum | 0.08 | (Damon et al., 2016a) | ||
| Benzoylecgonine | Hydrophobic | LTQ Velos Pro | Raw Human Urine | 0.2 | (Damon et al., 2016a) | ||
| Benzoylecgonine | Wax Printed | LTQ Velos Pro | Human urine | 0.21 | 0.45 | (Damon et al., 2016b) | |
| Benzoylecgonine | Wax Printed | LTQ Velos Pro | Fresh human urine | 0.97 | 2.50 | (Damon et al., 2016b) | |
| Citalopram | Silica Coated | Mini 15 | Bovine blood | 10 | (Zhang et al., 2012c) | ||
| Clozapine | UiO-66(Zr) Coated | TSQ Quantum Access Max | Human Blood | 0.04 | (Wang et al., 2016) | ||
| Clozapine | Polystyrene Microsphere Coated | TSQ Quantum Access Max | Bovine blood | 0.01–100 | 0.033 | (Wang et al., 2017a) | |
| Clozapine | Polystyrene Microsphere Coated | TSQ Quantum Access Max | Bovine serum | 0.065 | (Wang et al., 2017a) | ||
| Clozapine | Polystyrene Microsphere Coated | TSQ Quantum Access Max | Synthetic urine | 0.013 | (Wang et al., 2017a) | ||
| Clozapine | Silica Coated | TSQ Quantum Access Max | Bovine blood | 0.10 | (Zheng et al., 2015) | ||
| Clozapine | Silica Coated | TSQ Quantum Access Max | Human urine | 0.05–1000 | 0.05 | (Zheng et al., 2015) | |
| Clozapine | Zirconia Coated | TSQ Quantum Access Max | Bovine blood | 0.05–100 | 0.27 | (Zheng et al., 2016a) | |
| Cocaine | Hydrophobic | LTQ Velos Pro | Human Blood | 10–500 | 0.37 | 0.57 | (Damon et al., 2018) |
| Cocaine | Hydrophobic | LTQ Velos Pro | Raw Human Blood | 0.17 | (Damon et al., 2016a) | ||
| Cocaine | Hydrophobic | LTQ Velos Pro | Raw Human Serum | 0.06 | (Damon et al., 2016a) | ||
| Cocaine | Hydrophobic | LTQ Velos Pro | Raw Human Urine | 0.04 | (Damon et al., 2016a) | ||
| Cocaine | Wax Printed | LTQ Velos Pro | Human urine | 0.10 | 0.87 | (Damon et al., 2016b) | |
| Cocaine | Wax Printed | LTQ Velos Pro | Fresh human urine | 0.62 | 1.60 | (Damon et al., 2016b) | |
| Glucose | Paraffin barrier | Thermo LTQ | Neat | 1.8E5–4.5E7 | 5.E+05 | 1.67E+06 | (Colletes et al., 2016) |
| Lidocaine | Silica Coated | Mini 11 | Bovine blood | 20–1000 | 20 | (Zhang et al., 2012c) | |
| Methamphetamine | TFME-aptamer carbon nanotube | Homebuilt Ion Mobility | Saliva | 2–150 | 0.6 | (Zargar et al., 2018) | |
| Methamphetamine | TFME-aptamer carbon nanotube | Homebuilt Ion Mobility | Plasma | 1.5–200 | 0.45 | (Zargar et al., 2018) | |
| Methamphetamine | TFME-aptamer carbon dot | Homebuilt Ion Mobility | Saliva | 5–200 | 1.5 | (Zargar et al., 2018) | |
| Methamphetamine | TFME-aptamer carbon dot | Homebuilt Ion Mobility | Plasma | 3–250 | 0.9 | (Zargar et al., 2018) | |
| Methamphetamine | Hydrophobic | LTQ Velos Pro | Human Blood | 10–500 | 0.34 | 1.8 | (Damon et al., 2018) |
| Methamphetamine | Hydrophobic | LTQ Velos Pro | Raw Human Blood | 0.18 | (Damon et al., 2016a) | ||
| Methamphetamine | Hydrophobic | LTQ Velos Pro | Raw Human Serum | 0.24–500 | 0.09 | (Damon et al., 2016a) | |
| Methamphetamine | Hydrophobic | LTQ Velos Pro | Raw Human Urine | 0.05 | (Damon et al., 2016a) | ||
| Methamphetamine | Wax Printed | LTQ Velos Pro | Human urine | 3–250 | 0.13 | 0.60 | (Damon et al., 2016b) |
| Methamphetamine | Wax Printed | LTQ Velos Pro | Fresh human urine | 0.51 | 2.27 | (Damon et al., 2016b) | |
| Metolachlor | Silica Coated | TSQ Quantum Access Max | Milk | 100–10000 | 100 | (Wang et al., 2015) | |
| Myoglobin | Polystyrene Impregnated | TSQ Quantum Access Max | 0.1 M Tris Buffer | 5–1000 | 5 | (Li et al., 2018) | |
| Quetiapine | UiO-66(Zr) Coated | TSQ Quantum Access Max | Human Blood | 0.05 | (Wang et al., 2016) | ||
| Quetiapine | Polystyrene Microsphere Coated | TSQ Quantum Access Max | Bovine blood | 0.005–100 | 0.005 | (Wang et al., 2017a) | |
| Quetiapine | Polystyrene Microsphere Coated | TSQ Quantum Access Max | Bovine serum | 0.008 | (Wang et al., 2017a) | ||
| Quetiapine | Polystyrene Microsphere Coated | TSQ Quantum Access Max | Synthetic urine | 0.004 | (Wang et al., 2017a) | ||
| Quetiapine | Silica Coated | TSQ Quantum Access Max | Bovine blood | 0.10 | (Zheng et al., 2015) | ||
| Quetiapine | Silica Coated | TSQ Quantum Access Max | Human urine | 0.50 | (Zheng et al., 2015) | ||
| Quetiapine | Zirconia coated | TSQ Quantum Access Max | Bovine blood | 0.10 | (Zheng et al., 2016a) | ||
| Risperidone | UiO-66(Zr) Coated | TSQ Quantum Access Max | Human Blood | 0.41 | (Wang et al., 2016) | ||
| Risperidone | Polystyrene Microsphere Coated | TSQ Quantum Access Max | Bovine blood | 0.1–100 | 0.077 | (Wang et al., 2017a) | |
| Risperidone | Polystyrene Microsphere Coated | TSQ Quantum Access Max | Bovine serum | 0.079 | (Wang et al., 2017a) | ||
| Risperidone | Polystyrene Microsphere Coated | TSQ Quantum Access Max | Synthetic urine | 0.047 | (Wang et al., 2017a) | ||
| Risperidone | Silica Coated | TSQ Quantum Access Max | Bovine blood | 0.10 | (Zheng et al., 2015) | ||
| Risperidone | Silica Coated | TSQ Quantum Access Max | Human urine | 0.50 | (Zheng et al., 2015) | ||
| Risperidone | Zirconia Coated | TSQ Quantum Access Max | Bovine blood | 0.21 | (Zheng et al., 2016a) | ||
| Salicylic Acid | Urea Modified | LCQ Advantage Max | Urine | 1E3–1E5 | 4000 | 1000 | (Liu et al., 2017) |
| Sunitinib | Silica Coated | Mini 13 | Bovine blood | 10 | (Zhang et al., 2012c) | ||
| Verapamil | Silica Coated | Mini 14 | Bovine blood | 0.1–1000 | 10 | (Zhang et al., 2012c) | |
| Verapamil | Silica Coated | TSQ Quantum Access Max | Bovine blood | 0.10 | (Zheng et al., 2015) | ||
| Verapamil | Silica Coated | TSQ Quantum Access Max | Human urine | 0.05 | (Zheng et al., 2015) | ||
| Verapamil | Zirconia Coated | TSQ Quantum Access Max | Bovine blood | 0.42 | (Zheng et al., 2016a) |
Raw samples indicate samples were analyzed in a fresh, non-dried state
1. Chemically Modified Paper
Chromatography paper that underwent a gas-phase reaction with trichloro(3,3,3-trifluoropropyl) silane has a lowered surface energy, resulting in aqueous samples, such as biolfluid, to rest on the surface as a droplet. The immiscible solvent ethyl acetate applied between the liquid biofluid drop and the tip of the paper during analysis results in an online liquid/liquid extraction (Damon et al., 2016a). The sample can also be allowed to dry and then analyzed in an identical manner as a dried blood spheroid, as opposed to a dried blood spot (Damon et al., 2018). Ionization occurs via either an electrospray-based mechanism or an electrostatic spray, depending on the wetting state of the solvent on the paper. When blood is analyzed in the liquid phase, a lower LOD was found for four drugs of abuse: amphetamine, methamphetamine, cocaine, and benzoylecgonine of 0.06, 0.18, 0.17, and 0.13 ng/mL, respectively. In comparison, the dry-state study resulted in LODs of 0.12, 0.34, 0.37, and 0.48 ng/mL, respectively. These are lower than dry-state dried blood spot limits of detection by approximately 10-fold. Alanine transaminase was analyzed in fresh blood (Damon et al., 2016a), and the activity of the enzyme was quantified from 150–400 U/L with a LOD of 60 U/L. (Damon et al., 2018).
Chromatography paper modified with 1-[3-(trimethoxysilyl)propyl]urea, or urea-modified paper substate, was used to enhance sensitivity of small molecule analysis in the negative mode (Liu et al., 2017). Urea’s two adjacent NH groups contribute to small anion adsorption, resulting in the retention of anions such as Cl-, NO3-, SO4-, and also polar compounds like ferulic acid and quercetin. This retention decreases the competitive ionization that occurs in the droplets formed in the Taylor cone, increasing the target analyte signal in the negative mode. Another result of this retention is the decrease in electrical discharge, which is undesirable in some cases when analyzing compounds with strictly electrospray-based ionization, especially when a high signal-to-noise is required. This modification resulted in 2–109-fold increases in signal-to-noise of phytochemicals ferulic acid, quercetin, isorhamnetin, hyperoside, chrysophanol, verbascoside, and ginkgolide B, and LOD improvements for salicylic acid in urine and terpene lactones in Ginkgo biloba extract by 10 and 20-fold respectively.
2. Substrate Coated Paper
As opposed to chemically modifying the paper fiber, using addition substrates to coat the paper has become an attractive alternative. Commercially available Whatman grade SG81 is a silica gel-coated paper marketed for ion exchange partitioning. Zhiping Zhang et. al. instead used this paper for paper spray mass spectrometry analysis of blood spots (Zhang et al., 2012c). Blood was not able to penetrate the paper efficiently and instead rested on the surface with a small amount wetting through to the back, increasing analyte extraction from the blood. Lidocaine, amitriptyline, sunitinib, verapamil, and citalopram were analyzed using 10% isopropanol in dichloromethane as the spray solvent, and limit of quantification (LOQ) improvements of 5 – 50-fold decrease were found when using silica coated paper compared to Whatman grade 4 and ET31 chromatography paper with 9:1 methanol/water spray solvent.
Instead of relying on pre-fabricated modified paper substrates, a new method of preparing a substrate-coated paper was developed (Zheng et al., 2015). This method involves the vacuum filtration of commercially available materials, such as silica, metal oxides, organic polymers, metal powder, and carbon nanotubes, onto the surface of filter paper. The chosen material is suspended into an aqueous starch solution, sonicated, possibly heated to boiling (Wang et al., 2017a), and then added to the Buchner funnel containing a filter paper. Coating materials of silica particles (Wang et al., 2015), metal organic frameworks (Wang et al., 2016, 2017b; Dhummakupt et al., 2018), metal oxides (Zheng et al., 2016b, 2016a), and polystyrene (Wang et al., 2017a; Li et al., 2018) were studied. Silica particles were observed to fill pores and rest on the paper surface, adhered by the starch (Zheng et al., 2015). When analyzing blood and urine samples through silica-coated paper spray mass spectrometry, a decrease in limit of detection of 2–100 fold was observed for amitriptyline, clozapine, amisulpride, quetiapine, risperidone, aripiprazole, and verapamil when compared to normal filter paper. When analyzing myoglobin and lysozyme, normal filter paper shows the most abundant peak with a signal-to-noise ratio (S/N) of 1.5, but NH2 bonded silica microsphere coated paper has a S/N of 15.2 and 267.9 respectively. These improvements of paper could be attributed to the decrease of sample wetting through the paper fiber, increasing the concentration of sample on the paper surface and decreasing the sample interaction with free hydroxyl groups that may prevent elution and subsequent ionization.
Comparison of silica gel-coated paper (Whatman SG81) and prepared silica coated paper in-house showed the in-house paper had improved performance for the detection of azetochlor and metolachlor in milk of 11.3 and 5.7-fold respectively (Wang et al., 2015). This is due to the ability to optimize the amount of silica deposited onto the paper and increasing the silica amount provided a more favorable signal due to the decreased interaction between analyte and paper fiber.
Comparison of coating TiO2, Al2O3, ZnO, MgO, and ZrO2 on filter paper for the analysis of verapamil, amitriptyline, clozapine, amisulpride, quetiapine, risperidone, and aripiprazole (Zheng et al., 2016b). It was found that ZrO2 was optimal for the analysis of verapamil, as the other metal oxides adsorbed the analyte from a liquid solution more efficiently, leading to a lower MS signal. Additionally, it was found that the structure of the nanomaterial affected signal (Zheng et al., 2016a). Flower-like MgO nanoparticles exhibit a 6.2–21.9-fold increase in sensitivity compared to normal paper, while trapezoidal, needle-like, and nest-like structures have a lower increase in sensitivity, likely due to the flower-like MgO’s weak surface basicity.
Zirconium- and aluminum-based metal organic frameworks (MOF) were vacuum coated onto paper to extract drugs from dried blood spots (Wang et al., 2016, 2017b). Results showed that MOF contributed to a matrix suppression effect and a weaker interaction between analyte and the MOF surface compared to the normal analyte-paper interaction, increasing the sensitivity of verapamil detection in DBSs 3.5–7.8-fold.
By coating paper with carbon nanotubes, ionization can be achieved with only a 3V battery as opposed to the typical 3–5 kV required for paper spray (Narayanan et al., 2014). This was demonstrated with the ionization of triphenylphosphine. This is due to the protruding nanotube fibers concentrating charge rather than the macroscopic tip. Further utilization of carbon nanotube coated paper included the analysis of in-gel proteins (Han et al., 2015) and the probing of coordination complexes (Narayanan and Pradeep, 2017). Additional modification of carbon nanomaterials deposited on the paper with the covalent linkage of aptamers also increased sensitivity in the detection of methamphetamine when used as a thin film microextraction method (Zargar et al., 2018). Comparison of the aptamer conjugated carbon nanomaterials modified paper spray with typical extraction-ionization methods showed this new method is comparable to LC-MS, GC-MS combined with solid phase microextraction, and typical paper spray mass spectrometry methods in the detection of methamphetamine in plasma and saliva samples.
Paper also appears to be suitable for volatile molecule sampling (Sarkar et al., 2017). Paper coated with platinum (Pt) nanotubes (NT) was used to collect gaseous trinitrotoluene (TNT), and further addition of NaBH4 was used for the reduction to triaminotoluene. The addition of these nanotubes increases surface roughness and surface area, increasing catalytic activity. Paper spray analysis of the Pt NT paper was performed for the quantification of products and reactants, allowing for the study of reaction kinetics.
3. Non-Paper Substrates
Several new substrate-based ionization methods have emerged shortly after paper spray was reported. Some, including thread spray, coated blade spray and wooden tip spray, have advantages over paper spray, in that they provide the ability to perform extraction through a dipping method. Some also seek to perform ionization directly from the sample substrate, such as thread spray and leaf spray. Others, similar to many versions of modified paper spray, offer increased sensitivity with no other added benefits to traditional paper spray. A summary of these emerging substrate-based ambient ionization methods is shown in Figure 15. There is also high potential that some of the new substrates (e.g., thread) will offer microsampling and storage capabilities.
Figure 15.
Summary of several non-paper substrates used for spray ionization during ambient analysis.
3a. Polymers and Membranes
Replacing paper substrate with an organic-inorganic hybrid organosiloxane (OSX), polyester, and polyethylene materials is the logical next step in the field. The polymeric substrates cut to a triangle performs similarly to modified paper spray, in that the lack of hydroxyl groups results in a higher response of deposited analytes, increasing sensitivity (Dulay and Zare, 2017). The use of a conductive polymer, carbon nanotubes in poly(methyl methacrylate), eliminates the difficulty of extracting analyte from pores and showed a 2–25 times increase in sensitivity for organic molecules in plasma (Song et al., 2018).
Molecularly imprinted polymers (MIPs) generated atop cellulose membranes (but not paper) was demonstrated to act as a solid phase extraction platform for the analysis of dopamine, butyric acid, and sarcosine (Mendes et al., 2017). The MIPs triangle was soaked in the sample solution and then washed before analysis, allowing for elimination of matrix while retaining the target analyte (similar but not identical to the template molecule). Sensitivity of response increased when using MIPs compared to non-molecularly imprinted polymers, ESI, and traditional paper spray in urine. Further studies showed MIPs increased sensitivity of cocaine in oral fluids (Tavares et al., 2018) and agrochemicals atrazine, glyphosphate, and diuron in foodstuffs such as apples, bananas, and grapes (Pereira et al., 2018). Similarly, use of a graphene oxide membrane was used to analyze malachite green from carp tissue (Wei et al., 2018).
3b. Glass
Detection of CWA could benefit from paper spray for real-time response for first responders and civilians in war-ridden areas. Attempts to detect CWA simulants diisopropyl methylphosphonate, dimethyl methylphosphonate, and trimethyl phosphate by pumping aerosolized CWA simulants through the paper substrate and subsequent mass spectrometry detection resulted in unreliable capture efficiency and unsuitable chemical robustness (Dhummakupt et al., 2017b). When the paper substrate was replaced with glass fiber filter substrate, peak area counts increased by approximately 2 orders of magnitude, but still resulted in interaction between the glass and the simulants. By sulfonating the glass fiber, low mg/m3 LODs were achieved. Metal organic frameworks were also integrated onto glass substrates to increase analytical lifetimes of sarin, soman, and cyclosarin from less than 15 minutes on paper to greater than 50 minutes (Dhummakupt et al., 2018).
3c. Needles and Coated Blade Spray
Probe electrospray ionization (PESI) was developed as a method to directly sample and analyze liquids and wet samples using a non-porous metal needle (Chen et al., 2009). Because PESI uses a sharp tip, sample can be collected from a solid material by puncturing the surface and can be used for depth analysis. Similarly, touch spray uses a bent teasing needle for sample collection an ionization (Kerian et al., 2014). The bent tip promotes solvent flow, increasing reliability. Copper wire can be used as a direct probe (DP) for initiating ESI processes by droplet deflection. Analysis of various proteins using DP-ESI has shown that similar spectra are obtained when compared to that of conventional ESI. This technique also does not require a capillary or syringe pump, allows for rapid sample switching, increased analysis time, and is easy to clean and maintain (Hong et al., 1999).
Based on solid phase microextraction (SPME), coated blade spray (CBS) involves a stainless steel sheet cut into a blade (rectangle with one end cut to a tip) that is coated with the biocompatible polymer C18-polyacrylonitrile (Gómez-Ríos and Pawliszyn, 2014). By extracting target analytes from a liquid sample through dipping, or by depositing the sample directly onto the blade by pipette, followed by a rinsing step, the target analyte can be ionized with the applied solvent and voltage (Tascon et al., 2017b). Because stainless steel is conductive, and the blade is not porous, a more consistent electric field can be produced, which may help with a more consistent spray and greater sensitivity. Increased sensitivity is also largely due to the preconcentration of analyte and the decrease in matrix effects stemming from its SPME nature (Gómez-Ríos and Pawliszyn, 2014). In a 300 μL sample, a larger amount of analyte can be extracted compared to a 10 mL sample that may be deposited onto a paper triangle. Differential adsorption to a strategically chosen coating causes a larger absolute number of analyte molecules to adsorb, which increases the ultimate intensity of signal during mass spectrometry. A subsequent wash step releases matrix molecules and leaves the adsorbed analyte molecules preferentially. Additionally, the blade is re-usable, which may counteract the increase in price compared to traditional paper spray (Figure 16).
Figure 16.
Coated Blade Spray workflow and schematic (Reprinted with permission from Gómez-Rios et. al, 2014, copyright 2014 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.).
The coating used is not restricted to simple C18 polymer, as demonstrated by the use of hydrophilic lipophilic balance particles and polyacrylonitrile to analyze voriconazole (Tascon et al., 2017b), a mixture of anabolic molecules, β−2 agonists, diuretics, stimulants, narcotics, and β-blockers (Tascon et al., 2017a), residual wastewater contaminant pharmaceuticals including paroxetine, fluoxetine, diazepam, and sertraline (Poole et al., 2017), drugs of abuse such as methamphetamine, buprenorphine, fentanyl, clenbuterol, and oxycodone (Gómez-Ríos et al., 2017a), and immunosuppressive drugs tacrolimus, cyclosporine-A, sirolimus, and everolimus (Gómez-Ríos et al., 2018).
4. Other Cellulose-Based Materials
To better analyze plant samples, leaf spray was developed as a method which uses a living leaf as both ionization substrate and sample (Liu et al., 2011). Because leaves often naturally develop tips that are similar to those of paper triangles, applying a suitable solvent (or, in some cases, no solvent due to the plant’s high water content) and voltage resulted in the extraction and ionization of the compounds naturally found in the plant cells. Alternatively, the leaves can easily be cut into triangles if a natural tip is not present. Analysis of amino acids, carbohydrates, fatty acids, alkaloids, and lipids was demonstrated by utilizing both positive and negative mode. The utility of this method shows that often the native sample may be used directly in ionization rather than utilizing external extraction/separation procedures when analyzing with mass spectrometry during an initial survey. Although leaf spray is not useful for the analysis of the attractive biofluid, applications in agricultural impact could be quite significant. Without sample pretreatment such as grinding, offline extraction, or application to external sampling platforms, a relatively quick analysis of the native leaves on a plant can reveal insight to pesticide residues, glycosides, allergens, and potentially other molecules relevant to consumers for quality control that could impact entire populations, save those that are not able to be easily ionized (Malaj et al., 2012; Sarkar et al., 2012; Tadjimukhamedov et al., 2012; Zhang et al., 2012a, 2013; Snyder et al., 2015).
Among the first alternative materials used for electrospray-based ionization is wooden tip spray using a toothpick (Hu et al., 2011; Chen et al., 2013). Wooden tips can ionize samples either directly applied to the surface through pipette, or they can extract analytes through a process similar to blade spray from a solution. Wood is similar to paper, in that it has pores and hydroxyl groups on the surface that contribute to strong interactions with organic compounds. Because of this, modification of the surface with C18, NH2, SO3H to give a hydrophobic, basic, and acidic groups respectively was used to ionize a larger variety of molecules compared to unmodified wooden tip spray (Hu et al., 2018). Because toothpicks are cylindrical, investigation of separation along the z-axis showed the wood can be used as a solid phase for longitudinal extraction (Hu et al., 2013).
As an alternative to the typical Scoville method, thread spray was developed to detect capsaicinoid levels in pepper products and to be used as a general forensic detection method for thread-based clothing items (Jackson et al., 2018). Ionization directly from a thread that had been freed from an article of clothing and placed in a capillary with a solvent and applied voltage is possible, reducing sample preparation steps such as extraction and offline ionization. Other advantages of using thread include extended solvent lifetime and the natural sharp tip due to the cylindrical nature of thread and the small diameter. Extended solvent is due to the encapsulation of the thread in a capillary, reducing contact between solvent and air and thus limiting evaporation. A similar method has been developed to be paired with paper spray to extend solvent lifetime (Cao and Huang, 2018).
MICROFLUID DEVICES
Paper-based microfluidic devices are advantageous due to (i) the passive wicking of fluids due to capillary force without external pumps; (ii) the ability to analyze complex samples directly without sample preparation; (iii) the ability to easily pair the device to mass spectrometry through paper spray. The development of this field has led to point-of-care microfluidic devices that are inexpensive and easy to transport. Previous microfluidic paper-based devices have been paired with colorimetric, electrochemical, and chemiluminescent detection, providing only semi-quantitative results (Ellerbee et al., 2009; Steffens et al., 2009; Arena et al., 2010; Delaney et al., 2011; Ge et al., 2012; Lan et al., 2013; Thom et al., 2014). In contrast, pairing with a mass spectrometer increases reliability of results.
Through the use of a wax printer, hydrophobic barriers containing hydrophilic (paper) channels are easily designed and fabricated. This method was proposed to confine the spray solvent during PS by wax-printing the edges of the paper triangle to produce a narrow channel, resulting in an altered electric field (Damon et al., 2016b). This resulted in a lower DC voltage required to ionize amphetamine, methamphetamine, cocaine, and metaldehyde. The confined area also slowed solvent evaporation, resulting in an increase from 1.5 minutes on normal paper without wax to 10 minutes on wax printed paper when using 20 μL of 1:1 MeOH/H2O v/v. To further expand the microfluidic diagnostic device motif, the wax-printed triangle was paired with microfluidic wells that contain capture and detection antibodies for the detection of target antigens (Chen et al., 2016). The capture antibody, immobilized on the paper surface, was used to isolate the antigen Plasmodium falciparum histidine-rich protein 2 (PfHRP2), cancer antigen 125, and carcinoembryonic antigen. A complimentary detection antibody with a hydrolytically cleavable ionic probe was then applied to form a sandwich configuration. The microfluidic wells were then stacked on top of a paper triangle, and a basic solution was applied to the top of the microfluidic device, resulting in the cleavage, elution, and spray of the ionic probe into the mass spectrometer, producing a diagnostic peak if the antigen was present. This method of using ionic probes and antibodies without any detection enzymes was shown to be stable in the dry state after 30 days of storage.
Confining the wettable area of the PS device may contribute to increased sensitivity due to decreased dilution of the sample. This was demonstrated with wax printed paper (Damon et al., 2016b) and by using paraffin barriers (Colletes et al., 2016). Paraffin barriers are created by immersing a paper triangle into a paraffin solution, and then stacking the paraffined paper on a native, non-paraffined paper. A metal stamp in the design of the microfluidic channel barriers at 150°C was used to melt the paraffin onto the native paper, creating hydrophobic barriers in that design. When comparing the paraffin paper with microfluidic channels with normal paper, the microfluidic channels caused a decrease in the limit of detection and quantification by an order of magnitude when quantifying glucose. Results showed the paraffin barriers on paper spray had similar quantification capabilities compared to high performance liquid chromatography with an ultraviolet detector for glucose (Colletes et al., 2016). Similarly, wax printed paper showed an order of magnitude decrease in LOD and LOQ when quantifying amphetamine, methamphetamine, cocaine, and benzoylecgonine (Damon et al., 2016b). These results are likely due to the increase in electric field at the tip of the paper in addition to the limited solvent/analyte spreading, reducing redeposition of analyte onto the paper in areas not capable of ionization. The linear path forces the analyte forward toward the mass spectrometer, and the inaccessible rear corners of the paper cannot cause sample to be lost to ambient air.
EXTERNALLY PAIRED DEVICES
A. Cartridges
Use of cartridges paired with paper spray has previously been outlined extensively in a review by Manicke et al.(Manicke et al., 2016). Briefly, use of a cartridge in tandem with a paper triangle for ionization introduces the possibility of orthogonal sample pretreatment and further reduction of matrix effects in addition to those observed with paper spray alone. Additionally, a paper triangle alone cannot be used in an automated setup, increasing the expertise requirement of the operator and decreasing productivity. The encasement of the triangle in a cartridge introduces the possibility of loading the samples into an autosampler. A dual purpose cartridge that holds a paper triangle and solvent reservoir has been described (Salentijn et al., 2014). Continuous contact with the solvent was achieved with a hydrophilic wick, increasing spray time to tens of minutes. This continuous flow of solvent allows slowly eluted analytes to make their way to the tip of the triangle.
Further development of cartridges has externally paired paper spray with solid phase extraction (Zhang and Manicke, 2015; Zhang et al., 2017a). Biological sample was flowed through an HLB and cellulose-based solid phase extraction column where analyte was captured and pre-concentrated while the matrix was absorbed into a waste pad. The column was then moved above a paper triangle, and the analyte was washed out and ionized. Although a wash step is not used, it could be included to further increase sensitivity of this method. The simultaneous extraction and pre-concentration lead to a 14–70-fold decrease in limit of detection for carbamazepine, atenolol, sulfamethazine, diazepam, and alprazolam. A similar separation prior to paper spray was used to fractionate blood for plasma analysis (Bills and Manicke, 2016). A membrane composed of materials such as polymers or natural synthetic fibers were used to separate plasma in just a drop of blood.
B. Automation and High Throughput
Few methods of automation of paper spray have been proposed. A platform was introduced in which a moving state supporting several paper triangles as they come into contact with a metal electrode positioned in front of the mass spectrometer inlet was introduced, but the design did not gain much traction (Shen et al., 2013). Another contactless electrode design was recently introduced to induce a voltage on the paper surface (Zhu and Huang, 2018). The first commercially available paper spray automation platform is that of Prosolia, the Velox 360, where each paper triangle is contained in a cartridge, and a magazine containing these cartridges interfaces with the front of the mass spectrometer (Prieto Conaway et al., 2014; Zhang et al., 2017a). Solvent reservoirs wet the paper triangle, and a metal electrode in the cartridge supplies the voltage when it is in front of the mass spectrometer. After the paper triangles are analyzed, the cartridges drop down, and the next sample can be analyzed. More recently, Thermo Scientific developed a paper spray automation platform, the VeriSpray, capable of performing high throughput analysis without the need for sample preparation. To demonstrate the robustness of this automation platform, controlled substances and antifungal drugs in clinical samples were quantitatively measured. The results showed that sensitivity, accuracy, and precision are maintained for a large sample size, making this platform reliable and reproducible (Greta Ren et al., 2019b, 2019a, 2019c).
CONCLUSIONS
Paper is a versatile material that has been used extensively for biomedical applications, especially in newborn screening. The current trend to move towards a more personalized healthcare system and to open diagnosis to a wider group of people have increased interest in the use of paper in the development of low-cost, field-ready devices. Many methods have been used for signal transduction for analytes collected and processed on paper substrates, which include colorimetric, electrochemistry, conductivity, etc. By far, mass spectrometry detection has made the largest impact due to its sensitivity and selectivity, thereby reducing false-positive and false-negative outcomes. This capability has greatly expanded applicability of paper substrates from biomedical to food, environmental, and forensic applications as well as chemical reaction monitoring. Whereas this review focuses largely on dried blood spot sampling, storage and analysis, we have also dedicated substantial space in creating the awareness that the performance of the paper substrate can be greatly improved via simple chemical treatments that do not significantly change the physical properties (e.g., color or tensile strength) of the paper material. We have further discussed other material that are equally suitable for small sample collection and analysis. The analytical merits for different substrates in terms of small molecule analysis has been provided, alongside the mass spectrometer used for each study. Advancement of research in this area should culminate in the fabrication of devices that are deliverable to a wider group, including the underserved populations. In this regards, miniaturized instrumentation will play crucial roles.
Table 1.
Explanation of acronyms used in Figure 5.
| Acronym | Method | Comment |
|---|---|---|
| SLE | Solid-liquid extraction | Use of aqueous/organic mixtures to preferentially extract target analytes. |
| FIA | Flow injection analysis | A high throughput analysis method, often coupled to tandem MS for biomarker quantification; useful for screening of diseases. Potential for autosampler carry-over for performing online derivatizations. |
| MALDI | Matrix-assisted laser desorption ionization | High sample throughput for small pharmaceuticals, and not susceptible to ionization suppression by salts and buffers. Singly-charged species dominate for quantitation of biomolecules. |
| LDTD | Laser diode thermal desorption | High throughput (~9 s/sample), used for pharmaceutical discovery as alternative to plasma-based sources. |
| APCI | Atmospheric pressure chemical ionization | Enhanced detection of low-abundance molecules in small volume samples; capable of coupling to LC. |
| ICP | Inductively coupled plasma | Laser ablation allows ICP to be used for DBS analysis; causes atomization and ionization of gas-phase atoms. Typically used for metal analysis. |
| LC | Liquid chromatography | Allows highly sensitive detection when coupled to MS by reducing matrix effects. Pre-treatment of complex mixture required. |
| MS | Mass spectrometry | A versatile approach to chemical detection based on molecular weight measurements and fragmentation patterns. |
| GC | Gas chromatography | Allows highly sensitive detection of small molecules when coupled to MS. requires extensive sample preparation including but not limited to pre-derivatization and solid-liquid extractions. |
| SSSP | Sealing surface-sampling probe | Elimination of inhomogeneity by sampling entire DBS. Compatible with LC separations. |
| LMJ-SSP | Liquid microjunction surface-sampling probe | Better suited for non-porous/hard surfaces; incompatible with LC-separations. |
| CE | Capillary electrophoresis | Highly sensitive and does not require sample extraction; well suited for genotyping from small microvolumes. Reproducibility may be limited by adsorption of serum components and other matrix components in capillary wall. |
| ESI | Electrospray ionization | Commonly interfaced with LC for analysis of DBS, after analyte extraction from paper substrate |
| DESI | Desorption electrospray ionization | Uses ESI droplets to enable direct analysis of DBS without prior extraction or treatment. |
| DART | Direct analysis in real time | Uses metastable helium gas to enable direct analysis of DBS without prior treatment. |
| APTDCI | Atmospheric pressure thermal desorption chemical ionization | Enables qualitative and quantitative analysis of lipophilic compounds such as sterols in newborn screening. Works well for ionization of low proton-affinity compounds. |
| LA-ICP | Laser ablation inductively coupled plasma | Detection of trace metals (e.g., lead) in blood. Limited by matrix effects of sample but requires little to no sample preparation. Analysis of DBS in solid state. |
ACKNOWLEDGMENT
The authors thank the Ohio State University start-up funds and support by National Institute of General Medical Sciences (Award Number R35-GM-133647) and National Institute of Allergy and Infectious Diseases (Award Number R01-AI-143809).
ABBREVIATIONS
- APCI
atmospheric pressure chemical ionization
- CBS
coated blade spray
- CE
capillary electrophoresis
- CMS
capillary microsampling
- CWA
chemical warfare agents
- DART
direct analysis in real time
- DBS
dried blood spot
- DC
direct current
- DESI
desorption electrospray ionization
- DLD
deterministic lateral deviation
- DPS
dried plasma spot
- ESI
electrospray ionization
- FDA
Food and Drug Administration
- FIA
flow injection analysis
- GC
gas chromatography
- IS
internal standards
- LC
liquid chromatography
- TLC
thin layer chromatography
- LMJ-SSP
liquid microjunction surface sampling probe
- LOD
limit of detection
- LOQ
limit of quantification
- MIPs
molecularly imprinted polymers
- MOF
metal organic frameworks
- MRM
multiple reaction monitoring
- MS
mass spectrometry
- MS/MS
tandem MS
- NBS
newborn screening
- NT
nanotubes
- OSX
organic-inorganic hybrid organosiloxane
- PCR
polymerase chain reaction
- PESI
probe electrospray ionization
- PfHRP2
Plasmodium falciparum histidine-rich protein 2
- PK
pharmacokinetics
- PS
paper spray
- Pt
platinum
- RBCs
red blood cells
- S/N
signal-to-noise ratio
- SEC
size exclusion chromatography
- SPME
solid phase microextraction
- SRM
single reaction monitoring
- SSSP
sealing surface-sampling probe
- TK
toxicokinetic
- TNT
trinitrotoluene
- VAMS
volumetric absorptive microsampling
REFERENCES
- Adam BW, Alexander JR, Smith SJ, Chace DH, Loeber JG, Elvers LH, and Hannon WH 2000. Recoveries of Phenylalanine from Two Sets of Dried-Blood Spot Reference Materials: Predition from Hematocrit, Spot Volume, and Paper Matrix. Clinical Chemistry. 46:126–128. [PubMed] [Google Scholar]
- Adam BW, Hall EM, Sternberg M, Lim TH, Flores SR, O’Brien S, Simms D, Li LX, De Jesus VR, and Hannon WH 2011. The stability of markers in dried-blood spots for recommended newborn screening disorders in the United States. Clinical Biochemistry. 44:1445–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberici RM, Simas RC, Sanvido GB, Romão W, Lalli PM, Benassi M, Cunha IBS, and Eberlin MN 2010. Ambient mass spectrometry: bringing MS into the “real world.” Anal Bioanal Chem. 398:265–294. [DOI] [PubMed] [Google Scholar]
- Alfazil AA, and Anderson RA 2008. Stability of Benzodiazepines and Cocaine in Blood Spots Stored on Filter Paper. Journal of Analytical Toxicology. 32:511–515. [DOI] [PubMed] [Google Scholar]
- Aliaga-Aguilar H 2018. Characterization and Analysis of Paper Spray Ionization of Organic Compounds. Journal of The American Society for Mass Spectrometry. 29:17–25. [DOI] [PubMed] [Google Scholar]
- Allard P, Grenier A, Korson MS, and Zytkovicz TH 2004. Newborn screening for hepatorenal tyrosinemia by tandem mass spectrometry: analysis of succinylacetone extracted from dried blood spots. Clinical Biochemistry. 37:1010–1015. [DOI] [PubMed] [Google Scholar]
- Arena A, Donato N, Saitta G, Bonavita A, Rizzo G, and Neri G 2010. Flexible ethanol sensors on glossy paper substrates operating at room temperature. Sens Actuators B: Chem. 145:488–494. [Google Scholar]
- Bag S, Hendricks P, Reynolds JC, and Cooks R 2015. Biogenic aldehyde determination by reactive paper spray ionization mass spectrometry. Anal Chim Acta. 860:37–42. [DOI] [PubMed] [Google Scholar]
- Bain RM, Pulliam CJ, Raab SA, and Cooks RG 2016. Chemical Synthesis Accelerated by Paper Spray: The Haloform Reaction. Journal of Chemical Education. 93:340–344. [Google Scholar]
- Banerjee S, Basheer C, and Zare RN 2016. A Study of Heterogeneous Catalysis by Nanoparticle-Embedded Paper-Spray Ionization Mass Spectrometry. Angew Chem Int Ed. 55:12807–12811. [DOI] [PubMed] [Google Scholar]
- Barco S, Castagnola E, Moscatelli A, Rudge J, Tripodi G, and Cangemi G 2017. Volumetric adsorptive microsampling-liquid chromatography tandem mass spectrometry assay for the simultaneous quantification of four antibiotics in human blood: Method development, validation and comparison with dried blood spot. Journal of Pharmaceutical and Biomedical Analysis. 145:704–710. [DOI] [PubMed] [Google Scholar]
- Barfield M, Spooner N, Lad R, Parry S, and Fowles S 2008. Application of dried blood spots combined with HPLC-MS/MS for the quantification of acetaminophen in toxicokinetic studies. Journal of Chromatography B. 870:32–37. [DOI] [PubMed] [Google Scholar]
- Basuri P, Sarkar D, Paramasivam G, and Pradeep T 2018. Detection of Hydrocarbons by Laser Assisted Paper Spray Ionization Mass Spectrometry (LAPSI MS). Analytical Chemistry. 90:4663–4668. [DOI] [PubMed] [Google Scholar]
- Bellisario R, Colinas RJ, and Pass KA 2001. Simultaneous measurement of antibodies to three HIV-1 antigens in newborn dried blood-spot specimens using a multiplexed microsphere-based immunoassay. Early Human Development. 64:21–25. [DOI] [PubMed] [Google Scholar]
- Bills BJ, Kinkade J, Ren G, and Manicke NE 2018. The impacts of paper properties on matrix effects during paper spray mass spectrometry analysis of prescription drugs, fentanyl and synthetic canabinoids. Forensic Chemistry. 11:15–22. [Google Scholar]
- Bills BJ, and Manicke NE 2016. Development of a prototype blood fractionation cartridge for plasma analysis by paper spray mass spectrometry. Clinical Mass Spectrometry. 2:18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björkesten J, Enroth S, Shen Q, Wik L, Hougaard DM, Cohen AS, Sörensen L, Giedraitis V, Ingelsson M, Larsson A, et al. 2017. Stability of Proteins in Dried Blood Spot Biobanks. Molecular & Cellular Proteomics. 16:1286–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard S, Sadilek M, Scott CR, Turecek F, and Gelb MH 2008. Tandem Mass Spectrometry for the Direct Assay of Lysosomal Enzymes in Dried Blood Spots: Application to Screening Newborns for Mucopolysaccharidosis I. Clinical Chemistry. 54:2067–2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen CL, Licea-Perez H, Karlinsey MZ, Jurusik K, Pierre E, Siple J, Kenney J, Stokes A, Spooner N, and Evans CA 2013. A novel approach to capillary plasma microsampling for quantitative bioanalysis. Bioanalysis. 5:1131–1135. [DOI] [PubMed] [Google Scholar]
- Broek, van den I, Fu Q, Kushon S, Kowalski MP, Millis K, Percy A, Holewinski RJ, Venkatraman V, and Van Eyk JE 2017. Application of volumetric absorptive microsampling for robust, high-throughput mass spectrometric quantification of circulating protein biomarkers. Clinical Mass Spectrometry. 4–5:25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, and Huang G 2018. A facile approach to improve the spray time and stability of paper spray ionization mass spectrometry with a Teflon tube. Analytical Methods. 10:5540–5546. [Google Scholar]
- Capiau S, Stove VV, Lambert WE, and Stove CP 2013. Prediction of the Hematocrit of Dried Blood Spots via Potassium Measurement on a Routine Clinical Chemistry Analyzer. Anal Chem. 85:404–410. [DOI] [PubMed] [Google Scholar]
- Capiau S, Wilk LS, Aalders MCG, and Stove CP 2016. A Novel, Nondestructive, Dried Blood Spot-Based Hematocrit Prediction Method Using Noncontact Diffuse Reflectance Spectroscopy. Anal Chem. 88:6538–6546. [DOI] [PubMed] [Google Scholar]
- Carducci C, Santagata S, Leuzzi V, Carducci C, Artiola C, Giovanniello T, Battini R, and Antonozzi I 2006. Quantitative determination of guanidinoacetate and creatine in dried blood spot by flow injection analysis-electrospray tandem mass spectrometry. Clinica Chimica Acta. 364:180–187. [DOI] [PubMed] [Google Scholar]
- Carvalho TC, Oliveira IF, Tose LV, Vanini G, Kill JB, Neto AC, Machado LF, Ambrosio JCL, Lacerda V, Vaz BG, et al. 2016. Qualitative analysis of designer drugs by paper spray ionisation mass spectrometry (PSI-MS). Anal Methods. 8:614–620. [Google Scholar]
- Castilhos, de CD, Mezzalira J, Goldim MPS, and Coelho JC 2011. Influence of pre-analytical factors on α-galactosidase A, arylsulfatase B and α-glucosidase activities measured on dried blood spots on filter paper. Clinical Biochemistry. 44:922–926. [DOI] [PubMed] [Google Scholar]
- Castillo-Mancilla JR, Zheng J-H, Rower JE, Meditz A, Gardner EM, Predhomme J, Fernandez C, Langness J, Kiser JJ, Bushman LR, et al. 2013. Tenofovir, Emtricitabine, and Tenofovir Diphosphate in Dried Blood Spots for Determining Recent and Cumulative Drug Exposure. AIDS Research and Human Retroviruses. 29:384–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro A.C. de, Borges L.G. dos A., Souza R. da S. de, Grudzinski M, and D’Azevedo PA 2008. Evaluation of the human immunodeficiency virus type 1 and 2 antibodies detection in dried whole blood spots (dbs) samples. Revista Do Instituto de Medicina Tropical de São Paulo. 50:151–156. [DOI] [PubMed] [Google Scholar]
- Cernik AA, and Sayers MHP 1971. Determination of lead in capillary blood using a paper punched disc atomic absorption technique: Application to the supervision of lead workers. Occupational and Environmental Medicine. 28:392–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chace DH 2003. Use of Tandem Mass Spectrometry for Multianalyte Screening of Dried Blood Specimens from Newborns. Clinical Chemistry. 49:1797–1817. [DOI] [PubMed] [Google Scholar]
- Chalcraft KR, and Britz-McKibbin P 2009. Newborn Screening of Inborn Errors of Metabolism by Capillary Electrophoresis−Electrospray Ionization-Mass Spectrometry: A Second-Tier Method with Improved Specificity and Sensitivity. Analytical Chemistry. 81:307–314. [DOI] [PubMed] [Google Scholar]
- Chapman K, Chivers S, Gliddon D, Mitchell D, Robinson S, Sangster T, Sparrow S, Spooner N, and Wilson A 2014. Overcoming the barriers to the uptake of nonclinical microsampling in regulatory safety studies. Drug Discovery Today. 19:528–532. [DOI] [PubMed] [Google Scholar]
- Chen H-K, Lin C-H, Liu J-T, and Lin C-H 2013. Electrospray ionization using a bamboo pen nib. International Journal of Mass Spectrometry. 356:37–40. [Google Scholar]
- Chen LC, Yoshimura K, Yu Z, Iwata R, Ito H, Suzuki H, Mori K, Ariyada O, Takeda S, Kubota T, et al. 2009. Ambient imaging mass spectrometry by electrospray ionization using solid needle as sampling probe. Journal of Mass Spectrometry. 44:1469–1477. [DOI] [PubMed] [Google Scholar]
- Chen S, Chang Q, Yin K, He Q, Deng Y, Chen B, Liu C, Wang Y, and Wang L 2017. Rapid Analysis of Bisphenol A and Its Analogues in Food Packaging Products by Paper Spray Ionization Mass Spectrometry. Journal of Agricultural and Food Chemistry. 65:4859–4865. [DOI] [PubMed] [Google Scholar]
- Chen S, Wan Q, and Badu-Tawiah AK 2016. Mass Spectrometry for Paper-Based Immunoassays: Toward On-Demand Diagnosis. J Am Chem Soc. 138:6356–6359. [DOI] [PubMed] [Google Scholar]
- Chernetsova ES, and Morlock GE 2011. Ambient desorption ionization mass spectrometry (DART, DESI) and its bioanalytical applications. Bioanalytical Reviews. 3:1–9. [Google Scholar]
- Chipuk JE, Gelb MH, and Brodbelt JS 2010a. Rapid and Selective Screening for Sulfhydryl Analytes in Plasma and Urine Using Surface-Enhanced Transmission Mode Desorption Electrospray Ionization Mass Spectrometry. Analytical Chemistry. 82:4130–4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipuk JE, Gelb MH, and Brodbelt JS 2010b. Surface-Enhanced Transmission Mode Desorption Electrospray Ionization: Increasing the Specificity of Ambient Ionization Mass Spectrometric Analyses. Analytical Chemistry. 82:16–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb Z, Vries R. de, Spooner N, Williams S, Staelens L, Doig M, Broadhurst R, Barfield M, Merbel, van de N, Schmid B, et al. 2013. In-depth study of homogeneity in DBS using two different techniques: results from the EBF DBS-microsampling consortium. Bioanalysis. 5:2161–2169. [DOI] [PubMed] [Google Scholar]
- Cody RB, Laramée JA, and Durst HD 2005. Versatile New Ion Source for the Analysis of Materials in Open Air under Ambient Conditions. Anal Chem. 77:2297–2302. [DOI] [PubMed] [Google Scholar]
- Cody RB, Tamura J, and Downard KM 2018. Quantitation of anthocyanins in elderberry fruit extracts and nutraceutical formulations with paper spray ionization mass spectrometry. Journal of Mass Spectrometry. 53:58–64. [DOI] [PubMed] [Google Scholar]
- Colletes TC, Garcia PT, Campanha RB, Abdelnur PV, Romão W, Coltro WKT, and Vaz BG 2016. A new insert sample approach to paper spray mass spectrometry: a paper substrate with paraffin barriers. Analyst. 141:1707–1713. [DOI] [PubMed] [Google Scholar]
- Costa C, Webb R, Palitsin V, Ismail M, Puit M. de, Atkinson S, and Bailey MJ 2017. Rapid, Secure Drug Testing Using Fingerprint Development and Paper Spray Mass Spectrometry. Clinical Chemistry. 63:1745–1752. [DOI] [PubMed] [Google Scholar]
- Crawford E, Gordon J, Wu J-T, Musselman B, Liu R, and Yu S 2011. Direct analysis in real time coupled with dried spot sampling for bioanalysis in a drug-discovery setting. Bioanalysis. 3:1217–1226. [DOI] [PubMed] [Google Scholar]
- Cunha K.F. da, Eberlin MN, and Costa JL. 2018. Long-term stability of synthetic cathinones in dried blood spots and whole blood samples: a comparative study. Forensic Toxicology. [Google Scholar]
- Dajnoki A, Fekete G, Keutzer J, Orsini JJ, De Jesus VR, Chien Y-H, Hwu W-L, Lukacs Z, Mühl A, Zhang XK, et al. 2010. Newborn screening for Fabry disease by measuring GLA activity using tandem mass spectrometry. Clinica Chimica Acta. 411:1428–1431. [DOI] [PubMed] [Google Scholar]
- Dajnoki A, Muhl A, Fekete G, Keutzer J, Orsini J, DeJesus V, Zhang XK, and Bodamer OA 2008. Newborn Screening for Pompe Disease by Measuring Acid -Glucosidase Activity Using Tandem Mass Spectrometry. Clinical Chemistry. 54:1624–1629. [DOI] [PubMed] [Google Scholar]
- Damon DE, Davis KM, Moreira CR, Capone P, Cruttenden R, and Badu-Tawiah AK 2016a. Direct Biofluid Analysis Using Hydrophobic Paper Spray Mass Spectrometry. Anal Chem. 88:1878–1884. [DOI] [PubMed] [Google Scholar]
- Damon DE, Yin M, Allen DM, Maher YS, Tanny CJ, Oyola-Reynoso S, Smith BL, Maher S, Thuo MM, and Badu-Tawiah AK 2018. Dried Blood Spheroids for Dry-State Room Temperature Stabilization of Microliter Blood Samples. Anal Chem. 90:9353–9358. [DOI] [PubMed] [Google Scholar]
- Damon DE, Maher Yosef S., Yin Mengzhen, Jjunju Fred P. M., Young Iain S., Stephen Taylor, Simon Maher, and Badu-Tawiah Abraham K.. 2016b. 2D wax-printed paper substrates with extended solvent supply capabilities allow enhanced ion signal in paper spray ionization. Analyst. 141:3866–3873. [DOI] [PubMed] [Google Scholar]
- Daniel YA, Turner C, Haynes RM, Hunt BJ, and Dalton RN 2007. Quantification of Hemoglobin A2 by Tandem Mass Spectrometry. Clinical Chemistry. 53:1448–1454. [DOI] [PubMed] [Google Scholar]
- Davis KM, and Badu-Tawiah AK 2017. Direct and Efficient Dehydrogenation of Tetrahydroquinolines and Primary Amines Using Corona Discharge Generated on Ambient Hydrophobic Paper Substrate. Journal of The American Society for Mass Spectrometry. 28:647–654. [DOI] [PubMed] [Google Scholar]
- De Jesus VR, Zhang XK, Keutzer J, Bodamer OA, Mühl A, Orsini JJ, Caggana M, Vogt RF, and Hannon WH 2009. Development and Evaluation of Quality Control Dried Blood Spot Materials in Newborn Screening for Lysosomal Storage Disorders. Clin Chem. 55:158. [DOI] [PubMed] [Google Scholar]
- De Kesel PMM, Capiau S, Stove VV, Lambert WE, and Stove CP 2014. Potassium-based algorithm allows correction for the hematocrit bias in quantitative analysis of caffeine and its major metabolite in dried blood spots. Analytical and Bioanalytical Chemistry. 406:6749–6755. [DOI] [PubMed] [Google Scholar]
- Déglon J, Lauer E, Thomas A, Mangin P, and Staub C 2010. Use of the dried blood spot sampling process coupled with fast gas chromatography and negative-ion chemical ionization tandem mass spectrometry: application to fluoxetine, norfluoxetine, reboxetine, and paroxetine analysis. Analytical and Bioanalytical Chemistry. 396:2523–2532. [DOI] [PubMed] [Google Scholar]
- Déglon J, Versace F, Lauer E, Widmer C, Mangin P, Thomas A, and Staub C 2012. Rapid LC–MS/MS quantification of the major benzodiazepines and their metabolites on dried blood spots using a simple and cost-effective sample pretreatment. Bioanalysis. 4:1337–1350. [DOI] [PubMed] [Google Scholar]
- Delaney JL, Hogan CF, Tian J, and Shen W 2011. Electrogenerated Chemiluminescence Detection in Paper-Based Microfluidic Sensors. Anal Chem. 83:1300–1306. [DOI] [PubMed] [Google Scholar]
- Deng J, and Yang Y 2013. Chemical fingerprint analysis for quality assessment and control of Bansha herbal tea using paper spray mass spectrometry. Anal Chim Acta. 785:82–90. [DOI] [PubMed] [Google Scholar]
- Denniff P, and Spooner N 2014. Volumetric Absorptive Microsampling: A Dried Sample Collection Technique for Quantitative Bioanalysis. Analytical Chemistry. 86:8489–8495. [DOI] [PubMed] [Google Scholar]
- Dhummakupt ES, Carmany DO, Mach PM, Tovar TM, Ploskonka AM, Demond PS, DeCoste JB, and Glaros T 2018. Metal–Organic Framework Modified Glass Substrate for Analysis of Highly Volatile Chemical Warfare Agents by Paper Spray Mass Spectrometry. ACS Appl Mater Interfaces. 10:8359–8365. [DOI] [PubMed] [Google Scholar]
- Dhummakupt ES, Mach PM, Carmany D, Demond PS, Moran TS, Connell T, Wylie HS, Manicke NE, Nilles JM, and Glaros T 2017a. Direct Analysis of Aerosolized Chemical Warfare Simulants Captured on a Modified Glass-Based Substrate by “Paper-Spray” Ionization. Anal Chem. 89:10866–10872. [DOI] [PubMed] [Google Scholar]
- Dhummakupt ES, Mach PM, Carmany D, Demond PS, Moran TS, Connell T, Wylie HS, Manicke NE, Nilles JM, and Glaros T 2017b. Direct Analysis of Aerosolized Chemical Warfare Simulants Captured on a Modified Glass-Based Substrate by “Paper-Spray” Ionization. Anal Chem. 89:10866–10872. [DOI] [PubMed] [Google Scholar]
- Di Donna L, Taverna D, Indelicato S, Napoli A, Sindona G, and Mazzotti F 2017. Rapid assay of resveratrol in red wine by paper spray tandem mass spectrometry and isotope dilution. Food Chemistry. 229:354–357. [DOI] [PubMed] [Google Scholar]
- Domingos E, Carvalho T.C. de, Pereira I, Vasconcelos GA, Thompson CJ, Augusti R, Rodrigues RRT, Tose LV, Santos H, Araujo JR, et al. 2017. Paper spray ionization mass spectrometry applied to forensic chemistry – drugs of abuse, inks and questioned documents. Anal Methods. 9:4400–4409. [Google Scholar]
- Dulay MT, and Zare RN 2017. Polymer-spray mass spectrometric detection and quantitation of hydrophilic compounds and some narcotics. Rapid Communications in Mass Spectrometry. 31:1651–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelbroek PM, Heijden, van der J, and Stolk LML. 2009. Dried Blood Spot Methods in Therapeutic Drug Monitoring: Methods, Assays, and Pitfalls. Ther Drug Monit. 31. [DOI] [PubMed] [Google Scholar]
- Ellefsen KN, Costa J.L. da, Concheiro M, Anizan S, Barnes AJ, Pirard S, Gorelick DA, and Huestis MA 2015. Cocaine and metabolite concentrations in DBS and venous blood after controlled intravenous cocaine administration. Bioanalysis. 7:2041–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellerbee AK, Phillips ST, Siegel AC, Mirica KA, Martinez AW, Striehl P, Jain N, Prentiss M, and Whitesides GM 2009. Quantifying Colorimetric Assays in Paper-Based Microfluidic Devices by Measuring the Transmission of Light through Paper. Anal Chem. 81:8447–8452. [DOI] [PubMed] [Google Scholar]
- Elliott I, Mabey D, Peeling RW, Newton PN, and Smit PW 2014. An Overview of the Clinical Use of Filter Paper in the Diagnosis of Tropical Diseases. The American Journal of Tropical Medicine and Hygiene. 90:195–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elst, van der KCM, Span LFR, Hateren K. van, Vermeulen KM, Werf T.S. van der, Greijdanus B, Kosterink JGW, Uges DRA, and Alffenaar J-WC. 2013. Dried Blood Spot Analysis Suitable for Therapeutic Drug Monitoring of Voriconazole, Fluconazole, and Posaconazole. Antimicrobial Agents and Chemotherapy. 57:4999–5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espy RD, Manicke NE, Ouyang Z, and Cooks RG 2012a. Rapid analysis of whole blood by paper spray mass spectrometry for point-of-care therapeutic drug monitoring. Analyst. 137:2344–2349. [DOI] [PubMed] [Google Scholar]
- Espy RD, Muliadi AR, Ouyang Z, and Cooks RG 2012b. Spray mechanism in paper spray ionization. Int J Mass Spectrom. 325–327:167–171. [Google Scholar]
- Espy RD, Teunissen SF, Manicke NE, Ren Y, Ouyang Z, Asten, van A, and Cooks RG. 2014. Paper Spray and Extraction Spray Mass Spectrometry for the Direct and Simultaneous Quantification of Eight Drugs of Abuse in Whole Blood. Anal Chem. 86:7712–7718. [DOI] [PubMed] [Google Scholar]
- Fan L, and Lee JA 2012. Managing the effect of hematocrit on DBS analysis in a regulated environment. Bioanalysis. 4:345–347. [DOI] [PubMed] [Google Scholar]
- Fang K, Bowen CL, Kellie JF, Karlinsey MZ, and Evans CA 2018. Drug monitoring by volumetric absorptive microsampling: method development considerations to mitigate hematocrit effects. Bioanalysis. 10:241–255. [DOI] [PubMed] [Google Scholar]
- Feider CL, Krieger A, DeHoog RJ, and Eberlin LS 2019. Ambient Ionization Mass Spectrometry: Recent Developments and Applications. Analytical Chemistry. 91:4266–4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapierre Florian, Hon Wei Boon, David Melville, and Gooley Andrew A.. Collection and storage of volumetrically accurate and precise dried blood spots in a contained device: hemaPEN®. [Google Scholar]
- Forni S, Fu X, Palmer SE, and Sweetman L 2010. Rapid determination of C4-acylcarnitine and C5-acylcarnitine isomers in plasma and dried blood spots by UPLC–MS/MS as a second tier test following flow-injection MS/MS acylcarnitine profile analysis. Molecular Genetics and Metabolism. 101:25–32. [DOI] [PubMed] [Google Scholar]
- Fujita M, Brindle E, Shofer J, Ndemwa P, Kombe Y, Shell-Duncan B, and O’Connor KA 2007. Retinol-Binding Protein Stability in Dried Blood Spots. Clinical Chemistry. 53:1972–1975. [DOI] [PubMed] [Google Scholar]
- Gaissmaier T, Siebenhaar M, Todorova V, Hüllen V, and Hopf C 2016. Therapeutic drug monitoring in dried blood spots using liquid microjunction surface sampling and high resolution mass spectrometry. The Analyst. 141:892–901. [DOI] [PubMed] [Google Scholar]
- Ge L, Wang S, Song X, Ge S, and Yu J 2012. 3D Origami-based multifunction-integrated immunodevice: low-cost and multiplexed sandwich chemiluminescence immunoassay on microfluidic paper-based analytical device. Lab Chip. 12:3150–3158. [DOI] [PubMed] [Google Scholar]
- Gómez-Ríos GA, and Pawliszyn J 2014. Development of Coated Blade Spray Ionization Mass Spectrometry for the Quantitation of Target Analytes Present in Complex Matrices. Angew Chem Int Ed. 53:14503–14507. [DOI] [PubMed] [Google Scholar]
- Gómez-Ríos GA, Tascon M, Reyes-Garcés N, Boyacı E, Poole J, and Pawliszyn J 2017a. Quantitative analysis of biofluid spots by coated blade spray mass spectrometry, a new approach to rapid screening. Sci Rep. 7:16104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Ríos GA, Tascon M, Reyes-Garcés N, Boyacı E, Poole JJ, and Pawliszyn J 2018. Rapid determination of immunosuppressive drug concentrations in whole blood by coated blade spray-tandem mass spectrometry (CBS-MS/MS). Anal Chim Acta. 999:69–75. [DOI] [PubMed] [Google Scholar]
- Gómez-Ríos GA, Vasiljevic T, Gionfriddo E, Yu M, and Pawliszyn J 2017b. Towards on-site analysis of complex matrices by solid-phase microextraction-transmission mode coupled to a portable mass spectrometer via direct analysis in real time. The Analyst. 142:2928–2935. [DOI] [PubMed] [Google Scholar]
- Greenland P, Bewley NL, Melklejohn B, and Deane KL Blood Cholesterol Concentration: Fingerstick Plasma vs Venous Serum Sampling. 3. [PubMed] [Google Scholar]
- Ren Greta, Manicke Nicholas, Boeser Cornelia, and Wijeratne Neloni R.. 2019a. Detection of controlled substances in blood samples using the VeriSpray ion source with TSQ Altis MS for clinical research and forensic toxicology. [Google Scholar]
- Ren Greta, Manicke Nicholas, Boeser Cornelia, and Wijeratne Neloni R.. 2019b. Quantitative analysis of antifungal drugs using PaperSpray tandem mass spectrometry for clinical research. [Google Scholar]
- Ren Greta, Manicke Nicholas, Boeser Cornelia, and Wijeratne Neloni R.. 2019c. Robustness characterization of a quantitative whole blood assay using PaperSpray technology for clinical research. [Google Scholar]
- Guo T, Zhang Z, Yannell KE, Dong Y, and Cooks RG 2017. Paper spray ionization mass spectrometry for rapid quantification of illegal beverage dyes. Analytical Methods. 9:6273–6279. [Google Scholar]
- Guthrie R, and Susi A 1963. A Simple Phenylalanine Method for Detecting Phenylketonuria in Large Populations of Newborn Infants. Pediatrics. 32:338–343. [PubMed] [Google Scholar]
- Hajslova J, Cajka T, and Vaclavik L 2011. Challenging applications offered by direct analysis in real time (DART) in food-quality and safety analysis. TrAC Trends in Analytical Chemistry. 30:204–218. [Google Scholar]
- Hamers RL Drug-resistant HIV-1 in sub-Saharan Africa. Clinical and public health studies. 23. [Google Scholar]
- Hamid AM, Jarmusch AK, Pirro V, Pincus DH, Clay BG, Gervasi G, and Cooks RG 2014. Rapid Discrimination of Bacteria by Paper Spray Mass Spectrometry. Anal Chem. 86:7500–7507. [DOI] [PubMed] [Google Scholar]
- Hamilton J, Jones I, Srivastava R, and Galloway P 2012. A new method for the measurement of lysosomal acid lipase in dried blood spots using the inhibitor Lalistat 2. Clinica Chimica Acta. 413:1207–1210. [DOI] [PubMed] [Google Scholar]
- Han F, Yang Y, Ouyang J, and Na N 2015. Direct analysis of in-gel proteins by carbon nanotubes-modified paper spray ambient mass spectrometry. Analyst. 140:710–715. [DOI] [PubMed] [Google Scholar]
- Hannon WH, and Clinical and Laboratory Standards Institute. 2013. Blood collection on filter paper for newborn screening programs: approved standard. Wayne, PA: Clinical and Laboratory Standards Institute. [Google Scholar]
- Harper JD, Charipar NA, Mulligan CC, Zhang X, Cooks RG, and Ouyang Z 2008. Low-Temperature Plasma Probe for Ambient Desorption Ionization. Anal Chem. 80:9097–9104. [DOI] [PubMed] [Google Scholar]
- Heijden J. van der, Beer Y. de, Hoogtanders K, Christiaans M, Jong G.J. de, Neef C, and Stolk L 2009. Therapeutic drug monitoring of everolimus using the dried blood spot method in combination with liquid chromatography–mass spectrometry. Journal of Pharmaceutical and Biomedical Analysis. 50:664–670. [DOI] [PubMed] [Google Scholar]
- Heine R. ter, Alderden-Los CG, Rosing H, Hillebrand MJX, Gorp E.C.M. van, Huitema ADR, and Beijnen JH 2007. Fast and simultaneous determination of darunavir and eleven other antiretroviral drugs for therapeutic drug monitoring: method development and validation for the determination of all currently approved HIV protease inhibitors and non-nucleoside reverse transcriptase inhibitors in human plasma by liquid chromatography coupled with electrospray ionization tandem mass spectrometry. Rapid Communications in Mass Spectrometry. 21:2505–2514. [DOI] [PubMed] [Google Scholar]
- Heine R. ter, Hillebrand MJX, Rosing H, Gorp E.C.M. van, Mulder JW, Beijnen JH, and Huitema ADR. 2009a. Quantification of the HIV-integrase inhibitor raltegravir and detection of its main metabolite in human plasma, dried blood spots and peripheral blood mononuclear cell lysate by means of high-performance liquid chromatography tandem mass spectrometry. Journal of Pharmaceutical and Biomedical Analysis. 49:451–458. [DOI] [PubMed] [Google Scholar]
- Heine R. ter, Mulder JW, Gorp E.C. van, Wagenaar JF, Beijnen JH, and Huitema AD 2011. Clinical evaluation of the determination of plasma concentrations of darunavir, etravirine, raltegravir and ritonavir in dried blood spot samples. Bioanalysis. 3:1093–1097. [DOI] [PubMed] [Google Scholar]
- Heine R. ter, Rosing H, Gorp E.C.M. van, Mulder JW, Beijnen JH, and Huitema ADR 2009b. Quantification of etravirine (TMC125) in plasma, dried blood spots and peripheral blood mononuclear cell lysate by liquid chromatography tandem mass spectrometry. Journal of Pharmaceutical and Biomedical Analysis. 49:393–400. [DOI] [PubMed] [Google Scholar]
- Heine R. ter, Rosing H, Gorp E.C.M. van, Mulder JW, Steeg, van der WA, Beijnen JH, and Huitema ADR 2008. Quantification of protease inhibitors and non-nucleoside reverse transcriptase inhibitors in dried blood spots by liquid chromatography–triple quadrupole mass spectrometry. Journal of Chromatography B. 867:205–212. [DOI] [PubMed] [Google Scholar]
- Holodniy M, Groves E, and Merigan’ TC. 1991. Inhibition of Human Immunodeficiency Virus Gene Amplification by Heparin. 29:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holub M, Tuschl K, Ratschmann R, Strnadová KA, Mühl A, Heinze G, Sperl W, and Bodamer OA 2006. Influence of hematocrit and localisation of punch in dried blood spots on levels of amino acids and acylcarnitines measured by tandem mass spectrometry. Clinica Chimica Acta. 373:27–31. [DOI] [PubMed] [Google Scholar]
- Hong C-M, Lee C-T, Lee Y-M, Kuo C-P, Yuan C-H, and Shiea J 1999. Generating electrospray from solutions predeposited on a copper wire. Rapid Communications in Mass Spectrometry. 13:21–25. [Google Scholar]
- Hoogtanders K, Heijden, van der J, Christiaans M, Edelbroek P, Hooff, van JP, and Stolk LML 2007. Therapeutic drug monitoring of tacrolimus with the dried blood spot method. Journal of Pharmaceutical and Biomedical Analysis. 44:658–664. [DOI] [PubMed] [Google Scholar]
- Houbart V, Cobraiville G, Servais A-C, Napp A, Merville M-P, and Fillet M 2015. Hepcidin determination in dried blood by microfluidic LC–MS/MS: comparison of DBS and volumetric absorptive microsampling for matrix effect and recovery. Bioanalysis. 7:2789–2799. [DOI] [PubMed] [Google Scholar]
- Hu B, So P-K, Chen H, and Yao Z-P 2011. Electrospray Ionization Using Wooden Tips. Analytical Chemistry. 83:8201–8207. [DOI] [PubMed] [Google Scholar]
- Hu B, So P-K, Yang Y, Deng J, Choi Y-C, Luan T, and Yao Z-P 2018. Surface-Modified Wooden-Tip Electrospray Ionization Mass Spectrometry for Enhanced Detection of Analytes in Complex Samples. Analytical Chemistry. 90:1759–1766. [DOI] [PubMed] [Google Scholar]
- Hu B, So P-K, and Yao Z-P 2013. Analytical Properties of Solid-substrate Electrospray Ionization Mass Spectrometry. Journal of The American Society for Mass Spectrometry. 24:57–65. [DOI] [PubMed] [Google Scholar]
- Huang M-Z, Cheng S-C, Cho Y-T, and Shiea J 2011. Ambient ionization mass spectrometry: A tutorial. Analytica Chimica Acta. 702:1–15. [DOI] [PubMed] [Google Scholar]
- Ifa DR, Gumaelius LM, Eberlin LS, Manicke NE, and Cooks RG 2007. Forensic analysis of inks by imaging desorption electrospray ionization (DESI) mass spectrometry. The Analyst. 132:461. [DOI] [PubMed] [Google Scholar]
- Ifa DR, Wu C, Ouyang Z, and Cooks RG 2010. Desorption electrospray ionization and other ambient ionization methods: current progress and preview. Analyst. 135:669–681. [DOI] [PubMed] [Google Scholar]
- Ingels A-S, De Paepe P, Anseeuw K, Van Sassenbroeck D, Neels H, Lambert W, and Stove C 2011. Dried blood spot punches for confirmation of suspected γ-hydroxybutyric acid intoxications: validation of an optimized GC–MS procedure. Bioanalysis. 3:2271–2281. [DOI] [PubMed] [Google Scholar]
- Ingels A-SME, Lambert WE, and Stove CP 2010. Determination of gamma-hydroxybutyric acid in dried blood spots using a simple GC-MS method with direct “on spot” derivatization. Analytical and Bioanalytical Chemistry. 398:2173–2182. [DOI] [PubMed] [Google Scholar]
- Jackson S, Swiner DJ, Capone PC, and Badu-Tawiah AK 2018. Thread spray mass spectrometry for direct analysis of capsaicinoids in pepper products. Analytica Chimica Acta. 1023:81–88. [DOI] [PubMed] [Google Scholar]
- Javanshad R, and Venter AR 2017. Ambient ionization mass spectrometry: real-time, proximal sample processing and ionization. Analytical Methods. 9:4896–4907. [DOI] [PubMed] [Google Scholar]
- Jeong ES, Kim KH, Cha E, Kwon O-S, Cha S, and Lee J 2016. Direct and rapid quantitation of ephedrines in human urine by paper spray ionization/high resolution mass spectrometry. Journal of Chromatography B. 1028:237–241. [DOI] [PubMed] [Google Scholar]
- Jeong J-S, Kim S-K, and Park S-R 2013. Amino acid analysis of dried blood spots for diagnosis of phenylketonuria using capillary electrophoresis-mass spectrometry equipped with a sheathless electrospray ionization interface. Analytical and Bioanalytical Chemistry. 405:8063–8072. [DOI] [PubMed] [Google Scholar]
- Jett R, Skaggs C, and Manicke NE 2017. Drug screening method development for paper spray coupled to a triple quadrupole mass spectrometer. Anal Methods. 9:5037–5043. [Google Scholar]
- Jjunju FPM, Maher S, Damon DE, Barrett RM, Syed SU, Heeren RMA, Taylor S, and Badu-Tawiah AK 2016. Screening and Quantification of Aliphatic Primary Alkyl Corrosion Inhibitor Amines in Water Samples by Paper Spray Mass Spectrometry. Anal Chem. 88:1391–1400. [DOI] [PubMed] [Google Scholar]
- Jjunju Fred.P.M., Li A, Badu-Tawiah A, Wei P, Li L, Ouyang Z, Roqan IS, and Cooks RG 2013. In situ analysis of corrosion inhibitors using a portable mass spectrometer with paper spray ionization. Analyst. 138:3740–3748. [DOI] [PubMed] [Google Scholar]
- Johannessen A 2010. Dried blood spots in HIV monitoring: applications in resource-limited settings. Bioanalysis. 2:1893–1908. [DOI] [PubMed] [Google Scholar]
- Johnson B, Mascher H, Mascher D, Legnini E, Hung CY, Dajnoki A, Chien Y-H, Maródi L, Hwu W-L, and Bodamer OA 2013. Analysis of Lyso-Globotriaosylsphingosine in Dried Blood Spots. Annals of Laboratory Medicine. 33:274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DW, Gerace R, Ranieri E, Trinh M-U, and Fingerhut R 2007. Analysis of succinylacetone, as a Girard T derivative, in urine and dried bloodspots by flow injection electrospray ionization tandem mass spectrometry. Rapid Communications in Mass Spectrometry. 21:59–63. [DOI] [PubMed] [Google Scholar]
- Jonsson O, Steffen A-C, Sundquist VS, Janson J, Martinsson S, Määttä U, and Emanuelsson AB 2013. Capillary microsampling and analysis of 4-μl blood, plasma and serum samples to determine human α-synuclein elimination rate in mice. Bioanalysis. 5:449–462. [DOI] [PubMed] [Google Scholar]
- Jonsson O, Villar RP, Nilsson LB, Eriksson M, and Königsson K 2012a. Validation of a bioanalytical method using capillary microsampling of 8 μl plasma samples: application to a toxicokinetic study in mice. Bioanalysis. 4:1989–1998. [DOI] [PubMed] [Google Scholar]
- Jonsson O, Villar RP, Nilsson LB, Norsten-Höög C, Brogren J, Eriksson M, Königsson K, and Samuelsson A 2012b. Capillary microsampling of 25 μl blood for the determination of toxicokinetic parameters in regulatory studies in animals. Bioanalysis. 4:661–674. [DOI] [PubMed] [Google Scholar]
- Judd A, Parry J, Hickman M, McDonald T, Jordan L, Lewis K, Contreras M, Dusheiko G, Foster G, Gill N, et al. 2003. Evaluation of a modified commercial assay in detecting antibody to hepatitis C virus in oral fluids and dried blood spots. Journal of Medical Virology. 71:49–55. [DOI] [PubMed] [Google Scholar]
- Kar S, Maiti TK, and Chakraborty S 2015. Capillarity-driven blood plasma separation on paper-based devices. The Analyst. 140:6473–6476. [DOI] [PubMed] [Google Scholar]
- Kayiran SM, Ozbek N, Turan M, and Gurakan B 2003. Significant differences between capillary and venous complete blood counts in the neonatal period. Clinical and Laboratory Haematology. 25:9–16. [DOI] [PubMed] [Google Scholar]
- Keating JE, Minges JT, Randell SH, and Glish GL 2018. Paper spray mass spectrometry for high-throughput quantification of nicotine and cotinine. Analytical Methods. 10:46–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keevil BG 2011. The analysis of dried blood spot samples using liquid chromatography tandem mass spectrometry. Clinical Biochemistry. 44:110–118. [DOI] [PubMed] [Google Scholar]
- Kennedy JH, Palaty J, Gill CG, and Wiseman JM 2018. Rapid analysis of fentanyls and other novel psychoactive substances in substance use disorder patient urine using paper spray mass spectrometry. Rapid Communications in Mass Spectrometry. 32:1280–1286. [DOI] [PubMed] [Google Scholar]
- Kerian KS, Jarmusch AK, and Cooks RG 2014. Touch spray mass spectrometry for in situ analysis of complex samples. The Analyst. 139:2714–2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Ha SY, An JG, Cha S, Yim UH, and Kim S 2018a. Estimating degree of degradation of spilled oils based on relative abundance of aromatic compounds observed by paper spray ionization mass spectrometry. Journal of Hazardous Materials. 359:421–428. [DOI] [PubMed] [Google Scholar]
- Kim D, Lee J, Kim B, and Kim S 2018b. Optimization and Application of Paper-Based Spray Ionization Mass Spectrometry for Analysis of Natural Organic Matter. Analytical Chemistry. 90:12027–12034. [DOI] [PubMed] [Google Scholar]
- Kim D, Yim UH, Kim B, Cha S, and Kim S 2017. Paper Spray Chemical Ionization: Highly Sensitive Ambient Ionization Method for Low- and Nonpolar Aromatic Compounds. Analytical Chemistry. 89:9056–9061. [DOI] [PubMed] [Google Scholar]
- Kim J-H, Woenker T, Adamec J, and Regnier FE 2013. Simple, Miniaturized Blood Plasma Extraction Method. Analytical Chemistry. 85:11501–11508. [DOI] [PubMed] [Google Scholar]
- Kita K, and Mano Y 2017. Application of volumetric absorptive microsampling device for quantification of tacrolimus in human blood as a model drug of high blood cell partition. Journal of Pharmaceutical and Biomedical Analysis. 143:168–175. [DOI] [PubMed] [Google Scholar]
- Koal T, Burhenne H, Römling R, Svoboda M, Resch K, and Kaever V 2005. Quantification of antiretroviral drugs in dried blood spot samples by means of liquid chromatography/tandem mass spectrometry. Rapid Communications in Mass Spectrometry. 19:2995–3001. [DOI] [PubMed] [Google Scholar]
- Kok MGM, and Fillet M 2018. Volumetric absorptive microsampling: Current advances and applications. Journal of Pharmaceutical and Biomedical Analysis. 147:288–296. [DOI] [PubMed] [Google Scholar]
- Kouremenos Konstantinos, Ragunathan Kannan, Singh Pawanbir, Bowen Christopher, David de Souza, David Melville, Hon Wei Boon, Tull Dedreia, Lapierre Florian, Collins Anne, et al. Metabolomics/lipidomics of dried blood spots using a novel blood microsampling device (hemaPEN®). [Google Scholar]
- Koponen J, Rudge J, Kushon S, and Kiviranta H 2018. Novel volumetric adsorptive microsampling technique for determination of perfluorinated compounds in blood. Analytical Biochemistry. 545:49–53. [DOI] [PubMed] [Google Scholar]
- Koster RA, Greijdanus B, Alffenaar J-WC, and Touw DJ 2015. Dried blood spot analysis of creatinine with LC-MS/MS in addition to immunosuppressants analysis. Analytical and Bioanalytical Chemistry. 407:1585–1594. [DOI] [PubMed] [Google Scholar]
- Kovač J, Panic G, Neodo A, Meister I, Coulibaly JT, Schulz JD, and Keiser J 2018. Evaluation of a novel micro-sampling device, Mitra™, in comparison to dried blood spots, for analysis of praziquantel in Schistosoma haematobium-infected children in rural Côte d’Ivoire. Journal of Pharmaceutical and Biomedical Analysis. 151:339–346. [DOI] [PubMed] [Google Scholar]
- Kromdijk W, Mulder JW, Rosing H, Smit PM, Beijnen JH, and Huitema ADR 2012. Use of dried blood spots for the determination of plasma concentrations of nevirapine and efavirenz. Journal of Antimicrobial Chemotherapy. 67:1211–1216. [DOI] [PubMed] [Google Scholar]
- Lacher DA, Berman LE, Chen T-C, and Porter KS 2013. Comparison of dried blood spot to venous methods for hemoglobin A1c, glucose, total cholesterol, high-density lipoprotein cholesterol, and C-reactive protein. Clinica Chimica Acta. 422:54–58. [DOI] [PubMed] [Google Scholar]
- Lai C-C, Tsai C-H, Tsai F-J, Lee C-C, and Lin W-D 2001. Rapid monitoring assay of congenital adrenal hyperplasia with microbore high-performance liquid chromatography/electrospray ionization tandem mass spectrometry from dried blood spots. Rapid Communications in Mass Spectrometry. 15:2145–2151. [DOI] [PubMed] [Google Scholar]
- Lai P-H, Chen P-C, Liao Y-W, Liu J-T, Chen C-C, and Lin C-H 2015. Comparison of gampi paper and nanofibers to chromatography paper used in paper spray-mass spectrometry. International Journal of Mass Spectrometry. 375:14–17. [Google Scholar]
- Lan W-J, Maxwell EJ, Parolo C, Bwambok DK, Subramaniam AB, and Whitesides GM 2013. Paper-based electroanalytical devices with an integrated, stable reference electrode. Lab Chip. 13:4103–4108. [DOI] [PubMed] [Google Scholar]
- Lara-Ortega FJ, Beneito-Cambra M, Robles-Molina J, García-Reyes JF, Gilbert-López B, and Molina-Díaz A 2018. Direct olive oil analysis by mass spectrometry: A comparison of different ambient ionization methods. Talanta. 180:168–175. [DOI] [PubMed] [Google Scholar]
- Lebedev AT 2015. Ambient ionization mass spectrometry. Russian Chemical Reviews. 84:665–692. [Google Scholar]
- Lee MS, and Li W 2014. Dried Blood Spots: Applications and Techniques. Hoboken, New Jersey: Wiley. [Google Scholar]
- Lenk G, Hansson J, Beck O, and Roxhed N 2015. The effect of drying on the homogeneity of DBS. Bioanalysis. 7:1977–1985. [DOI] [PubMed] [Google Scholar]
- Li A, Wang H, Ouyang Z, and Cooks RG 2011. Paper spray ionization of polar analytes using non-polar solvents. Chemical Communications. 47:2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Wei P, Hsu H-C, and Cooks RG 2013. Direct analysis of 4-methylimidazole in foods using paper spray mass spectrometry. Analyst. 138:4624–4630. [DOI] [PubMed] [Google Scholar]
- Li H, Bigwarfe T, Myzithras M, Waltz E, and Ahlberg J 2019. Application of Mitra® microsampling for pharmacokinetic bioanalysis of monoclonal antibodies in rats. Bioanalysis. 11:13–20. [DOI] [PubMed] [Google Scholar]
- Li J, Zheng Y, Mi W, Muyizere T, and Zhang Z 2018. Polystyrene-impregnated paper substrates for direct mass spectrometric analysis of proteins and peptides in complex matrices. Analytical Methods. 10:2803–2811. [Google Scholar]
- Li W, Doherty JP, Kulmatycki K, Smith HT, and Tse FL 2012. Simultaneous LC–MS/MS quantitation of acetaminophen and its glucuronide and sulfate metabolites in human dried blood spot samples collected by subjects in a pilot clinical study. Bioanalysis. 4:1429–1443. [DOI] [PubMed] [Google Scholar]
- Li W, and Tse FLS 2010. Dried blood spot sampling in combination with LC-MS/MS for quantitative analysis of small molecules. Biomedical Chromatography. 24:49–65. [DOI] [PubMed] [Google Scholar]
- Li Y 2004. Tandem Mass Spectrometry for the Direct Assay of Enzymes in Dried Blood Spots: Application to Newborn Screening for Krabbe Disease. Clinical Chemistry. 50:638–640. [DOI] [PubMed] [Google Scholar]
- Liang X, Li Y, Barfield M, and Ji QC 2009. Study of dried blood spots technique for the determination of dextromethorphan and its metabolite dextrorphan in human whole blood by LC–MS/MS. Journal of Chromatography B. 877:799–806. [DOI] [PubMed] [Google Scholar]
- Liu H, Gao W, Tian Y, Liu A, Wang Z, Cai Y, and Zhao Z 2019. Rapidly detecting tetrabromobisphenol A in soils and sediments by paper spray ionization mass spectrometry combined with isotopic internal standard. Talanta. 191:272–276. [DOI] [PubMed] [Google Scholar]
- Liu J, He Y, Chen S, Ma M, Yao S, and Chen B 2017. New urea-modified paper substrate for enhanced analytical performance of negative ion mode paper spray mass spectrometry. Talanta. 166:306–314. [DOI] [PubMed] [Google Scholar]
- Liu J, Wang H, Cooks RG, and Ouyang Z 2011. Leaf Spray: Direct Chemical Analysis of Plant Material and Living Plants by Mass Spectrometry. Analytical Chemistry. 83:7608–7613. [DOI] [PubMed] [Google Scholar]
- Liu J, Wang H, Manicke NE, Lin J-M, Cooks RG, and Ouyang Z 2010. Development, Characterization, and Application of Paper Spray Ionization. Anal Chem. 82:2463–2471. [DOI] [PubMed] [Google Scholar]
- Liu Y, Zhou Y, Dai Y, Zhao Z, He L, and Zhang Q 2018. Quantitative analysis of finasteride tablets dissolution content with non-isotopically labelled internal standard by paper spray ionization mass spectrometry. Journal of Separation Science. [DOI] [PubMed] [Google Scholar]
- Lyon E, Laver T, Yu P, Jama M, Young K, Zoccoli M, and Marlowe N 2010. A Simple, High-Throughput Assay for Fragile X Expanded Alleles Using Triple Repeat Primed PCR and Capillary Electrophoresis. The Journal of Molecular Diagnostics. 12:505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Bai H, Li W, Wang C, Cooks RG, and Ouyang Z 2015. Rapid analysis of synthetic cannabinoids using a miniature mass spectrometer with ambient ionization capability. Talanta. 142:190–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald M, Ghani NAA, Wan Y, Cooper-White J, Dimeski G, and Punyadeera C 2014. Profiling of immunoglobulins in resting and mechanically stimulated saliva. Bioanalysis. 6:697–704. [DOI] [PubMed] [Google Scholar]
- Mach PM, Dhummakupt ES, Carmany DO, McBride EM, Busch MW, Demond PS, Rizzo GM, Hollinshead DE, and Glaros T 2018. On-Substrate Derivatization for Highly Volatile G-Series Chemical Warfare Agent Detection via Paper Spray Mass Spectrometry. Rapid Communications in Mass Spectrometry. [DOI] [PubMed] [Google Scholar]
- Maher S, Jjunju FPM, Damon DE, Gorton H, Maher YS, Syed SU, Heeren RMA, Young IS, Taylor S, and Badu-Tawiah AK 2016. Direct Analysis and Quantification of Metaldehyde in Water using Reactive Paper Spray Mass Spectrometry. Sci Rep. 6:35643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaj N, Ouyang Z, Sindona G, and Cooks RG 2012. Analysis of pesticide residues by leaf spray mass spectrometry. Analytical Methods. 4:1913. [Google Scholar]
- Manicke N, Abu-Rabie P, Spooner N, Ouyang Z, and Cooks RG 2011a. Quantitative Analysis of Therapeutic Drugs in Dried Blood Spot Samples by Paper Spray Mass Spectrometry: An Avenue to Therapeutic Drug Monitoring. J Am Soc Mass Spectrom. 22:1501–1507. [DOI] [PubMed] [Google Scholar]
- Manicke NE, Bills BJ, and Zhang C 2016. Analysis of biofluids by paper spray MS: advances and challenges. Bioanalysis. 8:589–606. [DOI] [PubMed] [Google Scholar]
- Manicke NE, Yang Q, Wang H, Oradu S, Ouyang Z, and Cooks RG 2011b. Assessment of paper spray ionization for quantitation of pharmaceuticals in blood spots. Int J Mass Spectrom. 300:123–129. [Google Scholar]
- Marques BLC, Brandão CU, Silva EF, Marques VA, Villela-Nogueira CA, Do Ó KMR, Paula M.T. de, Lewis-Ximenez LL, Lampe E, and Villar LM 2012. Dried blood spot samples: Optimization of commercial EIAs for hepatitis C antibody detection and stability under different storage conditions. Journal of Medical Virology. 84:1600–1607. [DOI] [PubMed] [Google Scholar]
- Martin NJ, Bunch J, and Cooper HJ 2013. Dried Blood Spot Proteomics: Surface Extraction of Endogenous Proteins Coupled with Automated Sample Preparation and Mass Spectrometry Analysis. Journal of The American Society for Mass Spectrometry. 24:1242–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzotti F, Di Donna L, Taverna D, Nardi M, Aiello D, Napoli A, and Sindona G 2013. Evaluation of dialdehydic anti-inflammatory active principles in extra-virgin olive oil by reactive paper spray mass spectrometry. International Journal of Mass Spectrometry. 352:87–91. [Google Scholar]
- McDade TW, Williams S, and Snodgrass JJ 2007. What a drop can do: Dried blood spots as a minimally invasive method for integrating biomarkers into population-based research. Demography. 44:899–925. [DOI] [PubMed] [Google Scholar]
- McKenna J, Dhummakupt ES, Connell T, Demond PS, Miller DB, Michael Nilles J, Manicke NE, and Glaros T 2017. Detection of chemical warfare agent simulants and hydrolysis products in biological samples by paper spray mass spectrometry. Analyst. 142:1442–1451. [DOI] [PubMed] [Google Scholar]
- McKenna J, Jett R, Shanks K, and Manicke NE 2018. Toxicological Drug Screening using Paper Spray High-Resolution Tandem Mass Spectrometry (HR-MS/MS). J Anal Toxicol. 42:300–310. [DOI] [PubMed] [Google Scholar]
- Meesters RJ, and Hooff GP 2013. State-of-the-art dried blood spot analysis: an overview of recent advances and future trends. Bioanalysis. 5:2187–2208. [DOI] [PubMed] [Google Scholar]
- Mei JV, Alexander JR, Adam BW, and Hannon WH 2001. Use of Filter Paper for the Collection and Analysis of Human Whole Blood Specimens. The Journal of Nutrition. 131:1631S–1636S. [DOI] [PubMed] [Google Scholar]
- Mei JV, Zobel SD, Hall EM, De Jesús VR, Adam BW, and Hannon WH 2010. Performance properties of filter paper devices for whole blood collection. Bioanalysis. 2:1397–1403. [DOI] [PubMed] [Google Scholar]
- Melgaço JG, Pinto MA, Rocha AM, Freire M, Gaspar LP, Lima SMB, Cruz OG, and Vitral CL 2011. The use of dried blood spots for assessing antibody response to hepatitis A virus after natural infection and vaccination. Journal of Medical Virology. 83:208–217. [DOI] [PubMed] [Google Scholar]
- Mendes TPP, Pereira I, Ferreira MR, Chaves AR, and Vaz BG 2017. Molecularly imprinted polymer-coated paper as a substrate for highly sensitive analysis using paper spray mass spectrometry: quantification of metabolites in urine. Anal Methods. 9:6117–6123. [Google Scholar]
- Mercolini L, Protti M, Catapano MC, Rudge J, and Sberna AE 2016. LC–MS/MS and volumetric absorptive microsampling for quantitative bioanalysis of cathinone analogues in dried urine, plasma and oral fluid samples. Journal of Pharmaceutical and Biomedical Analysis. 123:186–194. [DOI] [PubMed] [Google Scholar]
- Michely JA, Meyer MR, and Maurer HH 2017. Paper Spray Ionization Coupled to High Resolution Tandem Mass Spectrometry for Comprehensive Urine Drug Testing in Comparison to Liquid Chromatography-Coupled Techniques after Urine Precipitation or Dried Urine Spot Workup. Analytical Chemistry. 89:11779–11786. [DOI] [PubMed] [Google Scholar]
- Möller I, Thomas A, Geyer H, Schänzer W, and Thevis M 2012. Development and validation of a mass spectrometric detection method of peginesatide in dried blood spots for sports drug testing. Analytical and Bioanalytical Chemistry. 403:2715–2724. [DOI] [PubMed] [Google Scholar]
- Monge ME, Harris GA, Dwivedi P, and Fernández FM 2013. Mass Spectrometry: Recent Advances in Direct Open Air Surface Sampling/Ionization. Chem Rev. 113:2269–2308. [DOI] [PubMed] [Google Scholar]
- Narayanan R, and Pradeep T 2017. Probing Coordination Complexes by Carbon Nanotube-Assisted Low-Voltage Paper Spray Ionization Mass Spectrometry. Anal Chem. 89:10696–10701. [DOI] [PubMed] [Google Scholar]
- Narayanan R, Sarkar D, Cooks RG, and Pradeep T 2014. Molecular Ionization from Carbon Nanotube Paper. Angew Chem Int Ed. 53:5936–5940. [DOI] [PubMed] [Google Scholar]
- Nilghaz A, and Shen W 2015. Low-cost blood plasma separation method using salt functionalized paper. RSC Advances. 5:53172–53179.27019703 [Google Scholar]
- Nilsson LB, Ahnoff M, and Jonsson O 2013. Capillary microsampling in the regulatory environment: validation and use of bioanalytical capillary microsampling methods. Bioanalysis. 5:731–738. [DOI] [PubMed] [Google Scholar]
- Niu X, Beekhuijzen M, Schoonen W, Emmen H, and Wenker M 2016. Effects of Capillary Microsampling on Toxicological Endpoints in Juvenile Rats. Toxicological Sciences. 154:69–77. [DOI] [PubMed] [Google Scholar]
- Nys G, Kok MGM, Servais A-C, and Fillet M 2017. Beyond dried blood spot: Current microsampling techniques in the context of biomedical applications. TrAC Trends in Analytical Chemistry. 97:326–332. [Google Scholar]
- Oerton J, Khalid JM, Besley G, Dalton RN, Downing M, Green A, Henderson M, Krywawych S, Leonard J, Andresen BS, et al. 2011. Newborn screening for medium chain acyl-CoA dehydrogenase deficiency in England: Prevalence, predictive value and test validity based on 1.5 million screened babies. Journal of Medical Screening. 18:173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olshan AF 2007. Meeting Report: The Use of Newborn Blood Spots in Environmental Research: Opportunities and Challenges. Environmental Health Perspectives. 115:1767–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oradu SA, and Cooks RG 2012. Multistep Mass Spectrometry Methodology for Direct Characterization of Polar Lipids in Green Microalgae using Paper Spray Ionization. Anal Chem. 84:10576–10585. [DOI] [PubMed] [Google Scholar]
- Parker SL, Guerra Valero YC, Lipman J, Roberts JA, and Wallis SC 2016. Effect of time on recovery of plasma microsamples for the quantitative determination of vancomycin. Bioanalysis. 8:2235–2242. [DOI] [PubMed] [Google Scholar]
- Partington MW, and Sinnott B 1964. Case Finding in Phenylketonuria. Can Med Assoc J. 91:105–114. [PMC free article] [PubMed] [Google Scholar]
- Paula C. de, Jurisch M, Piccin E, and Augusti R 2018. Recognizing drug-facilitated crimes: Detection and quantification of benzodiazepines in beverages using fast liquid-liquid extraction with low temperature partitioning and paper spray mass spectrometry. Drug Testing and Analysis. [DOI] [PubMed] [Google Scholar]
- Pereira HV, Amador VS, Sena MM, Augusti R, and Piccin E 2016a. Paper spray mass spectrometry and PLS-DA improved by variable selection for the forensic discrimination of beers. Analytica Chimica Acta. 940:104–112. [DOI] [PubMed] [Google Scholar]
- Pereira I, Rodrigues MF, Chaves AR, and Vaz BG 2018. Molecularly imprinted polymer (MIP) membrane assisted direct spray ionization mass spectrometry for agrochemicals screening in foodstuffs. Talanta. 178:507–514. [DOI] [PubMed] [Google Scholar]
- Pereira I, Rodrigues SRM, Carvalho T.C. de, Carvalho VV, Lobón GS, Bassane JFP, Domingos E, Romão W, Augusti R, and Vaz BG 2016b. Rapid screening of agrochemicals by paper spray ionization and leaf spray mass spectrometry: which technique is more appropriate? Anal Methods. 8:6023–6029. [Google Scholar]
- Poole JJ, Gómez-Ríos GA, Boyaci E, Reyes-Garcés N, and Pawliszyn J 2017. Rapid and Concomitant Analysis of Pharmaceuticals in Treated Wastewater by Coated Blade Spray Mass Spectrometry. Environ Sci Technol. 51:12566–12572. [DOI] [PubMed] [Google Scholar]
- Prentice P, Turner C, Wong MC, and Dalton RN 2013. Stability of metabolites in dried blood spots stored at different temperatures over a 2-year period. Bioanalysis. 5:1507–1514. [DOI] [PubMed] [Google Scholar]
- Prieto Conaway MC, Manicke NE, and Kozak M 2014. Direct Analysis using Paper-Spray Mass Spectrometry: Method Development for the Rapid Screening of Drugs of Abuse for Forensic Toxicology. [Google Scholar]
- Protti M, Mandrioli R, and Mercolini L 2019. Tutorial: Volumetric absorptive microsampling (VAMS). Analytica Chimica Acta. 1046:32–47. [DOI] [PubMed] [Google Scholar]
- Qu Y, Brady K, Apilado R, O’Malley T, Reddy S, Chitkara P, Ibarra C, Alexander RV, and Dervieux T 2017. Capillary blood collected on volumetric absorptive microsampling (VAMS) device for monitoring hydroxychloroquine in rheumatoid arthritis patients. Journal of Pharmaceutical and Biomedical Analysis. 140:334–341. [DOI] [PubMed] [Google Scholar]
- Rashed MS, Al-Ahaidib LY, Al-Dirbashi OY, Al Amoudi M, Al-Sayed MMA, Rahbeeni Z, Al-Hassnan Z, Al-Dbaas A, Al-Owain M, and Luanaigh MN 2005. Tandem mass spectrometric assay of succinylacetone in urine for the diagnosis of hepatorenal tyrosinemia. Analytical Biochemistry. 339:310–317. [DOI] [PubMed] [Google Scholar]
- Reeber SL, Gadi S, Huang S-B, and Glish GL 2015. Direct analysis of herbicides by paper spray ionization mass spectrometry. Anal Methods. 7:9808–9816. [Google Scholar]
- Ren X, Paehler T, Zimmer M, Guo Z, Zane P, and Emmons GT 2010. Impact of various factors on radioactivity distribution in different DBS papers. Bioanalysis. 2:1469–1475. [DOI] [PubMed] [Google Scholar]
- Ren Y, Wang H, Liu J, Zhang Z, McLuckey MN, and Ouyang Z 2013. Analysis of Biological Samples Using Paper Spray Mass Spectrometry: An Investigation of Impacts by the Substrates, Solvents and Elution Methods. Chromatographia. 76:1339–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resende SF, Teodoro JAR, Binatti I, Gouveia RL, Oliveira BS, and Augusti R 2017. On-surface photocatalytic degradation of methylene blue: In situ monitoring by paper spray ionization mass spectrometry. Int J Mass Spectrom. 418:107–111. [Google Scholar]
- Ryona I, and Henion J 2016. A Book-Type Dried Plasma Spot Card for Automated Flow-Through Elution Coupled with Online SPE-LC-MS/MS Bioanalysis of Opioids and Stimulants in blood. Analytical Chemistry. 88:11229–11237. [DOI] [PubMed] [Google Scholar]
- Salentijn GIJ, Permentier HP, and Verpoorte E 2014. 3D-Printed Paper Spray Ionization Cartridge with Fast Wetting and Continuous Solvent Supply Features. Anal Chem. 86:11657–11665. [DOI] [PubMed] [Google Scholar]
- Santos H, Lima AS, Mazega A, Domingos E, Thompson CJ, Maldaner AO, Filgueiras PR, Vaz BG, and Romão W 2017. Quantification of cocaine and its adulterants (lidocaine and levamisole) using the Dragendorff reagent allied to paper spray ionization mass spectrometry. Anal Methods. 9:3662–3668. [Google Scholar]
- Sarkar D, Som A, and Pradeep T 2017. Catalytic Paper Spray Ionization Mass Spectrometry with Metal Nanotubes and the Detection of 2,4,6-Trinitrotoluene. Anal Chem. 89:11378–11382. [DOI] [PubMed] [Google Scholar]
- Sarkar D, Srimany A, and Pradeep T 2012. Rapid identification of molecular changes in tulsi (Ocimum sanctum Linn) upon ageing using leaf spray ionization mass spectrometry. The Analyst. 137:4559. [DOI] [PubMed] [Google Scholar]
- Scott CR, Elliott S, Buroker N, Thomas LI, Keutzer J, Glass M, Gelb MH, and Turecek F 2013. Identification of Infants at Risk for Developing Fabry, Pompe, or Mucopolysaccharidosis-I from Newborn Blood Spots by Tandem Mass Spectrometry. The Journal of Pediatrics. 163:498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelley JT, Badal SP, Engelhard C, and Hayen H 2018. Ambient desorption/ionization mass spectrometry: evolution from rapid qualitative screening to accurate quantification tool. Analytical and Bioanalytical Chemistry. 410:4061–4076. [DOI] [PubMed] [Google Scholar]
- Shen L, Zhang J, Yang Q, Manicke NE, and Ouyang Z 2013. High throughput paper spray mass spectrometry analysis. Clin Chim Acta. 420:28–33. [DOI] [PubMed] [Google Scholar]
- Shi H, Ma Y, Humphrey JH, and Craft NE 1995. Determination of vitamin A in dried human blood spots by high-performance capillary electrophoresis with laser-excited fluorescence detection. Journal of Chromatography B: Biomedical Sciences and Applications. 665:89–96. [DOI] [PubMed] [Google Scholar]
- Shi R-Z, El Gierari ETM, Faix JD, and Manicke NE 2016. Rapid Measurement of Cyclosporine and Sirolimus in Whole Blood by Paper Spray-Tandem Mass Spectrometry. Clin Chem. 62:295–297. [DOI] [PubMed] [Google Scholar]
- Shi R-Z, El Gierari ETM, Manicke NE, and Faix JD 2015. Rapid measurement of tacrolimus in whole blood by paper spray-tandem mass spectrometry (PS-MS/MS). Clin Chim Acta. 441:99–104. [DOI] [PubMed] [Google Scholar]
- Shimadzu. 2017. Introduction of SHIMADZU Microsampling Device.
- Silva LC, da Pereira I, Carvalho T.C. de, Allochio Filho JF, Romão W, and Gontijo Vaz B. 2019. Paper spray ionization and portable mass spectrometers: a review. Analytical Methods. 11:999–1013. [Google Scholar]
- Skogstrand K, Ekelund CK, Thorsen P, Vogel I, Jacobsson B, Nørgaard-Pedersen B, and Hougaard DM 2008. Effects of blood sample handling procedures on measurable inflammatory markers in plasma, serum and dried blood spot samples. Journal of Immunological Methods. 336:78–84. [DOI] [PubMed] [Google Scholar]
- Snyder DT, Schilling MC, Hochwender CG, and Kaufman AD 2015. Profiling phenolic glycosides in Populus deltoides and Populus grandidentata by leaf spray ionization tandem mass spectrometry. Analytical Methods. 7:870–876. [Google Scholar]
- Song X, Chen H, and Zare RN 2018. Conductive Polymer Spray Ionization Mass Spectrometry for Biofluid Analysis. Analytical Chemistry. 90:12878–12885. [DOI] [PubMed] [Google Scholar]
- Songjaroen T, Dungchai W, Chailapakul O, Henry CS, and Laiwattanapaisal W 2012. Blood separation on microfluidic paper-based analytical devices. Lab on a Chip. 12:3392. [DOI] [PubMed] [Google Scholar]
- Sosnoff CS, Ann Q, Bernert JT, Powell MK, Miller BB, Henderson LO, Hannon WH, Fernhoff P, and Sampson EJ 1996. Analysis of Benzoylecgonine in Dried Blood Spots by Liquid Chromatography-Atmospheric Pressure Chemical Ionization Tandem Mass Spectrometry*†. Journal of Analytical Toxicology. 20:179–184. [DOI] [PubMed] [Google Scholar]
- Spooner N, Denniff P, Michielsen L, De Vries R, Ji QC, Arnold ME, Woods K, Woolf EJ, Xu Y, Boutet V, et al. 2015. A device for dried blood microsampling in quantitative bioanalysis: overcoming the issues associated blood hematocrit. Bioanalysis. 7:653–659. [DOI] [PubMed] [Google Scholar]
- Spooner N, Lad R, and Barfield M 2009. Dried Blood Spots as a Sample Collection Technique for the Determination of Pharmacokinetics in Clinical Studies: Considerations for the Validation of a Quantitative Bioanalytical Method. Analytical Chemistry. 81:1557–1563. [DOI] [PubMed] [Google Scholar]
- Spreadborough MJ, Day J, Jackson-Addie K, and Wilson A 2013. Bioanalytical implementation of plasma capillary microsampling: small hurdles, large gains. Bioanalysis. 5:1485–1489. [DOI] [PubMed] [Google Scholar]
- Steffens C, Manzoli A, Francheschi E, Corazza ML, Corazza FC, Oliveira JV, and Herrmann PSP 2009. Low-cost sensors developed on paper by line patterning with graphite and polyaniline coating with supercritical CO2. Synthetic Metals. 159:2329–2332. [Google Scholar]
- Su Y, Wang H, Liu J, Wei P, Cooks RG, and Ouyang Z 2013. Quantitative paper spray mass spectrometry analysis of drugs of abuse. Analyst. 138:4443–4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suyagh MF, Kole PL, Millership J, Collier P, Halliday H, and McElnay JC 2010. Development and validation of a dried blood spot—LC–APCI–MS assay for estimation of canrenone in paediatric samples. Journal of Chromatography B. 878:769–776. [DOI] [PubMed] [Google Scholar]
- Swiner DJ, Jackson S, Durisek GR, Walsh BK, Kouatli Y, and Badu-Tawiah AK 2019. Microsampling with cotton thread: Storage and ultra-sensitive analysis by thread spray mass Spectrometry. Analytica Chimica Acta. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadjimukhamedov FK, Huang G, Ouyang Z, and Cooks RG 2012. Rapid detection of urushiol allergens of Toxicodendron genus using leaf spray mass spectrometry. The Analyst. 137:1082. [DOI] [PubMed] [Google Scholar]
- Takats Z 2004. Mass Spectrometry Sampling Under Ambient Conditions with Desorption Electrospray Ionization. Science. 306:471–473. [DOI] [PubMed] [Google Scholar]
- Takáts Z, Wiseman JM, and Cooks RG 2005. Ambient mass spectrometry using desorption electrospray ionization (DESI): instrumentation, mechanisms and applications in forensics, chemistry, and biology. Journal of Mass Spectrometry. 40:1261–1275. [DOI] [PubMed] [Google Scholar]
- Tascon M, Gómez-Ríos GA, Reyes-Garcés N, Poole J, Boyacı E, and Pawliszyn J 2017a. High-Throughput Screening and Quantitation of Target Compounds in Biofluids by Coated Blade Spray-Mass Spectrometry. Anal Chem. 89:8421–8428. [DOI] [PubMed] [Google Scholar]
- Tascon M, Gómez-Ríos GA, Reyes-Garcés N, Poole J, Boyacı E, and Pawliszyn J 2017b. Ultra-fast quantitation of voriconazole in human plasma by coated blade spray mass spectrometry. J Pharm Biomed Anal. 144:106–111. [DOI] [PubMed] [Google Scholar]
- Tavares LS, Carvalho TC, Romão W, Vaz BG, and Chaves AR 2018. Paper Spray Tandem Mass Spectrometry Based on Molecularly Imprinted Polymer Substrate for Cocaine Analysis in Oral Fluid. J Am Soc Mass Spectrom. 29:566–572. [DOI] [PubMed] [Google Scholar]
- Taverna D, Di Donna L, Mazzotti F, Policicchio B, and Sindona G 2013. High‐throughput determination of Sudan Azo‐dyes within powdered chili pepper by paper spray mass spectrometry. J Mass Spectrom. 48:544–547. [DOI] [PubMed] [Google Scholar]
- Taylor JM, Hughes AT, Milan AM, Rudge J, Davison AS, and Ranganath LR 2018. Evaluation of the Mitra microsampling device for use with key urinary metabolites in patients with Alkaptonuria. Bioanalysis. 10:1919–1932. [DOI] [PubMed] [Google Scholar]
- Teodoro JAR, Pereira HV, Correia DN, Sena MM, Piccin E, and Augusti R 2017. Forensic discrimination between authentic and counterfeit perfumes using paper spray mass spectrometry and multivariate supervised classification. Analytical Methods. 9:4979–4987. [Google Scholar]
- Teunissen SF, Fedick PW, Berendsen BJA, Nielen MWF, Eberlin MN, Graham Cooks R, and Asten A.C. van. 2017. Novel Selectivity-Based Forensic Toxicological Validation of a Paper Spray Mass Spectrometry Method for the Quantitative Determination of Eight Amphetamines in Whole Blood. Journal of The American Society for Mass Spectrometry. 28:2665–2676. [DOI] [PubMed] [Google Scholar]
- Thom NK, Lewis GG, Yeung K, and Phillips ST 2014. Quantitative fluorescence assays using a self-powered paper-based microfluidic device and a camera-equipped cellular phone. RSC Adv. 4:1334–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A, Geyer H, Schänzer W, Crone C, Kellmann M, Moehring T, and Thevis M 2012. Sensitive determination of prohibited drugs in dried blood spots (DBS) for doping controls by means of a benchtop quadrupole/Orbitrap mass spectrometer. Analytical and Bioanalytical Chemistry. 403:1279–1289. [DOI] [PubMed] [Google Scholar]
- Tosato F, Correia RM, Oliveira BG, Fontes AM, França HS, Coltro WKT, Filgueiras PR, and Romão W 2018. Paper spray ionization mass spectrometry allied to chemometric tools for quantification of whisky adulteration with additions of sugarcane spirit. Analytical Methods. 10:1952–1960. [Google Scholar]
- Tripathi S, Varun Kumar YVB, Prabhakar A, Joshi SS, and Agrawal A 2015. Passive blood plasma separation at the microscale: a review of design principles and microdevices. Journal of Micromechanics and Microengineering. 25:083001. [Google Scholar]
- Tsai C-W, Tipple CA, and Yost RA 2017. Application of paper spray ionization for explosives analysis. Rapid Communications in Mass Spectrometry. 31:1565–1572. [DOI] [PubMed] [Google Scholar]
- Turgeon C, Magera MJ, Allard P, Tortorelli S, Gavrilov D, Oglesbee D, Raymond K, Rinaldo P, and Matern D 2008. Combined Newborn Screening for Succinylacetone, Amino Acids, and Acylcarnitines in Dried Blood Spots. Clinical Chemistry. 54:657–664. [DOI] [PubMed] [Google Scholar]
- Turgeon CT, Magera MJ, Cuthbert CD, Loken PR, Gavrilov DK, Tortorelli S, Raymond KM, Oglesbee D, Rinaldo P, and Matern D 2010. Determination of Total Homocysteine, Methylmalonic Acid, and 2-Methylcitric Acid in Dried Blood Spots by Tandem Mass Spectrometry. Clinical Chemistry. 56:1686–1695. [DOI] [PubMed] [Google Scholar]
- Van Berkel GJ, and Kertesz V 2009. Application of a Liquid Extraction Based Sealing Surface Sampling Probe for Mass Spectrometric Analysis of Dried Blood Spots and Mouse Whole-Body Thin Tissue Sections. Analytical Chemistry. 81:9146–9152. [DOI] [PubMed] [Google Scholar]
- Van Berkel GJ, and Kertesz V 2013. Continuous-flow liquid microjunction surface sampling probe connected on-line with high-performance liquid chromatography/mass spectrometry for spatially resolved analysis of small molecules and proteins: LMJ-SPP on-line with HPLC/MS. Rapid Communications in Mass Spectrometry. 27:1329–1334. [DOI] [PubMed] [Google Scholar]
- Van Berkel GJ, Kertesz V, Koeplinger KA, Vavrek M, and Kong A-NT 2008. Liquid microjunction surface sampling probe electrospray mass spectrometry for detection of drugs and metabolites in thin tissue sections. Journal of Mass Spectrometry. 43:500–508. [DOI] [PubMed] [Google Scholar]
- Vandergrift GW, Hessels AJ, Palaty J, Krogh ET, and Gill CG 2018. Paper spray mass spectrometry for the direct, semi-quantitative measurement of fentanyl and norfentanyl in complex matrices. Clinical Biochemistry. 54:106–111. [DOI] [PubMed] [Google Scholar]
- Vega C, Spence C, Zhang C, Bills BJ, and Manicke NE 2016. Ionization Suppression and Recovery in Direct Biofluid Analysis Using Paper Spray Mass Spectrometry. J Am Soc Mass Spectrom. 27:726–734. [DOI] [PubMed] [Google Scholar]
- Verhaeghe T, Dillen L, Stieltjes H, Zwart L. de, Feyen B, Diels L, Vroman A, and Timmerman P 2017. The application of capillary microsampling in GLP toxicology studies. Bioanalysis. 9:531–540. [DOI] [PubMed] [Google Scholar]
- Vu DH, Bolhuis MS, Koster RA, Greijdanus B, Lange W.C.M. de, Altena, van R, Brouwers JRBJ, Uges DRA, and Alffenaar JWC. 2012. Dried Blood Spot Analysis for Therapeutic Drug Monitoring of Linezolid in Patients with Multidrug-Resistant Tuberculosis. Antimicrobial Agents and Chemotherapy. 56:5758–5763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu DH, Koster RA, Alffenaar JWC, Brouwers JRBJ, and Uges DRA 2011. Determination of moxifloxacin in dried blood spots using LC–MS/MS and the impact of the hematocrit and blood volume. J Chromatogr B. 879:1063–1070. [DOI] [PubMed] [Google Scholar]
- Vu DH, Koster RA, Bolhuis MS, Greijdanus B, Altena RV, Nguyen DH, Brouwers JRBJ, Uges DRA, and Alffenaar JWC 2014. Simultaneous determination of rifampicin, clarithromycin and their metabolites in dried blood spots using LC–MS/MS. Talanta. 121:9–17. [DOI] [PubMed] [Google Scholar]
- Wagner M, Tonoli D, Varesio E, and Hopfgartner G 2016. The use of mass spectrometry to analyze dried blood spots. Mass Spectrom Rev. 35:361–438. [DOI] [PubMed] [Google Scholar]
- Wang C, Zhu H, Cai Z, Song F, Liu Z, and Liu S 2013a. Newborn screening of phenylketonuria using direct analysis in real time (DART) mass spectrometry. Analytical and Bioanalytical Chemistry. 405:3159–3164. [DOI] [PubMed] [Google Scholar]
- Wang H, Liu J, Cooks RG, and Ouyang Z 2010. Paper Spray for Direct Analysis of Complex Mixtures Using Mass Spectrometry. Angew Chem Int Ed. 122:889–892. [DOI] [PubMed] [Google Scholar]
- Wang H, Manicke NE, Yang Q, Zheng L, Shi R, Cooks RG, and Ouyang Z 2011. Direct Analysis of Biological Tissue by Paper Spray Mass Spectrometry. Analytical Chemistry. 83:1197–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Ren Y, McLuckey MN, Manicke NE, Park J, Zheng L, Shi R, Cooks RG, and Ouyang Z 2013b. Direct Quantitative Analysis of Nicotine Alkaloids from Biofluid Samples using Paper Spray Mass Spectrometry. Anal Chem. 85:11540–11544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Zheng Y, Zhang X, Han X, Wang T, and Zhang Z 2015. A silica coated paper substrate: development and its application in paper spray mass spectrometry for rapid analysis of pesticides in milk. Analyst. 140:8048–8056. [DOI] [PubMed] [Google Scholar]
- Wang T, Zheng Y, Wang X, Austin DE, and Zhang Z 2017a. Sub-ppt Mass Spectrometric Detection of Therapeutic Drugs in Complex Biological Matrixes Using Polystyrene-Microsphere-Coated Paper Spray. Anal Chem. 89:7988–7995. [DOI] [PubMed] [Google Scholar]
- Wang X, Chen Y, Zheng Y, and Zhang Z 2017b. Study of Adsorption and Desorption Performances of Zr-Based Metal–Organic Frameworks Using Paper Spray Mass Spectrometry. Materials. 10:769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zheng Y, Wang T, Xiong X, Fang X, and Zhang Z 2016. Metal–organic framework coated paper substrates for paper spray mass spectrometry. Anal Methods. 8:8004–8014. [Google Scholar]
- Wei S-C, Fan S, Lien C-W, Unnikrishnan B, Wang Y-S, Chu H-W, Huang C-C, Hsu P-H, and Chang H-T 2018. Graphene oxide membrane as an efficient extraction and ionization substrate for spray-mass spectrometric analysis of malachite green and its metabolite in fish samples. Analytica Chimica Acta. 1003:42–48. [DOI] [PubMed] [Google Scholar]
- White S, Hawthorne G, Dillen L, Spooner N, Woods K, Sangster T, Cobb Z, and Timmerman P 2014. EBF: reflection on bioanalytical assay requirements used to support liquid microsampling. Bioanalysis. 6:2581–2586. [DOI] [PubMed] [Google Scholar]
- Wilhelm AJ, Burger, den JCG, and Swart EL 2014. Therapeutic Drug Monitoring by Dried Blood Spot: Progress to Date and Future Directions. Clinical Pharmacokinetics. 53:961–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiseman JM, Evans CA, Bowen CL, and Kennedy JH 2010. Direct analysis of dried blood spots utilizing desorption electrospray ionization (DESI) mass spectrometry. The Analyst. 135:720. [DOI] [PubMed] [Google Scholar]
- Wiseman JM, and Kennedy JH 2014. Analysis of Dried Blood Spots Using DESI Mass Spectrometry In: Raftery D, ed. Mass Spectrometry in Metabolomics. New York, NY: Springer New York; pp. 291–297. [DOI] [PubMed] [Google Scholar]
- Wiseman JM, and Manicke NE 2014. New Mass Spec Method for Blood and Urine Screening: The Advantages of Paper Spray MS over Conventional MS Include No Sample Prep. Genet Eng Biotechnol N. 34:20–21. [Google Scholar]
- Xie I, Xu Y, Anderson M, Wang M, Xue L, Breidinger S, Goykhman D, Woolf EJ, and Bateman KP 2018. Extractability-mediated stability bias and hematocrit impact: High extraction recovery is critical to feasibility of volumetric adsorptive microsampling (VAMS) in regulated bioanalysis. Journal of Pharmaceutical and Biomedical Analysis. 156:58–66. [DOI] [PubMed] [Google Scholar]
- Yan X, Augusti R, Li X, and Cooks RG 2013. Chemical Reactivity Assessment Using Reactive Paper Spray Ionization Mass Spectrometry: The Katritzky Reaction. ChemPlusChem. 78:1142–1148. [DOI] [PubMed] [Google Scholar]
- Yang Q, Manicke NE, Wang H, Petucci C, Cooks RG, and Ouyang Z 2012a. Direct and quantitative analysis of underivatized acylcarnitines in serum and whole blood using paper spray mass spectrometry. Analytical and Bioanalytical Chemistry. 404:1389–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Wang H, Maas JD, Chappell WJ, Manicke NE, Cooks RG, and Ouyang Z 2012b. Paper spray ionization devices for direct, biomedical analysis using mass spectrometry. Int J Mass Spectrom. 312:201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Wu J, Deng J, Yuan K, Chen X, Liu N, Wang X, and Luan T 2018. Rapid and on-site analysis of amphetamine-type illicit drugs in whole blood and raw urine by slug-flow microextraction coupled with paper spray mass spectrometry. Analytica Chimica Acta. 1032:75–82. [DOI] [PubMed] [Google Scholar]
- Yannell KE, Kesely KR, Chien HD, Kissinger CB, and Cooks RG 2017. Comparison of paper spray mass spectrometry analysis of dried blood spots from devices used for in-field collection of clinical samples. Analytical and Bioanalytical Chemistry. 409:121–131. [DOI] [PubMed] [Google Scholar]
- Ye Z, and Gao H 2017. Evaluation of sample extraction methods for minimizing hematocrit effect on whole blood analysis with volumetric absorptive microsampling. Bioanalysis. 9:349–357. [DOI] [PubMed] [Google Scholar]
- Youhnovski N, Bergeron A, Furtado M, and Garofolo F 2011. Pre-cut dried blood spot (PCDBS): an alternative to dried blood spot (DBS) technique to overcome hematocrit impact: Pre-cut dried blood spot: an alternative to dried blood spot. Rapid Commun Mass Spectrom. 25:2951–2958. [DOI] [PubMed] [Google Scholar]
- Youhnovski N, Mayrand-Provencher L, Bérubé E-R, Plomley J, Montpetit H, Furtado M, and Keyhani A 2017. Volumetric absorptive microsampling combined with impact-assisted extraction for hematocrit effect free assays. Bioanalysis. 9:1761–1769. [DOI] [PubMed] [Google Scholar]
- Yu M, Wen R, Jiang L, Huang S, Fang Z, Chen B, and Wang L 2018. Rapid analysis of benzoic acid and vitamin C in beverages by paper spray mass spectrometry. Food Chemistry. 268:411–415. [DOI] [PubMed] [Google Scholar]
- Zargar T, Khayamian T, and Jafari MT 2018. Aptamer-modified carbon nanomaterial based sorption coupled to paper spray ion mobility spectrometry for highly sensitive and selective determination of methamphetamine. Microchimica Acta. 185:103. [DOI] [PubMed] [Google Scholar]
- Zhang C, Bills BJ, and Manicke NE 2017a. Rapid prototyping using 3D printing in bioanalytical research. Bioanalysis. 9:329–331. [DOI] [PubMed] [Google Scholar]
- Zhang C, Glaros T, and Manicke NE 2017b. Targeted Protein Detection Using an All-in-One Mass Spectrometry Cartridge. J Am Chem Soc. 139:10996–10999. [DOI] [PubMed] [Google Scholar]
- Zhang C, and Manicke NE 2015. Development of a Paper Spray Mass Spectrometry Cartridge with Integrated Solid Phase Extraction for Bioanalysis. Anal Chem. 87:6212–6219. [DOI] [PubMed] [Google Scholar]
- Zhang JI, Li X, Ouyang Z, and Cooks RG 2012a. Direct analysis of steviol glycosides from Stevia leaves by ambient ionization mass spectrometry performed on whole leaves. The Analyst. 137:3091. [DOI] [PubMed] [Google Scholar]
- Zhang M, Liu H, Huang X, Shao L, Xie X, Wang F, Yang J, Pei P, Zhang Z, Zhai Y, et al. 2019. A novel LC-MS/MS assay for vitamin B1, B2 and B6 determination in dried blood spots and its application in children. Journal of Chromatography B. 1112:33–40. [DOI] [PubMed] [Google Scholar]
- Zhang N, Li Y, Zhou Y, Hou J, He Q, Hu X-G, Jia Y-M, Yu C-Y, and Nie Z 2013. Rapid detection of polyhydroxylated alkaloids in mulberry using leaf spray mass spectrometry. Analytical Methods. 5:2455. [Google Scholar]
- Zhang Y, Ju Y, Huang C, and Wysocki VH 2014. Paper Spray Ionization of Noncovalent Protein Complexes. Analytical Chemistry. 86:1342–1346. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Cooks RG, and Ouyang Z 2012b. Paper spray: a simple and efficient means of analysis of different contaminants in foodstuffs. Analyst. 137:2556–2258. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Xu W, Manicke NE, Cooks RG, and Ouyang Z 2012c. Silica Coated Paper Substrate for Paper-Spray Analysis of Therapeutic Drugs in Dried Blood Spots. Anal Chem. 84:931–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Wei Z, Zhao H, Jia J, Chen Z, Zhang S, Ouyang Z, Ma X, and Zhang X 2016. In Situ Ion-Transmission Mass Spectrometry for Paper-Based Analytical Devices. Analytical Chemistry. 88:10805–10810. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Wang Q, Wang X, Chen Y, Wang X, Zhang X, Bai Z, Han X, and Zhang Z 2016a. Development and Application of Zirconia Coated Paper Substrate for High Sensitivity Analysis of Therapeutic Drugs in Dried Blood Spots. Analytical Chemistry. 88:7005–7013. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Zhang X, Bai Z, and Zhang Z 2016b. Characterization of the surface properties of MgO using paper spray mass spectrometry: Characterization of the surface properties of MgO using paper spray mass spectrometry. Rapid Commun Mass Spectrom. 30:217–225. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Zhang X, Yang H, Liu X, Zhang X, Wang Q, and Zhang Z 2015. Facile preparation of paper substrates coated with different materials and their applications in paper spray mass spectrometry. Anal Methods. 7:5381–5386. [Google Scholar]
- Zhou X, Pei J, and Huang G 2015. Reactive paper spray mass spectrometry for in situ identification of quinones: Reactive paper spray mass spectrometry. Rapid Communications in Mass Spectrometry. 29:100–106. [DOI] [PubMed] [Google Scholar]
- Zhu H, and Huang G 2018. High-throughput Paper Spray Mass Spectrometry via Induced Voltage. Rapid Communications in Mass Spectrometry. [DOI] [PubMed] [Google Scholar]
- Zimmermann E 1939. The Dried Blood Test for Syphilis. Munchener Medizinische Wochenschrift. 86:1732–1733. [Google Scholar]
- Zytkovicz TH, Fitzgerald EF, Marsden D, Larson CA, Shih VE, Johnson DM, Strauss AW, Comeau AM, Eaton RB, and Grady GF 2001. Tandem Mass Spectrometric Analysis for Amino, Organic, and Fatty Acid Disorders in Newborn Dried Blood Spots. Clinical Chemistry. 47:1945–1955. [PubMed] [Google Scholar]
- 2018. Guideline on Bioanalytical Method Validation. London: European Medicines Agency; (EMEA/CHMP/EWP/192217/2009). [DOI] [PubMed] [Google Scholar]